A Festschrift in Honor of Edward M. Messing, MD, FACS
Excerpt from “Legends of Urology” recently published in the Canadian Journal of Urology.1

Edward M. Messing, MD, FACS
We thank the Editors of the journal for their kind permission to reproduce it here.
I am honored to be asked to write an article for The Canadian Journal of Urology about a Legend in Urology, but a bit intimidated about writing about myself. I was born in Brooklyn, NY in 1947 and lived there until I left for college shortly before my 18th birthday. My father was a dentist and my mother stayed at home raising my older brother and myself. I had a very warm and supportive upbringing, with a large extended family and frequent family gatherings. Being a true baby boomer, it is not surprising that I rarely wanted for companionship. There were always relatives or friends available for activities.
I am a product of the New York City public school system, with even my elementary school having 1,000 students and my high school almost 6 times as many. Classrooms were crowded, and occasionally students had to share a desk. Education was not exactly individualized, but like most of my fellow students, I learned how to “survive” in a crowd without withdrawing into anonymity. However this type of school experience also had its limitations, and while I enjoyed most classes, it wasn’t until I left for college at the University of Chicago (UC) that I realized how much pleasure I had in studying almost any subject.
UC was a serious institution with many more graduate, postdoctoral and professional students than undergrads, but professors were inspiring and seemed to take personal interest in every student. This may be a naïve impression, but it has stayed with me. Of course the mid-1960s was also a time of cultural and political change. This permeated UC, and I certainly participated in what might be considered “counter-culture” activities. But most of all, I loved studying almost any field. Debates about philosophers (a friend earnestly once told me that “Kierkegaard got down to the real ‘nitty-gritty”’), architecture’s influence on society, and James Madison‘s view on government and society in the Federalist Papers were not only assigned topics for reading or for essays, but were the subjects of late evening conversations.
At UC, I was also exposed to research. I spent time with a Botany professor, Manfred Rudat, defining one of the steps in the endogenous synthesis of a plant growth hormone, gibberellic acid. The opportunity for discovery, even if it was a very small step, made it clear to me that I wanted to do molecular research, and both Professor Rudat and other advisors convinced me that I could do that with a medical career and always have a profession to fall back on if research progress slowed. For a variety of personal reasons, I limited my medical school applications to New York City schools and chose (and was chosen by) New York University (NYU).
Early in my clinical years (M-3) I realized that I really loved surgery – but I also greatly enjoyed patient care and having long-term relationships with patients. These factors, and a wonderful experience on my Urology rotation in my senior year, led me to choose Urology as a career. I did my first two years of general surgery training at NYU/Bellevue Hospital – but by then I had already decided to enter Urology and had applied to, and been accepted by Stanford University for residency.
The 4 years in Tom Stamey’s program at Stanford were wonderful. It was not only the clinical and surgical experiences I had, but also trying to understand how someone as creative as Dr. Stamey viewed things. The great joy he took in patient care and detailed clinical studies was contagious. After a few brief case reports, my first longer article was written with Dr. Stamey on interstitial cystitis (IC) (an entity I still know little about). We described the large bladder capacity form of this condition, which accounts for the overwhelming majority of patients with IC, and reported at least some success using a series of intravesical clorpactin instillations (which Dr. Stamey had heard about from a colleague in the United Kingdom) as treatment. Success was modest, but long-term remissions did occur – and we still have limited information about the pathogenesis of this entity or mechanisms of action of clorpactin.
Upon finishing residency, I had a two-year commitment in the Public Health Service (PHS) – which I spent as Chief of Urology at the New Orleans PHS Hospital and as an adjunct faculty member at Tulane University. Both Tulane and the city were very welcoming. My wife, Susan and I love rock ‘n’ roll music and jazz – and of course there’s no place superior to New Orleans for this type of music or for its very unique food.
While in New Orleans, I also met Jean DeKernion, a native Louisianan– during his brief tenure on the Tulane faculty. By then I knew I wanted to have an academic career, and it was Jean who both inspired and convinced me that it should be in Urologic Oncology. Although at that time even in the strongest academic programs, the subspecialization we have today was uncommon (most urologic oncologists treated all cancers), it was obvious that large volumes of patients were required to carry out clinical and translational research. Both of us agreed I also needed fellowship training – mostly to develop research skills, so I joined him at UCLA when my two years in the PHS were completed.
At UCLA, although I was welcomed by the entire department, Dr. DeKernion clearly was my mentor. Very quickly after arriving he had me meet John Fahey, a world renowned immunologist. Dr. Fahey had developed a clinical immunology and immuno-oncology program, which had numerous fellows, and post-doctoral and graduate students taking classes and discussing research projects together. Kohler’s and Milstein’s paper on “hybridomas” – the cells needed to make monoclonal antibodies, had only recently been published, and it was considered a project worthy of NIH funding to generate monoclonal antibodies to tumor associated antigens expressed by specific cancers (with the idea of improving tumor diagnosis, classification, staging, and therapy). Indeed with close mentoring from Drs. Fahey and DeKernion, I generated a few monoclonal antibodies to antigens on human bladder cancer cell lines, learned a host of immunological, molecular and histologic techniques, and wrote and got funded an R01 grant on this topic (appropriately awarded to Drs. Fahey and DeKernion). Moreover, I took a variety of graduate courses, and by the time I was leaving, had redirected my interest to growth factors in cancer. Professor Harvey Hershman’s lab welcomed me to work in this area and I gained enough experience to be able to direct technicians and present at scientific conferences.
After completing two years of fellowship, I finally got my first “real” job, on the faculty of the Urology Division at the University of Wisconsin. I quickly became a busy clinician, but also had my own lab – provided by Paul Carbone, head of the University’s Cancer Center. Additionally, Wisconsin was the epicenter of the Eastern Cooperative Oncology Group (ECOG), also led by Dr. Carbone, who quickly ushered me into the group. In ECOG I met and worked with real stars in Urologic Oncology such as Paul Lange and Dick Williams in Urology, and Larry Einhorn, Pat Loehrer, Donald “Skip” Trump and later George Wilding in Medical Oncology.
Three years after coming to Wisconsin and joining ECOG, I was given the opportunity to write a randomized phase III clinical trial, EST 3886. This study was designed to challenge or confirm the controversial policy (championed by the Mayo Clinic) of starting androgen deprivation therapy (ADT) immediately on men who underwent radical prostatectomy (RP) and had positive lymph nodes, rather than withholding ADT until distant metastases developed (the more widely accepted approach, which was based on the findings of VA studies conducted 15–20 years earlier which indicated that while ADT delayed disease progression, it did not prolong survival). While EST 3886 accrued slowly, survival of men with positive nodes receiving immediate ADT following RP, was far superior to that of men who had ADT withheld until distant metastases developed. The study was published in the New England Journal of Medicine, and while initially controversial, has been incorporated into standard care.
My UCLA growth factor mentor, Harvey Hirschman, had taught me that the best experiment would answer a specific scientific question “yes or no” – and such is the case for randomized prospective clinical trials. I also learned that randomized phase III clinical trials in surgery with an oncologic endpoint (often survival) take a LONG time to conduct and complete. EST 3886 was approved by the NCI in 1986 and was not completed and published until 1999 (with a later follow up in 2006).
During this time, I maintained a very large surgical practice and continued lab work. I was fortunate enough to get NIH grants including a Program Project (P0-1) in selected methods in human bladder carcinogenesis. I frequently collaborated with Cathy Reznikoff, a cell biologist at Wisconsin who developed an in vitro carcinogenesis model using her immortalized “normal” human urothelial cell line, SV-HUC – which is still widely used as non-transformed human urothelial cells in many in vitro studies.
I also became convinced that early detection of bladder cancer, by detecting tumors destined to become muscle invading before they had actually done so, would reduce mortality and morbidity from this disease. I carried out large screening studies using home testing with chemical reagent strips for hematuria which strongly supported this hypothesis; but could never secure funding (or convince the NCI) to support a randomized prospective trial, which would be needed to confirm it.
By this time (1995) I had been at Wisconsin for 13 years and felt if I was to become a chairman – now was the time. The opportunity to replace Abraham Cockett at the University of Rochester was coming up (Dr. Cockett had just finished his term as president of the AUA) and I applied for and received the position, assuming the Chairmanship in mid-1995.
My 22½ years as chairman at Rochester has been a continuous learning experience. Our department has grown from 4 full-time adult urologists (including myself), and 2 pediatric urologists to 14 and 3, respectively. We’ve gone from no basic science researchers to 3, and under my successor Jean Joseph’s guidance, growth is continuing in several subspecialties. We now cover 4 hospitals “exclusively” and “share” 2 others, while we only worked in 1 when I arrived. While I no longer do hands on lab work, I collaborate with those who do (Yi Fen Lee, Chawnshang Chang, ShuYuan Yeh, and Hiroshi Miyamoto) on projects related to renal, bladder and prostate cancer.
About 7 years after coming to Rochester, our institution left ECOG and joined the Southwest Oncology Group (SWOG). I was immediately welcomed by the leadership of the SWOG GU Cancer Committee, Dave Crawford and later Ian Thompson (Urology), Nick Vogelzang (Medical Oncology), and Cathy Tangen (Statistics) and was asked to become co-chair of the Renal Cancer subcommittee, a position I continue to hold. Also, I was given the opportunity to design and conduct S 0337, a randomized prospective clinical trial testing immediate post TURBT intravesical gemcitabine versus saline for suspected low-grade urothelial cancer. The impetus for this study was that while a single immediate post TURBT instillation of a variety of chemotherapy agents has been repeatedly shown to be effective, and is part of both AUA and EAU guidelines, very few urologists perform it. This study was strongly positive for patients receiving gemcitabine and has recently been published in the Journal of the American Medical Association (JAMA). We are now conducting molecular studies to predict response to gemcitabine, performing a formal cost analysis, and are exploring ways to increase usage of this treatment throughout the country. Because clinical and translational research not only requires insight, but also large clinical volume, I continue to be active in the clinic and the operating room. I also continue to teach residents, medical students and graduate students.
Of course, none of this would have been possible without the enormous love and support of my wife, Susan and our two sons, Ross and James. Their understanding and extreme patience with my activities has been instrumental in my career.
I recently had the great fortune of being named the recipient of the Ramon Guiteras Award by the AUA, which by its own description is its highest honor. At the awards banquet, I said something that I believe deeply, that “we are truly privileged to be able to do things we love to do: teaching, patient care, surgery and clinical and translational research”. As I have throughout my career I intend to do what I advise my residents and younger colleagues: keep asking questions, and utilize the resources and infrastructure available to work with your colleagues to best answer those questions.
Edward M. Messing, MD, FACS
Professor of Urology
Professor of Oncology and Pathology
University of Rochester School of Medicine and Dentistry
Rochester, NY, USA
Foreword: Festschrift to Honor Edward M. Messing, MD, FACS
Jean Joseph, MD, MBA, FACS
Dr. Edward M. Messing has been a national and international leader in urologic oncology. His contributions as a physician, scientist, and mentor are most inspiring. It is fitting to celebrate his accomplishments in a Festschrift. In the pages that follow, a number of his colleagues pay tribute to his achievements, adding to the discussion on a number of topics related to urologic oncology, where Dr. Messing has certainly made his mark. Our gratitude to the editors of the “Bladder Cancer” journal for the opportunity to further commemorate the celebration of Dr. Messing’s outstanding career.
Dr. Messing arrived in Rochester in 1995 and served as the W.W. Scott Professor and Chair of the Department of Urology until 1997. He succeeded Abraham Cockett, who had just completed his term as President of the American Urological Association. He collaborated with Dr. Irwin Frank, a former Department Chair who later also served as President of the American Urological Association, along with other faculty members in the Department and the Cancer Center. As a resident in the program at the time, I recall the enthusiasm with which the faculty and trainees awaited Dr. Messing’s arrival. He was already a household name in urology, with his numerous research contributions.
Dr. Messing brought his energy and passion to further the multiple missions of the Medical Center and University. He served as Deputy Director of the Cancer Center, among many other leadership and administrative roles. He steadily increased the department’s activities in the region, providing urological care to the Greater Rochester Area and beyond. He also focused on expanding the department’s research infrastructure, recruiting world renown basic science researchers to help him address questions underpinning basic mechanisms of urologic cancers. His commitment to translational research has remained steadfast. Since we began planning the celebration of his career earlier this year, he has contributed another landmark article in the Journal of the American Medical Association (JAMA) where he showed the value of gemcitabine instillation after transurethral bladder tumor resection. He has published countless articles related to diagnostics and therapeutics, improving our deliverables as we seek to curb the emotional burden faced by patients and their families, and the economic impact on the health care system.
Dr. Messing has had an impact on urology regionally, nationally, and internationally. While this piece and others serve to introduce Dr. Messing, along with the celebrants whose presentations are summarized in the following pages, it does not serve justice to all of Messing’s contributions. Many with whom Dr. Messing has worked closely with over the years are not able to participate in the celebrations. It is also impossible to cover the various arenas in which he has excelled. Those who attended certainly heard the testaments from his colleagues, and mentees. His scientific publications exceed 300 articles, and dozens of book chapters. He has published extensively in urology related journals, and several high impact journals. His article published in the New England Journal of Medicine, assessing the impact of hormone therapy in patients with prostate cancer and nodal metastases, remains one of the most quoted urological articles.
Dr. Messing has contributed in every way possible as an academic urologist. He has been a major force influencing urology for over 4 decades. Whether through his various leadership roles, his publications, his work as a reviewer, his participation at study sections, or meetings, he is always present asking the most insightful questions benefiting both patients and urologists alike. Whether in the research or in the conference settings, he has provided even more answers. His work has added to guidelines, which have become standard of care. He is always available to colleagues and mentees near and far to help them solve complex clinical or research scenarios.
At the 2018 annual meeting of the American Urological Association, the association gave Messing its highest honor/most prestigious recognition, the Ramon Guiteras Award. He was recognized for his “outstanding contributions to the art and science of urology, specifically urologic oncology”. Those of us who have been privileged to work with him certainly know how deserving he is of such a recognition. Dr. Messing’s motivation, determination, and caring are certainly qualities we all seek to emulate. Few have achieved the level of accomplishments that he has. As he transitions to another phase in his splendid career, we look forward to his contributions and to share in his encyclopedic knowledge. He will continue to have an impact on the careers of many. Indubitably, Dr. Messing has had a remarkable career, well deserving of this international Festschrift celebration. Our thanks to Kim Ferrari, who has been Messing’s assistant since his arrival to Rochester, for her organizational skills, attending to every detail related to this successful Festschrift.
Festschrift Presentations
Personal Testimonies
Seymour Schwartz, MD
In 1994, the search for a new Chair of the Department of Urology was initiated by the Dean Marshall Lichtman. A committee was formed to seek out potential candidates and establish a list of preferences from which the Dean would make a selection. The Chair of the Urology Department, which had become an autonomous department in 1969 at the University of Rochester Medical Center, was a most attractive position - two members of the department had served as President of the American Urological Association and the gift of a generous endowment by a grateful patient to the Department in the 1960’s, was rivaled by few urology departments.
At the top of the list, which the committee presented to the Dean was an individual whose academic record was unrivaled: B.A. in Biology from the University of Chicago with election to Phi Beta Kappa, an MD from New York University School of Medicine with election to Alpha Omega Alpha, Residency in Urology at Stanford University, and a 2 year fellowship in Tumor Immunology and urologic Oncology.
In just 12 years after accepting his first faculty appointment in the Department of Urology at the University of Wisconsin – Madison, he had risen to Professor of Surgery and Chief of Urologic Oncology.
The committee indicated that among potential candidates, none could match Dr. Edward Messing regarding past productivity and potential for enhancing the excellence of the Department of Urology.
Dean Lichtman selected Dr. Messing and he accepted the position of Chairman of the Department of Urology in September 1995. Over the past 22 years, the academic visibility of the Department of Urology has intensified. Over 200 articles, authored or co-authored by Dr. Messing have appeared in peer-reviewed journals during those 22 years. Among the earliest of those publications is: “Chemoprevention of Bladder and Prostate Carcinoma” by Dr. Jean Joseph (the Department’s current Chair) and Dr. Messing – and so, the baton of leadership would be passed!
In 2018, the American Urological Association, for the first time, presented to a University of Rochester urologist the Ramon Guiteras Award. Edward Messing received the award, which is considered the American Urological Association’s most prestigious honor.
Kathy Rideout, EdD, PPCNP-BC, FNAP
I want to thank Dr. Joseph for allowing me to share my personal thoughts about Dr. Messing today. I have been relatively private about my health issues over the years but it was important for me to share publicly about the care that Dr. Messing has provided me for which I am eternally grateful.
Approximately 14 years ago, I developed significant urinary symptoms that necessitated a referral for a pelvic ultrasound. I will never forget leaving the imaging center where I was just told about this tumor in my bladder and the need to follow up soon with an urologist. Since I was a nurse practitioner at Strong Memorial Hospital, I knew to call the Strong Urology department – I called from the parking lot in tears. When I was told that I couldn’t be seen for a few weeks – I started to sob, telling the receptionist that I was a mother of 3 young children, (who at the time were 11, 12, and 13), I was panicked and I needed to see someone sooner than a few weeks. She put me on hold – and came back and said, Dr. Messing will see you tomorrow at 5:00 pm (which happened to be the Friday of the 4th of July holiday weekend).
Over the next 24 hours, I came to learn that Dr. Messing was the Chair of the Urology Department and was internationally renowned for his research on bladder cancer. My husband and I felt very blessed that this is who agreed to see me on such short notice.
Our first meeting with him was very matter of fact – where he confirmed that I most likely had bladder cancer – but that he would schedule my surgery the following week after I completed some additional pre-surgical testing. There was a kindness and a confidence I saw in his eyes that day – that I knew I would be ok.
So began our relationship! Over these 14 years, I have seen Dr. Messing from every 3 months to annually. The nursing staff that works with him have remained relatively unchanged throughout this time – which is a testament unto itself! I have had some re-occurrences and I have participated in a few of his research studies. With any setback or success – I knew I was in the best hands! He has called me at home in the evening to share with me test results or to check on how I am doing. He has calmed my fears and worries with his honesty but also with a strong assurance.
The amazing care that he has provided me from the beginning is not because I was an employee who became a senior leader at the UR School of Nursing – it’s because this is the care he provides everyone. He has been my lifesaver in more ways than one. My children are now grown, I continue to experience good health and I will be forever indebted to him!
Thank you Dr. Messing – from the bottom of my heart!
Session I: Diagnosis and Treatment of Bladder Cancer
Session Moderator: Ralph Brasacchio, MD
Systemic Treatments for Metastatic Urothelial Carcinoma
Kerry Schaffer, MD, Chunkit Fung, MD MSCE
Background
Among patients diagnosed with urothelial carcinoma (UC) of the bladder, approximately 4 percent present with metastatic disease [1]. For patients initially diagnosed with localized muscle invasive UC after definitive curative treatment, 22%, 24%, 39%, and 55% with T2, T3a, T3b, and T4 eventually recur with metastatic disease in 10 years, respectively [2]. During the past few years, the emergence of immune checkpoint inhibitors in addition to platinum-based chemotherapy has revolutionized the treatment landscape of UC.
First-line Systemic Therapy
Cisplatin-based chemotherapy is the standard first-line treatment for metastatic UC. A phase III trial of 455 patients with locally advanced or metastatic UC compared first-line chemotherapy with gemcitabine and cisplatin with methotrexate, vincristine, doxorubicin, and cisplatin (MVAC) and showed similar overall survival for both arms (hazard ratio [HR] 1.04; 95% CI 0.82–1.32, P = 0.75) [3]. However, gemcitabine and cisplatin had a superior toxicity profile than MVAC requiring fewer dose modifications as MVAC caused more grade 3 neutropenia (82% vs 71%) and mucositis (22% vs 1%).
For patients who are ineligible to receive cisplatin-based chemotherapy for metastatic UC (i.e. ECOG performance status >2, creatinine clearance <60 ml/min, grade >2 neuropathy or hearing loss, and class III heart failure), [4] treatment options include immune checkpoint inhibitors with pembrolizumab [5] and atezolizumab, [6] as well as systemic chemotherapy with gemcitabine and carboplatin [7]. The phase II trials of pembrolizumab [5] and atezolizumab [6] demonstrated an objective response rate (ORR) of approximately 23% to 24% in the first-line treatment of advanced or metastatic UC. The median duration of disease response was not reached at the time of data collection, reflecting a sustained disease response in the majority of responders (>70%) [5, 6]. The phase II/III EORTC trial 238 compared gemcitabine and carboplatin with methotrexate, carboplatin, and vinblastine for chemotherapy naïve patients with metastatic UC and showed similar median overall survival of 9.3 vs 8.1 months (P = 0.64) but less grade 3/4 toxicity with gemcitabine and carboplatin [7].
Recently, the FDA issued a safety warning against the use of first-line single-agent immune checkpoint inhibitors for patients with programmed death-ligand (PD-L)-1–low expressing cisplatin-eligible UC, following a demonstration of lower overall survival with pembrolizumab and atezolizumab compared with cisplatin-based chemotherapy [8].
Second-line Systemic Therapy
Second-line treatments for advanced and metastatic UC include two PD1 inhibitors (i.e. nivolumab [9] and pembrolizumab [10]) and three PD-L-1 inhibitors (i.e. durvalumab, [11] atezolizumab, [12] and avelumab [13]). These immune checkpoint inhibitors demonstrated an ORR of 14.8% to 24.4% with a complete disease response rate ranging from 2% to 11.4% [9–13]. Systemic chemotherapy now has a more limited role in the second-line setting for treatment of advanced UC and include regimens containing docetaxel, paclitaxel, pemetrexed, vinflunine, or gemcitabine.
Future Directions
Ongoing trials are evaluating novel targeted agents and combinations of immune checkpoint inhibitors with chemotherapy for treatment of advanced UC. Two novel treatments recently showed promising preliminary results in this disease setting, including enfortumab vedotin (EV) and fibroblast growth factor receptor (FGFR) inhibitor. EV is an antibody-drug conjugate which delivers microtubule disrupting agents to tumors that express Nectin-4 protein. A phase I trial of EV for patients who received at least one prior line of therapy for UC showed an ORR of 41% with a progression free survival of 23.1 weeks and a median overall survival of 12.5 months [14]. Similarly, a phase II study of an FGFR inhibitor (i.e. erdafitinib) demonstrated an ORR of 35% [15].
Non-Muscle Invasive Bladder Cancer: Paradox to Paradigm
Jay E. Reeder, PhD
Approximately 25% of bladder cancer patients present with muscle invasive or more advanced disease requiring definitive surgical, chemotherapy, or radiation treatment. Fortunately, for most individuals who experience bladder cancer the initial tumor or tumors will be confined to the urothelium and not invade into the detrusor muscle of the bladder. Non-muscle invasive bladder cancer (NMIBC) is initially treated by trans-urethral resection of bladder tumor (TURBT). This is not a cure; all patients are at risk of developing additional bladder tumors. Recurrent tumors may be NMIBC or may be invasive and life-threatening. Because of these risks the current standard of care is for periodic cystoscopies to check for new tumors. Monitoring may be augmented by cytological and molecular evaluation.
I have had the pleasure of working with Edward Messing since 1995 on various projects to better understand the biology behind recurrent bladder cancer and the development of detection techniques and potential therapeutic strategies to reduce the emotional, medical, and financial burdens of bladder cancer. The studies relied on a concerted effort by Dr. Messing, Irwin Frank, Abraham Cockett, and other members of the Department of Urology to diligently provide research specimens from bladder cancer patients seen in their clinics and operating rooms. Over 4,000 specimens were collected, analyzed and banked as part of these NIH funded studies.
Our early studies were focused on flow and image cytometry of exfoliated cells in urine and irrigation specimens and demonstrated that measurement of cellular DNA was a sensitive and specific assay to assess proliferation and aneuploidy in patients with bladder tumors. When fluorescence in situ hybridization technology became available, we demonstrated that it could be used to assay for the most common chromosomal aberration in bladder cancer, the loss of chromosome 9. When the CDKN2A locus was mapped to chromosome 9p21, we developed a FISH probe and showed that loss of this locus was predictive of bladder cancer recurrence in patients with no cystoscopic evidence of tumors.
Several paradoxes were revealed by analysis of multiple specimens over time from single patients. The usual assumption that cancer will accumulate genetic aberrations over time leading to ever more aggressive disease was not uniformly observed. DNA aneuploidy might be observed in an initial tumor, followed by a near normal diploid tumor. Patients had chromosome 9 monosomy followed by or preceded by tumors with chromosome 9 trisomy, suggesting cellular and nuclear fusion events. Patients were just as likely to regress in grade and stage as they were to progress. These data and the multichronotopic nature of bladder cancer recurrence support the paradigm that bladder cancer is both a monoclonal and polyclonal disease. Certainly fast growing and aggressive tumors display a linear clonal evolution, while low grade NMIBC cancers can be resected but may recur by seeding into new locations in the bladder. If resection is successful, other initiated cell populations may still exist in the urothelium and by nature of very low levels of DNA replication be insensitive to intravesical therapies that target DNA replication and DNA damage and repair.
The recently completed Southwest Oncology Group study “A Phase III Blinded Study of Immediate Post-TURBT Instillation of Gemcitabine Versus Saline in Patients with Newly Diagnosed or Occasionally Recurring Grade I/II Superficial Bladder Cancer” led by Dr. Messing and published in the Journal of the American Medical Association showed that a single intravesical instillation of gemcitabine following resection of NMIBC tumors reduced recurrence [16]. Dr. Messing and colleagues had the foresight to incorporate into the study design a plan to investigate basic cancer biology questions raised by our earlier studies and to develop predictors of response to the treatment. Banked specimens include initial and recurrent tumors and reference DNA from peripheral blood. These specimens will be used to evaluate the impact of gemcitabine on clonal and non-clonal recurrence and develop predictors of response to gemcitabine.
Radiation Therapy in the Management of Locally Advanced Bladder Cancer
Kevin Bylund, MD
Around 30% of patients diagnosed with Bladder Cancer present with muscle invasive or locally advanced disease (T2-T4). For these patients, radical cystectomy with neoadjuvant cisplatin-based chemotherapy remains the standard treatment.
However, with the median age at diagnosis at 73 years old and the fact that many patients are smokers and have other comorbidities, many patients are not candidates for radical cystectomy, and others have a strong wish to preserve their bladder. Particularly as patients age, radical cystectomy becomes more of a risk, with SEER data showing perioperative mortality at 5% for septuagenarians, and 9% for octogenarians [17]. Unfortunately, population based studies have found that over 25% of patients diagnosed with muscle invasive bladder cancer do not receive curative intent treatment [18].
For these patients, radiation therapy given over 5–7 weeks of treatment, particularly in combination with chemotherapy and transurethral resection of the bladder tumor (TURBT), also known as trimodality therapy (TMT) provides an attractive alternative. Although there have been no randomized trials comparing radical cystectomy to TMT, a large meta-analysis of over 9500 patients [19] did not show a difference in OS, DSS, or PFS at 5 or 10 years between cystectomy and TMT.
Good candidates for TMT are patients with urothelial histology, a unifocal tumor <5 cm in diameter without extensive CIS or macroscopic extravesicular disease, maximal TURBT before treatment, no tumor associated hydronephrosis, and adequate renal and bladder function. In addition, recent molecular studies have found that high MRE11 expression (a gene involved in double strand break repair) predicted improved outcomes with radiation therapy as compared to cystectomy [20].
TMT is a partnership between the disciplines of surgery, chemotherapy, and radiation therapy. A visibly complete TURBT is ideal, emphasized by a retrospective study showing 5 year OS improving from 43% to 57%, and 5 year DSS improving from 56% to 68% when a visibly complete TURBT was performed [21]. Patients undergoing TMT also require lifetime surveillance cystoscopies, as invasive or non-invasive recurrences are common, up to 50%. The importance of concurrent chemotherapy with surgery was emphasized in the randomized BC2001 trial, [22] with an improvement in 5 year overall survival from 35% to 48% when chemotherapy was added to radiation.
There is a low incidence of late side effects after radiation therapy for bladder cancer. Two large single institution studies [23, 24] show late Grade 3–4 GI toxicity at 2–7%, and late Grade 3–4 GU toxicity at 2–6%, with no grade 5 toxicity. In another study [25], 75% of patients were found to have normal functioning bladders by urodynamics studies after radiation therapy. Over 70% of survivors are able to keep their bladder long term after TMT.
Radiation therapy, particularly as part of TMT, is a valuable tool in the management of locally advanced bladder cancer.
Recent Advances in the Management of Metastatic Bladder Cancer
Deepak Sahasrabudhe, MD
Bladder cancer (BC) is the fourth most commonly diagnosed cancer in men. In 2018, it is estimated that there will be 81190 new cases (62380 men and 18810 women) and 17240 deaths (12520 men and 4720 women). The diagnosis of BC is associated with median loss of life expectancy of 2.7 years for men and 4.1 years for women [26].
Immunotherapy with intravesical instillation of BCG lowers the recurrence rate of non-muscle invasive bladder cancer (NMIBC). BCG is superior to the instillation of mitomycin C in lowering the hazard rate of first recurrence [27]. In 1985, Sternberg et al. reported that combination chemotherapy with MVAC yielded an overall response rate of 71% and CR rate of 50% in 25 patients with metastatic disease [28]. MVAC was superior to single agent cisplatin [29]. Subsequently, von der Maase et al. showed that the combination of gemcitabine plus cisplatin was comparable to MVAC and was better tolerated [30]. It is now commonly prescribed as neoadjuvant chemotherapy for muscle-invasive bladder cancer (MIBC) and for metastatic BC.
There were no advances in the management of metastatic BC for the next thirty years until the demonstration of activity of checkpoint inhibitors in previously treated metastatic disease [31–33]. The high mutational burden in BC offers a biologic explanation for the observed antitumor activity. Since February 2017, five checkpoint inhibitors have been approved for metastatic BC. They include anti-PD1 antibodies pembrolizumab, nivolumab and durvalumab and anti-PDL1 antibodies atezolizumab and avelumab. FDA approvals for this class of agents include second-line therapy for patients with metastatic BC who experience disease progression during or after first-line platinum-containing chemotherapy or within twelve months of perioperative chemotherapy with a cisplatin-containing regimen (pembrolizumab, nivolumab, durvalumab, and avelumab). For these indications PD-L1 testing is not required. FDA approvals also include first-line therapy for patients with metastatic BC who are ineligible for platinum-containing chemotherapy (pembrolizumab and atezolizumab). For this indication, PD-L1 testing is required.
There are nearly 50 active or soon to enroll trials of checkpoint inhibitors in bladder cancer currently listed in clinicaltrials.gov. These trials are testing the efficacy of checkpoint inhibitors in novel spaces in the treatment of BC, such as maintenance therapy in patients with response to front line chemotherapy (NCT02500121) or in combination with chemotherapy in frontline treatment of metastatic disease (NCT02853305) and as neoadjuvant therapy (NCT02690558). Coming full circle, checkpoint inhibitors are being evaluated in NMIBC that is refractory to intravesical therapy (SWOG1605 NCT02844816).
In addition, clinical trials of antibody drug conjugates are underway in BC. For example, enfortumab, an antibody against nectin 4, conjugated to vedotin is being tested in in metastatic BC after progression or lack of response to checkpoint inhibitors (NCT03219333). Novel combinations such as B701, an antibody against FGFR3, are being tested in combination with pembrolizumab (NCT03123055). Vaccines are also being evaluated in BC. These include a peptide vaccine from the cancer testis antigen, NY-ESO-1, in combination with BCG and sargamostim (NCT00070070) and DNA vaccine INO-5401, which targets WT1, H-TERT, PSMA, combined with INO-9012, a synthetic plasmid that expresses IL12 (NCT03502785). The plethora of immunotherapy clinical trials promises to improve the outcomes in advanced/metastatic bladder cancer. The famous quote “The Future is Now” (George Allen, coach of the NFL Washington Redskins) seems appropriate.
Differential Roles of Estrogen and ER in Different Stages of Bladder Cancer
Shuyuan Yeh, PhD
Epidemiological studies showed that women have a lower bladder cancer (BCa) incidence, yet higher muscle-invasive rates than men, suggesting that estrogen and the estrogen receptors, estrogen receptor alpha (ERα) and beta (ERβ), could be involved and may play differential roles in different stages of BCa progression.
We first applied gene knockout strategy to delete ERα or ERβ genes in BBN-induced mouse BCa models. Data from the in vivo knockout mouse model support that ERα plays a protective role and ERβ plays a promoting role in the initiation of BCa. The underlying mechanism showed that ERα could function via modulating the INPP4B/PI3K pathway and ERβ could at least function via up-regulating MCM5 to affect the tumor initiation and growth. In addition to affecting the BCa initiation, both of our in vitro and in vivo strategies showed that ICI182,780/Faslodex could increase integrin-α5β1 expression and IL-6 release to promote the recruitment of monocytes/macrophages toward BCa cells, which consequently increased TNF-α release to potentiate the anti-BCa effects of BCG treatment. Recently, we further delineated the roles of ERs in the BCa tumor microenvironment by recruiting the tumor associated immune cells (including macrophage, mast, and T cells) to promote the invasion and metastasis of BCa.
Predictive Validity of Patient Specific Surgical Rehearsals for Complex Minimal Invasive Renal Cancer Surgery
Ahmed Ghazi MD, MSc, FEBU
Surgical Treatment of Localized Renal Cell Carcinoma
Renal cell carcinoma is projected to be associated with 63,990 newly diagnosed kidney cancer cases and 14,400 cancer-related deaths in 2017 in the United States [34]. Renal tumor surgery has evolved from open radical nephrectomy to nephron sparing surgery for clinically localized renal masses. The indications for partial nephrectomy have also expanded from individuals with imperative indications (e.g. solitary kidney, bilateral tumors or high renal insufficiency risk) to all individuals whenever technically feasible, independent of tumor size, [35, 36] given the implications of preexisting chronic renal disease in these patients [37]. Furthermore, the broad acceptance of minimally invasive techniques has added to the increased utilization of procedures that are more technically demanding with steep learning curves [38, 39].
3D Printing Applications
Additive manufacturing, more commonly known as 3- dimensional (3D) printing, is a process that permits the rapid manufacturing of high-fidelity 3D models using a specially designed printer [40]. In Urology, various centers around the world have utilized this technology, to convert radiological imaging of patients with renal tumors into individualized physical models that aid the processes of informed consent, and preoperative partial nephrectomy planning [41, 42]. While the current practice is adequate for surgical planning, surgical models must advance beyond simply a visual aid to provide an interactive element and haptic feedback required to conduct an operation [43]. Unfortunately, none of the available printing polymers can be constituted to mimic human tissue properties. Utilizing a combination of 3D printing, hydrogel injection molding, and software that digitizes patients’ imaging data our laboratory was able to produce patient specific hydrogel models with the ability to accurately portray anatomical characteristics including individual patient variations, but also with the capacity to reproduce tissue characteristics and replicate the entire gestalt of the operative experience [44, 45].
Methods
We utilized our technique to develop patient specific kidney phantoms suitable for use as a simulation platform for preoperative rehearsal of 6 complex renal cancer surgery cases scheduled for MIPN (Table 1). Average nephrometry score was 9.3 (range 8–11). The patient specific kidney phantoms are mainly composed of polyvinyl alcohol (PVA) and include the tumor, parenchyma, artery, vein, and calyx. The correct composition that replicates the material properties of human kidneys was established by comparing the results of mechanical testing between multiple compositions of PVA and porcine kidneys. To determine the anatomical accuracy of the assembly process, the patient-specific PVA kidney phantoms were reimaged (C.T. scanner, Somatom; Siemens Healthcare) with 20% iodinated contrast. A detailed quantitative error analysis using the part comparison tool of 3-matic (Mimics 3-matic; Materialise Belgium) to detect any discrepancy in millimeters. The kidney phantom are then surrounded by the other hydrogel organs of relevant anatomy and placed in a laparoscopic trainer, where a surgical rehearsal is completed in a simulated setting. Surgical metrics (warm ischemia time-WIT, estimated blood loss-EBL. surgical margins) are gathered and results correlated between the rehearsal and real operation using a Wilcoxon test.
Table 1
Patient Demographics
| Age | Sex | Laterality | Tumor Location | Tumor Size | Nephrometry score | Previous Surgeries | |
| 1 | 62 yrs | M | Right | Polar | 2 cm | 8p | Ex-lap |
| 2 | 66 yrs | F | Right | Upper-midpole | 4.5 cm | 10p | Radical TAH-BSO |
| 3 | 22 yrs | M | Left | Upper pole | 4 cm | 9x | Right open partial |
| Hilar | 1 cm | ||||||
| Lower pole | 1.2 cm | ||||||
| midpole | 1.8 cm | ||||||
| 4 | 56 yrs | M | Left | Hilar | 6.5 cm | 10p | None |
| 5 | 76 yrs | F | Left | Hilar | 2.3 cm | 8a | None |
| 6 | 65 yrs | M | Left | Upper, midpole | 9 cm | 10x | None |
Analysis and Results
The PVA condition to best recreate the material properties of porcine kidneys was found to be 7% after completing 2 processing cycles (RMSE = 0.0003). Four patients’ anatomical geometry were analyzed, and the average mean discrepancy between the phantom and patient anatomy were –0.26 mm, –0.2 mm, 3.10 mm, 0.61 mm and 3.33 mm for kidney parenchyma, tumor, artery, vein and calyx respectively. Eight patients were consented to create a kidney phantom for rehearsal prior to the live surgery. Similar average WITs (16.5 & 16 minutes) and EBL (360–265 ml) were seen in the corresponding simulated and live surgeries. No positive margins were detected in any cases. A positive correlation was found for WIT and EBL (p-value, 0.103 & 0.396) between the simulated and live case.
Conclusion
Application of this versatile, inexpensive and reproducible method for creating patient-specific kidney phantoms has demonstrated its success in predicting the perioperative outcomes of live surgery which may be applicable as an effective surgical rehearsal tool for complex renal cases.
Session II: Imaging and Management of Urologic Malignancies
Session Moderator: Patrick Fultz, MD
Imaging in Urologic Oncology
Deborah Rubens MD, FACR, FAIUM, FSRU
Fifty years ago imaging of urologic tumors was performed in order to diagnose patient symptoms of pain or hematuria. Our options were limited to urography or retrograde pyelography which identified renal and bladder and collecting system masses or angiography which outlined tumor vascularity and provided an anatomic roadmap for surgery. The advent of computerized axial tomography and ultrasonography in the late 1970s, followed by MRI in the early 1980s permitted direct depiction of soft tissues without overlap, and provided unique information as to a tissue’s material content (fat, water, blood, calcium) as well as its perfusion patterns. We could diagnose simple renal cysts without aspiration or biopsy, identify angiomyolipomas by their fat content, and discriminate between solid renal neoplasms and hemorrhagic or proteinaceous cysts. We could visualize tumor invasion into adjacent tissues or vessels prior to surgery, and search for distant metastases. Imaging became routine for diagnosis (including biopsy guidance) and staging.
With the advent of effective hormonal and chemotherapeutic agents for testicular cancer and prostate cancer, a new imaging role arose to monitor for treatment response and identify asymptomatic recurrence. That role continues to expand as more chemotherapy and immunotherapy options have become available for renal and bladder cancer. As imaging evolved, therapeutic options grew to include partial nephrectomy and percutaneous tumor ablation, and targeted radiation therapy. With the organ left in situ, imaging was essential first to guide therapy, and subsequently to assess treatment effect and monitor for local or distant recurrence.
The ubiquitous applications of CT and MRI for general abdominal medical care also generated innumerable incidentally discovered asymptomatic lesions, mostly renal and adrenal. Some required immediate therapy, but most were benign, or of low malignant potential. As our understanding of various tumor behaviors advanced, therapeutic options grew to include active surveillance, as some prostate cancers and cystic renal cell carcinomas were found to be low risk for metastatic disease [46, 47]. CT, MRI and contrast enhanced ultrasound monitor asymptomatic slowly growing cystic renal lesions as active surveillance becomes an accepted paradigm for small lesions in patients with impaired renal function or other co-morbidities [48].
Prostatic MRI evolved from an anatomic staging exam to a multiparametric study; identifying the threshold for tumor aggressiveness, separating Gleason 6 from Gleason 7 lesions. Today MRI both guides and determines the necessity of biopsy and has become a mainstay of expectant prostate cancer management. The recent results of the Precision study (NEJM, 2018), showed an undeniable advantage in using MRI to both decrease unnecessary prostate biopsy, and obtain higher yield of more significant cancer with directed biopsy over standard random core prostate biopsy [49]. The next advance for prostate cancer will likely be metabolic imaging with Gallium mPSA PET-CT, which identifies more lesions and grades them better than MRI and also can detect distant metastases [50, 51]. As targeted therapies evolve for urologic cancer, it is likely that patients will undergo targeted PET or other metabolic studies with CT or MRI or Ultrasound, to see if their tumor is likely to respond to a particular agent. From detection to guided ablation, to monitoring, to assessment for specific tumor features and subsequent therapy response, we are clearly at a new horizon of imaging in urologic oncology.
Hereditary Kidney Cancer: Understanding the Cancer-Critical Genes
Guan Wu, MD, PhD
Hereditary kidney cancer accounts for approximately 5% of all kidney cancer. Several inherited kidney cancer syndromes have been well characterized, including von Hippel-Lindau disease (VH), hereditary papillary renal carcinoma (HPRC), Birt-Hogg-Dube (BHD), and hereditary leiomyomatosis and renal cell cancer (HLRCC). Each of these cancer syndromes exhibits a unique set of clinical manifestations and pathologic features. Mutations of VHL, MET, FCLN, and FH genes contribute to the development of these four hereditary kidney cancer syndromes respectively. Understanding these pathogenic cancer-critical genes in terms of their molecular mechanisms in enzymatic function, signal transduction, and metabolic process has enriched our knowledge about renal cell tumorigenesis and led to the development of a class of tyrosine kinase inhibitors for kidney cancer treatment. Our research lab at the Department of Urology, University of Rochester Medical Center has been focusing on functional analysis of VHL tumor suppressor-interacting proteins, MET signaling pathways, identification of the BHD tumor suppressor complex, and transgenic mouse models for kidney cancer. The following are highlights of our research efforts for the past twenty years.
One of the well-established functions of VHL is that VHL protein serves as a target-binding component in an E3 ubiquitin ligase, targeting HIF for degradation. However, although this could explain the highly vascularized nature of VHL-associated tumors, it does not explain the tumorigenic pathway of VHL mutations. Our research lab studied several VHL interacting partners and targets. During this investigation, we identified and characterized a subfamily of deubiquitinating enzymes (USP33 and USP20). Ubiquitination and deubiquitination are opposite post-translational protein modifications. These processes play important roles in cellular protein homeostasis, localization, and activity. This seminal research has led to significant interest in studying these enzymes’ roles in developmental biology, hormone activation, protein activity regulations, and cancer cell biology.
The MET gene product is a tyrosine-protein kinase, also called hepatocyte growth factor receptor. MET regulates many physiologic processes including cell proliferation, scattering, morphogenesis, and survival. MET mutations have been identified in a subset of hereditary papillary renal carcinoma (HPRC). Constitutive activation mutations appeared to be the trigger in developing this type of renal cell cancer. By studying interacting partners of the intracellular domains of c-MET, we discovered novel c-MET signaling pathways in cancer. Our work further characterized several intracellular signaling proteins, including RanBPM and SOCS box protein 1 that play roles in MET signal transduction.
The function of the tumor suppressor folliculin (FLCN) was unclear. Our research lab in collaboration with others has discovered the role of FLCN in cancer biology. We found that FCLN-deficient renal cancer cells exhibited higher radiosensitivity through autophagic cell death. Suppression of autophagy also enhances preferential toxicity of paclitaxel to FLCN-deficient renal cancer cells. We also created several FLCN renal tubule-specific knockout mouse models. These models allowed us to study renal tumor induction. We demonstrated that Flcn deficient renal cells are tumorigenic and sensitive to mTOR suppression. Flcn knockout mice also developed lung tumors and other neoplasia in multiple organs.
Accuracy of Nodal Staging and Outcomes of Lymphadenectomy for Non-metastatic Renal Cell Carcinoma: An Analysis of the National Cancer Database
Eric A. Singer, MD
Background
Lymph node dissection (LND) plays a critical role in multiple urologic malignancies [52–55]. Lymph node (LN) involvement in renal cell carcinoma (RCC) is typically associated with a poor prognosis [56]. While LND may provide diagnostic information in RCC, the therapeutic benefit remains controversial [57–61]. Several studies have suggested that LND does not confer a survival advantage in RCC patients, even in high-risk patients or those with clinical lymphadenopathy [58, 60, 61]. Conversely, other studies have found that a small subset of pN1M0 RCC patients with isolated nodal disease may have a durable long-term survival benefit after LND [57, 59]. Currently, the American Urologic Association (AUA) guideline on localized renal cancer recommends performing an LND in the setting of regional lymphadenopathy [62]. Thus, we sought to examine the accuracy of clinical staging by examining the relationship between clinical lymph node (cLN) status and pathologic lymph node (pLN) status among patients who received an LND at the time of kidney surgery. We also aimed to characterize contemporary LND practice patterns and to analyze survival outcomes after LND.
Study Population
The National Cancer Database (NCDB), a hospital registry database, was queried for patients with non–metastatic RCC who underwent either partial or radical nephrectomy from 2010–2014. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from this data. The data extracted for this institutional review board-exempt study was obtained from a de-identified NCDB file. A total of 173,834 patients from 2010–2014 were included in the kidney cancer data set. We excluded patients with metastasis (n = 36,870), non-surgical procedures (n = 24,001), and patients missing information (n = 2,000). The final sample size was 110,963. A total of 11,867 underwent surgery with concurrent LND coded at the time of their operation. Full nodal information (clinical and pathologic) was available on 11,300 patients.
Accuracy of Clinical Nodal Staging
Of the 11,300 evaluable patients who received an LND, 1725 were preoperatively staged as cLN positive. A total of 1895 patients were found to be pLN positive after LND. The sensitivity and specificity of cLN staging to detect positive pLN in patients who received LND were 67% and 95%, respectively. For all cLN negative patients, the NPV of LND was 94%. For all cLN positive patients, the PPV of LND was 74% [63].
Trends in LND
Among the entire cohort, LND was performed in 4523 (5%), 3625 (23%), 3280 (31%), and 321 (47%) patients with clinical T stage 1, 2, 3, and 4 tumors, respectively (p < 0.0001). Rates of LND have not significantly changed from 2010 to 2014 (p = 0.29). Three surgical approaches for LND were reported: robotic 2345 (20%), laparoscopic 2941 (25%), and open 6581 (55%) (p < 0.0001). Of 1725 patients who were cLN positive, 214 (12%), 359 (20%), and 1152 (67%), underwent robotic, laparoscopic, and open LND, respectively (p < 0.0001). Open surgery was associated with greater mean LN yield (5.9±7.1) compared to robotic (5.1±5.9) or laparoscopic surgery (3.9±4.9) (p < 0.0001) [64].
Impact of LND on Survival
To minimize selection bias, propensity score matching (PSM) was used to select one control for each LND case (9750 LND and 9750 no LND for total n = 19,500). Cox regression analyses were conducted to examine overall survival (OS) in patients who received LND compared to those who did not. Among the matched patients, no significant improvement in median OS was seen in patients who received LND (34.7 vs. 34.9 months, respectively; p = 0.98). Similarly, no significant improvement in median OS was found for the subset of cLN positive patients who underwent LND (p = 0.90). On Cox regression analysis, LND was not associated with an OS benefit (HR: 1.00; 95% CI 0.97–1.04) [64].
Conclusions
The clinical staging of lymph nodes in patients with RCC has a greater specificity than sensitivity. Improvements in preoperative imaging are needed to more accurately identify nodal disease. Among all RCC patients, LNDs were often performed for low stage disease, suggesting a potential overutilization of LND. Additional work will be needed to assess the impact of AUA guidelines on the management of cLN positive RCC. No OS benefit was seen in any subgroup of patients undergoing LND. Further investigation is needed to determine which patient populations may benefit from LND.
Funding
This work is supported by a grant from the National Cancer Institute (P30CA072720).
Elucidating Renal Cell Carcinoma Vasculogenesis During Bone Metastasis
Edward M. Schwarz, PhD
Although our ability to treat and manage various tumor metastases to bone has markedly improved, this is not the case for renal cell carcinoma (RCC), which remains a challenging surgical problem due to its great vascularity. Additionally, the unique mechanisms that mediate RCC vasculogenesis in bone are poorly understood. To the end of elucidating this process and developing selective drugs to target the large vessels that are unique to RCC, we established a xenograft model that recapitulates highly vascular RCC versus less vascular tumors that metastasize to bone. In these studies, we utilized human tumor cell lines of RCC (786-O), prostate cancer (PC3), lung cancer (A549), breast cancer (MDA-MB231), and melanoma (A375), which were transduced with firefly luciferase (Luc), injected into the tibiae of nude mice, and differences in growth, osteolysis, and vascularity were assessed by longitudinal bioluminescent imaging, micro-CT for measurement of calcified tissues and vascularity, and histology. Our results showed that while RCC-Luc has reduced growth and osteolytic potential versus several of the other tumor cell lines, it exhibited a significant increase in vascular volume (p < 0.05). Moreover, we found that this expansion was associated with a 3- and 5-fold increase in small and large vessel numbers respectively. To identify genes that could be responsible for the increased vascularity of RCC in our model, we performed in vitro gene expression profiling. The results showed that RCC-Luc expresses significantly (p < 0.05) more vegf-a (10-fold) and 20- to 30-fold less ang-1 versus the other lines. To expand on these findings, we performed a microarray analysis to compare the transcriptomes of RCC-Luc versus PC-Luc. Insulin-like mRNA binding protein-3 (IMP3) was among the genes that was differentially expressed. This finding was confirmed by real time RT-PCR, which demonstrated a significant 4-fold increase in imp-3 expression in RCC 786-O vs. PC3 cells in vitro (p < 0.001). To validate this observation, we studied retrospectively obtained contrast CTs from 72 patients with primary RCC, which were categorized as Low, Intermediate and High tumor vascularity based on a CT threshold analysis. Then we performed immunohistochemistry on paired histopathology specimens from 33 of these patients to correlate CT vascularity with IMP-3 expression. The results demonstrated that IMP-3 expression positively correlated with CT vascular enhancement (p < 0.01).
Additionally, IMP3 protein expression was strongly positive in all RCC tumors, but weak in PC bone metastases. Collectively, our findings demonstrate that quantitation of pre-operative CT is a feasible method to phenotype primary RCC vascularity, which correlates with IMP-3 expression. In situ and cell line gene expression studies demonstrate an association between high IMP-3 levels and RCC bone metastasis. Our future studies are aimed at defining the diagnostic potential of IMP-3 expression as a biomarker for RCC bone metastasis. We are also studying IMP-3 loss of function in vitro and in vivo to determine the functional significance of IMP-3 in RCC vascularity and tumor progression.
Improving Care Delivery and Outcomes for Older Patients with Genitourinary Malignancies
Supriya Mohile, MD, MS
The majority of patients with urologic malignancies are older. Older patients have a higher prevalence of comorbidity, disability, and geriatric syndromes. Due to these age-related health conditions, older patients are at more risk for adverse outcomes such as chemotherapy toxicity, surgical complications, and early mortality. Geriatric assessment (GA) can help identify older patients who are at highest risk of adverse outcomes. A recent American Society of Clinical Oncology (ASCO) guideline recommends GA in the decision-making process for chemotherapy for all older adults, including those with urologic malignancies [65]. A recent large multicenter cluster randomized study conducted in the University of Rochester NCI Community Oncology Research Program Research Base demonstrated that GA improves communication about age-related concerns and patient satisfaction [66]. Evidence is growing that physicians utilize GA to guide treatment decisions [67]. GA can also help guide non-oncologic interventions (e.g., cognition work up for those with cognitive impairment, physical therapy for those with balance issues and a history of falls) [68].
Session III: Advanced Diagnosis and Therapies in Bladder Cancer
Session Moderator: James Mohler, MD
The Biological Path to Novel Therapeutics in Cancer
Dan Theodorescu, MD, PhD
Our laboratory has used a combination of gene expression profiling and functional genomic screens to identify genes that suppress tumor growth and metastasis. One is RhoGDI2 [69, 70] a canonical GDP-dissociation inhibitor (GDIs) that decreases the rate of GDP dissociation from Ras-like GTPases. The other is AGL, a glycogen debranching enzyme deficient/nonfunctional in glycogen storage disease type 3 [71]. We have investigated the mechanism of action of these genes. Our early work revealed that RhoGDI2 suppressed bladder cancer metastasis but not primary tumor growth and this was mediated by Endothelin-1 [72]. Further investigation into the mechanism underlying this effect revealed that RhoGDI2 suppressed expression of the proteoglycan versican (VCAN, also known as chondroitin sulfate proteoglycan 2 [CSPG2]) with high versican levels associated with poor prognosis in patients with bladder cancer [73]. In establishing the functional importance of tumor expression of Endothelin and versican in promoting metastasis in in vitro and in vivo studies in mice, we implicated a role for the chemokine CCL2 and tumor-associated macrophages in this process. Later we found that macrophage-secreted osteopontin binds to CD44s on the tumor cells and promotes invasion and clonal growth [74]. These effects are RhoGDI2-sensitive and require CD44s binding to the Rac GEF TIAM1. Osteopontin expression also correlates with tumor aggressiveness and poor clinical outcome in patients. Inhibiting this pathway blocked early colonization of bladder cancer to the lung. In contrast, primary tumors and established metastasis were less sensitive. These data identified osteopontin-CD44-TIAM1-Rac1 axis as a RhoGDI2-sensitive pathway and potential therapeutic target in preventing the development of clinical bladder cancer metastasis. This mechanism revealed the specificity of RhoGDI2 in inhibiting colonization over established primary or metastatic tumors and in later work we found that it was shared with that of AGL, the other gene whose tumor and metastasis suppressor activity we discovered [71]. We showed that reduced AGL enhances tumor growth by inducing hyaluronic acid synthesis via induction of hyaluronic acid synthase 2 (HAS2) expression [75]. 4-Methylumbelliferone (4-MU), an inhibitor of HA synthesis, had similar effects to HAS2 depletion on tumor growth with 4-MU preferentially inhibiting growth of bladder tumor xenografts with low AGL expression. Interestingly, CD44 is the major receptor of both osteopontin and HA and hence this data suggests that targeting the molecular circuits responsible for the tumor-macrophage communication, such as with anti-CD44 or anti-HA therapies, may be an effective platform for biomarker and therapeutic development.
Extracellular Vesicles in Bladder Cancer: Small Vesicles with Big Roles
Yi-Fen Lee, PhD
Extracellular vesicles (EVs) are membrane-bound, nano-sized vesicles released by most cells which play key roles in cell-cell communication. Cancer cells release higher amounts of EVs which are involved in all steps of cancer progression and therapy response by communicating with the surrounding tumor microenvironment [76]. EVs can transfer oncogenic proteins to neighboring cells in a paracrine manner or travel through the body and fuse with specific cell types to deliver cargo in an endocrine manner, consequently affecting recipient cell behavior [77]. The focus of this talk will be on the roles of bladder cancer-derived EVs (BCEVs) and their cargo proteins in metastasis and in BCG therapy response in clinical and pre-clinical models.
Can We Find Evidence of Pre-Metastatic Niche Formation in Uninvolved Lymph Nodes?
Pre-metastatic niche (PMN) formation refers to the ability of tumors to educate microenvironments in distant organs to support the survival and outgrowth of future metastatic tumors [78]. Experimental evidence suggests that formation of PMNs can be induced by tumor-secreted factors, including tumor-derived extracellular vesicles (TEVs) [79–81]. During the evolution of metastasis, TEVs promote the formation of PMNs by recruiting non-resident progenitors and altering local resident cells such as fibroblasts, consequently inducing extracellular matrix remodeling and immune deregulation to support circulating tumor cell adhesion, colonization, and metastatic outgrowth [78, 82]. The difficulty of obtaining pre-metastatic tissues from cancer patients has limited clinical investigation of this phenomenon. To verify PMN formation in clinical samples and the role of TEVs, we collected lymph nodes, urine and blood from muscle-invasive BC patients. PMN formation was assessed by immuno-histochemical staining of tenascin C in uninvolved nodes in metastatic vs. non-metastatic cases. We also applied an MB49 syngeneic mouse model to demonstrate that pre-conditioning mice with EVs derived from metastases-prone MB49 sub-clones promoted lung metastases. Pro-metastatic BCEV cargo proteins were identified; their actions in fibroblast differentiation and matrix remodeling were investigated.
Can We Predict or Enhance the Response to BCG Immunotherapy?
Although BCG immunotherapy is the most successful therapy to prevent recurrence and progression of non-muscle invasive BC [83], many critical questions remain unanswered, and mismanaging BCG can prove disastrous for patients. Currently, there is no biomarker that can predict BCG response. Thus, there is a need to elucidate BCG’s mechanism of action and identify biomarkers to predict response and disease progression after BCG treatment.
EVs act as immune modulators that stimulate or suppress immune response by delivering proteins, cytokines, and nucleic acid to recipient cells. Immunologically active EVs were first reported in the EVs derived from Epstein-Barr virus transformed B cells [84] and dendritic cells [85] that harbor MHC-II antigen peptide complexes. In the context of BCG immunotherapy, communication between BC cells and the host immune system is necessary. We found that BC cells responded to BCG with elevated secretion of EVs enriched for immunologically active molecules, including MHC-I, MHC-II, and co-stimulatory molecules CD80 and CD86. We determined resulting EV immunogenicity using T cell phenotypic and functional analyses. Importantly, we collected and purified urinary EVs from patients before and after six BCG instillations to determine the correlation of BCG responsiveness with EV secretion patterns, including numbers and size distribution, and the contents of immunologically active EV molecules.
Hurdles
EVs have garnered substantial interest in recent years due to their involvement in numerous physiological and pathological processes and have great potential application to disease diagnosis, prognosis, and therapeutic guidance. However, major challenges in the EV field are the heterogeneous and dynamic nature of EVs and the lack of standardized protocols for their purification and characterization. The feasibility of high throughput EV isolation from biological fluids and identification of tissue- and disease-specific EV markers warrants the continued advance of EVs’ use in clinical applications.
Role of Entrepreneurial Science to Modern Academic: Mentorship by Ed Messing
Paul Okunieff, MD
When I arrived at the University of Rochester in 1997, Ed Messing was working with a talented physicist, Yan Yu, to develop a prostate implant planning system that was, and might still be, the best in the world. They licensed a patent, created a company, applied for and received a number of small business grants from the NCI, developed a product, and used it to improve human health. Although “bench to bedside” is good and “bench to bedside and back again” is good for grantsmanship, what we really need is more “bench to product”. It is only then, when an actual product is developed, that translational research impacts humankind. I will go through the steps that I learned from Ed and how I have tried to (and sometimes succeeded in) translating my science into real products. As grant funding in the sciences is reduced and becomes more and more difficult to obtain, especially for young and underrepresented scientists, the future of academic medicine might depend on the entrepreneurial science exemplified by Ed.
Chemoresistance in Muscle-Invasive Bladder Cancer
David J. McConkey, MD
Level 1 evidence supports the use of neoadjuvant chemotherapy (NAC) in the treatment of muscle-invasive bladder cancer (MIBC), but the clinical benefit afforded is judged to be modest by many investigators and only about half of eligible patients receive it. Recent genomic studies demonstrated that MIBCs can be grouped into basal and luminal molecular subtypes that are associated with different progression patterns and responses to conventional and targeted therapies. In particular, clinical benefit from NAC appears greatest in patients with basal/squamous tumors, whereas patients with luminal tumors appear to derive much less benefit. On an even more granular level, basal tumors that are more heavily infiltrated with T cells appear to be the most responsive to NAC, whereas luminal tumors that are infiltrated with cancer-associated fibroblasts (CAFs) appear to be the most resistant, and levels of fibroblast infiltration appear to increase even further after treatment with NAC. Ongoing studies within the context of the Southwest Oncology Group’s fully-accrued Phase II clinical trial of dose-dense MVAC versus gemcitabine plus cisplatin will provide the opportunity to validate these observations
The results of the Southwest Oncology Group’s landmark S8710 clinical trial demonstrated the clinical benefit of cisplatin-based combination chemotherapy in patients with MIBC going on to cystectomy [86]. However, the absolute impact of NAC on disease-specific survival in unselected patients can be considered modest, and NAC is therefore still under-utilized. Robust biomarkers that reliably distinguish chemo-sensitive from chemo-resistant tumors are desperately required, and aggressive efforts are underway to identify them. One of the most exciting recent findings has been the observation that MIBCs with inactivating mutations in certain DNA damage and repair (DDR) mutations are chemo-sensitive, [87, 88] prompting the design of clinical trials to examine whether patients whose tumors contain these alterations can be treated with NAC and avoid cystectomy.
Early work demonstrated that gene expression profiling could also be used to identify chemo-sensitive bladder cancers [89]. More recent studies demonstrated that MIBCs can be grouped into molecular subtypes [90–93] that exhibit different patterns of progression and differential responsiveness to chemotherapy. Rates of pathological downstaging were lowest in “p53-like” tumors characterized by cancer-associated fibroblast infiltration and extracellular matrix deposition in several clinical trial cohorts, [94, 95] although a subsequent study failed to observe such a relationship in another large cohort [11]. Interestingly, matched comparisons of pre- and post-treatment tumors revealed increased expression of the resistance signature post-therapy, [94, 95] suggesting that exposure to NAC could induce and/or select for chemo-resistance. Conversely, patients with basal tumors, [95, 96] and particularly basal tumors infiltrated with a certain subset of T cells [94] were the most chemo-sensitive and were associated with the greatest survival benefit.
Overall, it now appears that excellent candidate biomarkers are available that might be used to distinguish chemo-sensitive and chemo-resistant MIBCs. However, all of the work performed to date has been conducted retrospectively, and all of the current biomarkers require prospective validation. The Southwest Oncology Group (SWOG) designed a Phase II clinical trial (S1314) to prospectively validate the CoXEN algorithm’s performance, and the groups that were responsible for linking DDR mutations and molecular subtypes to NAC benefit have also integrated their analyses into the translational medicine plans for the trial. The trial is fully accrued, the DNA and RNA have been isolated, and the RNA expression profiling has already been performed, so these validation studies should be completed very soon.
Session IV: Insights into the Molecular and Surgical Management of Bladder Cancer
Session Moderator: Hani Rashid, MD
Androgen Receptor Roles in the Bladder Cancer Progression
Chawnshang Chang, PhD
Infiltrating macrophage may play a key role in impacting the progression of bladder cancer (BCa). Yet its detailed mechanism remains unclear. Here we found that co-culture of macrophages (THP-1) with BCa cells (J82 and TCCSUP cells) in transwells increased the numbers of migrating macrophages, and conditioned medium from co-culture of macrophages and BCa cells led to an increase in the BCa cell progression. Mechanism dissection indicated that conditioned medium from the co-culture of macrophages with BCa cells contains cytokines IL-8 responsible for macrophage recruitment, and interrupting the IL-8 led to suppressing the capacity of BCa cells to attract macrophages to tumor sites. Together, these results suggest that BCa cells may attract macrophages to tumor sites to increase tumor progression via altering the cytokines IL-8 secretion in tumor microenvironment.
To further study the androgen receptor (AR) roles in BCa progression, we developed a new combined therapy with Bacillus Calmette-Guérin (BCG) plus the AR degradation enhancer ASC-J9 or anti-androgen hydroxyflutamide (HF) to better suppress BCa progression. A mechanism study indicated that adding ASC-J9 led to increased BCG efficacy to suppress BCa cell proliferation through increasing the recruitment of macrophages that involved the increasing BCG attachment/internalization to BCa cells via increasing integrin-α5β1 expression and IL-6 release. The consequences may then increase BCG-induced BCa cell death via increased TNF-α release. We also found that adding ASC-J9 could directly increase BCG-induced HMGB1 release to promote the BCG cytotoxic effects for the suppression of BCa cell growth. Results from a preclinical study using an in vivo mouse model also proved that ASC-J9 could increase BCG efficacy to better suppress BCa progression in BBN-induced BCa mouse models. Together, these results suggest that a novel therapy may be developed via combining BCG plus ASC-J9 to better suppress BCa progression.
Immunotherapy for Bladder Cancer: A Revolution in the Making
Yves Fradet MD, Alain Bergeron PhD, Fanny Gaignier PhD and Marjorie Besançon PhD
Intravesical BCG was the first successful cancer immunotherapy approved by the FDA more than 30 years ago. A recent breakthrough is the remarkable response of metastatic bladder cancer patients to immune checkpoint (IC) inhibitor anti-PD1 in a phase III trial compared to second line systemic chemotherapy [97]. Although only 21% responded, 80% of responders were alive after 2 years and beyond, suggesting long-term durability of immune response [98]. Moreover, response rate appears to be higher in the earlier stages reaching 40% pT0 rate at cystectomy after only 3 cycles of anti-PD1 neoadjuvant treatment [99]. Immune checkpoints (ICs) refer to a series of pathways that can inhibit the immune response (CTLA-4, PD-1, TIM-3, LAG-3 and others), or activate it (TIGRIT, GITR, OX40 and others) [100]. Expression of these ICs is frequently dysregulated by tumors to escape immune recognition. In MIBC, many are co-expressed suggesting that combination therapies with several ICs may improve cancer eradication.
With so many possible combinations that may be effective in only subsets of patients, there is a need for pre-clinical data and predictive biomarkers to better tailor therapies. We have studied IC-based combination therapies in two immune-competent syngeneic murine BCa models: the MBT-2 (C3H mice) and the MB49 (C57BL/6 mice). These two models have reproducible and distinct profiles of IC expression in subsets of tumor infiltrating immune cells (TILs) as measured by multicolor flow cytometry (FCM). In the MBT-2 model the inhibitory IC TIM3 is expressed on many TIL sub-types and often co-expressed with PD-1. Unexpectedly, anti-TIM3 treatment and combination with anti-PD1 resulted in a dose-dependent activation of tumor growth, while treatment with the Gal-9 agonist inhibited tumor growth. The inhibitory LAG3 is more expressed in the MB49 model. Anti-LAG3 treatment of MB49 alone had little impact on survival, but the combination of anti-LAG3 and anti-PD1 doubled mice survival to 70% compared to 30% with anti-PD1 alone with strong memory upon re-challenge.
Activating ICs such as OX40 prevents T cell death and increases cytokine production. Surprisingly, the combination therapy with an agonist mAb to OX40 and anti-PD1mAb showed a striking 100% survival in repeated sets of experiments in both the MBT-2 and MB49 models with a strong memory upon repeat challenge in all mice. In the MB49 model, OX40 is expressed mostly on CD4 helper T cells and Natural Killer cells but not on effector CD8 T cells that strongly express PD-1. The combined treatment upon failure of primary anti-PD1 therapy also showed responses albeit at a more modest level. These results show the potential of pre-clinical models to identify combination and sequential IC therapy strategies that may improve survival of bladder cancer patients and suggest that a comprehensive immune profiling of bladder cancer cells and TILs may be a better predictor of response to IC. Multicolor FCM is limited by the requirement of fresh tumors, but this clinical limitation may be overcome by the development of a multiparameter Cytof platform allowing quantitative single cell measurement of up to 35 markers on fixed tissue sections.
Other biological factors may be synergistic to potentiate the immune response to IC inhibitors or activators such the individual microbiome and sex-steroid hormones. In the MBT-2 model, we showed that response to anti-PD-1 or BCG therapies was significantly better in female than in male mice. The combination of anti-androgen therapy with Enzalutamide with both anti-PD-1 or BCG therapies improved the response in male mice to the level of female mice. All these observations suggest that immunotherapy has the potential to cure even more advanced bladder cancers with the appropriate combination of personalized treatments.
A Decade of Robot-assisted Radical Cystectomy: Has it met our Expectations?
Khurshid Guru, MD
Robot-assisted surgical approach for pelvic urologic oncology has been around for almost two decades and the technique for robot-assisted radical cystectomy (RARC) with lymph node dissection has also been long established. Meanwhile the minimally invasive approach to cystectomy had already increased to 39% in 2013. Based on the National Cancer Database, patients who underwent a minimally invasive approach to cystectomy were more likely to receive neoadjuvant chemotherapy. Oncologic outcomes after RARC and lymph node dissection have proven to be safe and efficacious in both early and long term follow up. RARC has been one of the only newer minimally invasive techniques which have been evaluated in several randomized controlled trials which have proven oncologic efficacy. The Quality Cystectomy Score based on star-based criteria developed at Roswell Park Comprehensive Cancer Center has advocated for quality care criteria for patients undergoing RARC. These quality criteria range from neoadjuvant chemotherapy consultation to negative soft tissue surgical margins, adequate lymph node yield, readmission and 30-day mortality. Several perceived advantages of robot-assisted approaches for bladder cancer include less pain, minimal blood loss and earlier return of bowel function that ultimately help in quicker return to previous quality of life. Despite the slow adoption and early incorporation of this approach, rate of conversion (up to 5%) to open surgery has been reported. Early adoption of robot-assisted radical prostatectomy could have paved the way for RARC; meanwhile the negative impact of this could be suboptimal oncologic procedures during learning curve and performing urinary diversions which did not meet the standards set by open experts. Recent updates from the International Robotic Cystectomy Consortium database (IRCC) in 2018, show that incorporation of ICUD for the robotic focused group has increased from 9% in 2005 to 97% in 2016 with an annual increase of 11% [101].
Improving Recovery after Cystectomy
Janet Baack Kukreja, MD, MPH
How do we optimize outcomes and perioperative care to improve radical cystectomy outcomes?
• Current state of radical cystectomy outcomes
• Predicting perioperative outcomes
• Prospective studies for improvement in the radical cystectomy care
The focus of this talk will be on what is known regarding radical cystectomy outcomes, what patients are high risk for complications and how to improve ideal recovery for patients undergoing radical cystectomy.
Radical Cystectomy: A Complicated Road and Opportunity for Improvement
Often patients after radical cystectomy have poor outcomes and a failure to recover from their surgeries. Developments in perioperative care and possibly immunonutrition represent a promising new area to improve radical cystectomy morbidity. Despite significant changes in cystectomy care, 60% of patients experience a complication and 30% experience a readmission [102–104]. The occurrence of postoperative complications is associated with several risk factors including patient characteristics, e.g. age, as well as surgical factors. There is growing evidence that traumatic and surgical insults are associated with a period of relative immune-suppression, which may increase patient’s risk of infection and postoperative complications. Therefore, identifying at risk patients may allow for optimization and improved outcomes.
Surgical Stress Response
It is well known that major surgery and other types of trauma are associated with large changes in host defense mechanisms, making the patients highly susceptible to septic and inflammatory complications. The first surgical incision induces localized injury to tissues, afferent nerves, pain receptors and blood vessels. The surgical stress response has three key components: sympathetic nervous system activation, endocrine response with pituitary hormone secretion and insulin resistance, immunologic and hematologic changes including cytokine production, acute phase reactants, neutrophil leukocytosis, and lymphocyte proliferation [105, 106]. There is a delicate balance between the release of pro and anti-inflammatory cytokines. An exaggerated pro-inflammatory response and a compensatory anti-inflammatory response can have significant post-operative morbidity from immunosuppression [105]. The excessive inflammatory response followed by depression in cellular immunity is a significant underlying factor for postoperative complications [106].
Enhanced Recovery Pathways
The enhanced recovery program has the aims of decreasing perioperative stress by modifying the physiological and psychological response to stress. These goals are accomplished by decreasing postoperative pain, minimizing GI dysfunction and early mobilization leading to overall accelerated recovery and early discharge. These programs have been successful in decreasing length of stay, GI complications and opioid use after surgery [107]. There may be some opportunities to improve outcomes with further refinement of enhanced recovery programs and improved urologist implementation [108].
Immunonutrition
In high-risk surgical patients arginine and the omega-3 fatty acids, also known as immunonutrition, may adjust the balance of potent inflammatory mediators [109]. In both experimental and clinical settings, immunonutrition has been shown to up-regulate host immune response, to modulate the inflammatory response, shift protein synthesis from acute-phase reactants to constitutive proteins, thus improving gut oxygenation and barrier function after injury, and reducing septic morbidity and mortality. Immunonutrition is given to modulate the host response and to counteract the potentially adverse effect of postoperative immune changes [109].
A Cochrane review of immunonutrition of 13 studies for patients undergoing GI surgery concluded that immune modulation with supplements reduced infectious complications from 42% to 27% and total complications from 27% to 14% [110]. Meta-analysis of 27 different randomized clinical trials for recovery after immunonutrition found a decrease in infections of almost 60% [111].
Next Steps Towards Ideal Recovery
The next steps in radical cystectomy perioperative care include stratification of those who need to have inflammatory modulation. Ideal recovery can become realized with careful attention to patient factors. Further optimization beyond our current care for radical cystectomy patients will be necessary to reach the goal of further reducing perioperative morbidity and mortality.
Session V: Addressing the Bladder Cancer Burden
Session Moderator: Gerald Sufrin, MD
Screening for Bladder Cancer/Epidemiology
Yair Lotan, MD and Joshua Meeks, MD
Requirements for adoption of screening include that
• Disease can be detected earlier than if the cancer were detected by symptoms
• Treatment initiated early can improve outcome
• Prospective randomized studies show decrease in cause-specific mortality
The focus of this talk will be on what is known regarding bladder cancer epidemiology, what are the pros and cons of bladder cancer screening, what have we learned from screening trials and why screening is universally deemed unacceptable at this time.
Bladder Cancer is Common and Lethal
Urothelial carcinoma of the bladder (UCB) is the 5th most common cancer worldwide with an estimated 79,030 new cases and 16,870 deaths in the U.S. in 2017 [112]. At the time of diagnosis, 25% will have locally advanced (muscle-invasive or metastatic disease) cancer and almost 90% of those with advanced staged cancer were diagnosed at that higher stage [113]. The 5 year survival for metastatic UCB is only 5%. This high mortality should be contrasted with the greater than 90% survival for those with non-invasive UCB; [114] increased tumor volume and stage is directly related to decreased survival, such that those diagnosed with early cancer detected with microscopic hematuria have fewer advanced cancers than patients with gross hematuria [115]. Therefore, identifying bladder cancer at an earlier, asymptomatic stage improves survival.
Screening for Bladder Cancer May Have Widespread Benefits
Despite multiple advances is surgery, imaging and systemic therapy, the survival for patients with UCB has not changed in thirty-years, mostly due to the therapy-resistant nature of metastatic cancer. Patients diagnosed prior to muscle invasion (cT2) can usually keep their native bladder, avoid the morbidity of radical treatments (radical surgery or radiation therapy) and systemic toxicity from chemotherapy resulting in improved lifelong quality of life [116]. Diagnosis at an earlier stage of disease could decrease the extremely costly and frequently ineffective treatments for bladder cancer [117]. The costs of cystectomy and perioperative chemotherapy exceed $50000 with significantly higher costs for salvage therapies and dying of disease. Even a modest reduction in the risk of muscle invasion will impact thousands of patients each year in the U.S.
High Risk Populations
In order to develop rational screening policies, populations at risk need to be identified with sufficient incidence of disease. This has led to age cutoffs for screening of malignancies such as colon, prostate and breast cancer. There are known risk factors for UCB such as age, gender, ethnicity and smoking intensity [118]. We used data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) and National Lung Cancer Screening Trial (NLST) to stratify risk of UCB by overall population, gender, race, age at inclusion and smoking status and were able to identify populations with at least 2 cases per 1000 person years which is similar to yields for colorectal cancer per 1000 screened [119].
Prior Screening Trials
Screening using dipstick analysis for blood in the urine has been utilized in several large screening studies. Messing et al. tested 1,575 men (aged≥50 years) at home with hematuria dipsticks for 14 consecutive days and repeated at 9 months later (in those without hematuria) [120]. Men with abnormal urine tests underwent cystoscopy (n = 283) and 21 UCB were diagnosed (1.3%; including one with muscle invasion). Stage at diagnosis and survival were compared to 509 unscreened patients newly diagnosed with UCB from the Wisconsin cancer registry. Screened men were less likely to be diagnosed with muscle invasive cancer than non-screened men (4.8% vs 23.5%), and had a significantly lower disease specific mortality than unscreened men. No men with screen-detected UCB died of UCB, compared to 20.4% of non-screened men [121]. Britton et al. examined 2,356 men aged 60–85 for dipstick hematuria (weekly for 10 weeks) [122]. Urine testing was positive in 20% of men and UCB diagnosed in 17 men. No patient was diagnosed with muscle invasive disease, but more than half (9/17) had high-risk NMIBC.
Several studies have evaluated urine based tumor markers for bladder cancer screening. Urine based tumor markers have a higher sensitivity than hemoglobin testing when performed on a one-time basis and have a higher specificity but their cost is significantly higher [123]. Lotan et al. screened subjects at high-risk for UCB based on more than 10 years exposure to smoking or occupational carcinogen exposure with the NMP22 BladderChek urine test [124]. The cohort included 1,175 men and 327 women, of which 97% smoked and 34% worked for >15 years in a high-risk occupation. A positive NMP22 test was observed in 5.7% of the population, but only 2 non-invasive UCBs were found. All patients diagnosed with UCB were males and reported at least 40 pack years of smoking. Hedelin et al. screened 1,096 men (aged 60–70) using a combination of urinary dipstick hematuria testing and a bladder tumour marker (UBC) [125]. They found microscopic hematuria in about a quarter of men and restricted further investigation to those having an IPSS score above 10, a positive UBC test or more than 25 red blood cells/μl. Seven tumours were detected (0.6%), all in current or past smokers. In an attempt to reduce the number of cystoscopies performed, authors have suggested a more specific urinary test should be performed prior to cystoscopy. The Bladder Cancer Urine Marker Project screened 1,984 men aged 50–75 using home hematuria testing followed by molecular markers-nuclear matrix protein 22 (NMP22), microsatellite analysis (MA), fibroblast growth factor receptor 3 (FGFR3) mutation snapshot assay, and a custom methylation-specific (MLPA) test-to determine the need for cystoscopy [126]. Four UCBs and one kidney tumor were detected through this sequential protocol and this strategy reduced cystoscopy rate by 82.5%.
Burdens of Screening for Bladder Cancer are Minimal
Unlike screening for lung, breast and prostate cancers, there is almost no harm in screening for UCB. Screening for bladder cancer can be easily and safely performed using non-invasive urine-based assays and confirmed using cystoscopy with biopsy for those with abnormal lesions. The fact that bladder cancer is rarely found at autopsy suggests that almost all cases are associated with some symptoms, most commonly blood in the urine (hematuria), either microscopic or gross (visualizable). One concern with screening of other cancers, such as prostate cancer, is identification of clinically insignificant disease. However, nearly all bladder cancers eventually bleed and become symptomatic, as evidenced by the low incidence of undetected bladder cancer in autopsy series, suggesting that overdiagnosis with screening is unlikely. Overtreatment is also unlikely, given that most cancers become symptomatic.
Hurdles
The largest hurdle is scale and cost of randomized trials. Potential avenues are cohort studies with a large intervention arm and a silent control arm that is monitored electronically in large health care systems.
Bladder Cancer Prevention Strategies
Howard H Bailey, MD
Bladder cancer (BC) is the fourth most common cancer in men in the US with an incidence of approximately 20 cases per 100,000 people [127]. Muscle invasive BC accounts for 20–25% of newly diagnosed cases of BC whereas the remaining present as non-muscle invasive BC (“superficial” or NMIBC) with approximately half recurring and many of these advancing toward muscle-invasive disease [127]. The relatively high societal burden of BC (relatively common, potential lethality) support the pursuit of maneuvers or interventions to decrease the risk of developing muscle invasive BC. Understanding risk of developing BC but especially muscle invasive BC is critical to developing successful prevention strategies.
The various risk factors for BC can be thought of relative to a person’s ability to avoid or alter the contributing factor. Inherited genetic predispositions have been identified for BC, but interestingly the identified genes (e.g. N-acetyl transferase enzymes or solute carrier family 14) have a prominent role in controlling urothelial exposure to environmental carcinogens [128]. This highlights the importance of avoiding exposure to these carcinogens. Smoking is recognized as the most important risk factor for BC and is estimated to account for half of all BC tumors. There is a direct pathophysiologic link between tobacco and BC through tobacco smoke containing aromatic amines (e.g. β-naphthylamine, polycyclic aromatic hydrocarbons) that are renally excreted and exert a carcinogenic effect on the entire urinary system [128]. Additional environmental exposures (risk factors) which are associated with 20% of BC cases are other aromatic amines, polycyclic aromatic hydrocarbons, and chlorinated hydrocarbons related to industrial areas processing paint, dye, metal, and petroleum products and to a lesser extent arsenic exposure [128]. Dietary factors and BC are not consistently linked with data for alcohol, coffee, red meat and other dietary/nutrient factors showing equivocal results relative to BC risk [128]. Less pertinent to BC in North America, but a significant cause of BC in other parts of the world is Schistosomiasis, a common tropical disease affecting >200 million worldwide [129]. S. haematobium is the causative agent of urogenital schistosomiasis which is associated with a high incidence of squamous cell carcinoma of the bladder [129].
The most important risk factor for potentially lethal muscle invasive bladder cancer is the occurrence of non-muscle invasive BC [127]. This increased risk correlates with high grade disease, multiple tumors, tumors greater than 3 cm, recurrence within 1 year, and recurrence after previous intravesicular therapy [130]. In order to better focus risk reduction interventions, researchers continue to explore more accurate tools for prediction including risk stratification tables, scoring and better characterization of possible risk factors like diminished renal function (GFR < 60 ml/min), Diabetes Mellitus or tumor lymphovascular invasion [130].
Current uniformly accepted approaches for invasive Bladder Cancer prevention focus on diminishing tobacco exposure and intravesicular therapy for high risk non-muscle invasive bladder cancer. Intravesicular Bacillus Calmette-Guérin (BCG) exposure/infection is a nonspecific stimulant to the reticuloendothelial system and induces a local inflammatory response with the infiltration of immune effector cells and induction of a wide range of cytokines [131]. Animal studies have shown that effective BCG immunotherapy requires an immune competent host, an adequate dose of BCG, juxtaposition of BCG and tumor cells, and a limited number of cancer cells. These conditions are optimally met in bladder cancer, especially CIS.
Randomized clinical trials in non-muscle invasive BC have demonstrated the following: intravesicular therapy reduced tumor recurrence compared with surgery alone, BCG has usually produced better results than chemotherapy (doxorubicin, thiotepa, MMC), BCG administered at full dose via the 3 week, 3-year maintenance schedule used by SWOG has produced the best results, and a recent study has raised the profile of intravesicular gemcitabine for low grade non-muscle invasive BC [131, 132].
Ongoing or Planned Novel Considerations for Preventing Bladder Cancer
In addition to ongoing exploration of novel formulations, schedules or combinations of intravesical BCG or chemotherapy, multiple systemic approaches have been tried based on bladder cancer etiology or risk factors. Examples of agents recently tested in larger randomized phase 2 or 3 trials with little to no evidence of reducing risk of recurrent non-invasive or invasive BC include difluoromethylornithine (DFMO), fenretinide, celecoxib and selenium.
Activated epidermal growth factor receptor (EGFR) has long been implicated in/associated with urothelial carcinogenesis [133] leading to recent and ongoing NCI-sponsored early phase trials of genistein (which observed diminished phosphorylation of EGFR in urothelial tissue/tumor [134] and weekly erlotinib (personal communication T Downs and E Messing). Epidemiologic data and mechanistic targets of interest have continued early phase studies examining nutrients such as sulforaphanes or green tea polyphenols [135, 136]. Additional targets arising from urothelial carcinogenesis are agents targeting directly or indirectly the PTEN/PI3 kinase/AKT/mTOR pathway [133].
Results correlating non-muscle invasive BC to Diabetes Mellitus and less consistently to obesity have led to interest in Metformin, a biguanide approved as an oral hypoglycemic agent. Cohort and retrospective studies of diabetic patients with non-muscle and muscle invasive BC have observed strong associations between reduced risk and Metformin usage [137]. Proposed mechanisms of anticarcinogenic effects of metformin include regulation of Insulin-Like Growth Factors, alteration of oxidative stress and autophagy.
The success of intravesical BCG coupled with the ever-expanding knowledge of cancer immunology (increased PD-L1 expression in non-muscle invasive BC) is leading to the study of multiple additional immune-based approaches to BC prevention or treatment of non-muscle invasive BC [138, 139]. Interferon and the oral interferon inducer, bropirimine, have been studied alone and combined with BCG without clear evidence of added value. Keyhole limpet hemocyanin (KLH) is a high-molecular-weight glycoprotein, which induces both cell-mediated and humoral responses in animals and humans and is undergoing evaluation in “at risk” populations via both intravesical and dermal applications, as is intravesical Urocidin (mycobacterial DNA cell wall complex) [139]. Multiple immunotherapy approaches are being examined in non-muscle invasive BC including vaccines undergoing testing in BC therapy (NY-ESO-1 and MAGE-A4) and checkpoint inhibitors (ipilimumab, atezolizumab, pembrolizumab) [138, 139]. An area of increasing interest in cancer chemoprevention is epitope-specific vaccines based on known pathways of interest in tissue-specific carcinogenesis.
Based on the societal burden of BC and the relatively easy access to urothelial tissue, BC prevention should continue to be a priority.
Caring for Bladder Cancer – Can We Reduce the Cost Burden?
Ekaterina I. Noyes, PhD, MPH
Bladder cancer (BC) is the most costly cancer among the elderly, estimated at nearly $4 billion per year. Bladder cancer is responsible for 70,000 new diagnosed cases and over 15,000 deaths in the US annually. The cost of bladder cancer management includes costs of lifelong cystoscopic examinations, multimodal curative therapy, palliative care and supportive services. This financial burden of bladder cancer is further compounded by lack of adequate health insurance, out-of-pocket costs and emotional burden on patients and their families.
Given the aging of the population and the continued technological advances likely to occur over the next decade, such as new urinary markers for BC, improved endoscopy, and the evolving role of minimally invasive surgery, costs of managing patients with BC will likely continue to rise and each patient’s out-of-pocket costs will likely increase.
Researchers, providers and policy makers are working on identifying strategies to control the cost of bladder cancer management, especially the out-of-pocket patient costs. Analysis of intravesical chemotherapy after transurethral resection of bladder tumor (TURBT), neo-adjuvant therapy for cystectomy, and robot-assisted laparoscopic cystectomy suggested that these technologies are cost effective and should be implemented more widely for appropriate patients. Among other ways of reducing costs associated with BC are reduction of the rate of preventable post cystectomy complications, pre-and post-operative rehabilitation, and implementation of Enhanced Recovery After Surgery (ERAS) protocol. The primary post-operative complications and readmissions have the most significant impact on the health outcomes and cost of care in bladder cancer. Hence, multi-disciplinary strategies to reduce post-operative complications and facilitate patient recovery demonstrate high potential for being cost-effective. While all BC guidelines discuss the importance of post-treatment surveillance across all disease stages, imaging, however, which is often done via computerized tomography (CT), has both financial costs and a small but real risk of secondary malignancy from the ionizing radiation it uses. More research is needed to better understand overutilization of post-operative surveillance.
A growing body of evidence indicates that specially trained cancer patient navigators could be cost-effective in improving patient-provider communication, treatment adherence, and patient satisfaction, especially among vulnerable populations with limited health literacy and no regular source of care. Patient navigators often come from the same community as the patients themselves and understand the patient’s preferences and values. The navigator accompanies the patient throughout the entire treatment journey, across all care settings and providers, while helping to solve problems and overcome any barriers he/she may experience, both personally and clinically. Navigators can help patients communicate and build relationships with providers, educate patients about the importance of scheduled tests and procedures, and assist with adherence to complex treatment regimens. They can also help arrange appointments and transportation, complete disability paperwork and insurance enrollment forms, clarify discharge instructions, and link patients with available resources in the community, as needed.
In summary, overcoming economic barriers to high-quality bladder cancer care is an important priority for our healthcare system. This involves not only identifying cost-effective treatment alternatives whenever possible, but also understanding the role of social determinants of health and system-level barriers to care while working closely with patients, community partners, social services and other providers to help create seamless care transitions and improve patient experience and quality of life.
Session VI: Improving Diagnoses and Outcomes
Session Moderator: Gennady Bratslavsky, MD
A Clinical Trial for Localized Kidney Cancer: Is it Possible?
Steven C. Campbell, MD, Ithaar Derweesh, MD, and Edward Messing, MD
The list of randomized trials for localized RCC remains short, primarily consisting of 2 studies from the EORTC, namely 30881 (LND vs. no LND from 2009) [140] and 30904 (PN vs. RN from 2011) [141]. Beyond this there were a few small randomized trials comparing different approaches to minimally invasive radical nephrectomy which are no longer particularly impactful. The paucity of randomized trials for localized RCC is particularly striking when compared to the large database of randomized studies that have been completed for metastatic RCC.
EORTC 30904 is often criticized but it should be noted that it was conducted in exactly the population for which PN should have been most advantageous (median tumor size was 3.0 cm, and many patients likely had preexisting CKD, because “normal contralateral kidney” was assessed by review of imaging and serum creatinine levels, rather than more rigorous methods) [141]. Over 540 patients were randomized and followed for a median of 9.3 years, and an overall survival advantage for PN was not observed, despite better long-term renal function in the PN cohort [142]. Subsequent studies have demonstrated that CKD that is primarily due to surgical removal of nephrons tends to be much more stable (decline of function averages 0.7 ml/min/1.73m2 per year, similar to the aging process), and such patients have survival outcomes that are nearly identical to patients who do not have CKD [143].
Despite this, PN has become the standard of care for localized RCC, even for larger tumors that have increased oncologic potential. One argument has been that PN provides oncologic outcomes equivalent to RN, so we might as well prioritize the functional issues. Retrospective studies support this although selection bias has been a major concern. For instance, the recent study from Shah (2017) [144] included 1250 patients with cT1-2 RCC, including 336 managed with RN and 914 managed with PN. The 5-year unadjusted RFS was equivalent (89–90%) in both groups. However, the RN group included tumors with significantly increased tumor size and grade, more clear-cell and sarcomatoid histology, and more tumors upstaged to pT3. Overall, PN was mostly used for small renal masses, and RN for substantially more aggressive tumors, such as cT1b/T2. Keeping this in mind, the oncologic outcomes were very informative: 1) positive margins: 7% for PN and 0% for RN; 2) local recurrences: 4.4% with PN vs. 0.9% with RN; and 3) systemic recurrences were 3-fold more common for the RN cohort, reflecting more aggressive tumor biology [144]. Based on this, we can extrapolate that if PN was used for tumors with similarly increased oncologic potential, tumor recurrences would increase by approximately 3-fold, so rather than 90% RFS for PN at 5 years as seen in the Shah study, it would more likely be in the range of 70–80% for PN, substantially worse that what was reported for the RN group.
We are now proposing a randomized clinical trial for patients with cT1b/T2 tumors, with the hypothesis that RN will provide better 5-year RFS than the current standard of PN, and will also be associated with reduced perioperative morbidity and costs. Imprudent use of PN can have harmful consequences, [144] and such a trial will improve patient management and provide important information about the tumor biology of these tumors, which are understudied [145].
Equipoise about this topic has improved greatly over the past 5–6 years, and current estimates suggest that only about 400 total patients will be required for this study. Dr. Messing has played a critically important role as one of the leaders for our team that has been trying to initiate this study over the past several years [142, 146].
Novel Therapeutic Options for Non-Muscle Invading Bladder Cancer
Leonard G. Gomella, MD, FACS
Introduction
Bladder cancer is the fourth most common cancer in men and is less common in women. Non-muscle invasive bladder cancer (NMIBC) accounts for up to 70% of cases in the US. Through improved early diagnosis and treatments, 5 year survival for NMIBC is over 90% [147].
Low grade bladder cancer is usually treated by repeat ablations. Intermediate and high grade NMIBC requires additional intervention such as intravesical therapy. Studies have demonstrated the superiority of intravesical BCG over chemotherapy in high grade NMIBC in reducing recurrences and progression. The exact mechanism is still under investigation but BCG is one of the most effective immunotherapeutic agents in oncology.
While BCG has improved outcomes of high grade NIMBC, up to 1/3 of patients do not respond. With an initial response, over half will recur and progress long term [148]. The only FDA approved agent when BCG fails is intravesical valrubicin. This group of patients has limited options, with radical cystectomy the primary alternative.
Recognizing this is an area of unmet need, in 2018, the FDA issued guidance on developing treatments for BCG unresponsive NMIBC [149]. The FDA, working with bladder cancer experts, defined BCG-unresponsive disease as at least one of the following: (a) persistent or recurrent CIS or with recurrent Ta/T1 disease within 12 months of BCG, (b) recurrent high-grade Ta/T1 disease within 6 months of BCG or (c) T1 high-grade disease following BCG induction. An overview of the investigational approaches to BCG refractory NMIBC is presented here [150].
Intravesical Chemotherapy
Interest continues in intravesical single agent and combination chemotherapy, and in enhancements to improve the utility of chemotherapy. Valrubicin is the only FDA approved agent in the BCG refractory space and mitomycin is commonly administered following TURBT and is an alternative to BCG. Doxorubicin, epirubicin, gemcitabine, docetaxel, cisplatin and cabazitaxel have all been studied with limited success. A few combinations of these agents for NMIBC have shown promise [151]. Enhancements to improve intravesical chemotherapy include radiofrequency-induced thermochemotherapy with mitomycin C and a unique gemcitabine-releasing intravesical implant system.
BCG Based Approaches
Dose reduction to 1/3 dose is efficacious in intermediate-risk but not in high-risk NMIBC. Combining BCG with intravesical chemotherapy or adding cytokines to BCG (BCG + interferon (IFN) alpha) has not impacted recurrence or progression [152]. SWOG is revisiting percutaneous vaccination as a new intravesical strain of BCG is being brought to the US. SWOG 1602 is evaluating BCG strain differences and the role BCG vaccination in BCG-naïve high-grade NMIBC comparing Tokyo-172 BCG to TICE [153].
Novel Intravesical Immunotherapies
Bladder cancers have a high rate of somatic mutations similar to other tumors associated with environmental carcinogens [154]. This pattern suggests that other immunotherapy approaches beyond BCG are reasonable to investigate. Vicinium (VB4-845) fusion protein alone and in combination with durvalumab, vaccinia virus, CG0070 oncolytic adenovirus, Gp96 (pan-antigen cytotoxic therapy) intradermal vaccine, Mycobacterium cell wall-nucleic acid complex (MCNA), ALT-803 (IL-15 super agonist) and imiquimod (TMX-101) are a few of the novel immunomodulatory agents of interest [150].
A Phase III multicenter Society of Urologic Oncology trial has just completed enrollment. It investigated if recombinant adenovirus IFN alpha with Syn3 (rAd–IFNa/Syn3), a replication-deficient recombinant adenovirus gene transfer vector, can improve responses after BCG failure [155]. If this trial is positive, this is likely to be one of the next agents approved. A phase II trial using this agent after BCG failure demonstrated a 35% 1-year recurrence free survival [155].
Checkpoint Inhibitors
To capitalize on the approved checkpoint inhibitor immunotherapies for metastatic bladder cancer, attention is now being turned to using these agents in NMIBC [156]. Systemic monotherapy with pemrolizumab (KEYNOTE-057) and atezolizumab (SWOG 1605) are being investigated for BCG non-responsive NMIBC. Combination trials with systemic checkpoint inhibitors atezolizuzumab + intravesical 1/3 vs 2/3 vs full dose BCG (WO29635) and durvalumab plus intravesical BCG and durvalumab + EBRT (ADAPT-BLADDER) represent highly innovative NMIBC approaches.
Conclusion
Using our current management of high risk NMIBC many patients do well. However, as recognized by the FDA, there is a need to improve our treatments when BCG fails. Novel investigations with new and existing intravesical agents and exploring systemic immunotherapy are some of the approaches under study to manage the patient with high risk BCG refractory bladder cancer.
Ex-vivo Targeting of Upper Tract Urothelial Carcinoma Using Novel ICG-Var3 pHLIP Imaging Agent
Dragan Golijanin, MD
Upper tract urothelial cell carcinoma (UTUC) accounts for 5–10% of all urothelial neoplasms [157]. Over the past four decades, the incidence of UTUC in the USA increased by 10% [158]. The mainstay for diagnosis of UTUC includes clinical assessment of gross hematuria, hydronephrosis workup, increase in creatinine levels, flank pain, and upper tract assessment in patients with bladder cancer. Imaging approaches include CT and MRI with contrast, and cystoscopy or retrograde imaging followed by diagnostic ureteroscopy. These techniques are of limited accuracy in identifying upper tract lesions [159–161]. For example, white light URS for UTUC diagnosis is only 53% specific for malignant lesions, misdiagnosis can lead to unnecessary radical surgery or a missed opportunity for proper cure [160]. Incomplete and inaccurate findings by contemporary imaging modalities provide a challenge in patient care. There is a definitive need for improvement of diagnostic methods for UTUC.
The pH low insertion peptides (pHLIP) belong to a family of water soluble membrane peptides that target the acidic microenvironment of malignant cells based on the Warburg effect [162]. pHLIPs target low pH at the surface of cancer cells, where it is the lowest and independent of tumor perfusion, thus providing high specificity and sensitivity in tumor targeting [163]. In preclinical trials, pHLIP has been used for imaging and pH specific drug delivery [164, 165]. Among investigated pHLIPs variant 3 (Var3) demonstrated the best tumor targeting.
Our previous studies using a novel pH low insertion peptide (pHLIP) variant 3 (Var3) conjugated to indocyanine green (ICG) have demonstrated high sensitivity and specificity targeting of bladder urothelial carcinoma [166]. This time we used twelve fresh ex vivo upper tract specimens. Retrograde irrigation with ICG-Var 3 pHLIP was done for fifteen minutes. Near infrared fluorescent (NIRF) imaging was performed using Stryker 1588 AIM imaging system. Pathology findings were correlated with ICG-Var3 NIRF imaging and preoperative ureteroscopy. Urothelial ureteral, renal pelvis and calyceal mucosa from the patient who underwent radical nephrectomy for RCC, we used as negative control.
Twelve upper tract specimens were reviewed in the study: eleven with UTUC and one as a control (RCC). A total of 22 fluorescent lesions were identified by ICG-Var3 NIRF ex vivo imaging. All (n = 22) identified NIRF lesions had at least one major papillary component. Of these 22 lesions, seventeen were confirmed as high grade UTUC, eight as invasive and six as noninvasive UTUC.
Three lesions were low grade noninvasive UTUC. Of the nine invasive high grade lesions, two were presented with features of small cell carcinoma, and one with squamous differentiation.
Without the aid of ICG-Var3 pHLIP (white light assessment only), only fifteen lesions were grossly identified (68%). Of the seven lesions missed by white light assessment, six were high grade noninvasive papillary UCC and one low grade noninvasive UCC. ICG-Var3 pHLIP significantly improved detection of cancerous lesions (p = 0.01076). All regions stained by ICG-Var3 were confirmed as malignant by histopathology. ICG-Var3 successfully labelled high and low grade tumors of UT of various stages and subtypes. In all cases, the histology confirmed that non-involved, peri tumoral and distant, urothelium did not show any NIR signal. Ureter from a radical nephrectomy specimen for RCC was a true negative control (Fig. 1 j-l).
Fig.1
Demonstrates representative images of ICG-Var3 pHLIP targeting of high grade invasive (Fig. 1 a-c), high grade noninvasive (Fig. 1 d-f), and low grade noninvasive (Fig. 1 g-i) lesions.
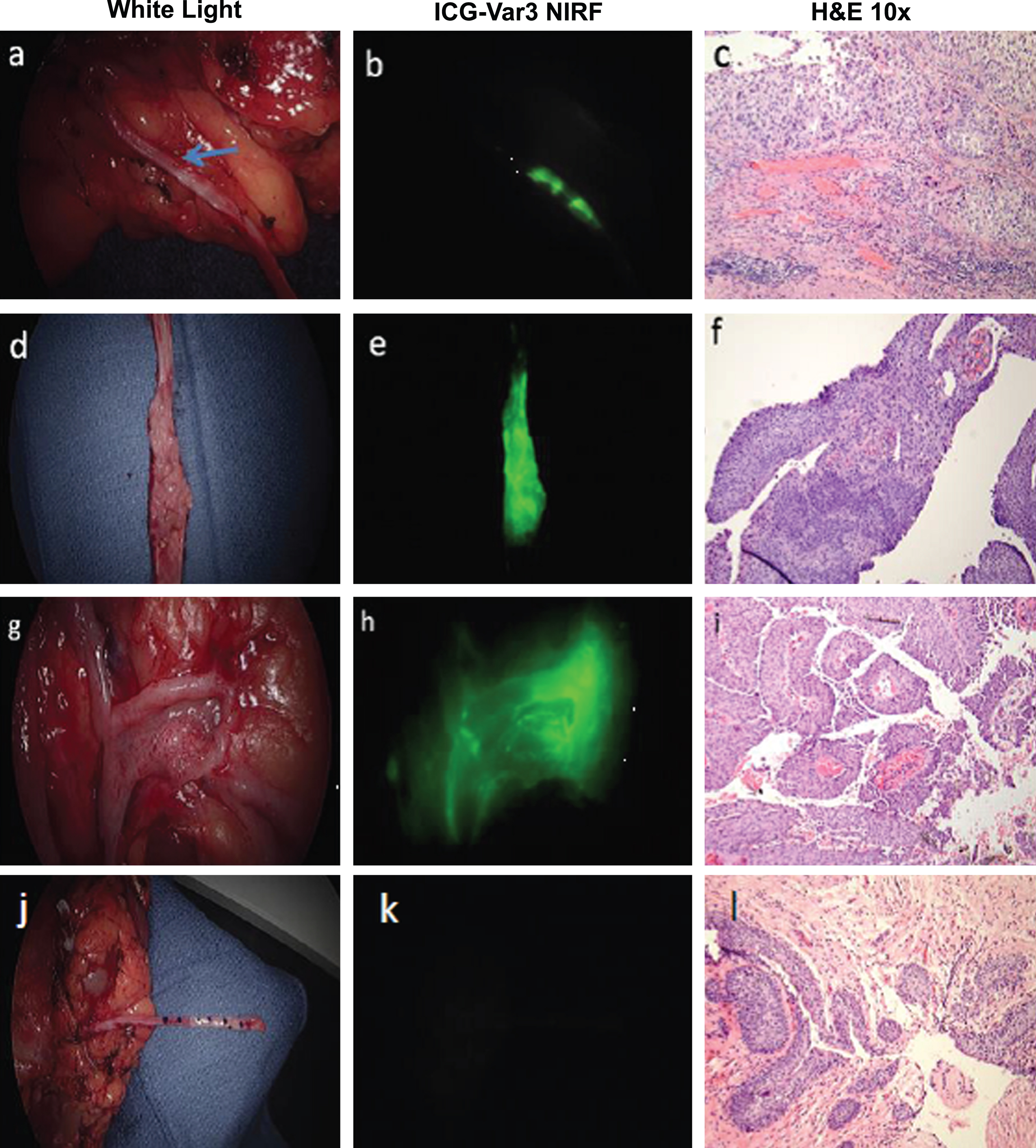
The ICG-Var3 imaging agent identified malignant upper tract urothelial lesions with 100% specificity and sensitivity. ICG-Var3 pHLIP NIRF diagnosed additional lesions (32% more) otherwise not seen with white light ex vivo cystoscopy or preoperative ureteroscopy.
The pHLIP-based agent offers promise in improving diagnostic accuracy for UTUC and has potential for targeted treatment of upper tract urothelial tumors.
Improving Efficacy of Intravesical Therapy
Robert S. Svatek, MD
Intravesical Bacillus Calmette-Guerin (BCG) is the standard of care treatment for the prevention of disease relapse in patients with papillary Ta and T1 bladder tumors following tumor resection and for the complete eradication of carcinoma in-situ (CIS). Following tumor removal, BCG is given weekly for 6 weeks, termed BCG induction. BCG is then given weekly for 3 weeks as maintenance cycles at 3 months, 6 months, then every 6 months (for 7 cycles or 3 years) following bladder tumor removal. This regimen is based on Level 1 evidence showing a significant benefit in terms of decreasing both recurrence and progression of bladder tumors [167, 168].
What are the current challenges and opportunities for BCG therapy? Despite long-standing and widespread use of BCG, we currently have no biomarkers that can help guide urologists for selecting patients for BCG therapy. Novel insights into disease pathogenesis and BCG responsiveness are expected to shed light on these areas and lead to improved personalization of BCG therapy. Second, how do we improve response rates for patients receiving BCG as first-line therapy? Since the first clinical use of BCG for treating bladder cancer in the 1970s, we have learned a considerable amount regarding the antitumor efficacy. Nevertheless, early experiments using preclinical models demonstrated key principles regarding BCG’s efficacy that still resonate today [169]. To be most effective, four conditions need to be met [170]:
1. Adequate number of viable organisms. Lower quantities of BCG and non-viable BCG components are generally ineffective. Thus intravesical instillations use extremely high quantities. For example, each vial of TICE BCG contains 100 to 800 million colony forming units. The vast majority of BCG is dispelled within hours of drug instillation. Thus agents to improve uptake and steady levels of BCG in the bladder mucosa could facilitate improved efficacy. For example, we conducted a phase I clinical trial which administered mitomycin C prior to BCG aiming to improved response rates and BCG uptake in patients with non-muscle invasive bladder cancer [171]. Other strategies to facilitate BCG attachment, adherence, or persistence could provide improved antitumor efficacy.
2. Close contact between tumor cells and BCG is required. The closer BCG is placed to the tumor, the more effective. The ability to instill BCG directly to the bladder surface contributes to its local effect and efficacy in bladder cancer. For tumors that arise in basal layers, BCG contact maybe limited. Tumor regression has been observed in cutaneous melanomas treated with BCG [172]. Therefore, could direct injection of BCG into more advanced tumors facilitate tumor regression?
3. Immunocompetence of the host – BCG does not work in models with deficient immune systems. In aging patients, many functional changes in immunity occur which could influence responses to immune therapy and BCG specifically. Further knowledge of BCG’s precise effector mechanisms of action will help guide clinical examinations of host deficiencies to identify potential non-responders. To improve host immunocompetence, one strategy being tested in S1602 is a phase III cooperative group trial testing the ability of intradermal BCG vaccination to boost response to intravesical BCG and testing the clinical efficacy between two different BCG strains (TICE versus Tokyo-172).
Small tumor burden – the efficacy of BCG wanes with increasing tumor size. Response rate to BCG was observed in 60% of guinea pigs with 100 mg tumor weights but in only 20% of guinea pigs with tumor weights of 500 mg [173]. Given this knowledge, could we identify other opportunities for BCG such as use in combination with radiotherapy for T2 disease either as an adjuvant post-radiation or concurrently during chemoradiation? Could BCG be used more strategically for upper tract disease through improved methods of instillation for low-volume disease.
In conclusion, the complete eradication of intravesical bladder cancer such as CIS by BCG remains one of the most successful and fascinating therapies in oncology. Historical observations of BCG’s preclinical activity offer key insights into opportunities for drug development and strategies to improve BCG response.
Early Stage Bladder Cancer – Risk Based Approach to Management
Mark S. Soloway, MD
The foundation for decision making for patients with urothelial cell carcinoma of the bladder (BC) is the cystoscopy and transurethral resection of the tumor (TURBT). The degree of difficulty of this underappreciated operation varies greatly. The goal of the urologist is to remove all tumors and submit bladder and sometimes prostatic urethra tissue that can be correctly interpreted by the pathologist. The extent of the TURBT depends on his/her perception of the grade and stage of the tumor(s). If the tumor is thought to be Ta one should avoid a deep resection; if the tumor appears to be high grade and possibly invasive beyond the basement membrane, detrusor muscle should be included in the specimen. The number, size, appearance (papillary or sessile), and location of the tumor(s) should be documented as this information is integral in assigning a risk category. Tumor grade is the most important prognostic factor. In performing the TURBT the urologist should minimize cautery artifact in acquiring the specimen.
The most common BC does not invade the basement membrane (i.e. pTa). Depending on various risk factors, e.g. grade, size, number, these patients are at risk of a subsequent tumor related to the carcinogen which caused the tumor, implantation following the TURBT, or incomplete resection. Importantly, patients with low-grade Ta tumors are at very low risk of stage progression and rarely die of BC [174]. Thus the goal of therapy should be to minimize the chance of a subsequent tumor and thus reduce the cost, inconvenience and morbidity of managing these patients [175, 176]. Post TURBT single dose intravesical chemotherapy, office fulguration, and active surveillance for small obvious LG Ta new tumors are methods which should be widely adopted to achieve these goals.
Urologists should be aware that there appears to be a grade migration for Ta bladder tumors [177]. As a result of the changes made by the International Society of Urologic Pathology the grade 1–3 system was changed to low and high grade. Many pathologists are diagnosing almost all papillary tumors with any degree of atypia as high grade. This implies that most of the prior grade 2 Ta tumors are now listed as high grade. This may lead to overtreatment of these biologically “benign” neoplasms [178]. For example, according to the EAU and AUA/SUO guidelines BCG is not indicated for LG Ta BC but is recommended for HG Ta/T1/CIS BC following a “complete” TURBT or, if needed, a confirmatory repeat TURBT for HG T1 BC. If the pathologist shifts his diagnosis from LG to HG for tumors which are grade 2 then this will likely lead to overutilization of BCG. This may also influence the use of more frequent cytology, surveillance cystoscopy, and upper tract monitoring as the clinician will manage these patients with biologically low risk tumors as if they are at intermediate or high risk for stage progression.
The use of the FISH test as a substitute for urinary cytology has increased the cost of management of patients with BC [179]. Since this is a chromosomal analysis the analysis is much more expensive then urine cytology. Voided and particularly bladder wash cytology is very sensitive for the identification of HG BC. Urologists should not be concerned with finding a LG BC since there is no urgency in identifying these biologically “benign” neoplasms.
Session VII: Challenges in Bladder Cancer Management
Session Moderator: Thomas Frye, DO
BCG Unresponsive Disease - A Roadmap for Drug Development
Seth P Lerner, MD, FACS
BCG unresponsive disease defines a disease state of non-muscle invasive bladder cancer for which BCG is no longer considered effective therapy [180]. Patients should have Ta or T1 high-grade (HG) disease or CIS and received at least one induction course plus one maintenance course of intravesical BCG and recurred with high-grade disease within 12 months of the last BCG treatment. Patients who recur with T1HG disease after induction only are also included. Guidelines indicate that radical cystectomy (RC) is standard of care for these patients who are medically fit and accepting of the surgical risks [181]. RC is associated with a high probability of long-term cancer control but at a cost of peri-operative morbidity and mortality risk. Valrubicin is currently the only US Food and Drug Administration (FDA) approved treatment for patients with BCG unresponsive CIS.
The FDA engaged in an active dialogue with the AUA and SUO to develop principles of trial design [182, 183] and published final guidance on a registration pathway in February, 2018 using a single-arm trial design, thus creating a huge opportunity for drug development and clinical trials in this disease state [https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
The underlying principle is that randomizing patients with BCG-unresponsive disease to a minimally effective drug as a concurrent control raises ethical concerns. Because effective drugs are not available and the alternative treatment is cystectomy, single-arm trials of patients with BCG unresponsive CIS disease with or without papillary disease are appropriate. The primary endpoint should be complete response where the lower bound of the 95 percent confidence interval should rule out a clinically unimportant CR rate and the median duration of CR is also meaningful.
Table 1
Active or recently completed clinical trials for patients with BCG unresponsive non-muscle invasive bladder cancer (clinicaltrials.gov accessed 8/11/2018)
| Ad-IFN gene therapy (FKD–SUO-CTC) |
| Atezolizumab (SWOG S1605 - Roche/GNE) |
| BCG + ALT-803 (Altor BioScience) |
| BGJ 398 FGFR targeted therapy – Ta but no CIS (MSKCC) |
| CG0070 (Cold Genesys) |
| Cabazitaxel, gemcitabine, and cisplatin Phase I (Columbia) |
| ChemoXRT for T1 (RTOG 0926) |
| Nab-Rapamycin-ABI-009 Phase I/II (AADi) |
| Nivolumab or Nivolumab Plus BMS-986205 (BMS) |
| Pembrolizumab (Merck) |
| sEphB4-HAS (USC) |
| Viccinium (Eleven Biotherapeutics) |
There are multiple clinical trials testing a variety of strategies including gene therapy, immune checkpoint inhibitors, novel cytokines, novel drug delivery and payloads, and combination therapies (Table 1). There are also several intravesical chemotherapy agents that can be used alone or in combination that show promise. Valrubicin is currently the only FDA approved drug with an indication for BCG unresponsive CIS. Dinney, et al. reported on 80 patients with CIS and the CR rate at 6 months was 18% but only 4% remained disease-free at 2 years [184] In SWOG S0353, patients were treated with induction intravesical gemcitabine followed by monthly maintenance for up to 10 months [185]. Among 47 evaluable patients, 47% were disease free at 3 months and 28 and 21%, respectively, remained disease free at 12 and 24 months. Gemcitabine has been used in combination with Mitomycin C or Docetaxel with 2-year disease free rates reported in 38% and 24–34%, respectively [186–188]. Device-assisted therapy may also be effective and is designed to deliver higher and sustained doses of intravesical chemotherapy. This can be achieved with microwave or electromotive therapy [189, 190].
Challenges remain in identifying those patients who should proceed to cystectomy vs. treatment with a different intravesical or systemic therapy approach, whether as a participant in a clinical trial or off trial treatment. The future is bright and it is highly likely we will see new treatment alternatives to cystectomy approved by the FDA for patients with BCG unresponsive disease.
Disease Progression- Utility of Prognostic Markers
Ganesh Palapattu, MD
Understanding the biological underpinnings of urologic cancers is critical to improving patient care. Unfortunately, as all of us who take care of patients with cancer understand, no two patients are alike. From clinical observation, we know that disease for disease, stage for stage and grade for grade, outcomes are not identical. To this end, biomarkers have been sought that allow prediction of disease trajectory (prognostic) and permit disease classification based upon anticipated treatment response (predictive).
Biomarker Development
Generally speaking, biomarker development has four phases [191]. The initial phase (phase I) consists of biomarker discovery. In this phase, laboratory science based on genomics, proteomics and/or metabolomics, identifies a putative biomarker. Typically, some initial assessment of test sensitivity and specificity is performed and the pre-clinical assay is moved forward to the next phase (phase II), clinical assay development. Here, the objective is to optimize the assay itself (analytic validity) and perform additional clinical testing on targeted disease positive samples. In phase III, criteria for a “positive” test are defined and more robust clinical testing on controls and cases of varying stage and grade in the context of relevant possible co-variates is done. Clinical testing in this phase is often composed of retrospective longitudinal studies. The goal of phase IV is to prospectively quantify the performance characteristics of a biomarker in the target population of interest. Here, sensitivity, specificity, positive, and negative predictive values are developed and clinical validity and utility defined. In phase V, the influence of the test on cancer control and population health is assessed. As one might imagine, moving a biomarker along the pathway from discovery to cancer control assessment is time consuming and costly. The Early Detection Resource Network (EDRN) program of the NCI Division of Cancer Prevention has been instrumental in getting clinically impactful biomarkers to the clinic via coordinating efforts among academic, industry and regulatory partners [192]. Such large scale collaborative programs are essential to translating laboratory discoveries into clinical reality.
Tumor Heterogeneity and Biomarkers
An inherent confounder surrounding the application of biomarkers to providing reliable information on disease progression is the issue of tumor heterogeneity [193]. Using sophisticated next generation sequencing approaches, we now know there are profound molecular differences among individual foci of cancer within a tumor, between tumors and across patients with the same histological type of tumor [194]. While the overall number of disease associated molecular alterations maybe relatively small for a given disease, such biological heterogeneity can make identifying clinically relevant and/or dominant foci challenging to discriminate and even harder to validate. Recognizing this, many teams have begun to combine multi-dimensional biomarkers (e.g., imaging, urine, serum, tissue based biomarkers) to capture as much biological information as possible [195].
In the field of bladder cancer, biomarkers have been avidly pursued to answer many important clinical questions [196], such as: How can the intensity of surveillance be optimally tailored to patient risk? Who will rapidly fail BCG therapy? In whom will neoadjuvant chemotherapy be helpful? In whom will adjuvant systemic therapy be most helpful? And, how can we better predict who will respond to systemic targeted and/or immune based therapies? The answers to these and many other similar questions will be needed to improve the care of our patients in the future.
Maximizing Immune Checkpoint Therapy for Bladder Cancer
George Wilding, MD
Immune checkpoint (IC) therapy represents a paradigm shift in oncology and has become a cornerstone for the treatment of patients with metastatic urothelial cancer (mUC) in the post-platinum and platinum-ineligible populations. Two monoclonal antibodies targeting programmed cell death protein-1 (PD-1) including nivolumab and pembrolizumab, [197–199] and three monoclonal antibodies targeting programmed death receptor ligand 1 (PD-L1) including atezolizumab, avelumab, and durvalumab, [200–203] have been approved by the FDA as first-line or second-line treatment of mUC.
Despite these historical successes, IC therapy only achieves clinical response rates of about 20%; the majority of patients do not benefit from IC therapy. Therefore, identification of biomarkers to guide IC therapy and understanding the mechanisms of resistance is important to maximize the clinical benefits of IC therapy. As a target for anti-PD1/PD-L1 therapy, tumor PD-L1 expression seems to be a natural predictive biomarker for patient selection. However, data from studies in this area have been inconsistent and disappointing. A number of recent findings indicate that defective interferon signaling inside tumor cells is an important resistance mechanism to IC therapy [204–206]. In addition, TGF-β produced from tumor-associated fibroblasts has been reported to attenuate anti-tumor response to PD-L1 blockade by exclusion of T cells from bladder tumors [207].
Multiple strategies are being investigated to improve therapeutic efficacy of IC therapy. One approach is to utilize combination immune checkpoint therapies. In this aspect, ongoing clinical trials are testing anti-PD1/PD-L1 in combination with anti-cytotoxic T lymphocyte-associated protein-4 (CTLA-4). In addition, anti-PD1 agents are being tested in combination with tumor immune microenvironment modulators such as the IDO inhibitor, epacadostat. Moreover, anti-PD1/PD-L1 and anti-CTLA-4 agents are being tested in combination with multiple regimens of chemotherapy. Furthermore, bi-specific molecules that target multiple negative immune regulatory pathways such as PD-L1 and TGF-β also appear to be appealing tactics to augment the efficacy of IC therapy. Recent clinical studies on target therapy agents such as FGFR3 inhibitors have also made significant progress. One of these FGFR3 inhibitors, erdafitinib, has recently gained the breakthrough status from the FDA for the treatment of mUC with FGFR3 mutations. An antibody drug conjugate, enfortumab vedotin, which targets Nectin-4 on bladder tumor cells, recently earned the FDA’s breakthrough designation as well. These two agents may also serve as combination partners for IC therapy.
Although these combination therapies appear to be rational and attractive approaches to overcome resistance to IC therapy, they can also be associated with life threatening toxicities termed as immune-related adverse events (irAEs) including hypophysitis, pneumonitis, hepatitis, and colitis, etc. Understanding the mechanisms of these irAEs in order to provide timely treatment is critical for maximizing the clinical efficacy of IC monotherapy and combination therapy.
Overall, IC therapy represents a remarkable development that brings new treatment options to patients with bladder cancer, although it has relatively low response rates. Future research to further understand mechanisms of resistance, identify biomarkers to guide therapy, develop novel combination strategies, and manage immune related toxicities will be essential to maximize the benefit of IC therapy. The next few years should see a dramatic growth spurt of immune checkpoint blockade to develop into a more mature and effective strategy in cancer therapy.
Neoadjuvant Chemotherapy for Urothelial Bladder Cancer: Observations, Opinions and Challenges
Michael J. Droller, MD
Patients with pathologically organ-confined muscle invasive bladder cancer who undergo cystectomy have a 5-year recurrence-free survival of 65–75% [208]. Those with non-organ-confined disease have a recurrence-free survival of only 30–50%. The majority of failures are with distant metastases (10–27% in the former; 19–35% in the latter) [209]. The presumed occult metastases at diagnosis in these patients as apparently accounting for these failures prompted several prospective randomized studies to determine whether neoadjuvant chemotherapy might attack these metastases and thereby improve both disease-free and overall survivals.
The Southwest Oncology Group (SWOG) trial, as the most widely referenced trial among these, reported a median survival of 77 months with neoadjuvant MVAC chemotherapy vs 46 months in cystectomy-only patients [210]. At 5 years, 57% in the combination therapy group were alive compared to 43% in the cystectomy-only group (stratified log rank p = 0.06). Disease-specific survivals were reflected in bladder cancer deaths in 77 of the cystectomy-only group vs 54 in the chemotherapy group (HR 1.66). Importantly, 38% of cystectomy specimens in the chemotherapy patients demonstrated no cancer (pT0) vs 15% in the surgery-only group (HR < 0.001). The apparent impact of this was seemingly manifest in the significantly longer survival of pT0 patients vs that in patients with any degree of residual disease (13.6 years vs 3.4 years, respectively). The interpretation was that down-staging to pT0 was reflective of the sensitivity of the primary cancer to chemotherapy, presumably indicative of a similar response by the occult metastases assumed to be present, this resulting in longer overall and disease-free survivals.
The confounding issue challenging this interpretation was the apparently equally advantaged survival in patients in the resection-only control group who also were pT0 at cystectomy [208, 210]. Thus, 85% and 82% of the pT0 patients in each group respectively were alive at 5 years. This was significantly longer than in all those patients in each treatment group who had residual disease.
Several considerations other than a presumed effect of chemotherapy could also explain these findings. For example, the distinctive developmental pathways of different forms of bladder cancer could reflect their intrinsic biologic potential with a greater or lesser likelihood of deep invasion and metastasis [208, 211, 212]. Additionally in this context, the number and rigor of transurethral resections during the course of a cancer’s development and/or recurrences could imply a less extensively invasive diathesis initially or a less aggressive biologic behavior during its developmental course, treatment outcomes reflecting “length bias sampling” [201, 206]. Alternatively, earlier diagnosis during a more prolonged progressive course and a resultant “lead time bias” might also have influenced treatment outcomes [208, 213].
A 5% absolute risk reduction in overall survival and an expected 9% reduction in cancer-specific mortality as reported in SWOG and the other randomized studies translates into the need to treat at least 20 patients with neoadjuvant chemotherapy regarding overall mortality and 11 for cancer-specific mortality to benefit only 1 patient in each category [208, 213]. The remaining 19 patients are then seemingly unnecessarily subjected to the potential morbidities of chemotherapy without apparent benefit but with the risk of toxicities and complications of chemotherapy that might delay or exclude patients from receiving cystectomy as definitive treatment for their disease [214]. In effect, the negative aspects of neoadjuvant chemotherapy might far outweigh its potential benefit in a majority if not all patients with muscle-invasive disease.
Taken together, several fundamental challenges remain: 1 - to characterize the developmental pathway of a given muscle-invasive bladder cancer and more accurately stage the disease; [215] 2 -to exploit those genetic and molecular features that determine the intrinsic biologic behavior of a particular malignancy and its chemo-sensitivity; [216, 217] 3 - to better identify and select those who may truly benefit from a more precisely directed treatment approach in reducing risk and optimizing treatment outcomes [211, 213].
Calcitriol (1,25 dihydroxycholecalciferol) and Cancer Therapy: A Missed Opportunity
Donald L. Trump, MD, FACP
Proteins important in vitamin D signaling (vitamin D receptor and vitamin D metabolizing enzymes) are expressed in most cancer cells and treatment with calcitriol (1,25dihydroxycholecalciferol) in vitro and in vivo inhibits tumor growth. This presentation reviews studies of calcitriol in the treatment of cancer.
The biochemical changes associated with anticancer effects of calcitriol include: [218] cell cycle arrest and modulation of CDK inhibitors (e.g. p21 and p27), [219] apoptosis induction, [220] suppression of “pro-proliferative” molecules such as P-MAPK (ERK1/2), P-AKT and MEKK-1. Complex cellular mechanisms may be disrupted following calcitriol exposure: [218] inhibition of angiogenesis, [219] inhibition of motility/invasion, [220] induction of differentiation, [221] modulation of growth factor production by stromal or tumor cells. Calcitriol impacts monocyte & macrophage differentiation, T cell function and cytokine production. Studies in non-Hodgkin lymphoma indicate that low levels of 25(OH)D3 are associated with poor outcome following therapy with cytotoxic chemotherapy + rituximab. Rituximab-mediated killing of lymphoma cells in vitro by an individual’s monocytes is substantially enhanced by restoration of normal serum vitamin D levels. Hsu and colleagues demonstrated that calcitriol enhances IL-8 secretion from bladder cancer cells (BCa) following Bacillus Calmette-Guerin (BCG) exposure; this enhances macrophage motility and BCa killing [222]. Intravesical application of calcitriol + BCG extends survival in mice with carcinogen (BBN)-induced bladder cancer. Calcitriol may inhibit tumor growth by modifying the activity of enzymes such as CYP3A4, AKR1C1-3, HSD17B2, CYP27A1 and SULT2B1b, which can disrupt sex steroid metabolism in prostate, breast and endometrial cells. In prostate models microRNA-98 suppresses cell growth and is induced following calcitriol exposure; enhanced expression of co-repressors NCoR1 and SMRT is associated with reduced responsiveness to calcitriol.
There have been extensive preclinical studies of calcitriol-based combination therapies. Synergy between calcitriol and glucocorticoids, inhibitors of CYP24A1, NSAIDs, retinoids and cytotoxic agents have been well described, in vitro and in vivo. Calcitriol potentiates the cytotoxicity of platinum compounds, anthracyclines, topoisomerase inhibitors, antimetabolites, and taxanes. These effects are most pronounced when calcitriol is administered before or simultaneously with the cytotoxic agent.
There have been many clinical trials seeking to define the clinical benefit of calcitriol in cancer therapy. None has provided strong evidence of benefit. Three factors have played an important role in this failure: [218] most trials have been single institution trials and such trials are usually underpowered; [219] most studies tested combinations of calcitriol and drugs with established activity, confounding the assessment of antitumor activity; [220] preclinical studies indicate that high exposure to calcitriol is important in its anticancer activity. There has not been one randomized trial testing the efficacy of the maximum possible dose of calcitriol + cytotoxic agent. Calcitriol (80–120mcg weekly i.v. plus cytotoxic agents) can be given safely to cancer patients. In these trials neither unusual toxicity nor potentiation of cytotoxic agent toxicity has been noted. The only toxicity of calcitriol described has been transient hypercalcemia. Another feature limiting development of this agent is the limited patent protection available for calcitriol. Novocea, a now defunct pharmaceutical company conducted 2 large clinical trials of docetaxel + a novel formulation of calcitriol in men with castration resistant prostate cancer (CRPC). The first was inadequately powered to detect a survival advantage though survival and PSA responses were better in the calcitriol arm of this randomized trial. The second trial was poorly designed, testing an inferior dose/schedule of docetaxel + calcitriol vs. a superior docetaxel dose/schedule + placebo. The placebo arm was superior in this trial of almost 1000 men with CRPC. In neither of these trials was an optimal dose of calcitriol used (45mcg weekly vs. the known MTD of >100mcg weekly). With the completion of these trials the community of investigators has concluded that calcitriol is not an effective cancer therapy, despite considerable preclinical data – and no robust clinical trials data.
Conflicts of Interest and Financial Disclosures
Jean V. Joseph, MD – Consultant: Intuitive Surgical
Ralph Brasacchio, MD - no COI or financial disclosures relevant to this presentation
Chunkit Fung, MD - Advisory Board: Exelixis, no COI or financial disclosures relevant to this presentation
Jay Reeder, PhD - Founder and Chief Technology Officer, EndoGlow. Scientific Review Officer, General Dynamics Information Technology. Relationship not relevant to the content of the article
Kevin Bylund, MD - no COI or financial disclosures relevant to this presentation
Deepak Sahasrabudhe, MD - no COI or financial disclosures relevant to this presentation
Shu Yuan Yeh, PhD - no COI or financial disclosures relevant to this presentation
Ahmed Ghazi, MD - Olympus America: consultant; Intuitive Surgical: research grant
Patrick Fultz, MD - no COI or financial disclosures relevant to this presentation
Deborah Rubens, MD - no COI or financial disclosures relevant to this presentation
Guan Wu, MD, PhD - no COI or financial disclosures relevant to this presentation
Eric Singer, MD, MA, FACS - Research support from Astellas/Medivation
Edward Schwarz, PhD - no COI or financial disclosures relevant to this presentation
Supriya Mohile, MD - no COI or financial disclosures relevant to this presentation
James Mohler, MD - no COI or financial disclosures relevant to this presentation
Dan Theodorescu, MD, PhD - available under Board Disclosures on the website: http://www.bladdercancerjournal.com
Yi-Fen Lee, PhD - no COI or financial disclosures relevant to this presentation
Paul Okunieff, MD - GainPep Inc – founder and stockholder and patent beneficiary through University of Rochester and NIH BAA grant–growth factor biobetter; DiaCarta Inc – founder and stockholder and consultant and an active SBIR’s – biomarkers, genomics; Entrinsic Health Inc – founder and stockholder and consultant and patent beneficiary through University of Florida and multiple grants (WHO, industry, NIH, NASA) – medicinal foods; PiPer Inc – No financial interest to me or my family, based on University of Rochester intellectual property; ViewRay Inc – No financial interest me or my family, based on University of Florida intellectual property
David McConkey, PhD - Grant support: Astra-Zeneca
Stock options: ApoCell, Inc
Hani Rashid, MD - no COI or financial disclosures relevant to this presentation
Chawnshang Chang, PhD - no COI or financial disclosures relevant to this presentation
Yves Fradet, MD - Grant from Astellas, consulting for Merck, Roche, AstraZeneca, Astellas, Sanofi.
Khurshid Guru, MD - no COI or financial disclosures relevant to this presentation
Janet Kukreja, MD, MPH - no COI or financial disclosures relevant to this presentation
Gerald Sufrin, MD - no COI or financial disclosures relevant to this presentation
Yair Lotan, MD - Consulting: AstraZeneca, Photocure, Pacific Edge, MDxHealthResearch: FKD, Abbott, Cepheid, Photocure, Pacific Edge, MDxHealth, Biocancell, Dannone, Profound, DSMC: Urogen, Equity ownership/stock options and consultant: Vessi Medical. Research: Genomedx
Howard Bailey, MD - Advisory Board – Capio Biosciences; Forte Research Systems, Inc.
No other potential COI.
Katia Noyes, PhD, MPH - no COI or financial disclosures relevant to this presentation
Seymour Schwartz, MD - no COI or financial disclosures relevant to this presentation
Kathy Rideout, EdD, PPCNP-BC, FNAP - no COI or financial disclosures relevant to this presentation
Gennady Bratslavsky, MD - no COI or financial disclosures relevant to this presentation
Stephen Campbell, MD - no COI or financial disclosures relevant to this presentation
Per-Anders Abrahamsson, MD, PhD - Cernelle AB / board member
CAMURUS AB / board member
IDL Biotech AB / board member
Mark Soloway, MD - no COI or financial disclosures relevant to this presentation
Leonard Gomella, MD - Advisory Board Astellas/Pfizer, Janssen, Merck, Starnd Diagnostics, MDx Health; FKD Industries Research support, Urology Chair NRG, Patents through Thomas Jefferson University
Dragan Golijanin, MD - no COI or financial disclosures relevant to this presentation
Robert S. Svatek, MD, MSCI - Participating in clinical trial of BCG partially sponsored by JBL BCG laboratories
Thomas Frye, DO - no COI or financial disclosures relevant to this presentation
Seth Lerner, MD - available under Board Disclosures on the website: http://www.bladdercancerjournal.com
Ganesh Palapattu, MD - no COI or financial disclosures relevant to this presentation
George Wilding, MD - no COI or financial disclosures relevant to this presentation
Michael Droller, MD - no COI or financial disclosures relevant to this presentation
Donald L. Trump, MD, FACP - Consultant for Bristol Myers Squibb
Notes
1 Messing EM. Legends in Urology Can J Urol 2018;25(4):9367-9369
REFERENCES
[1] | Institute NC: Cancer stat facts: Bladder cancer. Surveillance, Epidemiology, and End Results Program. (2018) . |
[2] | Stein JP , Lieskovsky G , Cote R , et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. Journal of Clinical Oncology. (2001) ;19: :666–75. |
[3] | von der Maase H , Hansen SW , Roberts JT , et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. (2000) ;18: :3068–77. |
[4] | Galsky MD , Hahn NM , Rosenberg J , et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. The Lancet Oncology. (2011) ;12: :211–4. |
[5] | Balar AV , Castellano D , O’Donnell PH , et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. (2017) ;18: :1483–92. |
[6] | Balar AV , Galsky MD , Rosenberg JE , et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. (2017) ;389: :67–76. |
[7] | De Santis M , Bellmunt J , Mead G , et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: Phase II—results of EORTC study 30986. Journal of Clinical Oncology. (2009) ;27: :5634–. |
[8] | FDA.gov: Keytruda (pembrolizumab) or Tecentriq (atezolizumab): FDA Alerts Health Care Professionals and Investigators: FDA Statement - Decreased Survival in Some Patients in Clinical Trials Associated with Monotherapy. 2018 Safety Alerts for Human Medical Products, (2018) . |
[9] | Sharma P , Retz M , Siefker-Radtke A , et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. (2017) ;18: :312–22. |
[10] | Bellmunt J , De Wit R , Vaughn DJ , et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New England Journal of Medicine. (2017) ;376: :1015–26. |
[11] | Powles T , O’donnell PH , Massard C , et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncology. (2017) ;3: :e172411-e172411. |
[12] | Rosenberg JE , Hoffman-Censits J , Powles T , et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. (2016) ;387: :1909–20. |
[13] | Patel MR , Ellerton J , Infante JR , et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. (2018) ;19: :51–64. |
[14] | Rosenberg JE , Sridhar SS , Zhang J , et al. Updated results from the enfortumab vedotin phase 1 (EV-101) study in patients with metastatic urothelial cancer (mUC), American Society of Clinical Oncology, (2018) . |
[15] | Loriot Y , Necchi A , Park SH , et al. Erdafitinib (ERDA; JNJ-42756493), a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRa): Phase 2 continuous versus intermittent dosing, American Society of Clinical Oncology, (2018) . |
[16] | Messing EM , Tangen CM , Lerner SP , Sahasrabudhe DM , Koppie TM , et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non–Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA. (2018) ;319: (18):1880–8. |
[17] | Liberman D , Lughezzani G , Sun M , Alasker A , Thuret R , Abdollah F , et al. Perioperative mortality is significantly greater in septuagenarian and octogenarian patients treated with radical cystectomy for urothelial carcinoma of the bladder. Urology. (2011) ;77: (3):660–6. (PMID:21256568). |
[18] | Gray PJ , Fedewa SA , Shipley WU , Efstathiou JA , Lin CC , Zietman AL , et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: Results from the National Cancer Data Base. European Urology. (2013) ;63: (5):823–9. (PMID: 23200811). |
[19] | Vashistha V , Wang H , Mazzone A , Liss MA , Svatek RS , Schleicher M , et al. Radical Cystectomy Compared to Combined Modality Treatment for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Int J Radiat Oncol Biol Phys. (2017) ;97: (5):1002–20. (PMID: 28332983). |
[20] | Choudhury A , Nelson LD , Teo MT , Chilka S , Bhattarai S , Johnston CF , et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle invasive bladder cancer. Cancer Research. (2010) ;70: (18):7017–26. (PMID: 20843819) |
[21] | Efstathiou JA , Spiegel DY , Shipley WU , Heney NM , Kaufman DS , Niemierko A , et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. European Urology. (2012) ;61: (4):705–11. (PMID: 22101114) |
[22] | James ND , Hussain SA , Hall E , Jenkins P , Tremlett J , Rawlings C , et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. New England J Med. (2012) ;366: (16):1477–88. (PMID: 22512481) |
[23] | Efstathiou JA , Bae K , Shipley WU , Kaufman DS , Hagan MP , Heney NM , et al. Late Pelvic Toxicity after Bladder-Sparing Therapy in Patients with Invasive Bladder Cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clinical Oncology. (2009) ;27: (25):4055–61. (PMID: 19636019) |
[24] | Rödel C , Grabenbauer GG , Kühn R , Papadopoulos T , Dunst J , Meyer M , et al. Combined-Modality Treatment and Selective Organ Preservation in Invasive Bladder Cancer: Long-Term Results. J Clinical Oncology. (2002) ;20: (14):2061–71. (PMID: 12118019) |
[25] | Zietman AL , Sacco D , Skowronski U , Gomery P , Kaufman DS , Clark JA , et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: Results of a urodynamic and quality of life study on long-term survivors. J Urology. (2003) ;170: (5):1772–6. (PMID: 14532773) |
[26] | Scosyrev E , Noyes K , Feng C , Messing EM . Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. (2009) ;115: ;68–74. |
[27] | Lamm DL , Blumenstein BA , Crawford ED , Crissman JD , Lowe BA , Smith JA , et al. Randomized Intergroup Comparison of Bacillus Calmette-Gubrin Immunotherapy and Mitomycin C Chemotherapy Prophylaxis in Superficial Transitional Cell Carcinoma of the Bladder. A Southwest Oncology Group Study Urol Oncol. (1995) ;I: :119–26. |
[28] | Sternberg CN , Yagoda A , Scher HI , Watson RC , Ahmed T , Weiselberg LR , et al. Preliminary Results of M-VAC (Methotrexate, Vinblastine, Doxorubicin and Cisplatin) for Transitional Cell Carcinoma of the Urothelium. J Urology. (1985) ;133: (3):403–7. |
[29] | Loehrer PJ , Einhorn LH , Elson PJ , Crawford ED , Kuebler P , Tannock I , et al. A Randomized Comparison of Cisplatin Alone or in Combination with Methotrexate, Vinblastine, and Doxorubicin in Patients With Metastatic Urothelial Carcinoma: A Cooperative Group Study. J Clin Oncol. (1992) ;10: :1066–73. |
[30] | von der Maase H , Sengelov L , Roberts JT , Ricci S , Dogliotti L , Oliver T , et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol. (2005) ;23: :4602–8. |
[31] | Plimack ER , Bellmunt J , Gupta S , Berger R , Chow LQM , Juco J , et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomized, open-label, phase 1b study. Lancet Oncology. (2017) ;18: :212–20. |
[32] | Massard C , Gordon MS , Sharma S , Rafii S , Wainberg ZA , Luke J , et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti–Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. (2016) ;34: (26):3119–25. |
[33] | Rosenberg JE , Hoffman-Censits J , Powles T , van der Heijden MS , Balar AV , Necchi A , et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. (2016) ;387: :1909–20. |
[34] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. (2017) ;67: (1):7–30. |
[35] | Campbell SC , Novick AC , Belldegrun A , Blute ML , Chow GK , Derweesh IH , et al. Guideline for management of the clinical T1 renal mass. Journal of Urology. (2009) ;182: (4):1271–9. [PUBMED: 19683266] |
[36] | Ljungberg B , Bensalah K , Canfield S , Dabestani S , Hofmann F , Hora M , et al. EAU guidelines on renal cell carcinoma: The 2014 update. European Urology. (2015) ;67: (5):913–24. |
[37] | Huang WC , Levey AS , Serio AM , et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. (2006) ;7: :735. |
[38] | Bianchi M , Becker A , Abdollah F , Trinh QD , Hansen J , Tian Z , et al. Rates of open versus laparoscopic and partial versus radical nephrectomy for T1a renal cell carcinoma: A population-based evaluation. International Journal of Urology. (2013) ;20: (11):1064–71. |
[39] | Ghani KR , Sukumar S , Sammon JD , Rogers CG , Trinh QD , Menon M . Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the nationwide inpatient sample. Journal of Urology. (1991) (4):907–12. |
[40] | AlAli AB , Griffin MF and Butler PE. Three-Dimensional Printing Surgical Applications. Journal ListEplastyv. 15: ;(2015) PMC4539849 |
[41] | Komai Y , Sakai Y , Gotohda N , Kobayashi T , et al. A novel 3-dimensional image analysis system for case-specific kidney anatomy and surgical simulation to facilitate clampless partial nephrectomy. Urology. (2014) ;83: :500e507. |
[42] | Silberstein JL , Maddox MM , Dorsey P , Feibus A , et al. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: A pilot study. Urology. (2014) ;84: :268e273. |
[43] | Scott DJ , Pugh CM , Ritter EM , Jacobs LM , Pellegrini CA , Sachdeva AK . New directions in simulation-based surgical education and training: Validation and transfer of surgical skills, use of non-surgeons as faculty, use of simulation to screen and select surgery residents, and long-term follow-up of learners. Surgery. (2011) ;149: :735–44. |
[44] | Stone J , Candela B , Alleluia V , Fazili A , Joseph J , Ghazi A . A novel technique for simulated surgical procedures using 3d printing technology. Journal of Urology. (2015) ;193: (4):e270. |
[45] | Ghazi A , Campbell T , Melnyk R , Feng C , Andrusco A , Stone J , Erturk E . Validation of A Full-immersion Simulation Platform For Percutaneous Nephrolithotomy Using 3D Printing Technology. J Endourol. (2017) ;31: (12):1314–20. |
[46] | Barrett T , Haider MA . The Emerging Role of MRI in Prostate Cancer Active Surveillance and Ongoing Challenges. AJR AM J Roentgenol. (2017) ;208: (1):131–9. doi: 10.2214/AJR.16.16355. Epub. 2016 Oct 11. PMID 27726415 |
[47] | Kashan M , Ghanaat M , Hotker AM , et al. Cystic Renal Cell Carcinoma: A Report on Outcomes of Surgery and Active Surveillance in Patients Retrospectively Identified on Pretreatment Imaging. J Urol. (2018) ;200: (2):275–82. doi: 10.1016/j.juro.2018.02.3087. Epub 2018 Mar 1. PMID:29496470 |
[48] | Barr RG , Peterson C , Hindi A . Evaluation of Indeterminate Renal Masses with Contrast-enhanced US: A Diagnostic Performance Study. Radiology. (2014) ;271: (1):133–42. doi: 10.1148/radiol.1313061 Epub 2013 Nov 18. PMID:24475802 |
[49] | Kasivisvanathan V , Rannikko AS , Borghi M , et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. (2018) 10;378: (19):1767–77. doi:10.1056/NEJMoa1801993. Epub 2018 Mar 18 PMID 29552975 |
[50] | Hope TA , Afshar-Oromieh A , Eiber M , et al. Imaging Prostate Cancer With Prostate-Specific Membrane Antigen PET/CT and PET/MRI: Current and Future Applications. AJR AmJ Roentgenol. (2018) ;211: (2):286–94. doi:10.2214/AJR.18.19957. Epub 2018 Jun 27. PMID 29949419 |
[51] | Berger I , Annabatttula C , Lewis J , et al. 68 Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: Correlation with final histopathology. Prostate Cancer Prostatic Dis. (2018) ;21: (2):204-11. doi:10.1038/s41391-018-0048-7. Epub 2018 Jun 1.PMID:29858591 |
[52] | Gordetsky J , Scosyrev E , Rashid H , Wu G , Silvers C , Golijanin D , et al. Identifying additional lymph nodes in radical cystectomy lymphadenectomy specimens. Mod Pathol. (2012) ;25: (1):140–4. |
[53] | Messing EM , Manola J , Sarosdy M , Wilding G , Crawford ED , Trump D . Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. (1999) ;341: (24):1781–8. |
[54] | Palapattu GS , Singer EA , Messing EM . Controversies surrounding lymph node dissection for prostate cancer. Urol Clin North Am. (2010) ;37: (1):57–65, Table of Contents. |
[55] | Tilki D , Brausi M , Colombo R , Evans CP , Fradet Y , Fritsche HM , et al. Lymphadenectomy for bladder cancer at the time of radical cystectomy. Eur Urol. (2013) ;64: (2):266–76. |
[56] | Shinder BM , Rhee K , Farrell D , Farber NJ , Stein MN , Jang TL , et al. Surgical Management of Advanced and Metastatic Renal Cell Carcinoma: A Multidisciplinary Approach. Front Oncol. (2017) ;7: :107. |
[57] | Bhindi B , Wallis CJD , Boorjian SA , Thompson RH , Farrell A , Kim SP , et al. The role of lymph node dissection in the management of renal cell carcinoma: A systematic review and meta-analysis. BJU Int. (2018) ;121: (5):684–98. |
[58] | Blom JH , van Poppel H , Marechal JM , Jacqmin D , Schroder FH , de Prijck L , et al. Radical nephrectomy with and without lymph-node dissection: Final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. (2009) ;55: (1):28–34. |
[59] | Gershman B , Moreira DM , Thompson RH , Boorjian SA , Lohse CM , Costello BA , et al. Renal Cell Carcinoma with Isolated Lymph Node Involvement: Long-term Natural History and Predictors of Oncologic Outcomes Following Surgical Resection. Eur Urol. (2017) ;72: (2):300–6. |
[60] | Gershman B , Thompson RH , Boorjian SA , Larcher A , Capitanio U , Montorsi F , et al. Radical Nephrectomy with or without Lymph Node Dissection for High Risk Nonmetastatic Renal Cell Carcinoma: A Multi-Institutional Analysis. J Urol. (2018) ;199: (5):1143–8. |
[61] | Ristau BT , Manola J , Haas NB , Heng DYC , Messing EM , Wood CG , et al. Retroperitoneal Lymphadenectomy for High Risk, Nonmetastatic Renal Cell Carcinoma: An Analysis of the ASSURE (ECOG-ACRIN 2805) Adjuvant Trial. J Urol. (2018) ;199: (1):53–9. |
[62] | Campbell S , Uzzo RG , Allaf ME , Bass EB , Cadeddu JA , Chang A , et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol. (2017) ;198: (3):520–9. |
[63] | Radadia KD , Rivera-Nunez Z , Kim S , Farber NJ , Sterling J , Falkiewicz M , Modi PK , Goyal S , Parikh R , Weiss RE , Kim IY , Elsamra SE , Jang TL , Singer EA . Accuracy of clinical nodal staging in patients undergoing surgery for renal cell carcinoma. 18th Annual Meeting of the Society of Urologic Oncology; (2017) ; Washington, D.C. |
[64] | Farber N , Rivera-Nunez Z , Kim S , Radadia KD , Modi PK , Goyal S , Parikh R , Weiss R , Kim IY , Elsamra SE , Jang TL , Singer EA . Outcomes of lymphadenectomy for non-metastatic renal cell carcinoma: A propensity score-weighted analysis. Journal of Urology. (2018) ;199: (4/Supplement):e456. |
[65] | Mohile SG , Dale W , Somerfield MR , et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. (2018) ;36: :2326–47. |
[66] | Mohile SG , Epstein RM , Hurria A , et al. Improving communication with older patients with cancer using geriatric assessment (GA): A University of Rochester NCI Community Oncology Research Program (NCORP) cluster randomized controlled trial (CRCT). Journal of Clinical Oncology. (2018) ;36: :LBA10003-LBA. |
[67] | Mohile SG , Magnuson A , Pandya C , et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. J Natl Compr Canc Netw. (2018) ;16: :301–9. |
[68] | Mohile SG , Velarde C , Hurria A , et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw. (2015) ;13: :1120–30. |
[69] | Wu Y , Moissoglu K , Wang H , Wang X , Frierson HF , Schwartz MA , et al. Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc Natl Acad Sci U S A. (2009) ;106: :5807–12. |
[70] | Gildea JJ , Seraj MJ , Oxford G , Harding MA , Hampton GM , Moskaluk CA , et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. (2002) ;62: :6418–23. |
[71] | Guin S , Pollard C , Ru Y , Ritterson Lew C , Duex JE , Dancik G , et al. Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease. J Natl Cancer Inst. (2014) ;106. |
[72] | Said N , Smith S , Sanchez-Carbayo M , Theodorescu D . Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest. (2011) ;121: :132–47. |
[73] | Said N , Sanchez-Carbayo M , Smith SC , Theodorescu D . RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. (2012) ;122: :1503–18. |
[74] | Ahmed M , Sottnik JL , Dancik GM , Sahu D , Hansel DE , Theodorescu D , et al. An Osteopontin/CD44 Axis in RhoGDI2-Mediated Metastasis Suppression. Cancer Cell. (2016) ;30: :432–43. |
[75] | Guin S , Ru Y , Agarwal N , Lew CR , Owens C , Comi GP , et al. Loss of Glycogen Debranching Enzyme AGL Drives Bladder Tumor Growth via Induction of Hyaluronic Acid Synthesis. Clin Cancer Res. (2016) ;22: :1274–83. |
[76] | Kanada M , Bachmann MH , Contag CH . Signaling by extracellular vesicles advances cancer hallmarks. Trends Cancer. (2016) ;2: (2):84–94. |
[77] | Kalluri R . The biology and function of exosomes in cancer. J Clin Invest. (2016) ;126: (4):1208–15. |
[78] | Peinado H , Zhang H , Matei IR , Costa-Silva B , Hoshino A , Rodrigues , et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat Rev Cancer. (2017) ;1: (5):302–17. |
[79] | Peinado H , Aleckovic M , Lavotshkin S , Matei I , Costa-Silva B , Moreno-Bueno G , et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. (2012) ;18: (6):883–91. |
[80] | Hoshino A , Costa-Silva B , Shen TL , Rodrigues G , Hashimoto A , et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015) ;527: (7578):329–35. |
[81] | Costa-Silva B , Aiello NM , Ocean AJ , Singh S , Zhang H , Thakur BK , et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. (2015) ;17: (6):816–26. |
[82] | Quail DF , Joyce JA . Microenvironmental regulation of tumor progression and metastasis. Nat Med. (2013) ;19: (11):1423–37. |
[83] | Hall MC , Chang SS , Dalbagni G , Pruthi RS , Seigne JD , Skinner EC , et al. Guideline for the management of non-muscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urology. (2007) ;178: (6):2314–30. |
[84] | Raposo G , Nijman HW , Stoorvogel W , Liejendekker R , Harding CV , MeliefCJ , et al. B lymphocytes secrete antigen-presenting vesicles. The Journal of Experimental Med. (1996) ;183: (3):1161–72. |
[85] | Thery C , Duban L , Segura E , Veron P , Lantz O , Amigorena S . Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature Immunology. (2002) ;3: (12):1156–62. |
[86] | Grossman HB , Natale RB , Tangen CM , Speights VO , Vogelzang NJ , Trump DL , et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) ;349: (9):859–66. |
[87] | Van Allen EM , Mouw KW , Kim P , Iyer G , Wagle N , Al-Ahmadie H , et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. (2014) ;4: (10):1140–53. |
[88] | Plimack ER , Dunbrack RL , Brennan TA , Andrake MD , Zhou Y , Serebriiskii IG , et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur Urol. (2015) ;68: (6):959–67. |
[89] | Lee JK , Havaleshko DM , Cho H , Weinstein JN , Kaldjian EP , Karpovich J , et al. A strategy for predicting the chemosensitivity of human cancers and its application to drug discovery. Proc Natl Acad Sci U S A. (2007) ;104: (32):13086–91. |
[90] | Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. (2014) ;507: (7492):315–22. |
[91] | Damrauer JS , Hoadley KA , Chism DD , Fan C , Tiganelli CJ , Wobker SE , et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. (2014) ;111: (8):3110–5. |
[92] | Robertson AG , Kim J , Al-Ahmadie H , Bellmunt J , Guo G , Cherniack AD , et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. (2017) ;171: (3):540–56. e25. |
[93] | Sjodahl G , Lauss M , Lovgren K , Chebil G , Gudjonsson S , Veerla S , et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. (2012) ;18: (12):3377–86. |
[94] | Choi W , Porten S , Kim S , Willis D , Plimack ER , Hoffman-Censits J , et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. (2014) ;25: (2):152–65. |
[95] | McConkey DJ , Choi W , Shen Y , Lee IL , Porten S , Matin SF , et al. A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naive Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Bevacizumab in Urothelial Cancer. Eur Urol. (2016) ;69: (5):855–62. |
[96] | Seiler R , Ashab HAD , Erho N , van Rhijn BWG , Winters B , Douglas J , et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol. (2017) ;72: (4):544–54. |
[97] | Bellmunt J , deWit R , Vaughn DJ , Fradet Y , Lee JL , Fong L , et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. (2017) ;376: (11):1015–26. |
[98] | Fradet Y , Bellmunt J , deWit R , Vaughn DJ , Lee JL , Fong L , et al. Pembrolizumab (pembro) versus investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer (UC): 2-year follow-up from the phase 3 KEYNOTE-045 trial ASCO 2018 (abstr 4521) J Clin Oncol (2018) . |
[99] | Necchi AB , Bianchi A , Raggi M , Giannatempo D , Luciano P , Colecchia R , et al. Preoperative pembrolizumab (pembro) before radical cystectomy (RC) for muscle-invasive urothelial bladder carcinoma (MIUC): Interim clinical and biomarker findings from the phase 2 PURE-01 study (Abstr 4507). ASCO; (2018) : J Clin Oncol 2018. |
[100] | Pardoll DM . The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) ;12: (4):252–64. |
[101] | Hussein AA , Ahmed YE , May P , Ali T , Ahmad B , Raheem S , Stone K , Hasasnah A , Rana O , Cole A , Wang D , Loud P , Guru KA . Natural History and Predictors of Parastomal Hernia after Robot-Assisted Radical Cystectomy and Ileal Conduit Urinary Diversion. J Urol. (2018) :766–73. |
[102] | Stimson CJ , Chang SS , Barocas DA , et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. (2010) ;184: (4):1296–300. |
[103] | Shabsigh A , Korets R , Vora KC , et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. (2009) ;55: (1):164–74. |
[104] | Kauffman EC , Ng CK , Lee MM , et al. Critical analysis of complications after robotic-assisted radical cystectomy with identification of preoperative and operative risk factors. BJU Int. (2010) ;105: (4):520–7. |
[105] | Desborough JP . The stress response to trauma and surgery. Br J Anaesth. (2000) ;85: (1):109–17. |
[106] | Dobson GP . Addressing the Global Burden of Trauma in Major Surgery. Frontiers in Surgery. (2015) ;2: :43. |
[107] | Baack Kukreja JE , Kiernan M , Schempp B , et al. Quality Improvement in Cystectomy Care with Enhanced Recovery (QUICCER Study). (2017) ;119: (1):38–49. doi: 10.1111/bju.13521. Epub 2016 Jun 3. |
[108] | Baack Kukreja JE , Messing EM , Shah J . Are we doing “better”? Urologic Oncology. (2016) ;34: (3):120.e17–21. doi: 10.1016/j.urolonc.2015.10.002. Epub 2015 Nov 14. |
[109] | Braga M , Gianotti L , Vignali A , Carlo VD . Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. (2002) ;132: (5):805–14. |
[110] | Burden S , Todd C , Hill J , Lal S . Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. (2012) ;11: :CD008879. |
[111] | Mauskopf JA , Candrilli SD , Chevrou-Severac H , Ochoa JB . Immunonutrition for patients undergoing elective surgery for gastrointestinal cancer: Impact on hospital costs. World J Surg Oncol. (2012) ;10: :136. |
[112] | Siegel RL , Miller KD , Jemal A . Cancer Statistics, 2017. CA Cancer J Clin. (2017) ;67: (1):7–30. |
[113] | Chang SS , Bochner BH , Chou R , et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. (2017) ;198: (3):552–559. |
[114] | |
[115] | Ramirez D , Gupta A , Canter D , et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. (2016) ;117: (5):783–6. |
[116] | Chang SS , Boorjian SA , Chou R , et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. (2016) ;196: (4):1021–9. |
[117] | Svatek RS , Sagalowsky AI , Lotan Y . Economic impact of screening for bladder cancer using bladder tumor markers: A decision analysis. Urol Oncol. (2006) ;24: (4):338–43. |
[118] | Burger M , Catto JW , Dalbagni G , et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) ;63: (2):234–41. |
[119] | Krabbe LM , Svatek RS , Shariat SF , Messing E , Lotan Y . Bladder cancer risk: Use of the PLCO and NLST to identify a suitable screening cohort. Urol Oncol. (2015) ;33: (2):65. e19–25. |
[120] | Messing EM , Young TB , Hunt VB , et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology. (1995) ;45: (3):387–96; discussion 396-387. |
[121] | Messing EM , Madeb R , Young T , et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. (2006) ;107: (9):2173–9. |
[122] | Britton JP , Dowell AC , Whelan P , Harris CM . A community study of bladder cancer screening by the detection of occult urinary bleeding. The Journal of Urology. (1992) ;148: (3):788–90. |
[123] | Lotan Y , Roehrborn CG . Sensitivity and specificity of commonly available bladder tumor markers versus cytology: Results of a comprehensive literature review and meta-analyses. Urology. (2003) ;61: (1):109–18; discussion 118. |
[124] | Lotan Y , Elias K , Svatek RS , et al. Bladder cancer screening in a high risk asymptomatic population using a point of care urine based protein tumor marker. Journal of Urology. (2009) ;182: (1):52–7; discussion 58. |
[125] | Hedelin H , Jonsson K , Salomonsson K , Boman H . Screening for bladder tumours in men aged 60-70 years with a bladder tumour marker (UBC) and dipstick-detected haematuria using both white-light and fluorescence cystoscopy. Scandinavian Journal of Urology and Nephrology. (2006) ;40: (1):26–30. |
[126] | Bangma CH , Loeb S , Busstra M , et al. Outcomes of a bladder cancer screening program using home hematuria testing and molecular markers. European Urology. (2013) ;64: (1):41–47, 1. |
[127] | Cormio L , Sanguedolce F , Cormio A , Massenio P , Pedicillo MC , Cagiano S , et al. Human epidermal growth factor receptor 2 expression is more important than Bacillus Calmette Guerin treatment in predicting the outcome of T1G3 bladder cancer. Oncotarget. (2017) ;8: (15):25433–41. |
[128] | Burger M , Catto JWF , Dalbagni G , Grossman HB , Herr H , Karakiewicz P , et al. Epidemiology and Risk Factors of Urothelial Bladder Cancer. European Urol. (2013) ;63: ;234–41. |
[129] | Feng M , Cheng X . Chapter 12 Parasite-Associated Cancers (Blood Flukes/Liver Flukes) Springer Nature Singapore Pte Ltd. 2017 Q. Cai et al. (eds.), Infectious Agents Associated Cancers: Epidemiology and Molecular Biology, Advances in Experimental Medicine and Biology. |
[130] | Eifler JB , Scarpato KR , Clark PE . Management of noninvasive bladder cancers. Curr Opin Oncol. (2015) ;27: :185–90. |
[131] | Gandhi NM , Morales A , Lamm DL . Bacillus Calmette-Guérin immunotherapy for genitourinary cancer. BJU International. (2013) ;112: :288–97. |
[132] | Messing EM , Tangen CM , Lerner SP , Sahasrabudhe DM , Koppie TM , Wood DP , et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non–Muscle-Invasive Bladder Cancer on Tumor Recurrence SWOG SRandomized Clinical Trial. JAMA. (2018) ;319: :1880–88. |
[133] | Czerniak B , Dinney C , McConkey D . Origins of Bladder Cancer. Annual Rev Pathol Mech Dis. (2016) ;11: :149–74. |
[134] | Messing E , Gee JR , Saltzstein DR , Kim K , diSant’Agnese A , Kolesar J , et al. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prev Res (Phila). (2012) ;5: (4):621–30. |
[135] | Yang L , Palliyaguru DL , Kensler TW . Frugal chemoprevention: Targeting Nrf2 with foods rich in sulforaphane. Seminars in Oncology. (2016) ;43: :146–153. |
[136] | Gee JR , Saltzstein DR , Kim K , Kolesar J , Huang W , Havighurst TC , et al. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev Res. (2017) ;10: (5):298–307. |
[137] | EL-Arabey AA . Genes and Environment. (2017) ;39: :13. |
[138] | Ho PL , Williams SB , Kamat AM . Immune Therapies in Non-Muscle Invasive Bladder Cancer. Current Treatment Options in Oncol. (2015) ;16: :5. |
[139] | Yu SS , Ballas LK , Skinner EC , Dorff TB , Sadeghi S , Quinn DI . Immunotherapy in Urothelial Cancer, Part Adjuvant, Neoadjuvant, and Adjunctive Treatment. Clinical Advances in Hematology & Oncology. (2017) ;15: (7):543. |
[140] | Blom JHM , van Poppel H , Mare'chal JM , et al. Radical Nephrectomy with and without Lymph-Node Dissection: Final Results of European Organization for Research and Treatment of Cancer (EORTC) Randomized Phase 3 Trial 1. European Urology. (2009) ;55: :28–34. |
[141] | Van Poppel H , Da Pozzo L , Albrecht W , et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. European Urology. (2011) ;59: :543–52. |
[142] | Scosyrev E , Messing EM , Sylvester R , Campbell SC , VonPoppel H . Renal Function after NSS vs. Radical Nephrectomy, results from the EORTC randomized trial 30904. Eur Urol. (2014) ;65: :372–7. |
[143] | Lane BR , Campbell SC , Demirjian S , Fergany AF . Surgically-induced chronic kidney disease may be associated with lesser risk of progression and mortality than medical chronic kidney disease. J Urol. (2013) ;189: :1649–55. |
[144] | Shah PH , Moreira DM , Patel VR , et al. Partial Nephrectomy is Associated with Higher Risk of Relapse Compared with Radical Nephrectomy for Clinical Stage T1 Renal Cell Carcinoma Pathologically Up Staged to T3a. J Urol. (2017) ;198: :289–96. |
[145] | Crane A , Suk-Ouichai C , Campbell JA , et al. Imprudent Utilization of Partial Nephrectomy. Urology. (2017) : doi:10.1016/j.urology.2017.12.009 |
[146] | Campbell SC , Derweesh IH , Lane BR , Messing EM . The management of a clinical T1b renal tumor in the presence of a normal contralateral kidney, role of radical nephrectomy. J Urology. (2013) ;289: :1198–202. |
[147] | https://seer.cancer.gov/csr/1975_2015/results_merged/sect_27_urinary_bladder.pdf (Accessed July 22, 2018). |
[148] | Cookson MS , Herr HW , Zhang ZF , et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. (1997) ;158: :62–67. |
[149] | https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm529600.pdf (Accessed July 22, 2018). |
[150] | Crijnen J , De Reijke TM . Emerging intravesical drugs for the treatment of non muscle-invasive bladder cancer. Expert Opin Emerg Drugs. (2018) ;23: (2):135–47. |
[151] | Steinberg RL , et al. Combination Intravesical Chemotherapy for Non–muscle-invasive Bladder Cancer. Eur Urol Focus. (2018) , in press. 10.1016/j.euf.2018.07.005 |
[152] | Shepherd ARH , Shepherd E , Brook NR . Intravesical Bacillus Calmette-Guérin with interferon-alpha versus intravesical Bacillus Calmette-Guérin for treating non-muscle-invasive bladder cancer. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No.: CD012112. DOI: 10.1002/14651858.CD012112.pub2 |
[153] | Meeks JJ , Lerner SP , Svatek RS . Bacillus Calmette-Guérin Manufacturing and SWOG S1602 Intergroup Clinical Trial. J Urol. (2017) ;197: (3 Pt 1):538–40. |
[154] | Lawrence MS , Stojanov P , Polak P , et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. (2013) ;499: (7457):214–8. |
[155] | Shore ND , Boorjian SA , Canter DJ , et al. Intravesical rAd-IFNα/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol. (2017) ;35: (30):3410–6. |
[156] | Bellmunt J , Powles T , Vogelzang NJ . A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat Rev. (2017) ;54: :58–67. |
[157] | Soria F , Shariat SF , et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. (2017) ;35: :379–87. |
[158] | Raman JD , Messer J , et al. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. (2011) ;107: :1059–64. |
[159] | Maruschke M , Kram W , et al. Upper Urinary Tract Tumors: Which Diagnostic Methods Are Needed? Urol Int. (2017) ;98: :304–11. |
[160] | Osman E , Alnaib Z , Kumar N . Photodynamic diagnosis in upper urinary tract urothelial carcinoma: A systematic review. Arab J Urol. (2017) ;15: :100–9. |
[161] | Aragon-Ching JB . Challenges and advances in the diagnosis, biology, and treatment of urothelial upper tract and bladder carcinomas. Urol Oncol. (2017) ;35: :462–4. |
[162] | Wyatt LC , Lewis JS , et al. Applications of pHLIP Technology for Cancer Imaging and Therapy. Trends Biotechnol. (2017) ;35: :653–64. |
[163] | Anderson M , Moshnikova M , et al. Probe for the measurement of cell surface pH in vivo and ex vivo. Proc Natl Acad Sci USA. (2016) ;113: :8177–81. |
[164] | Cheng CJ , Bahal R , Babar IA , et al. MicroRNA silencing for cancer therapy targeted to the tumor microenvironment. Nature. (2015) ;518: :107–10. |
[165] | Ozes AR , Wang Y , Zong X , Fang F , Pilrose J , Nephew KP . Therapeutic targetuibg using tumor specific peptides inhibitis long non-coding RNA HOTAIR activity in ovarian and breast cancer. Sci Rep. (2017) ;7: (1):894. |
[166] | Golijanin J , Amin A , Moshnikova A , et al. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc Natl Acad Sci USA. (2016) ;113: :11829–34. |
[167] | Lamm DL , Blumenstein BA , Crissman JD , et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol. (2000) ;163: (4):1124–9. |
[168] | Lamm DL . Preventing progression and improving survival with BCG maintenance. Eur Urol. (2000) ;37: (Suppl 1):9–15. |
[169] | Ratliff TL , Ritchey JK , Yuan JJ , Andriole GL , Catalona WJ . T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. (1993) ;150: (3):1018–23. |
[170] | Zbar B , Rapp HJ . Immunotherapy of guinea pig cancer with BCG. Cancer. (1974) ;34: (4 Suppl):1532–40. |
[171] | Svatek RS , Zhao XR , Morales EE , et al. Sequential Intravesical Mitomycin plus Bacillus Calmette-Guerin for Non-Muscle-Invasive Urothelial Bladder Carcinoma: Translational and Phase I Clinical Trial. Clin Cancer Res. (2015) ;21: (2):303–11. |
[172] | Morton DL , Eilber FR , Holmes EC , et al. BCG immunotherapy of malignant melanoma: Summary of a seven-year experience. Ann Surg. (1974) ;180: (4):635–43. |
[173] | Zbar and Rapp, Immunotherapy of Guinea Pig Cancer with BCG. Cancer. (1974) ;34: :1532–40. |
[174] | Linton KD , Rosario DJ , Thomas F , et al. Disease specific mortality in patients with low risk bladder cancer and the impact of cystoscopic surveillance. J Urol. (2013) ;189: :828–33. |
[175] | Soloway MS , Bruck DS , Kim SS . Expectant management of small, recurrent, non – invasive papillary bladder tumors. J Urol. (2003) ;170: :438–41. |
[176] | Soloway MS . Active surveillance or office fulguration for low-grade Ta bladder tumors: A win – win for patients and urologists. J Urol. (2018) ;199: :1120–2. |
[177] | Lokeshwar SD , Ruiz-Cordero R , Hup MC , Jorda M , Soloway MS . Impact of 2004 ISUP/WHO classification on bladder cancer grading. World J Urol. (2015) ;33: :1229–36. |
[178] | Klaassen Z , Soloway MS . European Association of Urology and American Urological Association/Society of Urologic Oncology Guidelines on risk categories for non-muscle invasive bladder cancer may lead to overtreatment for low – grade bladder tumors. Urology. (2017) ;105: :14–7. |
[179] | Nieder AM , Soloway MS , Herr HW . Should we abandon the FISH test? Eur Urol. (2007) ;51: :1469–71. |
[180] | Lerner SP , Dinney C , Kamat A , et al. Clarification of Bladder Cancer Disease States Following Treatment of Patients with Intravesical BCG. Bladder Cancer. (2015) ;1: (1):29–30. |
[181] | Chang SS , Bochner SS , Chou R , et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. (2017) ;198: (3):552–9. |
[182] | Jarow J , Maher VE , Tang S , et al. Development of Systemic and Topical Drugs to Treat Non-muscle Invasive Bladder Cancer. Bladder Cancer. (2015) ;1: (2):133–6. |
[183] | Jarow JP , Lerner SP , Kluetz PG , et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: Report of a Food and Drug Administration and American Urological Association public workshop. Urology. (2014) ;83: (2):262–4. |
[184] | Dinney CP , Greenberg RE , Steinberg GD . Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol. (2013) ;31: (8):1635–42. |
[185] | Skinner EC , Goldman B , Sakr W , et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. J Urol. (2013) ;190: (4):1200–4. |
[186] | Milbar N , Kates M , Chappidi MR , et al. Oncological Outcomes of Sequential Intravesical Gemcitabine and Docetaxel in Patients with Non-Muscle Invasive Bladder Cancer. Bladder Cancer. (2017) ;3: (4):293–303. |
[187] | Velaer KN , Steinberg R , Thomas LJ , et al. Experience with Sequential Intravesical Gemcitabine and Docetaxel as Salvage Therapy for Non-Muscle Invasive Bladder Cancer. Curr Urol Rep. (2016) ;17: (5):38. |
[188] | Lightfoot AJ , Breyer BN , Rosevear HM , et al. Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urol Oncol. (2014) ;32: (1):35.e15–9. |
[189] | Campodonico F , DiStasi S , Lev GM , et al. Intravesical Chemotherapy and Chemohyperthermia in Non-Muscle-Invasive Bladder Cancer; An Overview on Drug Administration Technologies and Pharmacokinetics. Curr Drug Metab. (2017) ;18: (7):657–65. |
[190] | Witjes JA . Topic issue on new treatments in bladder cancer. World J Urol. (2009) ;27: (3):285–7. |
[191] | Pepe MS , Etzioni R , Feng Z , et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. (2001) ;93: :1054–61. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11459866, accessed July 30, 2018. |
[192] | Prensner JR , Chinnaiyan AM , Srivastava S . Systematic, evidence-based discovery of biomarkers at the NCI. Clin Exp Metastasis. (2012) ;29: :645–52. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22868876, accessed July 30, 2018. |
[193] | Caswell DR , Swanton C . The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. (2017) ;15: :133. Available at:http://www.ncbi.nlm.nih.gov/pubmed/28716075, accessed July 30, 2018. |
[194] | Maley CC , Aktipis A , Graham TA , et al. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer. (2017) ;17: :605–19. Available at:http://www.ncbi.nlm.nih.gov/pubmed/28912577, accessed July 30, 2018. |
[195] | Perkins BA , Caskey CT , Brar P , et al. Precision medicine screening using whole-genome sequencing and advanced imaging to identify disease risk in adults. Proc Natl Acad Sci. (2018) ;115: :3686–91. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29555771, accessed July 30, 2018. |
[196] | Kluth LA , Black PC , Bochner BH , et al. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur Urol. (2015) ;68: :238–53. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25709027, accessed October 18, 2017. |
[197] | Sharma P , Retz M , Siefker-Radtke A , et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. (2017) ;18: (3):312–22. |
[198] | Bellmunt J , deWit R , Vaughn DJ , et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. New England Journal of Medicine. (2017) ;376: (11):1015–26. |
[199] | Balar AV , Castellano D , O’Donnell PH , et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. (2017) ;18: (11):1483–92. |
[200] | Rosenberg JE , Hoffman-Censits J . Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. The Lancet. (2016) ;387(10031):1909–20. |
[201] | Balar AV , Galsky MD , Rosenberg JE , et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. The Lancet. (2017) ;389: (10064):67–76. |
[202] | Boyerinas B , Jochems C , Fantini M , et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunology Research. (2015) ;3: (10):1148. |
[203] | Powles T , O’Donnell PH , Massard C , et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncology. (2017) ;3: (9):e172411. |
[204] | Gao J , Shi LZ , Zhao L , et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. (2016) ;167: (2):397–404.e9. |
[205] | Zaretsky JM , Garcia-Diaz A , Shin DS , et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. New England Journal of Medicine. (2016) ;375: (9):819–29. |
[206] | Patel SJ , Sanjang NE , Kishton RJ , et al. Identification of essential genes for cancer immunotherapy. Nature. (2017) ;548: :537. |
[207] | Mariathasan S , Turley SJ , Nickles D , et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. (2018) ;554: :544. |
[208] | Lavery HJ , Stensland KD , Niegisch G , et al. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: A useful surrogate. J Urol. (2014) ;191: :898–906. |
[209] | Bajorin DF , Dodd PM , Mazumdar M , et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. (1999) ;17: :3173–78. |
[210] | Grossman HB , Natalie RB , Tangen CM , et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. (2003) ;349: :859–65. |
[211] | Droller MJ . Bladder Cancer. Current Problems in Surgery. (1981) ;18: :205–79. |
[212] | Jones PA , Droller MJ . Pathways of development and progression in bladder cancer: New correlations between clinical observations and molecular mechanisms. Sem Urol. (1993) ;11: :177–92. |
[213] | Niegisch G , Lorch A , Droller MJ , et al. Neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: Which patients benefit? Eur Urol. (2013) ;64: :355–57. |
[214] | Lee CT , Madii R , Diagnault S , et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. (2006) ;175: :1262–67. |
[215] | Gofrit ON , Zorn KL , Steinberg GD , et al. The Will Rogers phenomenon in urologic oncology. J Urol. (2008) ;179: :28–34. |
[216] | Cordon-Cardo C . Molecular alterations associated with bladder cancer initiation and progression. Scand. J Urol Nephrol. (2008) ;42: :154–65. |
[217] | Goebell PJ , Knowles MA , et al. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urol Oncol. (2010) ;28: :409–28. |
[218] | Rubin D , Levij IS . Suppression by vitamins D2 and D3 of hamster cheek pouch carcinoma induced with 9,10-dimethyl-1,2-benzanthracene with a discussion of the role of intracellular calcium in the development of tumors. Pathol Microbiol (Basel). (1973) ;39: :446–60. |
[219] | Mazzilli SA , Hershberger PA , Reid ME , Bogner PN , Atwood K , Trump DL , et al. Vitamin D Repletion Reduces the Progression of Premalignant Squamous Lesions in the NTCU Lung Squamous Cell Carcinoma Mouse Model. Cancer Prev Res (Phila). (2015) ;8: ;895–904. |
[220] | Richards SE , Weierstahl KA , Kelts JL . Vitamin D effect on growth and vitamin D metabolizing enzymes in triple-negative breast cancer. Anticancer Res. (2015) ;35: (2):805–10. |
[221] | Lechner D , Kállay E , Cross HS . 1alpha,25-dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol Cell Endocrinol. (2007) ;263: (1-2):55–64. Epub 2006 Oct 9. |
[222] | Hsu JW , Yin PN , Wood R , Messing J , Messing E , Lee YF . 1 alpha, 25-dihydroxylvitamin D3 promotes Bacillus Calmette-Guérin immunotherapy of bladder cancer. Oncotarget. (2013) ;4: (12):2397–406. |




