Evaluation of Intraoperative Versus Postoperative Adjuvant Mitomycin C with Nephroureterectomy for Urothelial Carcinoma of the Upper Urinary Tract
Abstract
Background:
Results of randomized trials support a single dose of intravesical chemotherapy following radical nephroureterectomy (RNU) for urothelial carcinoma.
Objective:
To evaluate the impact of the timing of intravesical mitomycin C (MMC) administration on the rate of bladder tumor recurrence (BTR) following RNU.
Methods:
We performed a retrospective review of patients who underwent RNU for upper tract urothelial carcinoma (UTUC) and received intravesical MMC between 2008 and 2016. Patients were categorized into two separate groups based on the timing of MMC administration: patients who received MMC intraoperatively (IO) and patients who received MMC on post-operative day 1 or later (PO). Our primary endpoint was BTR rate within the first year after surgery.
Results:
Fifty-one patients met our inclusion criteria: (IO: n = 30; PO: n = 21). There were no statistically significant differences in baseline characteristics of age, gender, race, surgical approach, tumor grade, tumor stage, surgical margins, nodal status, concomitant CIS, or history of bladder cancer. The median length of follow-up for each group was 22 months for IO and 12 months for PO (P = 0.10). The estimated probability of 1-year BTR rates for the IO and PO groups were 16% and 33%, respectively (p = 0.09). Cox analysis noted that the IO patients had a significantly lower rate of BTR in the first year postoperatively (HR = 0.113, 95% CI = 0.28–0.63, p = 0.01).
Conclusions:
The use of intraoperative MMC at the time of RNU was associated with a decrease in the risk of 1-year recurrence within the bladder.
KEY DEFINITIONS AND ABBREVIATIONS
RNU = radical nephroureterectomy
UTUC = upper tract urothelial carcinoma
BTR = bladder tumor recurrence
MMC = Mitomycin C
IO = Intraoperative
PO = Postoperative
INTRODUCTION
Urothelial carcinoma is projected to be the sixth most common malignancy in the United States in 2018, affecting over 80,000 individuals [1]. The majority of urothelial carcinoma occurs in the bladder, while urothelial carcinoma involving the upper tract is relatively rare. Upper tract urothelial carcinoma (UTUC) accounts for less than 10% of malignancies of the upper urinary tract [2]. The incidence of UTUC has slowly increased over the past 30 years, and treatment options continue to evolve [3]. Radical nephroureterectomy (RNU) with bladder cuff excision remains the standard surgical management for high-risk UTUC and lower risk tumors not amenable to endoscopic management [2, 4]. Unfortunately recurrence in the bladder is common, occurring in 22–47% of cases [4]. Two separate prospective trials have demonstrated a single dose of adjuvant intravesical chemotherapy with either mitomycin C (MMC) or pirarubicin reduces the risk of bladder tumor recurrence (BTR) during the first year following RNU [5, 6]. These results were confirmed in a meta-analysis by Deng et al. in 2014, with a pooled hazard ratio of 0.38 for patients receiving intravesical chemotherapy [7]. Although the data are strong, the timing of intravesical chemotherapy administration varied significantly across these studies. Additionally, Lu et al. demonstrated underutilization of intravesical chemotherapy and significant heterogeneity of its timing among Society of Urologic Oncology (SUO) members [8]. It is clear that there is a paucity of evidence regarding the timing of intravesical chemotherapy and further investigation is necessary. The objective of our study was to evaluate the impact of the timing of post-operative intravesical MMC administration on the rate of BTR in patients undergoing RNU for UTUC.
MATERIALS AND METHODS
After obtaining institutional review board approval (University of Florida, IRB201602119), we performed a retrospective review of adult patients who underwent RNU for UTUC from 2008–2016 at our institution. Patients were included in the study if they received intravesical MMC during their perioperative course and had cystoscopic surveillance follow-up. Patients were excluded if they had a prior or simultaneous cystectomy, their surgery was performed for non-malignant causes, or they had non-urothelial cell carcinoma on final pathology. While there were a variety of surgical approaches to nephroureterectomy (open, laparoscopic, robotic), all procedures included excision of a bladder cuff with closure of the bladder defect. No patients received subsequent adjuvant intravesical therapy following RNU other than the single dose of MMC. Patients were categorized into two separate groups based on the timing of MMC administration: [1] patients who received MMC intraoperatively (IO), and [2] patients who received MMC on post-operative day 1 or later (PO). In IO patients, MMC was administered intraoperatively prior to bladder cuff excision and the bladder was irrigated with 100cc of sterile saline after draining MMC from the bladder. All patients in the PO group received MMC 24–72 hours after RNU. Timing of MMC was determined by the surgeon performing the RNU. The technique of bladder cuff excision was left to the discretion of the surgeon.
Our primary outcome was BTR within the first year of surgery. Our secondary outcome was BTR at any time after surgery. Surveillance for BTR following RNU included office cystoscopy and urine cytology every 3–6 months during the first 2 years and every 6–12 months thereafter. Pathologic confirmation was necessary to determine tumor recurrence. Baseline patient and tumor characteristics were compared across treatment groups using chi-square and Fisher’s two-sided exact tests for categorical parameters. One-way analysis of variance was used for continuous parameters. Associations between treatment groups and time to recurrence were assessed using Kaplan-Meier plots, log-rank test, and univariable and multivariable Cox proportional hazard models, adjusted for surgical approach, surgical margin status, and presence of concomitant carcinoma,using a backward elimination approach,. Statistical significance was defined as p < 0.05. All statistical analyses were performed using Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
A total of 51 patients met our inclusion criteria. The IO group consisted of 30 patients, and the PO group consisted of 21 patients. Of the patients in the PO group, all but two patients received MMC treatment on post-operative day one. The other two patients in the PO group received MMC treatment on postoperative day two and three. The median length of follow-up for each group was 22 months for IO and 12 months for PO (P = 0.10). Patient demographics are presented in Table 1. No statistically significant differences were noted in any of the patient characteristics evaluated. Pathologic tumor features noted on RNU are presented in Table 2. No differences in tumor stage, grade, presence of concomitant carcinoma in situ, or surgical margin status were noted between groups.
Table 1
Patient Characteristics
| IO, n (%) | PO, n (%) | p value* | |
| Patients | 30 (59) | 21 (41) | – |
| Race | |||
| White | 26 (87) | 19 (90) | 0.13 |
| Non-White | 4 (13) | 2 (10) | |
| Age (yrs), mean±SD | 71.5±10.9 | 72.5±9.0 | 0.73** |
| Gender | |||
| Male | 21 (70) | 18 (85) | 0.32 |
| Female | 9 (30) | 3 (15) | |
| Type of Surgery | |||
| Open | 5 (17) | 5 (24) | 0.72 |
| Minimally Invasive | 25 (83) | 16 (76) | |
| History of Bladder Cancer | |||
| Absent | 18 (60) | 14 (67) | 0.77 |
| Present | 12 (40) | 7 (33) | |
| History of Intravesical Therapy | |||
| Absent | 24 (80) | 17 (81) | 0.93 |
| Present | 6 (20) | 4 (19) |
*Fisher’s two-sided exact test unless otherwise noted. **ANOVA test.
Table 2
Tumor Characteristics
| IO, n (%) | PO, n (%) | p value* | |
| Tumor Grade | |||
| Low | 8 (27) | 8 (38) | 0.23** |
| High | 22 (73) | 13 (62) | |
| Tumor Stage | |||
| T0/Ta | 8 (27) | 11 (52) | 0.11 |
| T1 | 11 (37) | 2 (10) | |
| T2 | 3 (10) | 2 (10) | |
| T3/T4 | 8 (27) | 6 (29) | |
| Nodal Status | |||
| N0 | 15 (50) | 8 (38) | 0.66 |
| N+ | 2 (7) | 1 (5) | |
| Nx | 13 (43) | 12 (48) | |
| Surgical Margins | |||
| Negative | 25 (83) | 18 (86) | 1.0 |
| Positive | 5 (17) | 3 (14) | |
| Concomitant CIS | |||
| Present | 14 (47) | 15 (71) | 0.09 |
| Absent | 16 (53) | 6 (29) |
*Fisher’s two-sided exact test unless otherwise noted; **Chi-square test.
The estimated probability of 1-year BTR rates for the IO and PO groups were 16% and 33%, respectively (log rank test p value = 0.09). BTR rates noted at any time during follow-up were 21% and 33%, respectively (p = 0.17). Kaplan-Meier curves depicting BTR rates within 1 year is depicted in Fig. 1. Results of univariate and multivariate analyses for BTR within 1 year following RNU are reported in Table 3. No significant associations were noted between the variables evaluated and BTR within one year following RNU on univariate analysis. Multivariate analysis noted significant associations with the timing of MMC instillation, surgical approach, surgical margin status, and presence of concomitant carcinoma in situ for BTR within the first year following RNU. Notably, patients who received MMC on IO had a significantly reduced risk of BTR in the first year postoperatively (adjusted HR = 0.113, 95% CI = 0.028–0.63, p = 0.01). No significant associations were noted between the variables evaluated and BTR at any time following RNU on univariate or multivariate analysis (data not shown). Patients who received MMC on IO had a reduced, but statistically insignificant, risk of BTR at any time following RNU (adjusted HR = 0.28, 95% CI = 0.075–1.02, p = 0.053.)
Fig.1
Time to Bladder Tumor Recurrence within the First Year. Kaplan-Meier survival curves for time-to-recurrence of bladder tumors within the first year according to two treatment groups: 1) intraoperative mitomycin C and 2) post-operative day 1 or later mitomycin C.
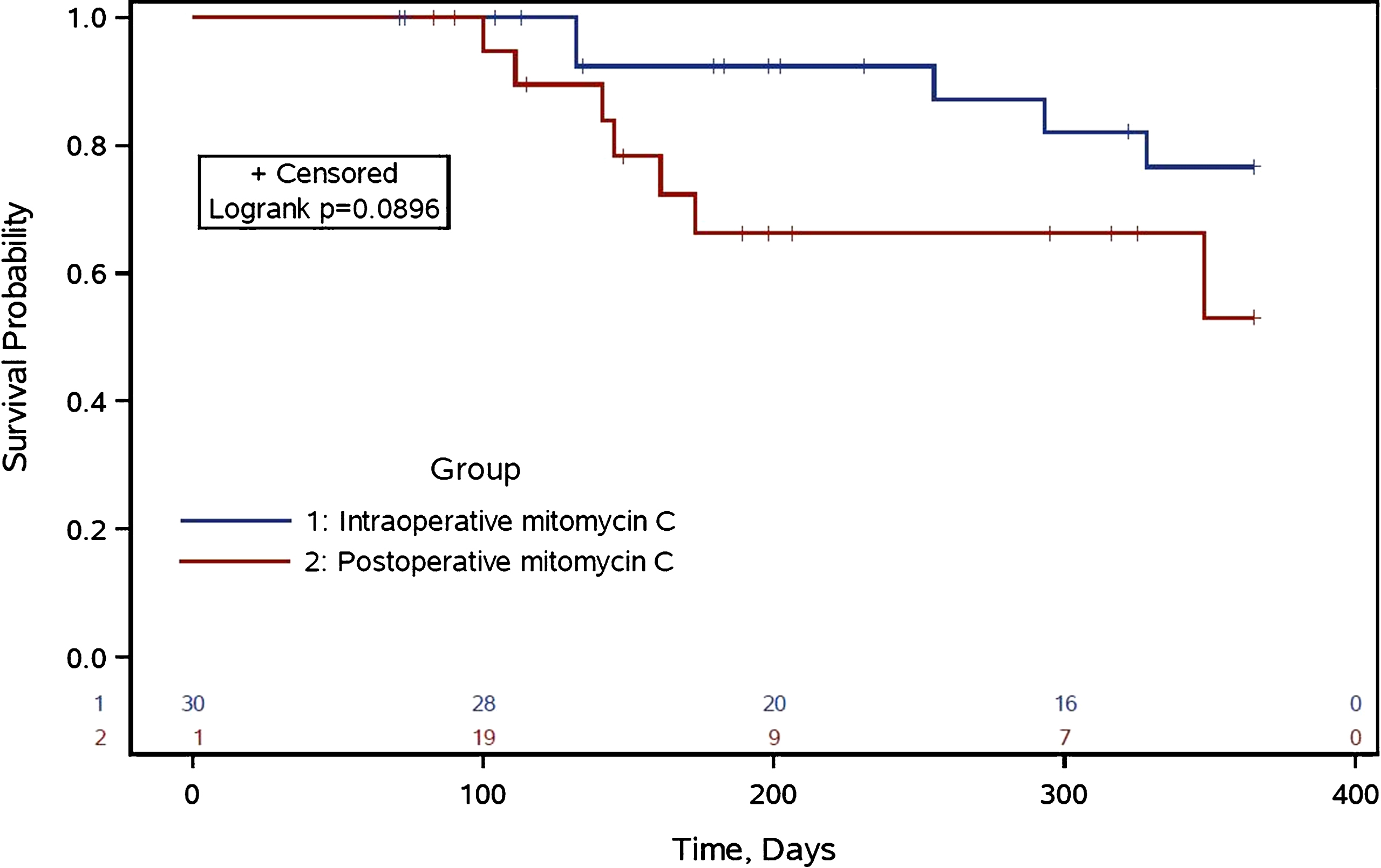
Table 3
Cox analysis of factors associated with bladder tumor recurrence following nephroureterectomy
| BTR 1st year | BTR at any time | |||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| IO MMC (vs. PO) | 0.13 | 0.03–0.63 | 0.01 | 0.28 | 0.08–1.01 | 0.053 |
| Open surgery | 5.08 | 1.16–22.34 | 0.03 | 2.82 | 0.73–10.85 | 0.13 |
| Surgical margins | 7.21 | 1.31–39.82 | 0.02 | 4.24 | 0.90–19.90 | 0.067 |
| Concomitant CIS | 5.18 | 1.18–22.72 | 0.03 | 2.39 | 0.66–8.60 | 0.18 |
DISCUSSION
BTR following RNU is common and requires routine cystoscopic surveillance. There are two potential explanations for this high rate of recurrence within the bladder following RNU. The first hypothesis is that the entire urothelium is affected by a molecular field change from widespread carcinogenic exposure, also known as field-cancerization [9]. The thought is that carcinogenic exposure can lead to independent, metachronous tumor development in the urinary tract. Alternatively, the second hypothesis is tumor seeding and implantation, in which malignant cells from a monoclonal genetic source spread throughout the lining of the urinary tract and seed the urothelium at various downstream sites [10, 11]. This theory has been supported by evaluation of tumor DNA showing similar mutations in BTR specimens compared to the original UTUC primary lesion and based upon location of BTR [12, 13]. Strategies aiming to reduce the rate of BTR following RNU target one or both of these hypotheses.
Evidence from randomized trials clearly supports the administration of a single dose of intravesical chemotherapy following RNU for UTUC. The first trial was the ODMIT-C trial by O’Brien et al, which is the largest prospective trial to investigate the impact of postoperative IVC following RNU [5]. Their study demonstrated a 40% relative risk reduction in BTR in patients receiving a single dose of intravesical MMC postoperatively following RNU. MMC administration in this study occurred on the day of urethral catheter removal, thus the timing of MMC administration varied between patients based upon duration of catheterization. According to the authors, this time point was “chosen to minimize the risk of extravasation,” and was most commonly performed during catheter removal 7–10 days after surgery. Of note, the ODMIT-C trial did not include patients with a previous history of bladder cancer. This is in contrast to our study cohort, in which a history of bladder cancer was present in a majority of both of the study groups. Nonetheless, this large, prospective trial paved the way for the routine use of MMC following RNU in clinical practice.
A second trial by Ito et al. confirmed O’Brien’s findings. In a relatively smaller cohort of patients, it was demonstrated that a single dose of intravesical pirarubicin (THP) had an even greater reduction in the rate of BTR (HR = 0.26) [6]. One significant difference between these studies was in the trial by Ito et al., THP was administered within 48 hours after surgery, compared to administration at 7–10 days postoperatively in the ODMIT-C trial. The implantation and seeding hypothesis could provide an explanation for the decreased recurrence risk with earlier administration of intravesical chemotherapy. One could hypothesize that if the urothelium is exposed to chemotherapy at or near the time of tumor cell release from surgical manipulation, tumor cell seeding and implantation would be inhibited. Based on this concept, even earlier administration of intravesical chemotherapy may be beneficial in further reducing BTR.
At our institution, MMC is administered most commonly at one of two different time points: [1] intraoperatively during RNU with drainage prior to bladder cuff removal, and [2] on postoperative day 1. Only two patients in our study received MMC outside of this time period (once administered on postoperative day 2 and once on postoperative day 3). For the patients receiving MMC intraoperatively, 40 mg (1 mg/mL) MMC is instilled via urethral catheter and allowed to dwell for 30–45 minutes followed by a bladder flush with 200 ml sterile saline prior to bladder cuff removal. Our current findings suggest that intraoperative administration of MMC may be associated with a lower rate of BTR when compared to administration on PO. One of the main concerns with early administration of IVC is its potential morbidity due to the toxicity of agents such as MMC and the risk of extravasation outside of the bladder lumen. However, there were no reported major complications related to IVC in either of the prospective trials listed above. A retrospective study by Moriarty et al. has demonstrated the safety of intraoperative instillation of intravesical chemotherapy at the time of RNU is a series of 51 patients [14]. Additionally, intravesical chemotherapy has been found to be safe when administered either prior to bladder cuff management or after appropriate bladder cuff closure.
Despite the reported safety of intravesical chemotherapy following RNU and the proven benefit in reducing BTR, intravesical chemotherapy remains underutilized in the United States. Lu et al. surveyed SUO members in 2016 to examine their practice patterns regarding intravesical chemotherapy following RNU [8]. Only 51% of respondents indicated they routinely administered intravesical chemotherapy following RNU. The most common reasons for not administering intravesical chemotherapy were lack of data, personal preference, and office infrastructure. This highlights the need for further education on the safety and benefits of intravesical chemotherapy following RNU to decrease BTR.
While our results are promising, the current study has multiple limitations that need to be addressed prior changing current practice. First, our study is limited by the relatively small sample size, relatively short follow up, and by its retrospective nature. Second, due to being a single site study patients with a history of bladder cancer were included in the current evaluation but were not included in the prior randomized trials [5, 6]. Third, the length of follow up differed significantly between patients in the IO and PO cohorts. However, the length of follow up was greater in the IO, putting these patients at greatest risk of having a BTR detected. Forth, the location and multifocality of UTUC was not considered in the current analysis. Prior studies have suggested that the rates of BTR may differed between UTUC located within the renal pelvis and ureter [15]. However, location of UTUC was not noted to impact BTR in the randomized trial by Ito et al. [6]. Additionally, while not significantly different between groups, concomitant CIS may have influenced the differences in BTR between groups. Fifth, due to the retrospective nature of the study the surveillance protocol was not standardized in the study population and we are unable to compare potential complications associated with MMC between groups. Finally, additional unmeasured cofounders which may influence BTR were not considered including ongoing smoking status and exposure to other carcinogens. These results should be viewed primarily as hypothesis generating and should not yet change clinical practice patterns. To our knowledge, this is the first study directly comparing outcomes between patients receiving intravesical MMC on IO and those receiving it on PO or later. A larger, prospective evaluation with standardized timing of intravesical chemotherapy administration and surveillance are needed. Further investigation will be essential to continue to improve our efforts in reducing BTR following RNU for UTUC.
CONCLUSIONS
Our results suggest the timing of intravesical chemotherapy administration may have an impact on the rate of BTR following RNU for UTUC. Developing a standardized, evidence-based approach for the timing of intravesical chemotherapy administration is an opportunity for urologists to improve BTR rates following RNU. Future prospective trials directly comparing the timing of intravesical chemotherapy are warranted.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2018. CA Cancer J Clin. (2018) ;68: (1):7–30. |
[2] | Rouprêt M , Babjuk M , Compérat E , Zigeuner R , Sylvester RJ , Burger M , et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol. (2015) ;68: (5):868–79. |
[3] | Raman JD , Messer J , Sielatycki JA , Hollenbeak CS . Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. (2011) ;107: (7):1059–64. |
[4] | Rouprêt M , Babjuk M , Compérat E , Zigeuner R , Sylvester RJ , Burger M , et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update, Eur Urol. (2018) ;73: (1):111–22. |
[5] | O’Brien T , Ray E , Singh R , Coker B , Beard R . Oncology BAoUSSo. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: A prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol. (2011) ;60: (4):703–10. |
[6] | Ito A , Shintaku I , Satoh M , Ioritani N , Aizawa M , Tochigi T , et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: The THP Monotherapy Study Group Trial, J Clin Oncol (2013) ;31: (11):1422–7. |
[7] | Deng X , Yang X , Cheng Y , Liu X , Wu B , Wang Z , et al. Prognostic value and efficacy valuation of postoperative intravesical instillation in primary urothelial carcinomas of upper urinary tract, Int J Clin Exp Med (2014) ;7: (12):4734–46. |
[8] | Lu DD , Boorjian SA , Raman JD . Intravesical chemotherapy use after radical nephroureterectomy: A national survey of urologic oncologists, Urol Oncol. (2017) ;35: (3):.113.e1–e7. |
[9] | Jones TD , Wang M , Eble JN , MacLennan GT , Lopez-Beltran A , Zhang S , et al. Molecular evidence supporting field effect in urothelial carcinogenesis, Clin Cancer Res. (2005) ;11: (18):6512–9. |
[10] | Habuchi T , Takahashi R , Yamada H , Kakehi Y , Sugiyama T , Yoshida O . Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet. (1993) ;342: (8879):1087–8. |
[11] | Hafner C , Knuechel R , Stoehr R , Hartmann A . Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies, Int J Cancer. (2002) ;101: (1):1–6. |
[12] | Sidransky D , Frost P , Von Eschenbach A , Oyasu R , Preisinger AC , Vogelstein B . Clonal origin of bladder cancer. N Engl J Med. (1992) ;326: (11):737–40. |
[13] | Ito A , Shintaku I , Satoh M , Ioritani N , Tochigi T , Numata I , et al. Intravesical seeding of upper urinary tract urothelial carcinoma cells during nephroureterectomy: An exploratory analysis from the THPMG trial. Jpn J Clin Oncol. (2013) ;43: (11):1139–44. |
[14] | Moriarty MA , Uhlman MA , Bing MT , O’Donnell MA , Brown JA , Tracy CR et al. Evaluating the safety of intraoperative instillation of intravesical chemotherapy at the time of nephroureterectomy. BMC Urol. (2015) ;15: :45. |
[15] | Seisen T , Granger B , Colin P , Léon P , Utard G , Renard-Penna R , et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol. (2015) ;67: (6):1122–33. |




