Early Complications and Mortality following Radical Cystectomy: Associations with Malnutrition and Obesity
Abstract
Background:
There are conflicting data regarding the impact of obesity on postoperative outcomes following radical cystectomy (RC) and how obesity and malnutrition interact in patients undergoing RC.
Objective:
To evaluate associations of body mass index (BMI), significant preoperative weight loss, and hypoalbuminemia with 30-day complications and mortality after RC.
Methods:
Review of the American College of Surgeons National Surgical Quality Improvement Program database identified 2,055 patients who underwent RC (2006-12). Univariate and multivariable logistic regression models were developed to assess associations between hypoalbuminemia (<3.5 g/dL), >10% preoperative weight loss, obesity as characterized by BMI (class I: 30–34.9, II: 35–39.9, III: ≥40 kg/m2), and 30-day complications and mortality.
Results:
The median BMI of the study cohort was 27.82 kg/m2 with 22.4% classified as having class I, 7.5% class II, and 4.2% class III obesity, respectively. Hypoalbuminemia and >10% weight loss were present in 16.7% and 3.5%, respectively. Among obese patients, 13.4% had hypoalbuminemia. On multivariable analysis, class I (OR 1.43, p = 0.01), class II (OR 1.92, p < 0.001), and class III (OR 2.32, p < 0.001) obesity and hypoalbuminemia (OR 1.47, p = 0.02) were independently associated with 30-day complications, and class III obesity (OR 2.96, p = 0.02) and hypoalbuminemia (OR 2.33, p = 0.03) were independently associated with 30-day mortality.
Conclusion:
Increasing class of obesity and hypoalbuminemia were independently associated with increased complications following RC. Hypoalbuminemia and class III obesity were associated with early mortality. This study highlights the fact that malnutrition may coexist in obese patients and underscores the need to identify patients with malnutrition who may be candidates for preoperative nutritional optimization.
INTRODUCTION
Radical cystectomy (RC) is the cornerstone of treatment for localized or metastatic muscle-invasive bladder cancer [1]. The association between preoperative malnutrition and greater postoperative complications and short-term mortality following major abdominal surgery is increasingly acknowledged [3–9]. According to the World Health Organization (WHO) criteria, malnutrition may be characterized by low body mass index (BMI < 18.5 kg/m2) and hypoalbuminemia (<3.5 g/dL) [10]. Severe malnutrition, frailty, and cachexia are also signified by rapid, unintentional weight loss [11]. Within the urologic literature, poor preoperative nutritional status has been demonstrated to be independently associated with inferior oncologic outcomes [10, 12–15].
With respect to obesity, as characterized by BMI, however, there are conflicting data regarding the impact of obesity on perioperative and oncologic outcomes following RC. Some authors failed to observe an association between obesity and perioperative morbidity and oncologic outcomes [16], while others have demonstrated that obesity is associated with increased risk of adverse perioperative and long-term oncologic outcomes [10, 17]. Conversely, other studies have observed that increasing obesity is counterintuitively associated with improved outcomes following surgery, a finding that has been termed “The obesity paradox” [18–20]. These conflicting reports may be related to the lack of specificity of the BMI measurement, in that it simply represents weight normalized by height and does not reflect true body composition [21].
Patients undergoing RC are at significant risk for malnutrition due to their age, comorbidities, and disease-related factors [22]. The interaction between obesity and malnutrition and the relative association of these two potentially modifiable risk factors with perioperative outcomes remains to be defined. As such, the objective of this study was to assess the associations between metrics of malnutrition including hypoalbuminemia, significant preoperative weight loss, and classes of obesity as defined by BMI, and 30-day complications and mortality within a contemporary national cohort.
MATERIALS AND METHODS
We queried the American College of Surgeons National Quality Improvement Program (ACS-NSQIP) for all patients who underwent RC between 2006 and 2012. The ACS-NSQIP is a prospectively collected, risk-adjusted, multi-institutional, national clinical registry database for general, urological, and vascular surgery patients. It includes 135 variables including the outcomes of 30-day complications and mortality following major surgical procedures [23]. This study is exempt from IRB approval, since it uses a publicly available deidentified registry.
RC cases performed between 2006 and 2012 were extracted from the dataset using Current Procedure Terminology codes for RC (51570, 51575, 51580, 51585, 51590, 51595, 51596). To minimize confounding associated with significant other major procedures that would not commonly be performed at the same time as RC, we excluded patients who underwent with multiple synchronous unrelated major procedures in addition to RC.
Preoperative nutrition and obesity-related factors that are available in the ACS-NSQIP dataset included preoperative albumin, weight loss >10% within 6 months prior to surgery and preoperative BMI (categorized as underweight (≥18.5), normal weight (18.5–24.9), overweight (25–29.9), class I (30–34.9), II (35–39.9), and III (≥40 kg/m2 obesity). Albumin was dichotomized as greater than or equal to versus below the threshold of 3.5 g/dL [10, 24–26]; <3.5 g/dL was classified as hypoalbuminemia.
Clinical, demographic, and treatment-related factors available included age, sex, race/ethnicity, medical comorbidities (including pulmonary comorbidity [dyspnea or chronic obstructive pulmonary disease], heart disease [congestive heart failure, history of myocardial infarction (MI), prior percutaneous catheterization, previous cardiac surgery, history of angina], baseline paralysis, steroid use, requirement for dialysis, history of stroke, peripheral vascular disease requiring revascularization or amputation, presence of ascites, American Society of Anesthesia (ASA) classification, history of substance abuse (smoking, alcohol use), functional status, exposure to preoperative chemotherapy or radiotherapy, and whether or not the patient had disseminated cancer at the time of surgery. Surgical factors included creation of a continent diversion (e.g. Neobladder) and concurrent lymphadenectomy. A subset of the medical comorbidities included in the analysis (history of MI, prior percutaneous catheterization, previous cardiac surgery, history of angina, paralysis, history of stroke, peripheral vascular disease, alcohol use, and chemotherapy or radiotherapy) were not included in NSQIP after 2011 and therefore only available for a subset of our cohort.
Statistical analysis
Continuous features were described with medians (interquartile range [IQR]). Categorical data were described with numbers (percentages). Chi-square tests assessed differences in hypoalbuminemia and preoperative weight loss by BMI category.
The outcomes of interest included major complications and mortality within 30 days of RC. The major complications reviewed included cardiac arrest, MI, pneumonia, ventilator for >48 hours, unplanned intubation, deep incision surgical site infection, organ space surgical site infection, sepsis, septic shock, deep vein thrombosis, pulmonary embolism, progressive renal insufficiency, acute renal failure, superficial surgical site infection, wound disruption, urinary tract infection, cerebrovascular accident/stroke, coma >24 hours, peripheral nerve injury, return to the operating room, and death.
Univariate and multivariable logistic regression were performed to evaluate associations between clinical, demographic, and surgical factors and the outcomes of interest. The final multivariable models were developed using forward selection of variables independently associated with the outcomes of interest, at the 5% level of significance; BMI category, preoperative weight loss, and hypoalbuminemia were forced to be included in the models. Due to the low prevalence of 30-day mortality, variables for the model of 30-day mortality were further selected based on clinical significance to avoid over-fitting.
Missing values were handled as follows: for descriptive statistics, only the patients for whom the variable was available were included in that variable’s distribution. In the univariate and multivariable modeling, for features with a significant number of patients with unknown values, missing data was included as a separate “unknown” category, or if only missing for a small number, was collapsed with the normal category and or excluded for continuous data, as specified in the multivariable tables.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary NC).
RESULTS
A total of 2,055 patients treated with RC between 2006–2012 were identified, with a median age of 69 (interquartile range [IQR]: 62, 76). The cohort included 1,667 (81.2%) males and 386 (18.8%) females and predominantly consisted of non-Hispanic white patients (n = 1,701 [82.8%]). Continent urinary diversions were undertaken in 426 (20.7%) patients and 1,437 (69.9%) underwent concurrent lymphadenectomy. In total, 1,494 (72.7%) were ASA class III-V and 45 (2.2%) were partially or totally dependent with respect to functional status. Clinical and demographic data for the study cohort is summarized in Table 1.
Table 1
| Feature (N available) | Number (%) or Median (Interquartile Range, IQR) |
| Age (N = 2055) | 69 (62,76) |
| Sex (N = 2053) | |
| Male | 1667 (81.2%) |
| Female | 386 (18.8%) |
| Not available | 2 |
| Race/Ethnicity (N = 2055) | |
| Non-Hispanic White | 1701 (82.8%) |
| Hispanic White | 29 (1.4%) |
| Black or African American | 68 (3.3%) |
| Asian, Native Hawaiian, or Pacific Islander | 18 (0.9%) |
| American Indian or Alaska Native | 2 (0.1%) |
| Other or Unknown | 237 (11.5%) |
| Year of Operation (N = 2055) | |
| 2006 | 9 (0.4%) |
| 2007 | 27 (1.3%) |
| 2008 | 88 (4.3%) |
| 2009 | 180 (8.8%) |
| 2010 | 207 (101%) |
| 2011 | 677 (32.9%) |
| 2012 | 867 (42.2%) |
| Neobladder (continent diversion) (N = 2055) | |
| No | 1629 (79.3%) |
| Yes | 426 (20.7%) |
| Concurrent Lymphadenectomy (N = 2055) | |
| No | 618 (30.1%) |
| Yes | 1437 (69.9%) |
| ASA Class (N = 2054) | |
| I/II | 560 (27.3%) |
| III/IV/V | 1494 (72.7%) |
| Not available | 1 |
| Current smoker within 1 year (N = 2055) | |
| No | 1513 (73.6%) |
| Yes | 542 (26.4%) |
| Diabetes mellitus (N = 2055) | |
| Insulin | 106 (5.2%) |
| Non-Insulin/Oral | 289 (14.1%) |
| None | 1660 (80.8%) |
| Hypertension requiring medication (N = 2055) | |
| No | 823 (40.0%) |
| Yes | 1232 (60.0%) |
| Preoperative hematocrit (N = 2004) | 38.5 (34.2, 42.0; range 11.8–53.8) |
| Preoperative creatinine (N = 1992) | 1.1 (0.9, 1.3; range 0.2–13.5) |
| Preoperative GFR (N = 1990) | 67.4 (52.0, 83.2; range 3.6–374.9) |
| Preoperative GFR Category | |
| Normal (eGFR > 90 ml/min/1.732) | 340 (17.1%) |
| CKD 2 (eGFR 60–89 ml/min/1.732) | 920 (46.2%) |
| CKD 3 (eGFR 30–59 ml/min/1.732) | 651 (32.7%) |
| CKD 4 (eGFR 15–29 ml/min/1.732) | 66 (3.3%) |
| CKD 5 (eGFR < 15 ml/min/1.732) | 13 (0.7%) |
| Not available | 65 |
| Chemotherapy for malignancy in < 30 days pre-op (N = 1112) | |
| No | 988 (88.8%) |
| Yes | 124 (11.2%) |
| Not available | 943 |
| Radiotherapy for malignancy in last 90 days (N = 1112) | |
| No | 1106 (99.5%) |
| Yes | 6 (0.5%) |
| Not available | 943 |
| Paralysis (hemiplegia, paraplegia, quadriplegia) (N = 1112) | |
| No | 1092 (98.2%) |
| Yes | 20 (1.8%) |
| Not available | 943 |
| Disseminated Cancer/Metastatic disease (N = 2055) | |
| No | 1964 (95.6%) |
| Yes | 91 (4.4%) |
| Steroid use for chronic condition (N = 2055) | |
| No | 1997 (97.2%) |
| Yes | 58 (2.8%) |
| Stroke (CVA, stroke with/without neurological deficits) (N = 1111) | |
| No | 1081 (97.3%) |
| Yes | 30 (2.7%) |
| Not available | 944 |
| History of revascularization/amputation for peripheral vascular disease (N = 1112) | |
| No | 1097 (98.7%) |
| Yes | 15 (1.3%) |
| Not available | 943 |
| Alcohol consumption >2 drinks/day in the 2 weeks prior to admission (N = 1117) | |
| No | 1070 (95.8%) |
| Yes | 47 (4.2%) |
| Not available | 938 |
| Dyspnea (N = 2055) | |
| At rest | 8 (0.4%) |
| With moderate exertion | 217 (10.6%) |
| No | 1830 (89.1%) |
| Functional status (N = 2052) | |
| Independent | 2007 (97.8%) |
| Partially Dependent | 38 (1.9%) |
| Totally Dependent | 7 (0.3%) |
| Not available | 3 |
| History of severe COPD (N = 2055) | |
| No | 1874 (91.2%) |
| Yes | 181 (8.8%) |
| Pulmonary comorbidity (N = 2055) | |
| No | 1722 (83.8%) |
| Yes | 333 (16.2%) |
| Ascites (N = 2055) | |
| No | 2054 (100.0%) |
| Yes | 1 (0.0%) |
| Heart disease (CHF, MI, previous PCI, previous cardiac surgery, angina) (N = 1122) | |
| No | 929 (82.8%) |
| Yes | 193 (17.2%) |
| Not available | 933 |
| History of angina in the 1 month prior to surgery (N = 1112) | |
| No | 1108 (99.6%) |
| Yes | 4 (0.4%) |
| Not available | 943 |
Note: Demographic, clinical, and treatment-related factors for the study cohort.
Nutritional and obesity-related features of interest (BMI, preoperative albumin level, and preoperative weight loss) are summarized in Table 2. The median BMI of the study cohort was 27.8 kg/m2 (IQR 24.4, 31.6), with 457 (22.4%) with class I, 153 (7.5%) with class II, and 85 (4.2%) with class III obesity. In total, 38 (1.9%) met criteria for underweight BMI (≤18.5 kg/m2). Significant (>10%) weight loss in the 6 months preceding surgery was observed in 71 (3.5%). The median preoperative serum albumin was 4.0 g/dL (IQR: 3.6, 4.3; range 1.4–9.7). Of note, albumin was available in 1,237 (60.2%) patients, overall. Among patients for whom albumin was available, hypoalbuminemia was observed in 207 (16.7%) patients. Among patients for whom preoperative albumin was available, hypoalbuminemia was present in 12.2%, 14.4%, and 17.7% of patients with class I, II, and III obesity, respectively. Figure 1A and 1B demonstrates the prevalence of hypoalbuminemia and significant preoperative weight loss, respectively, across BMI classes. Of note, >10% preoperative weight loss was extremely rare, occurring in 2.2%, 1.3%, and 1.2% of patients for class I, II, and III obesity, respectively.
Fig.1
(A) Note: Proportion of patients (%) with hypoalbuminemia (<3.5 g/dL) prior to radical cystectomy by body mass index category, among patients for whom preoperative albumin levels were available (n-1237). (B) Note: Proportion of patients (%) with >10% weight loss within 6 months prior to radical cystectomy by body mass index category.
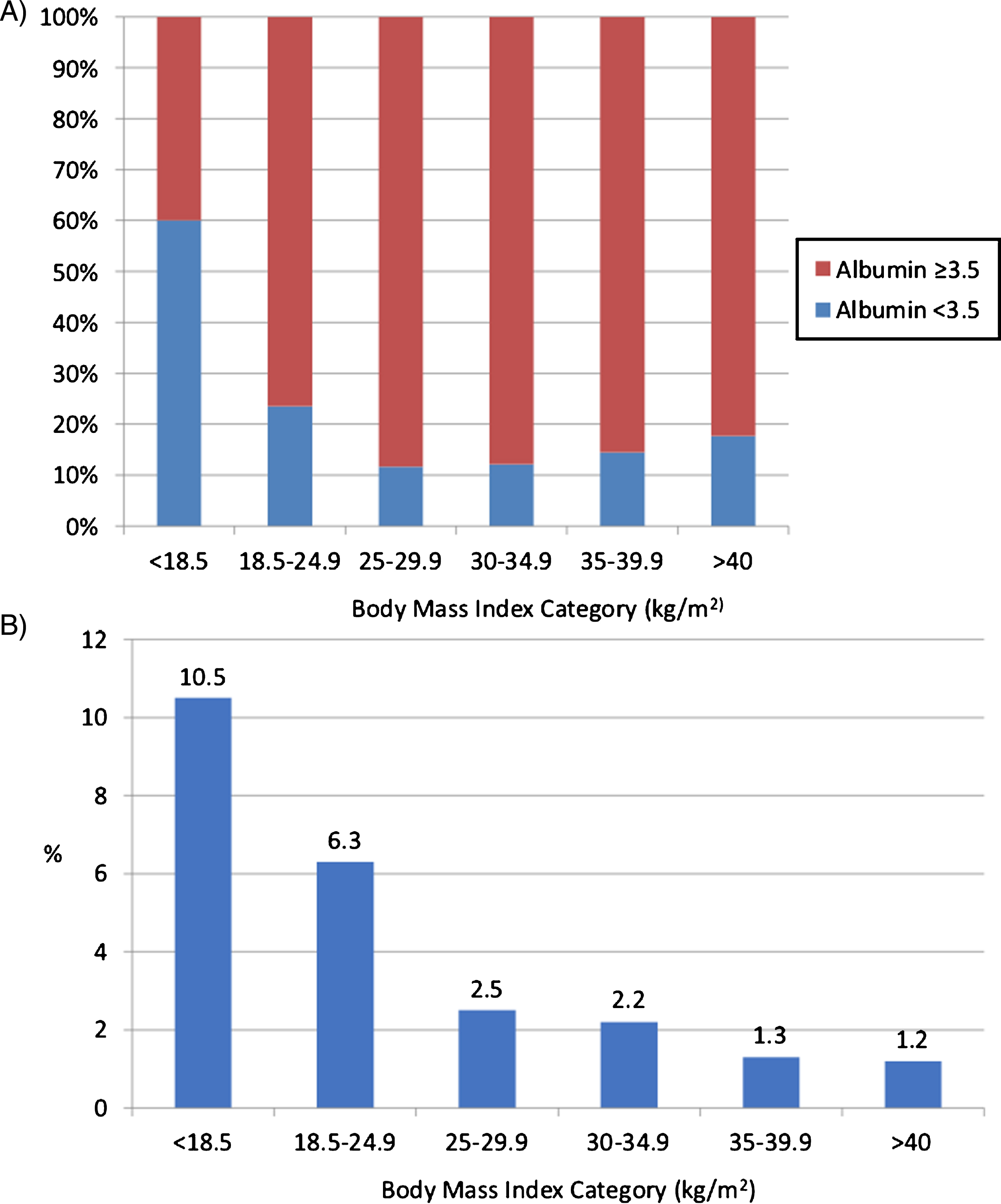
Table 2
| Nutritional Characteristic | Median (IQR; range) or N (%) |
| Body Mass Index (kg/m2; N = 2038) | 27.82 (24.44, 31.63; 10.80–70.85) |
| BMI Category (WHO Classification) (N = 2038) | |
| Underweight (BMI < 18.5 kg/m2) | 38 (1.9%) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 554 (27.2%) |
| Overweight (BMI 25.0–29.9 kg/m2) | 751 (36.8%) |
| Class I Obesity (BMI 30.0–34.9 kg/m2) | 457 (22.4%) |
| Class II Obesity (BMI 35.0–39.9 kg/m2) | 153 (7.5%) |
| Class III Obesity (BMI > 40 kg/m2) | 85 (4.2%) |
| Not available | 17 |
| >10% loss of body weight in the last 6 months (N = 2055) | |
| No | 1984 (96.5%) |
| Yes | 71 (3.5%) |
| Preoperative serum albumin (g/dL; N = 1237) | 4 (3.6, 4.3; 1.4–9.7) |
| Preoperative albumin category (N = 1237) | |
| <3.5 g/dL | 207 (16.7%) |
| ≥3.5 g/dL | 1030 (83.3%) |
| Not available | 818 |
Note: Distribution of Nutritional Factors and BMI Classifications.
In the study cohort, 705 of 2,055 patients (34.3%) experienced >1 complication within 30 days postoperatively, and a total of 63 (3.1%) died within 30 days of RC. Complications included sepsis and septic shock in 274 (13.3%) patients, respiratory complications (pneumonia, ventilator >48 hours, or reintubation) in 142 (6.9%), return to the operating room in 130 (6.3%), and superficial surgical site infection in 130 (6.3%), and 118 (5.7%) patients developed a deep vein thrombosis or pulmonary embolism. The prevalence of complications by type is detailed in Table 3. Blood transfusions were administered to 906 patients (44.1%). Excluding bleeding and transfusion, 332 (16.2%) experienced one complication, 180 (8.8%) experienced two complications, and 193 (9.4%) experienced three or more complications.
Table 3
| Operative Detail | Median (IQR) Or N(%) |
| Total Operative Time (minutes) | 329 (258, 419) |
| Transfusion | 906 (44.1%) |
| Days from cystectomy to discharge from hospital | 8 (7, 11) |
| Complications | N(%) |
| Any complication | 703 (34.3%) |
| Death within 30 days | 63 (3.1%) |
| Cardiac Arrest requiring CPR | 20 (1.0%) |
| Myocardial Infarction | 35 (1.7%) |
| Respiratory Pneumonia, ventilator >48 hours, reintubation | 142 (6.9%) |
| Pneumonia | 77 (3.7%) |
| Requirement for ventilation >48 hours | 55 (2.7%) |
| Unplanned intubation | 76 (3.7%) |
| Deep Incision Surgical Site Infection | 40 (1.9%) |
| Organ Space Surgical Site Infection | 98 (4.8%) |
| Sepsis or Septic Shock | 274 (13.3%) |
| Sepsis | 209 (10.2%) |
| Septic Shock | 70 (3.4%) |
| DVT or PE | 118 (5.7%) |
| Deep Vein Thrombosis/Thrombophlebitis/DVT Requiring Therapy | 81 (3.9%) |
| Pulmonary Embolism | 59 (2.9%) |
| Progressive Renal Insufficiency | 50 (2.4%) |
| Acute Renal Failure | 35 (1.7%) |
| Superficial Surgical Site Infection | 130 (6.3%) |
| Wound disruption | 65 (3.2%) |
| Urinary Tract Infection | 201 (9.8%) |
| Stroke/CVA with neurological deficit | 8 (0.4%) |
| Coma >24 hours | 0 (0.0%) |
| Peripheral nerve injury | 4 (0.2%) |
| Return to the Operative Room | 130 (6.3%) |
| Days from Principal Operative Procedure to Unplanned | 11 (5, 19) |
| Reoperation (2012 only) | |
| Multiple complications (excluding bleeding/transfusion) | |
| 0 | 1350 (65.7%) |
| 1 | 332 (16.2%) |
| 2 | 180 (8.8%) |
| 3 | 92 (4.5%) |
| 4 | 35 (1.7%) |
| 5 | 29 (1.4%) |
| 6 | 19 (0.9%) |
| 7 | 13 (0.6%) |
| 8 | 4 (0.2%) |
| 11 | 1 (0.0%) |
Note: Operative details and 30-day Complications and Mortality (N = 2055).
Univariate and multivariable logistic regression models detailing associations between the nutritional and clinical factors of interest with the outcomes of 30-day complications and 30-day mortality are presented in Tables 4A and 4B respectively. On multivariable analysis, increasing class of obesity, class I (Odds Ratio [OR] 1.43, 95% Confidence Interval [CI] 1.09–1.86; p = 0.01), class II (OR 1.92, 95% CI 1.32–2.79; p < 0.001), and class III (OR 2.32, 95% CI 1.46–3.71; p < 0.001) and hypoalbuminemia (OR 1.47, 95% CI 1.07–2.02; p = 0.02) were independently associated with increased risk of major complications within 30 days of surgery (Table 4A) after adjusting for >10% weight loss, functional status, and dyspnea.
Table 4A
| Univariate Models | Multivariable Model | |||
| Feature | Odds Ratio (95% Confidence Interval) | p-value | Odds Ratio (95% Confidence Interval) | p-value |
| BMI Category (ref = normal [18.5–24.9]) | ||||
| Underweight (<18.5) | 1.84 (0.95–3.58) | 0.07 | 1.66 (0.84–3.28) | 0.14 |
| Overweight (25.0–29.9) | 1.04 (0.82–1.32) | 0.72 | 1.13 (0.89–1.44) | 0.33 |
| Obese Class I (30.0–34.9) | 1.34 (1.03–1.74) | 0.03 | 1.43 (1.09–1.86) | 0.01 |
| Obese Class II (35.0–39.9) | 1.82 (1.26–2.63) | 0.001 | 1.92 (1.32–2.79) | <0.001 |
| Obese Class III (≥40.0) | 2.23 (1.40–3.53) | <0.001 | 2.32 (1.46–3.71) | <0.001 |
| Unknown | 0.70 (0.23–2.18) | 0.54 | 0.65 (0.20–2.17) | 0.49 |
| >10% loss of body weight within 6 months (ref = no) | 1.51 (0.93–2.43) | 0.09 | 1.44 (0.88–2.36) | 0.15 |
| Preoperative Serum Albumin (ref > 3.5) | ||||
| <3.5 | 1.53 (1.13–2.08) | 0.006 | 1.47 (1.07–2.02) | 0.02 |
| Unknown | 0.89 (0.74–1.09) | 0.26 | 0.88 (0.73–1.08) | 0.22 |
| Preoperative Serum Albumin (per 1 g/dL increase) | 0.81 (0.66–0.99) | 0.04 | ||
| Neobladder (ref = no) | 1.09 (0.88–1.37) | 0.43 | ||
| Lymphadenectomy (ref = no) | 0.91 (0.75–1.11) | 0.36 | ||
| Age (per 5-year increase) | 1.00 (0.95–1.04) | 0.85 | ||
| Year of Operation (ref = 2012) | ||||
| 2006 | 2.57 (0.68–9.63) | 0.16 | ||
| 2007 | 1.91 (0.88–4.11) | 0.10 | ||
| 2008 | 1.06 (0.67–1.69) | 0.80 | ||
| 2009 | 1.43 (1.03–1.99) | 0.03 | ||
| 2010 | 0.96 (0.69–1.33) | 0.81 | ||
| 2011 | 1.08 (0.87–1.33) | 0.49 | ||
| Male sex (ref = female) | 0.90 (0.72–1.14) | 0.38 | ||
| ASA Class 3–5 (ref = 1–2 or unknown) | 1.27 (1.03–1.56) | 0.03 | ||
| Current smoker within 1 year of surgery (ref = no) | 1.12 (0.91–1.37) | 0.29 | ||
| Diabetes Mellitus (ref = no) | ||||
| Insulin-dependent | 1.18 (0.79–1.78) | 0.41 | ||
| Treated with oral agents | 1.08 (0.83–1.41) | 0.55 | ||
| Preoperative Hematocrit (ref = normal or unknown: male 42+, female 38+) | ||||
| Very low (male < 32, female < 28) | 1.16 (0.85–1.58) | 0.35 | ||
| Low (male 32–41.9, female 28–37.9) | 1.02 (0.84–1.25) | 0.82 | ||
| Preoperative eGFR (ref = eGFR≥90 ml/min/1.73m2) | ||||
| CKD 2 (eGFR 60–89 ml/min/1.732) | 0.84 (0.65–1.09) | 0.19 | ||
| CKD 3 (eGFR 30–59 ml/min/1.732) | 0.95 (0.72–1.24) | 0.69 | ||
| CKD 4 (eGFR 15–29 ml/min/1.732) | 1.06 (0.62–1.83) | 0.83 | ||
| CKD 5 (eGFR < 15 ml/min/1.732) | 0.77 (0.23–2.57) | 0.68 | ||
| Unknown | 1.02 (0.59–1.77) | 0.94 | ||
| Chemotherapy for malignancy in < 30 days pre-op (ref = no) | ||||
| Yes | 0.68 (0.45–1.04) | 0.07 | ||
| Unknown | 1.01 (0.84–1.22) | 0.94 | ||
| Functional Status (ref = independent) | ||||
| Partially dependent | 1.58 (0.83–3.01) | 0.17 | 1.32 (0.68–2.58) | 0.41 |
| Totally dependent | 11.71 (1.41–97.45) | 0.02 | 12.37 (1.42–107.62) | 0.02 |
| Dyspnea (ref = no) | ||||
| At rest | 2.02 (0.50–8.10) | 0.32 | 2.24 (0.55–9.08) | 0.26 |
| With moderate exertion | 1.57 (1.18–2.09) | 0.002 | 1.49 (1.11–1.99) | 0.007 |
Note: Univariate and Multivariable Logistic Regression Models for Postoperative Complication within 30 days of surgery.
With regards to 30-day mortality, class III obesity (OR 2.96, 95% CI 1.18–7.42; p = 0.02) was independently associated with a nearly 3-fold increase in the risk of 30-day mortality. Hypoalbuminemia was associated with a 2.3-fold increase in the risk of death within 30 days (OR 2.33, 95% CI 1.10–4.95, p = 0.03). The full multivariable model is presented in Table 4B.
Table 4B
| Univariate Models | Multivariable Model | |||
| Feature | Odds Ratio (95% Confidence Interval) | p-value | Odds Ratio (95% Confidence Interval) | p-value |
| BMI Category (ref = normal [18.5–24.9]) | ||||
| Underweight (<18.5) | 3.72 (1.19–11.66) | 0.02 | 3.04 (0.95–9.74) | 0.0612 |
| Overweight (25.0–29.9) | 0.56 (0.27–1.16) | 0.12 | 0.60 (0.29–1.25) | 0.1685 |
| Obese Class I (30.0–34.9) | 1.00 (0.49–2.05) | 1.00 | 1.06 (0.51–2.19) | 0.8767 |
| Obese Class II (35.0–39.9) | 1.29 (0.50–3.33) | 0.60 | 1.36 (0.52–3.53) | 0.5291 |
| Obese Class III (≥40.0) | 2.84 (1.14–7.05) | 0.03 | 2.96 (1.18–7.42) | 0.0207 |
| Unknown | 4.21 (0.89–19.89) | 0.07 | 3.74 (0.78–17.91) | 0.0983 |
| >10% loss of body weight within 6 months (ref = no) | 1.42 (0.43–4.63) | 0.57 | 1.19 (0.35–4.04) | 0.7833 |
| Preoperative Serum Albumin (ref > 3.5) | ||||
| <3.5 | 2.82 (1.37–5.79) | 0.005 | 2.33 (1.10–4.95) | 0.0274 |
| Unknown | 1.68 (0.96–2.95) | 0.07 | 1.61 (0.91–2.83) | 0.1011 |
| Preoperative Serum Albumin (per 1 g/dL increase) | 0.49 (0.29–0.81) | 0.006 | ||
| Neobladder (ref = no) | 0.55 (0.26–1.16) | 0.12 | ||
| Lymphadenectomy (ref = no) | 1.68 (0.91–3.11) | 0.10 | ||
| Age (per 5-year increase) | 1.28 (1.11–1.46) | <0.001 | ||
| Male sex (ref = female) | 0.57 (0.33–0.99) | 0.047 | ||
| Year of Operation (ref = 2012) | ||||
| 2006–2008 | 1.12 (0.38–3.28) | 0.83 | ||
| 2009 | 0.96 (0.36–2.55) | 0.94 | ||
| 2010 | 2.07 (1.02–4.20) | 0.04 | ||
| 2011 | 0.87 (0.47–1.62) | 0.67 | ||
| ASA Class 3–5 (ref = 1–2 or unknown) | 2.30 (1.13–4.69) | 0.02 | ||
| Current smoker within 1 year of surgery (ref = no) | 0.79 (0.43–1.45) | 0.45 | ||
| Diabetes Mellitus (ref = no) | ||||
| Insulin-dependent | 1.29 (0.46–3.64) | 0.63 | ||
| Treated with oral agents | 1.18 (0.59–2.35) | 0.65 | ||
| Preoperative Hematocrit (ref = normal or unknown: male 42+, female 38+) | ||||
| Very low (male < 32, female < 28) | 1.74 (0.78–3.85) | 0.17 | ||
| Low (male 32–41.9, female 28–37.9) | 1.28 (0.71–2.29) | 0.42 | ||
| Preoperative eGFR (ref = eGFR≥90 ml/min/1.73m2) | ||||
| CKD 2 (eGFR 60–89 ml/min/1.732) | 1.43 (0.58–3.54) | 0.44 | ||
| CKD 3 (eGFR 30–59 ml/min/1.732) | 2.50 (1.03–6.10) | 0.04 | ||
| CKD 4 (eGFR 15–29 ml/min/1.732) or | ||||
| CKD 5 (eGFR < 15 ml/min/1.732) | 2.20 (0.54–8.90) | 0.27 | ||
| Unknown | 2.69 (0.66–11.06) | 0.17 | ||
| Disseminated Cancer (ref = no) | 3.35 (1.54–7.25) | 0.002 | ||
| Chemotherapy for malignancy in < 30 days pre-op (ref = no) | ||||
| Yes | 0.46 (0.11–1.94) | 0.47 | ||
| Unknown | 0.83 (0.50–1.38) | 0.29 | ||
| Functional Status (ref = independent) | ||||
| Partially dependent | 3.95 (1.36–11.51) | 0.01 | ||
| Totally dependent | 5.60 (0.66–47.27) | 0.11 | ||
| Dyspnea (ref = no) | ||||
| At rest or with moderate exertion | 2.18 (1.17–4.09) | 0.02 | ||
| Pulmonary comorbidity (dyspnea or COPD) (ref = no) | 2.13 (1.22–3.73) | 0.008 | ||
| Heart disease (ref = no) | ||||
| Yes | 1.83 (0.87–3.84) | 0.11 | ||
| Unknown | 0.96 (0.56–1.65) | 0.88 | ||
Note: Univariate and Multivariable Logistic Regressions for Death within 30 days.
DISCUSSION
Currently the morbidity after RC remains high, with a 28–64% 90-day complication rate [27, 28]. In this retrospective study, we utilized the NSQIP database to assess associations between increasing classes of obesity, as characterized by BMI, and markers of malnutrition and 30-day morbidity and mortality following RC in 2,055 patients with bladder cancer.
Our study had two main findings, which demonstrated that class I-III obesity and preoperative hypoalbuminemia (<3.5 g/dL) were independently associated with increased risk of moderate-severe complications within 30 days of RC. Additionally, class III obesity and hypoalbuminemia were independently associated with increased rate of mortality within 30 days.
The finding that hypoalbuminemia is associated with increased risk of adverse postoperative outcomes is well-supported in the urologic literature. Garg and colleagues conducted a retrospective review of 1,097 patients who underwent RC for bladder cancer between 1992–2005 [26]. After adjusting for both organ-confined disease and age-adjusted comorbidity index, a one-unit increase in preoperative albumin was associated with decreased risk of mortality (OR 0.33, 95% CI 0.14–0.75; p = 0.01) within 90 days of RC. A one-unit increase in preoperative albumin was further associated with a decreased risk of developing a postoperative complication (OR 0.61, 95% CI 0.42–0.90; p = 0.01). Furthermore, Gregg and colleagues demonstrated preoperative nutritional deficiency, defined by hypoalbuminemia (<3.5 g/dL), BMI < 18.5 kg/m2 or preoperative weight loss > 5%, significantly predicted increased mortality within 90 days of RC (HR 2.91, 95% CI 1.36–6.23; p < 0.01) [24]. In that study, 90-day survival was estimated to be 84.2% (95% CI 75.5–90; p < 0.01) in patients with nutritional deficiency (n = 103) vs. 94.9% (95% CI 92.3–96.6; p < 0.01) in patients with normal nutritional status (n = 431). Mortality within 90 days of RC was 16.5% (n = 17) vs. 5.1% (n = 22), respectively [24].
Johnson and colleagues similarly interrogated the NSQIP database with the objective of assessing associations between malnutrition, as defined by albumin < 3.5 g/dl, underweight (BMI < 18.5 kg/m2), and significant preoperative weight loss, and 30-day postoperative complications in a cohort of 1,213 patients [10]. After adjusting for age, sex, comorbidities, and year of operation, preoperative hypoalbuminemia (<3.5 g/dL) was the only predictor of developing a postoperative complication (OR 1.79, 95% CI 1.06–3.03; p = 0.03) compared to patients with a preoperative albumin of >3.5 g/dL. These results support the findings of the current study which similarly identified that hypoalbuminemia is independently associated with early complications while no association was observed for significant weight loss or underweight status. However, BMI was treated as a continuous variable. In this study, we evaluated markers of malnutrition including hypoalbuminemia and significant preoperative weight loss in the context of increasing classes of obesity and also assessed the associations between these potentially modifiable risk factors and the outcome of mortality.
There are limitations of the current study that must be acknowledged, primarily related to its retrospective study design and the use of a registry dataset to identify the study cohort. Indeed, limited variables are available when using registry data, and data may also be influenced by abstractor accuracy [31]. The NSQIP database does not include data on clinical staging, which impacts the complexity of the surgery which represents an important potential confounder in a study assessing risk factors for complications [32]. In this cohort, neoadjuvant chemotherapy data was only administered in 11% of the study cohort, which may limit generalizability to current practice where the standard of care is to administer neoadjuvant chemotherapy prior to RC for muscle-invasive bladder cancer. Perhaps the most significant limitation, however, was that serum albumin was only available in 60% of the study cohort as it is not standard of practice, although there is increasing emphasis on characterizing malnutrition in RC patients in the preoperative setting. Patients with missing data for albumin were included as a separate category in the multivariable models. Additionally, one measurement of albumin can be an unreliable indicator of nutritional status as it is an acute phase reactant and can fluctuate with inflammation [33]. Finally, we utilize BMI-based criteria to characterize obesity. However, as previously noted, BMI is nonspecific when assessing for true body composition [21]. As demonstrated by Psutka et al., BMI does not differentiate between excessive adipose tissue and lean muscle mass and may mask severe deficiencies in lean muscularity which has been associated with both decreased survival and increased complications following RC for bladder cancer [21, 33–37]. Occult paucity of lean muscularity, which is considered to be an extreme representation of cancer-associated malnutrition [3], is associated with both increased mortality and postoperative complications in the oncologic literature [38, 39–42]. Interestingly, in the current study, we observed that the prevalence of malnutrition, as characterized by hypoalbuminemia, remained significant among obese patients and increased in prevalence with increasing class of obesity. This finding underscores the importance of ensuring nutritional status is carefully assessed, even among the very obese.
In conclusion, assessment of preoperative nutritional status is imperative when assessing preoperative risk in patients with bladder cancer who are being prepared for RC. An accurate characterization of their overall nutrition, burden of cachexia, and assessment of body composition is important when counseling patients regarding expectations for perioperative complications. It also may inform the selection of patients for intensive nutritional pre-habilitation strategies in the time prior to surgery.
In the current study, using a national, prospectively collected registry, we observed that both increasing class of obesity and preoperative hypoalbuminemia are independently associated with increased risk of postoperative complications. Furthermore, class III obesity and hypoalbuminemia are independently associated with increased risk of death within 30 days of surgery. The current study emphasizes prior work regarding the clinical relevance of hypoalbuminemia in the RC patient and may further inform us on the selection of patients who would derive the greatest benefit from intensive preoperative nutritional optimization.
FUNDING SOURCES
None.
CONFLICT OF INTEREST DISCLOSURES
The authors have no conflict of interest to report.
REFERENCES
[1] | Clark PE , Spiess PE , Agarwal N , et al. NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer Version 2.2017. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. (2017) . |
[2] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2018. CA Cancer J Clin. (2018) ;68: :7–30. |
[3] | Arends J , Bachmann P , Baracos V , et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. (2017) ;36: :11–48. |
[4] | Buzby G , Mullen J , Matthews D , et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) ;139: :160–7. |
[5] | Gibbs J , Cull W , Henderson W , et al. Preoperative serum albumin level as a preditor of operative mortality and morbidity: Results from the National VA Surgical Risk Study. Arch Surg. (1999) ;134: :36–42. |
[6] | Burden S , Todd C , Hill J , et al. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. (2012) ;11: :CD008879. |
[7] | Sabzi F , Faraji R . Effect of body mass index on postoperative complications in beating coronary artery surgery. Ethiop J Health Sci. (2016) ;26: :509–16. |
[8] | Ihle C , Freude T , Bahrs C , et al. Malnutrition –An underestimated factor in the inpatient treatment of traumatology and orthopedic patients: A prospective evaluation of 1055 patients. Injury. (2017) ;48: :628–36. |
[9] | Ross F , Latham G , Joffe D , et al. Preoperative malnutrition is associated with increased mortality and adverse outcomes after pediatric cardiac surgery. Cardiology in the Young. (2017) ;1–10. |
[10] | Johnson DC , Riggs SB , Nielsen ME, , et al. Nutritional predictors of complications following radical cystectomy. World J Urol. (2015) ;33: :1129–37. |
[11] | Fried LP , Tangen CM , Walston J , et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) ;56: :M146–M156. |
[12] | Morgan TM , Tang D , Stratton KL , et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. (2011) ;59: :923–8. |
[13] | Abel EJ , Thompson RH , Margulis V , et al. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veings: A contemporary multicenter experience. Eur Urol. (2014) ;66: :584–92. |
[14] | Isac WE , Autorino R , Hillyer SP , et al. The impact of body mass index on surgical outcomes of robotic partial nephrectomy. BJU Int. (2012) ;110: :E997–E1002. |
[15] | Hua X , Ying-Ying C , Zu-Jun F , et al. Obesity, hypertension and diabetes mellitus affect complication rate of different nephrectomy techniques. Actas Urol Esp. (2014) ;38: :640–6. |
[16] | Hafron J , Mitra N , Dalbagni G , et al. Does body mass index affect survival of patients undergoing radical or partial cystectomy for bladder cancer? J Urol. (2005) ;173: :1513–7. |
[17] | Chromecki TF , Cha EK , Fajkovic H , et al. Obesity is associated with worse oncological outcomes in patients treated with radical cystectomy. BJU Int. (2013) ;111: :249–55. |
[18] | Choi Y , Park B , Jeong BC , et al. Body mass index and survival in patients with renal cell carcinoma: A clinical-based cohort and meta-analysis. Int J Cancer. (2013) ;132: :625–34. |
[19] | Rogde AJ , Gudbrandsdottir G , Hjelle KM , et al. Obesity is associated with an improved cancer-specific survival, but an increased rate of postoperative complications after surgery for renal cell carcinoma. Scand J Urol Nephrol. (2012) ;46: :348–57. |
[20] | Mullen JT , Davenport DL , Hutter MM . Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. (2008) ;15: :2164–72. |
[21] | Psutka SP , Moynagh M , Schmit G , et al. The impact of excess fat mass on mortality after radical nephrectomy for renal cell carcinoma: Beyond body mass index. J Urol. (2015) ;193: :e427. |
[22] | Tobert CM , Hamilton-Reeves JM , Norian LA , et al. Emerging impact of malnutrition on surgical patients: Literature review and potential implications for cystectomy in bladder cancer. J Urol. (2017) ;198: :511–19. |
[23] | Ingraham AM , Richards KE , Hall BL , et al. Quality improvement in surgery: The American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. (2010) ;44: :251–67. |
[24] | Gregg JR , Cookson MS , Phillips S , et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. (2011) ;185: :90–6. |
[25] | Lambert JW , Ingham M , Gibbs BB , et al. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology. (2013) ;81: :587–92. |
[26] | Garg T , Chen LY , Kim PH , et al. Preoperative serum albumin is associated with mortality and complications after radical cystectomy. BJU Int. (2014) ;113: :918–23. |
[27] | Shabsigh A , Korets R , Vora KC , et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. (2009) ;55: :164–74. |
[28] | Stein JP , Lieskovsky G , Cote R , et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. (2001) ;19: :666–75. |
[29] | Hensle TW , Askanazi J , Rosenbaum LH , et al. Metabolic changes associated with radical cystectomy. J Urol. (1985) ;134: :1032–6. |
[30] | Garg T , Chen LY , Kim PH , et al. Preoperative serum albumin is associated with mortality and complications after radical cystectomy. BJU Int. (2014) ;113: :918–23. |
[31] | Johnson EK , Nelson CP . Values and pitfalls of the use of administrative databases for outcomes assessment. J Urol. (2013) ;190: :17–8. |
[32] | Martinez A , Ngo C , Leblanc E , et al. Surgical complexity impact on survival after complete cytoreductive surgery for advanced ovarian cancer. Ann Surg Oncol. (2016) ;23: :2515–21. |
[33] | Psutka SP , Carrasco A , Schmit GD , et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer. (2014) ;120: :2910–8. |
[34] | Hirasawa Y , Nakashima J , Yunaiyama D , et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. (2016) ;23: :1048–54. |
[35] | Mayr R , Fritsche HM , Zeman F , et al. Sarcopenia predictors 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J Urol. (2018) . |
[36] | Mayr R , Gierth M , Zeman F , et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. (2018) . |
[37] | Wan F , Zhu Y , Chengyuan G , et al. Lower skeletal muscle index and early complications in patients undergoing radical cystectomy for bladder cancer. World J Surg Oncol. (2014) ;12: :14. |
[38] | Psutka SP , Boorjian SA , Moynagh MR , et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol. (2016) ;195: :270–6. |
[39] | Shachar SS , Deal AM , Weinberg M , et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. (2017) ;23: :658–65. |
[40] | Choi SY , Yoo S , You D , et al. Prognostic factors for survival in patients with synchronous or metachronous brain metastasis of renal cell carcinoma. Clin Genitourin Cancer. (2017) ;15: :717–23. |
[41] | Nakashima Y , Saeki H , Nakanishi R , et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg (2017) . |
[42] | Wendrich AW , Swartz JE , Bril SI , et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. (2017) ;71: :26–33. |




