Bladder Cancer Recovery Pathways: A Systematic Review
Abstract
Background:
Enhanced recovery pathways, also known as fast-track protocols, have been adopted since the early 2000s by various surgical specialties with the goal of improving patient outcomes and reducing the cost burden of major surgery on the health care system.
Objective:
To review the scientific literature on the origin of enhanced recovery pathways, track the contemporary utilization of such practices for patients undergoing radical cystectomy, and analyze the available data regarding their effect on morbidity, mortality, and treatment cost.
Methods:
A literature search of multiple electronic databases was undertaken. Manuscripts including patients undergoing radical cystectomy were chosen based on predefined criteria with an emphasis on randomized controlled trials and cohort studies. Strength of evidence for each study that met inclusion criteria was assessed based on the risk of bias, consistency, directness, and precision.
Results:
Database searches resulted in 1,236 potentially relevant articles. A total of 485 articles were selected for full-text dual review and 106 studies in 52 publications met the inclusion criteria.
Conclusion:
The utilization of enhanced recovery pathways with the goal of improving overall patient morbidity and mortality is well supported in the literature, however standardization of implementation and adherence across institutions is lacking, and their direct efficacy on reducing preventable treatment related expenditures is unconfirmed.
INTRODUCTION
There are 2.7 million people worldwide with a history of bladder cancer. It is estimated that in the United States alone, there will be 75,000 new cases and 15,000 deaths from bladder cancer in 2017 [1]. Contemporary demand for radical extirpative surgery is driven not only by the 20% of muscle-invasive bladder cancer (MIBC) cases diagnosed de novo each year, but also by the 15–30% of patients with non-muscle invasive bladder cancer (NMIBC) who progress to muscle invasion despite intravesical therapy. Radical cystectomy (RC) coupled with meticulous pelvic lymph node dissection is the gold standard treatment for MIBC as well as certain patients with high risk NMIBC [2]. Although RC in its current form has been undertaken since its first description by Marshall and Whitmore in 1962 [3], the procedure continues to carry a heavy burden of perioperative morbidity [4], hospital readmission [5], and mortality [6]. Recent advances in regionalization of care to high volume centers of excellence [7], and a more thorough understanding of patient risk stratification [8] have advanced our understanding of how to maximize effectiveness of therapy to patients while minimizing the financial costs to the health care system [9].
In the US, the annual cost of bladder cancer care in 2010 was estimated to be $3.98 billion and is expected to rise to $5.25 billion in 2020 [10]. Bladder cancer has the highest total Medicare payment cost from diagnosis to death with an estimated average lifetime cost of $77,720 per patient [11]. Roughly one-third of these costs can be attributed to complications, while surveillance and treatment of recurrences account for 60% [11]. Despite the heavy economic weight of bladder cancer through direct costs and loss of productivity [12], government-subsidized research into cost reduction is lacking relative to the impact of the disease [12, 13]. With both patient care and treatment value in mind, clinical pathways have emerged as a new frontier in the effort to reduce preventable treatment related expenditures and improve outcomes.
Standardized perioperative protocols, as they pertain to the management of patients who have undergone RC, work to ensure that patients receive the highest level of care though the most cost efficient means available. Adherence to clinical pathways decreases the variability of care between institutions and creates a culture of practice where physicians inform their decisions with the most up-to-date, evidence-based guidelines for care [14]. Additionally, clinical care pathways can serve to facilitate transparency and coherence of care planning thereby improving rates of patient satisfaction and reducing anxiety.
Enhanced Recovery After Surgery (ERAS) pathways are a specific subset of perioperative clinical care protocols that have been implemented by numerous surgical specialties with reported success [15]. Indeed, ERAS is being accepted by centers in the US for the treatment of bladder cancer patients [16]. Given their demonstrable efficacy, we evaluated contemporary trends in the adoption of radical cystectomy ERAS pathways, the detailed practices that comprise them, and the economic impact of ERAS on health-care associated cost.
METHODS
Data acquisition
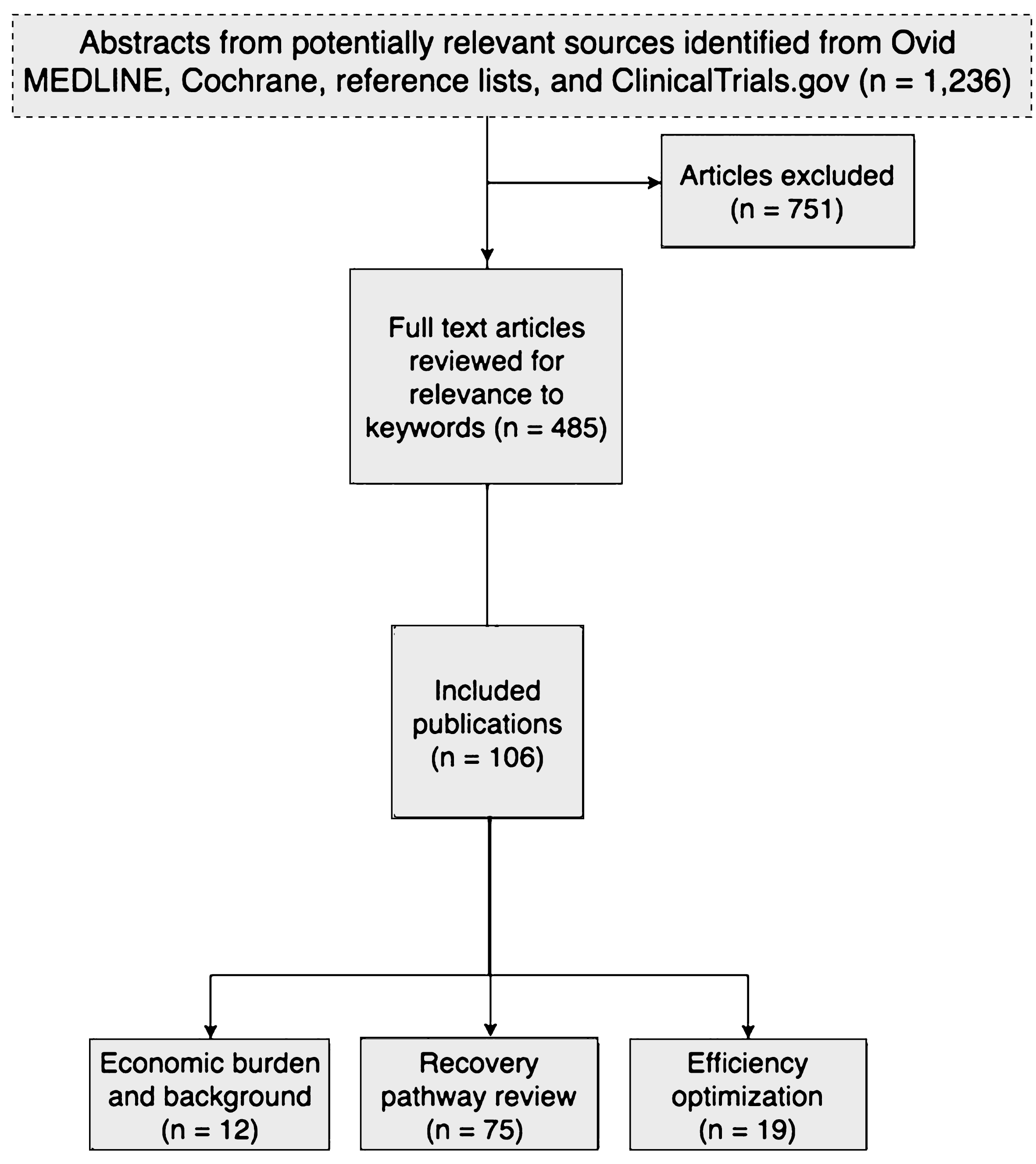
We performed a literature search of multiple electronic databases including Ovid MEDLINE from 1997–2017, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and Embase for relevant manuscripts using the terminology “enhanced recovery pathways and bladder cancer” (Fig. 1). The resulting studies were then crossed referenced with the following keywords: pathway, eras, enhanced recovery after surgery, postoperative, postoperative pathway, recovery protocol, length of stay, fast track, standard of care, clinical pathways, economics, and cost. Finally, the ClinicalTrials.gov website was queried for ongoing trials.
Fig.1
Flow diagram depicting the study design and selection of articles for inclusion in the analysis of enhanced recovery pathways for radical cystectomy.
Study selection
Two reviewers evaluated each study based on the predefined inclusion criteria noted above. The study population comprised adults with clinically localized bladder cancer undergoing RC. We included randomized trials and nonrandomized controlled clinical trials as well as cohort studies when randomized trials were not available. We restricted inclusion to studies published in or after 1997 to be more applicable to contemporary practice. Outcomes analyzed were cost, length of stay, 30 and 90 day readmission, complications, and mortality. Discrepancies were resolved through consensus.
Data synthesis
We assessed the strength of evidence for each study that met inclusion criteria based on the risk of bias, consistency, directness, and precision. Meta-analysis was unable to be performed due to an inadequate number of suitable trials.
RESULTS
Figure 1 summarizes the primary search results and selection of articles. Database searches resulted in 1,236 potentially relevant articles. A total of 485 articles were selected for full-text dual review and 106 studies in 52 publications met the inclusion criteria.
DISCUSSION
Recovery pathways: Historical perspectives
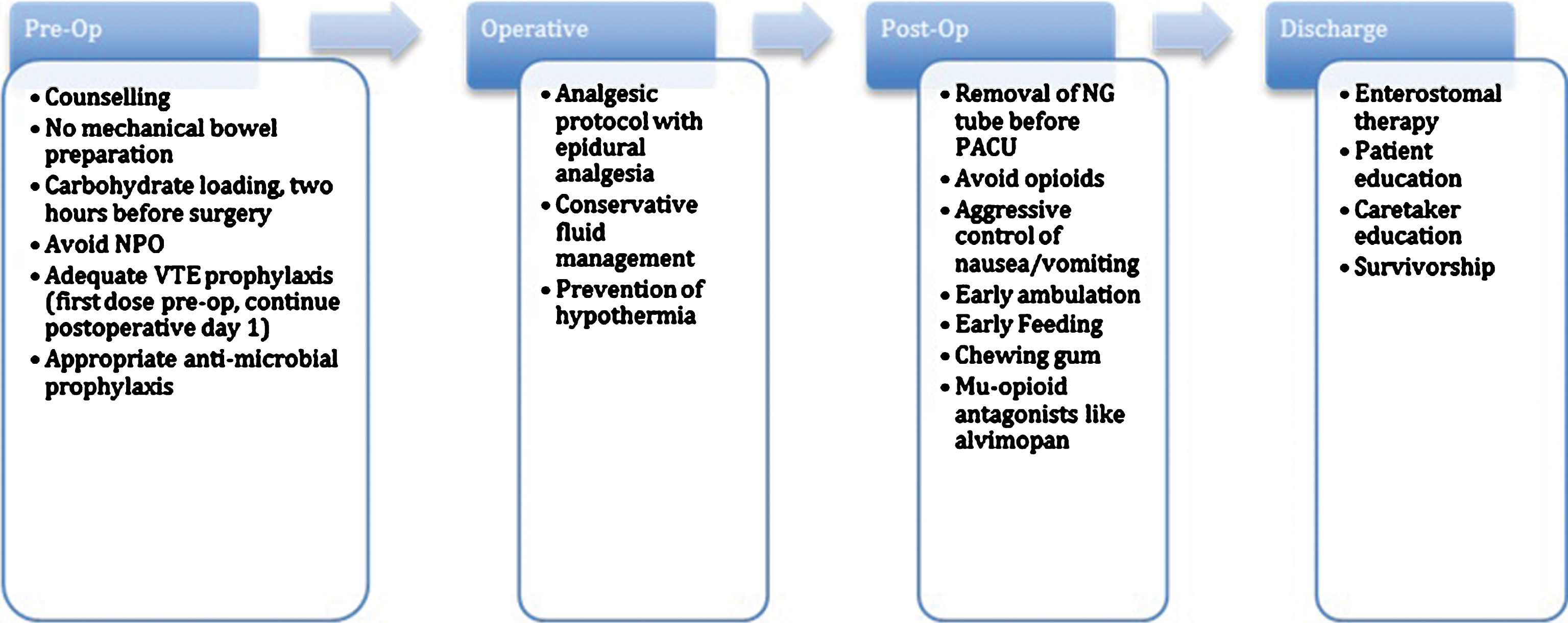
Fast track recovery pathways were conceptualized originally in the early 2000 s by investigators performing elective colorectal surgery. The initial studies by Kehlet et al. underscored the potential utility of this new form of management that could benefit patient outcomes and hospital costs alike [17]. Since their entrance into the literature, similar fast track pathways have become ubiquitous in the realm of colorectal surgery [18], and efforts to replicate this success have been undertaken for bladder cancer. Below is a summary of recommendations made after analysis of the literature regarding current practices and pathways in use today for RC (Fig. 2).
Fig.2
Flow diagram depicting various stages of perioperative treatment during radical cystectomy and the various enhanced recovery protocols for each.
Pre-operative pathways
1. Preoperative Counseling
Radical cystectomy, and the urinary diversion associated with it, can be an overwhelming concept to patients and their caretakers at first presentation. There is a paucity of data specifically evaluating the effect of preoperative patient counseling on RC treatment outcomes. However, there is evidence that patients who are able to retain a moderate level of comprehension regarding treatment implications on quality of life experience reduced anxiety, enhanced wound healing and postoperative recovery, and a decreased rate of complications [19]. For example, lack of adequate enterostomal education has been implicated as a factor directly resulting in delayed discharge [20]. An early focus on survivorship, in the form of disease-specific advocacy networks (www.bcan.org) can give patients the knowledge of what to expect during the active treatment and recovery phases of care and invite them to enter the community of other patients who have had similar treatment experiences. Participation in advocacy networks is increasing worldwide with the goal of reducing patient anxiety and improving treatment compliance both before and after surgery [21].
2. Mechanical Bowel Preparation
Mechanical bowel preparation has long been a standard practice prior to RC due to the use of bowel segments in continent and incontinent urinary diversion techniques. The rationale behind its use is attributed to a perceived reduction in enteroenteric anastomotic leaks, intra-abdominal infections, and wound complications [22]. However, there has been a contemporary trend towards avoidance of bowel preparation prior to cystectomy secondary to lack of demonstrable benefit and perhaps worse outcomes. For example, a recent clinical trial by Tabibi et al. of 30 candidates for urinary diversion who underwent a standard 3-day mechanical bowel prep vs 32 candidates who received no bowel prep demonstrated no significant difference in morbidity or length of stay (LOS) [23]. Additionally, a randomized controlled trial of 86 patients by Xu et al. suggests there is no advantage to be gained from performing bowel preparation, nor is there any statistical difference in time to first bowel movement between patients who received did not receive preparation [24]. Several studies have reported that mechanical bowel preparation could even be harmful, suggesting that the practice may result in an increased rate of wound infections and anastomotic dehiscence [25, 26].
3. Nutritional Considerations
It is estimated that roughly 30% of hospitalized patients are undernourished [27]. Cerantola et al. performed a prospective observational study of 220 patients undergoing major urologic surgery and found that those patients deemed to be at nutritional risk, as assessed preoperatively by a specialized study nurse, were found to have risk of complications three times higher than patients deemed to be nutritionally adequate [28]. Nutritional optimization is especially important in RC patients, since an increasing number are undergoing neoadjuvant chemotherapy prior to surgery [29]. Cisplatin-containing neoadjuvant chemotherapy regimens can be associated with adverse effects detrimental to nourishment in the interval between diagnosis and RC, although dose-dense regimens may improve this [30]. While no data exists regarding the specific effects of preoperative carbohydrate loading in cystectomy patients, studies evaluating its effects in colorectal surgery have shown that carbohydrate loading decreases thirst, insulin resistance, and helps to maintain both muscle strength and lean body mass [31].
Optimization of nutrient delivery at the cellular level is also under investigation. Postoperative insulin resistance can have profound effects on the outcomes of operative patients. Insulin resistance causes decreased glucose uptake and storage in skeletal muscle. The resulting loss of lean body mass can lead to a reduction in muscle function, thereby impairing mobility and progression of patients toward achieving baseline activities of daily living [32]. It can also play a role in the increased incidence of surgical complications. A Montreal study of 273 patients by Sato et al. found that the risk of serious postoperative infections was proportional to the degree of insulin resistance of the patient immediately after the surgery [33]. The ingestion of a carbohydrate-rich beverage 2–4 hours prior to surgery may enhance insulin action postoperatively and is directly related to a decrease in insulin-resistance related complications [34]. It is therefore recommended that 400 mL of a 12.5% carbohydrate beverage consisting mainly of maltodextrins be consumed preoperatively the morning of surgery for optimum efficacy [35].
Nil per os, the practice of abstaining from eating or drinking by mouth after midnight prior to general anesthesia is commonly enforced by providers out of fear of gastric content aspiration upon induction. Numerous studies have challenged this dogma and demonstrated that solid food intake up to 6 hours and liquid intake up to 2 hours before induction enhances patient comfort without increased risk of aspiration pneumonitis [36, 37]. Whereas it was previously believed that the limitation of preoperative fluids aided in the reduction of gastric volume, fluid intake up to 2 hours prior to surgery has been established by several studies to either maintain or decrease gastric volume [38, 39]. Limitations on the intake of food and liquids prior to surgery can also precipitate postoperative hypoglycemia, which in turn could have implications for postoperative recovery [40, 41].
4. Cardiopulmonary Optimization
Cardiovascular health is an important factor to monitor prior to surgery since patients with a poor aerobic threshold (<12 ml/min/kg), as determined through preoperative cardiopulmonary exercise screening, are more likely to experience major postoperative complications, a prolonged length of stay, and increased utilization of critical care following RC [42]. Similarly, smoking is associated with a worse prognosis after RC [43]. According to a 2013 study by Rink et al., the association between smoking and adverse events appears to be dose dependent and its effects are mitigated by smoking cessation [43]. Therefore, it is recommended that smokers be counselled in regard to the detrimental effects of smoking and the advantages of smoking cessation, particularly as they pertain to bladder cancer and their prognosis [44].
5. Thromboembolytic Prophylaxis
It is estimated that the incidence of clinically significant deep vein thrombosis (DVT) after cystectomy is 5% [45]. A retrospective analysis of cystectomy patients by Sun et al. found that symptomatic venous thromboembolism cases developed at a rate of 4.7%, with DVT and pulmonary embolism (PE) accounting for 2.1% and 2.6% respectively [46]. As is the case with other specialties, organizations such as the American Urological Association (AUA) have released best practice statements regarding risk stratification and optimal prophylactic measures to reduce the risk of DVT in patients undergoing urologic surgery [47]. Even with appropriate attention to DVT prevention in the index setting, Sun at el found that 57.8% of their reported thromboembolytic events occurred after discharge at an average of 20 days postoperatively (range 2 to 91) [46]. In another retrospective analysis by VanDlac et al. of 1,307 patients who underwent RC in the American College of Surgeons’ National Surgical Quality Improvement Program, more than half of all postoperative venous thromboembolisms (55%) occurred after discharge from the hospital, with a mean time to diagnosis of 15.2 days after surgery [48]. Based on this and standard practice for other invasive surgeries, including a double-blind randomized controlled trial by Bergqvist et al., extended duration pharmacological prophylaxis with low molecular weight heparin is recommended for 4 weeks postoperatively [49]. In an effort to reduce the rates of post-discharge and overall venous thromboembolism, Pariser et al. altered their regimen to include extended duration enoxaparin for patients undergoing radical cystectomy. Upon review of the data, they found that the change in regimen decreased the rate of venous thromboembolism from 12% to 5% and the rate of thromboembolism post-discharge from 6% to 2% [50]. These encouraging results must be considered with a degree of caution, however. Data from Fox Chase Cancer Center revealed that 13% of RC patients discharged on extended pharmacologic prophylaxis experience an estimated glomerular filtration rate (GFR) nadir less than 30 mL/min/1.73 m [2] within 90 days of surgery. This suggests a need for close postoperative follow up with careful attention to GFR in patients discharged on pharmacologic DVT prophylaxis to prevent supra-therapeutic serum levels and potential bleeding complications.
6. Microbial Prophylaxis
The optimal antibiotic combination for cystectomy patients should ideally be effective against both aerobes and anaerobes. The 2008 best practice statement from the AUA recommends as first line a single perioperative course of 2nd or 3rd generation cephalosporin or an aminoglycoside plus metronidazole or clindamycin, but the appropriate antimicrobial agent can only be selected following a comprehensive evaluation of circumstances specific to each patient [51]. For open or laparoscopic surgery involving entry into the urinary tract, the AUA found after comprehensive review of the literature that antimicrobial prophylaxis would reduce the rate of febrile UTI from 5–10% to 2–3% [52]. Antibiotics should be administered prior to skin incision and less than 1 hour before surgery, except for vancomycin which should be administered within two hours prior to incision [53]. Careful monitoring of institutional infection patterns and adoption of new protocols in response to evolving data can help lower rates of infection.
Intra-operative pathways
1. Analgesia Protocol
The goal of any perioperative analgesic plan should be to minimize the use of long acting opioids and opt instead for short acting agents so as to reduce paralytic ileus and opioid consumption after surgery. Thoracic epidurals are prominently featured in urologic and colorectal fast track protocols, and we recommend their use given the amount of evidence recognizing their utility. Maffezzini et al. noted improved functional outcomes in the form of rapid return of bowel function (median of 2 days) with the use of epidural analgesia (EDA) at level T9-11 until POD 3 [54]. In a different study investigating analgesia for colorectal surgery, Miedema et al. recommended epidural placement at level T7/8, noting that it would achieve both analgesia and sympathetic blockade, prevent gut paralysis, block stress hormone release, and attenuate postoperative insulin resistance [55, 56]. Toren et al. retrospectively studied 73 patients with EDA vs 58 patients with patient controlled morphine-based analgesia after RC and found improved pain control with activity in the patients with EDA [57]. Due to risk of respiratory depression from prolonged use of EDA, rectus sheath catheters have emerged as an alternative option in practice [58, 59]. Finally, transversus abdominus plane (TAP) blocks have shown to be an effective strategy to improve early and late pain control after surgery as well as reduce opioid requirement after laparoscopic surgical procedures [60].
2. Fluid Management
Individual goal-directed fluid therapy (IGDFT) with cardiac output monitoring allows for maintenance of the patient’s fluid requirements in a near physiologic state. This helps to avoid hypoperfusion of bowel and other organs, decrease incidence of sepsis, aid in the early return of bowel function, and improve renal function. Optimizing oxygen delivery to tissues and avoiding fluid shifts decreases the likelihood of paralytic ileus and delayed return of bowel function, both of which are associated with increased morbidity and LOS [61]. IGDFT using esophageal Doppler to achieve “near maximal stroke volume” has been recommended in colorectal surgery, and a randomized controlled trial of patients undergoing RC found that it reduced the incidence of ileus and postoperative nausea and vomiting at 24 and 48 hours [62].
3. Hypothermia Prevention
Prolonged exposure of the abdominal cavity to environmental temperature in combination with anesthesia can lead to perioperative hypothermia (<36°C/96.8°F). Hypothermic patients have been shown to have higher complication rates compared to patients kept closer to normal physiologic temperature [19]. Patient warming through the use of warm-air blankets and warmed IV fluids is associated with a decrease in surgical site infections and postoperative cardiac complications [63].
Post-operative pathways
1. Nasogastric (NG) Intubation
In a randomized controlled trial of 43 patients undergoing RC conducted by Adamakis et al., there was no significant difference in morbidity, LOS, or recovery of bowel function in patients who had early removal of NG tube versus those who had their NG tube removed only after first flatus [64]. A Cochrane meta-analysis of 33 randomized controlled trials focusing on patients undergoing major abdominal surgery demonstrated higher rates of postoperative complications, including increased time to return of bowel function, and higher incidences of pulmonary complications with no benefit over early NG removal in patients whose NG tubes were maintained after surgery [65]. Additionally, a strong trend existed between prolonged NG suction and wound complications and ventral hernia. Though these latter findings failed to reach statistical significance, the questions of whether NG tubes may lead to harm is still open for debate. Therefore, NG tube removal upon completion of surgery is recommended.
2. Prevention of Postoperative Ileus
Ileus prevention is crucial for patients undergoing bowel resection as this complication represents a significant contributor to delayed discharge and increasing cost. The incidence of postoperative ileus in RC patients has been estimated to be between 12–23% [13, 66]. As discussed previously, EDA has been shown to be highly efficient at preventing postoperative ileus compared to routine use of intravenous narcotic analgesia [17, 67]. Oral magnesium oxide has been shown to promote postoperative bowel function in a double-blinded randomized controlled trial of abdominal hysterectomy and in reports of recovery programs for colectomy [68, 69]. In a pooled analysis of phase III trials, Delaney et al. demonstrated that the μ-opioid receptor antagonist alvimopan, when taken orally after colorectal resection, significantly accelerated GI recovery in time to first flatus and first bowel movement [70]. Postoperative morbidity rates, LOS, and 30/90 day readmission rates were significantly reduced as well. Pertaining to RC, a Cochrane review [71] focusing heavily on data from a recent randomized control trial of alvimopan versus placebo [72] reported that alvimopan administration improves time to tolerance of solid food, return of bowel movements, and length of stay while maintaining a rate of adverse reactions similar to placebo.
3. Prevention of Postoperative Nausea and Vomiting
Inhalational anesthetics, nitrous oxide, and opioids contribute to postoperative nausea and vomiting (PONV). Patients at increased risk are candidates to be placed on multimodal anti-emetic prophylaxis. General recommendations include the liberal use of dexamethasone and ondansetron as needed. As noted above, Pillai et al. showed that fluid optimization through the use of esophageal Doppler monitoring significantly reduced nausea and vomiting in the early postoperative setting [62]. Mattei et al. demonstrated that stenting of the uretero-ileal anastomosis was effective at prevention of PONV as well [73].
4. Postoperative Pain Management
Opiate-related effects such as drowsiness, nausea, and ileus limit early mobilization and delay discharge, and therefore excessive use for postoperative pain management should be avoided. To reduce opiate reliance, minimal access surgery should be considered, as has been shown to significantly lower opiate requirements [74]. Meta-analyses have shown that EDA, preferably without opioids, for 2 to 3 days postoperatively provides more efficient analgesia with better effects on surgical stress response and fewer sedative effects compared to patient controlled opioid analgesia [67, 75]. Patients placed on a combination regimen of perioperative regional analgesia and oral paracetamol/NSAIDs have been shown to have the need for opiates eliminated almost entirely [76]. The use of pregablin or gabapentin in the early post-operative period has also been shown to limit opiate requirement and has become a mainstay in many ERAS protocols [77].
5. Early Mobilization
Extended periods of bedrest increase insulin resistance and muscle loss, decrease muscle strength, weaken pulmonary function, and worsen tissue oxygenation while increasing the risk of thromboembolism [78]. Therefore, the goal following RC for early mobilization is for 2 hours of ambulatory activity on postoperative day zero, with at least 6 hours out of the bed each subsequent hospital day starting postoperative day one until discharge. Pain relief through the use of ambulatory thoracic EDA can be an effective measure in encouraging early mobilization. A predetermined ambulation plan listing daily distance goals and targets for time out of bed improves patient motivation. All hindrances to mobilization should be avoided whenever possible [79].
6. Early Feeding
In conjunction with the consumption of carbohydrate-rich beverages prior to surgery, early feeding following surgery can aid in the reduction of insulin resistance, consequently improving muscle function and would healing while avoiding sepsis [80]. Meta-data analysis suggests that incidence of pneumonia, anastomotic dehiscence, wound infection, and death were all reduced in patients who receive early feeding, and additional benefits were observed in time to first flatus, time to first bowel movement, and reduction in LOS [81]. While oral fluids are preferable postoperatively, in circumstances where intravenous fluids are required balanced crystalloid solutions are preferable to normal saline solutions due to the minimization of hyperchloremic metabolic acidosis [82]. The use of chewing gum has been extensively studied in both colorectal and RC patients. There is a multitude of evidence that suggest chewing gum postoperatively results in a decrease in time to first flatus and return of bowel movement [83, 84].
Impact on cost
Of all cancers, bladder cancer has the highest lifetime treatment cost per patient diagnosed [11]. RC is a complex procedure associated with high rates of complications, readmissions, and overall morbidity. Given the consensus among experts that morbidity and readmissions have profound impacts on the high cost of treatment of bladder cancer, the reduction of both whenever possible should be of the utmost importance. Therefore, clinical trials designed to test the cost effectiveness of ERAS pathways are in demand.
The average cost of a RC including the 30 day postoperative period is about $30,000 [85]. The high cost of materials for newer robot-assisted laparoscopic radical cystectomy (RALRC) compared to the traditional open RC initially suggested a higher total cost for the RALRC. However, recent studies accounting for complication rates in the global treatment period report RALRC may lower overall costs [86–88]. Besides altering the operative approach with minimally invasive options, efforts to reduce cost have targeted the high price of perioperative complications. A recent study of a single institution in Sweden demonstrated that post-operative morbidity accounted for 3.1 times higher costs than uncomplicated surgeries [89]. One potential solution to this problem involves the centralization of care to high volume centers of excellence with experienced providers. Indeed, RC performed at high volume centers, defined as those performing more than 45 cystectomies per year [90], were associated with lower overall cost as well as more swift and appropriate response to adverse events following surgery [91].
Despite these data and the growing popularity of clinical fast track pathways being utilized in medical centers across the globe, there continues to be a lack of high level evidence in the literature examining the effect of ERAS pathways on cost for RC. To mitigate this, future trials should be designed to investigate the effects of ERAS pathways on: 1) Inpatient Stay, 2) Post-operative Home Care, and 3) Complication/Readmission Management.
1. Inpatient Stay:
Leow et al. analyzed discharge data from 11,225 patients who underwent radical cystectomy from 2003 to 2013 and found the mean 90-day direct hospital costs for each patient to be $32,261 [92]. Costs associated with radical cystectomy include those accumulated in preparation of the procedure as well as those accrued following the procedure. Preoperative costs include necessary imaging studies, laboratory evaluations, and consultations [93]. For patients with multiple risk factors, these consultations can be plentiful and significant. Therefore, risk calculators to identify low-risk patients who would not likely benefit from excessive consultations and high risk patients who would benefit from cardiac optimization could be implemented as both a cost-saving measure and a patient aid [94]. Postoperative costs include hospital stay and pharmacy charges, with complications and prolonged postoperative length of stay contributing substantially to cost increase and variability [92]. According to an annual survey by the American Hospital Association, the average cost of a single inpatient day in a US community hospital is $2,271 [95].
Radical cystectomy carries with it high perioperative risk, with reported complication rates ranging from 25% to 40% [96]. Each adverse event following cystectomy prolongs length of stay and significantly increases associated costs [97]. Konety et al. examined data from 6,577 radical cystectomy patients between 1998–2002 and found that at least 1 complication nearly doubled the odds of mortality and increased median cost and LOS by $15,000 and 4 days, respectively [96]. Sepsis contributes most significantly to cost increases attributable to adverse events, with septic patients incurring up to 3 times more cost and increased LOS versus non-septic patients [97, 98]. Postoperative paralytic ileus is another postoperative complication that contributes to increased cost. Given the substantial number of associated laboratory studies, imaging tests, and other indications associated with postoperative ileus, it has been implicated in twofold direct hospital costs and double the LOS [99]. The use of alvimopan to decrease this burden recently received US FDA approval. It was reported that its use results in the decrease of the median length of stay by one day at a cost of roughly $700 per day [100]. Compared to the cost of a day in the hospital, this method proves itself cost effective. With all of this information in mind, lessening recovery times and minimizing complication rates with prompt discharge when the patient can tolerate it should be the goal of every hospital’s enhanced recovery protocol. Given the fact that potentially preventable adverse events and nosocomial infections account for $15 billion and $5 billion respectively [97], bladder cancer seems to be a good place to start for increased scrutiny to bring overall costs down.
2. Postoperative Home Care:
One of the main drivers of cost in cystectomy care post-discharge is extended venous thromboembolism prophylaxis. However, VTE prophylaxis can be cost effective, especially when compared to the alternative of no prophylaxis and the potential for DVT or PE occurrence. The Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project estimates that the inpatient costs of a single DVT or PE are $10,000 and $20,000, respectively [101]. Cain et al. found that the average patient cost to complete VTE prophylaxis with enoxaparin for 28 days postoperatively was $62 (median $21, range $0–1210) [102]. In 2009, the US government dedicated $1.1 billion in funding for comparative effectiveness research that included cost effectiveness research [103]. As healthcare costs continue to rise, research such as this into achieving the most effective treatment outcomes for the lowest cost will only become more critical.
3. Complication/Readmission Management:
Post-discharge complications and readmissions factor heavily into the cost of treatment for bladder cancer. Wittig et al. examined a subset of 247 radical cystectomy patients during a 9-year period between 2003 and 2012 and determined a readmission rate of 40%, noting that patients readmitted to the hospital had direct costs that were 1.42 times greater than those who did not require readmission. Infection and dehydration were the most common reasons for readmission, accounting for 41% and 19% of initial readmissions respectively. Postoperative paralytic ileus was responsible for the highest total cost, due in large part to prolonged LOS (mean 16 days); ureteral stricture, sepsis, and pelvic abscesses were associated with the highest cost per day [104]. James et al. found that among 1,163 radical cystectomy patients who were readmitted between 2008 and 2011, 26% of cases were potentially modifiable and unrelated to inherent morbidity [105]. Krishnan et al. used a delay-time approach to maximize the probability of detecting patients at high risk of readmission and found that patients are most likely to exhibit concerning symptoms 4 to 5 days after discharge [106]. Similar models to optimize follow up care through early telephone calls or office visits could reduce readmission rates and drive costs down. This, in conjunction with the significant burden of risk and cost associated with readmissions following cystectomy, is grounds for an increased focus on optimizing all aspects of peri-cystectomy care to decrease morbidity for the patient and costs for all.
CONCLUSION
Enhanced recovery pathways for radical cystectomy that center on standardization of evidence-based care and disposal of outdated dogmatic practices are becoming more prevalent in the US, as evident by the rapidly growing body of literature that substantiates their use. While the rationale for the adoption of ERAS protocols pertaining to patient-oriented outcomes is well supported (decreased morbidity/mortality, reduced length of stay), their direct impact on cost-effectiveness on a macro-economic scale has yet to be investigated thoroughly. Additionally, a complete lack of published data exists across bladder cancer centers regarding implementation of ERAS pathways and adherence to various protocols, making comparisons of outcomes and cost analyses between institutions and other surgical specialties difficult, if not impossible. Future endeavors to incorporate caretaker education and survivorship into current protocols are needed to ensure that enhanced recovery continues to encompass not only the patient’s index hospitalization, but also their transition back into the primary care arena toward a sustained improvement in quality and quantity of life.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
None.
REFERENCES
[1] | Ploeg M , Aben KKH , Kiemeney LA . The present and future burden of urinary bladder cancer in the world. World J Urol (2009) ;27: (3):289–93. |
[2] | Chang SS , Bochner BH , Chou R , Dreicer R , Kamat AM , Lerner SP , et al. Treatment of non-metastatic muscle-invasive bladder cancer:AUA/ASCO/ASTRO/SUO guideline. J Urol (2017) . |
[3] | Whitmore WF Jr , Marshall VF . Radical Total Cystectomy for Cancer of the Bladder: 230 Consecutive Cases Five Years Later. J Urol (1962) ;87: (6):853–68. |
[4] | Patel MI , Bang A , Gillatt D , Smith DP . Contemporary radical cystectomy outcomes in patients with invasive bladder cancer: A population-based study. BJU Int (2015) ;116 Suppl 3: (Suppl. 1):18–25. |
[5] | Al-Daghmin A , Aboumohamed A , Din R , Khan A , Raza SJ , Sztorc J , et al. Readmission after robot-assisted radical cystectomy:Outcomes and predictors at 90-day follow-up. Urology (2014) ;83: (2):350–6. |
[6] | Quek ML , Stein JP , Daneshmand S , Miranda G , Thangathurai D , Roffey P , et al. A critical analysis of perioperative mortality fromradical cystectomy. J Urol (2006) ;175: (3 Pt1):886–9-discussion889-90. |
[7] | Barbieri CE , Lee B , Cookson MS , Bingham J , Clark PE , Smith JA , et al. Association of procedure volume with radical cystectomyoutcomes in a nationwide database. J Urol (1418) ;178: (4 Pt1):1418–21-discussion1421-2. |
[8] | Prasad SM , Ferreria M , Berry AM , Lipsitz SR , Richie JP , Gawande AA , et al. Surgical apgar outcome score: Perioperative riskassessment for radical cystectomy. J Urol (1046) ;181: (3):1056–52-discussion1052-3. |
[9] | DeSantis CE , Lin CC , Mariotto AB , Siegel RL , Stein KD , Kramer JL , et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin (2014) ;64: (4):252–71. |
[10] | Mariotto AB , Yabroff KR , Shao Y , Feuer EJ , Brown ML . Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst (2011) ;103: (2):117–28. |
[11] | Avritscher EBC , Cooksley CD , Grossman HB , Sabichi AL , Hamblin L , Dinney CP , et al. Clinical model of lifetime cost of treatingbladder cancer and associated complications. Urology (2006) ;68: (3):549–53. |
[12] | Sangar VK , Ragavan N , Matanhelia SS , Watson MW , Blades RA . The economic consequences of prostate and bladder cancer in the UK. BJU Int (2005) ;95: (1):59–63. |
[13] | Carter AJR , Nguyen CN . A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health (2012) ;12: (1):526. |
[14] | Azhar RA , Bochner B , Catto J , Goh AC , Kelly J , Patel HD , et al. Enhanced recovery after urological surgery: A contemporarysystematic review of outcomes, key elements, and research needs. Eur Urol (2016) ;70: (1):176–87. |
[15] | Karl A , Seitz M , Staehler M , Becker A , Weninger E , Rittler P , et al. Fast track approach in radical cystectomy. Urologe A (2011) ;50: (9):1072–5. |
[16] | Tyson MD , Chang SS . Enhanced recovery pathways versus standard care after cystectomy: A meta-analysis of the effect on perioperative outcomes. Eur Urol (2016) ;70: (6):995–1003. |
[17] | Kehlet H , Dahl JB . Anaesthesia, surgery, and challenges in postoperative recovery. Lancet (2003) ;362: (9399):1921–8. |
[18] | Sehgal R , Hill A , Deasy J , McNamara DA , Cahill RA . Fast-track for the modern colorectal department. World J Surg (2012) ;36: (10):2473–80. |
[19] | Gustafsson UO , Scott MJ , Schwenk W , Demartines N , Roulin D , Francis N , et al. Guidelines for perioperative care in electivecolonic surgery: Enhanced Recovery After Surgery (ERAS®)Society recommendations. Vol. 31: , Clinical nutrition (Edinburgh,Scotland). (2012) . pp. 783–800. |
[20] | Smart NJ , White P , Allison AS , Ockrim JB , Kennedy RH , Francis NK . Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: Early prediction model. Colorectal Dis (2012) ;14: (10):e727–34. |
[21] | Quale DZ , Bangs R , Smith M , Guttman D , Northam T , Winterbottom A , et al. Bladder cancer patient advocacy: A global perspective. Bladder Cancer. IOS Press (2015) ;1: (2):117–22. |
[22] | Hupe MC , Kramer MW , Merseburger AS . Preoperative and modifiable factors to lower postoperative complications after radical cystectomy. Curr Urol Rep (2015) ;16: (4):19. |
[23] | Tabibi A , Simforoosh N , Basiri A , Ezzatnejad M , Abdi H , Farrokhi F . Bowel preparation versus no preparation before ileal urinary diversion. Urology (2007) ;70: (4):654–8. |
[24] | Xu R , Zhao X , Zhong Z , Zhang L . No advantage is gained by preoperative bowel preparation in radical cystectomy and ileal conduit: A randomized controlled trial of 86 patients. Int Urol Nephrol 9 ed. (2010) ;42: (4):947–50. |
[25] | Platell C , Hall J . What is the role of mechanical bowel preparation in patients undergoing colorectal surgery? Dis Colon Rectum (1998) ;41: (7):875–82-discussion882-3. |
[26] | Ram E , Sherman Y , Weil R , Vishne T , Kravarusic D , Dreznik Z . Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg. American Medical Association (2005) ;140: (3):285–8. |
[27] | Kondrup J , Allison SP , Elia M , Vellas B , Plauth M , Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Vol 22: , Clinical nutrition (Edinburgh, Scotland) (2003) , pp. 415–21. |
[28] | Cerantola Y , Valerio M , Hubner M , Iglesias K , Vaucher L , Jichlinski P . Are patients at nutritional risk more prone to complications after major urological surgery? J Urol (2013) ;190: (6):2126–32. |
[29] | Zaid HB , Patel SG , Stimson CJ , Resnick MJ , Cookson MS , Barocas DA , et al. Trends in the utilization of neoadjuvant chemotherapy inmuscle-invasive bladder cancer: Results from the National CancerDatabase. Urology (2014) ;83: (1):75–80. |
[30] | van de Putte EEF , Mertens LS , Meijer RP , van der Heijden MS , Bex A , van der Poel HG , et al. Neoadjuvant induction dose-dense MVACfor muscle invasive bladder cancer: Efficacy and safety comparedwith classic MVAC and gemcitabine/cisplatin. World J Urol. 7 ed. Springer Berlin Heidelberg. (2016) ;34: (2):157–62. |
[31] | Nygren J , Thacker J , Carli F , Fearon KCH , Norderval S , Lobo DN , et al. Guidelines for perioperative care in elective rectal/pelvicsurgery: Enhanced Recovery After Surgery (ERAS®) Societyrecommendations. Vol. 31: , Clinical nutrition (Edinburgh,Scotland). (2012) . pp. 801–16. |
[32] | Ljungqvist O , Jonathan E . Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr. 4 ed (2012) ;36: (4):389–98. |
[33] | Sato H , Carvalho G , Sato T , Lattermann R , Matsukawa T , Schricker T . The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab (2010) ;95: (9):4338–44. |
[34] | Svanfeldt M , Thorell A , Hausel J , Soop M , Nygren J , Ljungqvist O . Effect of “preoperative” oral carbohydrate treatment on insulin action–a randomised cross-over unblinded study in healthy subjects. Clin Nutr (2005) ;24: (5):815–21. |
[35] | Hausel J , Nygren J , Lagerkranser M , Hellström PM , Hammarqvist F , Almström C , et al. A carbohydrate-rich drink reducespreoperative discomfort in elective surgery patients. AnesthAnalg (2001) ;93: (5):1344–50. |
[36] | Phillips S , Hutchinson S , Davidson T . Preoperative drinking does not affect gastric contents. Br J Anaesth (1993) ;70: (1):6–9. |
[37] | Brady M , Kinn S , Stuart P . Preoperative fasting for adults to prevent perioperative complications. Brady MC , editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd. (2003) ;44: (4):CD004423. |
[38] | Ljungqvist O , Søreide E . Preoperative fasting. Br J Surg (2003) ;90: (4):400–6. |
[39] | Søreide E , Strømskag KE , Steen PA . Statistical aspects in studies of preoperative fluid intake and gastric content. Acta Anaesthesiol Scand (1995) ;39: (6):738–43. |
[40] | Smith I , Kranke P , Murat I , Smith A , O’Sullivan G , Søreide E , et al. Perioperative fasting in adults and children: Guidelinesfrom the European Society of Anaesthesiology. Vol. 28: , EuropeanJournal of Anaesthesiology (2011) :556–69. |
[41] | American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Vol. 114: , Anesthesiology (2011) . pp. 495–511. |
[42] | Prentis JM , Trenell MI , Vasdev N , French R , Dines G , Thorpe A , et al. Impaired cardiopulmonary reserve in an elderly populationis related to postoperative morbidity and length of hospital stayafter radical cystectomy. BJU Int (2013) ;112: (2):E13–9. |
[43] | Rink M , Furberg H , Zabor EC , Xylinas E , Babjuk M , Pycha A , et al. Impact of smoking and smoking cessation on oncologic outcomes inprimary non–muscle-invasive bladder cancer. Eur Urol (2013) ;63: (4):724–32. |
[44] | Sosnowski R , Przewoźniak K . The role of the urologist in smoking cessation: Why is it important? Urologic Oncology: Seminars and Original ... (2015) . |
[45] | Novotny V , Hakenberg OW , Wiessner D , Heberling U , Litz RJ , Oehlschlaeger S , et al. Perioperative complications of radicalcystectomy in a contemporary series. Eur Urol (2007) ;51: (2):397–401-discussion401-2. |
[46] | Sun AJ , Djaladat H , Schuckman A , Miranda G , Cai J , Daneshmand S . Venous thromboembolism following radical cystectomy: Significant predictors, comparison of different anticoagulants and timing of events. J Urol (2015) ;193: (2):565–9. |
[47] | Forrest JB , Clemens JQ , Finamore P , Leveillee R , Lippert M , Pisters L , et al. AUA Best Practice Statement for the preventionof deep vein thrombosis in patients undergoing urologic surgery. Vol. 181: , The Journal of Urology (2009) . pp. 1170–7. |
[48] | VanDlac AA , Cowan NG , Chen Y , Anderson RE , Conlin MJ , La Rochelle JC , et al. Timing, incidence and risk factors for venousthromboembolism in patients undergoing radical cystectomyfor malignancy: A case for extended duration pharmacologicalprophylaxis. J Urol (2014) ;191: (4):943–7. |
[49] | Bergqvist D , Agnelli G , Cohen AT , Eldor A , Nilsson PE , LeMoigne-Amrani A , et al. Duration of prophylaxis against venousthromboembolism with enoxaparin after surgery for cancer. N Engl JMed. Massachusetts Medical Society (2002) ;346: (13):975–80. |
[50] | Pariser JJ , Pearce SM , Anderson BB , Packiam VT , Prachand VN , Smith ND , et al. Extended duration enoxaparin decreases the rate ofvenous thromboembolic events after radical cystectomy compared toinpatient only subcutaneous heparin. J Urol (2017) ;197: (2):302–7. |
[51] | Wolf JS , Bennett CJ , Dmochowski RR , Hollenbeck BK , Pearle MS , Schaeffer AJ , et al. Best practice policy statement on urologicsurgery antimicrobial prophylaxis. Vol. 179: , The Journal of Urology (2008) 1379–90. |
[52] | Prokocimer P , Quazza M , Gibert C , Lemoine JE , Joly ML , Dureuil B , et al. Short-term prophylactic antibiotics in patients undergoingprostatectomy: Report of a double-blind randomized trial with 2intravenous doses of cefotaxime. J Urol (1986) ;135: (1):60–4. |
[53] | Bratzler DW , Houck PM , Surgical Infection Prevention GuidelinesWriters Workgroup, American Academy of Orthopaedic Surgeons, American Association of Critical Care Nurses, American Associationof Nurse Anesthetists, et al. Antimicrobial prophylaxis forsurgery: An advisory statement from the National SurgicalInfection Prevention Project. (2004) . pp. 1706–15. |
[54] | Maffezzini M , Campodonico F , Capponi G , Manuputty E , Gerbi G . Fast-track surgery and technical nuances to reduce complications after radical cystectomy and intestinal urinary diversion with the modified Indiana pouch. Surg Oncol (2012) ;21: (3):191–5. |
[55] | Miedema BW , Johnson JO . Methods for decreasing postoperative gutdysmotility. Lancet Oncol (2003) ;4: (6):365–72. |
[56] | Uchida I , Asoh T , Shirasaka C , Tsuji H . Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. Br J Surg (1988) ;75: (6):557–62. |
[57] | Toren P , Ladak S , Ma C , McCluskey S , Fleshner N . Comparison of epidural and intravenous patient controlled analgesia in patients undergoing radical cystectomy. Can J Urol (2009) ;16: (4):4716–20. |
[58] | Smith J , Pruthi RS , McGrath J . Enhanced recovery programmes for patients undergoing radical cystectomy. Nat Rev Urol (2014) ;11: (8):437–44. |
[59] | Dutton TJ , McGrath JS , Daugherty MO . Use of rectus sheath catheters for pain relief in patients undergoing major pelvic urological surgery. BJU Int (2014) ;113: (2):246–53. |
[60] | De Oliveira GS , Castro-Alves LJ , Nader A , Kendall MC , McCarthy RJ . Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: A meta-analysis of randomized controlled trials. Anesth Analg (2014) ;118: (2):454–63. |
[61] | Bundgaard-Nielsen M , Secher NH , Kehlet H . ‘Liberal’ vs. “restrictive” perioperative fluid therapy–a critical assessment of the evidence. Acta Anaesthesiol Scand (2009) ;53: (7):843–51. |
[62] | Pillai P , McEleavy I , Gaughan M , Snowden C , Nesbitt I , Durkan G , et al. A double-blind randomized controlled clinical trial toassess the effect of Doppler optimized intraoperative fluidmanagement on outcome following radical cystectomy. J Urol (2011) ;186: (6):2201–6. |
[63] | Moola S , Lockwood C . Effectiveness of strategies for the management and/or prevention of hypothermia within the adult perioperative environment. Int J Evid Based Healthc (2011) ;9: (4):337–45. |
[64] | Adamakis I , Tyritzis SI , Koutalellis G , Tokas T , Stravodimos KG , Mitropoulos D , et al. Early removal of nasogastric tube isbeneficial for patients undergoing radical cystectomy with urinarydiversion. Int Braz J Urol (2011) ;37: (1):42–8. |
[65] | Nelson R , Edwards S , Tse B . Prophylactic nasogastric decompression after abdominal surgery. Nelson RL , editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd. (2007) ;8: (3):CD004929. |
[66] | Yuh BE , Nazmy M , Ruel NH , Jankowski JT , Menchaca AR , Torrey RR , et al. Standardized analysis of frequency and severity ofcomplications after robot-assisted radical cystectomy. Eur Urol (2012) ;62: (5):806–13. |
[67] | Marret E , Remy C , Bonnet F , Postoperative Pain Forum Group. Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg (2007) ;94: (6):665–73. |
[68] | Hansen CT , Sørensen M , Møller C , Ottesen B , Kehlet H . Effect of laxatives on gastrointestinal functional recovery in fast-track hysterectomy: A double-blind, placebo-controlled randomized study. Am J Obstet Gynecol (2007) ;196: (4):311.e1–7. |
[69] | Basse L , Raskov HH , Hjort Jakobsen D , Sonne E , Billesbølle P , Hendel HW , et al. Accelerated postoperative recovery programmeafter colonic resection improves physical performance, pulmonaryfunction and body composition. Br J Surg (2002) ;89: (4):446–53. |
[70] | Delaney CP , Wolff BG , Viscusi ER , Senagore AJ , Fort JG , Du W , et al. Alvimopan, for postoperative ileus following bowelresection: A pooled analysis of phase III studies. Ann Surg (2007) ;245: (3):355–63. |
[71] | Sultan S , Coles B , Dahm P . Alvimopan for recovery of bowel function after radical cystectomy. Sultan S , editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd. (2017) ;5: (4 suppl. 1):CD012111. |
[72] | Lee CT , Chang SS , Kamat AM , Amiel G , Beard TL , Fergany A , et al. Alvimopan accelerates gastrointestinal recovery after radicalcystectomy: A multicenter randomized placebo-controlled trial. Eur Urol (2014) ;66: (2):265–72. |
[73] | Mattei A , Birkhaeuser FD , Baermann C , Warncke SH , Studer UE . To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol (2008) ;179: (2):582–6. |
[74] | Nix J , Smith A , Kurpad R , Nielsen ME , Wallen EM , Pruthi RS . Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: Perioperative and pathologic results. Eur Urol (2010) ;57: (2):196–201. |
[75] | Zutshi M , Delaney CP , Senagore AJ , Mekhail N , Lewis B , Connor JT , et al. Randomized controlled trial comparing the controlledrehabilitation with early ambulation and diet pathway versus thecontrolled rehabilitation with early ambulation and diet withpreemptive epidural anesthesia/analgesia after laparotomy andintestinal resection. Am J Surg (2005) ;189: (3):268–72. |
[76] | Dudderidge TJ , Doyle P , Mayer EK , Taylor J , Agrawal S , Stolzenburg JU , et al. Evolution of care pathway for laparoscopic radicalprostatectomy. J Endourol (2012) ;26: (6):660–5. |
[77] | Schmidt PC , Ruchelli G , Mackey SC , Carroll IR . Perioperative gabapentinoids: Choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology (2013) ;119: (5):1215–21. |
[78] | Kehlet H , Wilmore DW . Multimodal strategies to improve surgical outcome. Am J Surg (2002) ;183: (6):630–41. |
[79] | Guenaga KKFG , Matos D , Wille-Jørgensen P . Mechanical bowel preparation for elective colorectal surgery. Guenaga KK , editor. Cochrane Database Syst Rev. Chichester, UK: John Wiley & Sons, Ltd. (2009) ;92: (1):CD001544. |
[80] | Kehlet H . Postoperative ileus|[mdash]|an update on preventive techniques. Nature Clinical Practice Gastroenterology & Hepatology. Nature Publishing Group (2008) ;5: (10):552–8. |
[81] | Osland E , Yunus RM , Khan S , Memon MA . Early versus traditionalpostoperative feeding in patients undergoing resectionalgastrointestinal surgery: A meta-analysis. JPEN J Parenter EnteralNutr. 8 ed. (2011) ;35: (4):473–87. |
[82] | Mythen MG , Swart M , Acheson N , Crawford R , Jones K , Kuper M , et al. Perioperative fluid management: Consensus statement fromthe enhanced recovery partnershi Perioper Med (Lond) (2012) ;1: (1):2. |
[83] | Kouba EJ , Wallen EM , Pruthi RS . Gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion. Urology (2007) ;70: (6):1053–6. |
[84] | Choi H , Kang SH , Yoon DK , Kang SG , Ko HY , Moon DG , et al. Chewinggum has a stimulatory effect on bowel motility in patients afteropen or robotic radical cystectomy for bladder cancer: Aprospective randomized comparative study. Urology (2011) ;77: (4):884–90. |
[85] | Nabhani J , Ahmadi H , Schuckman AK , Cai J , Miranda G , Djaladat H , et al. Cost Analysis of the Enhanced Recovery After Surgery Protocol in Patients Undergoing Radical Cystectomy for Bladder Cancer. Eur Urol Focus (2016) ;2: (1):92–6. |
[86] | Lee R , Chughtai B , Herman M , Shariat SF , Scherr DS . Cost-analysis comparison of robot-assisted laparoscopic radical cystectomy (RC) vs open RC. BJU Int (2011) ;108: (6 Pt 2):976–83. |
[87] | Lee R , Ng CK , Shariat SF , Borkina A , Guimento R , Brumit KF , et al. The economics of robotic cystectomy: Cost comparison of openversus robotic cystectomy. BJU Int (2011) ;108: (11):1886–92. |
[88] | Martin AD , Nunez RN , Castle EP . Robot-assisted radical cystectomy versus open radical cystectomy: A complete cost analysis. Urology (2011) ;77: (3):621–5. |
[89] | Berrum-Svennung I , Hedelin H , Holmäng S . Costs of radical cystectomy. Scandinavian Journal of Urology and Nephrology. Taylor & Francis (2009) ;39: (1):36–41. |
[90] | Vetterlein MW , Meyer CP , Leyh-Bannurah S-R , Mayr R , Gierth M , Fritsche H-M , et al. Effect of hospital and surgeon case volume onperioperative quality of care and short-term outcomes afterradical cystectomy for muscle-invasive bladder cancer: resultsfrom a european tertiary care center cohort. ClinicalGenitourinary Cancer (2017) ;15: (5):e809–17. |
[91] | Konety BR , Dhawan V , Allareddy V , O’Donnell MA . Association between volume and charges for most frequently performed ambulatory and nonambulatory surgery for bladder cancer. Is more cheaper? J Urol (2004) ;172: (3):1056–61. |
[92] | Cole AP , Leow JJ , Chang SL , Chung BI , Meyer CP , Kibel AS , et al. Surgeon and hospital level variation in the costs ofrobot-assisted radical prostatectomy. J Urol (2016) ;196: (4):1090–5. |
[93] | Svatek RS , Hollenbeck BK , Holmäng S , Lee R , Kim SP , Stenzl A , et al. The economics of bladder cancer: Costs and considerationsof caring for this disease. Eur Urol (2014) ;66: (2):253–62. |
[94] | Augoustides JGT , Neuman MD , Al-Ghofaily L , Silvay G . Preoperative cardiac risk assessment for noncardiac surgery: Defining costs and risks. J Cardiothorac Vasc Anesth (2013) ;27: (2):395–9. |
[95] | Association AH . TrendWatch chartbook 2007: Trends affecting hospitals and health systems. AHA; (2007) . |
[96] | Konety BR , Allareddy V . Influence of post-cystectomy complications on cost and subsequent outcome. J Urol (2007) ;177: (1):280–7-discussion287. |
[97] | Kim SP , Shah ND , Karnes RJ , Weight CJ , Frank I , Moriarty JP , et al. The implications of hospital acquired adverse events onmortality, length of stay and costs for patients undergoingradical cystectomy for bladder cancer. J Urol (2012) ;187: (6):2011–7. |
[98] | Gili-Ortiz E , González-Guerrero R , Béjar-Prado L , López-Mández J , Ramírez-Ramírez G . Surgical siteinfections in patients who undergo radical cystectomy: Excessmortality, stay prolongation and hospital cost overruns. ActasUrol Es (2015) ;39: (4):210–6. |
[99] | Asgeirsson T , El-Badawi KI , Mahmood A , Barletta J , Luchtefeld M , Senagore AJ . Postoperative ileus: It costs more than you expect. JAm Coll Surg (2010) ;210: (2):228–31. |
[100] | Hilton WM , Lotan Y , Parekh DJ , Basler JW , Svatek RS . Alvimopan for prevention of postoperative paralytic ileus in radical cystectomy patients: A cost-effectiveness analysis. BJU Int (2013) ;111: (7):1054–60. |
[101] | Schleyer AM , Robinson E , Dumitru R , Taylor M , Hayes K , Pergamit R , et al. Preventing hospital-acquired venous thromboembolism: Improving patient safety with interdisciplinary teamwork, quality improvement analytics, and data transparency. J Hosp Med (2016) ;11: (Suppl 2):S38–S43. |
[102] | Cain K , Schmeler KM , Langley G , Max O , Ramirez PT , Levenback CF . Patient cost associated with filling a prescription for extended-duration venous thromboembolism (VTE) prophylaxis following surgery for gynecologic cancer. Gynecol Oncol (2012) ;127: (1):18–21. |
[103] | Reinhardt UE . Cost-effectiveness analysis’ and US healthcare. New York Times (2009) . |
[104] | Wittig K , Ruel N , Barlog J , Crocitto L , Chan K , Lau C , et al. Critical analysis of hospital readmission and cost burden afterrobot-assisted radical cystectomy. J Endourol (2016) ;30: (1):83–91. |
[105] | James AC , Izard JP , Holt SK , Calvert JK , Wright JL , Porter MP , et al. Root causes and modifiability of 30-day hospitalreadmissions after radical cystectomy for bladder cancer. J Urol (2016) ;195: (4 Pt 1):894–9. |
[106] | Krishnan N , Liu X , Lavieri MS , Hu M , Helfand A , Li B , et al. Amodel to optimize followup care and reduce hospital readmissionsafter radical cystectomy. J Urol (2016) ;195: (5):1362–7. |




