Oncological Outcomes of Sequential Intravesical Gemcitabine and Docetaxel in Patients with Non-Muscle Invasive Bladder Cancer
Abstract
Background:
Bacillus Calmette-Guérin (BCG) unresponsive/relapsing patients with non-muscle invasive bladder cancer (NMIBC) who prefer bladder preservation over radical cystectomy (RC) or those who do not qualify for surgery may be offered intravesical therapies. Gemcitabine (GEM) combined with Docetaxel (DOCE) has been offered at Johns Hopkins Hospital (JHH).
Objective:
To evaluate experience with GEM/DOCE, to confirm safety of the regimen, to identify populations that may benefit most, and to consider the appropriate endpoints for judging efficacy of second line therapies.
Methods:
Thirty-three patients who received full induction GEM/DOCE since 2011, per the protocol adapted from U. Iowa, were identified and characterized. Multivariable logistic regression was used to determine factors associated with recurrence. Cox proportional hazard models evaluated risk factors for disease-free survival (DFS) and high-grade recurrence-free survival (HG-RFS).
Results:
There were no serious adverse effects of therapy. Across all patients, median follow-up time was 18.6 months with a median DFS of 6.5 months, 42% 1-year, and 24% 2-year DFS. Median HG-RFS was 17.1 months with 56% 1-year and 42% 2-year HG-RFS. Among patients initially presenting with HG-NMIBC, 46% (13/28) had HG recurrence. BCG unresponsive/relapsing patients (N = 25) displayed 49% 1-year HG-RFS and 34% 2-year HG-RFS. In total, there were 5 LG and 16 HG recurrences, with 5 progressions and 8 cystectomies among these.
Conclusions:
GEM/DOCE is a well-tolerated therapy that deserves further study as an alternative to immediate RC for highly selected patients with HG-NMIBC. BCG naïve patients responded more effectively than BCG unresponsive/relapsing patients. As anticipated, GEM/DOCE efficacy was improved for HG only patients.
INTRODUCTION
Bacillus Calmette-Guérin (BCG) following transurethral resection of bladder tumor (TURBT) remains the standard of care for patients diagnosed with intermediate and high risk non-muscle invasive bladder cancer (NMIBC) [1, 2]. After BCG, 30–77% of patients will experience recurrence within 5 years [3–5]. The standard of care for patients with high risk BCG-unresponsive NMIBC is radical cystectomy (RC) or additional intravesical BCG or chemotherapy. Some patients are not ideal candidates for surgery due to co-morbidities, while others may qualify for surgery but prefer bladder preservation, while others may lack access to BCG –all of these patients may consider alternative intravesical therapies. However, significant trade-offs exist for patients, namely the risk of progression associated with delaying or avoiding RC versus the potential morbidities and mortality associated with RC [6–8]. These considerations are part of the decision-making process that patients and physicians must undertake.
In the search for less invasive alternatives to RC, combinations of second-line intravesical chemotherapeutic agents have been assessed, including mitomycin C (MCC), valrubicin, gemcitabine, and docetaxel. Gemcitabine, a deoxycytidine nucleoside analog, has been shown to have antitumoral effect in both metastatic and NMIBC [9, 10]. Phase 2 trials in patients with BCG refractory/unresponsive disease have reported recurrence free-survival of up to 28% at 1 year [11]. Docetaxel, a microtubule depolymerization inhibitor, has shown antitumor efficacy in breast, prostate, and urothelial cancers [12]. In one study, fifty-four patients with BCG-refractory NMIBC were followed for a median of 39.1 months and found to have a 40% 1 year recurrence free survival [13, 14]. Despite this, the urological community continues to lack consensus regarding appropriate expectations from potential new therapies in the face of a curative option available with RC.
In 2015, Steinberg et al. published the first known study of sequential gemcitabine and docetaxel (GEM/DOCE) as salvage therapy for NMIBC patients who failed BCG, and demonstrated a 54% 1-year and 34% 2-year recurrence free survival, in addition to a 66% complete response at first surveillance [15]. At our institution, GEM/DOCE has been administered following the treatment protocol established by Steinberg et al. [15]. Our objective was to report our institutional experience with sequential gemcitabine and docetaxel for patients with NMIBC and to report on the regimen’s safety. Further, we examined the question of which endpoints are most appropriate for NMIBC patients receiving intravesical therapy after stratifying based on initial pathology and clinical characteristics.
MATERIALS AND METHODS
Approval of this research was secured from the Johns Hopkins Medicine Institutional Review Board as part of broader approval for studying perioperative and oncologic outcomes in cancers of the urinary tract in patients followed in the Johns Hopkins Cancer Registry.
Patient cohort
Patients receiving sequential Gemcitabine and Docetaxel (GEM/DOCE) were identified (N = 33) and retrospectively reviewed from the Johns Hopkins Non-Muscle Invasive Bladder Cancer database. Briefly, this database contains patients who received induction courses of intravesical therapy for NMIBC at Johns Hopkins Hospital between 2003 –2016.
Study variables
Clinicopathologic variables were collected from patient medical records. Collected variables included age at time of GEM/DOCE initiation, sex, race, smoking status, therapy initiation/end dates, previous NMIBC therapy history, surgeon responsible for care, American Joint Committee on Cancer TNM staging before GEM/DOCE induction (clinical stage), intolerance symptoms, follow-up dates and methods through entire study period, pathologic stage if follow-up biopsy obtained, American joint Committee on Cancer TNM staging at time of RC (pathologic stage) if obtained, and date/reasons for mortality.
GEM/DOCE instillation
Treatment protocol at our institution is based on the protocol established by Steinberg in collaboration with investigators at the University of Iowa, including O’Donnell and Nepple [15]. GEM/DOCE induction is given intravesically in sequential order once a week for six consecutive weeks. One gram of gemcitabine in 50 ml of sterile water is slowly instilled into the bladder and the catheter is clamped for 60 minutes. The bladder is then drained and 37.5 mg of docetaxel in 50 ml of NSS is slowly instilled in the bladder. The catheter is again clamped for 60 minutes. The docetaxel is then drained and catheter removed and the patient is questioned regarding discomfort, instructed to remain well-hydrated and to notify a physician with any adverse reactions, questions, or concerns.
Maintenance BCG for recurrent Ta, T1, and CIS NMIBC has been associated with significantly longer recurrence-free survival times than induction without maintenance [16]. A recent review in European Urology recommended at least a 3 year maintenance protocol for patients who have received BCG [17]. However, since GEM/DOCE remains an experimental approach to treating recurrent NMIBC patients, no protocol has been established for maintenance therapy. Since this study spans many years, is retrospective by nature, and patients were followed by several physicians, there was no standard protocol in place for patients to receive maintenance therapy at our institution. 7/33 (21%) of patients received some form of maintenance GEM/DOCE, namely intravesical GEM/DOCE monthly between cystoscopic exams. In an ideal future randomized study, a maintenance protocol would be emphasized as part of the treatment process and currently at our institution patients receive monthly intravesical GEM/DOCE.
Statistical analysis
The dual purpose of this study was to validate the safety of this protocol and to establish precedence for further study of the protocol in future controlled prospective studies. This was to be established by analyzing recurrence and progression rates.
The primary recurrence endpoints of interest were high-grade recurrence and any-grade recurrence. High-grade recurrence was defined as the finding of high-grade papillary carcinoma (HgTa), carcinoma in-situ (Tis), lamina propria invasion (T1), and any progression beyond these as diagnosed by tissue biopsy within 6 months of GEM/DOCE induction completion. Any-grade recurrence included recurrence with low-grade papillary carcinoma (LgTa) in addition to all previously defined high-grade recurrence. Progression included patients found to have muscle-invasive lesions (T2), invasion beyond bladder tissue (T3/T4), or metastatic disease by tissue biopsy, RC pathology, or imaging. Univariable and multivariable logistic regression was performed to determine clinical predictors (age, race, sex, pathological stage, method of follow-up, and history of BCG) of recurrence. Pathological stage was used as a surrogate for clinical tumor grade as previous studies have demonstrated high correlation between these characteristics [18, 19].
Next, univariable and multivariable Cox proportional hazards modeling was performed to determine predictors of high-grade recurrence free survival (HG-RFS) and disease-free survival (DFS). Patients were stratified by BCG history (unresponsive/relapsing vs. naïve) in order to separately assess these populations. Further, patients were grouped by whether they experienced high-grade recurrence and Kaplan-Meier curves with Wilcoxon tests were generated to assess for statistical differences in HG-RFS using STATA version 14 supported by the Johns Hopkins University School of Medicine.
RESULTS
Cohort background
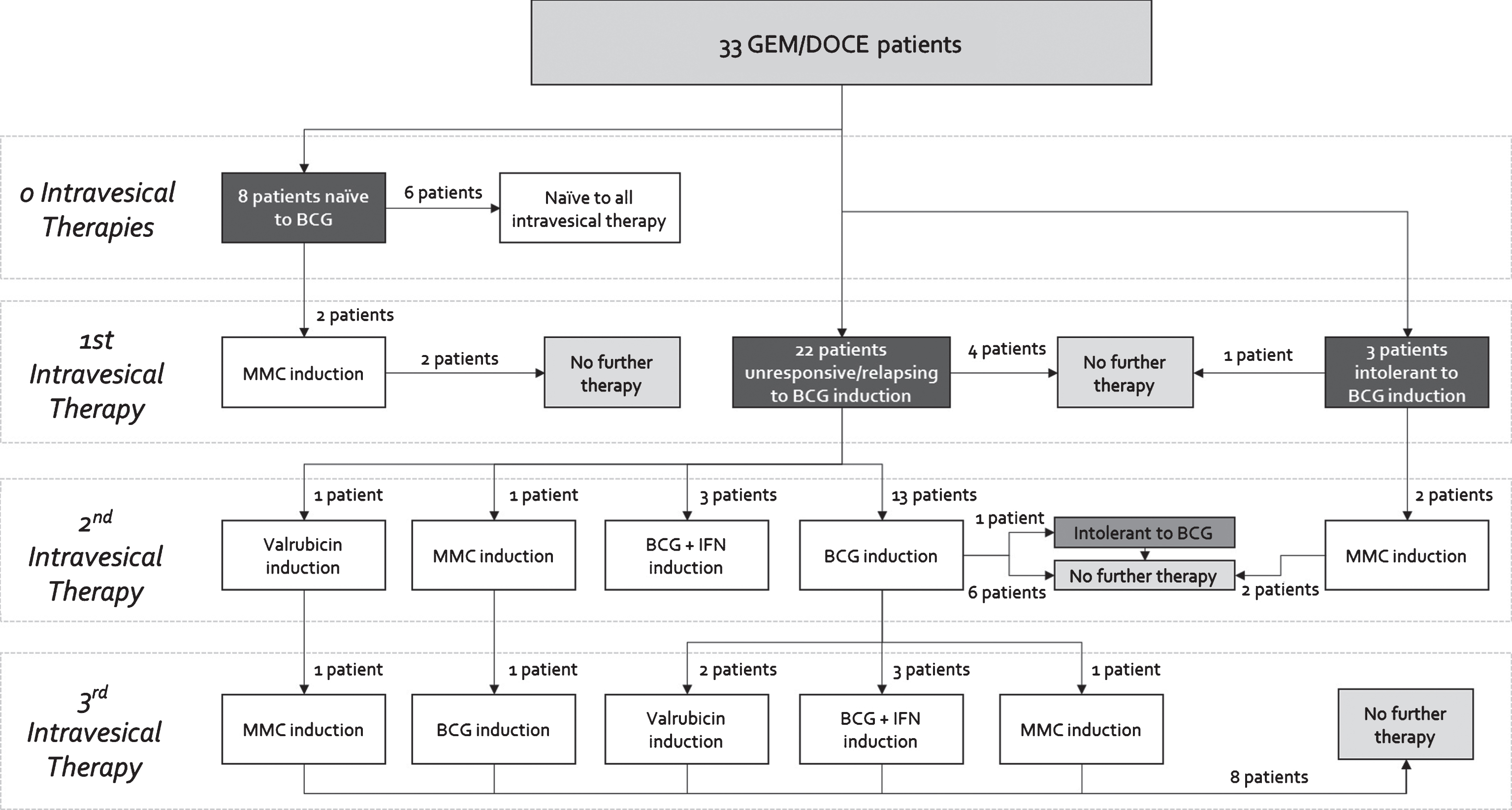
The 33 patient cohort was divided among 8 (24%) patients who were naïve to BCG, 3 (9%) patients who were BCG intolerant, and 22 (66%) patients who were BCG unresponsive/relapsing as defined by the 2015 Genitourinary Cancers Symposium taskforce [20] (Fig. 1). As evidenced by the complex and variable treatment histories in Fig. 1, this was a heavily pretreated population.
Fig.1
Previous intravesical therapies received by order of induction courses for patients receiving GEM/DOCE.
Treatment tolerance
Two patients who were initiated on GEM/DOCE with CIS pathology were unable to tolerate a full induction course. The first patient, who was previously BCG intolerant, received 4 weekly doses before developing hives. The patient then proceeded to receive only a one-half dose of gemcitabine and docetaxel for dose 5 and docetaxel only for dose 6. It was later determined that this patient’s reaction was likely to another medication the patient was on at the time. However, the patient did not go on to recur and eventually received a full monthly maintenance course of GEM/DOCE. The second patient, who was BCG unresponsive/relapsing, could not tolerate the initial instillation of the induction course and subsequently preferred to proceed directly to cystectomy, of which final pathology was TisN0.
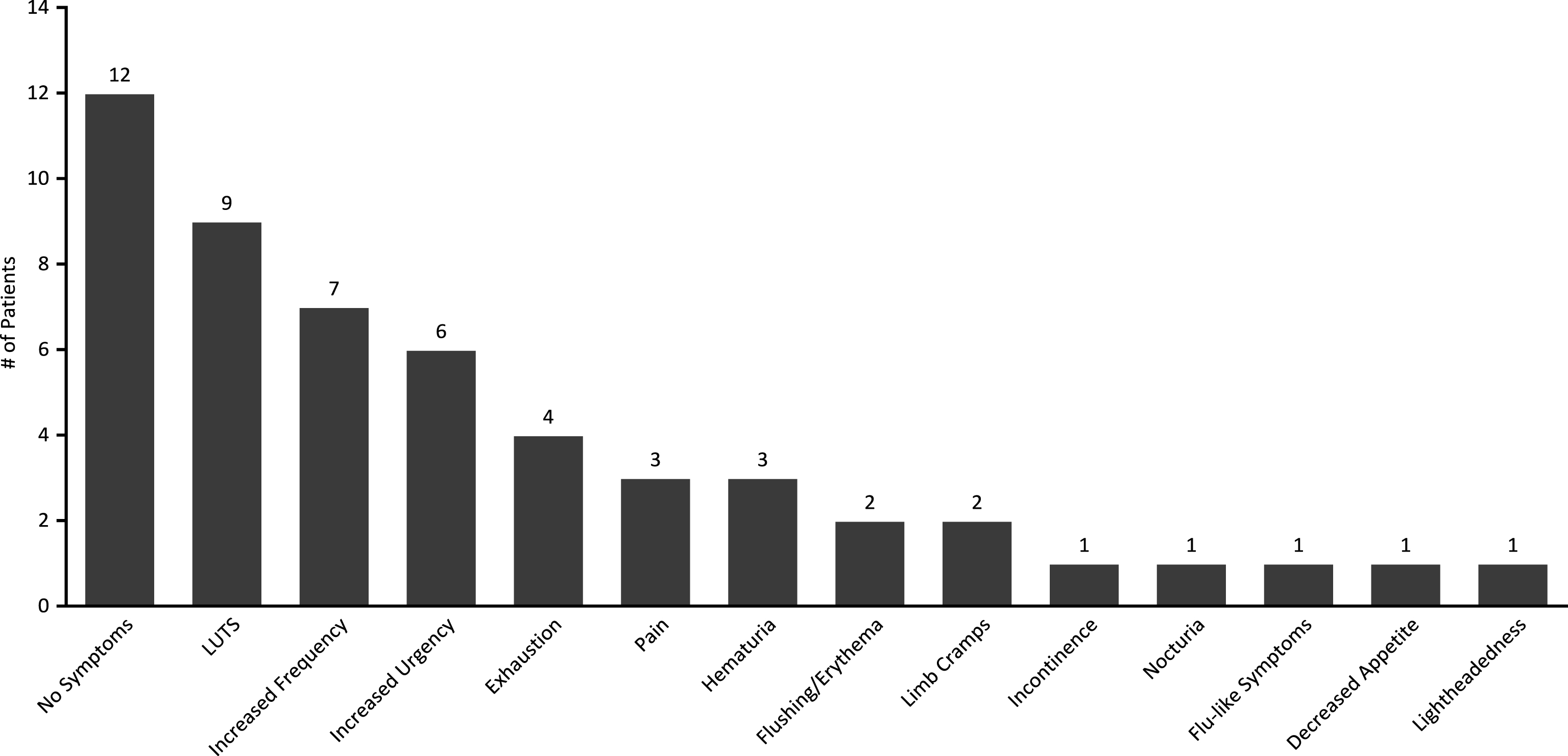
While only 2 patients experienced intolerance affecting treatment course, other patients experienced a wide range of mild symptoms including: LUTS (9/33, 27%), increased frequency (7/33, 21%), increased urgency (6/33, 18%), exhaustion (4/33, 12%), pain (3/33, 9%), hematuria (3/33, 9%), body flushing/erythema (2/33, 6%), limb cramps (2/33, 6%), incontinence (1/33, 3%), nocturia (1/33, 3%), general flu-like symptoms (1/33, 3%), decreased appetite (1/33, 3%) and lightheadedness (1/33, 3%). 12/33 (36%) patients experienced no symptoms throughout treatment (Fig. 2).
Fig.2
Prevalence of treatment symptoms experienced throughout induction courses of GEM/DOCE.
Treatment surveillance
In total, 23/33 (70%) of patients demonstrated response at first surveillance. Following treatment completion, patients were assessed for recurrence by one of three methods. Variation in follow-up method resulted from the advent and adoption during the study period of CysView in assessing recurrence following intravesical therapies [21, 22]. At first post-induction visit, 9/33 (27%) were followed up with traditional in-office cystoscopy with a flexible scope under white light at a median of 8.7 weeks. 1/9 (11%) patient presented with a suspicious lesion and was subsequently taken to the operating room for TURBT which confirmed CIS. Patients in the second group (8/33, 24%) were evaluated in the operating room at a median follow-up of 6.7 weeks with a confirmatory biopsy and resection using only white light cystoscopy. 3/8 (38%) of these were found to have high-grade recurrence at first follow-up. The third group (14/33, 42%) was followed up at a median of 6.8 weeks in the operating room using both white and blue light, followed by immediate confirmatory biopsy and TURBT if indicated. 4/14 (29%) of these were found to have recurrence at first follow-up, with two having LgTa and two having CIS. In total, 6/33 (18%) experienced HG recurrence at first surveillance while 2/33 (6%) experienced LG recurrence. 1/33 (3%) patient was lost to follow-up immediately after therapy completion and the 1/33 (3%) patient proceeded directly to RC.
BCG Naïve population
The distributions of demographic and baseline clinical characteristics were comparable between the BCG naïve and BCG unresponsive/relapsing populations (Table 1). The 8 BCG-naïve patients had a mean age of 71.9 years at induction initiation and were majority Caucasian (7/8, 88%) and male (6/8, 75%). 6/8 (75%) of patients had high grade disease (HgTa, HgT1, CIS). 3/8 (38%) of patients recurred after GEM/DOCE with high-grade disease (1 with CIS, 1 with HgT1, and 1 with HgT1 + CIS). 3/8 (38%) additional patients experienced LgTa recurrence.
Table 1
Baseline characteristics of patients who received GEM/DOCE for NMIBC stratified by BCG status
| BCG naïve | BCG Unresponsive/ | p-value | |
| Relapsing | |||
| Mean age | 71.9 (13.9) | 72.9 (10.8) | 0.83 |
| No. of patients | 8 | 25 | |
| Sex | 0.76 | ||
| Male | 6 (75%) | 20 (80%) | |
| Female | 2 (25%) | 5 (20%) | |
| Race | 0.81 | ||
| Caucasian | 7 (88%) | 21 (84%) | |
| Other | 1 (12%) | 4 (16%) | |
| Stage | 0.30 | ||
| CIS alone | 0 (0%) | 10 (40%) | |
| TaLG | 2 (25%) | 3 (12%) | |
| TaHG | 3 (38%) | 6 (24%) | |
| TaHG+CIS | 1 (12%) | 3 (12%) | |
| T1HG | 2 (25%) | 2 (8%) | |
| T1HG+CIS | 0 (0%) | 1 (4%) | |
| HG/LG Path | 0.37 | ||
| at Initiation | |||
| High-grade | 6 (21%) | 22 (79%) | |
| Low-grade | 2 (40%) | 3 (60%) |
The other 2 previously BCG naïve patients who recurred with high-grade disease after GEM/DOCE went on to receive induction and maintenance BCG and remained disease free at the end of the study period.
BCG Unresponsive/relapsing population
The BCG unresponsive/relapsing population had a mean age of 72.9 years and were also predominantly male (20/25, 80%) and Caucasian (21/25, 84%). Eighty-eight percent (22/25) had high-grade disease at GEM/DOCE initiation. For the purposes of our study, BCG intolerant patients were grouped with BCG unresponsive/relapsing patients. Fifty-two percent (13/25) of patients recurred with high-grade disease after GEM/DOCE (7 with CIS, 4 with HgTa, 2 with T1). Eight percent (2/25) of additional patients experienced LgTa recurrence. Of the BCG unresponsive/relapsing patients who recurred with high-grade disease after GEM/DOCE and then elected to again avoid cystectomy, 2 were found to have upper tract cancer, 1 was lost to follow-up, 1 received BCG and has yet to recur, and 1 was monitored with cystoscopies and has yet to recur as of the end of the study period.
High-Grade Recurrence Free Survival (HG-RFS) and Disease-Free Survival (DFS)
Mean (SD) and median follow-up for the overall cohort were 17.6 (9.5) months and 18.6 months respectively. Fifty percent (8/16) of HG events were in the initial 6 months of follow-up. One-year HG-RFS was 56% and 1-year DFS was 42%. Two-year HG-RFS was 42% and 2-year DFS was 24% (Fig. 4, Table 3). Median DFS was 6.5 months. In total, 23/33 (70%) of patients demonstrated response at first surveillance.
Fig.3
Cumulative number of cystectomies of patients who received GEM/DOCE since therapy initiation.
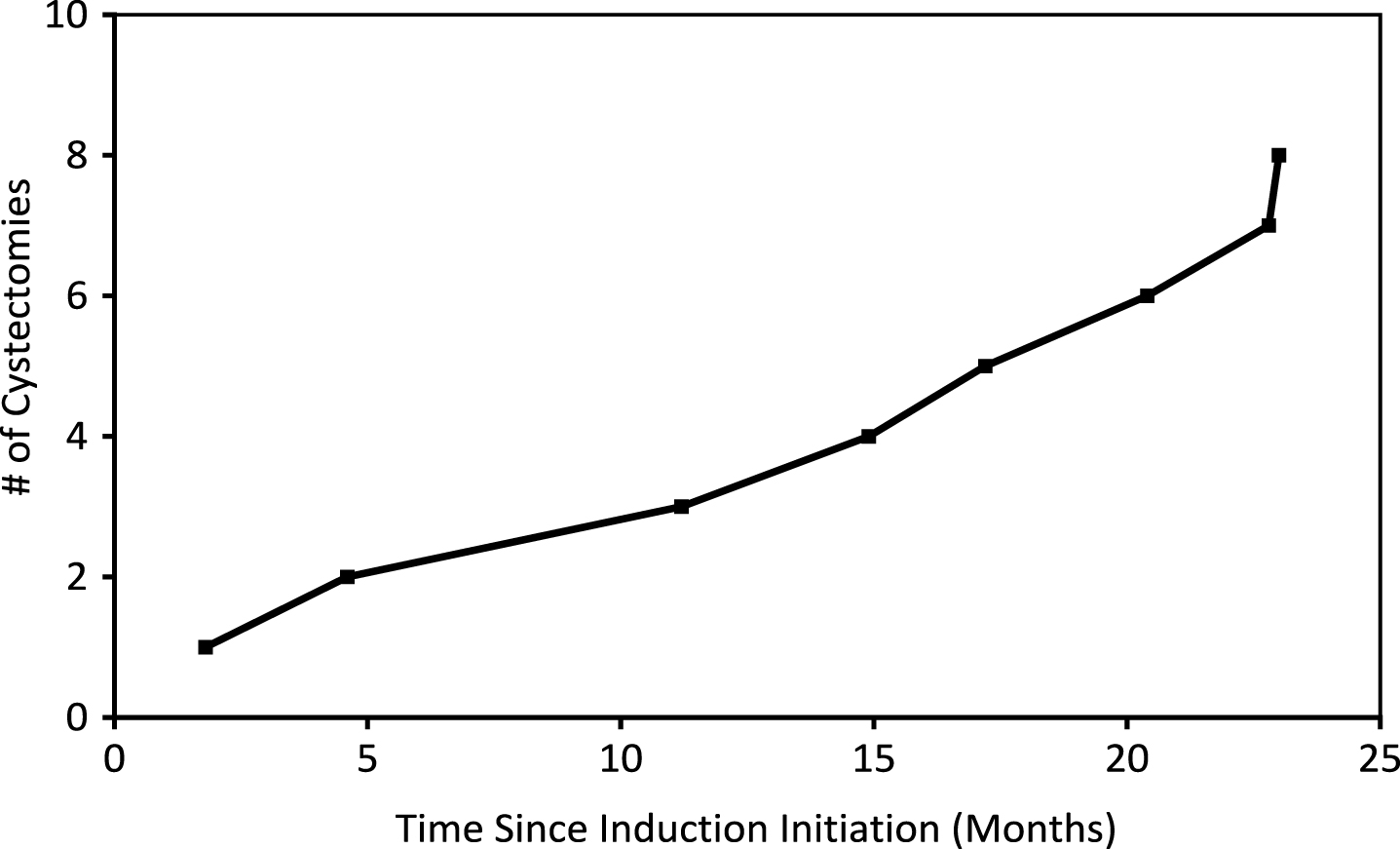
Fig.4
Kaplan-Meier plots of A) HG-RFS vs. DFS B) HG-RFS by HG vs LG pathology at therapy initiation C) HG-RFS by CIS vs. No CIS at therapy initiation D) HG-RFS by BCG-naïve vs. BCG-failed at therapy initiation.
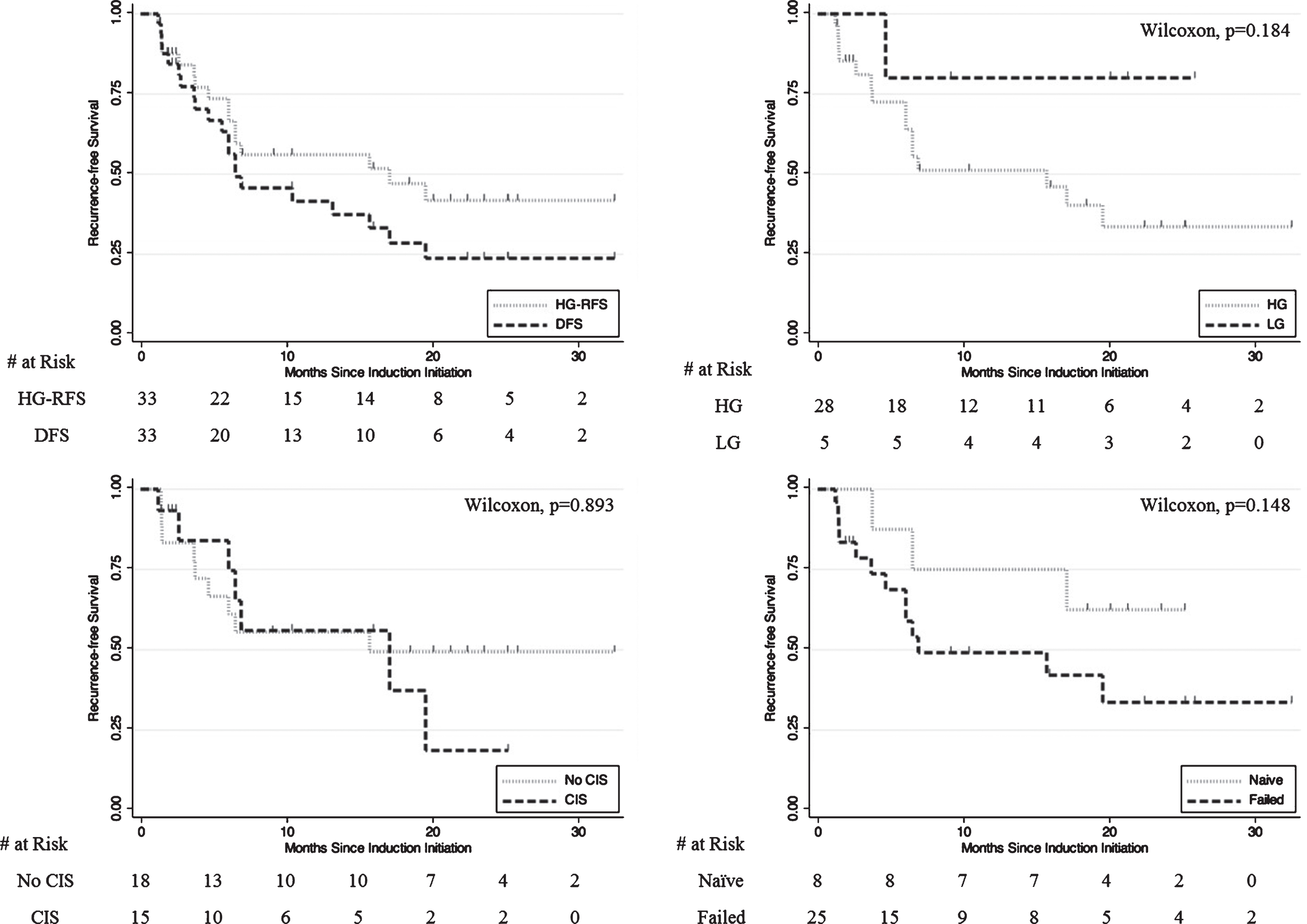
Table 2
Baseline characteristics of patients who received GEM/DOCE for NMIBC stratified by recurrence status
| No Recurrence | LG Recurrence | HG Recurrence | p-value | |
| Mean age | 73.0 (9.6) | 69.4 (14.6) | 73.4 (12.1) | 0.79 |
| No. of patients | 12 | 5 | 16 | |
| Sex | 0.92 | |||
| Male | 9 (75%) | 4 (80%) | 13 (81%) | |
| Female | 3 (25%) | 1 (20%) | 3 (19%) | |
| Race | 0.23 | |||
| Caucasian | 11 (92%) | 3 (60%) | 14 (88%) | |
| Other | 1 (8%) | 2 (40%) | 2 (12%) | |
| Stage | 0.001 | |||
| CIS alone | 7 (58%) | 0 (0%) | 3 (19%) | |
| TaLG | 0 (0%) | 4 (80%) | 1 (6%) | |
| TaHG | 3 (25%) | 1 (20%) | 5 (31%) | |
| TaHG+CIS | 0 (0%) | 0 (0%) | 4 (25%) | |
| T1HG | 1 (8%) | 0 (0%) | 3 (19%) | |
| T1HG+CIS | 1 (8%) | 0 (0%) | 0 (0%) | |
| HG/LG Path at Initiation | <0.001 | |||
| High-grade | 12 (43%) | 1 (3%) | 15 (54%) | |
| Low-grade | 0 (0%) | 4 (80%) | 1 (20%) |
Table 3
Key recurrence/cystectomy metrics of patients who received GEM/DOCE
| Metric | Result | Metric | Result |
| HG-RFS among all patients | HG-RFS among BCG Naïve patients | ||
| Median | 17.1 months | Median | – |
| 1-year | 56% | 1-year | 75% |
| 2-year | 42% | 2-year | 63% |
| DFS among all patients | HG-RFS among BCG Unresponsive/ | ||
| Relapsing patients | |||
| Median | 6.5 months | Median | 6.5 months |
| 1-year | 42% | 1-year | 49% |
| 2-year | 24% | 2-year | 34% |
| HG-RFS among initial HG patients | Cystectomies | ||
| Median | 15.7 months | Median time to | 16.1 months |
| 1-year | 51% | Among LG | 0 |
| presentation | |||
| 2-year | 34% | Among HG | 8 (10 rec.) |
| presentation |
Of patients who initiated GEM/DOCE therapy after high-grade presentation, 1-year HG-RFS was 51% and 2-year HG-RFS was 34% (Fig. 4). For these patients, median HG-RFS was 15.7 months. Median time to high-grade event was 6.0 months. 7/15 (47%) of HG events among initially HG patients were in the initial 6 months of follow-up.
BCG naïve patients had a median DFS of 6.5 months with a 50% 1-year DFS and 25% 2-year DFS while BCG unresponsive/relapsing patients had a median DFS of 6.5 months and a 1-year DFS of 38% and 2-year DFS of 24%. BCG naïve patients had a 75% 1-year HG-RFS and 63% 2-year HG-RFS while BCG unresponsive/relapsing patients had a 49% 1-year HG-RFS and 34% 2-year HG-RFS (median HG-RFS was 6.5 months).
Low grade recurrences
Fifteen percent (5/33) of patients initiated therapy with LgTa disease. One patient recurred with HgTa disease at 4.6 months. This patient was then followed with serial cystoscopies and did not recur as of 18.2 months. The other 4 patients experienced LgTa recurrence at 1.9 months, 2.7 months, 5.6 months, and 10.4 months respectively. None of these patients went on to RC. Additionally, one patient who initially presented with HgTa went on to have LgTa at 13.2 months of follow-up.
Clinical predictors of recurrence
The baseline characteristics stratified by eventual recurrence are listed in Table 2. A total of 12 (36%) patients experienced no recurrence, 5 (15%) patients experienced LG recurrence, and 16 (54%) patients experienced HG recurrence. There was no statistical significance in age (p = 0.79), sex (p = 0.92), race (p = 0.23), smoking status (p = 0.78), or previous BCG exposure (p = 0.13). Patients who initially presented with LG disease were more likely to recur with low-grade pathology (p < 0.001) and were less likely to have CIS at therapy initiation (p = 0.042).
There were no statistically significant demographic or clinical predictors of disease recurrence (HG or LG) in multivariable logistic regression models. The same was true when using multivariable Cox proportional hazard models for predicting DFS or HG-RFS.
Cystectomies and progression
All patients who were identified for cystectomy after treatment failure had a CT scan of chest/abdomen/pelvis four weeks prior to their scheduled cystectomy date. All but two patients did not have metastatic disease. Of the 10 patients who eventually underwent cystectomy (Fig. 3, Table 4), one (10%) was BCG-naïve. This patient was initiated on GEM/DOCE after presenting with HgT1 disease post-MMC induction. Following GEM/DOCE, the patient recurred with CIS at 3.7 months, and upon workup for cystectomy, was found to have metastatic disease. The patient died from disease 5.0 months after completing GEM/DOCE.
Table 4
Pathologic stage and timeline of patients who received GEM/DOCE and were recommended for RC
| # | Stage | Time to RC from therapy | Time to RC from therapy | BCG Status |
| initiation (Months) | completion (Months) | |||
| 1 | TisN0 | 1.8* | – | U/R |
| 2 | TisN0 | 4.6 | 3.5 | U/R |
| 3 | TisN0 | 11.2 | 10.0 | U/R |
| 4 | TisN0 | 14.9 | 13.8 | U/R |
| 5 | T2aN0 | 17.2 | 15.8 | U/R |
| 6 | T3aN2 | 20.4 | 19.3 | U/R |
| 7 | T3aN0 | 22.8 | 21.7 | U/R |
| 8 | TisN0 | 23.0 | 21.2 | U/R |
| 9 | N/A; Metastatic progression | Naïve | ||
| 10 | N/A; Metastatic progression | U/R |
Note: *Patient was intolerant to GEM/DOCE and did not undergo therapy beyond first instillation; U/R = BCG unresponsive/relapsing.
Nine (90%) patients were BCG unresponsive/relapsing. Eight (89%) eventually underwent cystectomy while the ninth individual was found to have metastatic disease upon workup. All 9 BCG unresponsive/relapsing patients who were identified for cystectomy initiated GEM/DOCE with high-grade disease (3/10 with CIS, 3/10 with HgTa, 2/10 with HgTa+CIS, 1/10 with HgT1). Sixty-three percent (5/8) of cystectomy patients had final pathology of TisN0. One (13%) had T2a pathology and two (25%) had T3a disease, one of which was N2 (2/23 pelvic nodes positive). Median time to cystectomy from initial instillation of GEM/DOCE was 16.1 months for BCG unresponsive/relapsing patients. One additional patient was completely intolerant to GEM/DOCE and elected to proceed to RC.
Mortality
All-cause and bladder cancer-specific mortality were 3% (1/33) at 1 year and 6% (2/33) at 2 years. The first patient was found to have metastatic disease on work-up for cystectomy and passed away 5.0 months after final intravesical instillation. The second patient underwent cystectomy and experienced a parastomal hernia post-operatively and underwent hernia repair. In the immediate post-operatively period, the patient had an acute coronary event and died 22.4 months after final intravesical instillation.
DISCUSSION
Sequential GEM/DOCE appears to be well tolerated in certain carefully selected patients who do not qualify for surgery due to co-morbidities or in others who prefer bladder preservation. This study primarily confirms the safety of GEM/DOCE in this population as evidenced by the lack of serious adverse events and the relative rarity of seriously concerning side-effects. It should be noted that 5 (15%) patients within the cohort progressed while on GEM/DOCE. Three (9%) patients eventually underwent cystectomy at 17.2 months (T2aN0), 20.4 months (T3aN2), and 22.8 months (T3aN0) post-GEM/DOCE initiation. Each of these patients had previously been BCG unresponsive/relapsing. The 2 (6%) remaining patients were found to have metastatic disease upon pre-operative imaging. One (3%) of these was BCG unresponsive/relapsing and the other was BCG naïve prior to GEM/DOCE. Unfortunately, the BCG naïve patient eventually died during the study period. It is well established that progression to muscle invasive bladder cancer or beyond is associated with poorer outcomes [23]. The existence of these progressions must be noted to explicitly state that GEM/DOCE therapy is not without risk. It is plausible that these patients could have been saved from progressive disease with alternative management.
Secondarily, this study establishes a need for further study of this regimen in a prospective and controlled manner. This is evidenced by the greater than 50% 1-year HG-RFS and greater than 30% 2-year HG-RFS among both low-grade and high-grade patients at therapy initiation, confirming data previously published at other institutions [5, 14]. The 25 BCG unresponsive/relapsing patients in particular had a 49% 1-year HG-RFS and 34% 2-year HG-RFS with a median HG-RFS of 6.5 months. Based on the Food and Drug Administration (FDA) and American Urological Association (AUA) 2014 Public Workshop’s recommendation that therapies require a 40–50% initial complete response rate at 6 months and a durable response rate of at least 30% for 18–24 months (with the lower bound of the 95% confidence interval excluding 20%), initiating clinical trials of GEM/DOCE would appear promising in the BCG unresponsive/relapsing population [24].
DFS among BCG unresponsive/relapsing patients, however, did not pass the 40–50% 1-year survival threshold (38%), 30% 2-year survival threshold (24%), or the 2-year lower bound of 20% (7%). Of note, this is higher than the 18–21% recurrence free survival of Valrubicin in the BCG failure population and in line with the 40% 1-year recurrence free survival of Docetaxel demonstrated by other studies [14]. This dichotomy between HG-RFS and DFS raises several important questions. First, should low-grade recurrence matter for 2nd-line therapies? As shown by detailed follow-up of our low-grade recurrent patients, none of these went on to need cystectomy, and moreover, none of these patients went on to have subsequent high-grade recurrence throughout the study period. We argue that low-grade recurrence should not be considered treatment failure in this population.
Given this logic, it may make sense to exclude patients with low-grade disease at GEM/DOCE initiation from the analysis to understand how treatment impacted only high-risk patients. Patients with high-grade cancer at GEM/DOCE initiation displayed a 51% 1-year HG-RFS, 34% 2-year HG-RFS, and 14% HG-RFS 2-year lower bound (<20% FDA/AUA recommendation). The only metric that fails to qualify is the 2-year lower bound of the 95% confidence interval, but much debate has centered around this recommendation [25, 26]. After publication of the thresholds by the FDA, Amrhein et al. published a response to the editor that urged the FDA/AUA panel to revisit excessive requirements that would eliminate opportunities for patients who cannot undergo a cystectomy [27]. Subsequently, the panel softened its language and stated that not all active studies are necessarily required to achieve the suggested response rates [28].
Further, we argue that 2nd line therapy studies examine alternatives to RC, such that the primary endpoint of interest should be necessity for RC or the development of metastatic disease. In our study, 18/28 (65%) high-risk patients avoided cystectomy throughout the follow-up period, and for those who underwent cystectomy or were recommended for RC, 6/10 (60%) had less than T2 disease. When looking at the BCG unresponsive/relapsing population, 16/25 (64%) of patients avoided RC. It is thus critical that the urologic community define both the type of outcomes that truly matter for NMIBC patients searching for alternatives to RC as well as the recurrence rates and endpoints that are appropriate in this population. Our study also raises the possibility that there may be a role for sequential GEM/DOCE in the BCG naïve population. Though standard of care indicates that BCG is a preferred therapy for NMIBC patients who have yet to be treated, 8/33 (24%) patients were naïve to BCG as a result of BCG shortage at the time of therapy. Three (38%) recurred with high-grade disease and 1-year and 2-year HG-RFS were 75% and 63% respectively. A previous phase II trial examining gemcitabine vs. BCG after 1 failure with BCG (as opposed to two) showed improved survival relative to gemcitabine after 2 failures and relative to BCG after 1 failure [29]. Given the continued shortage of BCG, the existence of a patient population for which BCG is not ideal (immunocompromised, genetics, etc.), and the fact that these rates are in line with success rates shown by first-line BCG, sequential GEM/DOCE may require further study as a first-line therapy [30–32]. Given the established importance of genetic susceptibility to certain treatments, an ideal future study would examine genetic expression differences between those who respond to GEM/DOCE vs. those who do not and compare this to those who respond to BCG [30, 33, 34].
With any discussion of a new therapeutic approach, the medical community must be cognizant of associated costs. At our institution, a 6-instillation induction course of Gemcitabine with Docetaxel translates to roughly 125% the cost of a 6-instillation induction course of BCG. More importantly, the regimen is significantly cheaper than other second-line therapies. For example, a 6-week course of GEM/DOCE is roughly a third of the cost of a 6-week course of Mitomycin (MMC) with Docetaxel making up the majority of that cost. Valrubicin has been shown to cost greater than $20,000 per induction course [14]. One recent study found the mean initial hospitalization cost for cystectomy to be $33,202 and mean readmission costs to be $14,417 among cystectomy patients [35]. Certainly the substantial cost savings of such an approach when addressing the most expensive cancer at a population level is important for patients, healthcare providers, and insurance companies, especially as the focus on improving the value of care provided continues to increase [36].
The limitations of this study include that it is retrospective by design. However, given the lack of consensus on clinical trial design, retrospective studies have contributed significantly to practice in the bladder cancer field. Nonetheless, the ideal controlled prospective study would assess this GEM/DOCE protocol vs. BCG as primary therapy in the high risk (HgTa, HgT1, CIS) NMIBC cohort. Additionally, the protocol should be explored for BCG unresponsive/relapsing and BCG intolerant cases in a prospective controlled manner. Another limitation is the small sample size, thereby reducing the power of any statistical analyses. As such, many potential relevant data points could not be controlled for, including comorbidities and recurrence/progression locations within the bladder. However, the study population in question is by nature one where most patients are not ideal candidates for cystectomy, indicating that there is at least some congruence in the level of morbidity within the cohort. Though we tracked the surgeon responsible for treatment, we cannot control for variations in follow-up by these physicians or variations in maintenance therapy dosing, which could have further contributed to recurrence/progression variation. In addition, we tracked history of previous intravesical therapies carefully and meticulously, but could not account for variations in history taking regarding these therapies. We therefore elected to describe a BCG unresponsive/relapsing population rather than separating this group into BCG unresponsive vs. BCG relapsing as would have been preferred by the International Bladder Cancer Group [20, 26].
In summary, intravesical Gemcitabine with Docetaxel is a well-tolerated therapy that deserves further study as an alternative to immediate RC for highly selected patients with HG-NMIBC. While admittedly patients with low-grade disease likely did not benefit from GEM/DOCE, no low-grade patients went on to cystectomy within the study period, indicating that the intervention did not cause harm. Moreover, most patients with high-grade disease who would have otherwise needed cystectomy avoided the morbid procedure. Further studies are needed to stratify the patient population that benefits most from GEM/DOCE as well as to understand whether previous intravesical therapy, maintenance regimen, follow-up methods, and other factors help explain the observed response for patients with NMIBC. Finally, the urologic community must critically re-examine appropriate outcomes and endpoints before ruling out potentially beneficial therapies for patients who have few other options.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
Persky Family Award, The James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions.
REFERENCES
[1] | Burger M , Capoun O , Cohen D , Babjuk M , Bo A , Herna V , et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol (2017) ;71: :447–61. |
[2] | Hall MC , Chang SS , Dalbagni G , Pruthi RS , Seigne JD , Skinner EC , et al. Guideline for the Management of Nonmuscle Invasive Bladder Cancer (Stages Ta, T1, and Tis): 2007 Update. J Urol (2007) ;178: :2314–30. |
[3] | Zlotta AR , Fleshner NE , Jewett MA . The management of BCG failure in non-muscle-invasive bladder cancer: An update. Can Urol Assoc (2009) ;3: :199–205. |
[4] | Sfakianos JP , Kim PH , Hakimi AA , Herr HW . The effect of restaging transurethral resection on recurrence and progression rates in patients with non-muscle invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin. J Urol (2014) ;191: :341–5. |
[5] | Kamat AM , Colombel M , Sundi D , Lamm D , Boehle A , Brausi M , et al. BCG-unresponsive non-muscle-invasive bladder cancer: Recommendations from the IBCG. Nat Rev Urol (2017) ;14: :244–55. |
[6] | Lawrentschuk N , Colombo R , Hakenberg OW , Lerner SP , Månsson W , Sagalowsky A , et al. Prevention and Management of Complications Following Radical Cystectomy for Bladder Cancer. Eur Urol (2010) ;57: :983–1001. |
[7] | Konety BR , Allareddy V , Herr H . Complications after radical cystectomy: Analysis of population-based data. Urology (2006) ;68: :58–64. |
[8] | Hautmann RE , Hautmann SH , Hautmann O . Complications associated with urinary diversion. Nat Rev Urol (2011) ;8: :667–77. |
[9] | Gontero P , Marini L , Frea B . Intravesical gemcitabine for superficial bladder cancer: Rationale for a new treatment option. BJU Int (2005) ;96: :970–6. |
[10] | Mohanty NK , Urol MC , Nayak RL , Vasudeva P , Arora RP . Intravesicle gemcitabine in management of BCG refractory superficial TCC of urinary bladder — our experience. Urol Oncol Semin Orig Investig (2008) ;26: :616–9. |
[11] | Skinner EC , Goldman B , Sakr WA , Petrylak DP , Lenz H , Lee CT , et al. SWOG S0353: Phase II Trial of Intravesical Gemcitabine in Patients with Nonmuscle Invasive Bladder Cancer and Recurrence after 2 Prior Courses of Intravesical Bacillus Calmette-Guerin. J Urol (2013) ;190: :1200–4. |
[12] | Mackler NJ , Pienta KJ . Drug insight: Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol (2005) ;2: :92–100. |
[13] | Barlow LJ , Mckiernan JM , Benson MC . Long-Term Survival Outcomes with Intravesical Docetaxel for Recurrent Nonmuscle Invasive Bladder Cancer After Previous Bacillus Calmette-Guérin Therapy. J Urol (2013) ;189: :834–9. |
[14] | Velaer KN , Steinberg RL , Thomas LJ , O’Donnell MA , Nepple KG . Experience with Sequential Intravesical Gemcitabine and Docetaxel as Salvage Therapy for Non-Muscle Invasive Bladder Cancer. Curr Urol Rep (2016) ;17: :1–5. |
[15] | Steinberg RL , Thomas LJ , O’Donnell MA , Nepple KG . Sequential Intravesical Gemcitabine and Docetaxel as Salvage Therapy for Non-Muscle Invasive Bladder Cancer. Bl Cancer (2016) ;17: :65–72. |
[16] | Lamm DL , Blumenstein BA , Crissman JD , Montie JE , Gottesman JE , Lowe BA , et al. Maintenance Bacillus Calmette-Guerin Immunotherapy for Recurrent Ta, T1, and Carcinoma in Situ Transitional Cell Carcinoma of the Bladder: A Randomized Southwest Oncology Group Study. J Urol (2000) ;163: :1124–9. |
[17] | Ehdaie B , Sylvester R , Herr HW . Maintenance Bacillus Calmette-Guerin Treatment of Non-muscle invasive Bladder Cancer: A Critical Evaluation of the Evidence. Eur Urol (2013) ;64: :579–85. |
[18] | Chappidi MR , Kates M , Johnson MH , Hahn NM , Bivalacqua TJ , Pierorazio PM . Lymph node yield and tumor location in patients with upper tract urothelial carcinoma undergoing nephroureterectomy affects survival: A U.S. population-based analysis (2004-2012). Urol Oncol Semin Orig Investig (2016) ;34: :331.e15–331.e24. |
[19] | Brown GA , Matin SF , Busby JE , Dinney CPN , Grossman HB , Pettaway CA , et al. Ability of Clinical Grade to Predict Final Pathologic Stage in Upper Urinary Tract Transitional Cell Carcinoma: Implications for Therapy. Urology (2007) ;70: :252–6. |
[20] | Lerner SP , Dinney C , Kamat A , Bivalacqua TJ , Nielsen M , O’Donnell M , et al. Clarification of Bladder Cancer Disease States Following Treatment of Patients with Intravesical BCG. Bl Cancer (2015) ;1: :29–30. |
[21] | Daneshmand S , Schuckman AK , Bochner BH , Cookson MS , Downs TM , Gomella LG , et al. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: Review of the clinical evidence and consensus statement on appropriate use in the USA. Nat Rev Urol (2014) ;11: :589–96. |
[22] | Geavlete B , Multescu R , Georgescu D , Jecu M , Stanescu F , Geavlete P . Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: Does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int (2012) ;109: :549–56. |
[23] | Sanli O , Dobruch J , Knowles MA , Burger M , Alemozaffar M , Nielsen ME , et al. Bladder cancer. Nat Rev Dis Prim (2017) ;3: :17022. |
[24] | Jarow JP , Lerner SP , Kluetz PG , Liu K , Sridhara R , Bajorin D , et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: Report of a food and drug administration and american urological association public workshop. Urology (2014) ;83: :262–4. |
[25] | Lerner SP , Bajorin DF , Dinney CP , Efstathiou JA , Groshen S , Hahn NM , et al. Summary and Recommendations from the National Cancer Institute’s Clinical Trials Planning Meeting on Novel Therapeutics for Non-Muscle Invasive Bladder Cancer. Bl Cancer (2016) ;2: :165–202. |
[26] | Kamat AM , Sylvester RJ , Bohle A , Palou J , Lamm DL , Brausi M , et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol (2016) ;34: :1–12. |
[27] | Amrhein J , Kamat AM , Morales A . Re: Jarow JP , et al. Clinical trial design for the development of new therapies for non-muscle-invasive bladder cancer: Report of a food and drug administration and american urological association public workshop (urology 2014;83:262-265). Urology (2014) ;84: :494–5. |
[28] | Jarow J , Kluetz PG , Lerner SP , Liu K , Sridhara R , Bajorin D , et al. Reply by the authors. Urology (2014) ;84: :495–6. |
[29] | Di Lorenzo G , Perdonà S , Damiano R , Faiella A , Cantiello F , Pignata S , et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: A multicenter prospective randomized trial. Cancer (2010) ;116: :1893–900. |
[30] | Zhang N , Jiang G , Liu X , Na R , Wang X , Xu J . Prediction of Bacillus Calmette-Guerin Response in Patients with Bladder Cancer after Transurethral Resection of Bladder Tumor by Using Genetic Variation Based on Genomic Studies. Biomed Res Int (2016) ;2016: :1–7. |
[31] | Ke H-L , Lin J , Ye Y , Wu W-J , Lin H-H , Wei H , et al. Genetic Variations in Glutathione Pathway Genes Predict Cancer Recurrence in Patients Treated with Transurethral Resection and Bacillus Calmette-Guerin Instillation for Non-muscle Invasive Bladder Cancer. Ann Surg Oncol (2015) ;22: :4104–10. |
[32] | Hwang M , Banerji J , Neill M . Letter. BJU Int (2013) ;112: :E435–E435. |
[33] | Chiong E , Kesavan A , Mahendran R , Huak Y , Hwei J , Koon Y , et al. NRAMP1 and hGPX1 Gene Polymorphism and Response to Bacillus Calmette-Guerin Therapy for Bladder Cancer. Eur Urol (2011) ;59: :430–7. |
[34] | Lima L , Oliveira D , Ferreira JA , Tavares A , Cruz R . The role of functional polymorphisms in immune response genes as biomarkers of bacille Calmette-Guérin (BCG) immunotherapy outcome in bladder cancer: Establishment of a predictive profile in a Southern Europe population. BJU I (2015) ;116: :753–63. |
[35] | Chappidi MR , Kates M , Stimson CJ , Johnson MH , Pierorazio PM , Bivalacqua TJ . Causes, Timing, Hospital Costs, and Perioperative Outcomes Of Index vs. Non-Index Hospital Readmissions Following Radical Cystectomy: Implications For Regionalization of Care. J Urol (2016) ;197: :296–301. |
[36] | Botteman MF , Pashos CL , Redaelli A , Laskin B , Hauser R . The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics (2003) ;21: :1315–30. |




