Therapeutic Effect on Bladder Cancer with a Conditionally Replicating Oncolytic Virus Derived from Type II Herpes Simplex Virus
Abstract
Purpose:
Despite recent improvements, resistance to traditional immunotherapy or chemotherapy is still common in patients with bladder cancer. We constructed an oncolytic virus from herpes simplex virus type II (HSV-2), which selectively targets tumor cells with an activated Ras signaling pathway. We evaluated the antitumor effect of this oncolytic HSV-2 (FusOn-H2) against bladder cancer, and compared with that of a first generation oncolytic virus derived from HSV-1 (Baco-1).
Materials and methods:
We established bladder tumor at the orthotopic site in C3H/He mice using the MBT-2 cells. Baco-1 or FusOn-H2 was instilled into the bladder through the urethra respectively. Tumor volume and weight were recorded by the end of the experiment. Animal spleens were also collected to determine if any anti-tumor immunity was elicited during virotherapy in this syngeneic bladder cancer model.
Results:
Two instillations of the oncolytic HSVs into bladder of tumor-bearing mice almost completely eradicated the tumor in majority of tumor bearing mice. The results of tumor-specific cytotoxic T lymphocyte activity assay showed that tumor destruction by oncolytic viruses in vivo, especially by the FusOn-H2, induced potent anti-tumor immune responses.
Conclusion:
Oncolytic virus derived from HSV-2 has potent anti-tumor activity against bladder cancer. Oncolytic effect of this virus in vivo induces tumor specific cellular immunity that further enhances the overall anti-tumor activity. Translating this novel virotherapy into the clinic could present an alternative intravesical therapy strategy for patients with bladder cancer.
INTRODUCTION
Bladder cancer is the second most frequent urological cancer in North America [1]. Patients with non-muscle invasive bladder cancer have a high rate of recurrence or progression after transurethral resection despite current intravesical chemotherapy and immunotherapy treatments [2, 3]. Relapse rate and metastatic risk are also high in patients with muscle-invasive bladder cancer. These data strongly support development of novel treatment strategies in order to improve the therapeutic benefit and prognosis in bladder cancer patients.
Genetically engineered viruses for tumor cell killing can provide an effective therapeutic approach for cancer treatment. Replication selective oncolytic viruses are genetically modified to infect, replicate in, and kill cancer cells, while replication is restricted in normal cells [4]. These viruses give an advantage over conventional gene-based cancer therapy, which is associated with inefficient delivery of therapeutic genes and reduced cancer cell killing activity. Several oncolytic viruses are in clinical trials for a variety of solid tumors of different tissue origins. Oncolytic herpes simplex viruses (HSVs) were initially designed and constructed for the treatment of brain tumors [5]. Subsequently, they were found to have activity against a variety of other human solid tumors, including colon cancer [6], breast cancer [7], prostate cancer [8], lung cancer [9], liver cancer [10], ovarian cancer [11], and bladder cancer [12–14].
The current oncolytic HSVs were constructed exclusively from type 1 virus (HSV-1), most commonly by deleting the γ34.5 gene, which encodes a neurovirulent factor, and/or insertional mutation of the ICP6 gene, which encodes the large submit of ribonucleotide reductase [5, 15–17]. Inactivation of either or both of these genes enables the virus to replicate selectively in dividing cells whereas sparing normal non-dividing cells [18–20]. We have constructed a new oncolytic virus from type 2 HSV (HSV-2) to exploit a unique feature of the viral ICP10 gene, which contains a well defined region in its NH2 terminus that seems to play an important role in initiating virus replication [21]. This domain can bind and phosphorylate the GTPase-activating protein Ras-GAP, leading to activation of the Ras/MEK/MAPK mitogenic pathway, and c-Fos induction and stabilization, a condition that is required for efficient HSV-2 replication [22, 23]. A mutant HSV-2 virus (FusOn-H2), deleted for its protein kinase (PK) domain, replicates selectively in tumor cells and lyses them.
In the present study, we investigated the anti-tumor effect of this oncolytic HSV (FusOn-H2) in an orthotopic murine bladder cancer model. Our results suggest that this mutant virus is a potent oncolytic agent against orthotopic bladder cancer. Two intravesical instillations of virus at a moderate dose completely eradicated the tumors in the majority of animals. Moreover, this virus induced a potent systemic immune response against native tumor antigens released from virus-infected tumor cells.
MATERIALS AND METHODS
Bladder cancer cell lines and viruses
The MBT-2 cells were originally provided by Dr. Timothy Ratliff (University of Iowa, Iowa City, IA). MBT-2 is a poorly differentiated murine bladder cancer cell line derived from a transplantable N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide induced bladder cancer in a female C3H/He mouse. The cells were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) at 37°C in an atmosphere humidified with 5% CO2. The human bladder cancer cell line 5637 was derived from a muscle-invasive bladder cancer and was obtained from the American Type Culture Collection (Rockville, MD). The cells were maintained in DMEM containing 10% FBS at 37°C in 5% CO2.
HSV-1 derived oncolytic virus, Baco-1 was constructed from a bacterial artificial chromosome (BAC) based construct that contains a mutated HSV genome. Baco-1 has both copies of the γ34.5 gene deleted and contains the green fluorescent protein (GFP) marker gene [11, 21]. For construction of FusOn-H2, the ICP10 left flanking region of the wild type HSV-2 strain 186 (wt186) genome, the ribonucleotide reductase domain, and the right flanking region were amplified by PCR. The N-terminal domain was deleted in the ICP10 gene. PCR-amplified DNA of enhanced GFP was cloned into the deleted N-terminal region. The modified ICP10 gene was inserted into the genome of wt186 by homologous recombination. Details of its construction have been described [24]. The purified viruses were titrated and stored at −80°C until use.
Phenotypic characterization and oncolytic activity of FusOn-H2 against bladder cancer cell lines
For in vitro phenotypic characterization, 5637 cancer cells were infected with either Baco-1 or FusOn-H2 at a dose of 0.1 pfu/cell. To evaluate the phenotypic character in the murine cancer cell line, MBT-2 cells were infected with either Baco-1 or FusOn-H2 at a dose of 10.0 pfu/cell. Cells were cultured in a maintenance medium (containing 1% FBS) and were incubated for up to two days to allow the fusion pattern and plaques to develop.
For measurement of in vitro oncolytic activity of the viruses, 5637 cells were seeded into 24-well plates and infected with Baco-1 or FusOn-H2 at 0.01 and 0.1 pfu/cell, or left without infection. Cells were harvested 24, 48, 72 h later by trypsinization, and the number of viable cells determined with a hemocytometer after Trypan blue staining. The percentage of viable cells was calculated by dividing the number of cells excluding Trypan blue in the infected well by the number excluding the stain in the well that was left uninfected. The experiments were repeated in triplicate, with mean cell numbers used for the final calculation. To test the in vitro killing effect against murine bladder cancer cells, MBT-2 cells were infected with Baco-1 or FusOn-H2 at 1.0 and 10.0 pfu/cell, or left without infection. The MBT-2 cells were infected with a two log fold increase in viral dose over 5637 cells. 5637 cells are human bladder cancer cells while MBT-2 cells are murine origin. So the former is significantly more permissive than the latter to the infection of Baco-1 or FusOn-H2, which is reflected by the different dose required for infection. The other steps were same as the experiment in 5637 cell line.
Creation of orthotopic murine bladder cancer models
Six to 8-week-old female C3H/He mice were purchased from Harlan Laboratory (Houston, TX). All animal experimental procedures were approved by the Baylor College of Medicine Animal Care and Use Committee. MBT-2 cells were harvested from subconfluent cultures by trypsinization and were washed in serum free medium. Single cell suspensions with >90% cell viability were determined by Trypan blue exclusion. The cells were resuspended in phosphate buffer saline (PBS) before injection. Orthotopic bladder cancer mice were established by direct injection of tumor cells into the bladder wall [25]. Animals were anesthetized with 20–30 mg/g avertin intraperitoneally. After shaving the lower quadrants of the abdomen, midline laparotomy was performed to expose the urinary bladder. 1×106 MBT-2 cancer cell suspension was injected into the anterior wall of the bladder via a 30 gauge needle. The wound was closed with a 3–0 suture.
Treatment by intravesical virus instillation and tumor measurements
The mice were randomly divided into three groups of five mice each. On day 8 and 15 after tumor injection, the mice in group 1 and 2 were treated with either Baco-1 or FusOn-H2 at a dose of 1×107 pfu in a total volume of 50 μl. Viral suspension was instilled via urethral catheter into the bladder. Then the urethra was clamped immediately after catheter removal using a micro-surgical clamp. The viruses were left to dwell within the bladder for 30 min and then the urethral clamp was removed and the mice voided spontaneously. Mice in group 3 treated with the same volume of PBS as the virus treated animals. On day 36 after tumor cell injection, all surviving mice were euthanized by CO2 exposure and tumor volume and weight were measured in each mouse. Tumor volume was calculated by the following formula: tumor volume (mm3) = [length (mm)]×[width (mm)]2×0.52.
Measurement of CTL activity and ELISPOT assay
Splenocytes were isolated from sacrificed mice in each treatment group. Effector cells were obtained by coculturing splenocytes (3×106) with irradiated (4,000 rad) MBT-2 cells (1×106), supplemented with recombinant human interleukin-2 (20 unit/ml). Four hour chromium release assay was performed to measure the ability of the effector cells to lyse target cells. The 51Cr labeled tumor cell suspension was put into 96 well, U-bottomed plates. Purified splenocytes were added as effector cells to the 96-well plate at various effector-to-target cell ratios (80:1, 40:1, 20:1, and 10:1) in a final volume of 200 μl/well and incubated at 37°C for 4 hours. RPMI medium used as a negative control. Supernatants were harvested and 51Cr release was measured with a scintillation counter. Spontaneous release was measured in wells containing target cells alone. Triton X-100 was used to lyse the target cells maximally. The percentage of specific lysis was calculated by the following formula: percent of specific lysis = [(experimental release 51Cr – spontaneous release 51Cr)/(maximum release 51Cr – spontaneous release 51Cr)]×100.
For ELISPOT assay, 96-well filter plates were precoated with anti-INF- γ monoclonal antibody (BD Bioscience, San Diego, CA) and incubated overnight at 4°C. Purified splenocytes were then dispensed into wells and stimulated with irradiated MBT-2 cells. After incubation 37°C for 24 hours followed by washing, biotinylated anti-INF- γ antibody (BD Bioscience) was added to each well and the plates were incubated for 1 hour at 37°C. A streptavidin-alkaline phosphatase conjugate was added to the wells. After incubation for 1 hour, the chromogenic alkaline phosphatase substrate was added and washing with tap water was done. After drying, the spots were counted.
Statistical analysis
Values are reported as means±standard deviation. The statistical analysis was done with Student’s t-test for the comparison of oncolytic activity between FusOn-H2 and Baco-1. One-way ANOVA test was used to evaluate the difference of tumor reduction among the three groups in vivo study. Statistical significance was defined as p < 0.05.
RESULTS
Phenotypic characterization of FusOn-H2 in bladder cancer cells
FusOn-H2 virus has been shown to induce syncytia formation in tumor cells of various tissue origins, including breast, ovary, prostate, and pancreas [11, 24, 26, 27]. To determine whether it can induce cell membrane fusion in human bladder cancer cells, we infected 5637, and MBT-2 cells, respectively in a six-well plate with either Baco-1 or FusOn-H2 virus, or left the cells uninfected as a control. Infection with Baco-1 induced a typical cytopathic effect, characterized by cell rounding and swelling. Syncytia were clearly visible in FusOn-H2 infected cells, but not in Baco-1 infected cells (Fig. 1). Hence, FusOn-H2 retains the fusogenic phenotype in bladder cancer cells.
Efficiency of FusOn-H2-induced killing of bladder cancer cells in vitro
To assess the cytolytic activity of oncolytic HSVs, we infected 5637 cells seeded in 24-well plates with Baco-1 or with FusOn-H2 at a dose of either 0.01 or 0.1 pfu/cell. The cells were harvested at 24, 48, or 72 hours after infection, and cell viability was determined by trypan blue staining. At a viral dose of 0.1 pfu/cell, FusOn-H2 killed significantly more tumor cells than Baco-1 did after 24 hours infection (p < 0.05). By 72 hours after infection, FusOn-H2 almost completely eradicated the tumor cells exposed to virus doses of 0.01 or 0.1 pfu/cell. Overall, anti-tumor activity of FusOn-H2 was significantly greater than that of Baco-1 (Fig. 2A and B).
We also infected MBT-2 cells seeded in 24-well plates with Baco-1 or with FusOn-H2 at a dose of either 1 or 10 pfu/cell to determine the oncolytic activity of FusOn-H2 against murine bladder cancer cell line. After infection with a dose of 10 pfu/cell, FusOn-H2 killed almost all tumor cells by 72 hours. At 10 pfu/cell, Baco-1 killed more than half of tumor cells after 24 hours infection, with similar oncolytic activity at 72 hours. The oncolytic effect of FusOn-H2 was superior to that of Baco-1(Fig. 2C and D).
Evaluation of anti-tumor effect of oncolytic HSV against orthotopic bladder cancer
We established an orthotopic bladder cancer model to evaluate the anti-tumor activity of oncolytic HSV against bladder cancer. Freshly harvested MBT-2 cells (1×106) were injected into the bladder wall of C3H/He mice. Two weeks later tumor cell implantation, mice were randomly divided into three groups, and were instilled with 1×107 pfu of either FusOn-H2 or Baco-1 or PBS (as a control) into the bladder through the urethra. A repeated intravesical instillation with the same dose of virus was performed one week later. Three weeks after the first treatment, all mice were euthanized and examined for orthotopic tumors. In the PBS control group, all specimens revealed large tumors indicating successful establishment of the orthotopic bladder cancer model. The mean tumor size in the PBS control group was 298.4±74.0 mm2 and much larger than those in oncolytic viral treatment groups (Fig. 3A and B). Intravesical instillation of FusOn-H2 had a significant therapeutic effect on the orthotopic bladder cancer. The mean volume of the remaining tumors was 10.2±3.9 mm2. Although Baco-1 instillation showed significant oncolytic effect compared to controls, the post-treatment mean tumor size was 27.7±14.5mm2 and larger than those in the FusOn-H2 instillation group (Fig. 3C) though the difference was not significant (p > 0.05). The mean weight of the tumor including BLADder were 538.7±222.1, 43.9±17.5, and 28.6±4.6 mg in control, Baco-1 infected, and FusOn-H2 infected group, respectively (Fig. 3D). Instillation of FusOn-H2 or Baco-1 led to complete eradication of orthotopic tumors in majority of the tumor bearing animals. All animals treated with viral instillation survived by the time of euthanasia, suggesting that FusOn-H2 or Baco-1 could be safely administered through the transurethral route.
Tumor-specific cytotoxic T lymphocyte (CTL) response induced by tumor destruction
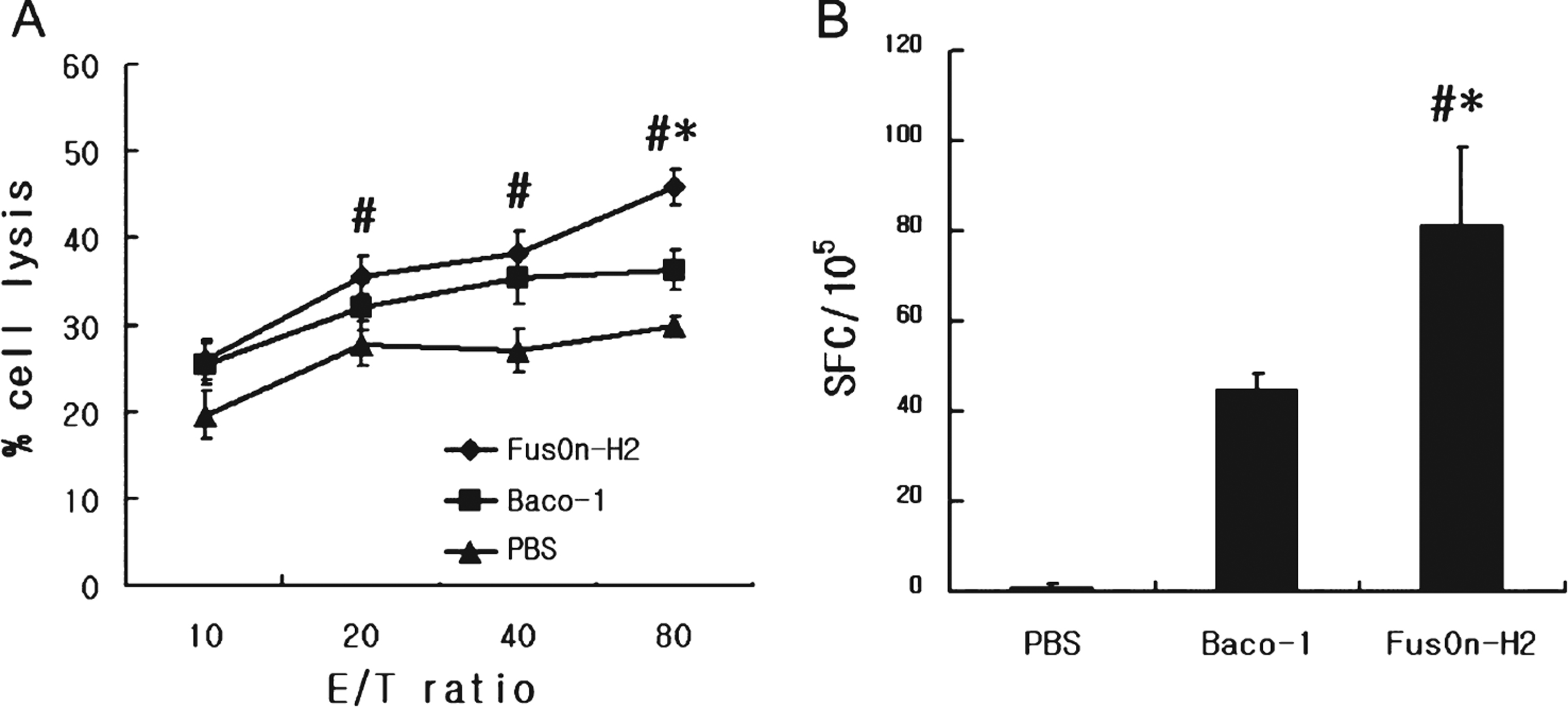
To evaluate the anti-tumor immunity of tumor destruction by oncolytic HSV, we quantified the activities of tumor specific CTLs collected from the spleens of mice used in the experiment. Both Baco-1 and FusOn-H2 induced CTL activity that could lyse MBT-2 cells. The CTL activity induced by FusOn-H2 was significantly higher than that induced by Baco-1 at the highest effector-to-target ratio. This suggests that FusOn-H2 induces more effective anti-tumor immunity than Baco-1(Fig. 4A).
The frequency of tumor-specific CTLs was measured in FusOn-H2 treated or Baco-1 treated group. The spot-forming cells per 105 splenocytes from FusOn-H2 treated group were greater than those from Baco-1 treated group (Fig. 4B).
DISCUSSION
The majority of urothelial bladder cancer is non-muscle invasive at initial diagnosis. Intravesical immunotherapy with BCG is the standard treatment for patients with high risk disease and in some cases of intermediate risk, particularly those that fail intravesical chemotherapy. However, BCG lacks long lasting, tumor-specific protective immunity [28, 29]. Despite repeat intravesical BCG instillation, up to 50% of patients experience recurrence or progression [3], and often require radical cystectomy to prevent progression and metastasis [30]. Novel treatment options such as oncolytic viral therapy are worth exploring. The immunocompetent orthotopic bladder cancer model we utilized in this study is proper to evaluate the pre-clinical therapeutic efficacy of oncolytic HSV. Intravesical instillation of adequate titers of viral suspension is deliverable by transurethral catheterization in this model.
Previous cancer gene therapies utilized a replication defective form of virus to deliver therapeutic genes. Several viruses including vaccinia, reovirus, and adenovirus have been previously examined as potential bladder cancer therapies [31–33]. These studies demonstrated potent activity against bladder cancer, but showed viral transduction in both normal and tumor cells, and low sensitivity to viral infection. Oncolytic adenoviruses showed a potent antitumor effect in vitro and in vivo. However, adenoviral uptake is mediated in part by the Coxsackie-adenoviral receptor (CAR) which is often absent on bladder cancer cells, diminishing the transduction efficiency of adenoviral mediated gene therapy with reduced cancer cell killing [25]. Therefore oncolytic HSV-2 was constructed and we investigated the anti-cancer efficacy of this virus.
Oncolytic viruses replicate conditionally in tumor cells and lyse them resulting in direct cell killing effects. The oncolytic HSVs were originally derived from HSV-1 for the treatment of brain tumors. Several oncolytic HSV-1 s including G207, NV1020, HF10 were investigated in murine bladder cancer models [12–14]. The oncolytic HSV-2 virus, FusOn-H2, was constructed by deletion of the N-terminal domain of the ICP 10 gene of HSV-2. This domain has been reported to activate the Ras mitogenic pathway which is required for efficient virus replication. So, deletion of this domain impairs virus growth in normal cells. The virus can replicate in tumor cells, because the Ras signaling pathway is aberrantly activated in most tumors. Bladder cancer is associated with expression of the Ras oncogene gene family including the P21 Ras oncogene [34, 35]. Therefore, bladder cancer is an attractive target for treatment with FusOn-H2. Oncolytic HSV-2 also shows more potent antitumor efficacy than oncolytic HSV-1, through fusogenic activity [36, 37], induction of apoptosis [38], and anti-tumor immune response [39].
We examined the phenotypic characterization following infection of human and murine bladder cancer cells in vitro and FusOn-H2 induced cell membrane fusion forming syncytia. Recent studies suggest that fusogenic activity of an oncolytic virus can significantly enhance its anti-tumor effect [36, 37]. Indeed, our in vitro data show that oncolytic activity of FusOn-H2 was significantly greater than that of the HSV-1 derived virus Baco-1. The fusogenic property of FusOn-H2 seems to potentiate its anti-tumor activity against bladder cancer cells.
Infection with oncolytic HSVs, especially FusOn-H2, in both human and murine bladder cancer cell line led to marked killing of tumor cells. In our preliminary study we investigated the anticancer effects of the FusOn-H2 against four human bladder cancer cell lines: T24, 5637, TCC-SUP, and RT4 cell lines. In all cell lines, infection with FusOn-H2 killed the tumor cells more and more as the time goes by. By 72 hours, FusOn-H2 almost completely eradicated the tumor cells exposed to virus doses of 0.1pfu/cell.
It is reported that the N-terminal domain of the ICP 10 gene of HSV-2 may block apoptosis in the host cell during infection [38]. Therefore, deletion of this domain from the HSV-2 may lead tumor cells into an apoptotic state after viral infection. This represents an additional antitumor mechanism associated with FusOn-H2, which may have contributed to the overall enhanced antitumor activity of this virotherapy against bladder cancer.
Tumor destruction by an oncolytic virus releases large amount of tumor antigens. These antigens can be presented to T cells via cross priming by antigen presenting cells. Exosomes released from tumor cells contain abundant native tumor antigen and molecules promoting antigen presentation. It was suggested that syncytia formation induced by viral fusogenic glycoproteins can induce the release of large amount of vesicles (exosomes), which can potentiate the anti-tumor immune response [39]. It was reported that anti-vector immune responses can reduce the transduction efficiency of subsequent administration of the same oncolytic HSV-1 vectors. Therefore, the therapeutic effect of this virus may be reduced in immune-competent animals [36]. The antitumor immune response of FusOn-H2 was sufficient however, to override the loss of oncolytic efficiency due to the effects of antiviral immunity [40]. Dr. Zhang’s group also described the inhibition of tumor growth after adoptive transfer of splenocytes from mice treated with FusOn-H2 virus. In our study, destruction of murine bladder cancer cells by the fusogenic FusOn-H2 induced a potent anti-tumor immune response against bladder cancer compared to Baco-1. Moreover, the inflammatory environment created by oncolytic viral infection may provide stimulatory signals to enhance the dendritic cell maturation [41], as well as promote the infiltration of tumor-specific immune cells to the tumor site.
We observed a marked reduction in tumor volume and weight after oncolytic viral treatment in vivo in an orthotopic bladder cancer model. The majority of tumors were eradicated in the FusOn-H2 treated group. The potent anti-tumor activity of FusOn-H2 is likely derived from the combined effect of direct cytolytic activity of viral replication, fusogenic ability, induction of apoptosis, and elicitation of the tumor specific cellular immune response.
There are several potential limitations of our study. The objective endpoint of our study was change in tumor volume and weight but we did not use direct tumor measurements which ma be more accurate. Quantitative measurement may also be done by labeling tumor cells with luciferase. We did not perform pathologic examination of the tumors or residual tumor bed which my have provided additional information regarding cytotoxicity and immune infiltrates.
In conclusion, our studies provide proof of concept in a pre-clinical model that intravesical instillation of an HSV-2-derived oncolytic virus has potent anti-tumor activity against orthotopic tumor derived from a murine and human bladder cancer cell line.. Additional pre-clinical studies are required in order to determine the specificity for cancer cells harboring ras pathway alterations. These data may support conducting a Phase I trial translating this novel oncolytic viral treatment in order to determine safety of FusOn-H2 intravesical therapy.
CONFLICT OF INTEREST
Xiaoliu Zhang is one of the inventors of FusOn-H2 that has been licensed to Onvi Biopharm, Inc.
ROLE OF THE FUNDING SOURCE
This work was supported in part by the Owens Foundation and the Partnership for Bladder Cancer Research, Scott Department of Urology, Baylor College of Medicine.
ACKNOWLEDGMENTS
We thank Xinping Fu (Baylor College of Medicine, Houston, TX, USA) for kind advice and support in experiment.
REFERENCES
1 | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T(2008) Cancer statistics, CA Cancer J Clin58: 7196 |
2 | Kim JC, Steinberg GD(2001) The limits of bacillus Calmette-Guerin for carcinoma in situ of the bladderJ Urol165: 745756 |
3 | Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ(1999) 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinomaSwedish-Norwegian Bladder Cancer Study GrouJ Urol161: 11241127 |
4 | Parato KA, Senger D, Forsyth PA, Bell JC(2005) Recent progress in the battle between oncolytic viruses and tumoursNat Rev Cancer5: 965976 |
5 | Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL(1995) Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomasNat Med1: 938943 |
6 | Carroll NM, Chiocca EA, Takahashi K, Tanabe KK(1996) Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virusAnn Surg; discussion224: 323329discussion 9-30 |
7 | Toda M, Rabkin SD, Martuza RL(1998) Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1Hum Gene Ther9: 21772185 |
8 | Walker JR, McGeagh KG, Sundaresan P, Jorgensen TJ, Rabkin SD, Martuza RL(1999) Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207Hum Gene Ther10: 22372243 |
9 | Toyoizumi T, Mick R, Abbas AE, Kang EH, Kaiser LR, Molnar-Kimber KL(1999) Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-in human non-small cell lung cancerHum Gene Ther10: 30133029 |
10 | Pawlik TM, Nakamura H, Yoon SS, Mullen JT, Chandrasekhar S, Chiocca EA(2000) Oncolysis of diffuse hepatocellular carcinoma by intravascular administration of a replication-competent, genetically engineered herpesvirusCancer Res60: 27902795 |
11 | Nakamori M, Fu X, Meng F, Jin A, Tao L, Bast RCJr(2003) Effective therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane fusion mechanismsClin Cancer Res9: 27272733 |
12 | Oyama M, Ohigashi T, Hoshi M, Nakashima J, Tachibana M, Murai M(2000) Intravesical and intravenous therapy of human bladder cancer by the herpes vector G207Hum Gene Ther11: 16831693 |
13 | Kohno S, Luo C, Goshima F, Nishiyama Y, Sata T, Ono Y(2005) Herpes simplex virus type 1 mutant HF10 oncolytic viral therapy for bladder cancerUrology66: 11161121 |
14 | Cozzi PJ, Malhotra S, McAuliffe P, Kooby DA, Federoff HJ, Huryk B(2001) Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and Nvis effective in the treatment of bladder cancer in an orthotopic syngeneic modelFASEB J15: 13061308 |
15 | Mineta T, Rabkin SD, Martuza RL(1994) Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutantCancer Res54: 39633966 |
16 | Randazzo BP, Kesari S, Gesser RM, Alsop D, Ford JC, Brown SM(1995) Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutantVirology211: 94101 |
17 | Andreansky SS, He B, Gillespie GY, Soroceanu L, Markert J, Chou J(1996) The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumorsProc Natl Acad Sci U S A93: 1131311318 |
18 | Pennisi E(1996) Will a twist of viral fate lead to a new cancer treatment? Science274: 342343 |
19 | Martuza RL(1997) Act locally, think globallyNat Med3: 1323 |
20 | Alemany R, Gomez-Manzano C, Balague C, Yung WK, Curiel DT, Kyritsis AP(1999) Gene therapy for gliomas: Molecular targets, adenoviral vectors, and oncolytic adenovirusesExp Cell Res252: 112 |
21 | Chung TD, Wymer JP, Smith CC, Kulka M, Aurelian L(1989) Protein kinase activity associated with the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10)J Virol63: 33893398 |
22 | Smith CC, Nelson J, Aurelian L, Gober M, Goswami BB(2000) Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growthJ Virol74: 1041710429 |
23 | Farassati F, Yang AD, Lee PW(2001) Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1Nat Cell Biol3: 745750 |
24 | Fu X, Tao L, Cai R, Prigge J, Zhang X(2006) A mutant type 2 herpes simplex virus deleted for the protein kinase domain of the ICP10 gene is a potent oncolytic virusMol Ther13: 882890 |
25 | Wang H, Satoh M, Abe H, Sunamura M, Moriya T, Ishidoya S(2006) Oncolytic viral therapy by bladder instillation using an E1A, E1B double-restricted adenovirus in an orthotopic bladder cancer modelUrology68: 674681 |
26 | Nakamori M, Fu X, Pettaway CA, Zhang X(2004) Potent antitumor activity after systemic delivery of a doubly fusogenic oncolytic herpes simplex virus against metastatic prostate cancerProstate60: 5360 |
27 | Fu X, Tao L, Li M, Fisher WE, Zhang X(2006) Effective treatment of pancreatic cancer xenografts with a conditionally replicating virus derived from type 2 herpes simplex virusClin Cancer Res12: 31523157 |
28 | Ratliff TL(2000) Role of animal models in understanding intravesical therapy with bacille Calmette-GuerinClin Infect Dis 31 SupplS3: 106108 |
29 | Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ(1993) T-cell subsets required for intravesical BCG immunotherapy for bladder cancerJ Urol150: 10181023 |
30 | Grossman HB, O’Donnell MA, Cookson MS, Greenberg RE, Keane TE(2008) Bacillus calmette-guerin failures and beyond: Contemporary management of non-muscle-invasive bladder cancerRev Urol10: 281289 |
31 | Gomella LG, Mastrangelo MJ, McCue PA, Maguire HJ, Mulholland SG, Lattime EC(2001) Phase i study of intravesical vaccinia virus as a vector for gene therapy of bladder cancerJ Urol166: 12911295 |
32 | Hanel EG, Xiao Z, Wong KK, Lee PW, Britten RA, Moore RB(2004) A novel intravesical therapy for superficial bladder cancer in an orthotopic model: Oncolytic reovirus therapyJ Urol172: 20182022 |
33 | Ramesh N, Ge Y, Ennist DL, Zhu M, Mina M, Ganesh S(2006) CGa conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancerClin Cancer Res12: 305313 |
34 | Oxford G, Theodorescu D(2003) The role of Ras superfamily proteins in bladder cancer progressionJ Urol170: 19871993 |
35 | Czerniak B, Cohen GL, Etkind P, Deitch D, Simmons H, Herz F(1992) Concurrent mutations of coding and regulatory sequences of the Ha-ras gene in urinary bladder carcinomasHum Pathol23: 11991204 |
36 | Fu X, Zhang X(2002) Potent systemic antitumor activity from an oncolytic herpes simplex virus of syncytial phenotypeCancer Res62: 23062312 |
37 | Ebert O, Shinozaki K, Kournioti C, Park MS, Garcia-Sastre A, Woo SL(2004) Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancerCancer Res64: 32653270 |
38 | Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L(2002) The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathwayJ Virol76: 14351449 |
39 | Bateman AR, Harrington KJ, Kottke T, Ahmed A, Melcher AA, Gough MJ(2002) Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cellsCancer Res62: 65666578 |
40 | Li H, Dutuor A, Fu X, Zhang X(2007) Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor modelJ Gene Med9: 161169 |
41 | Mellman I, Steinman RM(2001) Dendritic cells: Specialized and regulated antigen processing machinesCell106: 255258 |
Figures and Tables
Fig.1
Phenotypic characterization of oncolytic herpes simplex virus type 2 (FusOn-H2). (A) 5637 cells were infected with the indicated viruses at 0.1 pfu/cell or left uninfected as a control. (B) MBT-2 cells were infected with the indicated viruses at 10.0 pfu/cell or left uninfected as a control. Micrographs were taken 24 hour after infection. Syncytia were observed with FusOn-H2 infection but not Baco-1 or uninfected controls (original magnification×200).
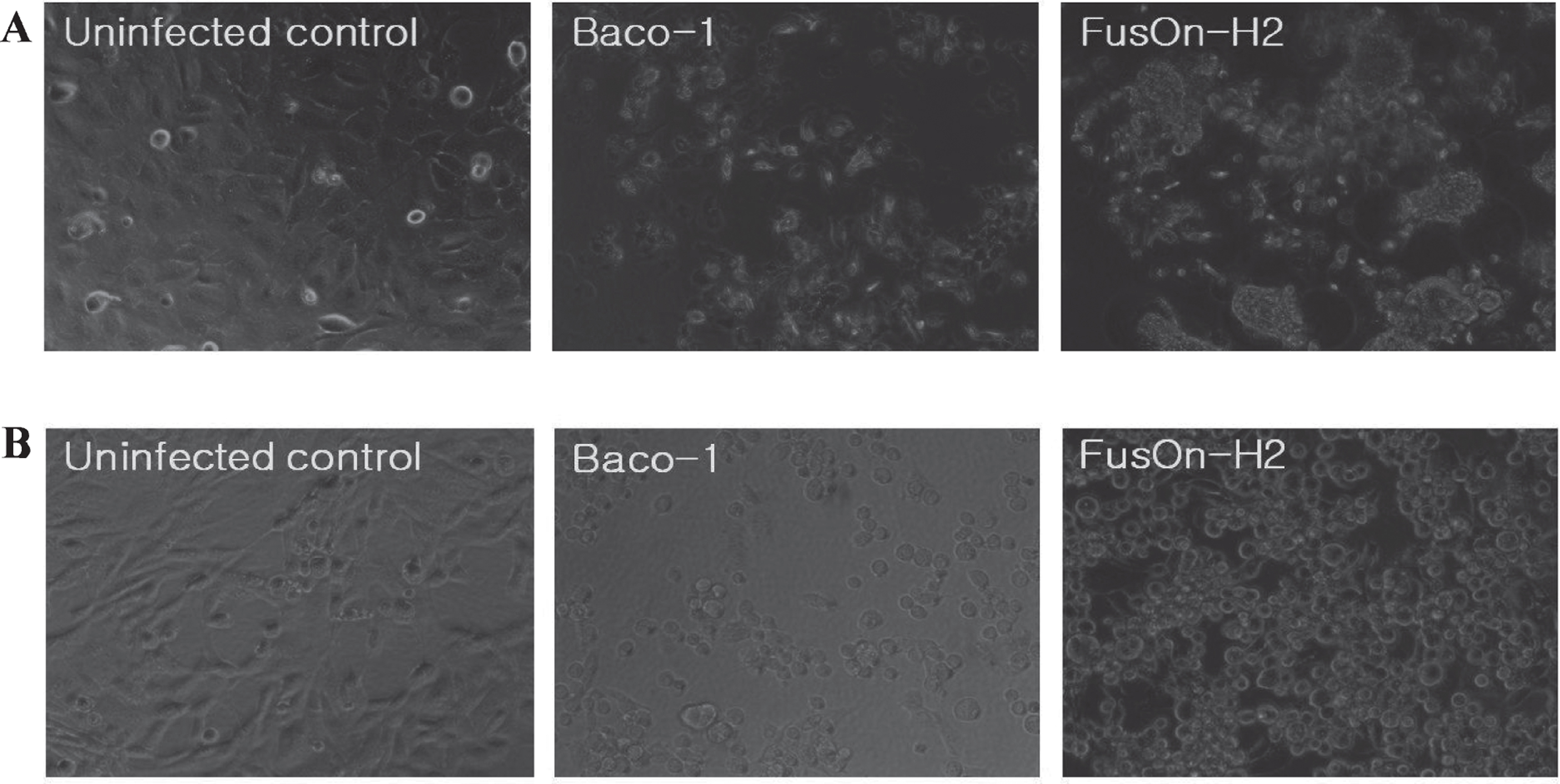
Fig.2
Oncolytic activity of FusOn-H2 against 5637 and MBT-2 cells in vitro. The 5637 cells were seeded in 24-well plates and infected with Baco-1 or with FusOn-H2 at a dose of either 0.01 pfu/cell (A) or 0.1 pfu/cell (B). The MBT-2 cells were seeded in 24-well plates and infected with Baco-1 or with FusOn-H2 at a dose of either 1.0 pfu/cell (C) or 10.0 pfu/cell (D). The cells were harvested at 24, 48, or 72 hours after infection, and cell viability was determined by trypan blue staining. Percent cell viability = [(number of viable cells in the infected wells)/ (number of viable cells in the uninfected wells)]×100. The bars represent mean±standard deviation. * p < 0.05.
![Oncolytic activity of FusOn-H2 against 5637 and MBT-2 cells in vitro. The 5637 cells were seeded in 24-well plates and infected with Baco-1 or with FusOn-H2 at a dose of either 0.01 pfu/cell (A) or 0.1 pfu/cell (B). The MBT-2 cells were seeded in 24-well plates and infected with Baco-1 or with FusOn-H2 at a dose of either 1.0 pfu/cell (C) or 10.0 pfu/cell (D). The cells were harvested at 24, 48, or 72 hours after infection, and cell viability was determined by trypan blue staining. Percent cell viability = [(number of viable cells in the infected wells)/ (number of viable cells in the uninfected wells)]×100. The bars represent mean±standard deviation. *
p < 0.05.](https://content.iospress.com:443/media/blc/2015/1-1/blc-1-1-blc150013/blc-1-1-blc150013-g002.jpg)
Fig.3
In vivo therapeutic effect of FusOn-H2 in the orthotopic bladder cancer model. After laparotomy incision, MBT-2 cells (1×106) were injected into the bladder wall of syngeneic C3H/He female mice. Two weeks following tumor cell implantation, mice were randomly divided into three groups, and were instilled with 1×107 pfu of either FusOn-H2 or Baco-1 or a similar volume of PBS (as a control) into the bladder via a transurethral catheter. A repeated intravesical instillation with same dose of virus was performed one week later. Three weeks after the first treatment, all mice were euthanized and examined for orthotopic tumors. (A, B) The photographs demonstrate the gross appearance of tumors with the arrows indicating tumors. (C) Comparison of tumor volume after oncolytic viral therapy. The bars represent mean±standard deviation. *Baco-1 versus PBS control (p < 0.05) and # FusOn-H2 versus PBS control (p < 0.01). (D) Comparison of tumor weight after oncolytic viral therapy. The bars represent mean±standard deviation. *Baco-1 versus PBS control (p < 0.05) and # FusOn-H2 versus PBS control(p < 0.05).
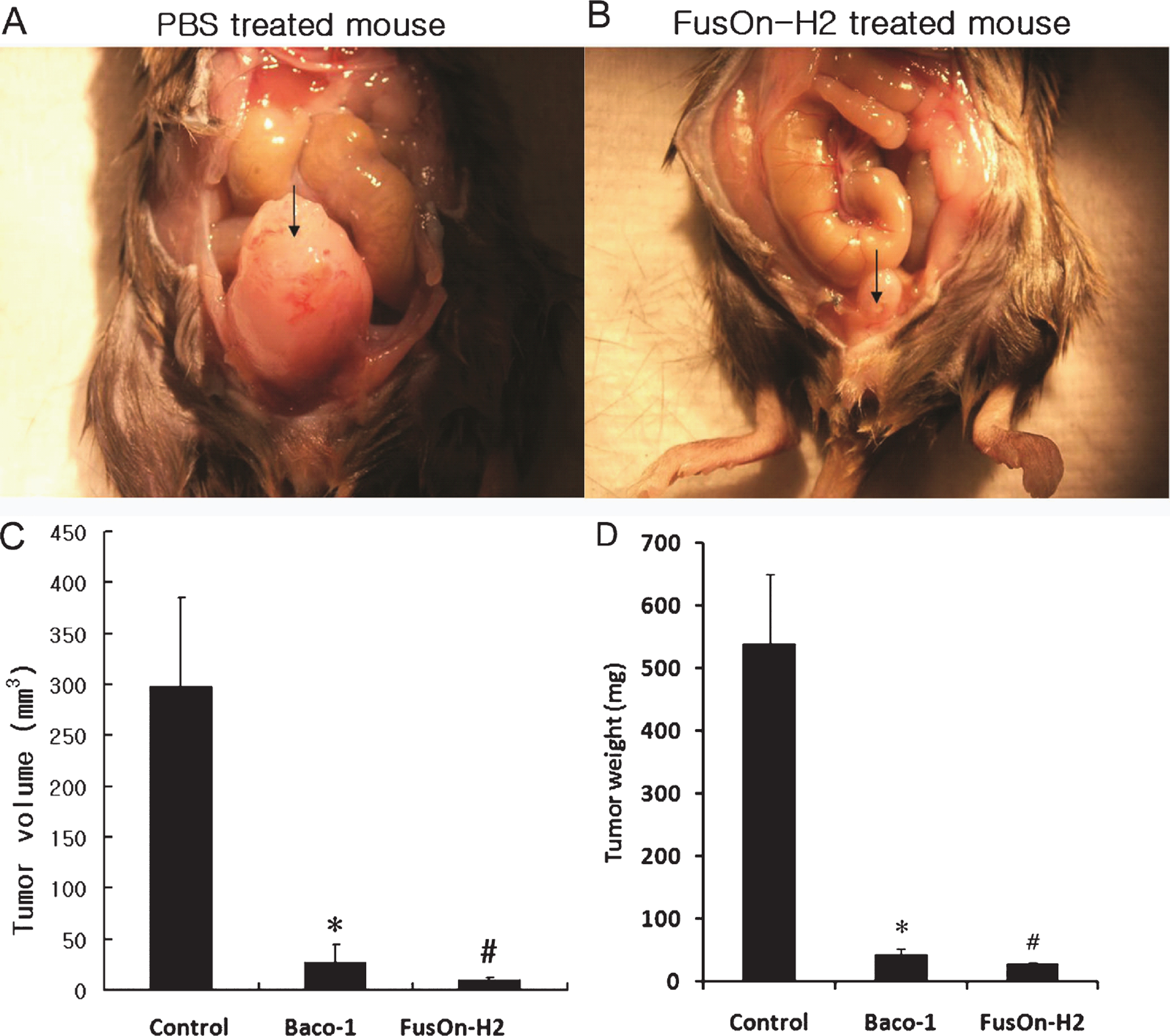
Fig.4
Tumor-specific cytotoxic T lymphocyte (CTL) activity after oncolytic viral therapy. (A) The cytotoxic activity of effector cells prepared from spleens was measured against MBT-2 cells. (B) Effector cells from the same preparation used in (A) were evaluated with the ELISPOT assay to determine the frequency of tumor-specific CTLs. The bars represent mean±standard deviation. *FusOn-H2 versus Baco-1 (p < 0.05) and # FusOn-H2 versus PBS (p < 0.05). E/T ratio, effector to target cell ratio; SFC, spot forming cells.