Patient experience, functional and quality of life outcomes in patients receiving surgical and non-surgical treatment for residual, recurrent, or new oropharyngeal cancer in a previously irradiated field: A systematic review
Abstract
BACKGROUND:
National guidance recommends counselling on functional (swallowing/ speech/ voice) and quality of life outcomes (QoL) for patients with recurrent, residual or new primary head and neck cancer (HNC) in a previously irradiated field (ReRuNeR).
AIM:
To investigate the measurement and reporting of function and QoL outcomes and patient/carer experience for ReRuNeR, focussing exclusively on oropharyngeal cancer (OPC).
METHODS:
Systematic narrative review of quantitative/qualitative studies.
RESULTS:
Seventeen articles reporting functional/ QoL outcomes following surgery were included. Gastrostomy dependence was the primary method of reporting function. Previously validated outcome measures (OMs) were used for reporting swallowing in four, speech in one and QoL in two trials. Qualitative data or non-surgical studies reporting function/QoL outcomes specific to ReRuNeR OPC were not identified. Methodological issues and heterogeneity noted across studies including absent baseline data, varying/ undefined timepoints for outcome measurement and the use of unvalidated tools. Patient-reported swallowing outcomes were mixed. Whereas instrumental assessment of swallowing showed a deterioration in safety/ efficiency. A post-surgical decline in speech was noted. Stable overall HR-QoL was reported but an increase in specific symptoms including speech, saliva and swallowing were noted. Pooled long term gastrostomy feeding rate was 23.42% (95% CI 10.2 to 36.6) (n = 108).
CONCLUSION:
A core dataset of patient and clinician-rated OMs is required to provide a comprehensive understanding of functional and QoL complications with ReRuNeR OPC. In combination with patient/carer experience data, these data can be used to inform pre-treatment counselling, rehabilitation and future clinical trial design.
1Introduction
Head and neck cancer (HNC) is the sixth most common cancer in the world and is increasing in incidence (Hardman et al., 2020). Squamous cell carcinomas (SCC) account for the majority of these tumours, with an increasing number of oropharyngeal cancers associated with the human papilloma virus (HPV). Treatments for primary disease include (chemo)radiation and/or surgery, with many patients undergoing multi-modality treatment. The impact of such treatments on function, including swallowing, speech, voice and overall quality of life (QoL), in the primary disease setting have been well documented (Patterson, McColl, Carding, & Wilson, 2018; Roe, Drinnan, Carding, Harrington, & Nutting, 2014).
Despite ongoing advancements in the treatment of primary oropharyngeal disease, rates of residual (diagnosed within 12 months of previous treatment), recurrent (diagnosed between 12 months and 5 years of previous treatment) and second primary disease (diagnosed > 5 years following previous treatment) remain high at approximately 20–30% (Hardman et al., 2020; Leeman et al., 2017; Mandapathil et al., 2014; Warnakulasuriya, 2009).
Residual, recurrent or new primary (ReRuNeR) oropharyngeal cancer (OPC) present some of the greatest challenges in HNC practice (Brady, Hardman, Paleri, Harrington, & Roe, 2020). Not only do these patients often present with functional issues such as difficulties with swallowing, speech and/or voice due to long-term/ late-onset effects from their prior treatment (Patterson et al., 2018), but the newly diagnosed disease and/ or the proposed treatment plan may further cause or compound such difficulties. In addition to disease and treatment-related issues, patients who have undergone treatments for HNC are also known to have a high level of psychological stressors, which can further add to symptom burden and overall QoL (Ringash et al., 2018).
The UK standard of care for ReRuNeR OPC is open surgery, an intervention associated with high morbidity (Mehanna, Kong, & Ahmed, 2016). Transoral robotic surgery (TORS) is now being considered as a minimally invasive option with encouraging oncological control (Paleri, Hardman, Brady, George, & Kerawala, 2020). There may be opportunities for re-irradiation; however, this has the potential for severe treatment toxicity. Non-curative options include palliative systemic treatments or best supportive care. More recently, immunotherapy is being utilised. In the UK, pembrolizumab, with or without platinum-based chemotherapy, is now used for untreatable metastatic or unresectable recurrent head and neck squamous cell carcinoma in patients whose tumours express a specific biomarker (PD-L1 with a combined positive score of 1 or more) (NICE, 2020).
Historically, patients with ReRuNeR OPC were considered to have a very poor prognosis with the majority of patients offered palliative treatments or best supportive care. However, over the past 20 years, there has been a paradigm shift in the treatment of recurrent HNC, with evidence to support increased survival rates for patients treated curatively using surgery, in particular for patients with ReRuNeR OPC (Jayaram et al., 2016). For those patients who are not suitable for surgical resection, the focus in recent literature has been on enhanced life-prolonging treatments such as the use of immunotherapy.
National guidelines suggest that patients diagnosed with ReRuNeR disease should be fully counselled on the likely functional and QoL impact of the available treatments (Mehanna et al., 2016).However, although there has been an apparent paradigm shift in studies investigating enhanced survival for patients with ReRuNeR OPC, there appears to be a lack of literature examining functional, QoL and patient experience outcomes for all treatment modalities in ReRuNeR OPC.
The aim of this systematic review is to identify if functional and QoL outcomes and patient experience data are reported, how they are measured and what are the functional and QoL outcomes and patient experience data reported for patients with ReRuNeR OPC. Working definitions for functional outcomes, QoL and patient experience as used in this review will be provided under methods.
2Methods
This systematic review used a population, intervention, comparison, outcome (PICO) framework (Schardt, Adams, Owens, Keitz, & Fontelo, 2007). This framework was divided as follows to report on the following: for patients with ReRuNeR OPC (Population), undergoing curative or non-curative treatment (Intervention), are functional, QoL and patient experience measures reported, and if so, how are they reported and what are the findings (Outcomes). A comparator was not a requirement for this review.
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were used to carry out the systematic review (Moher, Liberati, Tetzlaff, & Altman, 2010). The protocol was registered on PROSPERO an international prospective register of systematic reviews (CRD42021236540).
The main objectives of the systematic review were:
1. To report on the functional and QoL outcomes and patient experience data for patients with ReRuNeR OPC
2. To describe how functional, QoL outcomes and patient experience data are measured for patients with ReRuNeR OPC
2.1Eligibility criteria
2.1.1Study characteristics
All observational studies, including qualitative and experimental designs, both prospective and retrospective, were included. Only peer- reviewed articles published in English were included. No limitation was placed on year of publication. Abstracts and conference proceedings were excluded. Studies were excluded if cohorts of primary and ReRuNeR OPC were mixed unless a subgroup analysis of ReRuNeR OPC outcomes was available. Likewise, studies were excluded if they investigated head and neck disease recurrence rather than ReRuNeR OPC specifically, unless a subgroup analysis of findings for ReRuNeR OPC was available.
2.2Inclusion criteria
1. Adults aged 18 years or older with a diagnosis of recurrent, residual or new primary oropharyngeal disease in a previously radiated area
2. Participants undergoing treatment for ReRuNeR OPC including surgery (open or transoral robotic), re-irradiation, chemotherapy, and/or immunotherapy
3. Functional and/or QoL outcomes and/or patient experience data reported (validated or unvalidated tools/ methods including long-term feeding tube and tracheostomy usage/ qualitative findings)
4. English language
5. Full text available
2.3Primary and secondary outcome measures
The primary outcome measures included the reporting of either functional or QoL or patient experience outcomes. For the purposes of this systematic review functional outcome data includes any data presented in relation to swallowing and communication (including speech and voice) outcomes using previously validated or unvalidated measures. QoL outcomes include data measured using validated health related QoL patient-reported outcome measures. Patient and carer experience data includes any data that is collected with the intention to provide information about patients’ experiences with ReRuNeR OPC ‘including the impact of the disease or condition or related therapy or clinical investigation, and patient preferences with respect to treatment of the disease or condition’(FDA, 2016).
2.4Primary outcome measures
1. Functional outcomes including swallowing, speech and HR-QoL outcomes
2. Patient experience outcomes
2.5Secondary outcome measures
3. Gastrostomy dependence at > 6 months post treatment
4. Tracheostomy dependence at > 6 months post treatment
2.5Identification of studies
A systematic computer-based search was performed using the following electronic health databases: MEDLINE, CINAHL, and Embase. Additional searches were carried out on Web of Science and the meta-registries of Trials Databases (ClinicalTrials.gov and ISRCTN). The WHO International Clinical Trials Registry Platform (ICTRP) and the Australian New Zealand Clinical Trials Register (ANZCTR) were also included in the search.
Additional citation searches were completed for selected articles.
2.6Search strategy
Search terms included “oropharyngeal cancer” AND “recurrent” OR “residual disease” OR “second primary’ OR “radiation induced” AND “swallow” OR “dysphagia” OR “speech” OR “voice” OR “quality of life” OR “patient experience” OR “functional outcomes” AND “salvage surgery” OR “salvage therapy” OR “re-irradiation”. All search terms were exploded when possible and any subject headings relevant to each database were included.
2.7Selection of studies
The initial database searches were combined and 1,052 articles were imported into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). Following removal of 256 duplicates, the remaining 796 article titles and abstracts were screened for inclusion and exclusion criteria by authors GB, MW and PL.
These criteria were applied to the titles and abstracts, and articles that did not meet the inclusion criteria were excluded. Articles were included for full-text review where it was not possible to use the abstract to fully assess eligibility. The full texts of 47 articles were retrieved and assessed using the inclusion criteria. Senior author MW reviewed 10% of these articles also to ensure agreement with assessment of eligibility. Any ambiguities or discrepancies were resolved by discussion with author JR.
2.8Data extraction
A data extraction table was devised using Microsoft Excel. Data inputted included study location, patient characteristics, treatment modality, primary and secondary outcomes used, timepoints data collection, swallowing and QoL results, and feeding tube and tracheostomy use.
2.9Risk of Bias
A study-level risk of bias assessment was performed for all included studies by authors GB and MW. All included studies were observational in design so the Methodological Index for Non-Randomised Studies (MINORS) tool was utilised (Kim et al., 2013).
2.10Data synthesis
Quantitative data: It was anticipated from similar reviews in the primary disease setting (Roe et al., 2010) that the number of studies identified may be low and the heterogeneity between studies found high and so statistical analysis for comparing outcomes across modalities (meta-analysis) was not planned.
2.11Qualitative data
The intended method to synthesise any qualitative data findings was meta ethnography, a method of qualitative data synthesis which is gaining increasing focus on health care research (Atkins et al., 2008). This method aims to complete a secondary analysis to compare and contrast concepts across qualitative studies, to gain a deeper insight into the topic (Sattar, Lawton, Panagioti, & Johnson, 2021).
3Results
3.1Study selection
The literature search findings are summarised in Fig. 1 PRISMA flow diagram. Following the removal of duplicate records, 796 studies were identified and 47 included for full text review with 17 studies included for data extraction. The primary reason for exclusion was that only survival outcomes only reported (n = 16); this included studies focusing on surgery (n = 10), chemotherapy (n = 3), re-irradiation (n = 2) and immunotherapy (n = 1). Other reasons for exclusion included general HNC cohort where functional/ QoL or general toxicity outcomes were reported but with no subgroup analysis for patients with ReRuNeR (n = 10) or mixed primary/ ReRuNeR OPC cohorts (n = 1). One qualitative study was identified; however, it was excluded as it was not specific to ReRuNeR OPC but a more general recurrent HNC caseload.
Fig. 1
PRISMA diagram.
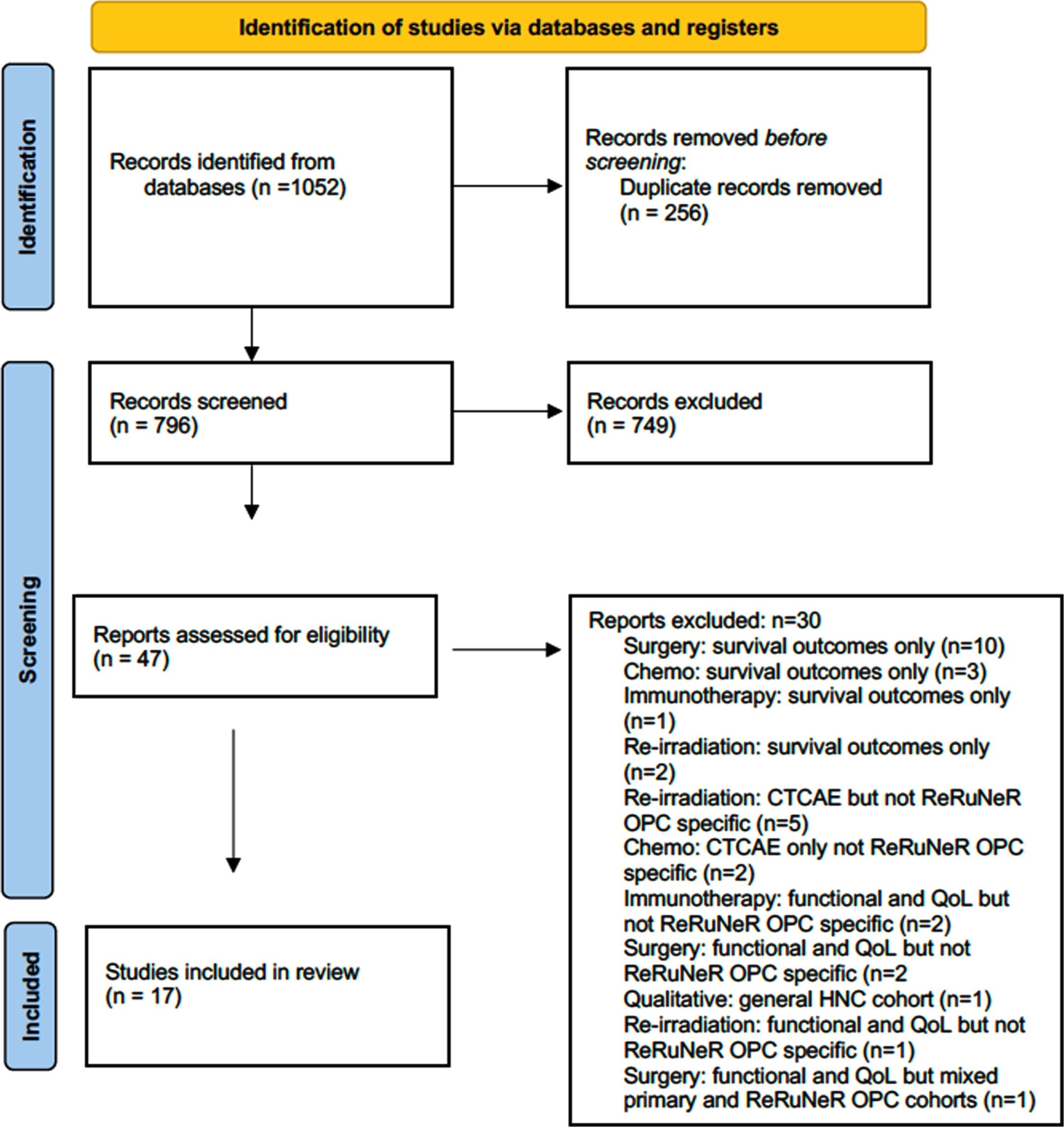
3.2Study characteristics
Study characteristics are summarised in Table 1. The geographic distribution of studies is as follows: US (n = 8), France (n = 4), UK (n = 2), Japan (n = 1), Australia (n = 1) and multicentre (n = 1). All studies were published between 2009 and 2022. Two studies included a prospective design, and the majority were single centre case series (n = 12). No qualitative/ patient experience studies were identified.
Table 1
Study characteristics
| Author | Year | Location | Study design | Intervention | Function/ Quality of Life outcomes primary or secondary, validated (V) or unvalidated (UV): | No of participants | Timepoints | Outcome measures used |
| (Charters et al., 2022) | 2022 | Australia | Case series | TORSa | Primary V | 3 | Baseline 3 months 12 months | FOIS b MDADI c SHId PASe, BRCSf, PSS-HN- NODg, PSS-HN-EIPh, PSS-HN-UoSi, Gastrostomy rate |
| (Culié et al., 2015) | 2015 | France | Case series | Open and TORS | Secondary V | 34 | Baseline 6 months | Speech intelligibility (unvalidated) DOSSj |
| (D’Andréa et al., 2022) | 2022 | France | Case series | Open and TORS | Primary V | 53 | Baseline, 3 months 1 year 2 years 3 years 4 years 5 years | MDADI EORTC-QLQC30k EORTC-QLQH & N35l Gastrostomy rate Tracheostomy rate |
| (Dean et al., 2010) | 2010 | United States | Case control study | Open and TORS | Secondary UV | 21 | No baseline 6 months | Gastrostomy rate Tracheostomy rate |
| (Hardman et al., 2022) | 2022 | Global | Case series | TORS | Secondary UV | 199 | No baseline 1 year | Gastrostomy rate Tracheostomy rate |
| (Kano et al., 2013) | 2013 | Japan | Case series | Open and TORS | Secondary UV | 11 | Baseline Undefined follow up | Gastrostomy rate Oral feeding (unvalidated) |
| (Kostrzewa et al., 2010) | 2010 | United Kingdom | Case series | Open surgery | Primary UV | 36 | No baseline Undefined follow up | Gastrostomy rate Oral feeding (unvalidated) |
| (Mazerolle et al., 2022) | 2022 | France | Case series | Open surgery | Secondary UV | 42 | No baseline Undefined follow up | Respiratory status Nutritional status |
| (Patel et al., 2016) | 2015 | United States | Case series | Open and TORS | Secondary UV | 34 | No baseline Undefined follow up | Gastrostomy rate Tracheostomy rate |
| (Philouze et al., 2017) | 2017 | France | Case series | Open and TORS | Secondary UV | 52 | No baseline Undefined follow up | Gastrostomy rate |
| (Pipkorn et al., 2019) | 2018 | United States | Case series | TORS | Secondary UV | 18 | No baseline Undefined follow up | Gastrostomy rate |
| (Sharma et al., 2022) | 2022 | United States | Case series | Open surgery | Secondary UV | 30 | No baseline Within 30 days of surgery | Gastrostomy rate |
| (Sweeny et al., 2016) | 2016 | United States | Case series | Open and TORS | Secondary UV | 69 | No baseline 1 year | Gastrostomy rate |
| (Tassone et al., 2022) | 2022 | United States | Case series | Open surgery | Primary UV | 89 | No baseline 1 year | Gastrostomy rate Tracheostomy rate |
| (White et al., 2013) | 2013 | United States | Case control study | Open and TORS | Secondary UV | 128 | No baseline 1 year | Gastrostomy rate Tracheostomy rate |
| (Williamson, Haywood, & Awad, 2021) | 2021 | United Kingdom | Case series | TORS | Primary V | 3 | Baseline Undefined follow up | UW-QOLm MDADI |
| (Zafereo et al., 2009) | 2009 | United States | Case control study | Other | Secondary UV | 41 | Undefined follow up | Oral intake (unvalidated) Speech mode (unvalidated) Speech intelligibility (unvalidated) Tracheostomy rate |
aTranosoral robotic surgery; bFunctional Oral Intake Scale; cMD Anderson Dysphagia Inventory; dSpeech Handicap Index; ePenetration Aspiration Scale; fBoston Residue and Clearance Scale; gPerformance Status Scale for Head and Neck Cancer - Normalcy of Diet; hPerformance Status Scale for Head and Neck Cancer - Eating in Public; iPerformance Status Scale for Head and Neck Cancer - Understandability of Speech; jDysphagia Outcome Severity Scale; kEuropean Organisation for Research and Treatment (EORTC) Quality of Life for Cancer Patients; lEuropean Organisation for Research and Treatment (EORTC) Quality of Life for Cancer Patients - Head and Neck; mUniversity of Washington Quality of Life Questionnaire.
3.3Patient characteristics
In total the studies included 863 patients with ReRuNeR OPC (Range 3–199). All studies investigated outcomes for surgical procedures, either open surgery or transoral robotic surgery. No oncological studies were identified reporting on functional or QoL outcomes for ReRuNeR OPC specifically. The authorship team of two large immunotherapy trials and one re-irradiation trial, who reported function and QoL data for a more general HNC cohort were contacted to see if a subgroup analysis was available for patients with ReRuNeR OPC; however, the data were not available for review at the time of writing.
3.4Risk of bias
MINORS risk of bias assessment findings are summarised in Table 2. All but one of the included studies did not involve a comparator group. For the non-comparative studies, the median MINORS score was 8 (out of a possible 12) with a range of 7–10. The one study which included a comparator group was rated with a MINORS score of 14 (out of a possible 24). Each item is scored from 0–2 where 0 indicates that the item was not reported, 1 indicates that it was reported but inadequately and 2 indicates that it was adequately reported within the article.
Table 2
Risk of bias assessment using MINORS (Kim et al. 2013)
| Study | Charters et al. | Culie et al. | D’Andrea et al. | Dean et al. | Hardman et al. | Kano et al. | Kostrzewa et al. | Mazerolle et al. | Patel et al. | Philouze et al. | Pipkorn et al. | Sharma et al. | Sweeney et al. | Tassone et al. | White et al. | Williamson et al. | Zafereo et al. |
| A clearly stated aim | 1 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive data | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Prospective collection of data | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Endpoints appropriate to the aim of the study | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Unbiased assessment of the study endpoint | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 |
| Assessment tests appropriate with the aim | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 |
| Loss of samples < 5% | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 1 |
| Prospecstive calculation of the study size | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| An adequate control group | 2 | ||||||||||||||||
| Contemporary groups | 2 | ||||||||||||||||
| Baseline equivalence of groups | 2 | ||||||||||||||||
| Adequate statistical analysis | 2 | ||||||||||||||||
| Total score: | 8 | 6 | 9 | 9 | 8 | 4 | 6 | 9 | 9 | 8 | 8 | 9 | 7 | 9 | 14 | 10 | 7 |
MINORS Scoring (Kim et al. 2013): 0: not reported; 1: reported but inadequately; 2: adequately reported.
3.5Results
Out of the 796 articles reporting outcomes for treatments for ReRuNeR OPC, specific functional and/or QoL data were available for patients with ReRuNeR OPC in 2% (n = 17). Within the 17 articles included, functional and/or QoL measures were reported as a primary outcome in 29% (n = 5), survival was reported as the primary measure in 71% (n = 12).
3.5.1Swallowing outcomes
Validated swallowing outcome measures were used in 23% of studies (n = 5). These measures included the MD Anderson Dysphagia Inventory (MDADI) (Chen et al., 2001) (n = 3), the Functional Oral Intake Scale (FOIS) (Crary, Mann, & Groher, 2005) (n = 1), the Performance Status Scale for Head and Neck Cancer (PSS-HN)(List, Ritter-Sterr, & Lansky, 1990) normalcy of diet (NOD) and eating in public (EIP) subscales (n = 1). Two studies reported on validated swallowing outcome measures obtained using an instrumental evaluation of swallowing. One study described the use of Flexible Endoscopic Evaluation of Swallowing (FEES) and the Penetration-Aspiration Scale (PAS)(Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996) and Boston Residue and Clearance Scale (BRCS) (Kaneoka et al., 2013). Another study described the use of videofluoroscopy and the Dysphagia Outcome and Severity Scale (DOSS) (O’Neil, Purdy, Falk, & Gallo, 1999).
Charters et al. (2022) reported on swallowing outcomes for 3 patients who underwent TORS for ReRuNeR OPC. Baseline and 12-month outcomes were reported for the FOIS, and PSS NOD scores. Here the FOIS score indicated a change from baseline where patients were managing a total oral diet with some diet modification (FOIS: 5) to being tube dependent with limited oral intake (FOIS: 3). PSS-NOD data showed a deterioration from soft chewable diet (PSS NOD: 50) to pureed diet (PSS NOD: 30). A number of 12 month swallowing outcomes were reported including the MDADI, PSS-EIP, Penetration-Aspiration Scale (PAS) score and the Boston Residue and Clearance Scale. No baseline data on these measures were included, however 12-month findings show impaired swallowing related QoL with an average MDADI composite score of 43 (a score of 100 indicates no swallowing difficulties) and median PSS EIP score of 25 (ordinal scale descriptor: eats only alone). PAS scores and Boston Residue and Clearance scales report that all 3 participants had unsafe swallowing with confirmed aspiration and inefficient swallowing, with confirmed post-swallow residue on instrumental evaluation of swallowing using FEES.
D’Andréa et al. (2022) also reported on the MDADI at 12 months for patients undergoing TORS and open salvage surgery. Overall, although a deterioration in mean composite MDADI scores from 71.4 at baseline to 64.3 at 1 year was noted, this change was not statistically significant (p = 0.13) nor did it reach the criterion for clinical significance (10-point change in MDADI composite score). The authors further clarify with individual patient data analysis that only 7 patients (30.4%) reported a 1-year clinically meaningful decrease of the MDADI score of more than ten points, while two patients (8.7%) reported a clinically meaningful increase in the MDADI composite score.
Williamson et al. (2021) report on 3 patients who underwent TORS with robotic assisted flap reconstruction and documented stable MDADI scores pre- and post-surgery with a composite score at baseline and follow up of 66.7. Here, the timepoint for post-surgical review was not defined.
Culié et al. (2015) reported on a case series of patients undergoing TORS and open salvage surgical procedures. Baseline and 6-month DOSS scores were reported and demonstrated a deterioration in swallowing function with increased aspiration of food/ fluids. At baseline 47% of participants were on full oral intake (DOSS score of 6-7), which reduced to 26% 6 months post-surgery. There was an increased number of patients requiring diet modification (DOSS score 3–5). However, between baseline and 6 months, the number of patients requiring non-oral feeding remained stable at 29%.
3.5.2Speech outcomes
Validated speech intelligibility measures, namely the Speech Handicap Index (SHI) (Rinkel et al., 2008) and the PSS Understandability of Speech (PSS-UoS) subscale (List et al., 1990) were both used in one single study. Other non-validated measures of speech intelligibility included a non-standardised/ validated rating of intelligibility or a comment on speech mode (n = 2).
Following TORS, speech outcomes were reported by Charters et al. (2022) using the PSS UoS and the SHI. No baseline data were reported but 12-month data demonstrated reduced speech intelligibility with a median score of 75 on the PSS UoS subscale (understandable most of the time but occasional repetition necessary) and 25 on the SHI (score from 0–120 with a higher score indicative of higher level of speech difficulties).
Speech outcomes following both open surgery and TORS were reported by Culie et al. (2015)_ using an unvalidated measure rating speech intelligibility from 0–3 (0 was severely altered/ unintelligible and 3 was normal/ near normal). No baseline data were reported, and 6-month data were available for 31/34 participants- here only 2 patients presented with normal/ near normal speech at 6 months post-surgery.
Zafereo et al. (2009) also reported unvalidated measures of speech intelligibility and in 41 patients reported that speech was > 80% intelligible. Again, baseline speech data were not available.
3.5.3HRQoL outcomes
Validated health-related QoL measures were used in two studies including the University of Washington QoL (UW-QoL) questionnaire (Rogers et al., 2002) (n = 1) and European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life for Cancer Patients (QLQ-30) and Quality of Life for Head and Neck Cancer (QLQ-H&N35) together (n = 1).
D’Andréa et al. (2022) reported EORTC-QLQ-C30 scores at baseline and 1 year showed non-significant change in global health status with preoperative mean score of 61.18 and 59.38 at 1 year (p = 0.96). The EORTC QLQ-H&N35 demonstrated some increased difficulties with sticky saliva, difficulty with social contact, eating and speech. Williamson et al. (2021) used the UW-QoL following TORS with free flap reconstruction. Mean scores for each symptom subscale at baseline and at an undefined timepoint post-surgery were reported. Increased mean scores indicating some level of improvement were noted in the following domains: pain, activity, chewing and anxiety. Reduction in mean scores, indicating deterioration, were reported in the remaining domains of appearance, recreation, swallowing, speech, shoulder, taste, saliva, and mood.
3.5.4Patient experience outcomes
No patient experience data were identified.
3.5.5Gastrostomy tube dependence
Where non-validated measures of swallowing function were reported, gastrostomy tube use was the most popular outcome reported in 88% of studies (n = 15). Some studies (n = 3) also specified oral versus non-oral feeding. Other studies (n = 2) focused on method of nutritional intake using three categories, gastrostomy with oral feeding, gastrostomy alone or oral feeding alone.
Long-term feeding tube rate (at least 6 months) was reported in 7/17 studies. A further 9 studies did not specify the timepoint or included data within 30 days of the surgery. Pooled data (n = 128) demonstrates a long-term gastrostomy dependence rate of 23.42% (95% CI 10.2 to 36.6).
3.5.6Long-term tracheostomy tube rate
Tracheostomy tube rate was reported in 47% of studies (n = 8). Long-term tracheostomy dependence (at least 6 months) was reported in 3/17 studies. A further 2 studies reported tracheostomy use post-surgery but did not define the timeframe. Pooled data (n = 309) demonstrates a long-term tracheostomy rate of 9.3% (95% CI 8.48 to 10.1).
4Discussion
This systematic review aimed to assess if function, QoL and patient experience are measured in patients undergoing treatment for ReRuNeR OPC. Only 17 articles were identified reporting functional and/or QoL outcomes for patients undergoing treatments for ReRuNeR OPC, five reporting functional/QoL as a primary measure. All 17 articles focused on surgical trials. No specific functional, QoL, patient experience of qualitative data for patients with ReRuNeR OPC undergoing non-surgical treatments were identified in this systematic review.
The measurement of HR-QoL in HNC has been recommended in both clinical practice and research over the past number of years (Rogers, Semple, Babb, & Humphris, 2016). The routine use of validated specific functional outcome measures to guide rehabilitation for communication and swallowing in HNC continues to emerge and has only recently been added as a recommendation in national guidelines (Schache et al., 2021). As a result, this review included previously validated measures of HR-QoL and all measures (validated and unvalidated) of communication and swallowing function were included.
Aside from reporting survival data only, many articles were excluded from this review due to the inclusion of mixed cohorts of HNC patients, with either general recurrent HNC cohorts or mixed primary and recurrent disease groups, with no subgroup analysis for ReRuNeR OPC. In comparison to other subtypes of head and neck cancer, the OPC subgroup are considered quite unique, in particular those with HPV related disease, who are often younger (less than 60 years) with low co-morbidity, absent or low smoking history (Ang et al., 2010). The incidence of OPC disease is increasing in the UK (Schache et al., 2016). This may be in part due to the emergence of new aetiological categories such as human papilloma virus (HPV), however, non-HPV-related disease which is associated with traditional causative factors including alcohol and tobacco consumption is also increasing (Schache et al., 2016). In the UK, many of these patients will have undergone organ-sparing treatment regimes with radiation therapy +/- chemotherapy. As a result, these patients will most likely be presenting with treatment side-effects in the form of changes to swallowing function, voice/speech and overall QoL (Patterson, McColl, Carding, & Wilson, 2018; Roe, Drinnan, Carding, Harrington, & Nutting, 2014). In addition, there can be a high level of psychological distress and emotional burden following previous treatment (Ringash et al., 2018). Further treatment for disease recurrence may cause further impairments or compound a unique set of baseline physical and psychological burden found in a potentially unique set of younger patients with OPC. With a growing number of patients with ReRuNeR OPC in the UK, specific functional and QoL data in relation to this unique cohort is required.
It is known from previous literature in the primary oropharyngeal disease that to accurately demonstrate the impact of treatment on function/ QoL, multidimensional assessment at pre-defined timepoints is required (Roe et al., 2010). In this review, 7 of the included studies did not report on baseline data and, a further 8 studies reported post-surgical functional and/or QoL data however did not define the follow up period. Pre-treatment baseline level of function has been cited as the key predictor for longer term swallowing outcomes for patients with HNC (Frowen, Cotton, Corry, & Perry, 2010). Given the risk of baseline functional difficulties in patients with ReRuNeR disease (Hardman et al. 2020), accurate baseline and longitudinal data measurements with pre-defined follow-up timepoints are imperative to accurately measure any potential change. Such methodological issues, in addition to the use of different outcome tools, make interpretation of the collective data difficult and any form of meta-analysis impossible.
This systematic review identified just three studies reporting speech outcomes. The standard of care for curative management of ReRuNeR OPC is at present open surgery. National guidelines highlight the potential functional morbidity including speech (Mehanna et al., 2016), however, based on this review, speech outcomes do not appear to be reported routinely. In the studies reporting speech outcomes, only one study used a previously validated speech outcome measure however, all studies did demonstrate altered speech in the post operative phase.
The majority of studies (n = 14) included data on gastrostomy use post-surgery. Eleven of these studies included gastrostomy tube dependence with or without tracheostomy tube dependence as the only measures of function post-surgery. In our review, pooled data (n = 128) demonstrates a long-term gastrostomy feeding rate of almost a quarter (23.42, 95% CI 10.2 to 36.6). In comparison to a previous systematic reviews reporting outcomes for TORS for recurrent HNC where long-term gastrostomy rate was recorded as 5% (Paleri et al., 2020), this estimate does seem to be higher. This may be due to the previous review including all HNC cohorts (not specific to ReRuNeR OPC) or only one treatment method (TORS rather than all surgery). Also, it is noted by the authors here that the definition of long-term use is not clear in the included studies. The current review defined long-term gastrostomy use as at least 6 months post-surgery. Given such ambiguities, the case for future studies to look at specific subgroups of HNC for more accurate/ clearly defined functional outcomes is strengthened. Gastrostomy tube use is widely used in the literature as a proxy for swallowing outcomes. As a unidimensional measure, it does not give an accurate/holistic measure of swallowing status. Many HNC patients may be having a combination of both oral intake and gastrostomy feeding. Also, without the provision of baseline data it is impossible to ascertain if gastrostomy tube presence is a consequence of primary disease management or because of the treatment of ReRuNeR disease.
There appears to be some variation in outcomes in relation to the studies using patient- and clinician-reported measures of swallowing function. It is known from the literature in relation to primary disease that clinician and patient-reported measures of toxicity are not always congruent (Falchook et al., 2016). Both within and across surgery types (TORS and open), mixed findings are reported using the MDADI. One study reports stable post-TORS findings (Williamson et al., 2021) and another reported a deterioration (Charters et al., 2022), although both of these studies included only 3 patients each. A further study, with a much larger cohort (n = 53) (D’Andréa et al., 2022) reports outcomes for both open and TORS surgery and details an overall mean deterioration in MDADI composite scores but that deterioration did not meet statistical or clinical significance. A previous study investigated the clinically meaningful difference in MDADI scores for patients with HNC using a combination of criterion standards including aspiration scores, feeding tube status and diet scores (Hutcheson et al., 2016). The methodology for this study however excluded patients with recurrent disease. It might thus be questioned if the MDADI and the critical 10-point clinically significant difference is suitable in the recurrent setting when we know that most of these patients will have baseline swallowing difficulties with lower diet scores, higher rates of gastrostomy tube usage and increased aspiration from their previous treatments +/- tumour related complications (Paleri et al., 2020).
With regards to QoL measurement, only 2 trials were identified where validated health-related quality of life tools were used, namely the UW-QoL questionnaire and the EORTC QLQ-C30 used in combination with the EORTC QLQ-H&N35. In an area of HNC practice where many treatment decisions are based on the potential impact of further treatments on QoL, one would expect a much greater focus within the literature. Although stable outcomes were reported by Williamson and colleagues (Williamson et al., 2021) it would appear that some aggregate data is missing in the publication. Scoring of the UW-QoL tool typically involves presenting the questionnaire data from two subscale scores, one for ‘Physical Function’ and another for ‘Social-Emotional Function.’ The Physical subscale score is computed as the simple average of 6 domain scores –those of chewing, swallowing, speech, taste, saliva and appearance. The Social-Emotional subscale score is also computed as the simple average of 6 domain scores - those of anxiety, mood, pain, activity, recreation and shoulder function. Such scores are not provided in this publication thus difficult to interpret. Also, a small sample size is noted in this study with just three patients. In a larger study by D’Andréa et al. (2022) non-significant changes in EORTC-QLQ-C30 scores at baseline and 1 year were reported. However, the EORTC QLQ-H&N35 demonstrated some increased difficulties with sticky saliva, difficulty with social contact, eating and speech. This echoes findings by Williamson and colleagues who, when reporting symptoms using the UW-QoL tool, noted increased symptoms in relation to swallowing, speech, taste, saliva, in addition to shoulder, appearance, recreation and mood. However, as noted in previous literature, the content validity of EORTC QLQ-C30 and QLQ-H&N35 (Degboe et al., 2018) has not been widely documented in relation to patients with disease recurrence. Similarly, much of the literature in relation to the development of the UW-QoL questionnaire has focused on patients treated for primary disease, who are now cancer free (Rogers, Lowe, Yueh, & Weymuller Jr, 2010).
This systematic review is not without its limitations. As noted, in preparing the protocol for this review it was hypothesised that literature regarding function and QoL data was in its infancy in the area of ReRuNeR. For this reason, meta-analysis was neither planned nor subsequently undertaken and instead, a narrative review of the evidence is presented. The data presented focuses solely on (potentially curative) surgical outcome data. A high level of bias was noted using the risk of bias assessment for included studies, which must be considered in the interpretation of the findings of this review. The majority of the studies were observational and retrospective in nature. Only two of the included studies were prospective, only one had a comparator group, and neither reported a sample size calculation.
Given the limited evidence on functional and QoL data in the recurrent HNC population, it may have been sensible to include all recurrent HNC studies rather than those specifically pertaining to OPC. For example, two large randomised controlled trials (Burtness et al., 2019; Cohen et al., 2019) reporting standardised QoL outcome measures for patients with recurrent and metastatic HNC including ReRuNeR OPC were excluded, as a subgroup analysis for ReRuNeR OPC disease was not presented in the published data. Although attempts were made to obtain the OPC data from the authors, this was not possible at the time of writing.
Previous consensus guidelines have been published on the conduct of trials in primary disease management to include swallowing and communication standardised outcome tools focusing on a range of clinician-reported, instrumental evaluation and patient-reported outcome measures (Lefebvre, Ang, & Panel, 2009). It is clear from this systematic review that similar standards are neither available nor applied in trials looking at ReRuNeR disease. Further research is required to identify, develop and validate outcome measures for use in the recurrent setting to guide the development of similar recommendations for the conduct of trials focusing on ReRuNeR OPC.
5Conclusion
This review has highlighted that only a small proportion of studies in ReRuNeR OPC report functional and QoL outcomes. These studies focus on surgical treatments, and functional and QoL outcomes for ReRuNeR OPC are not reported specifically in non-surgical trials. Patient experience or qualitative data have not been reported for patients with ReRuNeR OPC.
Where functional and QoL outcomes are reported in surgical trials, there does not appear to be consensus on the outcome tools which should be used, or the timepoints where data should be collected. Consequently, little or no comparison in data is possible across surgical trials. The reviewed literature does show that changes to function and QoL are likely to occur at least transiently. To accurately describe how ReRuNeR OPC and the available treatments impact on QoL and function, there needs to be further research driven by patient experience data highlighting areas of concern. Further research must focus on findings which could lead to a consensus on which tools, or combination of tools should be validated and used at which timepoints, to accurately provide a holistic and multidimensional profile of function and QoL for patients with ReRuNeR OPC.
Acknowledgments
Grainne Brady (Clinical Doctoral Research Fellow, NIHR300437) is funded by Health Education England (HEE) / NIHR for this research project. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care. Mary Wells and Justin Roe acknowledge the infrastructure and support of the NIHR Imperial Biomedical Research Centre. Grainne Brady, Vinidh Paleri and Justin Roe acknowledge support from the NIHR Biomedical Research Centre of the Royal Marsden NHS Foundation Trust and IReC. IReC has been made possible thanks to Charles Wilson CBE and Dr Rowena Olegario and Keith and Isabelle McDermott’s generous support of The Royal Marsden Cancer Charity.
Conflict of interest
Professor Vinidh Paleri is a proctor for Intuitive Medical Inc. The other authors have no conflicts of interest to disclose.
References
1 | Ang, K.K. , Harris, J. , Wheeler, R. , Weber, R. , Rosenthal, D.I. , Nguyen-Tân, P.F. ,... & Gillison, M.L. ((2010) ). Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. The New England Journal of Medicine, 363: (1), 24–35. doi: 10.1056/NEJMoa0912217 |
2 | Atkins, S. , Lewin, S. , Smith, H. , Engel, M. , Fretheim, A. , & Volmink, J. ((2008) ). Conducting a meta-ethnography of qualitative literature: Lessons learnt. BMC Medical Research Methodology, 8: (1), 21. doi: 10.1186/1471-2288-8-21 |
3 | Brady, G.C. , Hardman, J.C. , Paleri, V. , Harrington, K.J. , & Roe, J.W. ((2020) ). Changing paradigms in the treatment of residual/recurrent head and neck cancer: implications for dysphagia management. Current Opinion in Otolaryngology & Head and Neck Surgery, 28: (3), 165–171. |
4 | Burtness, B. , Harrington, K.J. , Greil, R. , Soulières, D. , Tahara, M. , de Castro, G. ,... & Bratland, Å. ((2019) ). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. The Lancet, 394: (10212), 1915–1928. |
5 | Charters, E. , Bogaardt, H. , Freeman-Sanderson, A. , Ballard, K. , Davies, S. , Oates, J. , & Clark, J. ((2022) ). Swallowing and communication outcomes following primary transoral robotic surgery for advanced or recurrent oropharyngeal cancer: Case series. International Journal of Speech-Language Pathology, 24: (4), 407–416. |
6 | Chen, A.Y. , Frankowski, R. , Bishop-Leone, J. , Hebert, T. , Leyk, S. , Lewin, J. , & Goepfert, H. ((2001) ). The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the MD Anderson dysphagia inventory. Archives of Otolaryngology–Head & Neck Surgery, 127: (7), 870–876. |
7 | Cohen, E.E. , Soulières, D. , Le Tourneau, C. , Dinis, J. , Licitra, L. , Ahn, M.-J. ,... & Mehra, R. ((2019) ). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-): a randomised, open-label, phase 3 study. The Lancet, 393: (10167), 156–167. |
8 | Crary, M.A. , Mann, G.D.C. , & Groher, M.E. ((2005) ). Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of Physical Medicine and Rehabilitation, 86: (8), 1516–1520. |
9 | Culié, D. , Benezery, K. , Chamorey, E. , Ettaiche, M. , Fernandez, J. , Poissonnet, G. ,... & Leysalle, A. ((2015) ). Salvage surgery for recurrent oropharyngeal cancer: post-operative oncologic and functional outcomes. Acta oto-Laryngologica, 135: (12), 1323–1329. |
10 | D’Andréa, G. , Bordenave, L. , Nguyen, F. , Tao, Y. , Paleri, V. , Temam, S. ,... & Gorphe, P. (2022). A prospective longitudinal study of quality of life in robotic-assisted salvage surgery for oropharyngeal cancer. European Journal of Surgical Oncology. |
11 | Dean, N.R. , Rosenthal, E.L. , Carroll, W.R. , Kostrzewa, J.P. , Jones, V.L. , Renee’ A.D. ,... & Magnuson, J.S. ((2010) ). Robotic-assisted surgery for primary or recurrent oropharyngeal carcinoma. Archives of Otolaryngology–Head & Neck Surgery, 136: (4), 380–384. |
12 | Degboe, A. , Knight, S.L. , Halling, K. , Trigg, A. , Al-Zubeidi, T. , Aldhouse, N. ,... & Rogers, S. N. ((2018) ). Patients’ experience of recurrent/metastatic head and neck squamous cell carcinoma and their perspective on the EORTC QLQ-C30 and QLQ-H&N35 questionnaires: a qualitative study. Journal of Patient-Reported Outcomes, 2: (1), 1–13. |
13 | Falchook, A.D. , Green, R. , Knowles, M.E. , Amdur, R.J. , Mendenhall, W. , Hayes, D.N. ,... & Chera, B.S. ((2016) ). Comparison of Patient- and Practitioner-Reported Toxic Effects Associated With Chemoradiotherapy for Head and Neck Cancer. JAMA Otolaryngology–Head & Neck Surgery, 142: (6), 517–523 10.1001/jamaoto.2016.0656 |
14 | FDA. (2016). Assessment of the Use of Patient Experience Data in Regulatory Decision-Making. https://www.fda.gov/drugs/development-approval-process-drugs/assessment-use-patient-experience-data-regulatory-decision-making |
15 | Frowen, J. , Cotton, S. , Corry, J. , & Perry, A. ((2010) ). Impact of demographics, tumor characteristics, and treatment factors on swallowing after (chemo) radiotherapy for head and neck cancer. Head & Neck: Journal for the Sciences and Specialties of the Head and Neck, 32: (4), 513–528. |
16 | Hardman, J.C. , Holsinger, F.C. , Brady, G.C. , Beharry, A. , Bonifer, A.T. , D’Andréa, G. ,... & Floros, P. ((2022) ). Transoral Robotic Surgery for Recurrent Tumors of the Upper Aerodigestive Tract (RECUT): An International Cohort Study. Journal of the National Cancer Institute, 114: (10), 1400–1409. |
17 | Hutcheson, K.A. , Barrow, M.P. , Lisec, A. , Barringer, D.A. , Gries, K. , & Lewin, J.S. ((2016) ). What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? The Laryngoscope, 126: (5), 1108–1113. |
18 | Jayaram, S.C. , Muzaffar, S.J. , Ahmed, I. , Dhanda, J. , Paleri, V. , & Mehanna, H. ((2016) ). Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: a systematic review and meta-analysis. Head & Neck, 38: (12), 1855–1861. |
19 | Kaneoka, A.S. , Langmore, S.E. , Krisciunas, G.P. , Field, K. , Scheel, R. , McNally, E. ,... & Cabral, H. ((2013) ). The Boston residue and clearance scale: preliminary reliability and validity testing. Folia Phoniatrica et Logopaedica, 65: (6), 312–317. |
20 | Kano, S. , Homma, A. , Hayashi, R. , Kawabata, K. , Yoshino, K. , Iwae, S. ,... & Shiga, K. ((2013) ).Salvage surgery for recurrent oropharyngeal cancer after chemoradiotherapy. International Journal of Clinical Oncology 18: (5), 817–823. |
21 | Kim, S.Y. , Park, J.E. , Lee, Y.J. , Seo, H.-J. , Sheen, S.-S. , Hahn, S. ,... & Son, H.-J. ((2013) ). Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. Journal of Clinical Epidemiology, 66: (4), 408–414. |
22 | Kostrzewa, J.P. , Lancaster, W.P. , Iseli, T.A. , Desmond, R.A. , Carroll, W.R. , & Rosenthal, E.L. ((2010) ). Outcomes of salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal cancer. The Laryngoscope, 120: (2), 267–272. |
23 | Lefebvre, J.-L. , Ang, K.K. , & Panel, L.P.C. ((2009) ). Larynx preservation clinical trial design: key issues and recommendations—a consensus panel summary. International Journal of Radiation Oncology* Biology* Physics, 73: (5), 1293–1303. |
24 | List, M.A. , Ritter-Sterr, C. , & Lansky, S.B. ((1990) ). A performance status scale for head and neck cancer patients. Cancer, 66: (3), 564–569. |
25 | Mazerolle, P. , Fuchsmann, C. , Schultz, P. , Benmoussa, N. , Malard, O. , Bozec, A. ,... & Lasne-Cardon, A. ((2022) ). Salvage total glossectomy and total glosso-laryngectomy: Are they worth it? A GETTEC French multicenter study. Oral Oncology, 130: , 105896. |
26 | Mehanna, H. , Kong, A. , & Ahmed, S.K. ((2016) ). Recurrent head and neck cancer: United Kingdom National Multidisciplinary Guidelines. Journal of Laryngology & Otology, 130: , S181–S190. doi: 10.1017/S002221511600061X |
27 | Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D.G. ((2010) ). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg, 8: (5), 336–341. |
28 | NICE. (2020). Pembrolizumab for untreated metastatic or unresectable recurrent head and neck squamous cell carcinoma. Retrieved from https://www.nice.org.uk/guidance/ta661/chapter/1-Recommendations |
29 | O’Neil, K.H. , Purdy, M. , Falk, J. , & Gallo, L. ((1999) ). The dysphagia outcome and severity scale. Dysphagia, 14: (3), 139–145. |
30 | Paleri, V. , Hardman, J. , Brady, G. , George, A. , & Kerawala, C. ((2020) ). Transoral Robotic Surgery for Residual and Recurrent Oropharyngeal Cancers. Otolaryngologic Clinics of North America, 53: (6), 1091–1108. doi: 10.1016/j.otc.2020.07.016 |
31 | Patel, S.N. , Cohen, M.A. , Givi, B. , Dixon, B.J. , Gilbert, R.W. , Gullane, P.J. ,... & Higgins, K.M. ((2016) ). Salvage surgery for locally recurrent oropharyngeal cancer. Head & Neck, 38: (S1), E658–E664. |
32 | Patterson, J. , McColl, E. , Carding, P. , & Wilson, J. ((2018) ). Swallowing beyond six years post (chemo) radiotherapy for head and neck cancer; a cohort study. Oral Oncology, 83: , 53–58. |
33 | Philouze, P. , Péron, J. , Poupart, M. , Pujo, K. , Buiret, G. , & Céruse, P. ((2017) ). Salvage surgery for oropharyngeal squamouscell carcinomas: A retrospective study from 2005 to 2013. Head& Neck, 39: (9), 1744–1750. |
34 | Pipkorn, P. , Sinha, P. , Kallogjeri, D. , Adkins, D. , Thorstad, W.T. , Rich, J.T. , & Jackson, R.S. ((2019) ). Outcomes of relapsed human papillomavirus-related oropharyngeal squamous cell carcinoma treated with curative intent. Head & Neck, 41: (5), 1312–1319. |
35 | Ringash, J. , Bernstein, L.J. , Devins, G. , Dunphy, C. , Giuliani, M. , Martino, R. , & McEwen, S. (2018). Head and neck cancer survivorship: learning the needs, meeting the needs. Paper presented at the Seminars in radiation oncology. |
36 | Rinkel RN, Verdonck-de Leeuw IM, van Reij EJ, Aaronson NK Leemans CR. Speech Handicap Index in patients with oral and pharyngeal cancer: better understanding of patients’ complaints. Head Neck. 2008 Jul;30(7):868-74. doi: 10.1002/hed.20795. PMID: 18302270. |
37 | Roe, J.W. , Carding, P.N. , Dwivedi, R.C. , Kazi, R.A. , Rhys-Evans, P.H. , Harrington, K.J. , & Nutting, C.M. ((2010) ). Swallowing outcomes following Intensity Modulated Radiation Therapy (IMRT) for head & neck cancer - a systematic review. Oral Oncol, 46: (10), 727–733. doi: 10.1016/j.oraloncology.2010.07.012 |
38 | Roe, J.W. , Drinnan, M.J. , Carding, P.N. , Harrington, K.J. , & Nutting, C.M. ((2014) ). Patient-reported outcomes following parotid-sparing intensity-modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncology, 50: (12), 1182–1187. |
39 | Rogers, S.N. , Gwanne, S. , Lowe, D. , Humphris, G. , Yueh, B. , & Weymuller, E.A. Jr ((2002) ). The addition of mood and anxiety domains to the University of Washington quality of life scale. Head & Neck: Journal for the Sciences and Specialties of the Head and Neck, 24: (6), 521–529. |
40 | Rogers, S.N. , Lowe, D. , Yueh, B. , & Weymuller E.A. Jr, ((2010) ). The physical function and social-emotional function subscales of the University of Washington Quality of Life Questionnaire. Archives of Otolaryngology–Head & Neck Surgery, 136: (4), 352–357. |
41 | Rogers, S.N. , Semple, C. , Babb, M. , & Humphris, G. ((2016) ). Qualityof life considerations in head and neck cancer: United KingdomNational Multidisciplinary Guidelines. J Laryngol Otol, 130: (S2), S49–s52. doi: 10.1017/s0022215116000438 |
42 | Rosenbek, J.C. , Robbins, J.A. , Roecker, E.B. , Coyle, J.L. , & Wood, J.L. ((1996) ). A penetration-aspiration scale. Dysphagia, 11: (2), 93–98. |
43 | Sattar, R. , Lawton, R. , Panagioti, M. , & Johnson, J. ((2021) ). Meta-ethnography in healthcare research: a guide to using a meta-ethnographic approach for literature synthesis. BMC Health Services Research, 21: (1), 50. doi: 10.1186/s12913-020-06049-w |
44 | Schache, A. , Kerawala, C. , Ahmed, O. , Brennan, P.A. , Cook, F. , Garrett, M. ,... & Mihai, R. ((2021) ). British Association of Head and Neck Oncologists (BAHNO) standards 2020. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology, 50: (3), 262–273. |
45 | Schache, A.G. , Powell, N.G. , Cuschieri, K.S. , Robinson, M. , Leary, S. , Mehanna, H. ,... & Junor, E. ((2016) ). HPV-related oropharynx cancer in the United Kingdom: an evolution in the understanding of disease etiology. Cancer Research, 76: (22), 6598–6606. |
46 | Schardt, C. , Adams, M.B. , Owens, T. , Keitz, S. , & Fontelo, P. ((2007) ). Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Medical Informatics and Decision Making, 7: (1), 1–6. |
47 | Sharma, B.K. , Contrera, K.J. , Jia, X. , Fleming, C. , Lorenz, R.R. , Koyfman, S.A. ,... & Fritz, M. (2022). Outcomes After Oral Cavity and Oropharyngeal Salvage Surgery. The Laryngoscope. |
48 | Sweeny, L. , Rosenthal, E.L. , Clemons, L. , Stevens, T.M. , McIntosh, E.R.C. , & Carroll, W.R. ((2016) ). Outcomes after surgical salvage for recurrent oropharyngeal squamous cell carcinoma. Oral Oncology, 60: , 118–124. |
49 | Tassone, P. , Wieser, M. , Givens, A. , Elliott, Z. , Philips, R. , Curry, J. ,... & Lamarre, E. (2022). Factors Leading to Gastrostomy Tube and Tracheostomy Requirements in Patients Treated Initially With Radiotherapy and Salvaged With Surgery and Free Flap Reconstruction. The Laryngoscope. |
50 | White, H. , Ford, S. , Bush, B. , Holsinger, F.C. , Moore, E. , Ghanem, T. ,... & Magnuson, J.S. ((2013) ). Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngology–Head & Neck Surgery, 139: (8), 773–777. |
51 | Williamson, A. , Haywood, M. , & Awad, Z. ((2021) ). Feasibility of free flap reconstruction following salvage robotic-assisted resection of recurrent and residual oropharyngeal cancer in 3 patients. Ear, Nose & Throat Journal, 100: (10_suppl), 1113S–1118S. |
52 | Zafereo, M.E. , Hanasono, M.M. , Rosenthal, D.I. , Sturgis, E.M. , Lewin, J.S. , Roberts, D.B. , & Weber, R.S. ((2009) ). The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer: Interdisciplinary International Journal of the American Cancer Society, 115: (24), 5723–5733. |




