Cough reflex testing in clinical dysphagia practice
Abstract
The addition of cough reflex testing (CRT) to a comprehensive clinical swallowing evaluation has the potential to substantially contribute to the management of patients with dysphagia, by providing insights into the integrity of the cough response to airway invasion and risk of silent aspiration. This “Spotlight on: Cough Reflex Testing in Clinical Dysphagia Practice” outlines the theoretical and practical considerations of using CRT as a screening tool to identify patients’ risk of silent aspiration. The following clinical questions are addressed: (1) What is coughing? (2) What is CRT? (3) What CRT method should I use? (4) How is the citric acid CRT conducted? (5) Can CRT be repeated to monitor changes in cough sensitivity? (6) What are the benefits of CRT in clinical dysphagia practice? This information is intended to support clinicians in implementing and interpreting CRT as a screening tool to identify patients’ risk of silent aspiration in clinical dysphagia practice.
1Introduction
Cough reflex testing (CRT) has been used in the field of respiratory medicine for over 60 years to evaluate the efficacy of cough medications. In the past 20 years, CRT has been adopted into the field of dysphagia. This clinical tutorial will outline (1) the theoretical underpinnings of CRT and (2) practical considerations of how to implement and interpret CRT as a screening tool to evaluate the integrity of the cough response and patients’ risk of silentaspiration.
2What is coughing?
Coughing is defined as “a forced expulsive maneuver against a closed glottis, that is associated with a characteristic sound” (Morice et al., 2007). However, not all coughing is the same. Coughing can occur with or without airway irritation and with varying levels of conscious control. An understanding of different types of coughing is important to draw accurate conclusions from CRT and to understand patients’ airway clearance abilities.
• Volitional (or voluntary) coughing has three phases: inspiration, compression, and expiration. It is preceded by an intention or command to cough, rather than airway irritation (Widdicombe et al., 2011). For example, a clinician may ask a patient to cough during a videofluoroscopic swallowing study (VFSS) upon observation of silent aspiration, or during a cranial nerve exam. In this case, coughing occurs without preceding sensory irritation. Volitional coughing can also be produced in response to mild, or sub-threshold (i.e., the point at which coughing can be suppressed) airway irritation (Eccles, 2009). For example, an individual may consciously perceive saliva in the airway and intentionally cough (or suppress) in response. In this case, coughing is preceded by sensory irritation, but occurs under high levels of conscious control.
• Reflexive Coughing is characterized by the same three phases as volitional coughing. However, it is preceded by upper or lower airway irritation (e.g., inflammation from upper respiratory tract infection) that reaches the cough threshold (i.e., the point at which coughing cannot be suppressed) (Eccles, 2009; Widdicombe et al., 2011). The initial inspiration provides air to expel materials from the lower airways (Widdicombe et al., 2011). However, it also draws material into respiratory tract and in this sense, represents a “pro-aspiration reflex” (Widdicombe et al., 2011).
• The laryngeal expiration reflex (LER) is characterized by rapid closing of the glottis and an expulsive phase in response to acute laryngeal irritation. The absence of an initial inspiration prevents inhalation of material into the airway. In this sense, it is an “anti-aspiration” mechanism (Widdicombe et al., 2011).
The precise pattern of coughing to penetration or aspiration of food/liquids is not well studied. It is likely to involve an initial LER, followed by reflexive and volitional coughing (Widdicombe et al., 2011). This pattern is effective to expel supra-glottic material (via the LER), followed by high velocity airflow to expel sub-glottic material (via reflexive and volitional coughing).
3What is CRT?
In clinical dysphagia practice, the CRT can be used as a screening tool to evaluate the integrity of the cough response to airway invasion and risk of silent aspiration. It involves inhalation of a cough evoking aerosol at a specific concentration via a nebulizer to induce coughing. Clinicians can record presence, absence and number of coughs elicited, as well as self-reported ratings of the perceived intensity of airway irritation (known as the urge-to-cough). In dysphagia research, aerodynamic measures of the cough response can also be quantified. Numerous studies have demonstrated the sensitivity and specificity of CRT in identifying patients with silent aspiration on instrumental assessment (Table 1). A sensitivity of 81% means that 81% of patients with silent aspiration will fail CRT (true positives), while 19% will go undetected (false negatives). A specificity of 65% means that 65% of patients without silent aspiration will pass CRT (true negatives), while 35% will fail (false positives). For screening tests, high sensitivity is preferable. However, CRT is not a test of silent aspiration per se. It provides information about the integrity of upper airway sensation that would otherwise go unassessed. Impaired upper airway sensation is one factor contributing to patients’ risk of silent aspiration. In the absence of impaired swallowing biomechanics, a patient with impaired upper airway sensation may not aspirate. Thus, the results of CRT must be interpreted in the context of the entire clinical swallowing evaluation. Only citric acid CRT has been validated as a screening tool to detect silent aspiration on instrumental assessment. Capsaicin CRT has been validated for detecting overt aspiration in patients with Parkinson’s Disease (Hegland et al., 2016). The differences in these aerosols are further discussed below.
Table 1
Studies reporting the sensitivity and specificity of citric acid CRT in identifying patients with silent aspiration on instrumental assessment
| Author (year) | Instrumental Assessment | Population | Sensitivity | Specificity | Method | ||||
| Citric acid concentration | Nebulizer | Inhalation duration(seconds) | Instruction | Pass criteria | |||||
| (Wakasugi et al., 2008) | VFSS FEES | n = 204 Cardiovascular disease (39%) Head and neck cancer (24%) Neuromuscular disease (17%) Respiratory disease (15%) Non-specified diseases (5%) | 67% 1 | 90% 1 | 1% (w/v) | Ultrasonic | 60 | “Subjects were directed to breathe through the mouth mask” (p, 365) | 5+ coughs |
| (Sato et al., 2012) | FEES | n = 141 Cerebrovascular disease Disuse syndrome Neuromuscular disease Respiratory Cancer Cervical spine injury Miscellaneous | 81% 2 | 65% 2 | 1% (w/v) | Mesh | 60 | “Patients were asked to inhale deeply through the mouth according to verbal instruction and inhale citric acid several times until the first cough occurred” (p, 1938) | 1 cough |
| (Miles et al., 2013) | VFSS FEES | n = 181 Stroke (38%) Cancer (10%) Respiratory (17%) Progressive neurological (10%) Other neurological (9%) Non-specified (16%) | 71% 2 (VFSS) | 60% 2 (VFSS) | 0.6 mol/L | Jet | 15 | “Patients were asked to breathe normally and cough if they felt the need to cough” (p, 26) | C2 cough (2 or more consecutive coughs) |
| 67% 3 (FEES) | 85% 3 (FEES) | ||||||||
| (Wakasugi et al., 2014) | VFSS FEES | n = 160 Cerebrovascular disease (36%) Neuromuscular disease (25%) Cancer (14%) Respiratory (8%) Other (17%) | 86% 1 | 71% 1 | 1% (w/v) | Handheld | 60 | “Subjects were directed to breathe nebulized mist through the mouth” (p, 77) | 5+ coughs |
| (Lee et al., 2014) | VFSS | n = 160 Ischemic stroke Intracerebral hemorrhage Traumatic brain injury Encephalitis Hypoxic brain damage Parkinson disease | 87.1% 2 | 70% 2 | 1% (w/v) | Portable | 604 | Not reported | 1 cough |
| (Guillén-Solà et al., 2015) | VFSS | n = 134 Sub-acute stroke5 | 19% 2 | 71% 2 | 1% (w/v) | Ultrasonic | 60 | “Cough as needed” (p, 1279) | 5+ coughs |
1Sensitivity and specificity of CRT in detecting silent aspiration for all patients (not only patients with aspiration). Trace silent aspiration was included. 2Sensitivity and specificity for the CRT in detecting silent aspiration for all patients (not only patients with aspiration). 3Trace silent aspiration was excluded. 4A cut of period of 28.12 seconds was used in the analysis. 5Patients who were considered low risk of aspiration were excluded from the study.
4What CRT method do I use?
A major challenge to implementing CRT into clinical practice is the variability of methods reported in the literature. A comprehensive discussion of CRT methods has been published elsewhere (Wallace, Guiu Hernandez, Ang, Hiew, et al., 2019). A feasible, rapid and easily interpretable method is advantageous in the clinical setting. If the goal is to screen patients for risk of silent aspiration, any validated method in Table 1 can be used. Clinicians should be aware that altering aspects of the method will influence the sensitivity and specificity of the test in identifying risk of silent aspiration. Some key methodological considerations are discussed below to support clinicians to make informed decisions.
4.1Cough-evoking aerosols
Both citric acid and capsaicin are commonly reported cough-evoking aerosols in the dysphagia literature. Other aerosols include tartaric acid and ultrasonically nebulised distilled water (also known as ‘fog’). The choice of aerosol should be carefully considered, as it has implications on the underlying neurophysiology of the induced cough. Citric acid preferentially stimulates neural pathways and rapidly adapting laryngeal receptors that play a role in coughing to aspiration (Canning et al., 2004; Mazzone & Undem, 2016). Thus, it is advantageous for assessing coughing in patients with dysphagia. Tartaric acid and ultrasonically nebulised distilled water mediate coughing via the same mechanisms (Canning et al., 2004; Mazzone & Undem, 2016). Capsaicin preferentially stimulates slowly adapting sensory receptors that mediate coughing to prolonged airway irritation (e.g., inflammation from an upper respiratory tract infection). A detailed discussion of neural pathways and cough receptor subtypes is provided elsewhere (Mazzone & Undem, 2016). Based on the advantages of citric acid in stimulating neural pathways and laryngeal receptors that play a role in coughing to airway invasion, and the research validating the use of citric acid CRT for this purpose, citric acid CRT is used by the authors. Thus, the following suggestions are made on citric acid CRT.
4.2Aerosol exposure –flow rate, exposure time and citric acid concentrations
Altering nebulizer flow rates, exposure times to citric acid or the citric acid concentration will influence the outcome of the test (Barber et al., 2005). Clinicians should pay close attention to these factors of their chosen protocol. Variations in citric acid concentrations are evident across protocols (Table 1). Most studies use a lower concentration (i.e. 1% w/v citric acid = 0.05 mol/L) for a longer exposure time (i.e. 60 seconds) (Guillén-Solà et al., 2015; Lee et al., 2014; Sato et al., 2012; Wakasugi et al., 2008; Wakasugi et al., 2014), compared to Miles et al., (2013) who used a higher concentration (0.6 mol/L = 11.5% w/v citric acid) for a shorter exposure time (15 seconds). Theoretically, a higher concentration and shorter exposure time would more likely mimic an aspiration event. Longer exposures will increase risk of tachyphylaxis (i.e., blunted sensation occurring from prolonged exposure to an irritant) (Morice et al., 2007). Regardless, clinicians should use the same citric acid concentration and exposure time of their chosen protocol to ensure validity. The way in which citric acid solutions are made is an important consideration. Citric acid solutions are made by dissolving citric acid (a colourless, odourless powder) in sterile 0.9% sodium chloride (Morice et al., 2007). For a 0.6 mol/L or 0.05 mol/L (i.e. 1%) citric acid solution, 115.3 g or 10 g of citric acid respectively is dissolved into 1 litre of sterile 0.9% sodium chloride. The use of 0.9% saline is important, as other solvents (e.g. distilled water) can confound effects of the citric acid and influence the overall validity of the test (Wallace, Guiu Hernandez, Ang, Hiew, et al., 2019). In addition, caution is warranted in using oxygen from the wall to nebulize citric acid solutions due to the potential confounding effects.
4.3CRT instructions
Instructions provided to patients vary across studies and change to reflect the type of cough being assessed. Some instructions prompt patients to cough (Guillén-Solà et al., 2015; Miles et al., 2013; Sato et al., 2012), while others do not (Wakasugi et al., 2008; Wakasugi et al., 2014). Clinicians are advised to use the instructions of their chosen CRT method to ensure validity and reliability. More recently, attention has shifted to evaluating a suppressed cough in patients with dysphagia, i.e., instructing patients to “try not to cough”; (Perry et al., 2019). Theoretically, suppressed coughing prevents individuals eliciting a volitional cough to a sub-threshold cough stimulus. It represents the point at which a cough cannot be suppressed and, in this sense, may more closely resemble coughing to aspiration (Monroe et al., 2014). However, it remains unknown whether a suppressed cough is superior in identifying risk of silent aspiration in patients with dysphagia. Instructing patients to “try not to cough” is a cognitively demanding task –a patient must perceive a stimulus, bring the cough behavior to consciousness, and suppress (Troche, Brandimore, Okun, et al., 2014). Research suggests that patients with dysphagia have blunted cognitive perception of airway irritation (discussed further below) (Troche, Brandimore, Okun, et al., 2014), and may be able to suppress coughing more easily.
4.4What is urge to cough (UtC)?
UtC is a measure of the perceived intensity of airway irritation that typically precedes an airway clearance response. To measure UtC, patients are asked to rate how much they felt the need to cough following inhalation of a cough-evoking aerosol on a modified Borg scale (Table 2) (Curtis & Troche, 2020; Hegland et al., 2011; Troche, Brandimore,Okun, et al., 2014). In patients with neurodegene-rative disease, UtC to capsaicin differentiated individuals with and without dysphagia (Curtis & Troche, 2020; Troche, Brandimore, Okun, et al., 2014) and with and without silent aspiration (Tabor-Gray et al., 2021), suggesting the cognitive perception of airway irritation is important for airway protection and clearance. Assessing UtC with CRT enables clinicians to quantify whether a patient perceived airway irritation but did not cough, or whether they had no perception of airway irritation whatsoever. UtC can also be assessed in response to penetration or aspiration on VFSS.
Table 2
Modified Borg scales for measuring Urge to Cough
| Modified Borg scale for measuring Urge to Cough | Modified Borg scale for measuring Urge to Cough | ||
| (Hegland et al., 2011) | (Curtis &Troche, 2020; Hegland et al., 2011) | ||
| 1 | No need to cough | 0 | None at all |
| 1.5 | Just noticeable urge to cough | 1 | Very slight |
| 2 | Slight urge to cough | 2 | Slight |
| 3 | Slight-to-moderate urge to cough | 3 | Moderate |
| 4 | Moderate urge to cough | 4 | Somewhat severe |
| 5 | Moderate-to-strong urge to cough | 5 | Severe |
| 6 | Strong urge to cough | 6 | |
| 7 | Strong-to-severe urge to cough | 7 | Very, very severe |
| 8 | Severe urge to cough | 8 | |
| 9 | Severe-to-maximum urge to cough | 9 | |
| 10 | Maximum urge to cough | 10 | Very, very, very severe (almost maximal) |
5How is CRT conducted?
The following citric acid CRT protocol is used by the authors to identify patients’ risk of silent aspiration in clinical dysphagia practice. This method is used by the authors as it was validated for this purpose on instrumental swallowing assessment (Miles et al., 2013). It is acknowledged however, that many different CRT methods exist and clinicians can use any validated method of CRT. For this method, a compressor, nebuliser, facemask, 0.9% saline and 0.6 mol/L citric acid solution are used. Different brands of instrumentation are available in different countries and instrumentation used in original research studies is often discontinued. For this reason, clinicians are advised to match the nebuliser specifications, i.e., nebuliser type (jet, ultrasonic, mesh) and flow rate, as close as possible to their chosen protocol to ensure validity and reliability.
Fig. 1
Instrumentation used by the authors for citric acid CRT.
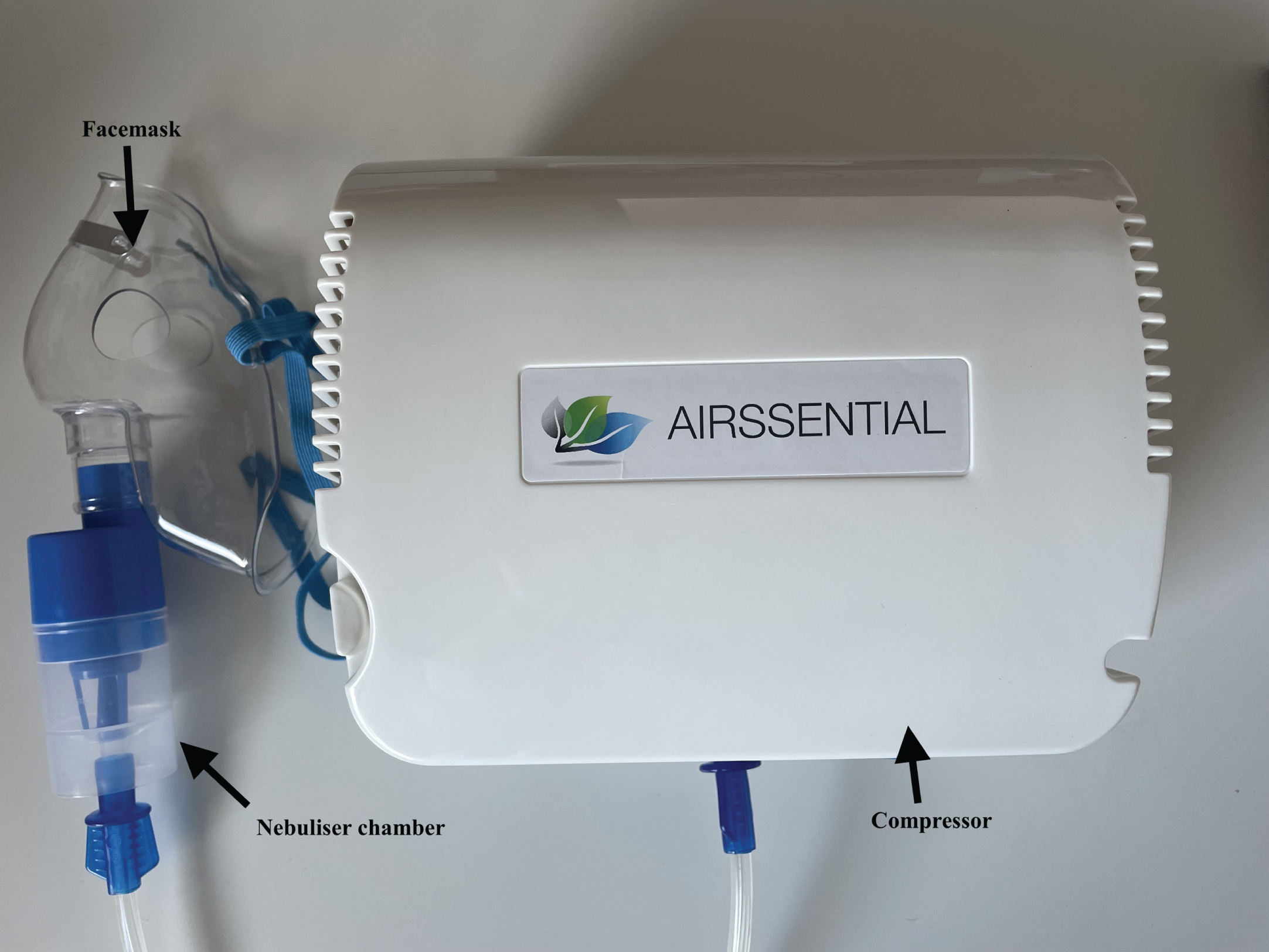
5.1Acclimatising the patient to the test
• Step 1: Approximately 20 ml of 0.9% saline solution is placed into the nebuliser chamber.
• Step 2: The mask is placed over the patient’s nose and mouth.
• Step 3: Instructions are provided to the patient “breathe normally through your mouth. Cough if you need to”.
• Step 4: The compressor is turned on, which begins nebulisation for a maximum of 15 seconds. 0.9% saline should not elicit coughing. It is used to acclimate the patient to the test.
5.2Assessing cough sensation to inhaled citric acid
• Step 5: The 0.9% saline is tipped out of the nebuliser and replaced with 20 ml 0.6 mol/L citric acid. Steps 2–4 are repeated.
• Step 6: The patient is observed for the production of a C2 cough response (i.e., 2 consecutive coughs) within 15 seconds.
• Step 7: After 1 minute break (which minimizes the risk of tachyphylaxis), steps 2–6 are repeated with 0.6 mol/L citric acid. A maximum of three trials are administered.
• In instances where it would be clinically meaningful to assess suppressed coughing, steps 1–7 are repeated with 1.2 mol/L citric acid. Patients are instructed to “breathe normally through your mouth. Try not to cough”.
5.3Interpretating the result
• Pass: If the patient produces a C2 cough response within 15 sec on two out of a maximum of three trials, the clinician can assume integrity of upper airway sensation, i.e., the patient is likely to cough in the event of penetration or aspiration during oral trials. This result should be integrated into the patient’s entire clinical swallowing evaluation to inform management, acknowledging that patients with intact airway sensation are still at risk of aspiration and aspiration pneumonia.
• Fail: If the patient does not produces a C2 cough response on two out of a maximum of three trials, the patient is likely to have blunted upper airway sensation. The clinician can assume that the patient may not respond to penetration or aspiration during oral trials. As above, the findings should be integrated into the patient’s entire clinical swallowing evaluation to inform management.
• Strong versus weak coughing: Auditory perceptual evaluation of the cough response to CRT has been reported in previous studies (Miles et al., 2013). However, numerous studies question its reliability and validity (Laciuga et al., 2016; Miles & Huckabee, 2013; Miles, McFarlane, et al., 2014; Wallace et al., 2021). Clinician training can improve the reliability of auditory perceptual cough evaluation (Miles, Huckabee, et al., 2014). Aerodynamic measures of coughing can provide more reliable and valid information to determine patients’ risk of aspiration and aspiration pneumonia (Bianchi et al., 2012; Curtis & Troche, 2020; Plowman et al., 2016; Smith Hammond et al., 2009).
• C2 or C5: Different opinions exist as to whether a C2 or C5 cough response should be used as the primary endpoint (Morice et al., 2007). It is recommended that clinicians choose the endpoint that is published in their chosen protocol. For patients with dysphagia, it’s likely that a range of airway clearance mechanisms should be recorded to reflect the continuum of airway clearance mechanisms elicited in response to airway invasion (Troche, Brandimore, Godoy, et al., 2014).
6Can CRT be repeated to monitor changes in cough sensation?
Currently, there are no studies validating recovery of airway sensation and cough response to CRT. Previous research in healthy individuals demonstrated that cough thresholds increase with repeated, alternate-day testing likely related to habituation to citric acid (Wallace, Guiu Hernandez, Ang, & Macrae, 2019). However, further research is needed to evaluate recovery of airway sensation and cough response to CRT in patients with dysphagia.
7What are the benefits of including CRT as part of the clinical swallowing evaluation?
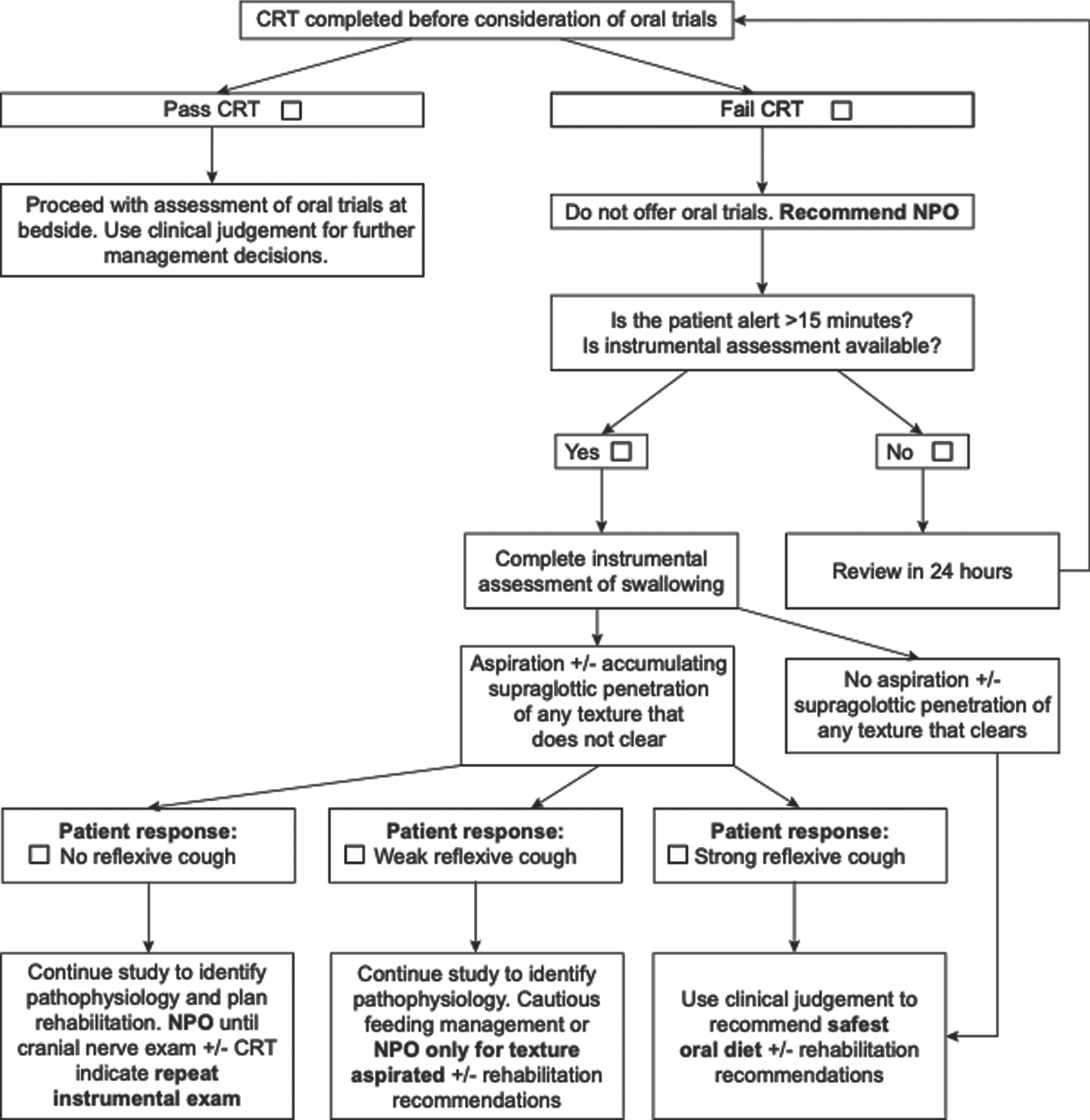
Numerous studies have shown that patients with effective coughing are less likely to develop aspiration pneumonia (Fujiwara et al., 2017; Hegland et al., 2014; Nakamori et al., 2020). Thus, assessing cough effectiveness can guide dysphagia management and clinical decision making. Effective coughing requires adequate cough sensation to sense misdirection of material into the airway, and adequate respiratory muscle strength to expel the material. CRT provides insights into the integrity of cough sensation. Cough strength can be objectively assessed using aerodynamic measures of coughing (Bianchi et al., 2012; Curtis & Troche, 2020; Min et al., 2018; Plowman et al., 2016; Smith Hammond et al., 2009). However, assessing cough strength or sensation will not prevent aspiration pneumonia, but rather, integration of that information into the wider clinical picture for each patient may inform management. Implementation of a Dysphagia in Stroke Protocol (Fig. 2) to guide acute stroke patient management reduced rates of aspiration pneumonia from 28% to 10% (Perry et al., 2019). In this case, patients identified with impaired cough sensation (i.e., those who failed CRT) were recommended no food or drink by mouth (i.e., nil per os) and referred for VFSS to identify swallowing pathophysiology and guide management. The Dysphagia in Stroke Protocol resulted in substantial reductions in rates of aspiration pneumonia. However, it is acknowledged that in some settings this approach may not be feasible due to difficulty accessing instrumental assessments. In such cases, integrating the results of CRT, the clinical swallowing evaluation and clinical intuition is essential in guiding decision making. Whether CRT is appropriate for use will also vary depending clinical setting and patient population, among other factors.
Fig. 2
The dysphagia in stroke protocol (Perry et al., 2019). NPO = Nil per os
8Conclusions
The addition of CRT into a comprehensive clinical swallowing evaluation greatly adds to the management of patients with dysphagia. It provides insight into cough sensation and potential risk of silent aspiration, which otherwise remain challenging to assess at bedside. CRT must be interpreted in the context of the patient’s entire clinical presentation. More research is needed to develop, refine, and expand our use of CRT in the management of patients with dysphagia.
Acknowledgments
The authors have no acknowledgements.
Conflict of interest
The authors have no conflict of interest to report.
References
1 | Barber C.M. , Curran, A.D. , Bradshaw, L.M. , Morice, A.H. , Rawbone, R. , Fishwick, D. , Reproducibility and validity of a Yan-style portable citric acid cough challenge. Pulmonary Pharmacology & Therapeutics, 18: (3), 177–180. https://doi.org/10.1016/j.pupt.2004.11.009 |
2 | Bianchi C , Baiardi, P , Khirani, S , Cantarella, G. , ((2012) ). Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am J Phys Med Rehabil, 91: (9), 783–788. https://doi.org/10.1097/PHM.0b013e3182556701 |
3 | Canning B.J , Mazzone, S.B. , Meeker, S.N. , Mori, N. , Reynolds, S.M. , Undem, B.J. , ((2004) ). Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. The Journal of physiology, 557: (Pt 2) 543–558. https://doi.org/10.1113/jphysiol.2003.057885 |
4 | Curtis J , Troche M ((2020) ). Handheld cough testing: A novel tool for cough assessment and dysphagia screening. Dysphagia, 35: , 1–8. https://doi.org/10.1007/s00455-020-10097-z |
5 | Eccles R , (2009). Central mechanisms IV: Conscious control of cough and the placebo effect. Handb Exp Pharmacol, (187), 241-262. https://doi.org/10.1007/978-3-540-79842-212 |
6 | Fujiwara K , Kawamoto, K , Shimizu, Y. , Fukuhara, T. , Koyama, S. , Kataoka, H. , Kitano, H. , Takeuchi, H. , ((2017) ). A novel reflex cough testing device. BMC Pulmonary Medicine, 17: (1), 19. https://doi.org/10.1186/s12890-017-0365-y |
7 | Guillén-Solà A. , Chiarella S.C. , Martínez-Orfila J. , Duarte, E. , Alvarado-Panesso, M.. , Figueres-Cugat, A.. , Bas, N. , Marco, E. , ((2015) ). Usefulness of citric cough test for screening of silent aspiration in subacute stroke patients: A prospective study. Arch Phys Med Rehabil, 96: (7), 1277–1283. https://doi.org/10.1016/j.apmr.2015.02.028 |
8 | Hegland K.W. , Okun M.S. , Troche M.S. , ((2014) ). Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung, 192: (4), 601–608. https://doi.org/10.1007/s00408-014-9584-7 |
9 | Hegland K.W. , Pitts T. , Bolser D.C. , Davenport P.W. , ((2011) ). Urge to cough with voluntary suppression following mechanical pharyngeal stimulation. Bratisl Lek Listy, 112: (3), 109–114. |
10 | Hegland K.W. , Troche M.S.. , Brandimore A. , Okun M.S. , Davenport P.W. , ((2016) ). Comparison of two methods for inducing reflex cough in patients with parkinson’s disease, with and without dysphagia. Dysphagia, 31: (1), 66–73. https://doi.org/10.1007/s00455-015-9659-5 |
11 | Laciuga H , Brandimore A.E. , Troche M.S. , Hegland K.W. . (2016). Analysis of clinicians’ perceptual cough evaluation. Dysphagia. https://doi.org/10.1007/s00455-016-9708-8 |
12 | Lee J.Y. , Kim D.-K , Seo K.M. , Kang S.H. . ((2014) ). Usefulness of the simplified cough test in evaluating cough reflex sensitivity as a screening test for silent aspiration. Annals of rehabilitation medicine, 38: (4), 476–484. https://doi.org/10.5535/arm.2014.38.4.476 |
13 | Mazzone S.B. , Undem B.J. , ((2016) ). Vagal afferent innervation of the airways in health and disease. Physiol Rev, 96: (3), 975–1024. https://doi.org/10.1152/physrev.00039.2015 |
14 | Miles A. , Huckabee M.-L. , ((2013) ). Intra- and inter-rater reliability for judgement of cough following citric acid inhalation. International Journal of Speech-Language Pathology, 15: (2), 209–215. https://doi.org/https://dx.doi.org/10.3109/17549507.2012.692812 |
15 | Miles A. , Huckabee M.-L. , McFarlane M. , ((2014) ). Inter-rater reliability for judgment of cough following citric acid inhalation after training. Speech, Language and Hearing, 17: , 204–209. https://doi.org/10.1179/2050572814Y.0000000040 |
16 | Miles A. , Huckabee M.-L. , McFarlane M. , ((2014) ). Inter-rater reliability for judgment of cough following citric acid inhalation after training [Article]. Speech, Language and Hearing, 17: (4), 204–209. https://doi.org/10.1179/2050572814Y.0000000040 |
17 | Miles A. , Moore S. , McFarlane M. , Lee F. , Allen J. , Huckabee M.L. , ((2013) ). Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav, 118: , 25–31. https://doi.org/10.1016/j.physbeh.2013.05.004 |
18 | Min S.W. , Oh S.H. , Kim G.C. , Sim Y.J. , Kim D.K. , Jeong H.J. , Allen J. , ((2018) ). Clinical importance of peak cough flow in dysphagia evaluation of patients diagnosed with ischemic stroke. Annals of rehabilitation medicine, 42: (6), 798–803. https://doi.org/10.5535/arm.2018.42.6.798 |
19 | Monroe M.D. , Manco K. , Bennett R. , Huckabee M.-L. , ((2014) ). Citric acid cough reflex test: Establishing normative data. Speech, Language and Hearing, 17: (4), 216–224. https://doi.org/10.1179/2050572814Y.0000000041 |
20 | Morice A.H. , Fontana G.A. , Belvisi M.G. , Birring S.S. , Chung K.F. , Dicpinigaitis P.V. , Kastelik J.A. , McGarvey L.P. , Smith J.A. , Tatar M. , Widdicombe J. , ((2007) ). ERS guidelines on the assessment of cough. European Respiratory Journal, 29: (6), 1256. https://doi.org/10.1183/09031936.00101006 |
21 | Nakamori M , Imamura E , Kuwabara M. , Ayukawa T. , Tachiyama K. , Kamimura T. , Hayashi Y. , Matsushima H. , Funai M. , Mizoue T. , Wakabayashi S. , ((2020) ). Simplified cough test can predict the risk for pneumonia in patients with acute stroke. PLOS ONE, 15: (9), e0239590. https://doi.org/10.1371/journal.pone.0239590 |
22 | Perry S.E. , Miles A. , Fink J.N. , Huckabee M.L. , ((2019) ). The Dysphagia in stroke protocol reduces aspiration pneumonia in patients with dysphagia following acute stroke: A clinical audit. Transl Stroke Res, 10: (1), 36–43. https://doi.org/10.1007/s12975-018-0625-z |
23 | Plowman E.K. , Watts S.A. , Robison R. , Tabor L. , Dion C. , Gaziano J. , Vu T. , Gooch C. , ((2016) ). Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia, 31: (3), 383–390. https://doi.org/10.1007/s00455-015-9687-1 |
24 | Sato M. , Tohara H. , Iida T. , Wada S. , Inoue M. , Ueda K. , ((2012) ). Simplified cough test for screening silent aspiration Archives of Physical Medicine and Rehabilitation, 93: (11), 1982–1986. https://doi.org/https://doi.org/10.1016/j.apmr.2012.05.016 |
25 | Smith Hammond C.A. , Goldstein L.B. , Horner R.D. , Ying J. , Gray L. , Gonzalez-Rothi L. , Bolser D.C. , ((2009) ). Predicting aspiration in patients with ischemic stroke: Comparison of clinical signs and aerodynamic measures of voluntary cough [Research Support, U.S. Gov’t, Non-P.H.S.]. Chest, 135: (3), 769–777. https://doi.org/http://dx.doi.org/10.1378/chest.08-1122 |
26 | Tabor-Gray L. , Vasilopoulos T. , Wheeler-Hegland K. , Wymer J. , Plowman E.K. , ((2021) ). Reflexive airway sensorimotor responses in individuals with amyotrophic lateral sclerosis. Dysphagia, 36: (4), 574–582. https://doi.org/10.1007/s00455-020-10171-6 |
27 | Troche M.S. , Brandimore A.E. , Godoy J. , Hegland K.W. . ((2014) ). A framework for understanding shared substrates of airway protection. Journal of Applied Oral Science: Revista FOB, 22: (4), 251–260. https://doi.org/10.1590/1678-775720140132 |
28 | Troche M.S. , Brandimore A.E. , Okun M.S. , Davenport P.W. . Hegland K.W. . ((2014) ). Decreased cough sensitivity and aspiration in Parkinson disease. Chest, 146: (5), 1294–1299. https://doi.org/10.1378/chest.14-0066 |
29 | Wakasugi Y. , Tohara H. , Hattori F. , Motohashi Y. , Nakane A. , Goto S. , Ouchi Y. , Mikushi S. , Takeuchi S. , Uematsu H. . ((2008) ). Screening test for silent aspiration at the bedside .Dysphagia, 23: (4), 364–370. https://doi.org/10.1007/s00455-008-9150-7 |
30 | Wakasugi Y. , Tohara H. , Nakane A. , Murata S. , Mikushi S. , Susa C. , Takashima M. , Umeda Y. , Suzuki R. , Uematsu H. . ((2014) ). Usefulness of a handheld nebulizer in cough test to screen for silent aspiration. Odontology, 102: (1), 76–80. https://doi.org/10.1007/s10266-012-0085-y |
31 | Wallace E. , Guiu Hernandez E. , Ang A. , Hiew S. , Macrae P. . ((2019) ). A systematic review of methods of citric acid cough reflex testing. Pulm Pharmacol Ther, 58: , 101827. https://doi.org/10.1016/j.pupt.2019.101827 |
32 | Wallace E. , Guiu Hernandez E. , Ang A. , Macrae P. , ((2019) ). Quantifying test-retest variability of natural and suppressed citric acid cough thresholds and urge to cough ratings. Pulm Pharmacol Ther, 58: , 101838. https://doi.org/10.1016/j.pupt.2019.101838 |
33 | Wallace E. , Macrae P. , Huckabee M. L. , ((2021) ). Objective measurement of acoustic intensity of coughing for clearance of penetration and aspiration on video-fluoroscopy. Int J Speech Lang Pathol, 23: (3), 313–320. https://doi.org/10.1080/17549507.2020.1784280 |
34 | Widdicombe J.G. , Addington W.R. , Fontana G.A. , Stephens R.E. ((2011) ). Voluntary and reflex cough and the expiration reflex; implications for aspiration after stroke. Pulm Pharmacol Ther, 24: (3). 312–317. https://doi.org/10.1016/j.pupt.2011.01.015 |




