Fiberoptic endoscopic evaluation of swallowing as a tool to facilitate dysphagia rehabilitation following a salvage hemi-glossectomy: Case report
Abstract
Background:
Dysphagia is a common consequence of tongue cancer and its treatment, with the possibility of long-term diet modification and feeding tube dependence. This is likely to have an impact on the activity, participation and psychological wellbeing of the individual.
Objective:
This case report presents the use of fiberoptic endoscopic evaluation of swallow (FEES) as a tool to support dysphagia rehabilitation through providing visual feedback following a salvage hemi-glossectomy for management of a recurrent squamous cell carcinoma (SCC) in the right anterolateral tongue.
Methods:
Clinician-rated and patient-reported outcome measures were used to assess the effectiveness of FEES as a tool to facilitate dysphagia rehabilitation. These measures include the: Performance Status Scale for Head and Neck (PSS-HN) cancer patients, 100 mL water swallow test (WST), Penetration-Aspiration Scale (PAS), the Yale Pharyngeal Residue Severity Rating Scale, and the Functional Intraoral Glasgow Scale (FIGS).
Results:
No Statistical tests were performed on this single case, however the results demonstrate a notable clinical improvement in all postoperative outcome measures at 12-months when compared with those taken two-months postoperatively.
Conclusion:
Use of sequential FEES could enhance patient engagement and inform dysphagia rehabilitation following hemi-glossectomy.
1Introduction
The development of increasingly aggressive treatments for oral cancer has resulted in reduced mortality in this population (Breen et al., 2017; Perry et al., 2016). However, treatment morbidity has increased with years lost to rising disability (Breen et al., 2017; Perry et al., 2016). Dysphagia remains the most common side effect of head and neck cancer treatment with those treated for oral and oropharyngeal cancers at most risk of swallowing difficulties with 50% of individuals less likely to achieve oral intake when compared with malignancies in other sites of the head and neck (Kalavrezos et al., 2013).
The primary treatment for tongue cancer is surgery with the need for adjuvant radiotherapy or chemoradiotherapy in larger tumours (Bhattacharya et al., 2021). Due to the critical function of the tongue in swallowing, surgical resection and reconstruction is likely to result in oral stage dysphagia with consequences for the pharyngeal stage of swallowing (Kalavrezos et al., 2013). The use of adjuvant radiotherapy is likely to further exacerbate oral and pharyngeal stage dysphagia (Kalavrezos et al., 2013). Speech and language therapy plays a critical role in swallow rehabilitation following treatment for tongue cancer (Ihara et al., 2021; Manikantan et al., 2009; Pyne at al., 2020). Videofluoroscopy (VFS) is the gold standard for the assessment of dysphagia following treatment for tongue cancer as it allows for visualisation of the oral stage of swallowing (Bhattacharya et al., 2021). Further, compensatory and rehabilitation exercises can be implemented to assess their effectiveness during the VFS procedure (Clark et al., 2016). In contrast, fiberoptic endoscopic evaluation of swallow (FEES) is limited in its assessment of the oral phase with only the base of tongue visible (Nacci et al., 2008). However, the literature suggests that in cases of advanced tongue malignancies, pharyngeal dysphagia may also occur due to the presence of oral stage dysphagia and other oncological interventions including neck dissection and chemo/radiotherapy (Bhattacharya et al., 2021; Ihara et al., 2021; Kalavrezos et al., 2013). In these instances, FEES can be used as an adjunct to VFS for diagnostic assessment of dysphagia. We were interested to explore whether the use of FEES had potential to be extended to support dysphagia rehabilitation following treatment for tongue cancer particularly due to the high prevalence of reduced sensation (Bhattacharya et al., 2021). FEES can be adjusted to provide live biofeedback, facilitating accurate execution of swallow exercises. It allows for ongoing assessment of dysphagia without the risks of increased radiation exposure. To our knowledge, the use of FEES as a tool to facilitate the management of dysphagia following major ablative surgery and reconstruction of the tongue has not been previously reported. This case report introduces the concept of using sequential FEES to support dysphagia rehabilitation following salvage surgery for a recurrent squamous cell carcinoma (SCC) in the anterolateral tongue.
2Methods
2.1Consent
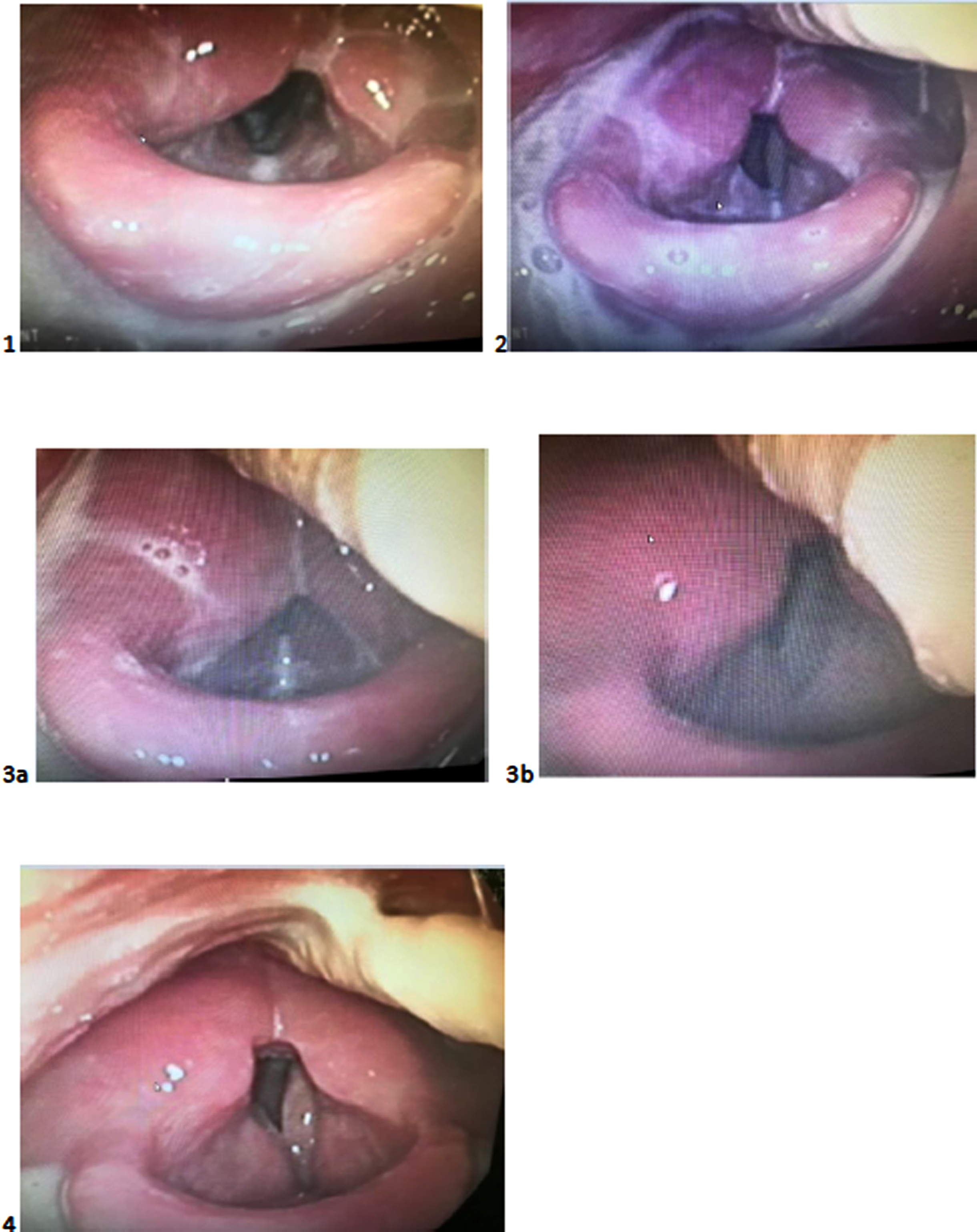
At the time that this article was submitted the patient had passed away and consent for publication and the images presented in Fig. 2 was therefore provided by the patient’s family. Figure 2 has been copyrighted.
2.2Patient information
This article presents a case study of a 57-year-old male patient who was seen for swallow rehabilitation at his local Head and Neck Cancer Service following salvage surgery for recurrent SCC in the right floor of mouth and anterolateral tongue. Two years prior he had been treated with chemoradiotherapy for a right tonsil SCC. The recurrent disease was treated with a right hemiglossectomy, right neck dissection and left anterolateral (ALT) free flap reconstruction and tonsillectomy. Postoperative complications required a salvage flap, due to accumulation of pus on the right internal jugular vein, with debridement and washout.
The classification system proposed by Bhattacharya and colleagues (Bhattacharaya et al, 2021) is used here. It considers the volume of the tumour and its location when assessing the extent of the glossectomy. The tumour volume was classified as grade III and the location as a lateral defect. The classification system is presented in appendix 1.
2.3Outcome measures
The outcome measures used to assess progress through rehabilitation were the Penetration Aspiration Scale (PAS) (Rosenbek et al., 1996), the Yale Pharyngeal Residue Severity Rating Scale (Neubaher et al., 2015), Performance Status Scale for Head and Neck (PSS-HN) cancer patients (List et al., 1990), the 100 mL Water Swallow Test (WST) (Wu et al., 2004), and the Functional Intraoral Glasgow Scale (FIGS) (Nicoletti et al., 2004). Diet and fluids were classified using the international dysphagia diet standardisation initiative (International dysphagia diet standardisation initiative, 2021).
The PAS denotes penetration and aspiration during VFS and FEES (Rosenbek et al., 1996) and the Yale Pharyngeal Residue Severity Rating Scale provides ratings of the presence and severity of post swallow vallecular and pyriform sinus residue during FEES (Neubaher et al., 2015). The 100 mL WST is used as an adjunct to a clinical assessment, providing a measure of swallow performance over time (Patterson et al., 2011; Wu et al., 2004). The PSS-HN includes three sub scales, however, only the normalcy of diet and public eating scales are presented (List et al., 1990). Normalcy of diet describes the diet an individual is managing (List et al., 1990). The public eating scale explores the impact of dysphagia on an individual’s ability to eat in public (List et al., 1990). The FIGS is a patient reported outcome measure used to assess the patient’s perception of their ability to speak, chew and swallow (Nicoletti et al., 2004). The results of the chew and swallow sub scales are presented.
The PSS-HN and the FIGS were completed during clinic appointments preoperatively and at two and twelve months postoperatively. The 100 mL WST was assessed preoperatively and twelve-months postoperatively. Due to the risk of aspiration the 100 mL WST was not completed two-months postoperatively. The PAS was implemented during all VFS and FEES assessments. The Yale Pharyngeal Residue Severity Rating Scale was assessed on all FEES.
2.4Patient directed swallow rehabilitation goals
Two months after surgery, the patient was transferred to his local hospital where he, his wife and the speech and language therapist (SLT) agreed the following long-term swallow rehabilitation goal: the patient would manage a meal at his daughter’s wedding in twelve months without the need for alternative feeding. The consistency of the diet was not specified at this stage.
2.5Swallow rehabilitation
A VFS completed at the tertiary Head and Neck Cancer Centre, two weeks postoperatively, demonstrated significant oropharyngeal dysphagia with impairment of safety and efficacy of the swallow. Recommendations following the VFS included nil by mouth with all nutrition to be provided via a nasogastric tube and intensive swallow rehabilitation to be commenced following transfer to the local Head and Neck Cancer Service.
Six weeks following the initial VFS the patient commenced swallow rehabilitation. A repeat VFS was completed to establish a baseline of swallow function and to guide rehabilitation. Results of the VFS demonstrated severe oropharyngeal dysphagia with salient features including impaired bolus preparation, reduced glossopalatal seal, reduced base of tongue retraction, reduced hyolaryngeal excursion, absent epiglottic deflection, reduced pharyngeal contraction and pre and post swallow aspiration. The patient was not sensate to aspiration. Compensatory and rehabilitation exercises were implemented to assess their effectiveness (Arrese & Lazarus, 2013; Logemann, 1998). A head turn to the non-affected side was implemented to reduce bolus pooling on the affected side of the oral cavity (Arrese & Lazarus, 2013; Logemann, 1998). The supraglottic swallow was implemented to manage pre-swallow spillover and reduced airway protection (Arrese & Lazarus, 2013; Logemann, 1998). The VFS demonstrated that the head turn was not effective however, the supraglottic swallow using a 2 mL bolus delivered by syringe was effective and did not compromise airway safety. The supraglottic swallow therefore formed the basis of the subsequent swallow rehabilitation.
The VFS results suggested the possibility of the patient needing long-term alternative feeding. Factors including the site and size of the tumour, the extent of the resection and preoperative radiotherapy increased the likelihood of long-term feeding tube dependence (Bhattacharya et al., 2021; Kalavrezos et al., 2013; Perry et al., 2016). On this basis, a referral was made to Gastroenterology for a percutaneous endoscopic gastrostomy (PEG).
At the same time, we also planned to commence an active swallow rehabilitation programme. We elected to use FEES to support swallow rehabilitation to determine whether it was helpful in providing biofeedback and/or increased patient understanding and engagement. FEES would also allow for ongoing assessment of pharyngeal dysphagia. In total, five FEES were completed within a four-month period. The initial FEES was completed three weeks after the second VFS with the remaining four procedures completed at intervals ranging from two to five weeks apart. Two FEES trained SLTs were present during each procedure. One adopted the role of the endoscopist and the other provided instructions and guided the patient during the session. The screen was positioned so that it was visible to the patient throughout the procedure. At the time of the initial FEES the anatomy was explained to both the patient and his wife. Milk was used on trials of level 0-normal fluids to ensure visibility. Controlled fluid boluses were delivered via syringe. The swallow exercises were described and demonstrated to the patient at the start of each FEES guided rehabilitation session to minimise the duration of each procedure.
Recommendations made following the VFS were implemented during the initial FEES. The supraglottic swallow was completed five times, facilitating establishment of accurate execution of this manoeuvre. The assessment demonstrated that a 3 mL bolus of level 0-normal fluids could be tolerated without airway compromise when the supraglottic swallow was implemented. A still image taken from the FEES completed on the 18th of January demonstrates level 0- normal fluids on the anterior commissure prior to implementation of the cough as part of the supraglottic swallow (Fig. 2, Image 1). On this basis, restricted 3 mL boluses delivered by syringe of level 0-normal fluids with the supraglottic swallow was recommended. The suggested frequency of these trials was five boluses, five times daily.
The aim of the second and third FEES was to introduce a level 4-pureed diet and to trial increasing the quantity of level 0- normal fluids using cup sips. FEES demonstrated that post swallow vallecular residue noted on the level 4-pureed diet was eliminated with a combination of a head turn to the right side and multiple effortful clearing swallows (Manikantan et al., 2009). FEES revealed extensive pre-swallow spillover on cup sips of level-0 normal fluids. This resulted in an increased volume of aspiration, compromising airway safety. The volume of pre-swallow spillover and passage of the bolus over the lateral channels is highlighted in the image taken on the 1st of February (Fig. 2, Image 2). Images taken on the 15th of March demonstrate the effectiveness of a post swallow cough in clearing aspiration on a restricted 3 mL bolus of level 0-normal fluids delivered via syringe (Fig. 2, Images 3a and 3b). Recommendations for level 0-normal fluids, therefore, remained the same as the initial FEES. The recommended frequency of the pureed diet was five teaspoons, five times daily with a head turn to the right and multiple effortful clearing swallows.
A significant improvement in pharyngeal contraction and airway protection was noted on the fourth and fifth FEES. FEES demonstrated that the supraglottic swallow on fluids was not required with an intermittent cough and throat clear effective in reducing trace, silent aspiration such that airway safety was not compromised. Following the fourth FEES two 125 g pots of custard/yoghurt per day was recommended. It was suggested that these pots could be eaten in part or as a whole. In addition, water with no limitations on volume was encouraged. One cup of tea per day was permitted as this was important for the patient’s quality of life. Figure 2 demonstrates the absence of post swallow pharyngeal residue and aspiration on cup sips of level 0-normal fluids on the 5th of April (Fig 2, Image 4). Following the final FEES, a level 4-pureed diet and level 0-normal fluids with no restrictions on quantity was recommended. An intermittent cough and multiple effortful dry swallows were encouraged on level 0-normal fluids and a level 4-pureed diet respectively.
A summary of recommendations made following each FEES is provided in Table 1.
Table 1
Rehabilitation programme
| Date | Fluid recommendations | Diet recommendations |
| 18th January | Consistency: level 0-normal fluids Quantity/frequency: 3 mL bolus via syringe; 5 boluses, 5 times daily Rehabilitation exercises: supraglottic swallow | |
| 1st February | Consistency: level 0-normal fluids Quantity: 3 mL bolus via syringe; 5 boluses, 5 times daily Rehabilitation exercises: supraglottic swallow | Consistency: level 4-pureed diet Quantity/frequency: 5 tsps, 5 times daily Rehabilitation exercises: effortful swallow and right head turn |
| 15th March | Consistency: level 0-normal fluids Quantity/frequency: 3 mL bolus via syringe, 5 boluses, 5 times daily Rehabilitation exercises: supraglottic swallow | Consistency: level 4-pureed diet Quantity/frequency: 5 tsps, 5 times daily Rehabilitation exercises: effortful swallow and right head turn |
| 5th April | Consistency: Level 0-normal fluids Quantity/frequency: unrestricted water and 1 cup of tea per day Rehabilitation exercises: intermittent post swallow cough | Consistency: level 4-pureed diet Quantity/frequency: 2,125 g pots of custard/yoghurt Rehabilitation exercises: effortful swallow and right head turn |
| 10th May | Consistency: Level 0-normal fluids Quantity/frequency: unrestricted Rehabilitation exercises: intermittent post swallow cough and effortful dry swallows | Consistency: level 4-pureed diet Quantity/frequency: Unrestricted Rehabilitation exercises: effortful swallow and intermittent post swallow cough |
Standard swallow rehabilitation appointments were attended in between FEES appointments. These appointments facilitated further practise of swallow exercises recommended during FEES appointments. Initially, these appointments were weekly, however, the duration between appointments increased as the patient progressed through rehabilitation. In addition to swallow exercises introduced during FEES appointments, thermotactile stimulation was implemented with the aim of improving sensory feedback (Manikantan et al., 2009). Tongue exercises targeting strength and range of movement were introduced to the dysphagia rehabilitation program (Manikantan et al., 2009).
3Results/Findings
3.1Outcome measures
The following preoperative outcomes measures were recorded at the tertiary Head and Neck Cancer Center. Results of the PSS-HN were as follows: normalcy of diet- 50 indicating soft chewable foods and public eating- 75 indicating no restriction of place, but restricts diet when in public (List et al., 1990). Results of the 100 mL WST were as follows: volume: 20 mL/swallow, capacity: 10 mL/second and speed: 2 second/swallow. The patient scored four on the chew and swallow sub scales of the FIGS. This indicates solid food with difficulty (Nicoletti et al., 2004). A PAS rating was not completed preoperatively.
The following outcome measures were recorded on discharge from the tertiary Head and Neck Cancer Center. Results of the PSS-HN were as follows: normalcy of diet -0, indicating non-oral feeding (tube feeding) and public eating-999 indicating inpatient (List et al., 1990). As discussed above, due to the high risk of aspiration, the 100 mL WST was not completed. The patient scored one on the chew and swallow subscales of the FIGS. This indicates cannot chew/swallow at all (Nicoletti et al., 2004). Following the initial postoperative VFS a PAS score of eight was recorded on level 0-normal fluids. This indicates aspiration with no effort to clear aspirated material (Rosenbek et al., 1996). Diet was not assessed on the initial VFS due to the risk of aspiration.
The following outcome measures were recorded at the point of discharge from swallow rehabilitation. Results of the PSS-HN were as follows: normalcy of diet-30, indicating pureed food and public eating-75, indicating no restrictions of place but restricts diet when in public (List et al., 1990). Results of the 100 mL WST were as follows: volume: 11.11 ml/swallow, capacity: 6.49 ml/sec and speed: 1.7 sec/swallow. The patient scored two and three on the chew and swallow sub scales of the FIGS respectively. This indicates semi-sold food with difficulty (Nicoletti et al., 2004). On the final FEES, a PAS score of eight was recorded on level-0 normal fluids when an intermittent cough was not implemented. On implementation of an intermittent cough a PAS score of six was recorded, indicating material enters the trachea and is spontaneously cleared into the larynx or pharynx (Rosenbek et., 1996). A PAS score of one was recorded on the level 4-pureed diet, indicating the absence of aspiration (Rosenbek et., 1996). Results of the PSS-HN, 100 mL WST, FIGS and PAS are summarised in Table 2.
Table 2
Results-PSS-HN, WST, FIGS, PAS
| PSS-HN | PSS-HN | WST | FIGS | PAS | |||||
| Normalcy of diet | Public eating | V | C | S | Ch | S | L0 | L4 | |
| Pre-operative | 50 | 75 | 20 | 10 | 2 | 4 | 4 | – | – |
| Two-months postoperative | 0 | 999 | – | – | – | 1 | 1 | 8 | – |
| 12-months postoperative | 30 | 75 | 11.11 | 6.49 | 1.7 | 2 | 3 | 6 | 1 |
Post swallow vallecular residue increased between the third and fourth FEES on level 0-normal fluids and was eliminated at the time of the final FEES. Post swallow vallecular residue on the level 4-pureed diet increased between the second and third FEES and then remained stable until discharge from swallow rehabilitation. Overall post swallow pyriform sinus residue remained stable over time. Results of the Yale Pharyngeal Residue Severity Rating Scale assessed on level 0-normal fluids and a level 4 pureed diet are summarised in Tables 3 and 4 respectively.
Table 3
Vallecular and pyriform sinus residue on level 0-normal fluids
| Vallecular | Pyriform sinuses | |
| 18th January | mild | mild |
| 1st February | mild | mild |
| 15th March | moderate | mild |
| 5th April | moderate | mild |
| 10th May | none | mild |
• PSS-HN:30-pureed diet, 50-soft chewable foods, 999-inpatient, 75-restrictions of place but restricts diet when in public
• WST: V: volume, C: chew, S: swallow, Ch: chew
• PAS: L0: Level 0 –normal fluids, L4: Level 4-pureed diet
Table 4
Vallecular and pyriform sinus on a level 4-pureed diet
| Vallecular | Pyriform sinuses | |
| 18th January | – | – |
| 1st February | mild | mild |
| 15th March | moderate | mild |
| 5th April | moderate | mild |
| 10th May | moderate | trace |
3.2Patient directed swallow rehabilitation goal
The patient reported managing a level 4-pureed diet and level 0-normal fluids at his daughter wedding. The PEG was removed twelve-months postoperatively.
4Discussion
Results of this case study demonstrate an initial postoperative deterioration in all patient and clinician reported outcomes measures when compared with those taken preoperatively. Several predictive factors are likely to have impacted on the immediate postoperative deterioration in swallow function. The patient discussed in this case study received preoperative radiotherapy leading to severe fibrosis and poor vascularity (Weert & Leemans, 2021). This increased the likelihood of postoperative complications and poor postoperative functional outcomes (Dzioba et al., 2017; Weert & Leemans, 2021). In addition, the patient had presented with a class III tumour volume, affecting the lateral tongue. Prior to surgery there were reports of swallowing difficulties and consequent diet modification. This was likely to be due to both radiotherapy dysphagia morbidity and large recurrent disease (Bhattacharya et al., 2021; Kalavrezos et al., 2013).
Findings of the VFSs completed at the tertiary and local Head and Neck Cancer Services were consistent with research that has demonstrated that class III defects and radiotherapy were significantly associated with pharyngeal dysphagia (Bhattacharya et al., 2021). It is likely that the neck dissection further exacerbated the pharyngeal dysphagia observed (Ihara et al., 2021). VFS was used to establish a baseline of swallow function as it allows for visualisation of the oral phase. This is critical in the assessment of dysphagia following treatment for tongue cancer. In addition, VFS enabled the assessment of the effectiveness of compensatory and rehabilitation swallow exercises (Clark et al., 2016; Logemann, 1998). While the rationale for implementing a head turn was reasonable, VFS demonstrated it was not functionally effective. VFS established the effectiveness of the supraglottic swallow and provided information about the bolus size that was therapeutic without compromising airway safety.
The extent of the dysphagia and the risk of aspiration evident on VFS indicated the need for frequent instrumental assessments. In addition, it was anticipated that biofeedback would be beneficial due to reduced sensation resulting in delayed swallow initiation, post-swallow pharyngeal residue and aspiration. Frequent VFS assessments were not a viable option due to the use of radiation and the requirement for pre-booking in radiology with limited availability of slots allocated to speech and language therapy services. Additionally, VFS does not allow for live biofeedback with the images reviewed with the patient following the assessment. Assessment of the oral phase is limited on FEES with only base of tongue retraction visible (Nacci et al., 2008). However, FEES allows for assessment of pharyngeal parameters likely to be affected by oral stage dysphagia, neck dissection and radiotherapy. FEES is cost-effective and provides increased accessibility as it can be completed in the clinic room (Nacci et al., 2008). Further, FEES does not use radiation and can therefore be used with increased frequency when compared with VFS (Nacci et al., 2008) and potentially to observe a whole meal. It was anticipated that the nasendoscope would be well tolerated due to reduced sensation.
This case report demonstrates the use of FEES as a tool to facilitate dysphagia rehabilitation. FEES allowed for live biofeedback, which is likely to have facilitated swallow rehabilitation particularly in the context of reduced sensation. Additionally, FEES was used to increase the patient’s understanding of pharyngeal and laryngeal anatomy, the presence and impact of the dysphagia and the rationale behind the swallow exercises selected. For example, FEES demonstrated the effectiveness of the cough as part of the supraglottic swallow in reducing and clearing aspiration on fluids. It also enabled the patient to ensure that his post swallow cough was timely. FEES demonstrated the extent of post swallow pharyngeal residue on the level-4 pureed diet. It re-enforced the potential for aspiration of post swallow residue and the need for multiple effortful clearing swallows. FEES enabled the patient to calibrate his effort when implementing the effortful swallow. Biofeedback allowed for self-monitoring on the number of effortful dry swallows required to clear pharyngeal residue.
Findings of this case report demonstrate an improvement in twelve-month postoperative outcome measures when compared with those taken two-months postoperatively. Preoperative swallow function was not attained, however, the patient was not expected to achieve this outcome due to factors including preoperative radiotherapy, preoperative diet modification, the location of the tumour and the extent of the resection (Bhattacharya et al., 2021; Kalavrezos et al., 2013). In addition, PEG removal was not an anticipated outcome given previous research which demonstrated increased risk of feeding tube dependence in this population (Kalavrezos et al., 2013). A fascio-cutaneous ALT free flap was used in the reconstruction. This type of free flap is thin and pliable and has therefore been demonstrated to result in improved swallow function (Kalavrezos et al., 2013). However, these benefits may not have been realised due to prior radiotherapy and postoperative complications (Weert & Leemans, 2021).
Twelve months postoperatively the only outcome measure to improve to that recorded preoperatively was the public eating rating. This finding may be due to patient acceptance of dysphagia morbidity as time post-treatment lapsed. Increased acceptance of postoperative functional outcomes may have enabled the patient to engage in personally meaningful activities, such as participating in his daughter’s wedding, within the limitations of his swallow impairment. The minimally clinically important difference (MCID) between scores on the PSS-HN is 10 points (Hutcheson et al., 2016). Therefore, the improvement in scores on the normalcy of diet and public eating scales achieved twelve months postoperatively when compared with two-month postoperative scores is clinically significant. It is not possible to determine whether changes in FIGS scores were significant as the MCID for this scale has not been established.
The increase in vallecular residue between the third and fourth FEES on level 0-normal fluids is consistent with the increase in bolus volume. The elimination of post-swallow vallecular residue on level 0-normal fluids at the time of the final FEES may be due to reduced oedema, increased sensation and improved pharyngeal contraction. The increase in post-swallow vallecular residue on the level 4-pureed diet between the second and the third FEES is consistent with the increase in bolus volume. Ratings of post swallow vallecular residue on a level 4-pureed diet remained stable over time. It is hypothesised that this is due to the viscosity of the consistency and ongoing impaired pharyngeal contraction. It is likely that pyriform sinus residue remained stable over time as this region was not affected by the surgical resection and reconstruction. PAS scores indicated persistent aspiration on level 0-normal fluids when swallow rehabilitation and compensatory strategies were not implemented. While results of the final FEES indicated that aspiration on level 0-normal fluids was eliminated with an intermittent cough, it was anticipated that this strategy was unlikely to be implemented with the frequency required to eliminate the risk of aspiration. Following discussions with the patient and the multidisciplinary team, level 0-normal fluids were recommended due to findings of the FEES, the absence of chest infections, good performance status and the patient’s wishes. The patient did not progress beyond a level 4-pureed diet due to the severity of the ongoing oral stage dysphagia.
It is likely that use of FEES as a tool to support dysphagia rehabilitation facilitated swallow recovery such that feeding tube independence was achieved. It is hypothesised that this was due to live biofeedback which facilitated self-monitoring of performance during swallow exercises. In addition, the frequency at which FEES could be performed contributed to timely diet and fluid upgrades. Reliance on VFS to assess the safety of diet and fluid upgrades would have delayed progress and increased the duration of feeding tube dependance. Increased patient understanding of swallow function and swallow exercises enabled by live visual feedback may have enhanced patient engagement in swallow rehabilitation, however, this hypothesis requires further testing. It should be noted that FEES is not recommended as a replacement of VFS in the diagnostic assessment of dysphagia following treatment for tongue cancer as visualisation of the oral phase is essential.
4.1Future research
This single case study describes the application of FEES as a tool to support dysphagia rehabilitation. However, further research is required to establish the efficacy and/or any additional benefit of FEES when used to support dysphagia rehabilitation. Sequential FEES was employed in this case example, however the optimal frequency of FEES use as a tool to facilitate dysphagia rehabilitation needs to be established. The development of a FEES intervention protocol would be of clinical benefit. A better understanding of the potential benefits of live biofeedback on patient engagement could also be explored through qualitative methods. In addition, the cost effectiveness of the sequential use of FEES to support dysphagia rehabilitation needs to be established. This could be explored through comparing patients who have undergone sequential FEES and those who receive standard swallow rehabilitation to establish whether the use of FEES has an impact on outcome measures such as the number of swallow rehabilitation sessions required to achieve feeding tube independence and feeding tube removal.
It would be useful for future research to consider the inclusion of alternative clinician and patient reported outcome measures. The MD Anderson Dysphagia Inventory (MDADI) could be used to assess swallowing related quality of life (Hutcheson et al., 2016). The MCID in MDADI scores is known and this could be used to assess whether swallow rehabilitation is having a clinically significant impact on quality of life (Hutcheson et al., 2016). In this case study, the normalcy of diet scale was used as a measure of swallow function as it was designed specifically for the head and neck cancer population and is an agreed outcome measure in our geographic region. For future large-scale research, we acknowledge that other scales such as the Functional Oral Intake Scale (FOIS) may also be useful. Similar to the PSS normalcy of diet, the FOIS, is time efficient, consistently reproducible and provides an accurate measure of swallow function in daily life (Crary et al., 2005). Use of the Dysphagia Outcomes and Severity Scale Score would be beneficial as a means of assessing dysphagia severity based on VFS results (O’Neil et al., 1999).
Thermotacile stimulation was implemented in the absence of an evidence base. As this is a single case study where multiple therapeutic approaches were implemented it is not possible to determine whether this contributed to an improvement in swallow function. Future research is required to establish its efficacy in this population. It would be beneficial to explore tongue pressure measurements and their impact on tongue function in addition to the role of surgical recovery at the oral preparatory phase.
5Conclusion
This case report presents the potential benefits of FEES as tool to facilitate the optimisation of swallow function following major ablative surgery and reconstruction of the tongue. The use of live biofeedback and the increased frequency at which FEES can be performed are potential factors that may contribute to enhanced swallow rehabilitation following multimodality treatment for tongue cancer.
Acknowledgments
The authors thank Ms. Deepti Sinha and Dr. Bernadette Henderson for their guidance when writing up this article. A special thanks to the patient presented in this case study for entrusting the authors with his dysphagia management and to his family for their generosity in consenting for this article to be published.
Declaration of interest
The authors have no conflict of interest to report. Given her role as an editorial board member, Roganie Govender had no involvement in the peer review of this article.
References
1 | Arrese L.C. , Lazarus C. ((2013) ). Special groups: head and Neck cancer. Otolaryngologic clinics of North America, 46: 6, 1123–1136.https://doi.org/10.1016/j.otc.2013.08.009. |
2 | Bhattacharya S , Thankappan, K , Joseph, S.T , Sukumaran, S.V. , Shetty, S. , Mayadevi, M. , Balasubramanian, D. , Iver, S. ((2012) ). Volume and location of the defect as predictors of swallowingoutcome after glossectomy: Correlation with a classification. Dysphagia, 36: 6, 974–983.https://doi.org/10.1007/s00455-020-10224-w. |
3 | Breen L.J. , O’Connor M. , Calder S. , Tai V. , Cartwright J. , &Bailey J.M. ((2017) ). The health professionals’ perspective ofsupport needs of adult head and neck cancer survivors and theirfamilies: a Delphi Study. Support Care Cancer, 25: , 2413–2420.https://doi.org/10.1007/s00520-017-3647-2. |
4 | Clark, P. , Radford, K. , Coffey M. , & Stewart M. ((2016) ). Speech and swallow rehabilitation in head and neck cancer: United Kingdom national multidisciplinary guidelines. The Journal of Laryngology & Otology, 130: S2, S176–S180.https://doi.org/10.1017/S0022215116000608. |
5 | Crary, M.A , Mann, G.D.C. , Groher M.E. , ((2005) ). Initial psychometric assessment of a functional oral intake scale for dysphagia in stoke patients. Arch Phys Med Rehabil,, 86: 8, 1516–1520.https://doi.org/10.1016/j.apmr.2004.11.049. |
6 | Dizioba A , Aalto D. , Papadopoulos-Nydam G. , Seikaly H. , Rieger J. , Wolfaardt J. , Osswald M. , Harris J.R. , O’Connell D.A , Lazarus C , Urken M. , Likterov I. , Chai R.L. , Rauscher E. , Buchbinder D. , Okay D. , Happonen R.P. , Kinnunen I. , Irjala H. , Socka T. , Laine J. , ((2017) ). Functional and quality of life outcomes after partial glossectomy: a multi institutional longitudinal study of the head and neck research network. Journal of otolaryngology- Head & Neck Surgery, 46: 1, 46–56.https://doi.org/10.1186/s40463-017-0234-y. |
7 | Hutcheson, K.A , Barrow, M.P , Lisec, A , Barringer D.A , Gries, K , Lewin, J.S. ((2016) ). What is a clinically relevant difference in MDADI scores between groups of head and neck Cancer patients? Laryngoscope, 126: 5, 1108–1113.https://doi.org/10.1002/lary.25778". |
8 | Ihara, Y , Tashimo, Y , Nozue, S , Iizumi, Y , Fukunishi, Y , Saito, Y , Shimane, T , Takahashi, K. ((2021) ). Changes in oral function and quality of life in tongue cancer patients based on resected area. Asian Pacific Journal of Cancer Prevention, 22: 8, 2549–2557.https://doi.org/10.31557/APJCP.2021.22.8.2549. |
9 | International dysphagia diet standardisation initiative. (2021, December). The IDDSI framework. International dysphagia diet standardisation initiative.https://iddsi.org/framework |
10 | Kalavrezos, N , Cotrufo, S , Govender, R , Rogers, P , Pirgousis, P , Balasundram, S , Lalabekyan, B , Liew, C. ((2013) ). Factors affecting swallow outcome following treatment for advanced oral and oropharyngeal malignancies. Head & Neck, 36: 1, 47–54.https://doi.org/10.1002/hed.23262. |
11 | List, M.A , Ritter-Sterr, C , Lansky, S.B. ((1990) ). A perforance status scale for head and neck cancer patients. Cancer, 66: 3, 564–163.https://doi.org/10.1080/02699050701481597. |
12 | Logemann, J.A. (1998). Evaluation and treatment of swallowing disorders. PRO-ED. |
13 | Manikantan, K , Khode, S , Sayed, S.I , Roe, J , Nutting, C.M , Rhys-Evans, P , Harrington, K.J , Kazi, R. ((2009) ). Dysphagia in head and neck cancer. Cancer Treatment Reviews,, 35: 8, 724–773.https://doi.org/10.1016/j.ctrv.2009.08.00815/10/2022. |
14 | Nacci, A , Ursino, F , La Vela R , Matteucci, F , Mallardi, V , Fattori, B. ((2008) ). Fiberoptic endoscopic evaluation of swallowing (FEES): proposal for informed consent. ACTA Otorhinolaryn gologica Italica, 28: 4, 206–211. |
15 | Neubauer, P.D , Rademaker, A.W , Leder, S.B. ((2015) ). The yale pharyngeal residue severity rating scale: An anatomically defined and image-base tool. Dysphagia, 30: , 512–528.https://doi.org/10.1007/s00455-015-9631-4 . |
16 | Nicoletti, G , Soutar, D.S , Jackson, M.S , Wrench, A.A , Robertson, G. ((2004) ). Objective assessment of speech after surgical treatment for oral cancer: experience from 196 cases. Plastic Reconstructive Surgery, 113: 1, 114–125.https://doi.org/10.1097/01.PRS.0000095937.45812.84. |
17 | O’Neil, K.H , Purdy, M , Falk, J , Gallo, L. ((1999) ). The dysphagia outcome and severity scale. Dysphagia, 14: 3, 139–145.https://doi.org/10.1007/PL00009595. |
18 | Patterson, J.M , Hildreth, A , McColl, E , Carding, P.N , Hamilton, D , Wilson, J.A. ((2011) ). The clinical application of the 100mLwater swallowtest in head and neck cancer. Oral Oncology, 47: 3, 180–184.https://doi.org/10.1016/j.oraloncology.2010.11.020718. |
19 | Perry, A , Cotton, S , Lee, S.H , Kennedy, C. ((2016) ). Therapeutic exercises for affecting post-treatment swallowing in people treated for advanced-stage head and neck cancers. Cochrane, 8: , 1–51.DOI:https://doi.org/10.1002/14651858.CD011112.pub2 . |
20 | Pyne, J.M , Dziegielewski, P.T , Constantinescu, G , Dzioba, A , O’Connell, D.A , Cote, D.W.J , Ansari, K , Harris, J , Conrad, D , Makki F.M , Hearn, M , Biron, V.L , Seikaly. ((2020) ). The functional & quality of life outcomes of total glossectomy with laryngeal preservation. Laryngoscope Investigative Otolaryngology, 5: , 853–859.https://doi.org/10.1002/lio2.435 . |
21 | Rosenbek, J.C , Robbins, J.A , Roecker, E.B , Coyle, J.L , Wood, J.L ((1996) ). A penetration-aspiration scale. Dysphagia, 11: 2, 93–98.https://doi.org/10.1007/BF00417897. |
22 | Weert, S.V , Leemans, C.R. ((2020) ). Salvage surgery in head and neck cancer. Oral Disease,, 27: , 117–154.https://doi.org/10.1111/odi.13582. |
23 | World Health Organisation. (2013). How to use the ICF: A practical manual for using the International Classification of Functioning, Disability and Health (ICF). Retrieved August 8, 2021, from https://cdn.who.int/media/docs/default-source/classification/icf/drafticfpracticalmanual2.pdf?sfvrsn=8a214b014741 |
24 | Wu, M.C , Chang, Y.C , Wang, T.G , Lin, L.C. ((2004) ). Evaluating swallowing dysfunction using a 100-mlwater swallowing test. Dysphagia, 19: 1, 43–47.DOI:https://doi.org/10.1007/s00455-003-0030. |
Appendices
Appendix 1
Fig. 1
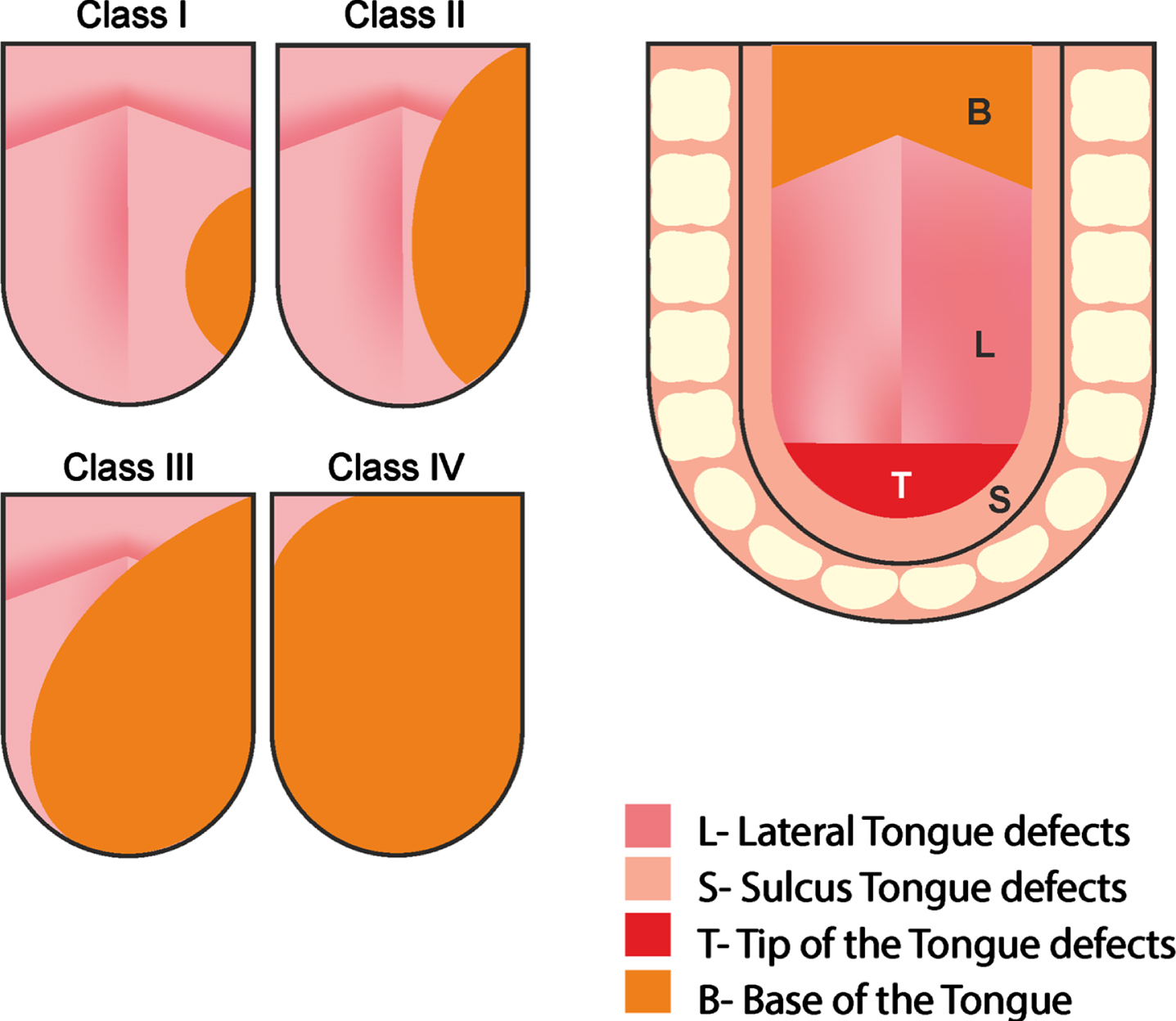
Note. This image was used to explain the defect assessment. From “Volume and location of the defect as predictors of swallowing outcome after glossectomy: Correlation with a classification”, by S. Bhattacharya, K. Thankappan, S. T. Joseph, S. V. Sukumaran, S. Shetty, M. Mayadevi, D. Balasubramanian and S. Iver, 2021, Dysphagia, 36(6), p. 976. Copyright 2021 by Springer Science+Business Media.
| Tumour volume | Description |
| Class I | Resection involving one third of the tongue |
| Class II | Resection involving one third to half of the tongue |
| Class III | Resection involving two thirds of the tongue |
| Class IV | Resection involving two thirds of the total tongue |
| Tumour location | Description |
| Lateral | Lateral tongue which may include the base of tongue |
| Tip | Tip defect anterior to the attachment of the frenulum linguae |
| Sulcus | Floor of mouth defect |
1) 18th of January: aspiration of level 0-normal fluids with the bolus visible on the anterior commissure and in the trachea.
2) 1st of February: level 0-normal fluids pooling in the vallecula and moving over the lateral channels into the supraglottis following cup sips.
3) 15th of March: aspiration of level 0-normal fluids (a) and effective clearance of aspiration following implementation of a cough as part of the supraglottic swallow (b).
4) 5th of April no evidence of aspiration following a cup sip of level 0-normal fluids when a throat clear/cough was implemented.
Fig. 2