Standardized Tapering off Subcutaneous Immunoglobulin in Chronic Inflammatory Demyelinating Polyneuropathy
Abstract
Background:
Attempting discontinuation of treatment in patients with chronic inflammatory demyelinating polyneuropathy (CIDP) is recommended. However, there is no evidence based regimen for tapering off subcutaneous immunoglobulin (SCIG). This trial investigated stepwise tapering off SCIG to detect remission and the lowest effective dosage. During tapering off, frequent vs less frequent clinical evaluation was compared.
Methods:
Patients with CIDP receiving a stable SCIG dosage followed a standardized tapering off regimen: 90%, 75%, 50%, 25% and 0% of the initial dose every 12th week, pending no deterioration occurred. In case of relapse during tapering off, the lowest effective dose was identified. Treatment with SCIG was registered for two years after participation. Disability score and grip strength were primary parameters. Participants were randomized to clinical evaluation every 6th week (frequent) or 12th week (less frequent).
Results:
Fifty-five patients were included of which thirty-five relapsed. Twenty patients (36%) were able to discontinue treatment without relapse. In relapsing patients, median dosage could be reduced by 10% (range, 0–75). After two years, 18 of 20 patients were still in remission without treatment. Frequent clinical evaluation did not detect deterioration more frequently than less frequent evaluation; RR 0.5 (95% CI, 0.2–1.2) (p = 0.17).
Conclusion:
In stable CIDP patients, SCIG could be completely tapered off in 36% of the patients and only in 10% of these patients relapse occurred during the following two years. More frequent evaluation was not superior to detect deterioration.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) can be treated with subcutaneous immunoglobulin (SCIG) leading to improved muscle strength and reduced disability [1, 2]. Follow-up studies have reported remission of symptoms in up to 30% of patients with CIDP over time [3, 4]. It is recommended to attempt dose reduction or discontinuation of immunoglobulin treatment regularly to detect clinical remission [5]. This will reduce unnecessary immunoglobulin treatment, reduce side effects and result in lower costs. Evidence supporting the efficacy of immunoglobulin treatment in CIDP is substantial, but there is a lack of knowledge on how tapering off is performed safely and effectively.
In CIDP, initial treatment is usually given as intravenous immunoglobulin (IVIG). After stabilization is obtained, treatment can be switched to SCIG, which is often done 1 : 1 according to the weekly IVIG dosage [1, 6–8] or with a standardized dosage of 0.2 to 0.4 g/kg/week [2]. Knowledge is lacking on how to detect remission in CIDP other than by abrupt discontinuation of treatment, which does not enable detection of the lowest effective dose [9].
Detection of partial or full remission of CIDP in patients treated with IVIG is usually done by dose reduction at each infusion or prolonging of the interval between infusions [10, 11]. As SCIG is often administered every week prolonging intervals between injections seems not feasible and dosage reduction is thus the most obvious way to detect remission. However, a study has shown bolus administration of SCIG every second week with an unaltered mean weekly dose to be effective [12].
The aim of our study was to determine the prevalence of patients with CIDP in remission after complete discontinuation of SCIG treatment using a standardized tapering off regimen. Moreover, we aimed to determine the average reduction of weekly SCIG dosage in patients relapsing during tapering off. We compared frequent versus less frequent clinical evaluation to detect deterioration of symptoms.
METHODS
Study design and study population
Patients were eligible for inclusion if they had a definite, probable, or possible diagnosis of CIDP [13] including multifocal-aquired demyelinating sensory and motor neuropathy (MADSAM) and neuropathy associated with monoclonal gammopathy with unknown significance (MGUS; IgA, IgG and IgM antibodies); patients had received SCIG for more than three months. Exclusion criteria were treatment with SCIG for less than three months or change of SCIG dosage within three months prior to enrollment, treatment with other immunomodulatory agents or immunosuppressants or presence of anti-myelin associated glycoprotein antibody (anti-MAG). Patients were treated with either Gammanorm® (Octapharma Pharmaceuticals AG, Austria) or Hizentra® (CSL Behring AB, Sweden).
Participants were recruited from four neurological departments treating all patients with CIDP in Denmark. The study was performed between March 2018 and June 2022.
Reduction in weekly dosage of SCIG was performed using a standardized regimen including stepwise reduction every 12th week pending clinical deterioration. SCIG dosage was reduced stepwise as follows: 90%, 75%, 50%, 25% and 0% of the initial, individual dosage before enrollment. Dosage was managed for 12 weeks each and participants completing the study were evaluated 12 weeks after discontinuation of treatment. This resulted in a maximal duration of participation of 60 weeks (Fig. 1). To secure the correct dose of immunoglobulin from inclusion and every 12th week subsequently, the exact dose of immunoglobulin was delivered to the participants every 12 weeks.
Fig. 1
Study design. Numbers in boxes indicate the SCIG dosage as a percentage of the individual total dose at enrollment. *Participants were evaluated every 6th week (grey numbers and broken arrows) (A) or every 12th week (black numbers and unbroken arrows) (B). #Medical treatment status was evaluated two years after last visit (maximum 164 weeks).
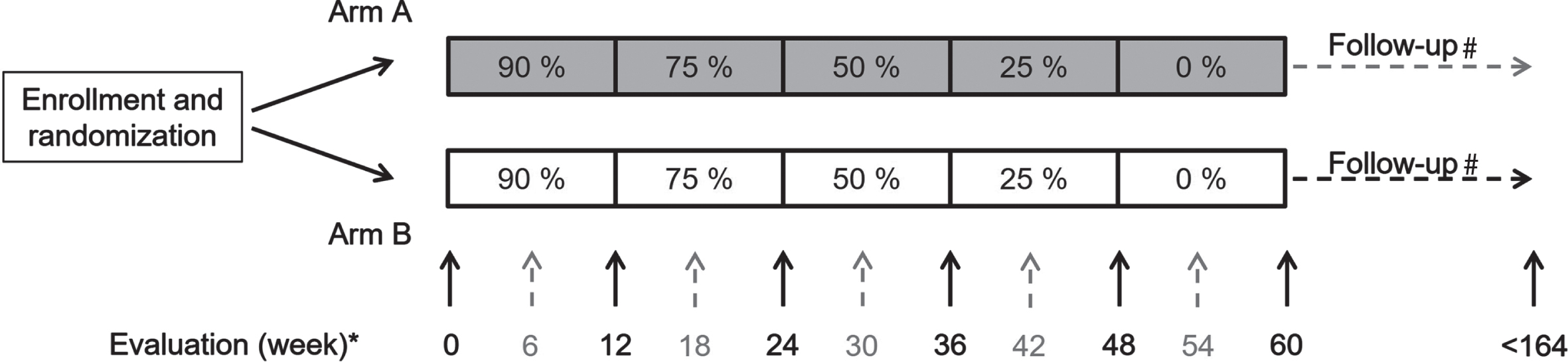
Figure 1 shows randomization 1 : 1 of participants at inclusion, participants to frequent clinical evaluation every 6th (black, unbroken arrow and grey, broken arrow) or less frequent clinical evaluation every 12th week (black, unbroken arrow).
In all participants, registration of ongoing immunoglobulin treatment and dosage was made two years after last follow-up visit.
Clinical evaluation
Disability was evaluated using the overall disability sum score (ODSS) with a range from 0 (not disabled) to 12 (severely disabled) and the Rasch built overall disability score (RODS) for CIDP (RODS-CIDP), consisting of 12 items describing usual activities of daily living. Each item is scored from 0 (no affection) to 2 (cannot perform), yielding a total range of 0 to 48 [14, 15].
Hand held grip strength was determined based on the average of a triple bilateral measurement using a JAMAR dynamometer [16].
Manual muscle strength was assessed using the modified Medical Research Council (MRC) sum score with bilateral evaluation of shoulder abduction, elbow flexion/extension, wrist flexion/extension, hip flexion, knee flexion/extension and ankle dorsal flexion each ranging from 0 to 5 (normal) resulting in a maximum score of 90 points.
Quality of life (QoL) was assessed using the European Quality of Life – 5-dimension – 5-level questionnaire (EQ-5D-5 L) including the Visual Analogue Scale (VAS) and calculating an index value based on a Danish population [17]. EQ-5D-5 L consists of five dimensions including mobility (MB), self care (SC), usual activities (UA), pain/discomfort (PD) and anxiety/depression (AD). Each domain is ranges from 1 to 5 according to level of influence. A total score (index value) is calculated ranging from 0 to 1. Moreover, a VAS determined the patient’s self-evaluated health on the day of examination using a range from 0 (worst health) to 100 (best health).
Dexterity was determined using the 9-hole peg test (9-HPT) and walking performance was evaluated using the 10-meter walk test (10-MWT). In both tests, double determination was done and the 9-HPT was performed bilaterally [18, 19].
Response to SCIG treatment
The clinical criteria for deterioration and study exclusion were an increase in the ODSS ≥ 1 point (except an isolated increase from 0 to 1 in either arm or leg) or a decrease in grip strength > 4 kg (8 kPa) measured as an average value of both hands compared to values obtained at the latest visit.
Participants who reported about clinical deterioration due to tapering off were evaluated before leaving the study. We registered whether or not the participants met the criteria for clinical deterioration. Moreover, participants could at any time during the study request an unscheduled evaluation and we registered whether decision of leaving the study was made due to a scheduled or unscheduled visit.
Statistics
At initiation of this study, approximately 120 Danish patients with CIDP received immunoglobulin of which 81 self-administered SCIG. We aimed to include 60 patients for this study. To our knowledge, no previous studies have evaluated standardized tapering off regimens; a power calculation could thus not be performed. However, we did a retrospective, non-inferiority, power calculation of a binary outcome comparing self-registration of deterioration versus clinical determination of deterioration in the two different follow-up regimens. Based on a proportion of 19% excluded due to self-registration in group A (6th week) versus 42% in group B (12th week), using a statistical power of 80%, a two-sided level of significance of 5%, and a non-inferiority level of 10% we found that 29 participants were needed in each group.
The primary outcomes were number of participants able to discontinue SCIG treatment completely and the overall reduction in the weekly SCIG dosage. We anticipated that approximately 5 to 10% of patients could successfully discontinue treatment and that the average dosage of immunoglobulin could be reduced by at least 10%.
The secondary outcome was the number of participants deteceted with clinical deterioration comparing frequent and less frequent clinical evaluation. Moreover, the number of self-registrations was compared to the number of clinical registrations at the outpatient clinic.
RESULTS
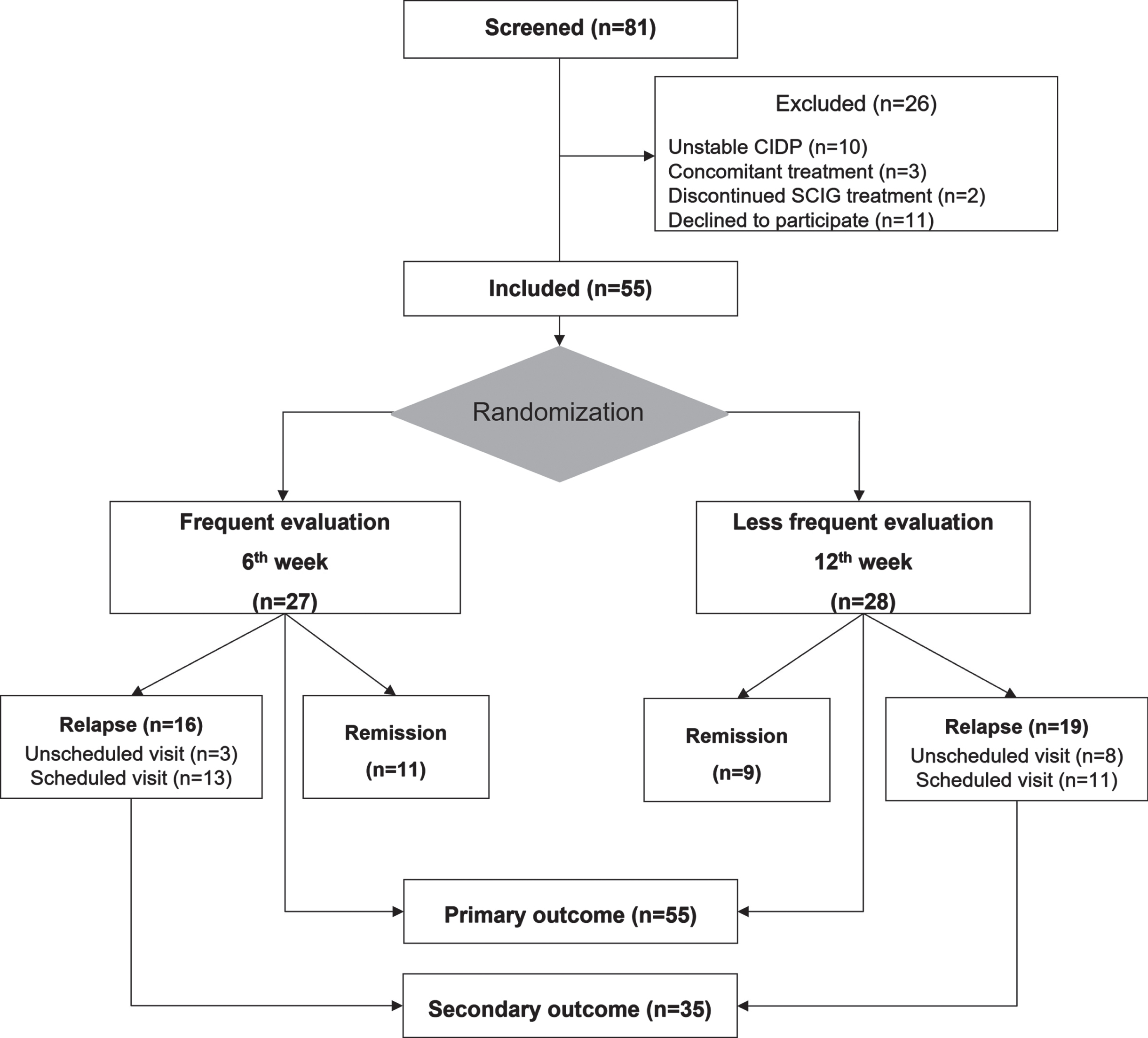
Eighty-one patients were screened for eligibility; 55 gave informed consent and were included. Of the 26 patients not included, 11 declined participation, 13 were excluded due to an unstable clinical condition or concomitant treatment, and two were no longer on SCIG therapy. Twenty-seven patients were randomized to frequent evaluation every 6th week and 28 patients to less frequent evaluation every 12th week. All participants (n = 55) were included in the analysis of primary outcomes and all participants with relapse (n = 35) were included for analysis of secondary outcomes (Fig. 2).
Fig. 2
Study flowchart.
Baseline charateristics according to the randomization are displayed in Table 1.
Table 1
Baseline characteristics for patients with relapse versus patients in remission
| Relapse (n = 35) | Remission (n = 20) | P-value | |
| Gender (M/F) | 27/8 | 12/8 | 0.22 |
| Age (years) | 65 (33–82) | 59 (32–79) | 0.10 |
| Weight (kg) | 81 (56–140) | 83 (49–121) | 0.91 |
| Duration of CIDP (months) | 96 (18–264) | 67.5 (13–228) | 0.29 |
| Duration of SCIG treatment (months) | 41 (5–96) | 38 (6–108) | 0.50 |
| Dosage of SCIG (g/week) | 30 (9.9–49.5) | 23.1 (9.9–56) | 0.047* |
Values are median (range: min– max); CIDP: chronic inflammatory demyelinating polyneuropathy; SCIG: subcutaneous immunoglobulin.
Comparison of continuous data between the two groups were analyzed by paired or unpaired t-tests or Wilcoxon’s signed rank test when appropriate. Binomial data were analyzed using Fisher’s exact test due to the low number of participants. Level of significance was 0.05.
Determination of lowest effective dosage of SCIG
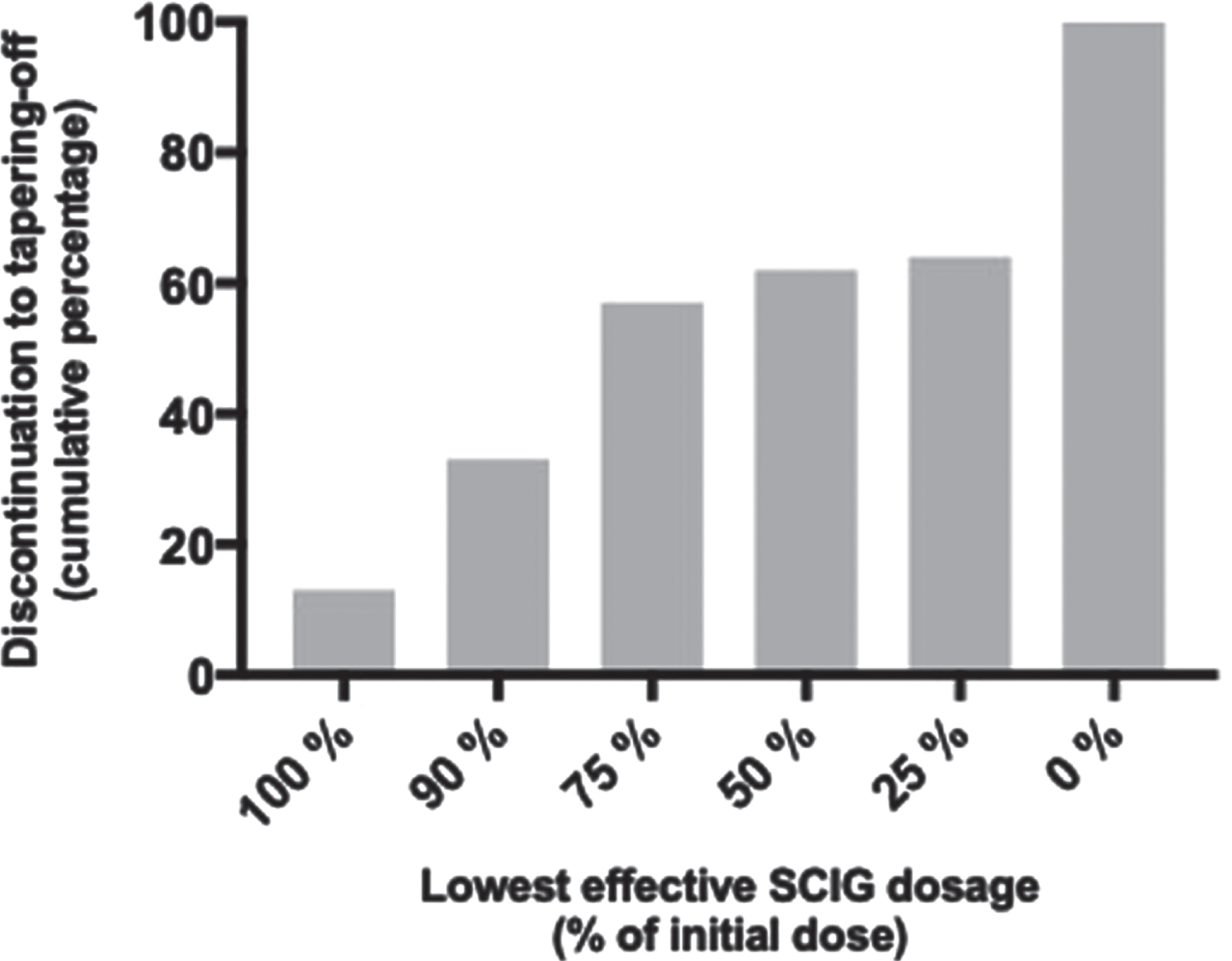
Overall, 20 participants (36%) completed the study protocol resulting in complete discontinuation of SCIG treatment and were thus defined to be in remission according to CDAS classification 2a and 2b [4]. Among the remaining 35 participants (64%) who relapsed during tapering off SCIG-treatment, seven participants (13%) did not tolerate any reduction in dosage whereas 11 participants (20%) tolerated a reduction of 10%, 13 participants (24%) tolerated a reduction of 25% and three participants (5%) tolerated halving the dosage. One participant (2%) deteriorated after complete discontinuation of treatment and recovered following treatment at a dosage of 25% (Fig. 3). Consequently, if a 50% reduction of dosage was tolerated, the relative risk (RR) of developing deterioration was 4.8 % (95CI, 0.7 to 20) (p < 0.0001). The median weekly dosage of SCIG could be reduced by 25% (range, 0 to 100) (p < 0.0001) in the entire cohort (n = 55), and by 10% (range, 0 to 75) (p < 0.0001) in participants relapsing during tapering off (n = 35).
Fig. 3
Cumulative percentage of discontinued participants according to the lowest effective dosage of SCIG.
Among relapsing participants, 97% could be stabilized by increasing SCIG to the last given dose prior to deterioration. Only one participant required an additional IVIG infusion to regain initial muscle strength and level of disability.
One participant was diagnosed with ulcerative colitis and treated with prednisolone during tapering off. The patient was in remission, and at the last visit prednisolone was withdrawn for three months. Another participant developed a rash during reduction of the dosage to 90% and further to 75% despite administering new batches of SCIG. Consent to participate was withdrawn but no clinical deterioration occured.
According to baseline data, patients with relapse received a higher dosage of SCIG, otherwise no differences were seen (Table 1).
ODSS deteriorated among patients with relapse (from 3.1 to 3.5 points, p = 0.002), whereas it improved in patients in remission (from 2.1 to 1.9 points, p = 0.02). Among other clinical measurements RODS, grip strength and 10-MWT deteriorated in relapsing patients, whereas it remained stable in remitted patients (Table 2).
Table 2
Clinical measurements in patients with relapse and in remission
| Relapse (n = 35) | Remission (n = 20) | Relapse vs remission (P-value) | ||||||
| Baseline | Final visit | P-value | Baseline | Final visit | P-value | Baseline | Final visit | |
| ODSS (point) | 3.1 (2.4) | 3.5 (2.2) | 0.002* | 2.1 (2.1) | 1.9 (1.9) | 0.02* | 0.31 | 0.01* |
| Grip strength (kg) | 28.2 (13.1) | 26.9 (14.4) | 0.02* | 30.4 (13.1) | 30.7 (13.8) | 0.14 | 0.76 | 0.33 |
| MRC (point) | 87.1 (4.9) | 86.7 (5.2) | 0.31 | 87.4 (3.3) | 87.9 (3.3) | 0.33 | 0.63 | 0.40 |
| RODS (point) | 37.9 (10.3) | 35.6 (10.8) | 0.002* | 40.1 (8.6) | 40.4 (9.4) | 0.21 | 0.6 | 0.12 |
| 9-HPT (sec) | 28.3 (11.6) | 26.3 (10.1) | 0.19 | 32.0 (35.1) | 30.5 (24.3) | 0.33 | 0.53 | 0.37 |
| 10-MWT (sec) | 7.1 (2.9) | 7.8 (3.7) | 0.008* | 7.5 (5.0) | 7.6 (6.4) | 0.59 | 0.85 | 0.88 |
| EQ-5D-5 L (index value) | 0.72 (0.15) | 0.69 (0.15) | 0.048* | 0.81 (0.1) | 0.86 (0.1) | 0.03* | 0.03* | 0.0002* |
| EQ-5D-5 L (VAS) | 69.1 (18) | 60.5 (19) | 0.02* | 74.8 (18) | 84.4 (15) | 0.0007* | 0.26 | <0.0001* |
Last column indicates P-values of comparisons of baseline and values at final visit in the two groups. Values are mean (SD); *P < 0.05.
At baseline, only the EQ-5D-5 L index value was different between patients with relapse, 0.71 (95CI: 0.66 to 0.77), compared to patients in remission, 0.80 (95CI: 0.74 to 0.87) (p = 0.03). In patients with relapse, the average VAS decreased from 74% to 60% (p = 0.02), whereas in patients in remission it improved from 80% to 88% (p = 0.0007). The index value improved in patients in remission (from 0.81 to 0.86, p = 0.03), and deteriorated in patients with relapse (from 0.71 to 0.68, p = 0.048) (Table 2).
At two-year follow-up, two patients were lost to follow-up. Eighteen of 19 patients in remission were still in remission after two years; one resumed SCIG treatment. Two of the 35 patients who relapsed during tapering off SCIG were in remission. In total, 20 patients were in remission after two years and 33 were still treated with immunoglobulin. The average SCIG dosage in patients with relapse had been reduced to 24.6 g/week (95CI: 20.3 to 28.8) from 29.5 g/week (95CI: 26.1 to 32.9) at enrollment (p < 0.007).
Frequent versus less frequent clinical evaluation
In the group with frequent evaluation (every 6th week), 11 participants (41%) discontinued SCIG treatment completely without clinical deterioration, whereas nine participants (32%) tolerated complete discontinuation in the group with less frequent evaluation (every 12th week) (p = 0.58). When comparing characteristics of the two groups at baseline and at the final visit, we found no differences (Table 3).
Table 3
Group characteristics and clinical measurements at baseline and final visit due to frequent (6th week) versus less frequent (12th week) evaluation
| Frequent evaluation | Less frequent evaluation | P-value | ||||||
| (6th week) | (12th week) | |||||||
| N | 27 | 28 | ||||||
| Gender (M/F) | 18 / 9 | 21 / 7 | ||||||
| Body weight (kg) | 81.3 (16.6) | 87.3 (20.8) | ||||||
| Duration of CIDP (months) | 86.6 (47.3) | 93.3 (64.7) | ||||||
| Duration of SCIG | 47.9 (27.0) | 42.3 (29.3) | ||||||
| treatment (months) | ||||||||
| Total dose (g/kg/week) | 0.36 (0.13) | 0.32 (0.12) | ||||||
| Total dose (g/week) | 30.5 (13.3) | 27.2 (9.5) | ||||||
| Baseline | Final visit | P-value | Baseline | Final visit | P-value | Freq. vs less freq. Baseline | Freq. vs less freq. Final visit | |
| ODSS (point) | 2.8 (2.2) | 2.9 (2.0) | 0.44 | 3.0 (2.5) | 2.9 (2.5) | 0.99 | 0.77 | 0.83 |
| Grip strength (kg) | 26.3 (12.4) | 25.7 (14.0) | 0.37 | 30.9 (13.1) | 31.0 (14.0) | 0.97 | 0.17 | 0.15 |
| MRC (point) | 87 (4.9) | 86.5 (5.9) | 0.14 | 87.5 (3.7) | 87.7 (3.0) | 0.86 | 0.69 | 0.62 |
| RODS (point) | 35.8 (12.8) | 37.0 (10.3) | 0.12 | 34.5 (13.8) | 37.4 (10.8) | 0.67 | 0.39 | 0.80 |
| 9-HPT (sec) | 32.2 (30.0) | 30.4 (21.0) | 0.04 | 27.4 (11.8) | 24.7 (11.6) | 0.08 | 0.38 | 0.30 |
| 10-MWT (sec) | 7.2 (4.0) | 7.9 (5.7) | 0.14 | 7.2 (3.3) | 7.6 (3.9) | 0.58 | 0.93 | 0.83 |
| EQ-5D-5 L (Index value) | 0.74 (0.14) | 0.73 (0.17) | 0.07 | 0.75 (0.15) | 0.75 (0.17) | 0.81 | 0.90 | 0.52 |
| EQ-5D-5 L (VAS) | 71.3 (20.3) | 64.7 (23.4) | 0.20 | 70.1 (15.3) | 71.8 (18.0) | 0.46 | 0.37 | 0.29 |
Last column indicates P-values of comparisons of baseline and values at final visit in the two groups. Values are mean (SD); *P < 0.05.
Overall, 23 of the 35 participants who had relapse during tapering off met at least one of the criteria for clinical deterioration. In the group with frequent evaluation (every 12th week), eleven out of 19 participants halted tapering at a regularly scheduled clinical visit; six met clinical criteria for deterioration and five because the patient wanted to halt despite not meeting clinical criteria. In those participants who were seen more frequently (every 6 weeks), thirteen of the 16 participants halted tapering at a scheduled visit; eight met the criteria for deterioration and five wanted to halt despite not meeting clinical criteria. In eight of these 13 participants, halting occurred at one of the intermediate visits not occurring for the other group. Eleven unscheduled visits were conducted and were all initiated by participants concern for deterioration. Three participants were from the group evaluated every 6th week and they were alle meeting criteria for deterioration. Eight were from the group evaluated every 12th week and six met the exclusion criteria.
Comparing the two regimens, the ability to detect deterioration at unscheduled versus scheduled visits was similar with a relative risk (RR) of 0.5 (95CI, 0.2 to 1.2) (p = 0.17). Neither merging of the intermediate visit (n = 8) with unscheduled visit (n = 3) in the frequent evaluation group disclosed a significant difference, RR 1.9 (95CI, 0.9 to 4.4) (p = 0.18).
Clinical evaluations at the final visit in (scheduled versus unscheduled) showed no differences. According to the clinical criteria for deterioration, there was no difference between the number of participants meeting the criteria at a scheduled versus an unshcduled visit, RR 1.6 (95CI, 0.9 to 2.7) (p = 0.14).
DISCUSSION
In this cohort study of patients with CIDP, we applied a standardized regimen of stepwise reduction of SCIG to sidentify patients in remission as well as to detect the lowest effective dosage in patients still requiring treatment. Stepwise reduction of SCIG was safe and feasible. In 36% of the patients, SCIG could be completely discontinued without clincal relapse at short term follow-up (12 weeks). In 90% of these patients, no relapse occurred during the following two years. Furthermore, in patients still requiring SCIG, the median dosage could be reduced by 10% without clinical progression. Thus, using a stepwise reduction, clinical evaluation every 12th week as self-registration of deterioration in performance level is as safe and effective as clinical evaluation every 6th week.
In CIDP, Gorson et al reported a prognosis based on a long-term follow-up of 106 patients. Eleven percent were in permanent remission, 19% were in remission < 5 years, 40% were stable on immunemodulating therapy, and 30% were severily disabled with a high disability score despite immunemodulating treatment [4]. Furthermore, in a Danish cohort, 53% received no treatment 15 years after their CIDP diagnosis [20] and in a recent meta-analysis Al-Zuhairy et al found that 40% of CIDP patients were in remission [21]. For SCIG treatment, the long-term prognosis and the response to tapering off are sparsely studied. For IVIG, EAN/PNS recommends considering a stepwise reduction once every year or every second year to detect possible remission [5], but no standardized tapering off regimen has been suggested. In a previous study comparing steroid therapy with IVIG, complete discontinuation of IVIG treatment resulted in relapse in 85% of patients after a median of 4.5 months [9].
Our findings support the findings by Gorson et al reporting approximately 30% of patients with CIDP to be in remission enabling successful tapering off all medical treatment.
Among the relapsing participants, 35% of patients did not accept further tapering off although not fulfilling the criteria for relapse. The main reasons for this were fear of getting worse or deterioration apart from grip strength and disability. We found that ODSS, RODS and MRC scores were more abnormal in patients who met the criteria for deterioration compared to those who did not. For ODSS, and to a lesser extent RODS, this is not surprising as deterioration in ODSS was one of the exclusion criteria and RODS is closely correlated to ODSS [22]. However, for MRC it seems that this parameter could be more useful than grip strength, probably because it includes more muscle groups. In this way, deterioration in patients with unaffected hands could be detected. In previous studies, disability scores have been widely used to detect deterioration or improvement in CIDP [2, 23]. Our study confirmed this and our findings suggested that gait performance can be used to monitor patients. Also, grip strength has been used as a marker of response to treatment in CIDP with a level of 8 kPa corresponding to 4 kg being the threshold for a clinically meaningful difference [24, 25]. Moreover, grip strength can easily be assessed providing an objective measurement of muscle strength. However, many patients with CIDP have deficits primarily in the lower limbs; therefore other evaluation methods than grip strength are needed to detect significant clinical deterioration. Also, grip strength has been shown to correlate poorly with muscle strength in the lowers limbs [26] whereas walking performance correlates with QoL in CIDP [22]. Therefore, we suggest that evaluation of disability and walking performance should be included in the evaluation of responsiveness to medical treatment of CIDP.
A recent study by Adrichem et al evaluated stepwise withdrawal of IVIG in CIDP in a randomized, controlled, double-blind study. This study found 59% of patients relapsed due to withdrawal, and 42% of patients relapsed in the IVIG continuing group; the difference was not statistically significant. Moreover, 28% of withdrawal patients were stable 60 weeks after the last treatment with IVIG and 97% of patients with relapse were restablished within 12 weeks [27]. Compared to our results, we found relapse in 64% of the patients, 36% of the patients were in remission during long term follow-up, and 97% were restabilized after increasing SCIG dose to the level when they were last stable. As we did not include a group of patients without withdrawal, we could not evaluate the rate of patients with relapse despite continuous treatment in our cohort.
Considering the costs of SCIG, identification of patients in remission is highly relevant [28]. With a price of 45€ per gram of immunoglobulin and reduced total weekly dose of 700 grams in our 55 patients, direct costs were reduced by 31,500€ per week equal to 1,638,000€ per year. Moreover, savings in equipment resulted in further savings of approximately 60,000€ per year.
By randomizing participants to frequent or less frequent clinical evaluation (at 6 or 12 weeks), we were able to compare any difference in risk of clinical deterioration. However, it could be speculated that if a patient had a scheduled clinical evaluation every 6th week they would be more reluctant to complain about clinical deterioration between visits compared with patients evaluated only every 12th week. Therefore, we cannot exclude bias in favour of more patients being excluded due to a scheduled visit. A sub-analysis evaluating the number of participants in the frequent evaluation group who discontinued participation at an intermediate visit (e.g., in week 6, 18, 30 or 42), we found no difference in relative risk. This suggests that clinical evaluation every 12th week is sufficient. To obtain further knowledge, a study comparing no planned clinical follow-up examination with frequently planned evaluations could be relevant.
QoL did not change in relation to randomization and there was no difference between participants excluded at a scheduled versus unscheduled visit or between those who met exclusion criteria and those who did not.
Our study has some limitations. First of all, dosage reduction was not blinded neither to the patients nor to the clinicians due to the design of the study including the same treatment regimen in both groups. To secure adherence to the scheduled reduction, only the exact amount of SCIG required was delivered to the participants. Moreover, a treatment diary was kept and at every visit, blood samples were drawn analyzing immunoglobulin G level. Secondly, patients eligible for participation had the opportunity to decline participation according to the Helsinki criteria for clinical studies. This has probably resulted in a higher frequency of participation of patients in remission, as patients unwilling to undergo dose reduction are more likely to relapse. It could be argued that patients who have previously tried tapering off or discontinued immunoglobulin treatment and relapsed would decline participation. This could also be the case in patients who have the impression of a day-to-day or week-to-week variation. In contrast, patients with few or no symptoms of CIDP would be more willing to participate. Likely, both conditions would have increased the proportion of patients in remission in this study and according to this scenario, it could be argued that only 20 of 81 patients (25%) were in remission, however 15 of the 81 screened were not able to participate according to the inclusion criteria but not due to unwillingness to participate. Therefore, if one should give a more sceptic interpretation of percentage of patients in remission it should more likely be a total of 66 patients, including the 11 patients who declined, and then 20 of 66 (30%) were in remission. We consider this to be a small difference from our conclusion of 35% being in remission as 11 of 66 patients (16,7%) declined participation. This was considerably less than in the study by Adchrim et al in which 36 of 90 eligible patients (37.5%) declined to participate [27]. Thirdly, as patients could withdraw consent to continue participation, we were unable to further reduce dosage in some patients despite not fulfilling our predefined criteria for meaningful deterioration. In the group of relapsing patients, 34% of the participants did not fulfill the criteria, although the majority complained about deterioration of other functions than muscle strength (e.g., deterioration in walking performance, more paresthesias or neuropathic pain). In future studies, criteria for clinical deterioration could also include deterioration in walking performance; however, so far the effect of immunoglobulin in CIDP has been based on the effect on muscle strength and disability [2, 23].
CONCLUSION
Stepwise reduction of SCIG was found to be safe and feasible in patients with CIDP. We found that 35% patients with stable CIDP receiving SCIG as mono-therapy were in remission and that 32% remained in remission during two years of follow-up. If a 50% reduction of the dose of immunoglobulin was tolerated, the risk of clinical deterioration was very low (<5%). In patients still requiring immunoglobulin, the overall dose of SCIG could be reduced by 10%. Finally, frequent (every 6th week) and less frequent clinical evaluation (every 12th week) was equally effective to detect clinical deterioration. Moreover, the number of participants excluded following an unscheduled visit versus a scheduled visit was similar.
AVAILABILITY OF DATA
The dataset used and analyzed in this study will be made available upon request by the corresponding author to qualified researchers (i.e., affiliated with a university, research institution or hospital).
ACKNOWLEDGMENTS
The study nurses Dorthe Welch, Anette Anberg, Tine Leth, Elma Budalica, Mette Magnussen and Derya Özen are thanked for their support in this study.
CONFLICT OF INTEREST
Dr. Markvardsen received fees from Octapharma, Alexion, Alnylam, and CSL Behring and travel support from Alnylam; Dr. Sindrup has no conflicts of interest; Dr. Christiansen has no conflicts of interest; Ms. Sheik has no conflicts of interest; Dr. Holbech has no conflicts of interest; Dr. Andersen received fees from Alexion, Sanofi Genzyme, UCB and Zealand Pharma and served as member of advisory boards at NMD Pharma and Amicus Pharmaceuticals.
CONTRIBUTIONS
Lars K. Markvardsen: Drafting/revision of the manuscript for content, including medical writing for content. Major role in the acquisition of data. Study concept and design. Analysis and interpretation of data.
Søren H. Sindrup: Major role in the acquisition of data and revision of the manuscript for content, including medical writing for content.
Ingelise Christiansen: Major role in the acquisition of data and revision of the manuscript for content, including medical writing for content.
Aisha M. Sheikh: Minor role in the acquisition of data and revision of the manuscript for content, including medical writing for content.
Jakob V. Holbech: Major role in the acquisition of data and revision of the manuscript for content, including medical writing for content.
Henning Andersen: Major role in the acquisition of data and revision of the manuscript for content, including medical writing for content. Study concept and design. Interpretation of data.
ETHICAL CONSIDERATIONS, REGISTRATIONS, AND PATIENT CONSENT
All participants gave written informed consent before the screening procedure to participate. The study was approved by the Central Denmark Region Committee on Health Research Ethics (1-10-72-177-17) and the Danish Medicines Agency (2017113751) and registered in the EU Clinical Trials Register (EudraCT 2017-002024-24) by 9 June 2017.
REFERENCES
[1] | Markvardsen LH , Debost JC , Harbo T , Sindrup SH , Andersen H , Christiansen I , et al. Subcutaneous immunoglobulin in responders tointravenous therapy with chronic inflammatory demyelinatingpolyradiculoneuropathy. European Journal of Neurology: The OfficialJournal of the European Federation of Neurological Societies. (2013) ;20: (5):836–42. |
[2] | van Schaik IN , Bril V , van Geloven N , Hartung HP , Lewis RA , Sobue G , et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. (2018) ;17: (1):35–46. |
[3] | Kuwabara S , Misawa S , Mori M , Tamura N , Kubota M , Hattori T . Long term prognosis of chronic inflammatory demyelinating polyneuropathy: A five year follow up of 38 cases. J Neurol Neurosurg Psychiatry. (2006) ;77: (1):66–70. |
[4] | Gorson KC , van Schaik IN , Merkies IS , Lewis RA , Barohn RJ , Koski CL , et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: Recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst. (2010) ;15: (4):326–33. |
[5] | van den Bergh PYK , van Doorn PA , Hadden RDM , Avau B , Vankrunkelsven P , Allen JA , et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. Eur J Neurol. (2021) ;28: (11):3556–83. |
[6] | Hadden RD , Marreno F . Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: Improved tolerability and patient satisfaction. Ther Adv Neurol Disord. (2015) ;8: (1):14–9. |
[7] | Christiansen I , Markvardsen LH , Jakobsen J . Comparisons in fluctuation of muscle strength and function in patients with immune-mediated neuropathy treated with intravenous versus subcutaneous immunoglobulin. Muscle Nerve. (2018) ;57: (4):610–4. |
[8] | Cocito D , Merola A , Peci E , Mazzeo A , Fazio R , Francia A , et al. Subcutaneous immunoglobulin in CIDP and MMN: A short-term nationwide study. J Neurol. (2014) ;261: (11):2159–64. |
[9] | Nobile-Orazio E , Cocito D , Jann S , Uncini A , Messina P , Antonini G , et al. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg Psychiatry. (2015) ;86: (7):729–34. |
[10] | Lunn MP , Ellis L , Hadden RD , Rajabally YA , Winer JB , Reilly MM . A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. J Peripher Nerv Syst. (2016) ;21: (1):33–7. |
[11] | Kuitwaard K , Fokkink WJR , Brusse E , Vrancken AFJE , Eftimov F , Notermans NC , et al. Maintenance IV immunoglobulin treatment in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. (2017) ;22: (4):425–32. |
[12] | Cocito D , Peci E , Romagnolo A , Rigaldo S , Rosso M , Lopiano L , et al. Subcutaneous “bolus” immunoglobulin dose in CIDP: A proof-of concept study. J Neurol Sci. (2017) ;380: , 54–7. |
[13] | Joint Task Force of the E, the PNS European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society–First Revision. J Peripher Nerv Syst. (2010) ;15: (1):1–9. |
[14] | van Nes SI , Vanhoutte EK , van Doorn PA , Hermans M , Bakkers M , Kuitwaard K , et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. (2011) ;76: (4):337–45. |
[15] | Merkies IS , Schmitz PI , van der Meche FG , Samijn JP , van Doorn PA , Inflammatory Neuropathy C, et al. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry. (2002) ;72: (5):596–601. |
[16] | Rajabally YA , Narasimhan M . Jamar hand-held grip dynamometry in chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. (2013) ;325: (1-2):36–8. |
[17] | Herdman M , Gudex C , Lloyd A , Janssen M , Kind P , Parkin D , et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. (2011) ;20: (10):1727–36. |
[18] | Goodkin DE , Hertsgaard D , Seminary J . Upper extremity function in multiple sclerosis: Improving assessment sensitivity with box-and-block and nine-hole peg tests. Arch Phys Med Rehabil. (1988) ;69: (10):850–4. |
[19] | Adonis A , Taylor GP . Assessing Walking Ability in People with HTLV-1-Associated Myelopathy Using the 10 Meter Timed Walk and the 6 Minute Walk Test. PLoS One. (2016) ;11: (6):e0157132. |
[20] | Al-Zuhairy A , Sindrup SH , Andersen H , Jakobsen J . A population-based study of long-term outcome in treated chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. (2020) ;61: (3):316–24. |
[21] | Al-Zuhairy A , Jakobsen J . Outcome in chronic inflammatory demyelinating polyneuropathy: A systematic review and meta-analysis. Muscle Nerve. 2023. |
[22] | Ryltoft AK , Al-Zuhairy A , Sindrup SH , Andersen H , Markvardsen LK . Quality of life in chronic inflammatory demyelinating polyneuropathy patients treated with subcutaneous immunoglobulin. Acta Neurol Scand. (2020) ;142: (6):637–40. |
[23] | Hughes RA , Donofrio P , Bril V , Dalakas MC , Deng C , Hanna K , et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurology. (2008) ;7: (2):136–44. |
[24] | Vanhoutte EK , Latov N , Deng C , Hanna K , Hughes RAC , Bril V , et al. Vigorimeter grip strength in CIDP: A responsive tool that rapidly measures the effect of IVIG–the ICE study. Eur J Neurol. (2013) ;20: (5):748–55. |
[25] | Doneddu PE , Hadden RDM . Daily grip strength response to intravenous immunoglobulin in chronic immune neuropathies. Muscle Nerve. (2020) ;62: (1):103–10. |
[26] | Knak KL, Andersen LK, Christiansen I, Markvardsen LK. Does grip strength reflect isokinetic muscle strength in lower limbs in patients with chronic inflammatory demyelinating polyneuropathy? Muscle Nerve. (2018) ;58: (3):449–52. doi: 10.1002/mus.26136. |
[27] | Adrichem ME , Lucke IM , Vrancken AFJE , Goedee HS , Wieske L , Dijkgraaf MGW , et al. Withdrawal of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Brain. (2022) ;145: (5):1641–52. |
[28] | Lazzaro C , Lopiano L , Cocito D . Subcutaneous vs intravenousadministration of immunoglobulin in chronic inflammatorydemyelinating polyneuropathy: An Italian cost-minimization analysis. Neurol Sci. (2014) ;35: (7):1023–34. |



