Single-Blind, Randomized, Controlled Clinical Trial of Exercise in Ambulatory Spinal Muscular Atrophy: Why are the Results Negative?
Abstract
Background:
The benefits of exercise on long-term health and well-being are well established. The possible benefits of exercise in Spinal Muscular Atrophy (SMA) have not been explored in a controlled clinical trial format.
Objective:
To assess the effects of exercise on measures of function, strength, and exercise capacity in ambulatory SMA patients.
Methods:
Fourteen participants, ages 10–48 years, were randomized to control and exercise cohorts after a 1 month lead-in period. The exercise group received 6 months of intervention. Thereafter, both groups received the intervention for the remaining 12 months. Participants were monitored for a total of 19 months. Exercise included individualized home-based cycling and strengthening. The primary outcome measure was distance walked during the six-minute walk test (6MWT). Secondary outcomes included strength, function, exercise capacity, quality of life and fatigue.
Results:
Twelve participants completed the first 7 months of the study, and 9 completed all 19 months. At baseline, the groups were similar on all clinical variables. There were no group changes at any time point in the 6MWT, fatigue, or function. Percent-predicted VO2 max improved 4.9% in all participants in 6 months (p = 0.036) (n = 10).
Conclusion:
Daily exercise is safe in ambulatory SMA and should be encouraged. We did not uncover any deleterious effects on strength, function, or fatigue. Our study documented a reduction in oxidative capacity and a blunted conditioning response to exercise possibly representing an important insight into underlying pathophysiological mechanisms. These findings also may be linked causally to mitochondrial depletion in SMA and warrant further study.
INTRODUCTION
Spinal Muscular Atrophy (SMA) is an autosomal recessive motor neuron disease manifested typically by infantile- or childhood-onset progressive muscle weakness. SMA is caused by degeneration of lower motor neurons in the spinal cord and brainstem. [1] Despite identification of a single mutation in the Survival Motor Neuron (SMN1) gene located on chromosome 5q13, SMA is phenotypically heterogeneous ranging from a life threatening to life altering disease due, in part, to a disease modifying homologue gene, SMN 2. The mild SMA phenotype, Type 3, also termed Kugelberg Welander disease, typically presents after age 18 months with symptoms of proximal muscle weakness, mostly involving the lower limbs. SMA Type 3 patients are able to walk unaided but have difficulty rising from the floor or low surfaces, running, and negotiating stairs. The muscle weakness is largely symmetrical and the severity of weakness is positively correlated with disease duration. [2, 3]
SMA causes significant disability, and there is currently no effective drug treatment. Maximizing function, endurance, general health and well-being, in an effort to modulate disease morbidity, is the focus of supportive treatment modalities. In the general population, the benefits of exercise on long-term health and well-being are well established. [4] Despite promising pre-clinical and pilot reports, the effects of exercise in SMA have not been addressed in a controlled trial. Among healthy individuals as well as individuals with neuromuscular disorders, exercise has been shown to improve cardiorespiratory endurance, functional strength, and quality of life. [4–10] SMA 3 patients represent an ideal target population because their residual strength permits endurance and strengthening programs.
The overall goal of this project is to evaluate the effectiveness of aerobic and strengthening exercise to improve function in SMA, and to explore the physiology underlying the effects. Moreover, by studying the effects of exercise in human patients in a controlled trial, we hope to provide evidence for improved standard of care.
METHODS
Study design
This was an evaluator-blinded, randomized, controlled trial of aerobic and strengthening exercise in 14 ambulatory SMA patients ranging in age from 8 to 50 years. [11] The exercise protocol included an individualized muscle strengthening program in combination with a home based recumbent cycle ergometry aerobic exercise program. The aerobic exercise was performed five times weekly with target duration of 30 minutes per day. The strengthening exercise was performed three times weekly with a similar target duration. Patients were randomized either to an exercise arm or control arm after a one month lead in period. During months 2 to 7, the exercise group received the exercise intervention while the other group served as the control. The participants assigned to the control group were asked to continue their usual care during this period. After month 7, both groups received the exercise intervention. During the first 13 months of the study, participants were evaluated in-clinic every 3 months. Months 14 to 19 of the study were designed to mimic real-world conditions, during which time all participants were encouraged to continue on their own without formal monitoring or clinic visits, until month 19.
Study population
All participants or guardians of participants signed informed consent approved by the Columbia University Medical Center Institutional Review Board. The study was registered with ClinicalTrials.gov (NCT01166022). The participant characteristics and performance on the outcome measures at baseline have been previously described [11] and are summarized below. There were more males than females enrolled. The age range was 10–48 years old. All of the patients in the exercise arm were described as having SMA type 3b, the milder phenotype of ambulatory SMA patients. In the control group, 4 patients had SMA type 3b and the other 3 had type 3a. At baseline, all participants had normal pulmonary function and substantially attenuated exercise capacity. On average, the exercise capacity was 35.3% of predicted for age and gender. Both groups were insufficiently active, spending on average 83.5% of waking hours in sedentary activities. The exercise and control groups were similar on all measured variables.
Intervention - cycle and strengthening
The initial design of the exercise regimen was structured based on participant performance on the exercise tolerance test and strength assessments collected by the blinded evaluator according to widely accepted recommendations for exercise prescription. [12] Video-conferencing visits using Skype were utilized to ensure that the participants were performing the exercise correctly, to problem solve or change or advance their program according to their responses to exercise, and to enhance exercise safety and adherence. During these virtual visits, interim medical histories, concomitant medications and adverse events also were reviewed.
There were several different assessments incorporated in the study to measure participant adherence during the exercise intervention. These assessments included exercise diaries, heart rate monitors, and frequently scheduled electronic correspondence with the study team.
Outcome measures
The primary outcome measure was distance walked during the six-minute walk test (6MWT). Percent-predicted distance on the six-minute walk test distance was computed from normative values. [13, 14] Physiologic fatigue was determined by the difference in the distance walked in the first minute compared with the sixth minute and expressed as a percent, with a positive value representing fatigue. [15]
Secondary outcome measures
Secondary outcomes included exercise capacity, strength and functional measures, quality of life and fatigue. Measurement of maximal oxygen uptake (VO2 max) is considered the gold standard measure of exercise tolerance. [16] Exercise tolerance is determined by the functioning of multiple body systems including cardiovascular, respiratory, and neuromuscular. [17] A limitation in one or more body systems will result in reduced exercise capacity, as identified in a lower than normal VO2 max. [17] Because maximal exercise testing including measurement of maximal oxygen uptake had not been administered previously to persons with SMA, the feasibility was uncertain at the start of the study.
Manual muscle testing (MMT) is performed as part of a routine neurological exam and is often included as a secondary outcome measure in clinical trials in neuromuscular disorders. We assessed 28 bilateral muscle groups of the arms and legs using MMT according to the Medical Research Council (MRC) 10-point grading scale. [18] Quantitative muscle strength assessments of 10 arm and leg muscle groups using a handheld dynamometer were also included.
Gross motor function was assessed using the Hammersmith Functional Motor Scale Expanded (HFMSE), [19] 10 meter walk /run, [15] and the Timed Up and Go Test (TUG). [20] Pulmonary function was assessed by measuring forced expiratory vital capacity (FVC) as percent predicted for age and height. [21]
Quality of life and fatigue
Quality of life and perceived fatigue were captured at each visit using standard questionnaires. Child participants and parents were asked to complete the “PedsQL™ Generic Quality of Life Inventory” and “Multidimensional Fatigue Scale,” a proprietary test, to measure quality of life and perceived fatigue. [22] The SF-36, a widely used health-related quality of life measure [23] and the Fatigue Severity Scale (FSS) a perceived fatigue severity questionnaire, [24] were completed by patients age 18 years and older.
Statistical methods
Efficacy was determined by comparing the mean difference in distance walked on the 6MWT over 6 months between the exercise and control group using an independent samples t-test. Similar analyses were performed on all other clinical variables collected. Repeated measures analyses of variance were used to evaluate the effects of exercise on the primary and secondary outcomes for all participants over 12 and 18 months in the control and exercise groups respectively. For all analyses, differences were considered statistically significant at alpha of p≤0.05.
RESULTS
Participant characteristics
Twelve participants completed the first 7 months of the study, and 9 completed all 19 months. The two participants who dropped out during the first 7 months of the study lived more than 1,000 miles from the study site and found the travel too burdensome. One participant was enrolled in another clinical trial after completing the wait list control portion of the study and the other two participants completed at least 12 months of the study and chose not to continue during the unmonitored period. In general, participant compliance with the prescription was excellent based on patient diaries, interim Skype calls and clinic study visits. Most participants were at least 90% compliant with the exercise prescription over the 6 month intensive monitoring period of the study (n = 9).
Exercise ability, particularly aerobic exercise, improved slowly in the participants. Time spent during aerobic exercise was reported in the patient diaries and confirmed during video conferencing and in-person clinic visits. After 6 months, 50% of the participants had achieved the target volume of 150 minutes per week of aerobic exercise. The other participants’ aerobic exercise intensity ranged from 24 – 91 minutes per week at the same time point. Only one participant achieved 150 minutes of aerobic exercise per week at month 3. Strengthening exercise was reported in amount of time spent to perform the exercise and as the number of individual exercises completed. This was also included in the patient diaries and confirmed during video conferencing and in-person clinic visits. Unlike aerobic exercise, most patients reported performing all of the 5-6 strengthening exercises, for about 20–30 minutes, 3 times weekly as prescribed throughout the intervention period.
Exercise was well tolerated in all participants. Adverse events were collected during clinic and video-conferencing visits. An un-blinded study monitor reviewed reports monthly. All adverse events reported throughout the duration of the trial are included in Table 1. Migraine headaches were the most frequently reported other medical adverse event however all of the 25 events occurred in two participants with a history of this condition. Of the musculoskeletal events, falls were the most frequent, followed by musclesoreness and low back pain (Table 1). There were no group difference in the frequency of falls or muscle soreness, during the controlled period (Table 2). During the controlled period, low back pain was reported 5 times in one individual in the exercise group with a known history of this condition. Hip pain was reported only 3 times during the controlled period in one individual. The remaining musculoskeletal adverse events were reported infrequently over the entire 19 month study, occurring on 3 or less occasions, and therefore not included in a formal analysis. There were no study-related serious adverse events.
There were no significant group changes after 6 months in the primary outcome measure (distance walked on the 6MWT), or the measures of strength or motor function (Table 3). Similarly, there were no changes on any of the spatial or temporal gait variables collected during the 6MWT. Some but not all participants in the exercise arm showed improvements in function over 6 months (Fig. 1). Although the changes were not statistically significant, all of these changes in the exercise arm were positive. Even with exercise over 12 and 18 months, there were no statistically significant changes on clinical measures of motor function and strength.
Participants performed an exercise tolerance test using an electronically-braked recumbent cycle ergometer. Oxygen uptake and related variables were measured during the test. All 14 subjects were able to complete the exercise tolerance test without injury or adverse events. The average VO2 max achieved was 15.16 mL/kg/min which is 34.1% of the predicted value for age and gender. Most patients (64% ) achieved maximal performance defined as a respiratory exchange ratio (RER) of >1.
In all participants, there was a significant improvement in VO2 max within 6 months of exercise, and this improvement was even greater in the most compliant participants. These compliant participants were defined as those achieving 90% of the recommended exercise per week and not missing more than 2 weeks of exercise in a 3 month period. Percent-predicted VO2 max improved 4.9% in all participants (p = 0.036) (n = 10). Even greater changes (6.6% ) were seen in the most compliant participants (p = 0.012) (n = 5).
Quality of life and perceived fatigue
Baseline comparisons between groups showed similar measures of quality of life and fatigue. [11] There were no significant changes in quality of life or perceived fatigue between or within the exercise and control groups over the 6 month control period or the duration of the study (Table 4).
DISCUSSION
Daily exercise, at a volume recommended for all Americans, including those with disability [25], appears to be safe in ambulatory SMA children and adults, and should be encouraged. Our study suggests that sustained aerobic exercise, at a volume recommended for healthy individuals, improves overall fitness in SMA patients. We did not uncover any deleterious effects on strength, function, or fatigue. All testing and intervention procedures were well tolerated without any serious adverse events.
Our study demonstrates that exercise performance in SMA improves very slowly, and this rate of change must be considered when designing timelines for future clinical trials. Despite eventually achieving the target exercise intensity, there were no significant changes in clinical measures of motor function and strength over the study duration. Similarly, there were no observed changes in perceived or physiologic fatigue in this study. Notably, our patients were able to maintain a regular, exercise regimen that met the U.S. targets for exercise without any adverse effects for up to 18 months, which would normally be expected to result in long-term general health benefits.
Although no significant improvements were observed in clinical measures of function and strength, modest benefits were achieved in aerobic capacity. However, when compared to other neuromuscular disorders undergoing similar training paradigms,SMA patients were less responsive to aerobic conditioning possibly representing an important clue to pathophysiological differences. Exercise capacity measured by peak oxygen uptake (VO2 max) appears to be lower in ambulatory SMA patients compared to other myopathic [5, 7–10] and denervating disorders [26, 27]. We speculate that this blunted response reflects an SMA specific mitochondrial dysfunction. In this study involving ambulatory SMA patients, baseline VO2 max ranged from 8.6 – 25.1 mL/kg/min compared to a range of 17 – 36 mL/kg/min found in adult populations with other neuromuscular conditions. Remarkably, comparable studies in most other disease groups reported significant positive training effects (up to 47% ) following 12 weeks of exercise using similar training protocols. [5, 7–10, 26, 27]
In a recent open-label pilot study, SMA patients made improvements in aerobic capacity with 12 weeks of intensive exercise training, without concomitant improvements in strength and function. [28] This form of study design and treatment paradigm provides limited opportunity for gradual increases in exercise intensity and frequency. Unfortunately, for SMA patients, this intervention resulted in debilitating adverse effects such as complaints of overwhelming subjective fatigue, that made maintaining the exercise regimen difficult. [29] In our study in similar ambulatory SMA patients, we noted only modest improvements (6.6% ) in exercise capacity (V02 max) over 6 months even when the observations were limited to those patients who were considered compliant with the exercise prescription. Based on our clinical experience and these pilot studies, we believe SMA patients are vulnerable to incapacitating fatigue with overexertion. Maintaining a moderate level of exercise intensity and avoiding steep increases in activity in short time periods was thought to be sufficient to protect the SMA participants from this experience. Larger studies are necessary to determine the optimal titration of exercise dosing and explore relationships between slope of improvement in exercise ability and disease severity.
SMN is widely expressed in all tissues, yet the clinical phenotype associated with SMN deficiency is that of a motor neuron disease. It remains unclear why the alpha-motor neurons are unduly vulnerable. In fact, current evidence supports dysfunction of the sensory-motor spinal reflex circuit as a significant contributory disease mechanism. [30] Deficiency in transmission at the neuromuscular junction, [31] muscle, [32] as well as proprioceptive neurons and interneurons [30] in SMA confirms our understanding that SMA is not a cell autonomous disease and that structures other than motor neurons, in fact, contribute to the phenotype.
The apparent reduced oxidative capacity and blunted response to aerobic exercise training, observed in the patients enrolled in this study, may have genetic underpinnings. Our clinical findings may support the hypothesis that mitochondrial biogenesis is vulnerable to SMN insufficiency but further studies are necessary. In addition to the central role of mitochondria in motor neuron function, down-regulation of mitochondrial biogenesis has been suggested in SMA, [33, 34] and other neurodegenerative disorders. [35] Muscle tissue from SMA patients showed reduced mitochondrial DNA content and reduced PGC1-alpha, the primary cofactor of mitochondrial biogenesis, and these findings were positively correlated with SMA disease severity. [34] We, therefore, speculate that impaired mitochondrial biogenesis is a downstream consequence of SMN insufficiency analogous to other recently reported downstream consequences of SMN insufficiency. [36, 37] Hence, the clinical consequences of reduced mitochondrial biogenesis may account for the blunted response of SMA patients to aerobic conditioning exercise.
The results of this prospective, single (examiner) blinded, randomized and controlled clinical trial of the effect of exercise on established functional outcome measures provides important guidance to clinical management of SMA patients and future research study design. Moreover, the lack of observed improvement in functional measures and the blunted response in overall oxidative capacity suggests a new disease model that involves mitochondrial depletion throughout the whole motor unit. Weakness and fatigue, the clinical signatures of SMA, together with apparent reduced oxidative capacity, as documented in this study, suggest a vulnerability of mitochondrial biogenesis to SMN insufficiency and warrant further research.
ACKNOWLEDGMENTS
This study is supported, in part, by the Department of Defense; USAMRAA Grant/Cooperative award number: 09131005(W81XWH-10-1-0127), and the Spinal Muscular Atrophy Foundation. The sponsors had no role in the conduct of this study. We gratefully acknowledge the research participants and their families for their generous gift of time and effort.
The authors have no conflicts of interests to report.
REFERENCES
1 | Darras BT, Markowitz JA, Monani UR, De Vivo DC (2015) Chapter 8 - Spinal Muscular Atrophies Basil T, Darras HRJ, Ryan Monique M, De Vivo Darryl C Neuromuscular Disorders of Infancy, Childhood, and Adolescence (Second Edition) 117 145 San Diego Academic Press |
2 | Deymeer F, Serdaroglu P, Poda M, Gulsen-Parman Y, Ozcelik T, Ozdemir C (1997) Segmental distribution of muscle weakness in SMA III: Implications for deterioration in muscle strength with time Neuromuscul Disord 7: 8 521 528 |
3 | Deymeer F, Serdaroglu P, Parman Y, Poda M (2008) Natural history of SMA IIIB Neurology 71: 664 449 |
4 | Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise Med Sci Sports Exerc 43: 7 1334 1359 |
5 | Sveen ML, Jeppesen T, Hauerslev S, Kober L, Krag TO, Vissing J (2008) Endurance training improves fitness and strength in patients with becker muscular dystrophy Brain 2824 2831 |
6 | Haller RG, Wyrick P, Taivassalo T, Vissing J (2006) Aerobic conditioning: An effective therapy in McArdle’s disease Ann Neurol 59: 6 922 928 |
7 | Olsen DB, Orngreen MC, Vissing J (2005) Aerobic training improves exercise performance in facioscapulohumeral muscular dystrophy Neurology 64: 6 1064 1066 |
8 | Sveen ML, Jeppesen T, Hauerslev S (2007) E. Endurance training: An effective and safe treatment for patients with LGMD2I Neurology 68 59 61 |
9 | Johnson L, Collier K, Edwards D, Philippe D, Eastwood P, Walters S (2009) Improvement in aerobic capacity after an exercise program in sporadic inclusion body myositis Journal of Clinical Neuromuscular Disease 10: 1 178 181 |
10 | Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Duno M (2006) Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy Brain 129: Pt 12 3402 3412 |
11 | Montes J, Ewing-Garber C, Kramer SS, Montgomery MJ, Dunaway S, Kamil-Rosenberg S (2014) A Randomized, Controlled Clinical Trial of Exercise in Patients with Spinal Muscular Atrophy: Methods and Baseline Characteristics Journal of Neuromuscular Diseases 1: 2 151 161 |
12 | Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM (2011) Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise Med Sci Sports Exerc 43: 7 1334 1359 |
13 | Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V (2007) Six-minute walk test in children and adolescents J Pediatr 150: 4 395 399 9 e1-2 |
14 | Gibbons WJ, Fruchter N, Sloan S, Levy RD (2001) Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years J Cardiopulm Rehabil 21: 2 87 93 |
15 | Montes J, McDermott MP, Martens WB, Dunaway S, Glanzman AM, Riley S (2010) Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy Neurology 74: 10 833 838 |
16 | ACSM’s guidelines for exercise testing and prescription. 9th edition, North american ed ed. American college of sports M, editor. Philadelphia, PA : LippincottWilliams & Wilkins; 2013 2013. 480 pages : Illustrations (black and white); 21cm p |
17 | Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA (2013) Exercise standards for testing and training: A scientific statement from the american heart association Circulation 128: 8 873 934 |
18 | Florence JM, Pandya S, King WM, Robison JD, Baty J, Miller JP (1992) Intrarater reliability of manual muscle test (Medical Research Council scale) grades in Duchenne’s muscular dystrophy Phys Ther 72: 2 115 122 discussion 22-6 |
19 | O’Hagen JM, Glanzman AM, McDermott MP, Ryan PA, Flickinger J, Quigley J (2007) An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients Neuromuscul Disord 17: 9-10 693 697 |
20 | Dunaway S, Montes J, Garber CE, Carr B, Kramer SS, Kamil-Rosenberg S (2013) Performance of the timed “up & go” test in spinal muscular atrophy Muscle Nerve |
21 | Wang X, Dockery DW, Wypij D, Fay ME, Ferris BGJr (1993) Pulmonary function between 6 and 18 years of age Pediatr Pulmonol 15: 2 75 88 |
22 | Varni JW, Rode CA, Seid M, Katz ER, Friedman-Bender A, Quiggins DJ (1999) The pediatric cancer quality of life inventory-32 (PCQL-32). II. Feasibility and range of measurement J Behav Med 22: 4 397 406 |
23 | Ware JJr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity Medical Care 34: 3 220 233 |
24 | Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus Arch Neurol 46: 10 1121 1123 |
25 | Physical Activity Guidelines for Americans. [[Internet]]. Washington, D.C.: U.S. department of health and human services; 2008 cited 2008. Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf |
26 | Mhandi L, Millet G, Calmels P, Richard A, Oullion R, Gautheron V (2008) Benefits of interval-training on fatigue and funtional capacities in Charcot-Marie-Tooth disease Muscle & Nerve 37: 601 610 |
27 | Preisler N, Andersen G, Thogersen F, Crone C, Jeppesen TD, Wibrand F (2009) Effect of aerobic training in patients with spinal and bulbar muscular atrophy (Kennedy disease) Neurology 72: 4 317 323 |
28 | Madsen KL, Hansen RS, Preisler N, Thogersen F, Berthelsen MP, Vissing J (2015) Training improves oxidative capacity, but not function, in spinal muscular atrophy type III Muscle Nerve 52: 2 240 244 |
29 | Madsen KL, Hansen RS, Preisler N, Thogersen F, Berthelsen MP, Vissing J Training improves oxidative capacity, but not function in spinal muscular atrophy type III Muscle Nerve 2014 |
30 | Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD (2012) SMN Is Required for Sensory-Motor Circuit Function in Drosophila Cell 151: 2 427 439 |
31 | Kariya S, Park GH, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS (2008) Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy Hum Mol Genet 17: 16 2552 2569 |
32 | Bricceno KV, Martinez T, Leikina E, Duguez S, Partridge TA, Chernomordik LV (2014) Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics Hum Mol Genet 23: 18 4745 4757 |
33 | Acsadi G, Lee I, Li X, Khaidakov M, Pecinova A, Parker GC, Hüttemann M (2009) Mitochondrial dysfunction in a neural cell model of spinal muscular atrophy Journal of Neuroscience Research 87: 12 2748 2756 |
34 | Ripolone M, Ronchi D, Violano R, Vallejo D, Fagiolari G, Barca E (2015) Impaired Muscle Mitochondrial Biogenesis and Myogenesis in Spinal Muscular Atrophy JAMA Neurology 72: 6 666 675 |
35 | Christos K, Koutzaki S, Melvin J (2013) Mitochondrial Dysfunction in Neuromuscular Disorders Seminars in Pediatric Neurology 20: 202 215 |
36 | Lotti F, Imlach WL, Saieva L, Beck ES, Hao le T, Li DK (2012) An SMN-Dependent U12 Splicing Event Essential for Motor Circuit Function Cell 151: 2 440 454 |
37 | Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L (2011) Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy Neuron 69: 3 453 467 |
Figures and Tables
Fig.1
Percent change in scores over 6 months for strength and functional measures for the exercise (black bars) and control (gray bars) groups.
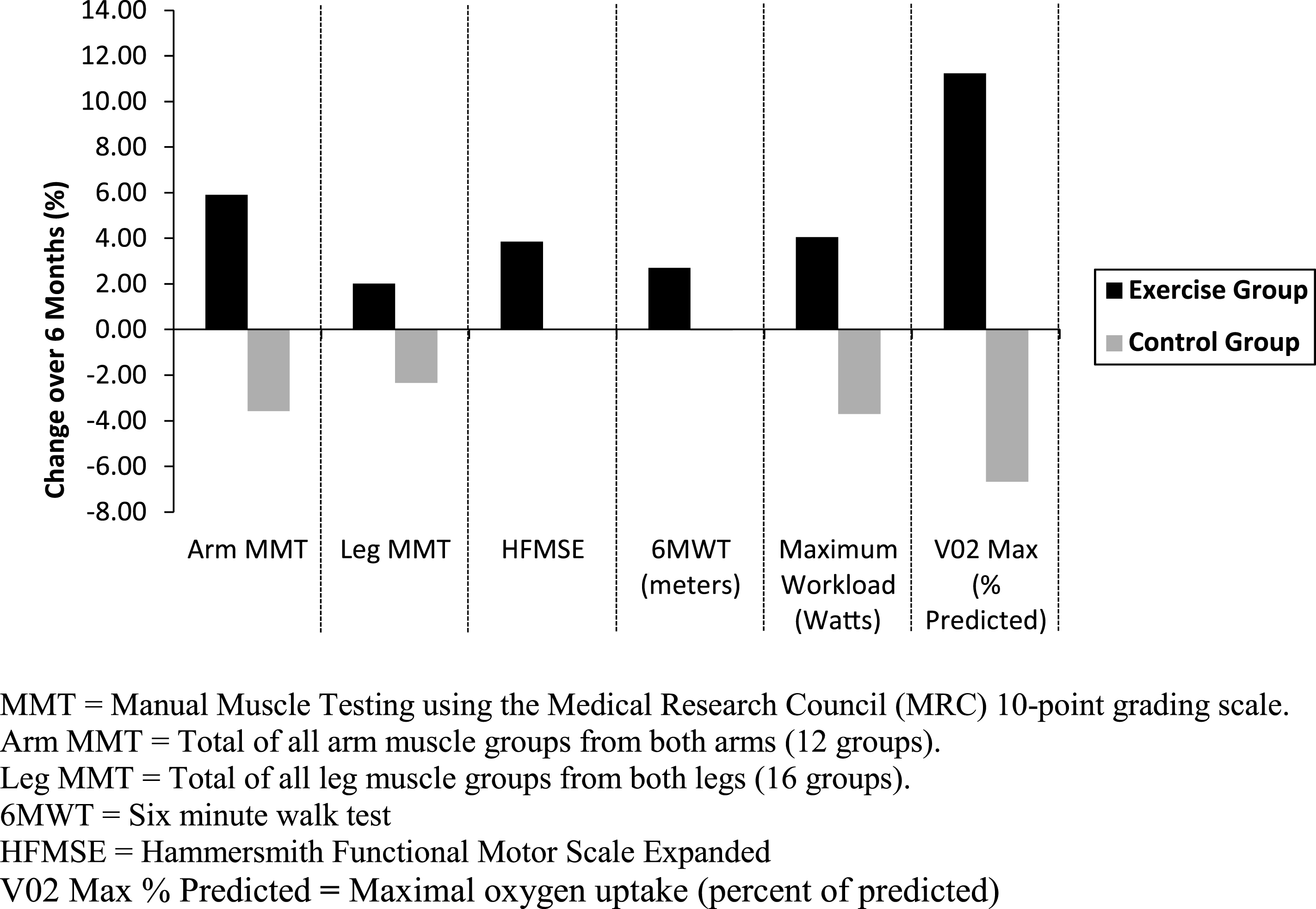
Table 1
The frequency of all non-serious musculoskeletal and other adverse events reported from all participants (n = 14) during the entire study period
| Non-Serious Adverse Events | |||
| Musculoskeletal | Frequency | Other | Frequency |
| Fall | 156 | Migraine Headache | 25 |
| Muscle Soreness | 18 | Fatigue | 10 |
| Low Back Pain | 11 | URI | 8 |
| Hip Pain | 3 | Fever | 4 |
| Knee Pain | 3 | Nasal Congestion | 3 |
| Leg Weakness | 2 | Paresthesia | 2 |
| Neck/Shoulder Pain | 1 | Cough | 2 |
| Generalized Weakness | 1 | Ear Infection | 2 |
| Upper Leg Pain | 1 | Diarrhea | 1 |
| Lower Leg Cramp | 1 | Sty (Right Eye) | 1 |
| Arm Pain | 1 | Sinus Infection | 1 |
| Total | 198 | Earache | 1 |
| Sore Throat | 1 | ||
| Strep Throat | 1 | ||
| Malaise | 1 | ||
| Ingrown Toenail | 1 | ||
| Gastroenteritis | 1 | ||
| CMV Infection | 1 | ||
| Nosebleed | 1 | ||
| Vertigo | 1 | ||
| Nausea | 1 | ||
| Elevated BP | 1 | ||
| Influenza | 1 | ||
| Total | 71 | ||
| Overall Total | 269 | ||
Table 2
Comparison of the frequency of non-serious musculoskeletal adverse events by group during the 6-month controlled period
| Adverse Event | Exercise | Control | p-value |
| Fall | 23 | 35 | 0.488 |
| Muscle Soreness | 6 | 4 | 0.690 |
Table 3
Comparisons of change in clinical outcome measures between the exercise and control groups over the 6-month controlled period
| Outcome Variable | Exercise (n = 5) | Control (n = 7) | P | ||
| Baseline | Month 6 | Baseline | Month 6 | ||
| 6MWT Distance (m) | 337.60 (81.46) | 347.00 (79.00) | 331.57 (133.00) | 331.43 (111.96) | 0.844 |
| Fatigue (% ) | 21.82 (13.20) | 21.80 (10.13) | 25.99 (19.82) | 24.53 (18.35) | 0.791 |
| HFMSE | 50 (8.16) | 52 (6.63) | 54 (8.16) | 54 (7.07) | 0.357 |
| VO2Max (mL/kg/min) | 12.72 (4.07) | 12.60 (4.00) | 15.94 (5.10) | 14.60 (3.08) | 0.496 |
| VO2Max (% predicted) | 31.80 (5.97) | 35.83 (5.07) | 41.92 (12.22) | 39.30 (7.92) | 0.218 |
| 10 meter walk/run (sec) | 8.29 (2.62) | 7.95 (2.61) | 7.80 (3.00) | 8.11 (2.72) | 0.308 |
| TUG (sec) | 14.68 (6.25) | 15.90 (8.23) | 15.69 (17.47) | 12.63 (9.76) | 0.302 |
| Total MMT | 174.00 (18.40) | 180.80 (21.37) | 185.00 (33.40) | 179.86 (32.69) | 0.159 |
| Arm MMT | 76.40 (6.88) | 81.20 (9.04) | 78.71 (14.17) | 76.00 (13.18) | 0.080 |
| Leg MMT | 97.60 (12.24) | 99.60 (15.90) | 106.29 (22.33) | 103.86 (21.86) | 0.409 |
| HHD knee extension (kg) | 4.44 (1.58) | 3.54 (1.52) | 2.33 (1.02) | 2.16 (0.93) | 0.560 |
| HHD knee flexion (kg) | 6.64 (1.42) | 5.34 (2.06) | 4.71 (2.10) | 4.20 (1.75) | 0.340 |
| HHD shoulder abduction (kg) | 3.48 (1.15) | 3.02 (1.31) | 4.06 (1.68) | 4.00 (1.52) | 0.165 |
| HHD elbow extension (kg) | 2.90 (2.30) | 2.86 (1.66) | 3.99 (2.26) | 3.66 (2.31) | 0.485 |
| HHD elbow flexion (kg) | 10.52 (4.40) | 9.60 (5.71) | 10.89 (4.90) | 10.31 (4.47) | 0.716 |
| FVC (% predicted) | 103.20 (13.10) | 104.00 (13.19) | 102.86 (14.10) | 101.43 (14.62) | 0.476 |
Values are presented as mean (standard deviation) unless otherwise specified.6MWT = Six minute walk test. HFMSE = Hammersmith Functional Motor Scale Expanded, TUG = Timed Up and Go Test, MMT = Manual Muscle Testing using the Medical Research Council (MRC) 10-point grading scale. Total MMT = Total of all arm and leg muscle groups (28 groups). Maximum possible score = 280. Arm MMT = Total of all arm muscle groups from both arms (12 groups). Maximum possible score = 120. Leg MMT = Total of all leg muscle groups from both legs (16 groups). Maximum possible score = 160. HHD = Handheld dynamometry. FVC = Forced vital capacity.
Table 4
Assessments of quality of life and perceived fatigue during the 6-month controlled period in (A) adult participants on the SF-36 and FSS, and (B) child participants and their parents on the PedsQL™ Neuromuscular and Fatigue Scales
| Outcome Variable | Exercise (n = 4) | Control (n = 2) | ||
| Baseline | Month 6 | Baseline | Month 6 | |
| (A) | ||||
| SF-36 | ||||
| Physical Health | 35.6 (6.88) | 36.1 (7.50) | 39.4 (.29) | 37.8 (1.60) |
| Mental Health | 60.3 (25.06) | 60.5 (23.65) | 54.2 (2.32) | 55.0 (2.74) |
| Exercise (n = 4) | Control (n = 4) | |||
| FSS | 4.6 (1.69) | 4.6 (1.51) | 5.0 (.85) | 5.4 (1.04) |
Values are presented as mean (standard deviation) unless otherwise specified. SF-36 = Short Form 36. FSS = Fatigue Severity Scale.
| (B) | ||||
| Outcome Variable | Exercise (n = 1) | Control (n = 3) | ||
| Baseline | Month 6 | Baseline | Month 6 | |
| Neuromuscular (Peds QL)* | ||||
| Child | 90.0 | 89.0 | 85.3 (13.61) | 85.4 (9.40) |
| Parent | 68.0 | 73.0 | 83.0 (10.81) | 85.7 (9.61) |
| Fatigue (Peds QL)** | ||||
| Child | 88.0 | 90.0 | 81.7 (15.49) | 85.2 (12.12) |
| Parent | 51.0 | 57.0 | 75.9 (18.86) | 83.24 (14.43) |
Values are presented as mean (standard deviation) unless otherwise specified. *PedsQL™ Quality of Life Neuromuscular Module. **PedsQL™ Multidimensional Fatigue Scale



