Costs and Resource Use Associated with Community-Dwelling Patients with Alzheimer’s Disease in Japan: Baseline Results from the Prospective Observational GERAS-J Study
Abstract
Background:
As the Japanese population ages, caring for people with Alzheimer’s disease (AD) dementia is becoming a major socioeconomic issue.
Objective:
To determine the contribution of patient and caregiver costs to total societal costs associated with AD dementia.
Methods:
Baseline data was used from the longitudinal, observational GERAS-J study. Using the Mini-Mental State Examination (MMSE) score, patients routinely visiting memory clinics were stratified into three groups based on AD severity. Health care resource utilizationwas recorded using the Resource Utilization in Dementia questionnaire. Total monthly societal costs were estimated using Japan-specific unit costs of services and products (patient direct health care use, patient social care use, and informal caregiving time). Uncertainty around mean costs was estimated using bootstrapping methods.
Results:
Overall, 553 community-dwelling patients withADdementia (28.3% mild[MMSE21-26], 37.8% moderate[MMSE 15-20], and 34.0% moderately severe/severe [MMSE < 14]) and their caregivers were enrolled. Patient characteristics were: mean age 80.3 years, 72.7% female, and 13.6% living alone. Caregiver characteristics were: mean age 62.1 years, 70.7% female, 78.8% living with patient, 49.0% child of patient, and 39.2% sole caregiver. Total monthly societal costs of AD dementia (Japanese yen) were: 158,454 (mild), 211,301 (moderate), and 294,224 (moderately severe/severe). Informal caregiving costs comprised over 50% of total costs.
Conclusion:
Baseline results of GERAS-J showed that total monthly societal costs associated with AD dementia increased with AD severity. Caregiver-related costs were the largest cost component. Interventions are needed to decrease informal costs and decrease caregiver burden.
INTRODUCTION
Worldwide, people are living longer [1]. At the current rate, the world’s population aged≥60 years is expected to increase from 900 million in 2015 to 2 billion in 2050. Likewise, in 2050 there will be 434 million persons aged≥80 years worldwide. With aging, the incidences of Alzheimer’s disease (AD) and other dementias increase [2, 3]. It is estimated that in 2050, 131.5 million persons worldwide will suffer from dementia [4]. The estimated global cost of dementia was 818 billion United States dollars (USD) in 2015. Of these costs, 40.4% (330.8 billion USD) were attributed to informal (unpaid) patient care. Informal caregiving costs include the value of caregiving time, lost income of caregivers, costs related to the purchase of formal care (e.g., hired helper, home health care), and caregiver health care costs related to the giving of informal care [5]. Thus, informal care places a considerable burden on family caregivers. Given demographic trends that indicate a declining overall population and a growing population of those over 65 years of age, Japan may experience a larger impact of rising dementia prevalence rates and a greater burden of providing informal care than other countries [6, 7].
Japan has the highest life expectancy in the world [8]. This, combined with a simultaneous decline in birth rates has created a “super-aging society” with approximately 33% and 20% of persons predicted to be older than 65 and 75 years of age, respectively, by 2030 [9]. The rates of all-cause and AD dementia are increasing in Japan [10–12] and these conditions are becoming major health issues. In 2010, 2.5 million adults in Japan were estimated to suffer from dementia, ranking Japan among 9 countries with the highest number of sufferers [13]. In a national survey of people 65 years of age and older in Japan, the prevalence of AD dementia was 15.8% [14]. In addition to medical aspects, AD dementia is a socioeconomic issue impacting the family and society, as patients with advanced AD dementia are not able to care for themselves.
In response to changing age demographics, Japan implemented a unique insurance program mandating social long-term care insurance (LTCI) as a universal entitlement for people aged over 40 years [15]. This program provides comprehensive evaluation of the elderly and financial assistance via national insurance benefits for family caregivers. Subsequently, reforms were introduced to prevent senior citizens from becoming dependent on others while their needs are still relatively low, and to reduce economic incentives for institutionalization [16]. This shifted care to the community and family.
The cost of dementia to society is considerable. Using the Japanese government statistics, Sado and colleagues estimated 2014 costs associated with dementia to be 14.5 trillion Japanese Yen (JPY); of these costs, 6.16 trillion JPY was associated with informal care [17]. In that study, time spent on informal care over a 1-week period was based on surveys distributed to caregivers. A replacement cost approach and an opportunity cost approach was applied to the time spent providing activities of daily living (ADL)-type care and instrumental ADL-type care, respectively. Likewise, using Japanese statistics, Hanaoka and colleagues estimated total costs associated with dementia to be 3.78 to 5.51 trillion JPY in 2014 with informal care comprising 1.42 to 3.15 trillion JPY [18]. In that study, the Comprehensive Cost of Illness method was used to calculate costs. Informal costs were calculated based on time spent caregiving and the cost of caregiver time based on three case models.
With the exception of Japanese government statistics, there is little real-world, observational data on the socioeconomic burden of AD dementia in Japan. Because care arrangements and resource use patterns change over time and are dependent on culture, health care systems, and social norms, data from other countries cannot be extrapolated to Japan. Furthermore, local cost data would be more informative for Japan’s decision makers. The GERAS-Japan (GERAS-J) study was designed to provide domestic cost data under the Japanese health care system. GERAS-J is an 18-month prospective observational study [19] similar to studies conducted in European Union countries, GERAS-I (UK, France, and Germany) [20] and GERAS-II (Italy [21] and Spain [22]). The GERAS studies were designed to determine resource use and total costs associated with AD dementia and its impact on caregivers, stratified by AD severity (mild, moderate, and moderately severe/severe) at study entry. Data on resource usage and cost in Asia is limited. Therefore, the current study is highly valuable as input to subsequent economic evaluations. The objective of this study was to determine the contribution of patient and caregiver costs to total societal costs associated with AD dementia, using baseline data from GERAS-J.
METHODS
Study design
GERAS-J is a longitudinal prospective, multicenter, observational, cohort study reflecting routine care of community-dwelling patients with AD dementia with an 18-month follow-up of resource utilization in Japan. Patients and their primary caregivers were enrolled from November 2016 to December 2017 at 30 Japanese study sites (memory clinics, including 13 university hospitals, 12 hospitals, and 5 clinics) by 49 investigators.
Patients
Male and female outpatients at least 55 years old were eligible if they were currently being treated as outpatients at hospitals/clinics where patients are routinely seen for diagnosis and follow-up for AD dementia, and their treatment decisions were made solely at the discretion of the treating physician. Patients met criteria for probable AD according to the National Institute on Aging and Alzheimer’s Association Alzheimer’s criteria [23], and had a Mini-Mental State Examination (MMSE) [24] score of 26 or less. Patients with a history, clinical signs, or evidence via imaging of stroke, transient ischemic attack, or Parkinson’s disease prior to or at the start of AD onset, or probable Lewy body dementia were excluded. Additionally, patients without a primary caregiver and those concurrently participating in an interventional trial were excluded.
Patients were classified into three groups (mild, moderate, moderately severe/severe) based on the AD severity at baseline using MMSE [24] criteria consistent with the GERAS-I and United Kingdom (UK) National Institute for Health and Care Excellence (NICE) guidelines: mild, MMSE 21– 26 points; moderate, MMSE 15– 20 points; and moderately severe/severe, MMSE≤14 points [20, 25].
This study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, and was consistent with Good Pharmacoepidemiology Practices and applicable laws and regulations of Japan. The protocol was reviewed by a central ethical review board (ERB). Some sites required site-specific ERB approval following the rules of each site. Written consent of both patient (and his/her representative) and caregiver was required.
Assessments and outcome measures
Personnel at each study site collected baseline data and administered assessments. Collected data included baseline demographics, comorbidities, and drug use of both patient and caregiver, AD diagnosis, living conditions, and AD treatment of patients. Patients’ and caregivers’ clinical outcomes, health care resource utilization, health-related quality of life, financial assistance, out-of-pocket expenses, and burden of AD dementia were also collected at baseline.
Clinical outcomes of patients were assessed by researchers in an interview-based manner using the MMSE [24], Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL) [26], Neuropsychiatric Inventory (NPI) [27], and Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) [28, 29]. The MMSE and ADAS-Cog measure patient cognition [24, 29]. The ADAS-Cog was only applied to patients with a MMSE > 14 points. The NPI assesses patient behavior and the ADCS-ADL measures patient function [26, 27]; information for both was obtained from the caregiver in an interview conducted by a researcher. For the ADCS-ADL, separate scores for the basic ADL and instrumental ADL were reported.
Health outcomes and health care resource utilization were assessed using the EuroQol-5 Dimensions 5 Levels (EQ-5D-5 L Visual Analog Scale [VAS] and Health Index) [30, 31], Zarit Burden Interview (ZBI) [32], and the Resource Utilization in Dementia (RUD) questionnaire [33]. The EQ-5D-5 L and ZBI questionnaires were self-administered under supervision by researcher. The RUD questionnaire was administered by researchers in an interview-based manner. Caregivers answered a proxy version of the EQ-5D-5 L on the patients’ behalf and also answered the questionnaire for themselves. Caregivers provided information for the ZBI and RUD questionnaire. Caregivers provided information on time spent assisting patients’ basic ADLs such as using the toilet, eating, dressing, grooming, walking, and bathing; assisting patients’ instrumental ADLs such as shopping, cooking, housekeeping, laundry, transportation, taking medication, and managing finances; and providing supervision.
Study size
Sample size was estimated assuming exponential cost distribution and assuming that 23.1%, 30.5%, and 45.7% of patients with mild, moderate, and moderately severe/severe AD, respectively, would be lost to follow-up or have follow-up inadequate for cost-estimation at 18 months. The expected dropout rates were derived from GERAS-I [20]. To obtain a 95% confidence interval (CI) of±9.0% of the mean cost, a minimum of 550 (precision of 18%) patients were required to be enrolled. This assumed equal numbers for the three AD subgroups (mild, moderate, and moderately severe/severe). The observed samples sizes gave an approximate precision of 16%, 14%, and 14% for the three groups mild, moderate, and moderately severe/severe, respectively.
Cost estimation
Total monthly societal costs associated with AD dementia were estimated for both patient and caregiver during the last 30 days from the baseline visit by applying Japan-specific unit costs (2016 values) of services and products to recorded resource use (RUD questionnaire and additional data collected on treatments including pharmacotherapy and neuropsychological assessments) (Supplementary Table 1).
Costs were broken down into three cost components: 1) patient health care costs (included cost of patient medications, nights in hospital [hospital admissions], emergency room visits, and outpatient visits); 2) patient social care costs (included costs of community care services, structural adaptations to patient living accommodation, and consumables; 3) caregiver informal care costs (included costs of caregiver time and of the caregiver missing work). The caregiver time in hours was calculated from the RUD with active caregiving time for basic living activity and instrumental living activity. Supervision time was excluded. Informal care costs were calculated using the higher cost of either caregivers’ time or caregivers’ missed work (same unit cost was applied to both items) [17]. For working caregivers, the unit cost for caregiver time was the value of lost production time, with the cost based on the national average wage (see Supplementary Table 1). For non-working caregivers, the unit cost for caregiver time was the value of lost leisure time, and this was costed at 35% of the national average wage per country population as in previous studies [20, 34].
Statistical methods
All patients who fulfilled study entry criteria were included in the analyses. The differences in patient and caregiver characteristics and reported assessments (i.e., MMSE, ADCS-ADL, ADAS, NPI, EQ-5D-5 L, ZBI, and RUD) between AD severity groups were tested with an analysis of variance (ANOVA) or the Brown-Mood median test (exact p-value from the Monte Carlo estimate) for continuous variables and Fisher’s Exact Test or Monte Carlo Estimate were used for categorical variables. Tests of significance were based at the 5% level.
The distribution of cost data was assessed for the total group and each group (mild, moderate, moderately severe/severe) separately. Mean costs are reported with 95% CIs which were calculated using a bootstrapping approach with the lower CI coming from the 2.5 centile and the upper CI from the 97.5 centile. The bootstrap resampling of the original data was performed 10,000 times. For each of 10,000 samples with replacement, the median and mean were calculated.
Factors associated with total societal costs were analyzed using generalized linear models using the gamma distribution with a log-link function with total societal cost as the dependent variable, and independent variables selected through a backward selection method (p > 0.05 for removal), with patient age, patient gender, and MMSE severity group all forced to remain in the model. For predictor variables, if a continuous covariate was missing, the population median was imputed. If a categorical covariate was missing, the population mode was imputed. There was no baseline missing cost information. Patient variables used for the backward selection were: AD severity group, age, gender, LTCI certified (Yes/No), level of education, time since AD diagnosis, living location (urban; rural), living arrangements (living alone; not living alone+married; not living alone+not married), number of comorbidities, number of patients experiencing a fall in the past 3 months, baseline ADCS-ADL total score, and baseline NPI score. Caregiver variables used for the backwards selection approach were: number of caregivers caring for the patient, age, gender, relationship to patient, number of comorbidities, caregiver in employment, and baseline ZBI score. Model assumptions were assessed using residual diagnostics and model fit was determined using deviance.
SAS 9.4 (SAS Institute, Cary, NC, USA) was used for analyses.
RESULTS
Overall, 560 patients were screened at 30 Japanese study sites by 49 investigators. Of these, 7 withdrew (5 did not meet inclusion criteria; 1 met exclusion criteria [Lewy body dementia]; 1 discontinued before completing questionnaires). A total of 553 community-dwelling patients with AD dementia and their caregivers were enrolled in GERAS-J (Table 1). Most patients were female (72.7%) and the mean age was 80.3 years old. Of all patients, 28.3%, 37.8%, and 34.0% had mild, moderate, and moderately severe/severe AD, respectively. The moderately severe/severe AD group had a longer time since AD diagnosis and fewer patients living alone at home. Overall, 53.8% of mild, 67.9% of moderate, and 88.3% of moderately severe/severe AD patients had received a certification of care needs under the LTCI, which is the Japan-specific care support system.
Table 1
Patient characteristics at baseline
| Overall (N = 553) | Mild AD (N = 156) | Moderate AD (N = 209) | Moderately Severe/Severe AD (N = 188) | p | |
| Age, mean (SD), median [Min-Max] (years) | 80.3 (7.3), 81.0, [57-100] | 79.6 (6.7), 80.5, [60-96] | 81.0 (6.9), 82.0, [61-100] | 80.1 (8.2), 81.0, [57-97] | 0.3331 |
| Female, n (%) | 402 (72.7%) | 108 (69.2%) | 156 (74.6%) | 138 (73.4%) | 0.5022 |
| Time since AD diagnosis, mean (SD), median (years) | 2.8 (2.5), 2.2 | 1.8 (2.1), 1.2 | 2.5 (2.1), 2.2 | 3.9 (2.6), 3.5 | <0.0011 |
| Marital status: married/ cohabitating, n (%) | 321 (58.0%) | 93 (59.6%) | 117 (56.0%) | 111 (59.0%) | 0.2073 |
| Patient lives alone at home, n (%) | 75 (13.6%) | 36 (23.1%) | 34 (16.3%) | 5 (2.7%) | <0.0012 |
| Patient lives in own home, n (%) | 546 (98.7%) | 153 (98.1%) | 207 (99.0%) | 186 (98.9%) | 0.9312 |
| Education, n (%) | 0.6282 | ||||
| ≤12 years4 | 466 (84.3%) | 126 (80.8%) | 178 (85.2%) | 162 (86.2%) | |
| >12 years5 | 87 (15.7%) | 30 (19.2%) | 31 (14.8%) | 26 (13.8%) | |
| Patients taking medication indicated for AD treatment, n (%) | 523 (94.6%) | 142 (91.0%) | 202 (96.7%) | 179 (95.2%) | 0.0662 |
| LTCI certified, n (%) | 392 (70.9%) | 84 (53.8%) | 142 (67.9%) | 166 (88.3%) | <0.0012 |
Percentages are based on the overall population at baseline (i.e., no missing data). 1p-value is from Brown-Mood median test. 2p-value is from Fisher’s Exact Test. 3p-value is from Pairwise Comparisons of Means (Bonferroni Correction applied). 4Primary/Junior/High School. 5Vocational/Junior College/Higher Professional School/University/Graduate School. AD, Alzheimer’s disease; LTCI, long-term care insurance; Max, maximum; Min, minimum; N, population size; n, number in group; SD, standard deviation.
Table 2 shows baseline characteristics of the caregivers. Most caregivers were female (70.7%) and the mean age was 62.1 years. Children were the most common caregivers (49.0%), followed by a spouse (37.1%). The percent of caregivers living with the patient increased with AD severity (67.9% [mild] to 94.1% [moderately severe/severe]). Overall, 39.2% of caregivers were the sole caregiver. Approximately half (47.7%) of caregivers had paid work in addition to caregiving.
Table 2
Caregiver characteristics at baseline
| Overall (N = 553) | Mild AD (N = 156) | Moderate AD (N = 209) | Moderately Severe/Severe AD (N = 188) | p | |
| Age, mean (SD), median, [Min-Max] (years) | 62.1 (12.5), 60.0 [28-93] | 61.9 (13.4), 60.0 [28-90] | 62.4 (12.5), 60.0 [32-93] | 62.0 (11.8), 60.5 [30-89] | 0.9361 |
| Female, n (%) | 391 (70.7%) | 113 (72.4%) | 150 (71.8%) | 128 (68.1%) | 0.6372 |
| Caregiver’s relation to patient, n (%) | 0.6243 | ||||
| Spouse | 205 (37.1%) | 62 (39.7%) | 71 (34.0%) | 72 (38.3%) | |
| Sibling | 8 (1.4%) | 4 (2.6%) | 2 (1.0%) | 2 (1.1%) | |
| Child | 271 (49.0%) | 74 (47.4%) | 105 (50.2%) | 92 (48.9%) | |
| Other | 69 (12.5%) | 16 (10.3%) | 31 (14.8%) | 22 (11.7%) | |
| Living with patient, n (%) | 436 (78.8%) | 106 (67.9%) | 153 (73.2%) | 177 (94.1%) | <0.0012 |
| Sole caregiver, n (%) | 217 (39.2%) | 64 (41.0%) | 83 (39.7%) | 70 (37.2%) | 0.6843 |
| Caregiver has paid work, n (%) | 264 (47.7%) | 77 (49.4%) | 106 (50.7%) | 81 (43.1%) | 0.2882 |
Percentages are based on the overall population at baseline (i.e., no missing data). 1p-value is from analysis of variance. 2p-value is from Fisher’s Exact Test. 3p-value is from Fisher’s Exact Test (Monte Carlo Estimate). AD, Alzheimer’s disease; Max, maximum; Min, minimum; N, population size; n, number in group; SD, standard deviation.
As expected, all clinical characteristics of patients were worse as AD severity increased (Table 3). Caregiver distress and burden were greater when caregiving for patients with moderately severe/severe AD than patients with mild AD. Quality of life (EQ-5D VAS and Health Index) was worse in patients with moderately severe/severe AD than in patients with mild and moderate AD, but there was no significant difference for caregivers.
Table 3
Patient and caregiver-reported assessment
| Overall (N = 553) | Mild AD (N = 156) | Moderate AD (N = 209) | Moderately Severe/Severe AD (N = 188) | p | |
| Patient assessment | |||||
| MMSE | |||||
| Mean (SD) Median | 16.4 (6.1) 17.0 | 22.9 (1.6) 22.0 | 17.8 (1.7) 18.0 | 9.5 (4.5) 11.0 | <0.0011 |
| ADAS-Cog11 Mean (SD) | 22.3 (6.6) | 18.3 (5.8) | 25.1 (5.5) | - | <0.0012 |
| ADCS-ADL basic | |||||
| Mean (SD) Median | 18.0 (5.3) 20.0 | 20.6 (2.8) 22.0 | 19.4 (3.4) 20.0 | 14.2 (6.4) 16.0 | <0.0011 |
| ADCS-ADL instrumental | |||||
| Mean (SD) | 28.6 (14.2) | 38.7 (11.2) | 30.6 (12.1) | 17.9 (11.1) | <0.0012 |
| ADCS-ADL total | |||||
| Mean (SD) Median | 46.5 (18.5) 49.0 | 59.4 (13.1) 62.0 | 50.0 (14.5) 52.0 | 32.0 (16.5) 32.0 | <0.0011 |
| NPI-12 total | |||||
| Mean (SD) Median | 12.4 (12.5) 8.0 | 8.8 (10.9) 6.0 | 11.6 (13.8) 7.0 | 16.2 (11.3) 16.0 | <0.0011 |
| EQ-5D index (proxy) | |||||
| Mean (SD) Median | 0.73 (0.19) 0.77 | 0.81 (0.16) 0.83 | 0.78 (0.16) 0.80 | 0.62 (0.20) 0.62 | <0.0011 |
| EQ-5D VAS (proxy) | |||||
| Mean (SD) Median | 69.6 (19.1) 70.0 | 74.7 (15.3) 80.0 | 71.2 (18.2) 75.0 | 63.5 (21.3) 60.0 | <0.0011 |
| Caregiver assessment | |||||
| NPI-12 caregiver distress | |||||
| Mean (SD) Median | 5.6 (6.6) 3.0 | 4.5 (6.1) 2.0 | 5.3 (7.3) 3.0 | 6.8 (6.0) 5.0 | 0.0011 |
| ZBI total score | |||||
| Mean (SD) | 29.4 (15.6) | 27.2 (16.1) | 27.2 (15.3) | 33.6 (14.8) | <0.0012 |
| EQ-5D index | |||||
| Mean (SD) | 0.90 (0.12) | 0.90 (0.13) | 0.90 (0.13) | 0.90 (0.13) | 0.9212 |
| EQ-5D VAS | |||||
| Mean (SD) | 80.5 (15.1) | 80.5 (15.2) | 80.6 (16.1) | 80.3 (13.8) | 0.9842 |
1 p-value is from Brown-Mood median test. 2 p-value is from analysis of variance. Data were missing for 0.2% to 0.7% of patients for NPI-12 and EQ-5D, and 11.8% of patients for ADAS-Cog11. There were no missing data for MMSE, ADCS-ADL, or ZBI. AD, Alzheimer’s disease; ADCS-ADL, Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory; ADAS-Cog 11, Alzheimer’s Disease Assessment Scale-Cognitive Subscale 11; EQ-5D, EuroQol-5 Dimension; MMSE, Mini-Mental State Examination; N, population size; NPI-12, Neuropsychiatric Inventory-12; SD, standard deviation; VAS, visual analog scale; ZBI, Zarit Burden Interview.
Over half of patients with AD received social care services (Table 4). Daycare was the most commonly used community service with the proportion of patients using daycare increasing with AD severity: 35.9% mild; 47.4% moderate, 62.8% moderately severe/severe (p < 0.001).
Table 4
Social service use by patients in the month prior to baseline1
| Overall (N = 553) | Mild AD (N = 156) | Moderate AD (N = 209) | Moderately Severe/Severe AD (N = 188) | p2 | |
| Patient received community services, n (%) | 320 (57.9%) | 71 (45.5%) | 115 (55.0%) | 134 (71.3%) | <0.001 |
| District nurse visit | 10 (1.8%) | 4 (2.6%) | 3 (1.4%) | 3 (1.6%) | 0.723 |
| Home aid/orderly | 38 (6.9%) | 13 (8.3%) | 13 (6.2%) | 12 (6.4%) | 0.705 |
| Food delivery | 13 (2.4%) | 3 (1.9%) | 6 (2.9%) | 4 (2.1%) | 0.828 |
| Daycare | 273 (49.4%) | 56 (35.9%) | 99 (47.4%) | 118 (62.8%) | <0.001 |
| Transportation | 61 (11.0%) | 15 (9.6%) | 24 (11.5%) | 22 (11.7%) | 0.808 |
| Other3 | 22 (4.0%) | 5 (3.2%) | 7 (3.3%) | 10 (5.3%) | 0.561 |
1Data are from the Resource Utilization in Dementia Questionnaire (RUD) questionnaire. Percentages are based on the overall population at baseline (i.e., no missing data). 2p-value is from Fisher’s exact test. 3Other includes rehabilitation, volunteer/helper, and etc. AD, Alzheimer’s disease; N, population size; n, number in group.
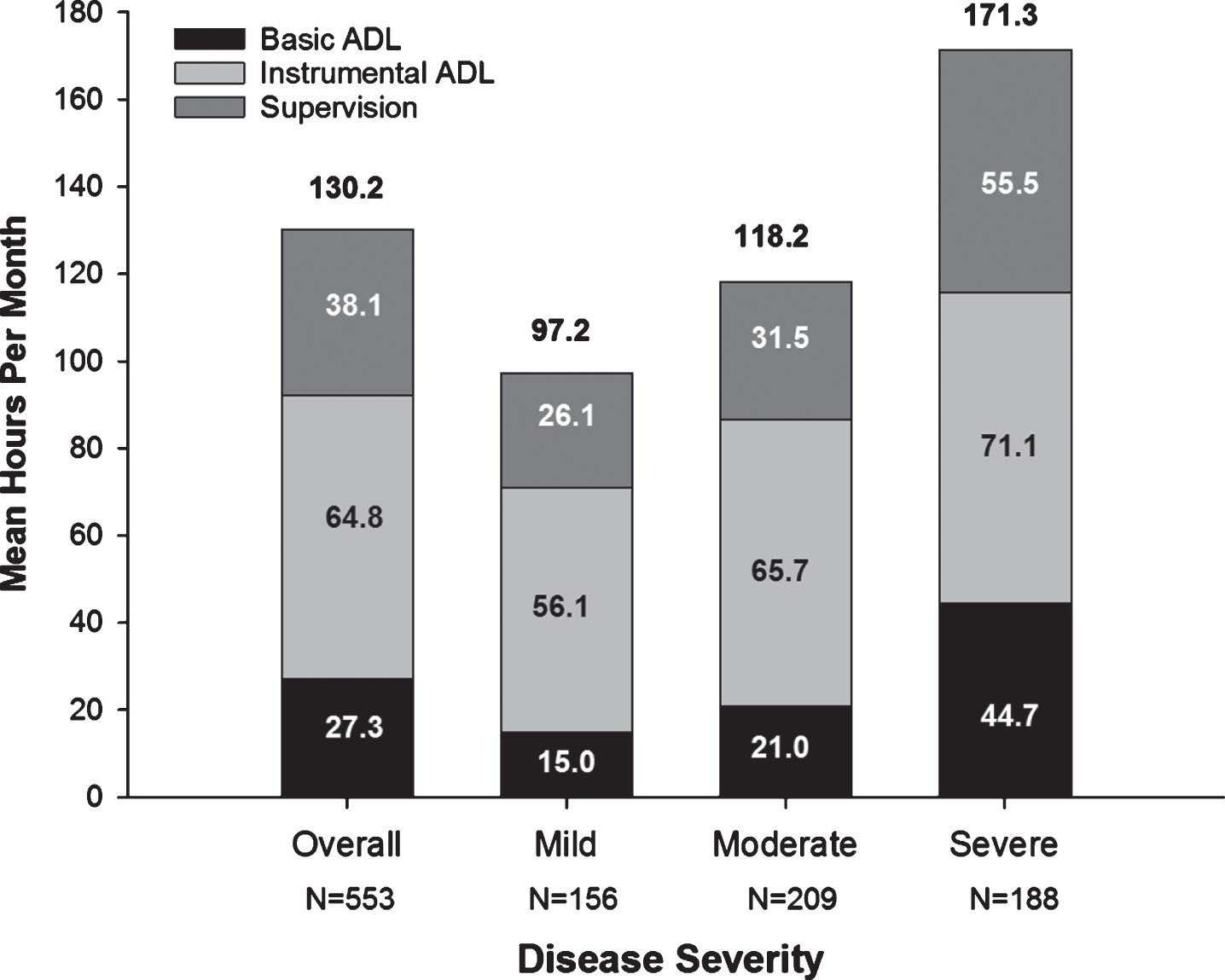
Total caregiver time increased with AD severity, as did the subcategories of caregiving time (basic and instrumental ADL, and supervision) (Fig. 1). Supplementary Table 2 shows medical resource use of patients and caregivers.
Fig. 1
Caregiver Time for Activities of Daily Living (Hours/Month) at Baseline. All values are based on data provided for the last 30 days prior to the baseline visit. The N value is the number of respondents that spent any time supervising in the last 30 days. The numbers above the graphs are total caregiver time (mean hours per month). ADL, activities of daily living; Severe, moderately severe/severe.
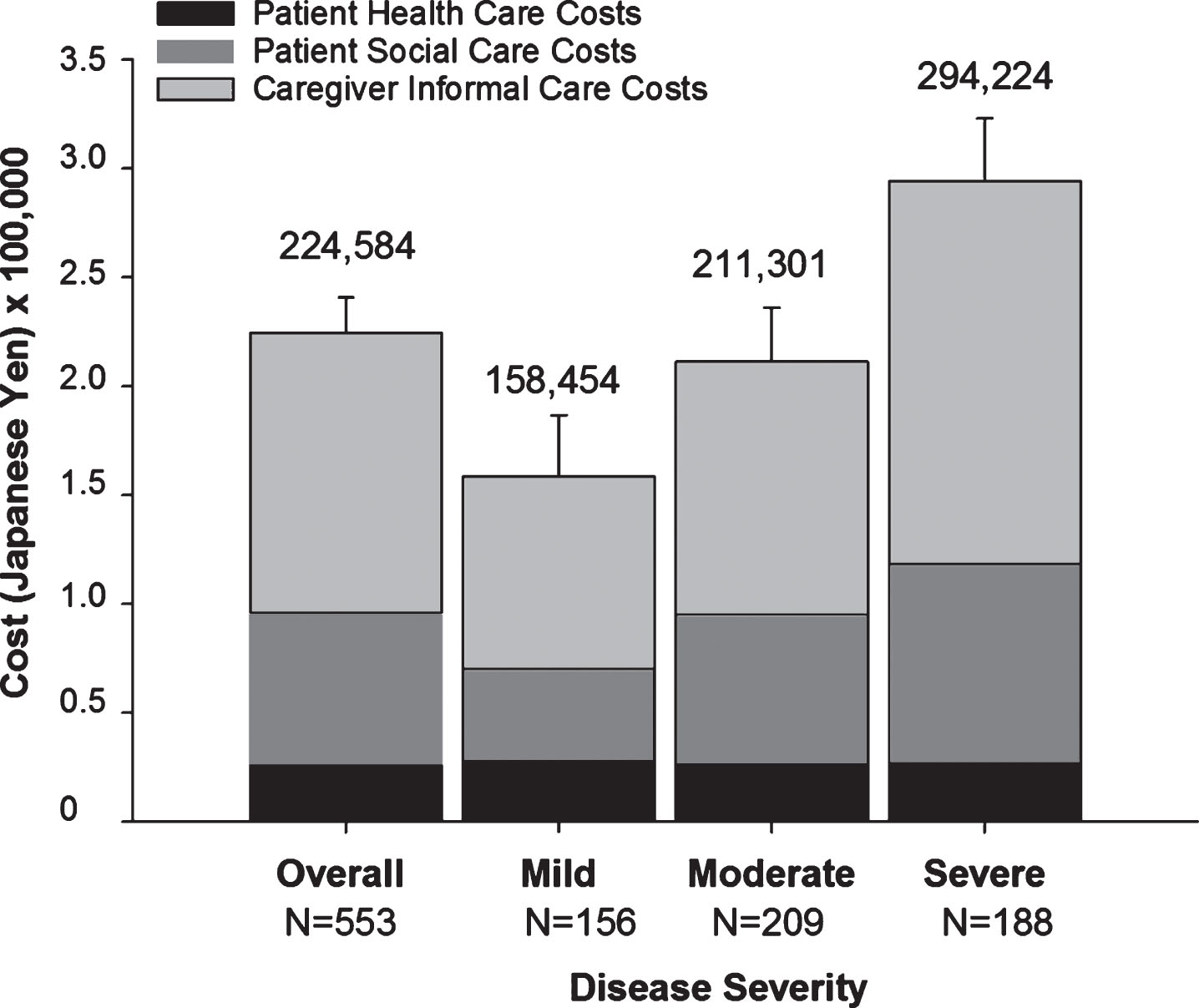
Total monthly societal costs associated with AD dementia increased with AD severity (Fig. 2 and Supplementary Table 3). Patients with moderately severe/severe AD had significantly higher total costs than patients with mild and moderate AD, that is, the CIs for moderate severe/severe AD did not overlap with those of mild and moderate AD. Likewise, both patient social care costs (patient direct non-medical costs) and caregiver informal care costs (caregiver indirect non-medical costs) increased with AD severity (Supplementary Table 3). Informal caregiver costs were the largest cost component accounting for over half of total societal costs (Fig. 2 and Supplementary Table 3).
Fig. 2
Total Monthly Societal Costs (JPY) Associated with the Treatment of Alzheimer’s Disease at Baseline by Severity. Mean costs per month in JPY are shown. The numbers above the graphs are total societal costs and error bars indicate the upper CI of the mean of total societal costs. The bootstrap method was used to calculate the 95% CI for the mean. The exchange rate used to convert JPY to USD is 0.0093572 (source: https://www.bloomberg.com/quote/USDJPY:CUR; date 06-APR-2018). In USD, mean total societal costs were: 2,101 overall; 1,483 mild; 1,977 moderate; and 2,753 severe. CI, confidence interval; JPY, Japanese Yen; N, population size; Severe, moderately severe/severe; USD, United States Dollars.
In GERAS-J, independent factors associated with lower total societal costs were higher ADL total score (better functioning of patient), patient age, higher number of caregivers, and married/cohabitating and not living alone. Increased time since AD diagnosis, LTCI certification, and increased ZBI score (increased caregiver burden) were associated with higher costs (Table 5).
Table 5
Factors associated with total monthly societal cost at baseline
| Factor | Estimate (95% CL) | Total societal costs LS mean (95% CI) | p |
| Patient factors | |||
| MMSE severity | 0.1222 | ||
| Mild | –0.0717 (–0.2717; 0.1284) | 172,750 (150,014; 198,933) | 0.4826 |
| Moderate | 0.0903 (–0.0762; 0.2568) | 203,124 (179,564; 229,775) | 0.2878 |
| Moderately severe/severe* | 185,583 (159,325; 216,168) | ||
| Age | –0.0129 (–0.0223; –0.0036) | 0.0066 | |
| Gender | 0.6070 | ||
| Female | –0.0386 (–0.1855; 0.1084) | 183,175 (167,465; 200,358) | |
| Male* | 190,376 (164,783; 219,945) | ||
| ADCS-ADL Total Score | –0.0129 (–0.0178; –0.0079) | <0.0001 | |
| Living arrangements | <0.0001 | ||
| Living alone | 0.1104 (–0.1027; 0.3234) | 222,628 (182,819; 271,106) | 0.3100 |
| Not living alone and married | –0.3066 (–0.4618; –0.1515) | 146,718 (134,252; 160,341) | 0.0001 |
| Not living alone and not married* | 199,368 (174,044; 228,376) | ||
| Time since AD diagnosis | 0.0307 (0.0023; 0.0591) | 0.0341 | |
| LTCI certified | <0.0001 | ||
| No | –0.5607 (–0.7212; –0.4003) | 141,084 (121,725; 163,522) | |
| Yes* | 247,173 (224,594; 272,021) | ||
| Caregiver factors | |||
| Number of other caregivers involved | –0.1324 (–0.1971; –0.0678) | <0.0001 | |
| ZBI Total Score | 0.0150 (0.0103; 0.0196) | <0.0001 |
Generalized linear models were used with the Gamma Distribution and a log-link function. Exclusion Criterion: p-value of Ward Statistic for the covariate > 0.05. The number of patients in population = 553. The number of patients used = 553. P-values in bold are significant. If a continuous covariate was missing then the population median was imputed. If a categorical covariate was missing then the population mode was imputed. MMSE, patient age, and patient gender were all forced into the final model. Model statistics: R2DEV was 0.359. *Reference category for the estimates. AD, Alzheimer’s disease; ADCS-ADL, Alzheimer’s Disease Co-operative Study activities of daily living; CI, confidence interval; CL, confidence limit; LS, least squares; LTCI, long-term care insurance; MMSE, Mini-Mental State Examination; ZBI, Zarit Burden Interview.
DISCUSSION
Here, we report the baseline demographics and real-costs associated with care of community-dwelling patients with AD dementia in Japan. This is the first prospective observational report evaluating the socioeconomic burden of AD dementia on both patients and their caregivers in Japan. The total monthly societal costs increased from 158,454 JPY (1,483 USD) to 294,224 JPY (2,753 USD) with increasing AD severity. Informal caregiver costs were the largest cost component accounting for over half of total societal costs.
The key results presented here are consistent with baseline results from GERAS-I (UK, France, and Germany) [20] and GERAS-II (Spain) [22]. The total societal costs associated with AD in GERAS-J rose with increasing AD severity and were approximately 1.9-fold higher among patients with moderately severe/severe than with mild AD. This trend is consistent with previous reports examining AD severity and costs regardless of the method used to define AD severity and of the country where the study was conducted [35–39]. In GERAS-J, patient direct medical costs remained relatively constant across AD severity groups, consistent with GERAS-I [20] and GERAS-II (Spain) [22].
Our multivariate analyses showed a common pattern of independent factors associated with total societal costs among Japan and EU countries. Better patient functioning (higher ADL score) was one of the important independent factors associated with lower costs similar to GERAS-I [40]. The patient’s living arrangements and caregiver burden were also shown to be independent factors associated with total costs in both GERAS-J and GERAS-I [40]. One of the Japan-specific independent factors associated with higher costs was LTCI certification, which reflects patients’ worsening ADL and care needs. There is a strong association between cognitive and functional impairment [41], and ADL had a significant relationship with societal costs.
Time since diagnosis was also identified as a Japan-specific factor significantly associated with total costs. This variable was assessed for the association with societal cost in previous studies in EU/Germany/France [40] and Sweden [37], but was not identified as a significant factor in either of those studies. Perhaps the time since diagnosis variable was significant in Japan because the Japanese health- and social-care system has multiple access points in the dementia care pathway and caregivers of patients with longer time since diagnosis had more experience seeking health and societal care [42]. Long-term caregiving creates a greater burden and the informal caregivers are more likely to seek outside social support.
In GERAS-J, GERAS-I, and GERAS-II, informal care costs (caregiver time, costs associated with the caregiver missing work) was the largest driver of total societal costs [20–22]. In GERAS-J, caregiver informal costs comprised 56%, 55%, and 60% of total societal costs each among patients with mild, moderate, and moderately severe/severe AD, respectively. This is consistent with GERAS-I where informal costs comprised approximately 50% to 60% of total societal costs depending on the country [20]. The drivers of informal care costs are likely country-specific, depending on payers, community resources, and social and family support systems. For example, in GERAS-J, 53.8%, 67.9%, and 88.3% of patients with mild, moderate, and moderately severe/severe AD were LTCI certified. Increased LTCI certification was associated with AD severity, suggesting a greater need for appropriate social care support in accordance with disease progression, which reduces the burden to the family and keeps patients dwelling in the community. It is important to understand these drivers in a country-specific manner so economic and social relief can be given to caregivers [20].
In GERAS-J, total caregiver time (comprised as basic ADL, instrumental ADL, and supervision to prevent dangerous events) ranged from 97.2 (mild AD) to 171.3 hours per month (moderately severe/severe AD) with instrumental ADL being the largest component. Because instrumental ADL may be culture-specific [43], it is difficult to make between-country comparisons. However, it is important to note that the average total time spent caring for a person with mild AD was 24.3 hours per week, whereas that for a person with moderately severe/severe AD was 43 hours per week, equivalent to part-time and full-time paid employment, respectively, for which the caregiver is not paid. In contrast to GERAS-I [20], where most caregiving was performed by the spouse (65.9%), most caregivers in GERAS-J were adult children followed by the spouse (49.0% child; 37.1% spouse). This may be because fewer patients in GERAS-J were married or cohabitating (59%) compared to GERAS-I (72%). Additionally, 38% of AD patients in GERAS-J were widowed with only 0.9% never married. The lack of a spouse may necessitate an adult child moving into the home to care for a single parent. In an analysis of GERAS-I, unmarried patients living with nonspousal caregivers had higher costs than patients either living alone or married and living with spousal/nonspousal caregivers [40].
While the informal caregiver was the spouse of the patient less frequently in GERAS-J as compared to GERAS-I, the proportion of informal care costs was similar between the studies. The analysis of informal care costs in both GERAS-J and GERAS-I was calculated from active caregiving time for basic and instrumental ADL. The proportion of caregivers living with patients was similar between GERAS-J (78.8%) and GERAS-I (76.0%) and the mean hours of providing ADL type care were not different. This caused similar informal care costs between the two studies. The average age of the caregiver in GERAS-J (62.1±12.5 years; range 28-93) was lower than in GERAS-I (67.3±12.03) [20], suggesting that many caregivers in GERAS-J were still of working age. Indeed, 47.7% of caregivers in GERAS-J had at least some paid work, whereas only 23.8% of caregivers in GERAS-I had paid work [20]. It is possible that costs could be driven by the inability of an adult child caregiver living with a patient to work. Thus, adult children caregivers could cause loss of productivity in Japan.
In GERAS-J, daycare was the most popular social service, whereas in GERAS-I home-aids/orderlies were used the most often [20]. Almost 50% of patients used daycare in Japan. The use of daycare likely reduced the time spent on caregiving, perhaps allowing the caregiver to engage in paid work and leisure time. However, despite the availability of community resources, such as daycare, caregiver stress and burden increased with AD severity in GERAS-J. Thus, current community resources in Japan are unable to completely address the sources of caregiver burden.
In GERAS-J, 94.6% of patients were taking medication indicated for AD treatment. Currently, the efficacy of AD medication is evaluated by cognition and daily functioning [44, 45]. The development of new medications that slow the decline of community-dwelling patients with mild AD may lead to cost savings by reducing caregiver time [46]. Because patients with advanced AD may require long-term care in nursing facilities, delaying disease progression may have other economic advantages such as allowing the patient to live at home longer. The evaluation of new AD treatments from the perspective of delayed progression and socioeconomic burden will likely become important for these treatments to be accepted by payers [20], and the data collected in the present study can help estimate the long-term impact of new treatments.
This study does have some limitations. Because this is a cohort of community-dwelling patients using memory clinics and with an informal, dedicated primary caregiver, the GERAS-J population may not be completely representative of AD patients living in the community who visit primary care physicians. The MMSE was used to stratify costs by disease severity, but different cut-offs for AD severity have been used in other studies [36]. In this study, the GERAS-I and NICE categories were used. Thus, it may be difficult to extrapolate studies using these MMSE groupings to studies using different MMSE cut-offs. This study does not examine the socioeconomic impact of shifting care from residential nursing facilities to family caregivers. Finally, the cost and time information was based on responders’ memory and recall may not be accurate.
A strength of GERAS-J is the inclusion of patients across the spectrum of AD dementia which will inform assumptions about how further deterioration in the later stage of AD dementia may be associated with a subsequent need for residential nursing facility care. GERAS-J adds to existing evidence about understanding the costs and burden for AD care, and it provides important information needed to make policy decisions regarding the socioeconomic challenges of community-dwelling patients with AD. Data from GERAS-J will increase understanding of the impact of AD dementia on patient and caregiver burden, and on related societal costs in Japan. The cross-sectional data from the baseline analysis is important to help us understand differences between severity stages of disease and potential care needs.
In conclusion, at baseline, total societal costs increased with AD severity. Informal care costs were the largest component of societal costs.
ACKNOWLEDGMENTS
We firstly wish to thank the participating patients and their caregivers, site investigators, and study personnel who participated in this study. The authors would like to thank Drs. Joel Raskin and Catherine Reed, both of Eli Lilly and Company, for protocol review and consultancy. This study was supported by Eli Lilly and Company, and medical writing and editorial assistance was provided by Lori Kornberg, PhD, and Antonia Baldo, BA, who are full-time employees of Syneos Health (Raleigh, NC).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0811r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-190811.
REFERENCES
[1] | WorldHealthOrganization.Aging and Health. https://www.who.int/news-room/fact-sheets/detail/ageingand-health, Last updated 5 February 2018, Accessed on 21 June 2019. |
[2] | van der Flier WM , Scheltens P ((2005) ) Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry 76: , Suppl 5, v2–v7. |
[3] | Meguro K , Ishii H , Kasuya M , Akanuma K , Meguro M , Kasai M , Lee E , Hashimoto R , Yamaguchi S , Asada T ((2007) ) Incidence of dementia and associated risk factors in Japan: The Osaki-Tajiri Project. J Neurol Sci 260: , 175–182. |
[4] | Prince M , Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf, Last updated October 2015, Accessed on 21 June, 2019. |
[5] | Moore MJ , Zhu CW , Clipp EC ((2001) ) Informal costs of dementia care: Estimates from the National Longitudinal Caregiver Study. J Gerontol B Psychol Sci Soc Sci 56: , S219–228. |
[6] | Ministry of Health, Labor and Welfare ((2016) ) Population trends of Japan. Available at: http://www.stat.go.jp/english/data/nenkan/pdf/yhyou02.pdf, last accessed October 2019. |
[7] | Ministry of Health, Labor and Welfare ((2010) ) Population trends of Japan. Available at: https://www.mhlw.go.jp/english/wp/wp-hw4/dl/general_welfare_and_labour/P5.pdf, last accessed October 2019. |
[8] | Muramatsu N , Akiyama H ((2011) ) Japan: Super-aging society preparing for the future. Gerontologist 51: , 425–432. |
[9] | Ministry of Health, Labor and Welfare ((2006) ) National Institute of Population and Social Security Research, Population Projections for Japan. Available at: https://www.mhlw.go.jp/english/social security/dl/social security6-g.pdf, last accessed October 2019. |
[10] | Dodge HH , Buracchio TJ , Fisher GG , Kiyohara Y , Meguro K , Tanizaki Y , Kaye JA ((2012) ) Trends in the prevalence of dementia in Japan. Int J Alzheimers Dis 2012: , 956354. |
[11] | Sekita A , Ninomiya T , Tanizaki Y , Doi Y , Hata J , Yonemoto K , Arima H , Sasaki K , Iida M , Iwaki T , Kanba S , Kiyohara Y , ((2010) ) Trends in prevalence of Alzheimer’s disease and vascular dementia in a Japanese community: The Hisayama Study. Acta Psychiatr Scand 122: , 319–325. |
[12] | Montgomery W , Ueda K , Jorgensen M , Stathis S , Cheng Y , Nakamura T ((2017) ) Epidemiology, associated burden, and current clinical practice for the diagnosis and management of Alzheimer’s disease in Japan. Clinicoecon Outcomes Res 10: , 13–28. |
[13] | Duthey B. , Background paper 6.11: Alzheimer disease and other dementias. A Public Health Approach to Innovation. http://www.who.int/medicines/areas/priority_medicines/BP6_11Alzheimer.pdf, Last updated 20 February, 2013, Accessed 16 April 2019. |
[14] | Asada T ((2012) ) [Prevalence of dementia in Japan: Past, present and future]. Rinsho Shinkeigaku [Clinical Neurology] 52: , 962–964. |
[15] | Matsuda S , Yamamoto M ((2001) ) Long-term care insurance and integrated care for the aged in Japan. Int J Integr Care 1: , e28. |
[16] | Tsutsui T , Muramatsu N. ((2007) ) Japan’s universal long-term care system reform of 2005: Containing costs and realizing a vision. J Am Geriatr Soc 55: , 1458–1463. |
[17] | Sado M , Ninomiya A , Shikimoto R , Ikeda B , Baba T , Yoshimura K , Mimura M ((2018) ) The estimated cost of dementia in Japan, the most aged society in the world. PloS One 13: , e0206508. |
[18] | Hanaoka S , Matsumoto K , Kitazawa T , Fujita S , Seto K , Hasegawa T ((2019) ) Comprehensive cost of illness of dementia in Japan: A time trend analysis based on Japanese official statistics. Int J Health Care 31: , 231–237. |
[19] | Ueda K , Sato M , Tanji Y , Brnabic AJM , Treuer T , Aprospective observational study of Alzheimer’s disease patients and their caregivers: Design and objectives of GERAS-J. Presented at the 36th Annual Meeting of Japan Society for Dementia Research, Kanazawa, November 24 – 26, 2017. |
[20] | Wimo A , Reed CC , Dodel R , Belger M , Jones RW , Happich M , Argimon JM , Bruno G , Novick D , Vellas B , Haro JM ((2013) ) The GERAS Study: A prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries–study design and baseline findings. J Alzheimers Dis 36: , 385–399. |
[21] | Bruno G , Mancini M , Bruti G , Dell’Agnello G , Reed C ((2018) ) Costs and resource use associated with Alzheimer’s Disease in Italy: Results from an observational study. J Prev Alzheimers Dis 5: , 55–64. |
[22] | Olazaran J , Aguera-Ortiz L , Argimon JM , Reed C , Ciudad A , Andrade P , Dilla T ((2017) ) Costs and quality of life in community-dwelling patients with Alzheimer’s disease in Spain: Results from the GERAS II observational study Int Psychogeriatr 29: , 2081–2093. |
[23] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[24] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[25] | National Institute of Health and Clinical Excellence. NICE technology appraisal guideline 217. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. http://www.nice.org.uk/guidance/TA217, Last updated 20 June, 2018, Last accessed on 21 June, 2019. |
[26] | Galasko D , Bennett D , Sano M , Ernesto C , Thomas R , Grundman M , Ferris S ((1997) ) An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 11: , Suppl 2 S33–39. |
[27] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[28] | Mohs RC , Knopman D , Petersen RC , Ferris SH , Ernesto C , Grundman M , Sano M , Bieliauskas L , Geldmacher D , Clark C , Thal LJ ((1997) ) Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 11: Suppl 2, S13–21. |
[29] | Rosen WG , Mohs RC , Davis KL ((1984) ) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141: , 1356–1364. |
[30] | Kind P ((1996) ) The EuroQol instrument: An index of health-related quality of life. In Quality of Life and Pharmacoeconomics in Clinical Trials, Spilker B, ed. 2nd edition. Lippincott-Raven Publishers, Philadelphia, pp. 191-201. |
[31] | Naglie G , Tomlinson G , Tansey C , Irvine J , Ritvo P , Black SE , Freedman M , Silberfeld M , Krahn M ((2006) ) Utility-based Quality of Life measures in Alzheimer’s disease. Qual Life Res 15: , 631–643. |
[32] | Zarit SH , Reever KE , Bach-Peterson J ((1980) ) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20: , 649–655. |
[33] | Wimo A , Wetterholm AL , Mastey V , Winblad B ((1998) ) Evaluation of resource utilization and caregiver time in anti-dementia drug trials- a quantitative battery. In The Health Economics of Dementia, Wimo A, Jonsson B, Karlsson G, Winblad B, eds. John Wiley & Sons, London. |
[34] | Gustavsson A , Brinck P , Bergvall N , Kolasa K , Wimo A , Winblad B , Jönsson L ((2011) ) Predictors of costs of care in Alzheimer’s disease: A multinational sample of 1222 patients. Alzheimers Dement 7: , 318–327. |
[35] | Leicht H , Heinrich S , Heider D , Bachmann C , Bickel H , van den Bussche H , Fuchs A , Luppa M , Maier W , Mösch E , Pentzek M , Rieder-Heller SG , Tebarth F , Werle J , Weyerer S , Wiese B , Zimmermann T , König HH ; AgeCoDe study group ((2011) ) Net costs of dementia by disease stage. Acta Psychiatr Scand 124: , 384–395. |
[36] | Mauskopf J , Racketa J , Sherrill E ((2010) ) Alzheimer’s disease: The strength of association of costs with different measures of disease severity. J Nutr Health Aging 14: , 655–663. |
[37] | Mesterton J , Wimo A , By A , Langworth S , Winblad B , Jonsson L ((2010) ) Cross sectional observational study on the societal costs of Alzheimer’s disease. Curr Alzheimer Res 7: , 358–367. |
[38] | Quentin W , Riedel-Heller SG , Luppa M , Rudolph A , Konig HH ((2010) ) Cost-of-illness studies of dementia: A systematic review focusing on stage dependency of costs. Acta Psychiatr Scand 121: , 243–259. |
[39] | Hux MJ , O’Brien BJ , Iskedjian M , Goeree R , Gagnon M , Gauthier S ((1998) ) Relation between severity of Alzheimer’s disease and costs of caring. CMAJ 159: , 457–465. |
[40] | Dodel R , Belger M , Reed C , Wimo A , Jones RW , Happich M , Argimon JM , Bruno G , Vellas B , Haro JM ((2015) ) Determinants of societal costs in Alzheimer’s disease: GERAS study baseline results. Alzheimers Dement 11: , 933–945. |
[41] | Liu-Seifert H , Siemers E , Sundell K , Mynderse M , Cummings J , Mohs R , Aisen P ((2018) ) Analysis of the relationship of cognitive impairment and functional impairment in mild Alzheimer’s disease in EXPEDITION 3. J Prev Alzheimers Dis 5: , 184–187. |
[42] | Nakanishi M , Nakashima T ((2014) ) Features of the Japanese national dementia strategy in comparison with international dementia policies: How should a national dementia policy interact with the public health- and social-care systems? . Alzheimers Dement 10: , 468–476. |
[43] | Wimo A , Prince M ((2010) ) World Alzheimer Report 2010. The Global Economic Impact of Dementia. Alzheimer’s Disease International, http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf, Last updated June, 2011, Accessed on 16 April 2019. |
[44] | Matsunaga S , Kishi T , Iwata N ((2015) ) Memantine monotherapy for Alzheimer’s disease: A systematic review and meta-analysis. PloS One 10: , e0123289. |
[45] | Posner H , Curiel R , Edgar C , Hendrix S , Liu E , Loewenstein DA , Morrison G , Shinobu L , Wesnes K , Harvey PD ((2017) ) Outcomes assessment in clinical trials of Alzheimer’s disease and its precursors: Readying for short-term and long-term clinical trial needs. Innov Clin Neurosci 14: , 22–29. |
[46] | Lenox-Smith A , Reed C , Lebrec J , Belger M , Jones RW ((2018) ) Potential cost savings to be made by slowing cognitive decline in mild Alzheimer’s disease dementia using a model derived from the UK GERAS observational study. BMC Geriatr 18: , 57. |



