Low Serum High-Density Lipoprotein Cholesterol Levels Associate with the C9orf72 Repeat Expansion in Frontotemporal Lobar Degeneration Patients
Abstract
Decreased levels of serum high-density lipoprotein (HDL) cholesterol have previously been linked to systemic inflammation and neurodegenerative diseases, such as Alzheimer’s disease. Here, we aimed to analyze the lipoprotein profile and inflammatory indicators, the high-sensitivity C-reactive peptide (hs-CRP) and glycoprotein acetyls (GlycA), in sporadic and C9orf72 repeat expansion-associated frontotemporal lobar degeneration (FTLD) patients. The C9orf72 hexanucleotide repeat expansion is the most frequent genetic etiology underlying FTLD. The concentrations of different lipid measures in the sera of 67 FTLD patients (15 C9orf72 repeat expansion carriers), including GlycA, were analyzed by nuclear magnetic resonance spectroscopy. To verify the state of systemic inflammation, hs-CRP was also quantified from patient sera. We found that the total serum HDL concentration was decreased in C9orf72 repeat expansion carriers when compared to non-carriers. Moreover, decreased concentrations of HDL particles of different sizes and subclass were consistently observed. No differences were detected in the very low- and low-density lipoprotein subclasses between the C9orf72 repeat expansion carriers and non-carriers. Furthermore, hs-CRP and GlycA levels did not differ between the C9orf72 repeat expansion carriers and non-carriers. In conclusion, the HDL-related changes were linked with C9orf72 repeat expansion associated FTLD but were not seen to associate with systemic inflammation. The underlying reason for the HDL changes remains unclear.
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) is the second most common cause of dementing diseases in working-age people and accounts for approximately 10% of all progressive dementias [1]. FTLD is clinically divided into two main subcategories, namely behavioral variant frontotemporal dementia (bvFTD) [2] and primary progressive aphasias (PPAs) [3]. PPAs are further divided into the following subcategories: nonfluent variant primary progressive aphasia (nfvPPA) and semantic variant primary progressive aphasia (svPPA). In addition, the logopenic variant of primary progressive aphasia (lvPPA) is clinically considered a subtype of PPA, but is neuropathologically associated with Alzheimer’s disease (AD) [3].
FTLD presents autosomal dominant inheritance in up to 50% of patients [4, 5]. The most common genetic etiology underlying FTLD is the hexanucleotide repeat expansion (GGGGCC) on the short arm of chromosome 9 open reading frame 72 (C9orf72) [6, 7]. This repeat expansion accounts for approximately 25% of familial FTLD cases in Europe and the USA [8]. Besides FTLD, the C9orf72 repeat expansion also causes up to 40% of familial amyotrophic lateral sclerosis (ALS) cases in these populations [8]. Investigations in induced pluripotent stem cell-derived neurons from C9orf72 repeat expansion carriers and different animal models have suggested that both toxic gain-of-function and loss-of-function mechanisms underlie C9orf72 repeat expansion-associated FTLD and ALS [9]. Transcription and aberrant repeat-associated non-ATG (RAN) translation of the expanded C9orf72 hexanucleotide repeat in both sense and antisense directions have been shown to lead to the formation and accumulation of expanded repeat-containing RNA foci and dipeptide-repeat proteins (DPRs) and result in neurotoxicity and neurodegeneration. In addition, several studies have shown that C9orf72 repeat expansion carriers display an approximately 50% decrease in the levels of normal C9orf72 RNA and protein, indicating haploinsufficiency as another potential contributor to disease pathogenesis [9].
Dysfunction in brain lipid homeostasis is suggested to be a risk factor for different neurodegenerative disorders [10, 11]. Altered blood lipid metabolism is known to associate with cardiovascular diseases, well-known risk factors for neurodegenerative diseases, but also with neurodegenerative diseases themselves, even though it is presently unclear if the blood and brain lipid levels correlate with each other. Lowered serum high-density lipoprotein (HDL) cholesterol has been indicated to be linked to AD [12, 13]. In addition, a decreased HDL concentration is related to systemic inflammation [14]. Recent studies C9orf72 knockout mice have shown drastic systemic inflammation and autoimmune disease-like phenotypes. These examinations together with human studies suggest a potential role for inflammation in C9orf72 repeat expansion-associated disease pathogenesis [15–17]. So far only a few studies have provided insight into the lipid metabolism in FTLD patients and these studies have not contained analyses of the genetic background of the patients [18, 19]. However, the examination of lipid and cholesterol changes in ALS, a close pathological analogue to C9orf72 repeat expansion-associated FTLD, has been more extensive [20–30]. Dyslipidemia in ALS has also been acknowledged [21, 29, 30].
Here, our aim was to examine potential alterations in the serum lipoprotein levels in FTLD patients carrying or not the C9orf72 repeat expansion. To our knowledge, these are the first reported findings that compare lipoprotein alterations in C9orf72 repeat expansion carriers to non-carriers.
MATERIALS AND METHODS
Ethical considerations
The study was performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from the participants. The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District.
Patients
A cohort comprising a total of 67 patients with FTLD, diagnosed between the years 1996–2017 at the memory outpatient clinics of Kuopio University Hospital, was utilized in this study (Table 1). An experienced neurologist, specialized in cognitive and dementing disorders, examined all of the patients. The patients with bvFTD were diagnosed according to the latest diagnostic criteria by Rascovsky and colleagues [2], and patients with PPAs were diagnosed according to the Gorno-Tempini diagnostic criteria [3]. A retrospective review based on these same criteria was used for patients who had been originally diagnosed before the Rascovsky or Gorno-Tempini criteria were published. All patients with bvFTD, nfvPPA, or svPPA fulfilled the criteria with either a probable or a definite diagnosis. Patients with FTLD-MND had at least a probable diagnosis of bvFTD, nfvPPA, or svPPA and the clinical manifestation of motoneuron disease (MND). None of the patients in our cohort were diagnosed with lvPPA. The serum samples were collected at the time of diagnosis. Collected samples were aliquoted and frozen (–80°C) for future analyses.
Table 1
Demographic data and prevalence of the C9orf72 repeat expansion and APOE ɛ4 alleles among diagnosed FTLD patients
| bvFTD | PPA (nfv + sv) | FTLD-MND | Total | |
| Cases | 49 | 12 | 6 | 67 |
| Sex, f/m | 30/19 (61/39%) | 7/5 (58/32%) | 4/2 (67/33%) | 41/26 (61.2/38.8%) |
| Mean age at diagnosis, y | 65.0 | 69.8 | 61.8 | 65.6 |
| C9orf72 carriers/non-carriers | 14/21 (40/60% *) | 1/10 (1/10% *) | 0/5 (0/100% *) | 15/36 (29/61% *) |
| APOE ɛ4 carriers/non-carriers | 15/22 (40.5/ 59.5% *) | 2/8 (20/80% *) | 0/3 (0/100% *) | 17/33 (34/66% *) |
| C9orf72 repeat | C9orf72 repeat | |||
| expansion carriers | expansion non-carriers | |||
| APOE ɛ4 carriers/non-carriers | 3/9 (25/75% *) | 9/14 (39.1/60.9% *) |
*Ratio of genotyped cases. APOE ɛ4, Apolipoprotein E ɛ4; bvFTD, behavioral variant frontotemporal dementia; C9orf72, Chromosome 9 open reading frame 72; FTDL-MND, Frontotemporal lobar degeneration – motoneuron disease; PPA (nfv + sv), Primary progressive aphasia (non-fluent variant + semantic variant).
Patients with FTLD were further divided into two subgroups based on their C9orf72 repeat expansion status: C9orf72 repeat expansion carriers (N = 15) and C9orf72 repeat expansion non-carriers (N = 36). Within the clinical subgroups, 14 bvFTD and one nfvPPA patient carried the C9orf72 repeat expansion. None of the FTLD-MND or svPPA patients carried the C9orf72 repeat expansion. Genotyping data of the C9orf72 repeat expansion was not available for 23.9% (16/67) FTLD patients (13 bvFTD, one nfvPPA, and one FTLD-MND). Demographic information of the study patients is presented in Table 1.
Genetic analyses
The C9orf72 repeat expansion was analyzed using the repeat-primed polymerase chain reaction assay [6]. One patient with the C9orf72 expansion carried an intermediate expansion (28 repeats) and the rest had the full expansion (>40 repeats). The patients classified as C9orf72 repeat expansion non-carriers had less than five repeats. Other genes associated with FTLD were not analyzed as mutations in these genes have previously been observed to be extremely rare in the Finnish population [31, 32].
The apolipoprotein E (APOE) alleles were genotyped from extracted genomic DNA with polymerase chain reaction using TaqMan genotyping assays (rs429358 and rs7412 polymorphisms, Applied Biosystems, Foster City, CA, USA) and allelic discrimination (ABI 7500 platform) [33]. The QIAamp DNA blood mini extraction kit (QIAGEN) was used to extract genomic DNA from blood.
Lipoprotein analysis
The concentrations for lipoprotein particles, phospholipids, free cholesterol, cholesterol esters, and triglycerides in the sera for each lipoprotein size and density were quantified using a nuclear magnetic resonance spectroscopy platform [34, 35]. The value for total lipids in each lipoprotein subclass is calculated by summing phospholipids, total cholesterol, and triglycerides. The concentration for total cholesterol is the sum of free cholesterol and cholesterol esters. This platform has been used in multiple genetic and epidemiological setups [36–39]. Lipoprotein data was missing from one C9orf72 repeat expansion carrier and from one non-carrier (both diagnosed with bvFTD). This same method was used to quantify glycoprotein acetyls (mainly a1-acid glycoprotein) (GlycA) from the patient sera. Circulating GlycA are a prospective and novel biomarker for systemic inflammation [40].
High sensitivity C-reactive protein
To analyze the state of inflammation in the study cohort, high sensitivity C-reactive protein (hs-CRP) was quantified from patient serum with Cobas 8000 (Hitachi High Technology Co, Tokyo, Japan), using a high sensitive assay for CRP (Latex CRPHS, cat# 04628918 190, Cobas c systems, Roche Diagnostics GmbH, Mannheim, Germany).
Statistics
An independent two sample t-test was used to test the significance of differences between the C9orf72 repeat expansion carriers and non-carriers. Hs-CRP median levels were compared with independent samples Mann-Whitney U-test.
The normality of distributions was tested by calculating the skewness of data and comparing it to the skewness value after logarithmic transformation. Abnormal distribution of a variable was corrected using logarithmic transformation if the absolute difference in skewness between logarithmic and native data was greater than one. The threshold for statistical significance was p < 0.05.
Statistical analysis was conducted using SPSS (v23 and v25, IBM Analytics, Armonk, NY, USA). Figure 1 was generated with R/Rstudio (v3.4.1, Boston, MA, USA) and ggplot2 third-party R package (v2.2.1).
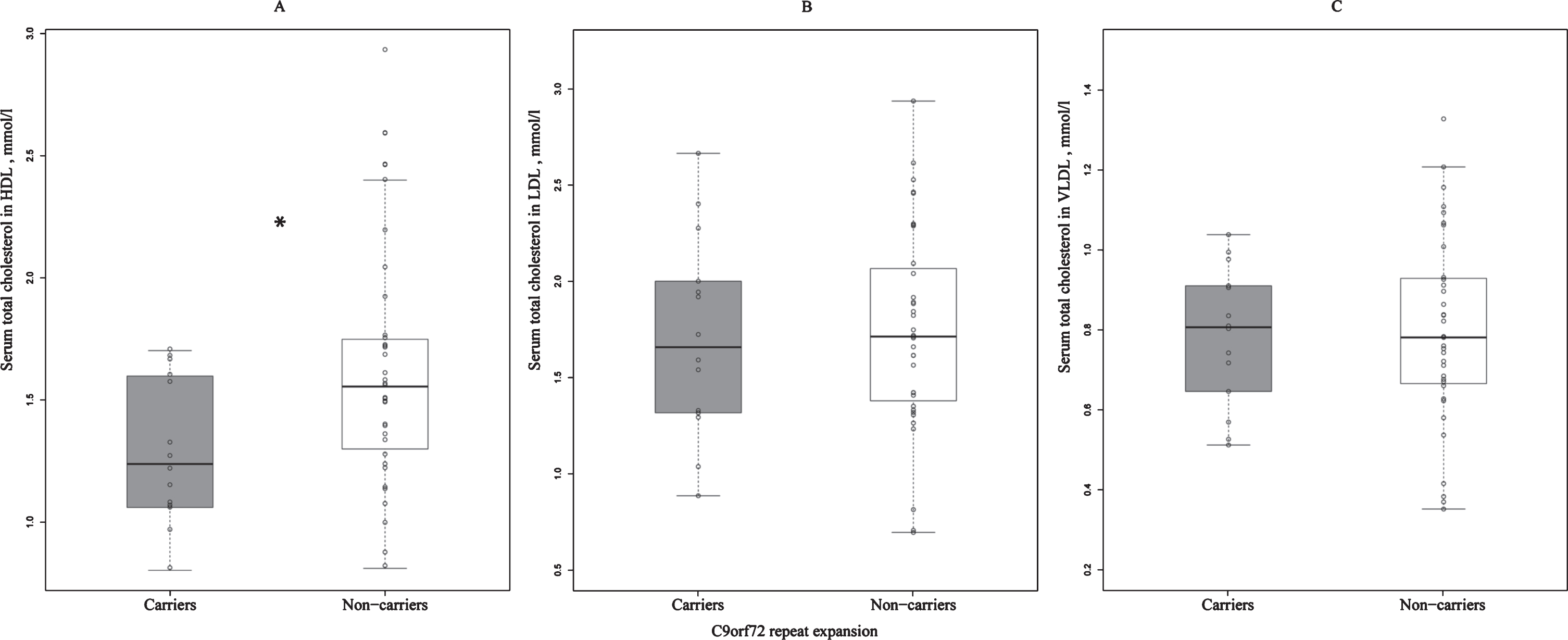
Fig.1
Differences in the concentrations of serum total cholesterol in HDL (A), LDL (B), and VLDL (C) between C9orf72 repeat expansion carriers and non-carriers. Total serum cholesterol concentrations in HDL are significantly lower in the repeat expansion carriers when compared to non-carriers. *statistically significant difference, p = 0.030; C9orf72, Chromosome 9 open reading frame 72; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
RESULTS
We found that the concentration of total HDL cholesterol in the sera of C9orf72 repeat expansion carriers was lower when compared to non-carriers (p = 0.030) (Fig. 1A). Interestingly, there were no differences in the serum concentrations of total LDL and VLDL cholesterol between the C9orf72 carriers and non-carriers (Fig. 1B, C).
Similar findings were observed when the subclasses of HDL, LDL, and VLDLs of different particle sizes were analyzed. The concentration of total cholesterols in very large HDLs was lower in the C9orf72 repeat expansion carriers when compared to non-carriers (p = 0.004). Moreover, the concentrations of phospholipids (p = 0.010) and free cholesterol (p = 0.007) as well as total lipids (p = 0.047) in very large HDLs were also lower. Particle concentration, and concentrations of cholesterol esters and triglycerides in very large HDLs did not differ between the C9orf72 repeat expansion carriers and non-carriers. In addition, differences in the concentrations of subclasses in large HDLs were similar to those in the very large HDL subclasses. The C9orf72 repeat expansion carriers showed lower concentrations of total lipids (p = 0.046), phospholipids (p = 0.041), free cholesterol (p = 0.046), cholesterol esters (p = 0.031), and large HDL particles (p = 0.033) compared to non-carriers. However, the concentrations of total cholesterol and triglycerides in large HDLs were not altered between the C9orf72 repeat expansion carriers and non-carriers. In the medium and small HDL subclasses, only the concentration of free cholesterol in small HDLs was lower in the C9orf72 repeat expansion carriers compared to non-carriers (p = 0.037) (Table 2). Concentrations of LDL subclasses were similar between the C9orf72 repeat expansion carriers and non-carriers. Likewise, there were no differences in the VLDL subclasses between the C9orf72 repeat expansion carriers and non-carriers. The ratio of free cholesterol and cholesterol esters in very large HDLs were lower in the C9orf72 repeat expansion carriers (p = 0.010). There were no other clear differences in this ratio in any other HDL subclass or in any VLDL or LDL subclasses.
Table 2
Differences in particle concentration, total lipids, phospholipids, total cholesterol, cholesterol esters, free cholesterol, and triglycerides in very large, large, medium, and small high-density lipoproteins between carriers and non-carriers of the C9orf72 repeat expansion
| HDL particle size | HDL subclass | Mean. C9orf72 carriers, mmol/l | Mean. C9orf72 non-carriers, mmol/l | p |
| Very large | Particle concentration | 3.56e-04 | 5.28e-04 | 0.055 |
| Total lipids | 0.36 | 0.54 | 0.047 | |
| Phospholipids | 0.14 | 0.23 | 0.010 | |
| Total cholesterol | 0.20 | 0.29 | 0.004 | |
| Cholesterol esters | 0.16 | 0.21 | 0.073 | |
| Free cholesterol | 0.049 | 0.081 | 0.007 | |
| Triglycerides | 0.020 | 0.023 | 0.406 | |
| Large | Particle concentration | 1.01e-03 | 1.42e-03 | 0.033 |
| Total lipids | 0.64 | 0.89 | 0.046 | |
| Phospholipids | 0.30 | 0.43 | 0.041 | |
| Total cholesterol | 0.30 | 0.43 | 0.052 | |
| Cholesterol esters | 0.23 | 0.32 | 0.031 | |
| Free cholesterol | 0.068 | 0.10 | 0.046 | |
| Triglycerides | 0.034 | 0.041 | 0.357 | |
| Medium | Particle concentration | 1.81e-03 | 2.08E-03 | 0.112 |
| Total lipids | 0.77 | 0.89 | 0.106 | |
| Phospholipids | 0.37 | 0.42 | 0.108 | |
| Total cholesterol | 0.37 | 0.44 | 0.094 | |
| Cholesterol esters | 0.29 | 0.34 | 0.100 | |
| Free cholesterol | 0.077 | 0.095 | 0.091 | |
| Triglycerides | 0.036 | 0.033 | 0.359 | |
| Small | Particle concentration | 4.69e-03 | 4.90e-03 | 0.374 |
| Total lipids | 1.04 | 1.09 | 0.333 | |
| Phospholipids | 0.57 | 0.60 | 0.376 | |
| Total cholesterol | 0.43 | 0.45 | 0.374 | |
| Cholesterol esters | 0.32 | 0.33 | 0.731 | |
| Free cholesterol | 0.10 | 0.12 | 0.037 | |
| Triglycerides | 0.047 | 0.046 | 0.735 | |
| Total cholesterol in HDL | 1.3 | 1.6 | 0.030 | |
| HDL particle diameter | 9.9 nm | 10.1 nm | 0.035 |
C9orf72, Chromosome 9 open reading frame 72; HDL, high-density lipoprotein. The p-values are presented as native values and are not subjected to multiple test.
The mean diameter of HDL particles was smaller in patients carrying the C9orf72 repeat expansion in comparison to non-carriers (p = 0.035). The mean diameters of VLDL and LDL particles were similar between the C9orf72 repeat expansion carriers and non-carriers. Moreover, the concentrations of phosphatidylcholine and conjugated linoleic acid were lower in cases carrying the C9orf72 repeat expansion compared to non-carriers (p = 0.023 and p = 0.040, respectively). We observed that the concentration of triglycerides in small and medium HDL particles was higher in carriers of two APOE ɛ4 alleles when compared to patients with only one or no APOE ɛ4 alleles. The APOE ɛ4 genotype did not affect the concentrations of HDLs in any other subclass. In addition, we saw that serum HDL concentrations were consistently lower in men in nearly every subclass. Furthermore, serum triglycerides in large and small LDL were also lower in men.
The median hs-CRP concentration in the C9orf72 repeat expansion carriers was 0.60 pg/ml. There was no difference between the median hs-CRP concentrations between C9orf72 repeat expansion carriers and non-carriers (median hs-CRP in non-carriers was 0.65 pg/ml). The non-carriers showed slightly higher mean value, but this was due to one bvFTD patient with hs-CRP concentration of 36.40 pg/ml. In addition, there were three non-carriers whose hs-CRP concentrations were under the quantification range of the assay (0.15 pg/ml). These values were set to zero. The hs-CRP levels did not correlate with HDL or LDL concentrations in any lipoprotein subclass in carriers and non-carriers combined, but were observed to negatively correlate with extremely large, very large, and large VLDLs in multiple subclasses. We also observed a non-significant trend of slightly depressed GlycA in FTLD patients with the C9orf72 repeat expansion when compared to those without (p = 0.064). The mean concentration of GlycA in the C9orf72 repeat expansion carriers was 1.49 mmol/l and 1.62 mmol/l in non-carriers.
DISCUSSION
We found here that C9orf72 repeat expansion carriers show lower concentrations of HDL category lipids as compared to non-carriers. This alteration in serum lipids appears to be specific for HDL as no changes were observed in the concentrations of VLDL or LDL between these groups. To our knowledge, this is the first study reporting blood lipoprotein alterations in C9orf72 repeat expansion associated FTLD patients. Dyslipidemia has been suggested to associate with brain atrophy and is thought to occur in neurodegenerative diseases, i.e., AD, but not much information is available on lipid alterations in FTLD. Growing data suggest that a low concentration of serum HDL may be associated with an increased risk of certain neurodegenerative diseases. For instance, a lowered HDL serum cholesterol has been described to associate with AD [12, 13]. However, contradictory evidence has also been presented, linking an increased risk of AD and cholesterol esters relative to total lipids in large HDL [41].
Our results are in line with previous findings related to lipoprotein changes in FTLD [18, 19, 42]. It has been previously reported that bvFTD patients have a lower blood HDL cholesterol concentration when compared to AD patients [18]. The same study also reported an increased total cholesterol/HDL ratio in bvFTD patients as compared to a healthy control group. In addition blood triglycerides were elevated in both bvFTD and SD patients compared to a control group [18]. In a more recent study, the same group reported increased triglycerides and a total cholesterol/HDL ratio, and a lowered HDL cholesterol concentration in ALS and FTD patients when compared to controls [43]. Furthermore, a recent lipidomics analysis from plasma showed alterations in different lipid species in bvFTD as compared to AD patients or controls and suggested hypertriglyceridemia and hypoalphalipoproteinemia in bvFTD [19]. Several studies also associate the incidence and risk of ALS to changes in LDL category cholesterols and lipids [20, 21, 23, 27, 29]. The information regarding the involvement of HDL in ALS is inconclusive. None of these studies, however, have contained genetic analyses to allow comparisons between C9orf72 repeat expansion carriers and non-carriers. In the study by Wuolikainen et al., the C9orf72 repeat expansions were confirmed for eleven ALS patients, but the cholesterol variables were not examined on the basis of the repeat expansion carrier status [22].
Chronic inflammation has also been reported to decrease serum HDL levels [14]. However, the mechanism by which inflammation may alter lipid metabolism is not fully understood. As HDL has been shown to possess anti-inflammatory and antioxidant properties and is a central transporter of cholesterol from the tissues to the liver, inflammation might compromise this reverse transporter function and thus promote cholesterol accumulation and exacerbate inflammatory responses, creating a vicious cycle (see, e.g., the review by Feingold and Grunfeld in 2016 [44]). Recently, a plausible association of inflammatory disturbances and FTLD has been proposed. First, an increased prevalence of autoimmune diseases has been reported in FTLD patients compared to controls [45–47] and second, FTLD seems to be inversely linked to cancer [48], which may suggest alterations in the immune system or responses. Interestingly, the contribution of autoimmune mechanisms and genetic variation in loci associated with the immune system in FTLD were also suggested in recent studies [15, 49–51]. In line with these patient-derived data, C9orf72 repeat expansion has been linked to disturbances in the immune system [52], e.g., in mouse models with loss of function of the C9orf72 gene, which indicate a severe autoimmune phenotype, high mortality rate, and increased levels of proinflammatory cytokines and signs of neuroinflammation [16, 17, 52, 53]. On the other hand, a remarkable amount of FTLD patients display dietary changes [2], which may partly be related to changes in serum lipid concentrations. The C9orf72 repeat expansion associated FTLD patients have been reported to exhibit less alterations in eating habits than repeat expansion non-carriers. In particular the acquired preference for sweet foods was lower in C9orf72 carriers [54]. As we did not detect alterations in lipid particles other than HDL, we suggest that eating habits do not underlie the decreased serum HDL levels in our study.
Connections between HDLs and inflammation are not possible to evaluate with our findings. Based on the hs-CRP and GlycA levels there were no differences in relation to the C9orf72 repeat expansion in the FTLD patients. This could, at least partly be due to the low sample size, but could also reflect the mechanistic complexity underlying the HDL-related changes and chronic inflammation. It is also well known that certain common autoimmune disorders, e.g., rheumatoid arthritis, may not necessarily increase CRP levels. Furthermore, there are no previous reports regarding hs-CRP or GlycA in FTLD or their correlations with the C9orf72 repeat expansion. Lunette and colleagues connected ALS progression with hs-CRP [55], but also inconclusive results have been presented [56]. In the latter study, Nagel et al. did not see an association between hs-CRP levels and the risk of ALS. Currently, GlycA has been associated with systemic inflammation [40, 57] but has not been as extensively studied in the context of neurodegeneration. A study by Tynkkynen et al. assessed inflammation in AD and incident dementia by quantifying GlycA but reported only weak associations [41]. Further research is warranted to better elucidate these apparently complex associations.
Of the tested cofounding factors, sex significantly affected the concentrations of lipoprotein variables in the sera. Men showed lower concentrations of multiple HDL category lipids when compared to women. However, the prevalence of the APOE ɛ4 allele was only affecting these HDL levels in two subclasses. Considering the similarity in sex distributions among C9orf72 repeat expansion carriers and non-carriers, we do not suspect that the reported changes in HDL category lipids between C9orf72 repeat expansion carriers and non-carriers are due to sex-related differences in HDL or lipoprotein metabolism.
Strengths and weaknesses
The strengths of this study include utilization of a clinically and genetically well-characterized FTLD patient cohort with a large number of C9orf72 repeat expansion carriers. However, even though the proportion of C9orf72 expansion carriers in the cohort is high, the size of the total cohort is limited. Thus, larger sample sets are needed to confirm the results obtained in the study. Lipoprotein quantification provides absolute concentrations, instead of relying on relative concentrations or signal intensities. The serum metabolomics platform is thoroughly validated and used extensively. Another limiting factor is the fact that the provided p-values were not subjected to multiple testing. After applying multiple correction, the p-values depicted non-significant findings, due to the small sample size. However, a clearly discernable trend is obvious and warrants further research to thoroughly validate the presented association.
Conclusions
We conclude that total serum HDL cholesterol was lower in FTLD patients with the C9orf72 repeat expansion. This was consistent in several subclasses of very large and large HDLs. There were no significant differences in the concentrations of VLDL or LDL cholesterol subclasses between C9orf72 repeat expansion carriers and non-carriers. We did not find an association between these lipoprotein changes and quantifiable markers of systemic inflammation, hs-CRP or GlycA. Thus, the background behind the presented HDL changes remains unclear and needs further investigations.
ACKNOWLEDGMENTS
Technical laboratory assistance was provided by Tarja Kauppinen, Päivi Räsänen, and Anne Kaikko. Proofreading of the manuscript was conducted by Elsa Language Services (Kuopio, Finland). Olli Jääskeläinen has received a personal grant from Emil Aaltonen Foundation. Olli Jääskeläinen, Sanna-Kaisa Herukka, Annakaisa Haapasalo, Mikko Hiltunen, and Hilkka Soininen are part of the EU H2020-TWINN SynaNet, ‘Neurologic and Psychiatric Disorders: from synapses to network’ consortium, funded by the European Horizon 2020 research and innovation program (grant number 692340). Annakaisa Haapasalo and Anne M. Remes are supported by the Academy of Finland (grant numbers 315459 and 2315460). Mika Ala-Korpela is supported by a Senior Research Fellowship from the National Health and Medical Research Council (NHMRC) of Australia (APP1158958). He also works in a unit that is supported by the University of Bristol and UK Medical Research Council (MC_UU_12013/1). The Baker Institute is supported in part by the Victorian Government’s Operational Infrastructure Support Program.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0132r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190132.
REFERENCES
[1] | Ratnavalli E , Brayne C , Dawson K , Hodges JR ((2002) ) The prevalence of frontotemporal dementia. Neurology 58: , 1615–1621. |
[2] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EGP , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini M-L , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[3] | Gorno-Tempini ML , Hillis AE , Weintraub S , Kertesz A , Mendez M , Cappa SF , Ogar JM , Rohrer JD , Black S , Boeve BF , Manes F , Dronkers NF , Vandenberghe R , Rascovsky K , Patterson K , Miller BL , Knopman DS , Hodges JR , Mesulam MM , Grossman M ((2011) ) Classification of primary progressive aphasia and its variants. Neurology 76: , 1006–1014. |
[4] | Rohrer JD , Guerreiro R , Vandrovcova J , Uphill J , Reiman D , Beck J , Isaacs AM , Authier A , Ferrari R , Fox NC , Mackenzie IRA , Warren JD , de Silva R , Holton J , Revesz T , Hardy J , Mead S , Rossor MN ((2009) ) The heritability and genetics of frontotemporal lobar degeneration. Neurology 73: , 1451–1456. |
[5] | Benussi A , Padovani A , Borroni B ((2015) ) Phenotypic heterogeneity of monogenic frontotemporal dementia. Front Aging Neurosci 7: , 171. |
[6] | Renton AE , Majounie E , Waite A , Simón-Sánchez J , Rollinson S , Gibbs JR , Schymick JC , Laaksovirta H , van Swieten JC , Myllykangas L , Kalimo H , Paetau A , Abramzon Y , Remes AM , Kaganovich A , Scholz SW , Duckworth J , Ding J , Harmer DW , Hernandez DG , Johnson JO , Mok K , Ryten M , Trabzuni D , Guerreiro RJ , Orrell RW , Neal J , Murray A , Pearson J , Jansen IE , Sondervan D , Seelaar H , Blake D , Young K , Halliwell N , Callister JB , Toulson G , Richardson A , Gerhard A , Snowden J , Mann D , Neary D , Nalls MA , Peuralinna T , Jansson L , Isoviita V-M , Kaivorinne A-L , Hölttä-Vuori M , Ikonen E , Sulkava R , Benatar M , Wuu J , Chiò A , Restagno G , Borghero G , Sabatelli M , Heckerman D , Rogaeva E , Zinman L , Rothstein JD , Sendtner M , Drepper C , Eichler EE , Alkan C , Abdullaev Z , Pack SD , Dutra A , Pak E , Hardy J , Singleton A , Williams NM , Heutink P , Pickering-Brown S , Morris HR , Tienari PJ , Traynor BJ , Traynor BJ ((2011) ) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72: , 257–268. |
[7] | DeJesus-Hernandez M , Mackenzie IR , Boeve BF , Boxer AL , Baker M , Rutherford NJ , Nicholson AM , Finch NA , Flynn H , Adamson J , Kouri N , Wojtas A , Sengdy P , Hsiung G-YR , Karydas A , Seeley WW , Josephs KA , Coppola G , Geschwind DH , Wszolek ZK , Feldman H , Knopman DS , Petersen RC , Miller BL , Dickson DW , Boylan KB , Graff-Radford NR , Rademakers R ((2011) ) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72: , 245–256. |
[8] | Majounie E , Renton AE , Mok K , Dopper EG , Waite A , Rollinson S , Chiò A , Restagno G , Nicolaou N , Simon-Sanchez J , van Swieten JC , Abramzon Y , Johnson JO , Sendtner M , Pamphlett R , Orrell RW , Mead S , Sidle KC , Houlden H , Rohrer JD , Morrison KE , Pall H , Talbot K , Ansorge O , Hernandez DG , Arepalli S , Sabatelli M , Mora G , Corbo M , Giannini F , Calvo A , Englund E , Borghero G , Floris GL , Remes AM , Laaksovirta H , McCluskey L , Trojanowski JQ , Van Deerlin VM , Schellenberg GD , Nalls MA , Drory VE , Lu C-S , Yeh T-H , Ishiura H , Takahashi Y , Tsuji S , Le Ber I , Brice A , Drepper C , Williams N , Kirby J , Shaw P , Hardy J , Tienari PJ , Heutink P , Morris HR , Pickering-Brown S , Traynor BJ , Morris HR , Pickering-Brown S , Traynor BJ ((2012) ) Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: A cross-sectional study. Lancet Neurol 11: , 323–330. |
[9] | Balendra R , Isaacs AM ((2018) ) C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat Rev Neurol 14: , 544–558. |
[10] | Yadav RS , Tiwari NK ((2014) ) Lipid integration in neurodegeneration: An overview of Alzheimer’s disease. Mol Neurobiol 50: , 168–176. |
[11] | Vance JE ((2012) ) Dysregulation of cholesterol balance in the brain: Contribution to neurodegenerative diseases. Dis Model Mech 5: , 746–755. |
[12] | Merched A , Xia Y , Visvikis S , Serot JM , Siest G ((2000) ) Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol Aging 21: , 27–30. |
[13] | Zuliani G , Cavalieri M , Galvani M , Volpato S , Cherubini A , Bandinelli S , Corsi AM , Lauretani F , Guralnik JM , Fellin R , Ferrucci L ((2010) ) Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. The InChianti Study. J Gerontol A Biol Sci Med Sci 65A: , 559–564. |
[14] | Feingold KR , Grunfeld C ((2000) ) The Effect of Inflammation and Infection on Lipids and Lipoproteins, MDText.com, Inc. |
[15] | Ferrari R , Hernandez DG , Nalls MA , Rohrer JD , Ramasamy A , Kwok JBJ , Dobson-Stone C , Brooks WS , Schofield PR , Halliday GM , Hodges JR , Piguet O , Bartley L , Thompson E , Haan E , Hernández I , Ruiz A , Boada M , Borroni B , Padovani A , Cruchaga C , Cairns NJ , Benussi L , Binetti G , Ghidoni R , Forloni G , Galimberti D , Fenoglio C , Serpente M , Scarpini E , Clarimón J , Lleó A , Blesa R , Waldö ML , Nilsson K , Nilsson C , Mackenzie IRA , Hsiung G-YR , Mann DMA , Grafman J , Morris CM , Attems J , Griffiths TD , McKeith IG , Thomas AJ , Pietrini P , Huey ED , Wassermann EM , Baborie A , Jaros E , Tierney MC , Pastor P , Razquin C , Ortega-Cubero S , Alonso E , Perneczky R , Diehl-Schmid J , Alexopoulos P , Kurz A , Rainero I , Rubino E , Pinessi L , Rogaeva E , St George-Hyslop P , Rossi G , Tagliavini F , Giaccone G , Rowe JB , Schlachetzki JCM , Uphill J , Collinge J , Mead S , Danek A , Van Deerlin VM , Grossman M , Trojanowski JQ , van der Zee J , Deschamps W , Van Langenhove T , Cruts M , Van Broeckhoven C , Cappa SF , Le Ber I , Hannequin D , Golfier V , Vercelletto M , Brice A , Nacmias B , Sorbi S , Bagnoli S , Piaceri I , Nielsen JE , Hjermind LE , Riemenschneider M , Mayhaus M , Ibach B , Gasparoni G , Pichler S , Gu W , Rossor MN , Fox NC , Warren JD , Spillantini MG , Morris HR , Rizzu P , Heutink P , Snowden JS , Rollinson S , Richardson A , Gerhard A , Bruni AC , Maletta R , Frangipane F , Cupidi C , Bernardi L , Anfossi M , Gallo M , Conidi ME , Smirne N , Rademakers R , Baker M , Dickson DW , Graff-Radford NR , Petersen RC , Knopman D , Josephs KA , Boeve BF , Parisi JE , Seeley WW , Miller BL , Karydas AM , Rosen H , van Swieten JC , Dopper EGP , Seelaar H , Pijnenburg YAL , Scheltens P , Logroscino G , Capozzo R , Novelli V , Puca AA , Franceschi M , Postiglione A , Milan G , Sorrentino P , Kristiansen M , Chiang H-H , Graff C , Pasquier F , Rollin A , Deramecourt V , Lebert F , Kapogiannis D , Ferrucci L , Pickering-Brown S , Singleton AB , Hardy J , Momeni P ((2014) ) Frontotemporal dementia and its subtypes: A genome-wide association study. Lancet Neurol 13: , 686–699. |
[16] | Atanasio A , Decman V , White D , Ramos M , Ikiz B , Lee H-C , Siao C-J , Brydges S , LaRosa E , Bai Y , Fury W , Burfeind P , Zamfirova R , Warshaw G , Orengo J , Oyejide A , Fralish M , Auerbach W , Poueymirou W , Freudenberg J , Gong G , Zambrowicz B , Valenzuela D , Yancopoulos G , Murphy A , Thurston G , Lai K-MV ((2016) ) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6: , 23204. |
[17] | Burberry A , Suzuki N , Wang J-Y , Moccia R , Mordes DA , Stewart MH , Suzuki-Uematsu S , Ghosh S , Singh A , Merkle FT , Koszka K , Li Q-Z , Zon L , Rossi DJ , Trowbridge JJ , Notarangelo LD , Eggan K ((2016) ) Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci Transl Med 8: , 347ra93. |
[18] | Ahmed RM , MacMillan M , Bartley L , Halliday GM , Kiernan MC , Hodges JR , Piguet O ((2014) ) Systemic metabolism in frontotemporal dementia. Neurology 83: , 1812–1818. |
[19] | Kim WS , Jary E , Pickford R , He Y , Ahmed RM , Piguet O , Hodges JR , Halliday GM ((2018) ) Lipidomics analysis of behavioral variant frontotemporal dementia: A scope for biomarker development. Front Neurol 9: , 104. |
[20] | Delaye JB , Patin F , Piver E , Bruno C , Vasse M , Vourc’h P , Andres CR , Corcia P , Blasco H ((2017) ) Low IDL-B and high LDL-1 subfraction levels in serum of ALS patients. J Neurol Sci 380: , 124–127. |
[21] | Chen X , Yazdani S , Piehl F , Magnusson PKE , Fang F ((2018) ) Polygenic link between blood lipids and amyotrophic lateral sclerosis. Neurobiol Aging 67: , 202.e1–202.e6. |
[22] | Wuolikainen A , Acimovic J , Lövgren-Sandblom A , Parini P , Andersen PM , Björkhem I ((2014) ) Cholesterol, oxysterol, triglyceride, and coenzyme Q homeostasis in ALS. Evidence against the hypothesis that elevated 27-hydroxycholesterol is a pathogenic factor. PLoS One 9: , e113619. |
[23] | Mariosa D , Hammar N , Malmström H , Ingre C , Jungner I , Ye W , Fang F , Walldius G ((2017) ) Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol 81: , 718–728. |
[24] | Rafiq MK , Lee E , Bradburn M , McDermott CJ , Shaw PJ ((2015) ) Effect of lipid profile on prognosis in the patients with amyotrophic lateral sclerosis: Insights from the olesoxime clinical trial. Amyotroph Lateral Scler Front Degener 16: , 478–484. |
[25] | Huang R , Guo X , Chen X , Zheng Z , Wei Q , Cao B , Zeng Y , Shang H ((2015) ) The serum lipid profiles of amyotrophic lateral sclerosis patients: A study from south-west China and a meta-analysis. Amyotroph Lateral Scler Front Degener 16: , 359–365. |
[26] | Dorst J , Kühnlein P , Hendrich C , Kassubek J , Sperfeld AD , Ludolph AC ((2011) ) Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol 258: , 613–617. |
[27] | Zeng P , Zhou X ((2019) ) Causal effects of blood lipids on amyotrophic lateral sclerosis: A Mendelian randomization study. Hum Mol Genet 28: , 688–697. |
[28] | Chio A , Calvo A , Ilardi A , Cavallo E , Moglia C , Mutani R , Palmo A , Galletti R , Marinou K , Papetti L , Mora G ((2009) ) Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 73: , 1681–1685. |
[29] | Yang JW , Kim S-M , Kim H-J , Kim J-E , Park KS , Kim S-H , Lee K-W , Sung J-J ((2013) ) Hypolipidemia in patients with amyotrophic lateral sclerosis: A possible gender difference? J Clin Neurol 9: , 125. |
[30] | Dupuis L , Corcia P , Fergani A , Gonzalez De Aguilar J-L , Bonnefont-Rousselot D , Bittar R , Seilhean D , Hauw J-J , Lacomblez L , Loeffler J-P , Meininger V ((2008) ) Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70: , 1004–1009. |
[31] | Krüger J , Kaivorinne A-L , Udd B , Majamaa K , Remes AM ((2009) ) Low prevalence of progranulin mutations in Finnish patients with frontotemporal lobar degeneration. Eur J Neurol 16: , 27–30. |
[32] | Kaivorinne A-L , Krüger J , Kuivaniemi K , Tuominen H , Moilanen V , Majamaa K , Remes AM ((2008) ) Role of MAPT mutations and haplotype in frontotemporal lobar degeneration in Northern Finland. BMC Neurol 8: , 48. |
[33] | De la Vega FM , Lazaruk KD , Rhodes MD , Wenz MH ((2005) ) Assessment of two flexible and compatible SNP genotyping platforms: TaqMan SNP Genotyping Assays and the SNPlex Genotyping System. Mutat Res 573: , 111–135. |
[34] | Soininen P , Kangas AJ , Wurtz P , Tukiainen T , Tynkkynen T , Laatikainen R , Jarvelin M-R , Kahonen M , Lehtimaki T , Viikari J , Raitakari OT , Savolainen MJ , Ala-Korpela M ((2009) ) High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 134: , 1781–1785. |
[35] | Soininen P , Kangas AJ , Wurtz P , Suna T , Ala-Korpela M ((2015) ) Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 8: , 192–206. |
[36] | Kettunen J , Tukiainen T , Sarin A-P , Ortega-Alonso A , Tikkanen E , Lyytikainen L-P , Kangas AJ , Soininen P , Wurtz P , Silander K , Dick DM , Rose RJ , Savolainen MJ , Viikari J , Kahonen M , Lehtimaki T , Pietilainen KH , Inouye M , McCarthy MI , Jula A , Eriksson J , Raitakari OT , Salomaa V , Kaprio J , Jarvelin M-R , Peltonen L , Perola M , Freimer NB , Ala-Korpela M , Palotie A , Ripatti S ((2012) ) Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 44: , 269–276. |
[37] | Kettunen J , Demirkan A , Wurtz P , Draisma HHM , Haller T , Rawal R , Vaarhorst A , Kangas AJ , Lyytikainen L-P , Pirinen M , Pool R , Sarin A-P , Soininen P , Tukiainen T , Wang Q , Tiainen M , Tynkkynen T , Amin N , Zeller T , Beekman M , Deelen J , van Dijk KW , Esko T , Hottenga J-J , van Leeuwen EM , Lehtimaki T , Mihailov E , Rose RJ , de Craen AJM , Gieger C , Kahonen M , Perola M , Blankenberg S , Savolainen MJ , Verhoeven A , Viikari J , Willemsen G , Boomsma DI , van Duijn CM , Eriksson J , Jula A , Jarvelin M-R , Kaprio J , Metspalu A , Raitakari O , Salomaa V , Slagboom PE , Waldenberger M , Ripatti S , Ala-Korpela M ((2016) ) Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun 7: , 11122. |
[38] | Kujala UM , Makinen V-P , Heinonen I , Soininen P , Kangas AJ , Leskinen TH , Rahkila P , Wurtz P , Kovanen V , Cheng S , Sipila S , Hirvensalo M , Telama R , Tammelin T , Savolainen MJ , Pouta A , O’Reilly PF , Mantyselka P , Viikari J , Kahonen M , Lehtimaki T , Elliott P , Vanhala MJ , Raitakari OT , Jarvelin M-R , Kaprio J , Kainulainen H , Ala-Korpela M ((2013) ) Long-term leisure-time physical activity and serum metabolome. Circulation 127: , 340–348. |
[39] | Würtz P , Kangas AJ , Soininen P , Lawlor DA , Davey Smith G , Ala-Korpela M ((2017) ) Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: A primer on -omic technologies. Am J Epidemiol 186: , 1084–1096. |
[40] | Duprez DA , Jacobs DR ((2019) ) GlycA, a composite low-grade inflammatory marker, predicts mortality: Prime time for utilization? J Intern Med. doi:10.1111/joim.12961 |
[41] | Tynkkynen J , Chouraki V , Lee SJ van der , Hernesniemi J , Yang Q , Li S , Beiser A , Larson MG , Sääksjärvi K , Shipley MJ , Singh-Manoux A , Gerszten RE , Wang TJ , Havulinna AS , Würtz P , Fischer K , Demirkan A , Ikram MA , Amin N , Lehtimäki T , Kähönen M , Perola M , Metspalu A , Kangas AJ , Soininen P , Ala-Korpela M , Vasan RS , Kivimäki M , Duijn CM van , Seshadri S , Salomaa V ((2018) ) Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimers Dement 14: , 723–733. |
[42] | Ahmed RM , Irish M , Piguet O , Halliday GM , Ittner LM , Farooqi S , Hodges JR , Kiernan MC ((2016) ) Amyotrophic lateral sclerosis and frontotemporal dementia: Distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol 15: , 332–342. |
[43] | Ahmed RM , Highton-Williamson E , Caga J , Thornton N , Ramsey E , Zoing M , Kim WS , Halliday GM , Piguet O , Hodges JR , Farooqi IS , Kiernan MC ((2018) ) Lipid metabolism and survival across the frontotemporal dementia-amyotrophic lateral sclerosis spectrum: Relationships to eating behavior and cognition. J Alzheimers Dis 61: , 773–783. |
[44] | Feingold KR , Grunfeld C ((2016) ) Effect of inflammation on HDL structure and function. Curr Opin Lipidol 27: , 521–530. |
[45] | Miller ZA , Rankin KP , Graff-Radford NR , Takada LT , Sturm VE , Cleveland CM , Criswell LA , Jaeger PA , Stan T , Heggeli KA , Hsu SC , Karydas A , Khan BK , Grinberg LT , Gorno-Tempini ML , Boxer AL , Rosen HJ , Kramer JH , Coppola G , Geschwind DH , Rademakers R , Seeley WW , Wyss-Coray T , Miller BL ((2013) ) TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry 84: , 956–962. |
[46] | Miller ZA , Sturm VE , Camsari GB , Karydas A , Yokoyama JS , Grinberg LT , Boxer AL , Rosen HJ , Rankin KP , Gorno-Tempini ML , Coppola G , Geschwind DH , Rademakers R , Seeley WW , Graff-Radford NR , Miller BL ((2016) ) Increased prevalence of autoimmune disease within C9 and FTD/MND cohorts. Neurol Neuroimmunol Neuroinflamm 3: , e301. |
[47] | Katisko K , Solje E , Koivisto AM , Krüger J , Kinnunen T , Hartikainen P , Helisalmi S , Korhonen V , Herukka S-K , Haapasalo A , Remes AM ((2018) ) Prevalence of immunological diseases in a Finnish frontotemporal lobar degeneration cohort with the C9orf72 repeat expansion carriers and non-carriers. J Neuroimmunol 321: , 29–35. |
[48] | Katisko K , Haapasalo A , Koivisto A , Krüger J , Hartikainen P , Korhonen V , Helisalmi S , Herukka S-K , Remes AM , Solje E ((2018) ) Low prevalence of cancer in patients with frontotemporal lobar degeneration. J Alzheimers Dis 62: , 789–794. |
[49] | Borroni B , Stanic J , Verpelli C , Mellone M , Bonomi E , Alberici A , Bernasconi P , Culotta L , Zianni E , Archetti S , Manes M , Gazzina S , Ghidoni R , Benussi L , Stuani C , Di Luca M , Sala C , Buratti E , Padovani A , Gardoni F ((2017) ) Anti-AMPA GluA3 antibodies in frontotemporal dementia: A new molecular target. Sci Rep 7: , 6723. |
[50] | Katisko K , Kokkonen N , Krüger J , Hartikainen P , Koivisto AM , Helisalmi S , Korhonen VE , Kokki M , Tuusa J , Herukka S-K , Solje E , Haapasalo A , Tasanen K , Remes AM ((2018) ) The association between frontotemporal lobar degeneration and bullous pemphigoid. J Alzheimers Dis 66: , 743–750. |
[51] | Broce I , Karch CM , Wen N , Fan CC , Wang Y , Hong Tan C , Kouri N , Ross OA , Höglinger GU , Muller U , Hardy J , Momeni P , Hess CP , Dillon WP , Miller ZA , Bonham LW , Rabinovici GD , Rosen HJ , Schellenberg GD , Franke A , Karlsen TH , Veldink JH , Ferrari R , Yokoyama JS , Miller BL , Andreassen OA , Dale AM , Desikan RS , Sugrue LP , Sugrue LP ((2018) ) Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies. PLoS Med 15: , e1002487. |
[52] | O’Rourke JG , Bogdanik L , Yáñez A , Lall D , Wolf AJ , Muhammad AKMG , Ho R , Carmona S , Vit JP , Zarrow J , Kim KJ , Bell S , Harms MB , Miller TM , Dangler CA , Underhill DM , Goodridge HS , Lutz CM , Baloh RH ((2016) ) C9orf72 is required for proper macrophage and microglial function in mice. Science 351: , 1324–1329. |
[53] | Sudria-Lopez E , Koppers M , de Wit M , van der Meer C , Westeneng H-J , Zundel CAC , Youssef SA , Harkema L , de Bruin A , Veldink JH , van den Berg LH , Pasterkamp RJ ((2016) ) Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects. Acta Neuropathol 132: , 145–147. |
[54] | Snowden JS , Rollinson S , Thompson JC , Harris JM , Stopford CL , Richardson AMT , Jones M , Gerhard A , Davidson YS , Robinson A , Gibbons L , Hu Q , DuPlessis D , Neary D , Mann DMA , Pickering-Brown SM ((2012) ) Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 135: , 693–708. |
[55] | Lunetta C , Lizio A , Maestri E , Sansone VA , Mora G , Miller RG , Appel SH , Chiò A ((2017) ) Serum C-reactive protein as a prognostic biomarker in amyotrophic lateral sclerosis. JAMA Neurol 74: , 660. |
[56] | Nagel G , Peter RS , Rosenbohm A , Koenig W , Dupuis L , Rothenbacher D , Ludolph AC ((2017) ) Adipokines, C-reactive protein and amyotrophic lateral sclerosis – results from a population- based ALS registry in Germany. Sci Rep 7: , 4374. |
[57] | Gruppen EG , Kunutsor SK , Kieneker LM , van der Vegt B , Connelly MA , de Bock GH , Gansevoort RT , Bakker SJL , Dullaart RPF ((2019) ) GlycA, a novel pro-inflammatory glycoprotein biomarker is associated with mortality: Results from The PREVEND study and meta-analysis. J Intern Med. doi: 10.1111/joim.12953 |



