Safety and improvement of movement function after stroke with atomoxetine: A pilot randomized trial
Abstract
Background: Intensive, task-oriented motor training has been associated with neuroplastic reorganization and improved upper extremity movement function after stroke. However, to optimize such training for people with moderate-to-severe movement impairment, pharmacological modulation of neuroplasticity may be needed as an adjuvant intervention.
Objective: Evaluate safety, as well as improvement in movement function, associated with motor training paired with a drug to upregulate neuroplasticity after stroke.
Methods: In this double-blind, randomized, placebo-controlled study, 12 subjects with chronic stroke received either atomoxetine or placebo paired with motor training. Safety was assessed using vital signs. Upper extremity movement function was assessed using Fugl-Meyer Assessment, Wolf Motor Function Test, and Action Research Arm Test at baseline, post-intervention, and 1-month follow-up.
Results: No significant between-groups differences were found in mean heart rate (95% CI, –12.4–22.6; p = 0.23), mean systolic blood pressure (95% CI, –1.7–29.6; p = 0.21), or mean diastolic blood pressure (95% CI, –10.4–13.3; p = 0.08). A statistically significant between-groups difference on Fugl-Meyer at post-intervention favored the atomoxetine group (95% CI, 1.6–12.7; p = 0.016).
Conclusion: Atomoxetine combined with motor training appears safe and may optimize motor training outcomes after stroke.
1Introduction
Stroke is a major public health concern, as there are over 795,000 new strokes annually in the United States at a cost of $34 billion (Mozaffarian et al., 2015). Interventions to minimize tissue damage in acute stroke have had some success (Eissa, Krass, & Bajorek, 2012). However, there is still a crucial need for interventions to maximize recovery of movement function after neurologic damage has occurred (Nudo, Plautz, & Frost, 2001). These interventions can capitalize on neuroplastic change (the capacity of the brain to reorganize). Neuroplastic change correlateswith recovery of movement function after brain lesions (Nudo et al., 2001; Sawaki et al., 2014). Intensive, task-oriented motor training is an example of a therapeutic intervention that adheres to principles of neuroplastic change (Kleim & Jones, 2008). This intervention has been shown to improve mildly impaired movement function after stroke (Wolf et al., 2006). However, it has not been established as effective to mitigate chronic, severe impairment of upper extremity (UE) movement function after stroke (Bonifer & Anderson, 2003). In general, further research is needed to advance the effectiveness of interventions targeting chronic, severe impairment of UE movement function after stroke. In particular, optimizing intensive, task-oriented training for people with severe impairment may require adjuvant interventions to upregulate neuroplasticity (Hayward, Barker, & Brauer, 2010).
For more than a decade, the research community has viewed pharmacological intervention as a potentially valuable tool for optimizing therapy after stroke. Specifically, a variety of drugs have been examined as potential interventions to promote recovery of movement and speech functions (Floel et al., 2005; Foster, Good, Fowlkes, & Sawaki, 2006; Walker-Batson et al., 2001). Research in animals and humans indicates that some medications, such as amphetamine, may enhance outcomes of motor training (Feeney & Sutton, 1987). One of the proposed mechanisms by which amphetamine may enhance outcomes of motor training is by increasing central levels of norepinephrine (Feeney & Sutton, 1987), a neurotransmitter that appears to serve a major role in the plasticity process (Feeney & Sutton, 1987). Drugs that stimulate central levels of norepinephrine have been shown to promote recovery of movement and cognitive function, while drugs that inhibit noradrenergic pathways yield a deleterious effect in this regard (Feeney & Sutton, 1987; Wang et al., 2011). At the same time, the number of patients in the several clinical trials who have received motor training paired with amphetamine after stroke is very small, due to amphetamine’s potential interaction with many other medications as well as its potential for addiction. Furthermore, the existing research about amphetamine has shown it to have inconsistent results as well as significant unwanted effects (Wang et al., 2011).
Atomoxetine, a potent and selective inhibitor of the presynaptic norepinephrine transporter (Simpson & Perry, 2003), has been approved by the Food and Drug Administration for treatment of attention deficit hyperactivity disorder (Simpson & Perry, 2003). Unlike amphetamine, atomoxetine lacks affinity for dopaminergic and serotonergic receptors and therefore has no potential for abuse (Simpson & Perry, 2003). A small study using atomoxetine has been shown to enhance motor memory formation in 10 healthy participants (Foster et al., 2006) using an established laboratory paradigm (Classen, Liepert, Wise, Hallett, & Cohen, 1998). However, atomoxetine has not been systematically evaluated for improvement of movement function after stroke. The present, double-blind, sham-controlled study was the first to address this evidence gap by evaluating the effects of atomoxetine on cardiovascular safety and movement function in subjects with chronic, moderate-to-severe impairment in movement function after stroke. The central hypothesis was that subjects who received atomoxetine paired with intensive, task-oriented UE motor training would have similar changes in blood pressure and heart rate, as well as similar rate of adverse events, as a placebo group (ie, subjects who received a placebo paired with intensive, task-oriented UE motor training). Additionally, it was hypothesized that the atomoxetine group would show initial indications of more improved movement function than the placebo group.
2Methods
In accordance with the Declaration of the World Medical Association (www.wma.net), this study was approved by the authorized institutional human research review boards at the institutions governing the research (ie, Wake Forest University, Winston-Salem, NC; the University of Kentucky, Lexington, KY; Cardinal Hill Hospital, Lexington, KY). All study procedures were in accordance with these institutions’ guidelines. The research setting was a neurorehabilitation research lab. Subjects were recruited from Cardinal Hill Rehabilitation Hospital, University of Kentucky, Wake Forest University, and local communities.
2.1Eligibility criteria
To remove the potential confound of spontaneous motor recovery, only individuals at least 6 months from the onset of stroke were recruited. To ensure safety and minimize potential confounding variables, the following exclusion criteria were selected: a) history of traumatic head injury; b) history of severe alcohol or drug abuse; c) history of psychiatric illness; d) unstable cardiac dysrhythmia; e) untreated hypertension (systolic pressure >160 mm Hg and/or diastolic pressure >100 mm Hg); f) history of myocardial infarction or unstable angina; g) positive pregnancy test or being of childbearing age and not using contraception; h) glaucoma; i) history of hypersensitivity or idiosyncrasy to sympathomimetic drugs; j) within 3 months of recruitment, addition or change in the dosage of drugs known to exert detrimental effects on recovery of movement function (Boroojerdi, Ziemann, Chen, Butefisch, & Cohen, 2001; Butefisch et al., 2002; Goldstein & Davis, 1990; Sawaki et al., 2002; Sawaki, Werhahn, Barco, Kopylev, & Cohen, 2003); or k) aphasia or cognitive deficit severe enough to preclude informedconsent.
As required by the authorized institutional human research review boards at the institutions where the research was conducted, all subjects provided written informed consent after receiving a verbal and written explanation of the purposes, procedures, and potential hazards of this study, which used a parallel-group block design within the conceptual framework of a superiority trial. After screening and informed consent, a convenience sample of 12 subjects who were 21 years of age or older was enrolled. All subjects had moderate-to-severe UE deficit in movement function (the inability to extend the affected metacarpophalangeal joints at least 10°; and the wrist, 20° (Wolf et al., 2006)). After enrollment, subjects participated in a baseline evaluation of movement function, 10 consecutive weekdays of intervention, 1 evaluation immediately after the intervention period, and a 1-month follow-up evaluation. After baseline evaluation, the principal investigator (PI) used a computerized experimental design generator and randomizer program to govern group assignment. This program generated a simple random allocation sequence (1 : 1) for assigning subjects into 2 equal-sized groups (ie, either the atomoxetine group or the placebo control group). The PI generated the random allocation sequence, enrolled subjects, and assigned subjects to interventions. Subjects were ordered by the randomizer in strict accordance with the order of enrollment.
2.2Intervention component 1: Atomoxetine (real or placebo)
The initial recommended and safe dose of atomoxetine (Strattera, by Eli Lilly) in subjects over 70 kg is 40 mg/day; therefore this dose was administered in this study (Simpson & Perry, 2003), for the intervention group. Placebo capsules were identical to atomoxetine capsules. Drug condition (ie, real versus placebo) was the only independent variable. In each intervention session (10 total sessions), dosing occurred just prior to intensive, task-oriented UE motor training.
2.3Intervention component 2: Intensive, task-oriented UE motor training
In each intervention session, 2 hours of intensive, task-oriented UE motor training commenced 60 minutes after atomoxetine or placebo intake to maximize peak plasma drug concentration during motor training (Simpson & Perry, 2003). All subjects participated in motor training, which was delivered in a 1 : 1 ratio between an occupational therapist and a subject. Training focused on skill acquisition through the use of unilateral (ie, impaired hand) and bilateral activities to improve movement function of the impaired hand. Tasks targeted functional goals (eg, activities of daily living) or goal subcomponents (eg, pinching, grasp/release, or functional reach patterns). Tasks were repeated at rate of approximately 10 to 50 repetitions each session according to the demands of the task. Tasks had progressive difficulty, meaning that task demands elicited progressively more skilled performance over time (shaping) (Wolf et al.,2006).
2.4Evaluation and outcome measures
To evaluate drug effects and monitor for adverse events, heart rate and blood pressure were measured and recorded by the PI prior to administration of atomoxetine or placebo. Drug administration proceeded if no abnormalities were present. Blood pressure and heart rate were then continuously monitored every 30 minutes until 30 minutes after the session had ended. A daily log of each subject’s reported sense of well-being or complaints associated with each session was recorded by the PI before and aftereach session.
To evaluate movement function, the Fugl Meyer Assessment (FMA), the Action Research Arm Test (ARAT), and the WMFT were administered at baseline, after the intervention period, and at 1-month follow-up. The FMA is a quantitative measure of motor recovery, balance, sensation, coordination and speed. It is based on the principle that recovery of movement function occurs in a predictable progression (Duncan, Propst, & Nelson, 1983). The FMA is feasible for use after stroke and has been extensively applied in this condition (Duncan et al., 1983). The inter-rater reliability (0.886 0.984 according to the subset for lower or UE) and test-retest reliability (= 0.99) of FMA are high (Duncan et al., 1983). The total possible score for UE movement function is 66. The highest possible FMA motor score for a tested UE is 66. The minimal clinically important difference (MCID) for FMA is 9 to 10 points; and the minimal detectable change (MDC) is 5.2 (Pandian & Arya, 2014). Based on its responsiveness and validity, FMA was used as the primary outcomemeasure.
The ARAT was developed specifically to measure UE grasp, grip, pinch, and gross movement using 4 specific tests (van der Lee, Beckerman, Lankhorst, & Bouter, 2001). Each item is graded on a 4-point ordinal scale with a total possible score of 57. The inter-rater reliability (=0.99) and test-retest reliability (=0.98) of ARAT are extremely high. The highest possible ARAT score for a tested UE is 57. The MCID for ARAT is 5.7 points; and the MDC is 3.5 (Pandian & Arya, 2014).
The WMFT is a time- and function-based evaluation and encompasses a battery of 17 tasks (Wolf et al., 2006). It is performed using a 16″ × 43″ laminated template on a standardized table. All tasks simulate functional tasks and are applied sequentially according to the task complexity. The WMFT has established reliability and validity and has been extensively applied in several studies to evaluate UE motor capacity after stroke (Wolf et al., 2006). The inter-rater reliability (0.93∼0.99) and test-retest reliability (=0.97) of WMFT are considered excellent (Whitall, Savin, Harris-Love, & Waller, 2006). The MCID for WMFT is 1.5 to 4 seconds; and the MDC is 12 seconds (Pandian & Arya, 2014). Time-based measures were collected for the paretic (ie, more affected) and non-paretic (is, lessaffected) UEs.
2.5Blinding
Subjects, care providers, and assessors of movement function were blinded to group assignment in that they were not made aware of which condition any subject was assigned to. Additionally, personnel administering atomoxetine (real or placebo) did not administer intensive, task-oriented UE motor training. Furthermore, atomoxetine and placebo capsules were visually identical, as prepared bya pharmacy.
2.6Statistics
Analyses of vital signs utilized linear mixed effects models with random intercept and slope to account for the 8 repeated measurements over time from each subject. Similarly, for FMA, ARAT, and WMFT, a longitudinal repeated measures model was used that accounts for time, trial arm, and their interaction. These models incorporate an unstructured working covariance matrix, and the Kenward and Roger degrees of freedom method was used for inference (Kenward & Roger, 1997). These analyses correspond to the use of repeated measures MANOVA, but with the allowance of missing data. Primary interest was in the comparison of mean changes in outcomes from baseline to immediately post-intervention and to 1-month follow-up for the 2 trial arms. For more detail, the separate impacts of each trial arm on the mean change of each outcome are presented. Longitudinal repeated measures ANCOVA models were also fit for each outcome due to slight imbalances at baseline. However, results were very similar and thus are not presented. All available data were utilized for analyses. All tests were 2-sided, with statistical significance pre-specified as P < 0.05. Furthermore, all tests were pre-specified. Multiple testing corrections were not directly utilized due to their known limitations (Altman, 2000; Perneger, 1998). Information provided in Table 4 can be utilized to obtain formal multiple testing corrections. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
3Results
Figure 1 shows participant flow. Table 1 shows clinical characteristics and demographic data for the sample. A total of 12 subjects (6 women) with chronic stroke, mean age of 55 years (range, 35–66 years), and mean interval of 25 months after stroke (range 6–52 months) were enrolled. Recruitment and all evaluations took place between April 2006 and June 2010. The study ended when projected enrollment was reached. Twelve subjects completed all intervention sessions, baseline evaluation, and evaluation at immediately post-intervention. Nine subjects completed the 1-month follow-up evaluation (n = 3 in the atomoxetine group and n = 6 in the placebo group). Losses to 1-month follow-up occurred due to either transportation constraints or subjects’ enrollment in other research studies immediately after post-intervention evaluation.
Effects of atomoxetine on vital signs were not significantly different when compared with placebo (Fig. 2). The interaction was not significant (p = 0.23) for the mean heart rate over time between the 2 trial arms. After taking out the interaction, there was not a significant trial arm difference (p = 0.53). Specifically, adjusting for time, the estimated mean heart rate was 5.1 [95% CI: (–12.4, 22.6)] beats per minute higher for atomoxetine relative to placebo. The interaction was not significant (p = 0.21) for changes in mean of systolic blood pressure over time between the 2 trial arms. After taking out the interaction, there was no statistical difference between the 2 trial arms (p = 0.08). Specifically, adjusting for time, the estimated mean systolic blood pressure was 14.0 [95% CI: (–1.7, 29.6)] mm Hg lower for atomoxetine relative to placebo. The interaction was not significant (p = 0.995) for changes in mean diastolic blood pressure over time between the 2 trial arms. After taking out the interaction, there was not a significant trial arm difference (p = 0.79). Specifically, adjusting for time, the estimated mean diastolic blood pressure was 1.4 [95% CI: (–10.4, 13.3)] mm Hg higher for atomoxetine relative toplacebo.
Table 2 shows the each subject’s reportedsense of well-being or complaints. While there were reports of mental fatigue, increased sleepiness, andexhaustion associated with both conditions, there was no clear indication of unwanted effects related to atomoxetine.
Table 3 summarizes baseline scores on outcome measures for the 2 trial arms. Table 4 shows changes in UE movement function associated with intervention. From baseline to immediately post-intervention, within-group changes for the atomoxetine group indicated significant improvement on FMA (95% CI, 5.4–13.2; p = 0.0003) and ARAT (95% CI, 4.6–10.7; p = 0.0002). Within-group changes for the placebo group indicated significant improvement on ARAT (95% CI, 2.3–8.4; p = 0.003) and WMFT paretic UE (95% CI, –0.35 ––0.03; p = 0.02). From baseline to 1-month follow-up, within-group changes in the atomoxetine group indicated significant improvement on FMA (95% CI, 5.9–17.9; p = 0.002) and ARAT (95% CI, 5.1–15.1; p = 0.001). Within-group changes for the placebo group indicated significant improvement on FMA (95% CI, 1.5–10.2; p = 0.015), ARAT (95% CI, 2.6–10.4; p = 0.005) and WMFT paretic UE (95% CI, –0.37 ––0.04; p = 0.02). Between-groups comparison of changes from baseline to immediately post-intervention revealed significantly more improvement on FMA (95% CI, 1.6–12.7; p = 0.016) for the atomoxetine group compared with the placebo group. No between-groups differences were found at 1-month follow-up.
4Discussion
The present study was the first-ever investigation of the safety and efficacy of atomoxetine paired with motor training for people with moderate-to-severe deficit in movement function after stroke. Findings constituted novel evidence that atomoxetine has a safe cardiovascular profile and preliminary efficacy to support clinically meaningful outcomes of intensive task-oriented UE motor training after stroke, even in cases of chronic, moderate-to-severe impairment.
A previous case series study of 3 subjects with chronic, mild movement deficit after stroke showed that atomoxetine paired with not only motor training but also non-invasive neuromodulation (repetitive transcranial magnetic stimulation (rTMS)) may improve FMA and WMFT, as evident at post-intervention and at 1-month follow-up (Kinoshita et al., 2016). However, atomoxetine was delivered concomitantly with other interventions; and there was no control group. Additionally, there was no systematic evaluation of cardiovascular or safety data. In contrast, the present study demonstrated that the effects of atomoxetine on blood pressure and heart rate were not significantly different from placebo; and no serious adverse events occurred in either group. Additionally, although both groups showed significantly improved movement function immediately after intervention, the atomoxetine group showed significantly more improvement on the primary outcome measure (ie, FMA) than the placebo group. Notably, the estimated mean FMA and ARAT change exceeded MCID only for the atomoxetine group, both at post-intervention and at 1-month follow-up.
The present study’s limitations included small sample size. A larger sample size is recommended for future studies. Additionally, examining the effects of atomoxetine beyond 1 month could substantiate the drug’s long-term efficacy. Also, as the present study administered a daily dose of 40 mg during the 10-day intervention, future studies should conduct a dose-escalation study of atomoxetine in order to achieve the optimal dose according to each participant’s weight. The optimal dose for treatment of ADHD is 1.2 mg/kg/ day, which in a 70 kg individual corresponds to 80 mg (Simpson & Perry, 2003).
Future studies are recommended to expand thescope of the present study. For example, variablesaffecting functional recovery may be particularlyamenable to change during the sub-acute stage of stroke recovery. Given the positive influence of early therapy on UE movement function after stroke (Wolf et al., 2006), as well as the efficacy of atomoxetine to improve UE movement function in healthy subjects (Foster et al., 2006), it would be worthwhile to explore the impact of atomoxetine paired with motor training during sub-acute stroke recovery. Other recommended research should examine effects of drugs other than atomoxetine that increase central levels of norephinephrine. For example, reboxetine is a norepinephrine reuptake inhibitor that has exhibited positive short-term effects on movement function in cases of chronic stroke (Wang et al., 2011; Zittel, Weiller, & Liepert, 2007), but further research is needed to establish that these benefits are long-lasting. The alpha 2 antagonist atipamezole has been associated with improvement of movement function in acute post-stroke stages, but research on its effects in chronic stroke is lacking (Beltran, Papadopoulos, Tsai, Kartje, & Wolf, 2010). Future research that systematically compares the effects of these drugs (which have a similar selective mechanism of action as atomoxetine) with the effects of atomoxetine would help to establish which of these drugs may have the most beneficial and safe impact on outcomes of intensive task-oriented UE motor training for people with chronic, moderate-to-severe impairment after stroke.
Finally, neuroimaging evidence may be crucial to advance understanding of how the noradrenergic effects of atomoxetine may influence neuroplastic change after stroke. However, there is a scant evidence base in this regard. Yamada and colleagues evaluated the effects of atomoxetine combined with intensive speech therapy in 4 participants with post-stroke aphasia. All participants demonstrated improved language function measured by the Western Aphasia Battery and the Token Test. Improvements were associated with increased perilesional blood flow using single photon emission computed tomography (Yamada, Kakuda, Yamamoto, Momosaki, & Abo, 2016). Separate studies have evaluated off-label use of atomoxetine in subjects with Parkinson’s disease. For instance, Rae and colleagues conducted a double-blind, placebo-controlled, randomized crossover study in 10 participants with moderate idiopathic Parkinson’s disease. While there was inter-individual variability, increased functional brain network activity indicatedimproved modulation of frontal-subcortical areas for response inhibition. Additionally, investigators found increased sensitivity of the inferior frontal gyrus to afferent inputs originating from the pre-supplementary motor cortex. The investigators suggested that the selective effects of atomoxetine may have partially restored noradrenergic denervation that may play a role in impaired inhibitory responses in Parkinson’s disease (Rae et al., 2016). Borchert and colleagues performed an fMRI study to evaluate the effects of atomoxetine in 33 participants with idiopathic Parkinson’s disease. They found that atomoxetine increased connectivity from the right inferior frontal gyrus to the dorsal anterior cingulate. Change was associated with change in a simple measure of executive function (Borchert et al.,2016).
In conclusion, findings of the present study indicated that atomoxetine appears safe to pair with motor training in the context of stroke rehabilitation. Furthermore, atomoxetine appears to have potential to optimize clinical outcomes of intensive task-oriented training for people with chronic, moderate-to-severely impaired movement function after stroke. Overall, given the promising results of the present feasibility study, a full-scale investigation with expanded outcome measures, including measures of neuroplastic change, is warranted.
Disclosures
This study was funded by the Dana Foundation and the Cardinal Hill Endowment for Stroke and Spinal Cord Injury Research (1215375670). There are no financial benefits to the authors. There are no conflicts of interest related to this research or this manuscript. The registration number with clinicaltrials.gov is NCT02788357.
Acknowledgments
We extend our heartfelt appreciation to our study participants, to Dr. David Jackson for referrals, and to our research assistants, Daniel Aken and Candy Pettry.
References
1 | Altman D.G. ((2000) ). Statistics in medical journals: Some recent trends. Statistics in Medicine, 19: (23), 3275–3289. |
2 | Beltran E.J. , Papadopoulos C.M. , Tsai S.Y. , Kartje G.L. , & Wolf W.A. ((2010) ). Long-term motor improvement after stroke is enhanced by short-term treatment with the alpha-2 antagonist, atipamezole. Brain Research, 1346: , 174–182. doi: 10.1016/j.brainres.2010.05.063 |
3 | Bonifer N. , & Anderson K.M. ((2003) ). Application of constraint-induced movement therapy for an individual with severe chronic upper-extremity hemiplegia. Physical Therapy, 83: (4), 384–398. |
4 | Borchert R.J. , Rittman T. , Passamonti L. , Ye Z. , Sami S. , Jones S.P. ,... & Rowe J.B. ((2016) ). Atomoxetine Enhances Connectivity of Prefrontal Networks in Parkinson’s Disease. Neuropsychopharmacology, 41: (8), 2171–2177. doi: 10.1038/n2016.18 |
5 | Boroojerdi B. , Ziemann U. , Chen R. , Butefisch C.M. , & Cohen L.G. ((2001) ). Mechanisms underlying human motor system plasticity. Muscle & Nerve, 24: (5), 602–613. |
6 | Butefisch C.M. , Davis B.C. , Sawaki L. , Waldvogel D. , Classen J. , Kopylev L. , & Cohen L.G. ((2002) ). Modulation of use-dependent plasticity by d-amphetamine. Annals of Neurology, 51: (1), 59–68. |
7 | Classen J. , Liepert J. , Wise S.P. , Hallett M. , & Cohen L.G. ((1998) ). Rapid plasticity of human cortical movement representation induced by practice. Journal of Neurophysiology, 79: (2), 1117–1123. |
8 | Duncan P.W. , Propst M. , & Nelson S.G. ((1983) ). Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Physical Therapy, 63: (10), 1606–1610. |
9 | Eissa A. , Krass I. , & Bajorek B.V. ((2012) ). Optimizing the management of acute ischaemic stroke: A review of the utilization of intravenous recombinant tissue plasminogen activator (tPA. Journal of Clinical Pharmacy and Therapeutics, 37: (6), 620–629. doi: 10.1111/j.1365-2710.2012.01366.x |
10 | Feeney D.M. , & Sutton R.L. ((1987) ). Pharmacotherapy for recovery of function after brain injury. Critical Reviews in Neurobiology, 3: , 135–197. |
11 | Floel A. , Breitenstein C. , Hummel F. , Celnik P. , Gingert C. , Sawaki L. ,... & Cohen L.G. ((2005) ). Dopaminergic influences on formation of a motor memory. Annals of Neurology, 58: (1), 121–130. |
12 | Foster D.J. , Good D.C. , Fowlkes A. , & Sawaki L. ((2006) ). Atomoxetine enhances a short-term model of plasticity in humans. Archives of Physical Medicine and Rehabilitation, 87: (2), 216–221. doi: 10.1016/j.apmr.2005.08.131 |
13 | Goldstein L.B. , & Davis J.N. ((1990) ). Restorative neurology. Drugs and recovery following stroke. Stroke; A Journal of Cerebral Circulation, 21: (11), 1636–1640. |
14 | Hayward K. , Barker R. , & Brauer S. ((2010) ). Interventions to promote upper limb recovery in stroke survivors with severe paresis: A systematic review. Disability and Rehabilitation, 32: (24), 1973–1986. doi: 10.3109/09638288.2010.481027 |
15 | Kenward M.G. , & Roger J.H. ((1997) ). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics, 53: (3), 983–997. doi: 10.2307/2533558 |
16 | Kinoshita S. , Kakuda W. , Yamada N. , Momosaki R. , Okuma R. , Watanabe S. , & Abo M. ((2016) ). Therapeutic administration of atomoxetine combined with rTMS and occupational therapy for upper limb hemiparesis after stroke: A case series study of three patients. Acta Neurologica Belgica, 116: (1), 31–37. doi: 10.1007/s13760-015-0503-3 |
17 | Kleim J.A. , & Jones T.A. ((2008) ). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech Language and Hearing Research, 51: (1), S225–S239. doi: 10.1044/1092-4388(2008/018) |
18 | Mozaffarian D. , Benjamin E.J. , Go A.S. , Arnett D.K. , Blaha M.J. , Cushman M. ,... & Stroke Statistics S. ((2015) ). Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation, 131: (4), e29–e322. doi: 10.1161/CIR.0000000000000152 |
19 | Nudo R.J. , Plautz E.J. , & Frost S.B. ((2001) ). Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle & Nerve, 24: (8), 1000–1019. doi: 10.1002/mus.1104 |
20 | Pandian S. , & Arya K.N. ((2014) ). Stroke-related motor outcome measures: Do they quantify the neurophysiological aspects of upper extremity recovery?. Journal of Bodywork and Movement Therapies, 18: (3), 412–423. doi: http://dx.doi.org/10.1016/j.jbmt.2013.11.006 |
21 | Perneger T.V. ((1998) ). What’s wrong with Bonferroni adjustments. Bmj, 316: (7139), 1236–1238. |
22 | Rae C.L. , Nombela C. , Rodriguez P.V. , Ye Z. , Hughes L.E. , Jones P.S. ,... & Rowe J.B. ((2016) ). Atomoxetine restores the response inhibition network in Parkinson’s disease. Brain: A Journal of Neurology, 139: (Pt 8), 2235–2248. doi: 10.1093/brain/aww138 |
23 | Sawaki L. , Boroojerdi B. , Kaelin-Lang A. , Burstein A.H. , Butefisch C.M. , Kopylev L. ,... & Cohen L.G. ((2002) ). Cholinergic influences on use-dependent plasticity. Journal of Neurophysiology, 87: (1), 166–171. |
24 | Sawaki L. , Butler A.J. , Leng X. , Wassenaar P.A. , Mohammad Y.M. , Blanton S. ,... & Wittenberg G.F. ((2014) ). Differential patterns of cortical reorganization following constraint-induced movement therapy during early and late period after stroke: A preliminary study. NeuroRehabilitation. doi: 10.3233/NRE-141132 |
25 | Sawaki L. , Werhahn K.J. , Barco R. , Kopylev L. , & Cohen L.G. ((2003) ). Effect of an alpha(1)-adrenergic blocker on plasticity elicited by motor training. Experimental Brain Research, 148: (4), 504–508. |
26 | Simpson D. , & Perry C.M. ((2003) ). Atomoxetine.; discussion. Paediatric Drugs, 5: (6), 416–407. |
27 | van der Lee J.H. , Beckerman H. , Lankhorst G.J. , & Bouter L.M. ((2001) ). The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. Journal of Rehabilitation Medicine, 33: (3), 110–113. |
28 | Walker-Batson D. , Curtis S. , Natarajan R. , Ford J. , Dronkers N. , Salmeron E. ,... & Unwin D.H. ((2001) ). A double-blind, placebo-controlled study of the use of amphetamine in the treatment of aphasia. Stroke; A Journal of Cerebral Circulation, 32: (9), 2093–2098. |
29 | Wang L.E. , Fink G.R. , Diekhoff S. , Rehme A.K. , Eickhoff S.B. , & Grefkes C. ((2011) ). Noradrenergic enhancement improves motor network connectivity in stroke patients. Annals of Neurology, 69: (2), 375–388. doi: 10.1002/ana.22237 |
30 | Whitall J. , Savin D.N. Jr , Harris-Love M. , & Waller S.M. ((2006) ). Psychometric properties of a modified Wolf Motor Function test for people with mild and moderate upper-extremity hemiparesis. Archives of Physical Medicine and Rehabilitation, 87: (5), 656–660. doi: 10.1016/j.apmr.2006.02.004 |
31 | Wolf S.L. , Winstein C.J. , Miller J.P. , Taub E. , Uswatte G. , Morris D. ,... & Nichols-Larsen D. ((2006) ). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA: The Journal of the American Medical Association, 296: (17), 2095–2104. doi: 10.1001/jama.296.17.2095 |
32 | Yamada N. , Kakuda W. , Yamamoto K. , Momosaki R. , & Abo M. ((2016) ). Atomoxetine administration combined with intensive speech therapy for post-stroke aphasia: Evaluation by a novel SPECT method. The International Journal of Neuroscience, 126: (9), 829–838. doi: 10.3109/00207454.2015.1074226 |
33 | Zittel S. , Weiller C. , & Liepert J. ((2007) ). Reboxetine improves motor function in chronic stroke. A pilot study. Journal of Neurology, 254: (2), 197–201. doi: 10.1007/s00415-006-0326-5 |
Figures and Tables
Fig.1
Participant flow. Each stage of the study is represented, from eligibility assessment through analysis of final evaluation data. The number of randomly assigned subjects who received intended treatment in each group is shown, along with losses to follow-up.
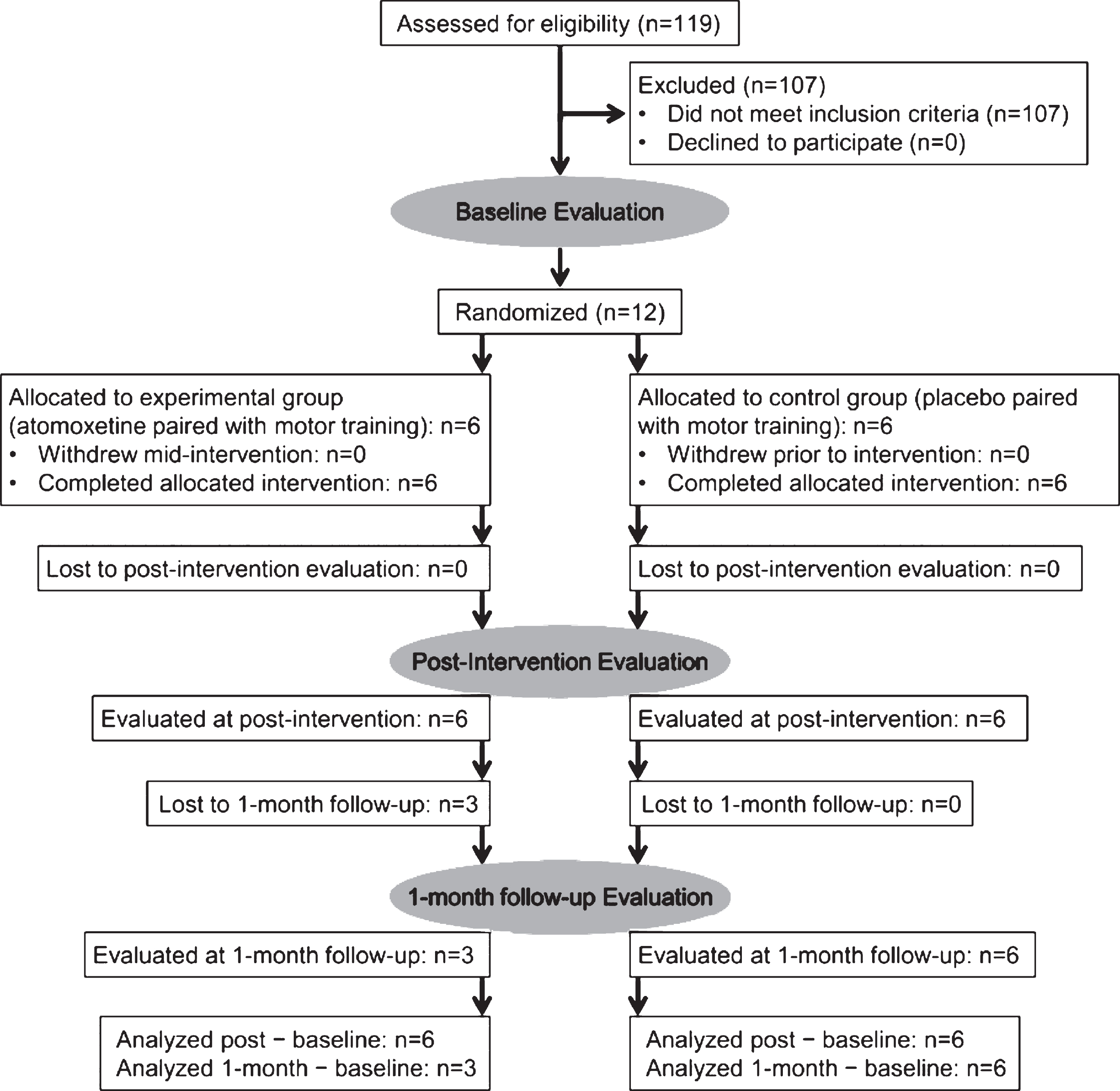
Fig.2
Vital signs by group as a function of time post dose. No significant between-groups differences were evident for (A) heart rate, (B) systolic blood pressure, or (C) diastolic blood pressure.
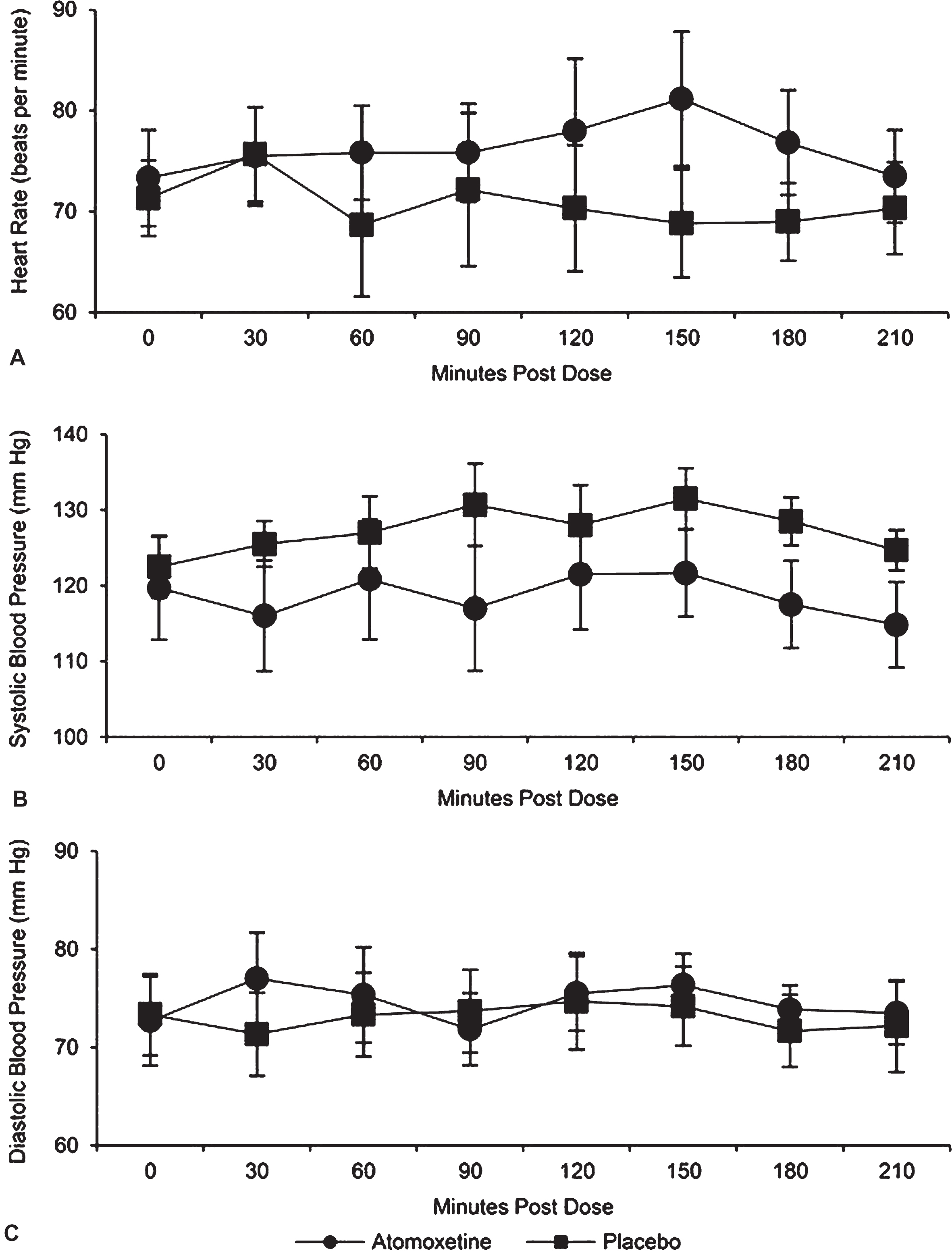
Table 1
Clinical characteristics and demographic data
| Subject | Age (years) | Group | Sex | Affected Hemisphere | Stroke Type | Months Post-Stroke |
| 1 | 39 | ATM | M | Right | Ischemic | 52 |
| 2 | 35 | ATM | F | Left | Ischemic | 28 |
| 3 | 50 | ATM | F | Left | Ischemic | 25 |
| 4 | 65 | ATM | F | Left | Ischemic | 19 |
| 5 | 49 | ATM | M | Left | Ischemic | 19 |
| 6 | 65 | ATM | M | Left | Ischemic | 24 |
| ATM | 50.5 ± 12.6 | 3F/3M | 5Left/1Right | 6 ischemic | 27.7 ± 12.4 | |
| 7 | 58 | Placebo | M | Left | Ischemic | 16 |
| 8 | 53 | Placebo | M | Right | Ischemic | 37 |
| 9 | 66 | Placebo | F | Left | Ischemic | 6 |
| 10 | 66 | Placebo | F | Right | Ischemic | 48 |
| 11 | 58 | Placebo | M | Left | Hemorrhagic | 8 |
| 12 | 58 | Placebo | F | Right | Hemorrhagic | 13 |
| Placebo | 59.8 ± 5.2 | 3F/3M | 3Left/3Right | 4 ischemic/2 Hemorrhagic | 21.3 ± 17.1 |
Abbreviations: ATM, Atomoxetine; F, Female; M, Male.
Table 2
Daily log of subjects’ complaints
| Subject | Group | Reported Complaints |
| 1 | Atomoxetine | Mental fatigue on days 6 and 8 |
| (ATM) | ||
| 2 | ATM | None |
| 3 | ATM | Sleepiness and fatigue during first 4 days |
| 4 | ATM | Slightly fatigued on days 2 and 5 |
| 5 | ATM | None |
| 6 | ATM | Slightly fatigued at the end of all sessions |
| 7 | Placebo | Headache on day 9 |
| 8 | Placebo | Fatigued day 4 |
| 9 | Placebo | Dizziness on day 1 |
| 10 | Placebo | None |
| 11 | Placebo | None |
| 12 | Placebo | None |
Table 3
Group scores at baseline (sample mean±standard deviation). Sample size was 6 in each group
| Outcome measures for upper | Baseline scores | Baseline scores |
| extremity (UE) movement | atomoxetine | for placebo |
| function | group | group |
| Fugl-Meyer Assessment | 29.3±11.3 | 39.8±21.3 |
| (points) | ||
| Action Research Arm Test | 19.2±12.8 | 29.3±23.0 |
| (points) | ||
| Wolf Motor Function Test | ||
| (log[sec]): | ||
| more affected UE | 1.53±0.48 | 1.20±0.75 |
| less affected UE | 0.53±0.39 | 0.56±0.35 |
Table 4
Estimated means, 95% confidence intervals, and p-values corresponding to mean change from fitting a single repeated measures model for each variable
| Outcome measures of | Post-intervention – Baseline | 1-month follow-up – Baseline | |||||
| upper extremity (UE) | Atomoxetine | Placebo | ATM– | ATM | Placebo | ATM– | |
| movement function | (ATM) | Placebo | Placebo | ||||
| Fugl-Meyer Assessment | 9.3 | 2.2 | 7.2 | 11.9 | 5.8 | 6.1 | |
| (points) | (5.4, 13.2) | (–1.7, 6.1) | (1.6, 12.7) | (5.9, 17.9) | (1.5, 10.2) | (–1.3, 13.5) | |
| p = 0.0003 | p = 0.24 | p = 0.016 | p = 0.002 | p = 0.015 | p = 0.10 | ||
| Action Research Arm | 7.7 | 5.3 | 2.3 | 10.1 | 6.5 | 3.6 | |
| Test (points) | (4.6, 10.7) | (2.3, 8.4) | (–1.9, 6.6) | (5.1, 15.1) | (2.6, 10.4) | (–2.8, 10.0) | |
| p = 0.0002 | p = 0.003 | p = 0.25 | p = 0.001 | p = 0.005 | p = 0.24 | ||
| Wolf Motor Function Test | –0.10 | –0.19 | 0.09 | –0.11 | –0.20 | 0.10 | |
| (WMFT) more affected | (–0.26, 0.06) | (–0.35, –0.03) | (–0.13, 0.31) | (–0.28, 0.06) | (–0.37, –0.04) | (–0.14, 0.34) | |
| UE (log[sec]) | p = 0.18 | p = 0.02 | p = 0.39 | p = 0.19 | p = 0.02 | p = 0.40 | |
| WMFT less affected | 0.01 | –0.13 | 0.13 | 0.01 | –0.07 | 0.09 | |
| (log[sec]) | (–0.13, 0.15) | (–0.26, 0.01) | (–0.06, 0.33) | (–0.22, 0.25) | (–0.28, 0.14) | (–0.23, 0.40) | |
| p = 0.91 | p = 0.07 | p = 0.16 | p = 0.90 | p = 0.46 | p = 0.56 | ||
For Fugl-Meyer Assessment and Action Research Arm test, an increase represents improvement. For WMFT, a decrease represents improvement.



