Pulmonary adenocarcinoma of low malignant potential defines indolent NSCLC associated with overdiagnosis in the national lung screening trial
Abstract
BACKGROUND:
The national lung screening trial (NLST) demonstrated a reduction in lung cancer mortality with lowdose CT (LDCT) compared to chest x-ray (CXR) screening. Overdiagnosis was high (79%) among bronchoalveolar carcinoma (BAC) currently replaced by adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) and adenocarcinoma of low malignant potential (LMP) exhibiting 100% disease specific survival (DSS).
OBJECTIVE:
Compare the outcomes and proportions of BAC, AIS, MIA, and LMP among NLST screendetected stage IA NSCLC with overdiagnosis rate.
METHODS:
Whole slide images were reviewed by a thoracic pathologist from 174 of 409 NLST screen-detected stage IA LUAD. Overdiagnosis rates were calculated from follow-up cancer incidence rates.
RESULTS:
Most BAC were reclassified as AIS/MIA/LMP (20/35
CONCLUSIONS:
AIS/MIA/LMP proportionally matches the overdiagnosis rate among stage IA NSCLC in the NLST, exhibiting 100% 7-year DSS. Biomarkers designed to recognize AIS/MIA/LMP preoperatively, would be useful to prevent overtreatment of indolent screen-detected cancers.
1.Introduction
The national lung screening trial (NLST) demonstrated an overall mortality reduction of 20% at a median of 6.5-years after three annual lowdose CT-screenings (LDCT) compared to the control arm screened by chest X-ray (CXR) among high-risk smokers [1]. Mortality reduction was associated with a stage shift towards the detection of early-stage disease whereby the majority of NSCLC are curable by surgery alone. Two follow-up studies have reported the frequency of overdiagnosis, defined as the excess cancers in the CXR-arm presenting clinically after screening cessation compared to the LDCT-arm at median time points of 6.4-years and 11.3-years [2, 3]. The overdiagnosis rate for all cancers was reported as 18.5% at 6.4-years and 3.1% at 11.3-years; NSCLC as 22.5% at 6.4-years (not specifically reported at 11.3-years); and BAC as 78.9% at both the 6.4-year and 11.3-year time period.
Since the study period of the NLST, BAC was abandoned as a pathologic entity and replaced by the more specifically defined entities of adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), lepidic predominant adenocarcinomas, and invasive mucinous adenocarcinoma (IMA) [4]. AIS and MIA together exhibit long-term 100% DSS whereas lepidic predominant and IMA are regarded as low and intermediate grade cancers respectively [5, 6]. While BAC comprised 27% of all screen-detected adenocarcinoma in the CTLS-arm, AIS and MIA together comprise
While no central pathologic review was performed in the NLST, H&E-stained slides were subsequently digitized from a subcomponent of the NLST. Using this digitized subset, the aims of this study are to determine the frequency of AIS/MIA/LMP among screen-detected stage IA NSCLC in the CTLS and CXR-arms of the NLST and determine their relationship to the historically classified entity BAC. We further seek to determine the prognostic significance of BAC as it relates to more specifically defined pathologic entities and to better understand its association with overdiagnosis among NSCLC.
2.Methods
2.1Patients and samples
Details of the NLST have been published previously [1, 2, 3]. Briefly adults aged 55–74 years of age with a minimum of 30 pack-years of cigarette smoking and who were either current or former smokers who had quit within the past 15-years were enrolled between 2002–2004 at 33 United States based medical institutions and randomized to receive three annual protocol screens of either LDCT (26,722 participants) or single-view CXR (26,730 participants). CXR does not reduce lung cancer mortality compared to routine community care and was therefore deemed an appropriate control arm [10]. Participants were excluded prior to randomization if they had unexplained weight loss or hemoptysis in the prior year, a CT-scan 18-months prior to enrollment, or a history of lung cancer. Screendetected lung cancers were distinguished from non-screen detected cancers if they were diagnosed within 1-year of a positive screen with no intervening negative screens or
Screen-detected cancers were staged using the 6th edition of the American Joint Committee on Cancer (AJCC). There were 649 screen-detected cancers in the LDCT-arm of which 591 were NSCLC (95 BAC & 496 non-BAC NSCLC) and 279 screen-detected cancers in the CXR-arm of which 247 were NSCLC (13 BAC & 234 non-BAC NSCLC). Cases of SCLC (LDCT 49 & CXR 28) and carcinoid (LDCT 5 & CXR 1) were excluded. We additionally excluded all stage IB-IV, cases of NSCLC of unknown stage (LDCT 5 & CXR 2), stage IA carcinomas of unknown histologic type designated by ICD-0-3 code 8000 (LDCT 2 & CXR 1) and cases where neither histologic type nor stage were known (LDCT 1 & CXR 0); yielding 325 and 84 stage IA NSCLC for analysis in the LDCT and CXR-arms respectively. Whole slide images (WSI) were previously generated from archived FFPE tumor blocks from 463 patients derived from one of the NLST screening networks (Lung Screening Study Network, LSS) which included 10 screening centers enrolling 34,612 participants that detected lung cancer in 1,284 participants. The WSI and linked but anonymized clinical and pathologic annotations were made available for download from the National Cancer Institute Cancer Data Access System after an approved application (Project ID NLST-867). All participants enrolling in the NLST signed an informed consent developed and approved by the institutional review board (IRB) at each screening site. Among screen-detected stage IA NSCLC, there were 182 with WSI of which 174 had tumor sufficient for classification. There were a median of 2 digitized tumor slides per patient (range 1–5).
2.2Histopathological analysis
All WSI were reviewed by a single experienced thoracic pathologist (EJB). Adenocarcinoma in situ (AIS) was rendered for purely lepidic tumors
2.3Survival, overdiagnosis and statistical analysis
Disease-specific survival (DSS), defined as time from surgery to death from lung cancer or time of last follow-up (unrelated deaths censored at time of event) was estimated using the Kaplan-Meier method comparing groups with the log-rank test. The probability of a screen-detected cancer being an overdiagnosis was calculated as previously described [2] where the excess number of total cancers (screen & non-screen detected) in the LDCT compared to CXR-arms previously reported at the end of two time periods (2009 and 2014) are divided by the number of screen-detected cancers from the original report. Statistical analyses were performed using SPSS version 28 (IBM). Chi Square Test for Homogeneity or the Fisher’s Exact test were used for categorical variables, as appropriate. Post hoc analysis involved pairwise comparisons using the z-test of two proportions with a Bonferroni correction. Continuous variables were compared between groups using Welch’s
3.Results
3.1Cohort comparison
Table 1
Clinicopathologic characteristics of NLST stage IA screen-detected NSCLC
| Variable | LDCT | CXR |
|
|---|---|---|---|
| Number | 325 | 84 | |
| Male sex | 180 (55) | 47 (56) | 0.926 |
| Age, median (IQR) | 63 (59–67) | 64 (60–68) | 0.266 |
| Current smoker | 168 (52) | 48 (57) | 0.372 |
| History of COPD | 37 (11) | 6 (7) | 0.259 |
| Race | 0.417 | ||
| White | 301 (93) | 76 (91) | |
| Black | 15 (5) | 7 (8) | |
| Asian | 5 (1) | 1 (1) | |
| Other | 4 (1) | 0 | |
| Historic classification | 0.024 | ||
| BAC | 74 (23) | 7 (8) | |
| LUAD | 141 (43) | 40 (48) | |
| LUSC | 67 (21) | 23 (27) | |
| LC | 14 (4) | 3 (4) | |
| NSCLC & Other | 29 (9) | 11 (13) | |
| Time to Cancer Dx, years, median (IQR) | 1.3 (0.3–2.3) | 1.1 (0.2–2.1) | 0.233 |
| Follow up, years, median (IQR) | 6.6 (6.2–7.0) | 6.5 (5.2–6.9) | 0.034 |
| 7-year DSS, %, (95% CI) | 87 (82–90) | 79 (68–87) | 0.046 |
Note: The data are shown as the number and (%) unless otherwise indicated. Abbreviations: BAC, bronchoalveolar carcinoma; DSS, disease specific survival; LC, large cell carcinoma; LUAD, lung adenocarcinoma, LUSC, lung squamous cell carcinoma, NSCLC & Other, non-small cell lung carcinoma and cases coded as other rare subtypes of NSCLC. Small cell lung carcinoma, carcinoid tumors, and those with unknown histologic type (ICD-O-3 8000) or stage excluded. Stage IA as per AJCC 6th edition.
Table 2
Clinicopathologic comparison of stage IA screen-detected NSCLC stratified by histology
| LDCT | CXR | |||||
| Variable | Histology | No-Histology |
| Histology | No-Histology |
|
| Number | 142 | 183 | 32 | 52 | ||
| Male sex | 77 (54) | 103 (56) | 0.711 | 20 (63) | 27 (52) | 0.343 |
| Age, median (IQR) | 63 (59–67) | 63 (59–68) | 0.671 | 65 (59–68) | 64 (61–67) | 0.809 |
| Current smoker | 83 (58) | 85 (46) | 0.032 | 18 (56) | 30 (58) | 0.897 |
| History of COPD | 12 (8) | 25 (14) | 0.142 | 0 | 6 (12) | 0.078 |
| Race | 0.492 | 1.0 | ||||
| White | 133 (94) | 168 (92) | 29 (91) | 47 (90) | ||
| Black | 4 (3) | 11 (6) | 3 (9) | 4 (8) | ||
| Asian | 3 (2) | 2 (1) | 0 | 1 (2) | ||
| Other | 2 (1) | 2 (1) | 0 | 0 | ||
| Historic classification | 0.018 | 0.061 | ||||
| BAC | 31 (22) | 43 (23) | 4 (12) | 3 (6) | ||
| LUAD | 65 (46) | 76 (42) | 15 (47) | 25 (48) | ||
| LUSC | 28 (20) | 39 (21) | 12 (38) | 11 (21) | ||
| LC | 11 (7) | 3 (2) | 3 (6) | |||
| NSCLC & Other | 7 (5) | 22 (12) | 1 (3) | 10 (19) | ||
| Time to Cancer Dx, years, median (IQR) | 1.3 (0.3–2.2) | 1.3 (0.3–2.3) | 0.432 | 0.9 (0.2–2.1) | 1.1 (0.2–2.2) | 0.534 |
| Follow up, years, median (IQR) | 6.7 (6.2–7.1) | 6.5 (6.1–6.9) | 0.289 | 6.6 (5.9–7.0) | 6.2 (4.2–6.9) | 0.307 |
| 7-year DSS, %, (95% CI) | 87 (80–92) | 87 (80–91) | 0.993 | 81 (63–91) | 78 (62–88) | 0.837 |
Note: The data are shown as the number and (%) unless otherwise indicated. Abbreviations: BAC, bronchoalveolar carcinoma; DSS, disease specific survival; LC, large cell carcinoma; LUAD, lung adenocarcinoma, LUSC, lung squamous cell carcinoma, NSCLC & Other, non-small cell lung carcinoma and cases coded as other rare subtypes of NSCLC. Small cell lung carcinoma, carcinoid tumors, and those with unknown histologic type (ICD-O-3 8000) or stage excluded. Stage IA as per AJCC 6th edition.
Table 3
NLST screen detected stage IA NSCLC with histology stratified by classification
| New classification | Historic classification | ||||||
| LDCT | CXR | BAC | LUAD | LUSC | LC | NSCLC & other | |
| Total | 142 | 32 | 35 | 80 | 40 | 11 | 8 |
| AIS/MIA | 5 (3.5) | 0 | 5 | 0 | 0 | 0 | 0 |
| LMP | 18 (12.7) | 1 (3.1) | 15 | 4 | 0 | 0 | 0 |
| IMA | 4 (2.8) | 2 (6.3) | 3 | 3 | 0 | 0 | 0 |
| LUAD | 80 (56.3) | 15 (46.9) | 12 | 68 | 4 | 6 | 5 |
| LUSC | 33 (23.2) | 14 (43.8) | 0 | 4 | 36 | 4 | 3 |
| LCNEC | 2 (1.4) | 0 | 0 | 1 | 0 | 1 | 0 |
Note: The data are shown as the number and (%) and unless otherwise indicated. Abbreviations: AIS, adenocarcinoma in situ; BAC, bronchoalveolar carcinoma; LC, large cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; LMP, low malignant potential; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MIA, minimally invasive adenocarcinoma; NSCLC & Other, non-small cell lung carcinoma and cases coded as other rare subtypes of NSCLC. Small cell lung carcinoma, carcinoid tumors, and those with unknown histologic type (ICD-O-3 8000) or stage excluded. Stage IA as per AJCC 6th edition.
Table 4
Overdiagnosis calculations of NSCLC by stage and historic classification
| LDCT screen detected | Total cancers median 6.4-years | Total cancers median 11.3-years | ||||||||||
| Stage All | Stage IA | LDCT | CXR | Diff | Overdx all stage | Overdx Stage IA | LDCT | CXR | Diff | Overdx all stage | Overdx Stage IA | |
| Total NSCLC | 591 | 325 | 926 | 793 | 133 | 22.5% | 40.9% | 1397 | 1343 | 54 | 9.1% | 16.6% |
| BAC | 95 | 74 | 111 | 36 | 75 | 78.9% | 101.4% | 121 | 46 | 75 | 78.9% | 101.4% |
| NSCLC no BAC | 496 | 251 | 815 | 757 | 58 | 11.7% | 23.1% | 1276 | 1297 | |||
Abbreviations: BAC, bronchoalveolar carcinoma; CXR, chest X-ray arm; Diff, difference of LDCT – CXR arms at specified time points; LDCT, low-dose CT-arm; NSCLC, non-small cell lung cancer; Overdx, overdiagnosis. Stage IA as per AJCC 6th edition. All stage screen-detected NSCLC and total cancers at median of 6.4-years and 11.3-years from published reports as described in the methods section.
The proportion of screen-detected stage IA NSCLC among total screen-detected NSCLC was significantly greater in the LDCT-arm than the CXR-arm (325/591
3.2Histologic classification comparison
Table 3 shows the proportions of tumors histologically classified as indolent by the historic compared to the newer pathologic classification. AIS/MIA comprised a minority of stage IA cancers in the LDCT-arm (3.5%) and were not observed in the CXR-arm. LMP comprised 12.7% of the LDCT-arm but only 3.1% of the CXR-arm. The majority of BAC were AIS/MIA/LMP (20/35
Table 5
Overdiagnosis pathologic correlation among stage IA screen-detected NSCLC
| Pathologically predicted | Actual at median 11.3-years | |||||
|---|---|---|---|---|---|---|
| Category | 7-year DSS | Denominator | Overdiagnosis | Denominator | Overdiagnosis |
|
| AIS/MIA | 100% | 142 | 5 (3.5) | 325 | 54 (16.6) | |
| AIS/MIA/LMP | 100% | 142 | 23 (16.2) | 325 | 54 (16.6) | 0.911 |
| BAC | 94% | 325 | 74 (22.8) | 325 | 54 (16.6) | 0.049 |
Note: The data are shown as the number and (%). Abbreviations: AIS, adenocarcinoma in situ, BAC, bronchoalveolar carcinoma, LMP, low malignant potential, MIA, minimally invasive adenocarcinoma. Stage IA as per AJCC 6th edition.
Figure 1.
Kaplan Meier Curves showing disease specific survival of screen-detected Stage IA NSCLC. (A) Total non-BAC NSCLC stratified by historic classification, (B) Total BAC vs. non-BAC NSCLC, (C) WSI BAC vs. non-BAC NSCLC, (D) WSI excluding AIS/MIA/LMP and remaining BAC vs. non-BAC NSCLC.
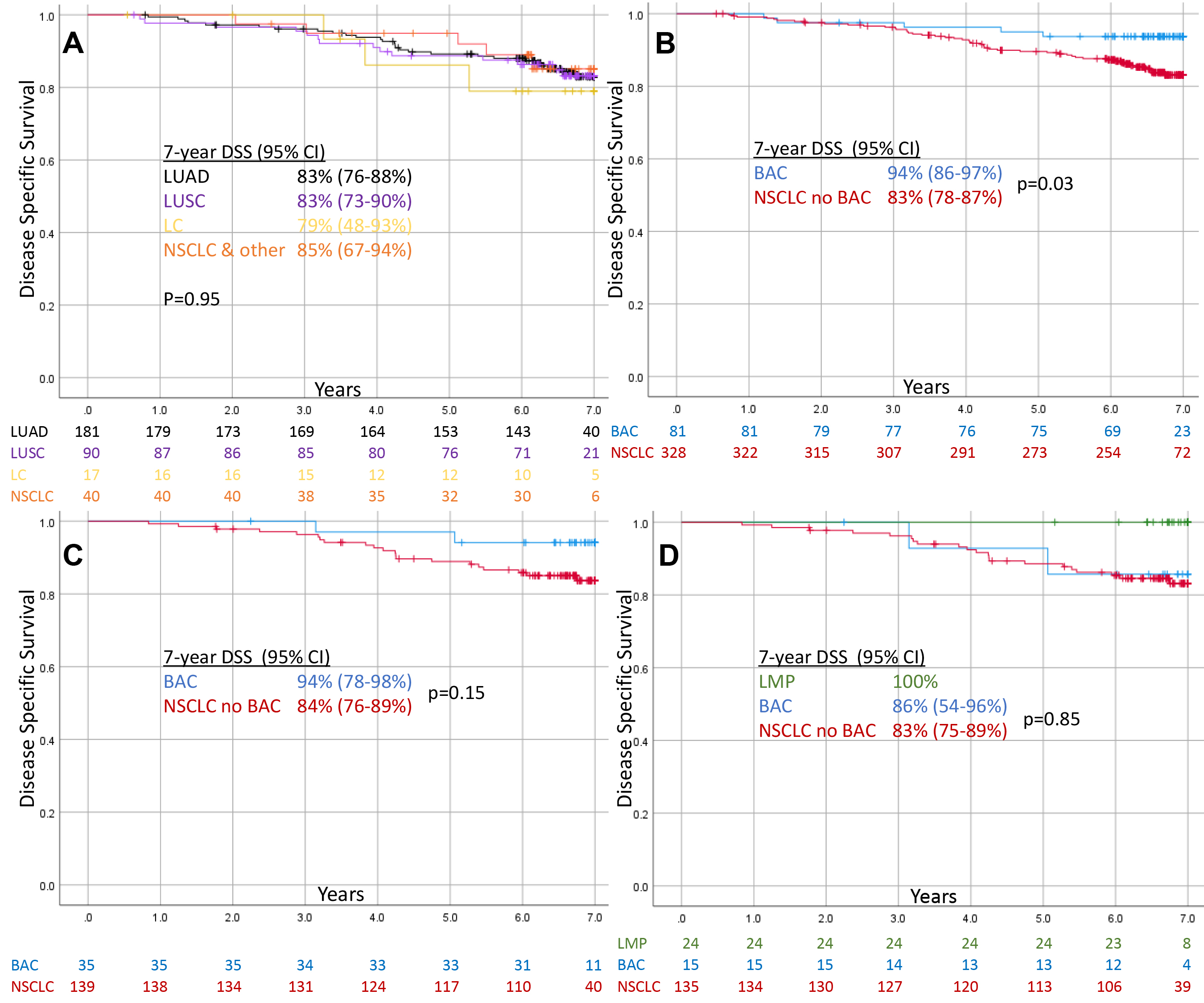
3.3Lung cancer specific survival
Figure 1 shows DSS for AJCC 6th ed. Stage IA NSCLC. Historic classifications of LUAD, LUSC, LC, and NSCLC & Other showed similar 7-year DSS (79–85%,
3.4Overdiagnosis rate and pathologic associations
The overdiagnosis rates of NSCLC and the data from which they are derived from are shown in Table 4. The calculated overdiagnosis rate for all stage NSCLC (22.5%) and non-BAC NSCLC (11.7%) at 6.4-years and BAC (78.9%) at both 6.4- and 11.3-years are identical to previously published results [2, 3]. Using the same formula, we calculate overdiagnosis rate for AJCC 6th ed. stage IA tumors as these were the group most overrepresented in the LDCT compared to CXR-arms given the size of these tumors limits their detection by CXR alone. The overdiagnosis rate for stage IA NSCLC dropped from 40.9% at 6.4-years to 16.6% at 11.3-years. No excess BAC were detected from 6.4- to 11.3-years, remaining at 75 in spite of the fact that this number exceeded the total excess NSCLC of 54. A similar erroneous observation of 21 fewer non-BAC NSCLC in the LDCT compared to CXR-arm raise concerns about the comparability of these diagnostic categories over the follow-up period of this study. As such, the calculated overdiagnosis rates of BAC vs. non-BAC NSCLC (stage IA: 101.4% vs.
Table 5 shows the proportion of pathologically predicted indolent screen-detected NSCLC in the LDCT-arm compared to the observed overdiagnosis rate. The combined proportion of tumors classified as AIS/MIA/LMP closely approximates the calculated overdiagnosis rate of stage IA NSCLC (16.2% vs. 16.6%) and importantly exhibited 100% 7-years DSS. In contrast, the combined proportions of AIS/MIA fell short of the observed overdiagnosis rate (3.5% vs. 16.6%,
4.Discussion
Overdiagnosis is a major problem in cancer screening programs as it leads to patient anxiety associated with a malignant diagnosis, unnecessary procedures with associated risks, and costs to the health care system [11, 12]. It has been proposed that indolent screen-detected cancers be reclassified as indolent lesions of epithelial origin (IDLE) [13]; however such terminology is challenging for pathologists to incorporate given that precise classification generally requires complete excision of a tumor which alters the natural history of the disease, i.e. 100% DSS after resection is not equivalent to 100% DSS without treatment. Aiming at the intent of the IDLE proposal, we proposed to expand the histologic spectrum of indolent adenocarcinoma beyond AIS/MIA to include a group of tumors termed LMP; a subset with identical behavior as determined by 100% long-term DSS after surgery shown in two previously published cohorts [7, 14]. In the present study, we sought to advance this concept by determining the rate of AIS/MIA/LMP among a subset of screen-detected stage IA NSCLC from the LDCT-arm of the NLST with digitized images, confirm their indolent behavior after surgery, and compare this to the calculated overdiagnosis rate derived epidemiologically with extended follow-up. AIS/MIA/LMP tumors in the NLST exhibited 100% 7-year DSS and were nearly identical in proportion to the epidemiologically calculated overdiagnosis rate of stage IA NSCLC at median follow-up of 11.3-years (16.2% vs. 16.6%).
Overdiagnosis rates for all lung cancer at median of 6.4-years has been reported as 18.5% and 3.1% at 11.3-years [2, 3]. Given the rapid growth rate of SCLC, the goal of annual lung cancer CT-screening is early detection and treatment of clinically significant NSCLC. As such, the overdiagnosis rates of NSCLC are most relevant for cancer screening and has been reported at 22.5% at 6.4-years, dropping to 9.1% at 11.3-years for all stage disease. Given that overdiagnosis must correlate with early stage (non-metastatic) lesions visible by CT-scans but not routine CXR, the AJCC 6th ed. Stage IA (
In the screening period and initial follow-up of the NLST cohorts, BAC was the category of NSCLC most associated with indolent behavior. As such, overdiagnosis rates for all stage BAC have been reported as 78.9% at both 6.4 and 11.3-years of median follow-up [2, 3]. There are at least three problems with this conclusion. First, in 2011 – during the period of extended (passive) follow-up of the NLST cohorts – the IASLC/ATS/ERS proposed a new histologic classification for lung adenocarcinoma [15]in which BAC was abandoned and replaced with AIS, MIA, IMA, and lepidic predominant adenocarcinoma. As such, the low frequency of additional BAC in both the LDCT and CXR-arms (10 cases each) during the extended follow-up between 2009 and 2014 may reflect adoption of a new classification rather than a true change in BAC incidence. Second, not all BAC behaved indolently, with 6% of stage IA BAC dying of lung cancer at 7-years after surgery and thus precluding such an entity defining indolent/non-progressive cancer. Third, the most consistently applied histologic feature separating BAC from LUAD was the presence of a lepidic component, confirmed in this cohort (
AIS/MIA/LMP exhibited 100% 7-year DSS and occurred in a proportion nearly identical to the overdiagnosis rate (16.2% vs. 16.6%). In contrast, the proportion of AIS/MIA was significantly less than (3.5%) and the proportion of BAC was significantly more than (22.8%) than the observed overdiagnosis rate (16.6%). Moreover, we show that once AIS/MIA/LMP are removed, BAC behaves similarly to all other NSCLC (7-year DSS: 86% vs. 83%,
Herein, we document an additional 19 cases of LMP which when combined with our previous reports totals 110 cases of LMP with long-term 100% DSS. More recently, LMP exclusive of AIS/MIA was found to comprise 12.4% of an Italian cohort of 274 stage IA LUAD [8]. Of the 34 LMP described, 5 recurred (14.7%) with 2 dying of lung cancer. Molecular confirmation of matched driver mutation was confirmed in only 1 case while 3 cases were late recurrences (6–9 years after surgery) raising the possibility of second primary lung cancers which are known to occur in 15–18% of patients within 7-years of treatment for a primary lung cancer [9, 16, 17]. Alternatively, some of these may be slow-growing local recurrences. We have observed only a single recurrence of an LMP, occurring at the staple line 1-year after wedge resection with a close surgical margin. The patient is alive and without metastatic spread
One goal of pathologically defining IDLE’s is for the development of biomarkers which would allow their identification prior to treatment [18]. To this aim, prior investigators have shown by radiomic analysis of CT-images that 18% of screen-detected stage I adenocarcinoma in the LDCT-arm can be predicted as “good-risk”, showing 100% 7-year DSS, using Computer-Aided Nodule Assessment and Risk Yield (CANARY) [19]. This would imply that many AIS/MIA/LMP might be predicted by radiomic features preoperatively (see Steiner et al. in this edition). Additionally, we have previously demonstrated by gene expression profiling the ability to predict aggressive histologic features, and thus exclude LMP, which we hope to apply as a tissue based biomarker [14]. A combined radiomic and tissue-based biomarker suitable for presurgical biopsies, might together provide even greater sensitivity and specificity for preoperative prediction of cancers likely to represent overdiagnosis. This knowledge could predict patients who might benefit from tissue sparing surgical approaches (wedge or segmentectomy) or those who might be better treated by non-invasive approaches such as stereotactic body radiation therapy (SBRT), cryoablation, or radiofrequency ablation. Alternatively, some of these patients might be better managed by active surveillance protocols similar to the management of low-grade prostate cancer [20].
This study has several limitations. First, the histologic assessment was only possible on the subset of screen-detected stage IA NSCLC with WSI (44% in the LDCT-arm) which were derived from a minority (10/33) of screening sites, but which enrolled the majority (34,612/53,452) of participants. Given the similar proportions of historically classified BAC and LUAD and lack of outcome differences between those with and without WSI, we believe our histologic findings are generalizable to the overall group of screen-detected NSCLC. Histologic classification was limited to representative tumor blocks (median of 2) rather than slides from the entire tumor which generally includes 3–4 tissue blocks for tumors of this size (
5.Conclusion
The combined pathologic subgroup of AIS/MIA/LMP in NLST correlates well with the epidemiologically observed rate of overdiagnosis among stage IA NSCLC (
Acknowledgments
The authors thank the National Cancer Institute for access to NCI’s data collected by the NLST (Project ID NLST-867).
This work was supported in part by 1R01CA275015-01A1.
Author contributions
Conception: EJB, TBS, KMRC.
Interpretation or analysis of data: EJB, TBS, KMRC.
Preparation of the manuscript: EJB, TBS.
Revision for important intellectual content: EJB, TBS, KMRC.
Supervision: EJB, KRC.
References
[1] | National Lung Screening Trial Research Team, D.R. Aberle, A.M. Adams, C.D. Berg, W.C. Black, J.D. Clapp, R.M. Fagerstrom, I.F. Gareen, C. Gatsonis, P.M. Marcus and J.D. Sicks, Reduced lung-cancer mortality with low-dose computed tomographic screening, N Engl J Med. 365: ((2011) ), 395–409. |
[2] | E.F. Patz, P. Pinsky, C. Gatsonis, J.D. Sicks, B.S. Kramer, M.C. Tammemägi, C. Chiles, W.C. Black, D.R. Aberle and NLST Overdiagnosis Manuscript Writing Team, Overdiagnosis in low-dose computed tomography screening for lung cancer, JAMA Intern Med. 174: ((2014) ), 269–274. |
[3] | W.C. Black, C. Chiles, T.R. Church, I.F. Gareen, D.S. Gierada, I. Mahon, E.A. Miller, P.F. Pinsky and J.D. Sicks, Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial National Lung Screening Trial Writing Team 1, J Thorac Oncol. 14: ((2019) ), 1732–1742. |
[4] | W.D. Travis, E. Brambilla, A.G. Nicholson, Y. Yatabe, J.H.M. Austin, M.B. Beasley, L.R. Chirieac, S. Dacic, E. Duhig, D.B. Flieder, K. Geisinger, F.R. Hirsch, Y. Ishikawa, K.M. Kerr, M. Noguchi, G. Pelosi, C.A. Powell, M.S. Tsao, I. Wistuba and WHO Panel, The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification, J Thorac Oncol. 10: ((2015) ), 1243–1260. |
[5] | H.Y. Lee, M.J. Cha, K.S. Lee, H.Y. Lee, O.J. Kwon, J.Y. Choi, H.K. Kim, Y.S. Choi, J. Kim and Y.M. Shim, Prognosis in Resected Invasive Mucinous Adenocarcinomas of the Lung: Related Factors and Comparison with Resected Nonmucinous Adenocarcinomas, J Thorac Oncol. 11: ((2016) ), 1064–1073. |
[6] | WHO Classification of Tumours Editorial Board, Thoracic tumours, 5th ed., Lyon (France): International Agency for Research on Cancer, S.l., (2021) . |
[7] | I. Yambayev, T.B. Sullivan, K. Suzuki, Q. Zhao, S.E. Higgins, O.H. Yilmaz, V.R. Litle, P. Moreira, E.L. Servais, C.T. Stock, S.M. Quadri, C. Williamson, K.M. Rieger-Christ and E.J. Burks, Pulmonary Adenocarcinomas of Low Malignant Potential: Proposed Criteria to Expand the Spectrum Beyond Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma, Am J Surg Pathol. 45: ((2021) ), 567–576. |
[8] | A. Pittaro, F. Crivelli, G. Orlando, F. Napoli, V. Zambelli, F. Guerrera, S. Sobrero, M. Volante, L. Righi and M. Papotti, Pulmonary Low Malignant Potential Adenocarcinoma: A Validation of the Proposed Criteria for This Novel Subtype, Am J Surg Pathol. 48: ((2024) ), 204–211. |
[9] | L. Ma, T.B. Sullivan, K.M. Rieger-Christ, I. Yambayev, Q. Zhao, S.E. Higgins, O.H. Yilmaz, L. Sultan, E.L. Servais, K. Suzuki and E.J. Burks, Vascular invasion predicts the subgroup of lung adenocarcinomas = 2.0 cm at risk of poor outcome treated by wedge resection compared to lobectomy, JTCVS Open. 16: ((2023) ), 938–947. |
[10] | M.M. Oken, W.G. Hocking, P.A. Kvale, G.L. Andriole, S.S. Buys, T.R. Church, E.D. Crawford, M.N. Fouad, C. Isaacs, D.J. Reding, J.L. Weissfeld, L.A. Yokochi, B. O’Brien, L.R. Ragard, J.M. Rathmell, T.L. Riley, P. Wright, N. Caparaso, P. Hu, G. Izmirlian, P.F. Pinsky, P.C. Prorok, B.S. Kramer, A.B. Miller, J.K. Gohagan, C.D. Berg and PLCO Project Team, Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial, JAMA. 306: ((2011) ), 1865–1873. |
[11] | H.G. Welch and W.C. Black, Overdiagnosis in cancer, J Natl Cancer Inst. 102: ((2010) ), 605–613. |
[12] | Y. Shieh, M. Eklund, G.F. Sawaya, W.C. Black, B.S. Kramer and L.J. Esserman, Population-based screening for cancer: hope and hype, Nat Rev Clin Oncol. 13: ((2016) ), 550–565. |
[13] | L.J. Esserman, I.M. Thompson, B. Reid, P. Nelson, D.F. Ransohoff, H.G. Welch, S. Hwang, D.A. Berry, K.W. Kinzler, W.C. Black, M. Bissell, H. Parnes and S. Srivastava, Addressing overdiagnosis and overtreatment in cancer: a prescription for change, Lancet Oncol. 15: ((2014) ), e234–242. |
[14] | E.J. Burks, J. Zhang, T.B. Sullivan, X. Shi, J.M. Sands, S.M. Regis, B.J. McKee, A.B. McKee, S. Zhang, H. Liu, G. Liu, A. Spira, J. Beane, M.E. Lenburg and K.M. Rieger-Christ, Pathologic and gene expression comparison of CT-screen detected and routinely detected stage I/0 lung adenocarcinoma in NCCN risk-matched cohorts., Cancer Treatment and Research Communications. 29: ((2021) ), 100486. |
[15] | W.D. Travis, E. Brambilla, M. Noguchi, A.G. Nicholson, K.R. Geisinger, Y. Yatabe, D.G. Beer, C.A. Powell, G.J. Riely, P.E. Van Schil, K. Garg, J.H.M. Austin, H. Asamura, V.W. Rusch, F.R. Hirsch, G. Scagliotti, T. Mitsudomi, R.M. Huber, Y. Ishikawa, J. Jett, M. Sanchez-Cespedes, J.-P. Sculier, T. Takahashi, M. Tsuboi, J. Vansteenkiste, I. Wistuba, P.-C. Yang, D. Aberle, C. Brambilla, D. Flieder, W. Franklin, A. Gazdar, M. Gould, P. Hasleton, D. Henderson, B. Johnson, D. Johnson, K. Kerr, K. Kuriyama, J.S. Lee, V.A. Miller, I. Petersen, V. Roggli, R. Rosell, N. Saijo, E. Thunnissen, M. Tsao and D. Yankelewitz, International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma, J Thorac Oncol. 6: ((2011) ), 244–285. |
[16] | H. Saji, M. Okada, M. Tsuboi, R. Nakajima, K. Suzuki, K. Aokage, T. Aoki, J. Okami, I. Yoshino, H. Ito, N. Okumura, M. Yamaguchi, N. Ikeda, M. Wakabayashi, K. Nakamura, H. Fukuda, S. Nakamura, T. Mitsudomi, S.-I. Watanabe, H. Asamura and West Japan Oncology Group and Japan Clinical Oncology Group, Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial, Lancet. 399: ((2022) ), 1607–1617. |
[17] | N. Altorki, X. Wang, D. Kozono, C. Watt, R. Landrenau, D. Wigle, J. Port, D.R. Jones, M. Conti, A.S. Ashrafi, M. Liberman, K. Yasufuku, S. Yang, J.D. Mitchell, H. Pass, R. Keenan, T. Bauer, D. Miller, L.J. Kohman, T.E. Stinchcombe and E. Vokes, Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer, N Engl J Med. 388: ((2023) ), 489–498. |
[18] | S. Srivastava, E.J. Koay, A.D. Borowsky, A.M. De Marzo, S. Ghosh, P.D. Wagner and B.S. Kramer, Cancer overdiagnosis: a biological challenge and clinical dilemma, Nat Rev Cancer. 19: ((2019) ), 349–358. |
[19] | F. Maldonado, F. Duan, S.M. Raghunath, S. Rajagopalan, R.A. Karwoski, K. Garg, E. Greco, H. Nath, R.A. Robb, B.J. Bartholmai and T. Peikert, Noninvasive Computed Tomography-based Risk Stratification of Lung Adenocarcinomas in the National Lung Screening Trial, Am J Respir Crit Care Med. 192: ((2015) ), 737–744. |
[20] | R. Bernardino, R.K. Sayyid, R. Leão, A.R. Zlotta, T. van der Kwast, L. Klotz and N.E. Fleshner, Using active surveillance for Gleason 7 (3+4) prostate cancer: A narrative review, Can Urol Assoc J. ((2023) ). |
[21] | K. Kadota, J. Nitadori, N. Rekhtman, D.R. Jones, P.S. Adusumilli and W.D. Travis, Reevaluation and reclassification of resected lung carcinomas originally diagnosed as squamous cell carcinoma using immunohistochemical analysis, Am J Surg Pathol. 39: ((2015) ), 1170–1180. |
[22] | A. Jemal and S.A. Fedewa, Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015, JAMA Oncol. 3: ((2017) ), 1278–1281. |
[23] | J. Huo, C. Shen, R.J. Volk and Y.-C.T. Shih, Use of CT and Chest Radiography for Lung Cancer Screening Before and After Publication of Screening Guidelines: Intended and Unintended Uptake, JAMA Intern Med. 177: ((2017) ), 439–441. |
[24] | I. Yambayev, T.B. Sullivan, K.M. Rieger-Christ, E.L. Servais, C.T. Stock, S.M. Quadri, J.M. Sands, K. Suzuki and E.J. Burks, Vascular invasion identifies the most aggressive histologic subset of stage I lung adenocarcinoma: Implications for adjuvant therapy, Lung Cancer. 171: ((2022) ), 82–89. |
[25] | M.C. Aldrich, S.F. Mercaldo, K.L. Sandler, W.J. Blot, E.L. Grogan and J.D. Blume, Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers, JAMA Oncol. 5: ((2019) ), 1318–1324. |
[26] | C.A. Haiman, D.O. Stram, L.R. Wilkens, M.C. Pike, L.N. Kolonel, B.E. Henderson and L. Le Marchand, Ethnic and racial differences in the smoking-related risk of lung cancer, N Engl J Med. 354: ((2006) ), 333–342. |
[27] | US Preventive Services Task Force, A.H. Krist, K.W. Davidson, C.M. Mangione, M.J. Barry, M. Cabana, A.B. Caughey, E.M. Davis, K.E. Donahue, C.A. Doubeni, M. Kubik, C.S. Landefeld, L. Li, G. Ogedegbe, D.K. Owens, L. Pbert, M. Silverstein, J. Stevermer, C.-W. Tseng and J.B. Wong, Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement, JAMA. 325: ((2021) ), 962–970. |
[28] | R. Kakinuma, Y. Muramatsu, H. Asamura, S.-I. Watanabe, M. Kusumoto, T. Tsuchida, M. Kaneko, K. Tsuta, A.M. Maeshima, G. Ishii, K. Nagai, T. Yamaji, T. Matsuda and N. Moriyama, Low-dose CT lung cancer screening in never-smokers and smokers: results of an eight-year observational study, Transl Lung Cancer Res. 9: ((2020) ), 10–22. |
[29] | H.-R. Kang, J.Y. Cho, S.H. Lee, Y.J. Lee, J.S. Park, Y.-J. Cho, H.I. Yoon, K.W. Lee, J.H. Lee and C.-T. Lee, Role of Low-Dose Computerized Tomography in Lung Cancer Screening among Never-Smokers, J Thorac Oncol. 14: ((2019) ), 436–444. |
[30] | S. Lam, Lung Cancer Screening in Never-Smokers, J Thorac Oncol. 14: ((2019) ), 336–337. |




