CT imaging phenotypes linked to CA125 and HE4 biomarkers are highly predictive in discriminating between hereditary and sporadic ovarian cancer patients
Abstract
BACKGROUND:
Hereditary ovarian cancers (HOC) represent about 23% of ovarian cancer (OC) cases: they are most frequently related to germline mutations in the BRCA genes.
OBJECTIVE:
We aimed to compare CA125/HE4 serum levels and Computed Tomography (CT) features at time of ovarian cancer (OC) diagnosis in two populations: BRCA mutant and BRCA wild-type (WT) OC, and to investigate the relationship between this laboratory and radiological biomarker and BRCA mutation status.
METHODS:
This retrospective study included 60 newly diagnosed OC patients with FIGO stage IIIC-IV disease, tested for BRCA1/2 germline mutation status of which preoperative CT scan and serum tumor marker assay were available.
RESULTS:
The median level of CA125 (708 U/mL) was significantly higher (p < 0.002) in BRCA1/2 mutated patients than in WT patients (176 U/mL), whereas the median level of HE4 (492 pmol/L) was significantly higher (p < 0.002) in WT than in BRCA-mutated patients (252 pmol/L). BRCA mutation carriers showed a higher incidence of bilateral ovarian masses (p = 0.0303) characterized by solid structures (p < 0.00001), higher peritoneal tumor load, macronodular implants >2 cm (p = 0.000099), increased frequency of lymphadenopathies (p = 0.019), and metastasis (p = 0.052) compared to patients with BRCA WT.
CONCLUSIONS:
Tumor markers and CT patterns may help in identifying BRCA mutation status in OC directing patients towards a personalized treatment.
1Introduction
Ovarian cancer (OC) is one of the deadliest malignancies in gynecologic cancers and the seventh most common cancer in women, nonetheless its early detection is still elusive due to the spectrum of non-specific symptoms and lack of valid screening strategies [1–5]. High-grade serous ovarian carcinoma (HGSC) is the most common OC subtype, accounting for 75% of all OC cases [6].
Hereditary ovarian cancers (HOC) represent about 23% of OC cases: they are most frequently related to germline mutations in the BRCA gene [7–10]. Genetic testing for BRCA1/2 mutation carriers has been demonstrated to be a key point in risk evaluation and clinical management of ovarian cancer [11–13].
In the latest decades, several studies have investigated the correlation between OC and the levels of serum tumor markers, demonstrating the well-established importance of the role of Carbohydrate antigen 125 (CA125) as a current benchmark for OC detection [7, 14, 15]. Particular importance in this context has gained HE4, a glycoprotein mapped on WFDC2 gene and overexpressed in epithelial ovarian cancer (EOC), and several studies demonstrated its efficacy as tumor serum marker for early detection of OC, remission monitoring and detecting relapse [5, 16]. Moreover, in a comparative study with CA125, HE4 showed better results in distinguishing benign from malignant tumors in women with a pelvic mass because, in general, HE4 levels are not increased in benign conditions, endometriosis or pelvic inflammatory disease [17].
Several studies reported that BRCA mutations were associated with the levels of serum tumor markers including carcinoembryonic antigen (CEA), Carbohydrate antigen 19-9 (CA 19.9), CA125 and Human Epididymis protein 4 (HE4) [5, 13, 18, 19].
Nonetheless, limited data exist regarding biomarker capability to improve the early detection of OC in BRCA mutation carriers, although promising results have been reported using the combination of HE4 with CA125 [14, 20].
Lately, HE4 has been demonstrated to play a significant role in OC identification and it has been reported to be superior to CA125 in diagnosing early stage sporadic ovarian cancer (SOC); however, HE4 behavior as well as its usefulness in HOC has not been unambiguously determined [20].
Recently, some studies suggested that BRCA mutation status may influence also Computed Tomography (CT) disease presentation [21]; CT features could help to recognize OC related to BRCA mutation status [22, 23]. In particular, qualitative and quantitative differences regarding peritoneal carcinosis and visceral metastasis were shown between BRCA-mutated and BRCA wild type (WT) genotype [21, 24]. Furthermore, it has been demonstrated that identification of BRCA mutation status can be associated with a different therapeutic choice and prognosis prediction [25, 26].
Therefore, the inclusion of predictive/prognostic biomarkers in clinical trials should be considered to improve patient selection and to avoid unnecessary toxic treatment for patients who would not optimally benefit [27, 28].
In this study, we aimed to compare CA125/HE4 serum levels and CT features at time of OC diagnosis in two different populations: BRCA-mutant OC and BRCA WT OC, and to investigate the relationship between this laboratory and radiological biomarker and BRCA mutation status.
2Patients and methods
2.1Eligibility criteria
This was a retrospective study designed and conducted at laboratory of Tumor Markers-Laboratory Department of Experimental Medicine and Radiological sciences of Sapienza University of Rome; patients admitted to our Institutions from 2017 to 2019 were evaluated.
All procedures performed were in accordance with the clinical setting and ethical standards of the our institutional board and with the 1964 Helsinki declaration and its later amendments; for this type of study formal consent was not required.
In this study, we included 60 newly diagnosed OC patients (median age, 58 years; range, 25–85 years) following the subsequent criteria: (a) FIGO stage III or IV HGSOC, (b) genetic counseling and BRCA mutation testing, (c) preoperative abdominal-pelvic CT before and after administration of intravenous contrast medium (c) almost 7 days between CT and serum collection. Patients undergoing neoadjuvant chemotherapy were not included in the study.
The patients were divided into two groups based upon their genetic status: 30 women carrying BRCA1/2 mutations constituted Group 1 while Group 2 was composed of 30 BRCA WT women. According to previous studies, BRCA-mutated patients were on average 10 years younger than BRCA WT patients (average age of BRCA-mutated: 50 years old; average age of BRCA WT: 58 years, p = 0.001).
We also included in the protocol a control group composed by 80 healthy patients age-matched non affected by OC: 40 healthy women carrying BRCA1/2 mutation and 40 healthy BRCA WT women; their CA125 and HE4 serum levels were measured and compared with serum data of patients affected by OC enrolled in our study.
All patients were included to investigate the associations between BRCA mutation status, biomarker serum levels and CT imaging features in patients affected by OC.
2.2Biomarker detection and CT technique
CA125 and HE4 serum levels were measured on LUMIPULSE G1200 (Fujirebio-Europe, Belgium), an automated assay system based on chemiluminescent enzyme immunoassay (CLEIA) technology by a two-step sandwich in immunoreaction cartridges. All assays were performed according to the manifacturer’s instructions, and the normal levels were considered at <35 U/mL and <150 pmol/L for CA125 and HE4, respectively.
Contrast-enhanced high-resolution multi-detector row CT (Somatom Sensation 64; Siemens Medical System, Erlangen, Germany) was utilized. Images were acquired during breath hold by applying the following parameters: automatic milliampere setting with a range of 240–400 mA (calculated based on the patient’s size), 120 kVp, 0.6×64-mm2 collimation, 1 mm section thickness, and 0.8–1.5-mm reconstruction interval of coronal and sagittal images; and itch less than 1. Patients were required to ingest 2 L of water 20 min before the CT acquisition in order to achieve optimal distension of stomach and bowel; few minutes before the scans, an intravenous injection of 20 mg of butyl-scopolamine (Buscopan, Boehringer) was administered in order to reduce peristaltic bowel movement.
CT images were acquired before and after administration of contrast medium, cranio-caudally, from the diaphragm’s dome to the pelvis. The images were postprocessed (MPR) with reconstructions in sagittal and coronal plane sections with a 1 mm interval to improve the anatomical analysis, particularly of the surface lesions.
Non-ionic iodinated contrast medium (350 mg I/mL, Iomeron, Bracco, Milan, Italy) was administered intravenously utilizing an automatic injector (Stellant DCT, Medrad, Indianola, PA) at an infusion rate of 3–3.5 mL/s for a total volume of 90–120 mL, it was acquired a venous phase (time delay from contrast agent injection approximately to 70 seconds).
2.3CT analysis
All preoperative CT scans were retrospectively reviewed by senior radiologist with 20 years of experience in pelvic MRI, with particular interest and expertise in oncologic female pelvic imaging.
2.3.1Ovarian lesions
The ovarian lesions were split on the basis of size in two groups (<6 cm and >6 cm) and evaluated on the basis of structure: predominantly cystic/ multicystic and predominantly solid/ solid (solid components in predominantly cystic/ multicystic lesion were up to 50% and predominantly solid/solid more than 50%) and on the basis of unilateral or bilateral tumor presence.
2.3.2Extraovarian tumor spread
Peritoneal carcinomatosis implants: We evaluated the presence/absence of micronodular carcinomatosis (characterized by small 1–5 mm peritoneal nodularities diffusely affecting the serosa and sub-serosal fat), of macronodular carcinomatosis (peritoneal implants with a diameter >5 mm that may flow into each other in an irregular thickening of the sub-serosal fat and serosa, assuming the so-called “plaque” appearance) and of omental cake (infiltration of the omental fat by carcinosis implants that assumes plaque-like aspect). Peritoneal implants were also evaluated based on morphologic pattern and were labeled as either nodular or infiltrative: nodular implants showed predominantly definite or rounded or “pushing” margins; while infiltrative morphology was characterized by ill-defined or infiltrative borders. The carcinosis was assessed quantitatively according to Peritoneal Cancer Index (PCI) which assigns a score based on the entity of the carcinosis diffusion and greatness; PCI score divided and renamed the abdomen into 12 regions and assigned to each region a score relating to the size of carcinosis implants (LS0-3) (Fig. 1, Tables 1 and 2).
Fig. 1
PCI score dived and renamed the abdomen in nine regions by two transverse planes and two sagittal planes. Nine regions are numbered in clockwise direction with 0 at the umbilicus and 1 defining the space beneath the right hemidiaphragm.
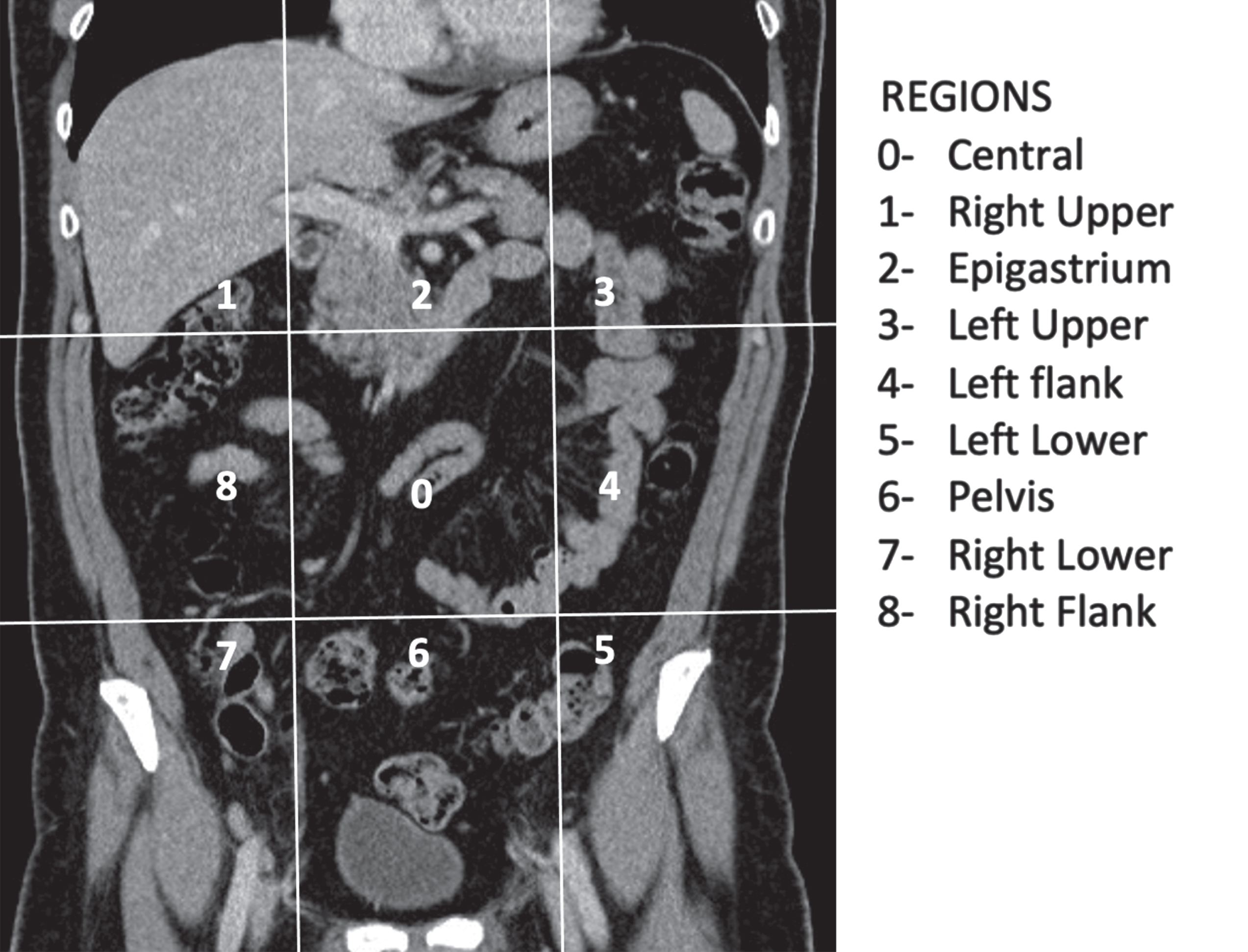
Table 1
Peritoneal Cancer Index (PCI)
| REGIONS | LESION SCORE |
| 0 | Central |
| 1 | Right Upper |
| 2 | Epigastrium |
| 3 | Left Upper |
| 4 | Left flank |
| 5 | Left Lower |
| 6 | Pelvis |
| 7 | Right Lower |
| 8 | Right Flank |
| 9 | Upper Jejunum |
| 10 | Lower Jejunum |
| 11 | Upper Ileum |
| 12 | Lower Ileum |
| 13 | |
| PCI |
Table 2
Lesion Size Score
| LS 0 | No tumor seen |
| LS 1 | Tumor up to 0.5 cm |
| LS 2 | Tumor up to 5 cm |
| LS 3 | Tumor >5 cm or confluence |
Lympho-nodes involvement: Lympho-nodes involvement of the following sites was also considered, the following short-axis dimensions were used to define their pathological meaning: (a) supradiaphragmatic lymph nodes >5 mm, (b) lombo-aortic >10 mm (c) mesenteric >10 mm and (d) pelvic >8 mm.
Ascites, pleurisy and distal metastasis: The reader noted also the presence/absence of ascites, pleurisy, pleural effusion and distal metastasis.
2.4Statistical analysis
2.4.1Biomarkers
Continuous variables are expressed as mean±standard deviation (SD). Categorical data are expressed as percentages. Continuous variables were tested for normality with Kolmogorov-Smirnov test. Differences between groups were tested with the one-way analysis of variance (ANOVA), for normally distributed continuous variables, or with the Kruskal-Wallis H test, for not normally distributed variables. As appropriate, a post hoc test was subsequently performed on each group pair. Categorical variables between groups were compared by Fisher’s exact test. A p of <0.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS Statistics software version 23 (Chicago, IL, USA).
2.4.2CT
Statistical analysis was performed using the SPSS statistical software (ver. 26.0 SPSSinc, Chicago,IL).
Chi-squared (χ2) test was performed to study the correlation between different CT features (ovarian masses, PD, lymphadenopathies, distal metastasis, ascites, pleurisy) in two groups: BRCA-mutant OC (Group 1) and 30 BRCA WT OC (Group 2).
A P value of less than 0.05 was considered statistically significant.
3Results
3.1Laboratory
Among women of the two control groups, no pathological values of CA125 and HE4 were found. Serum levels of CA125 and HE4 were determined and compared between BRCA-mutated and BRCA WT patients. The median level of CA125 (708 U/mL, range 190-2751,) was significantly higher (p < 0.002) in BRCA-mutated patients than in WT patients (176 U/mL, range 81–451):
The proportion of patients with high serum CA125 level (>35 U/mL) was significantly higher in BRCA1/2 mutated patients (29/30) than in BRCA WT ones (24/30) while, on the contrary, all WT women (30/30) had elevated HE4 serum levels, increased only in 19/30 BRCA1/2 mutated women (p < 0.002) Fig. 2.
Fig. 2
Percentage of HOC and SOC patients with high CA125 and He4 values. A statistically positive difference is observed in the positive percentages of HE4 and CA125 in the different patient groups. (p < 0.002).
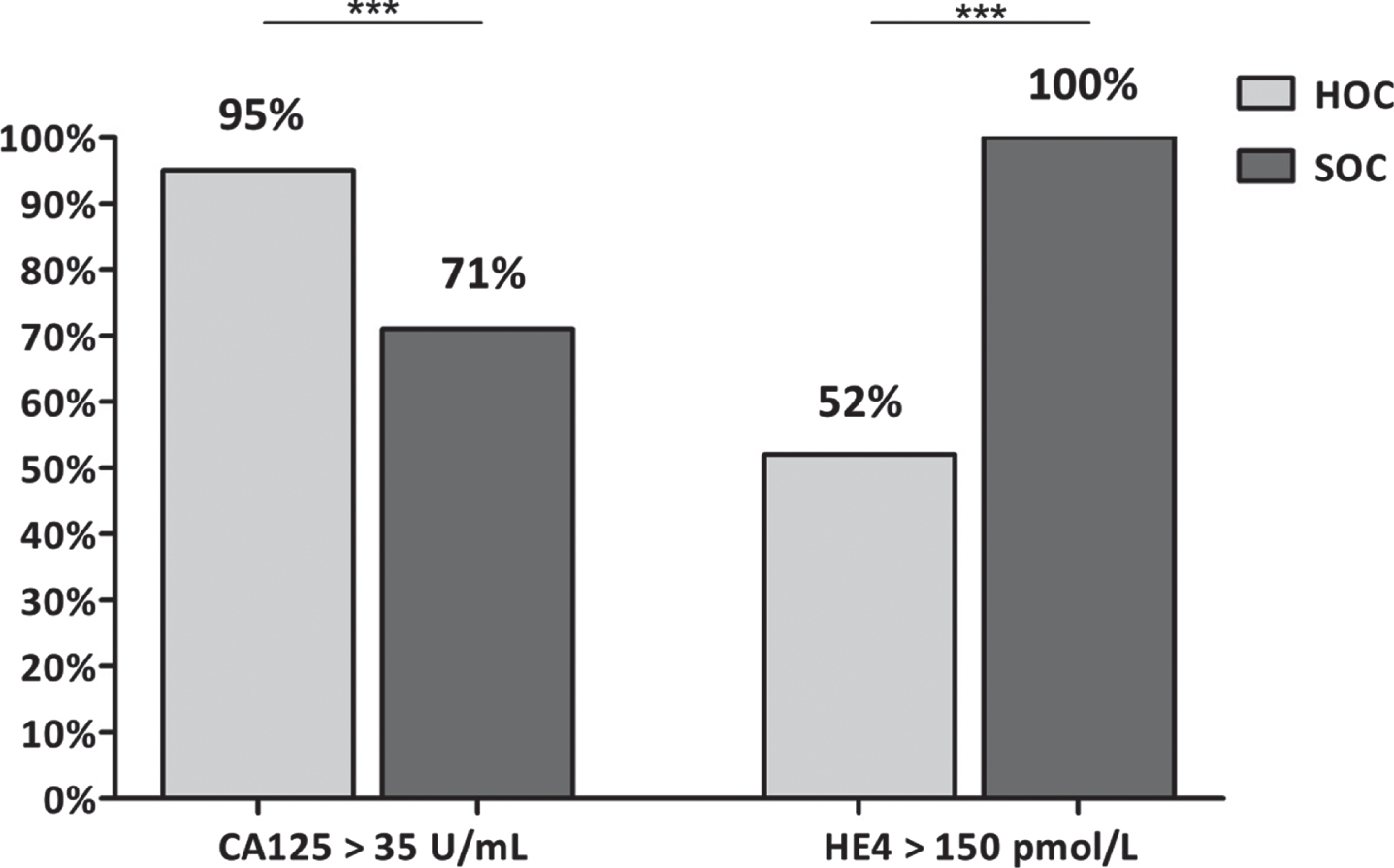
In addition, the median HE4 level (492 pmol/L, range 157–2232) was significantly higher(p < 0.002) in BRCA WT than in BRCA1/2 mutated patients (252 pmol/L, range 35–650).
In the 40 healthy women carrying BRCA1/2 mutation we observed the following results: (HE4 35 pmol/L, range 42–50; CA125 28 U/mL range 8–29) and in 40 healthy BRCA WT women (HE4 33 pmol/L, range 41–55; CA125 25 U/mL, range 10–32)
3.2CT
Below, we describe the associations between BRCA mutation status and CT pattern of disease, summarized in Tables 3–6.
Table 3
Association between ovarian lesions CT features and BRCA mutation status
| BRCA mutant status | |||
| CT features | BRCA mutant | BRCA Wild Type | P value |
| OVARY | |||
| Ovarian mass | |||
| monolateral | 6 | 15 | 0.014851 |
| bilateral | 24 | 15 | |
| Architecture | |||
| cystic/predominantly cystic | 3 | 27 | <0.00001 |
| solid/ predominantly solid | 27 | 3 | |
| Dimension | |||
| >6 cm | 21 | 24 | 0.371093 |
| <6 cm | 9 | 6 | |
Table 4
Association between PD CT features and BRCA mutation status
| BRCA mutant status | |||
| CT features | BRCA mutant | BRCA Wild Type | P value |
| PERITONEUM | |||
| Macronodular carcinosis | |||
| >2 cm | 24 | 9 | 0.000099 |
| <2 cm | 6 | 21 | |
| Micronodular carcinosis | |||
| present | 9 | 21 | 0.001946 |
| absent | 21 | 9 | |
| Omental cake | |||
| present | 15 | 12 | 0.436275 |
| absent | 15 | 18 | |
| PD pattern | |||
| nodular | 24 | 6 | <0.00001 |
| infiltrative | 6 | 24 | |
| Sites | |||
| Right subdiaphragmatic (1) | |||
| present | 15 | 15 | 1 |
| absent | 15 | 15 | |
| Left subdiaphragmatic (3) | |||
| present | 21 | 18 | 0.416793 |
| absent | 9 | 12 | |
| Right parietocolic space (8) | |||
| present | 24 | 21 | 0.371093 |
| absent | 6 | 9 | |
| Left parietocolic space (4) | |||
| present | 18 | 15 | 0.436275 |
| absent | 12 | 15 | |
| Pelvis (6) | |||
| present | 29 | 9 | <0.00001 |
| absent | 1 | 21 | |
Table 5
Association between metastasis, ascites and pleurisy and BRCA mutation status
| BRCA mutant status | |||
| CT features | BRCA mutant | BRCA Wild Type | P value |
| METASTASIS | |||
| present | 9 | 3 | 0.052808 |
| absent | 21 | 27 | |
| ASCITES | |||
| present | 21 | 27 | 0.052808 |
| absent | 9 | 3 | |
| PLEURISY | |||
| present | 12 | 15 | 0.436275 |
| absent | 18 | 15 | |
Table 6
Association between lymphadenopathies and BRCA mutation status
| BRCA mutant status | |||
| CT features | BRCA mutant | BRCA Wild Type | P value |
| LYNPHADENOPATY | |||
| mesenteric | |||
| present | 3 | 12 | 0.00729 |
| absent | 27 | 18 | |
| pericardiophrenic | |||
| present | 21 | 12 | 0.019517 |
| absent | 9 | 18 | |
| lombo-aortic | |||
| present | 9 | 15 | 0.113846 |
| absent | 21 | 15 | |
| pelvic | |||
| present | 12 | 12 | 1 |
| absent | 18 | 18 | |
3.2.1Ovarian lesions
A statistically significant correlation between BRCA mutational status and ovarian mass structure (P < 0.00001) was detected. In particular, Group 1 showed a solid/predominantly solid architecture in 27/30 patients while Group 2 had cystic/predominantly cystic architecture in 27/30 patients. Moreover, the presence of bilateral ovarian masses was more frequent in Group 1 (24/30) than in Group 2 (15/30) (p = 0.014851).
No significant associations were found between BRCA mutation status and ovarian masses size (< />6 cm) (p = 0.371093).
3.2.2Peritoneal carcinomatosis implants
Some statistically significant correlations were observed between BRCA mutational status and PD. First, in Group 1 was observed a higher tumor load than in Group 2, quantified by PCI score. In fact, Group 1 showed a mean PCI score of 11.3 (range 4–19) while Group 2 showed a mean PCI score of 7.2 (range 0–11).
Second, Group 2 was statistically correlated to micronodular PD (p = 0.001946) mostly associated with omental distribution and mesenteric lymphadenopathy involvement (p = 0.00729). On the other hand, Group 2 showed a higher incidence of macronodular PD characterized by nodular size >2 cm (p = 0.000099) and a trend toward a higher frequency of peritoneal and diaphragmatic carcinomatosis than Group 1. Regarding the macronodular implants distribution, the presence of peritoneal implants in pelvis was statistically associated with Group 1 (p < 0.00001).
Last, significant associations were found between morphology of peritoneal implants and BRCA mutational status. In particular, nodular PD pattern were significantly associated with Group 1 whereas Group 2 showed a higher incidence of infiltrative peritoneal implants (p < 0.00001).
Lympho-nodes involvement: the presence of mesenteric and pericardiophrenic lymphadenopathy remained significantly associated with BRCA mutational status. In particular, pericardiophrenic lymphadenopathies were statistically associated with Group 1 (p = 0.019517) while mesenteric lymph nodes with Group 2 (p = 0.00729).
Ascites, pleural effusion and distant metastases: presence of distant metastases in Group 1 (p = 0.052808) and presence of ascites in Group 2 (p = 0.052808) were associated with lower statistical significance with BRCA mutational status.
No significant associations were observed between BRCA mutational status and CT hallmark of pleural effusion (p = 0.436275).
Figures 3 and 4 illustrate the main features of each group.
Fig. 3
BRCA-mutated OC typical features consist in: bilateral ovarian masses with a solid/predominantly solid structures (Fig. 2a-b); macronodular carcinomatosis with predominant nodular morphologic pattern characterized by well-defined/ rounded /“pushing” borders; PD composed of macronodular implants which confluence each other in a plaque-like thickenings of omental fat assuming the so called “omental cake” aspect (Fig. 2c-d); presence of pericardiophrenic lymphadenopathy (short-axis greater than 0.5 cm) (Fig. 2e); presence of liver metastasis (Fig. 2f).
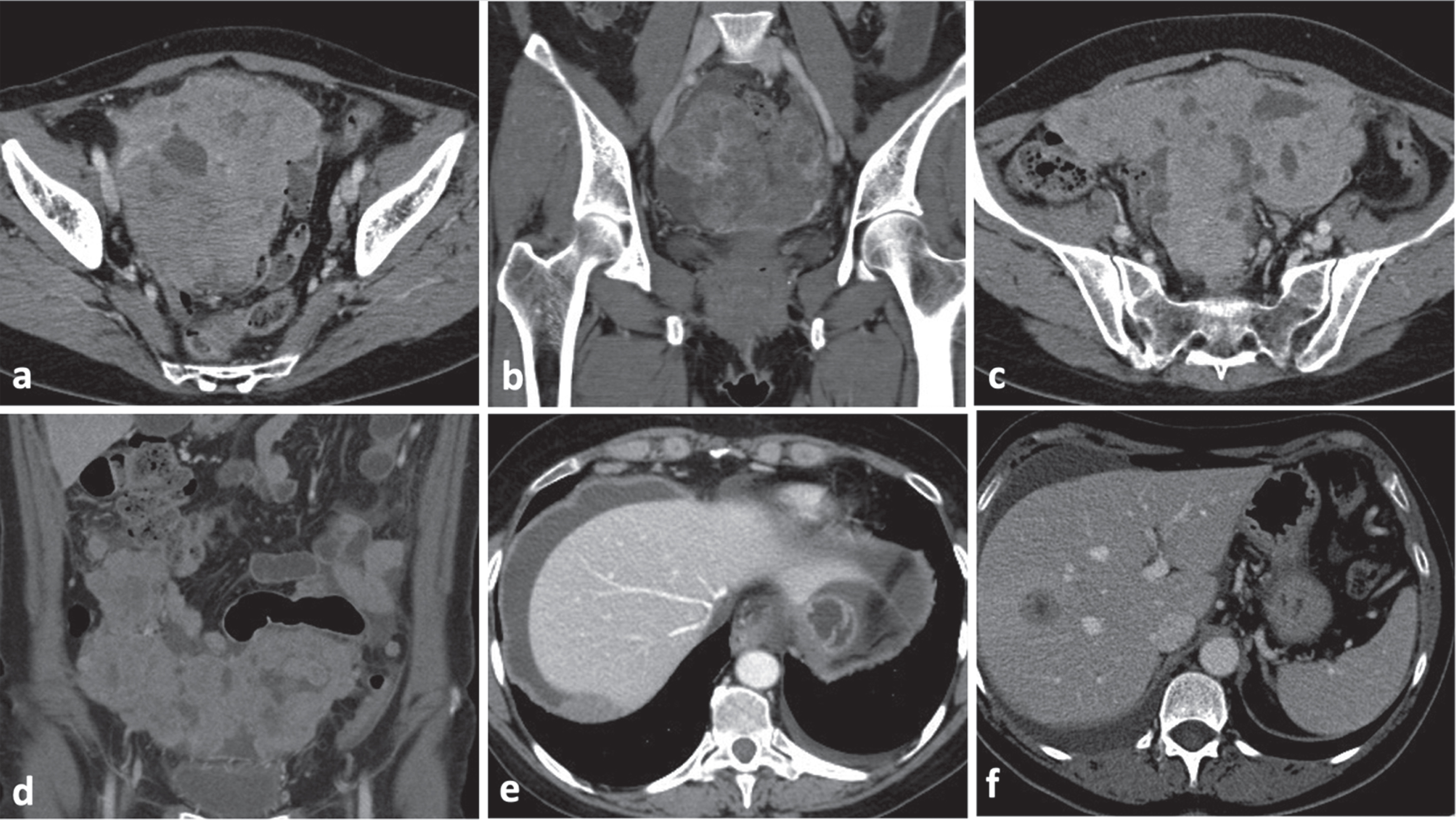
Fig. 4
BRCA WT OC typical features consist in: ovarian masses with cystic/predominantly cystic structures (Fig. 3a-b); micronodular carcinomatosis and macronodular carcinomatosis with infiltrative morphologic pattern characterized by mostly poorly defined borders (Fig. 3c-e); presence of mesenteric lymphadenopathy (short-axis greater than 1 cm) (Fig. 3f).
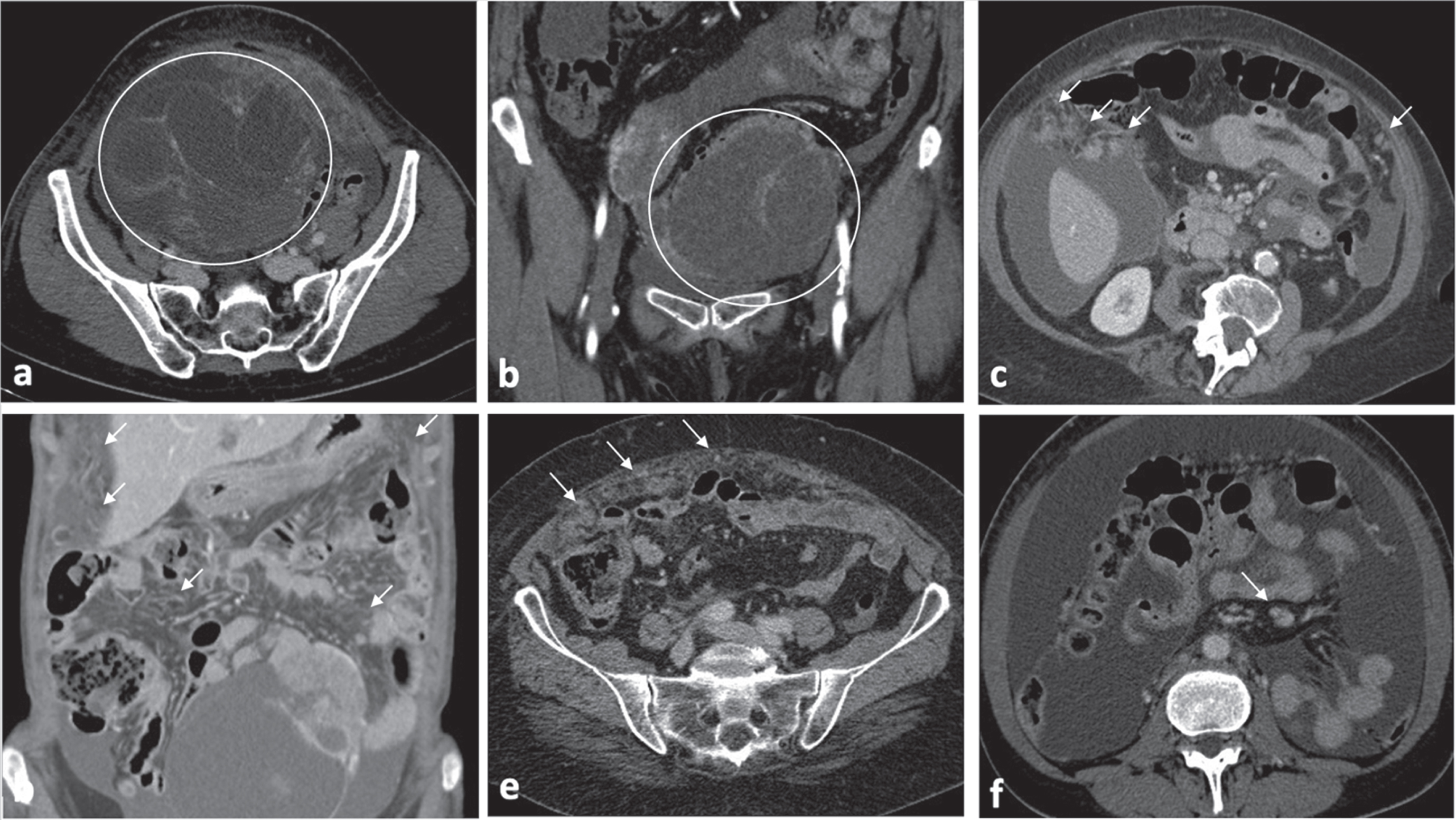
4Discussion
In the last few decades, several efforts have been made to detect valid OC-related biomarkers in order to stratify prognosis and establish personalized treatments for patients affected by OC. In this context, particular relevance has assumed identification of BRCA mutational status [26].
BRCA1 and BRCA2 mutations, causing chromosomal instability, increase sensitivity to platinum and to new drugs that inhibit the poly (ADP-ribose) polymerase (PARP) enzyme [29, 30]. In these patients, chemotherapy causes disruption of the double stranded DNA that cannot be repaired.
In this context, our study aims to highlight the associations between serum biomarkers, HE4 and Ca125, and the phenotypic features of CT presentation of disease with BRCA1/2 gene mutations in OC patients.
We observed a significantly higher median level of CA125 (708 U/mL) (p < 0.002) in patients with BRCA mutation than in patients with WT (176 U/mL). Several studies conducted on mutated and wild type BRCA patients found no significant differences in CA125 concentration at the time of OC diagnosis [31, 32].
In line with our results, Liu et al. confirmed elevated serum level of CA125 to be associated with the mutation status of BRCA1 and BRCA2 [33].
Serum CA125 is the gold standard biomarker in epithelial OC, although it shows low sensitivity in the early stages of neoplastic pathology [34] and low specificity [5], being also elevated in some non-malignant gynecological disorders, such as endometrial diseases, uterine myoma and pregnancy, and in other non-gynecological disorders, such as pancreatitis, liver cirrhosis, hepatitis and congenital heart disease [35–39].
In postoperative monitoring, serum CA125 levels have been shown to be effective in assessing response to treatment and disease [15, 40]. Indeed, in post-chemotherapy monitoring, a steady increase in serum CA125 values is associated with a poor response to treatment and a shorter progression-free survival (PFS) and overall survival time (OS) [41–43]. In the light of these observations, the association of increased values of CA125 and the BRCA1/2 mutation, could strengthen the genetic investigation in OC women with high levels of this tumor marker. On the other hand, CA125 demonstrated high sensitivity (92%) and high specificity when used within a screening algorithm (Ovarian Cancer Screening Study, ROCA) to monitor women with a family history of ovarian or breast cancer or with BRCA mutations [44].
The median level of HE4 (492 pmol/L) in BRCA WT patients was significantly higher (p < 0.002) than in patients with BRCA1 mutations (252 pmol/L). In the study on the role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer, Plotti et al. also reported a higher concentration of HE4 in non-mutated patients than in BCRA patients. Furthermore, they suggest a possible reason for this difference in HE4 value could be higher levels of microscopic disease patients with BRCA WT. They observed a greater micronodular carcinomatosis in BCRA WT patients compared to carriers of mutation and explains the poorer prognosis in BRCA WT patients [45]. We confirmed this finding in our study.
Our findings were similar to the results of Sala et al. regarding PD morphological features [8]. In fact, they affirmed that BRCA-mutated patients had peritoneal implants characterized by nodular PD pattern, whereas all BRCA WT OC patients had infiltrative PD pattern. Breast imaging literature support the outcomes concerning the associations between morphological pattern of PD presentation and BRCA mutation status [46, 47]. Furthermore, in accordance with Sala at al., our study showed that supradiaphragmatic lymphadenopathy was associated with BRCA-mutant OC (P = 0.019517). Finally, in accordance with Gourley at al. and to Petrillo et al., our study suggests that the presence of BRCA mutations could be associated with increased incidence of parenchymal lesions in BRCA1/2- mutated patients (p = 0.052808) [21, 24]. Recent literature data show that identification of BRCA mutation status in recurrent ovarian cancer (ROC) appears to have a key role in guiding surgical therapy. Gallotta at al. highlighted how the assessment of germline and somatic BRCA mutational status could be considered as positive predictive factor of post-hepatic resection progression free survival (PHR-PFS) [48]. Furthermore, they also investigated the prognostic role of BRCA mutational status in ovarian cancer patients with isolated lymph-node recurrence (ILNR) who underwent salvage lymphadenectomy (SL); they showed that BRCA mutational status did not appear to correlate with post-salvage lymphadenectomy progression free survival (PSL-PFS), differently from other recurrence sites in ROC patients [49].
In conclusion, we highlight that the use of a combined model of serum tumor biomarkers and CT imaging, is able to differentiate patients with BRCA mutations from WT patients assisting oncologists in selecting the most appropriate therapy.
In addition, recent studies have investigated how quantitative analysis of texture features on radiological images can lead to non-invasive preoperative diagnosis of a suspicious tissue. Thus, integration of serum biomarkers, radiological and texture feature analysis could lead to a non-invasive virtual biopsy of ovarian cancer helping to direct patients early towards personalized treatment and more appropriate therapy.
The main weakness of our study is the small number of patients and the retrospective nature of the study. We believe that serum concentrations of HE4 CA125 and features of CT disease should be tested in a larger population of EOC patients in prospective, multicentric and randomized studies, in order to improve diagnostic strategies and treatment options.
Acknowledgments
We thank Barbara Colaprisca and Giuseppina Gennarini for their technical assistance.
Authors’ contributions
LM and EA were responsible for project administration and supervision. VC, EB, TG, ST and VV contributed to the acquisition of the data. LM, EA, CC and AA analyzed the data. LM, VC and EA wrote the first manuscript draft. All authors critically revised the article and approved the final manuscript to be published.
Conflict of interest
None.
Abbreviations
OC | Ovarian Cancer |
HGSC | High-Grade Serous Ovarian Carcinoma |
HOC | Hereditary Ovarian Cancers |
SOC | Sporadic Ovarian Cancer |
CT | Computed Tomografy |
CEA | Carcinoembryonic Antigen |
CA 19.9 | Carbohydrate Antigen 19-9 |
CA125 | Carbohydrate Antigen 125 |
HE4 | Human Epididymis Protein 4 |
WT | Wild Type |
CLEIA | Chemiluminescent Enzyme Immunoassay |
PD | Peritoneal Disease |
PCI | Peritoneal Cancer Index |
SD | Standard Deviation |
HR | Homologous Recombination |
PARP | The Enzyme Poly (ADP-Ribose) Polymerase |
PFS | Progression-Free Survival |
OS | Overall Survival |
PLCO | Prostate, Lung, Colorectal and Ovarian |
UKC-TOCS | United Kingdom Collaborative Trial of Ovarian Cancer Screening |
CGN | Cancer Genetics Network |
GOG | Gynaecologic Oncology Group |
ROCA | Risk Ovarian Cancer Algorithm |
RMI | Risk of Malignancy Index |
ROMA | Risk of Ovarian Malignancy Algorithm |
LPS-PIV | Laparoscopic Intraperitoneal Diffusion of Disease |
ROC | Recurrent Ovarian Cancer |
PHR-PFS | Post-Hepatic Resection Progression Free Survival |
ILNR | Isolated Lymph-Node Recurrence |
SL | Salvage Lymphadenectomy |
PSL-PFS | Post-Salvage Lymphadenectomy Progression Free Survival |
References
[1] | Bowtell DD , Böhm S , Ahmed AA , Aspuria P-J , Bast RC , Beral V , et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. (2015) ;15: :668–79. doi:10.1038/nrc4019. |
[2] | Matulonis UA , Sood AK , Fallowfield L , Howitt BE , Sehouli J , Karlan BY . Ovarian cancer. Nat Rev Dis Primers. (2016) ;2: :16061. doi:10.1038/nrd2016.61. |
[3] | Lheureux S , Gourley C , Vergote I , Oza AM . Epithelial ovarian cancer. The Lancet. (2019) ;393: :1240–53. doi:10.1016/S0140-6736(18)32552-2. |
[4] | Lisio M-A , Fu L , Goyeneche A , Gao Z , Telleria C . High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. IJMS. (2019) ;20: :952. doi:10.3390/ijms20040952. |
[5] | Le Page C , Chung J , Rahimi K , Köbel M , Provencher D , Mes-Masson A-M . Exploring the clinical impact of predictive biomarkers in serous ovarian carcinomas. CDT. (2020) ;21: :974–95. doi:10.2174/1389450120666191016143836. |
[6] | Singh N , McCluggage WG , Gilks CB . High-grade serous carcinoma of tubo-ovarian origin: Recent developments. Histopathology. (2017) ;71: :339–56. doi:10.1111/his.13248. |
[7] | Russo A , Calò V , Bruno L , Rizzo S , Bazan V , Di Fede G . Hereditary ovarian cancer. Critical Reviews in Oncology/Hematology. (2009) ;69: :28–44. doi:10.1016/j.critrevonc.2008.06.003. |
[8] | Nougaret S , Lakhman Y , Gönen M , Goldman DA , Miccò M , D’Anastasi M , et al. High-grade serous ovarian cancer: Associations between BRCA mutation status, CT imaging phenotypes, and clinical outcomes. Radiology. (2017) ;285: :472–81. doi:10.1148/radiol.2017161697. |
[9] | Hirsh-Yechezkel G , Chetrit A , Lubin F , Friedman E , Peretz T , Gershoni R , et al. Population attributes affecting the prevalence of BRCA mutation carriers in epithelial ovarian cancer cases in Israel. Gynecologic Oncology. (2003) ;89: :494–8. doi:10.1016/S0090-8258(03)00152-5. |
[10] | Risch HA , McLaughlin JR , Cole DEC , Rosen B , Bradley L , Kwan E , et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. The American Journal of Human Genetics. (2001) ;68: :700–10. doi:10.1086/318787. |
[11] | Pan Z , Xie X . BRCA mutations in the manifestation and treatment of ovarian cancer. Oncotarget. (2017) ;8: :97657–70. doi:10.18632/oncotarget.18280. |
[12] | Paradiso AV , Digennaro M , Patruno M , De Summa S , Tommasi S , Berindan-Neagoe I . BRCA germline mutation test for all woman with ovarian cancer? BMC Cancer. (2019) ;19: :641. doi:10.1186/s12885-019-5829-4. |
[13] | Deng H , Chen M , Guo X , Heng J , Xu X , Peng L , et al. Comprehensive analysis of serum tumor markers and BRCA1/2 germline mutations in chinese ovarian cancer patients. Mol Genet Genomic Med. (2019) ;7: (6):e672. doi:10.1002/mgg3.672. |
[14] | Anastasi E , Gigli S , Ballesio L , Angeloni A , Manganaro L . The complementary role of imaging and tumor biomarkers in gynecological cancers: An update of the literature. Asian Pac J Cancer Prev. (2018) ;19: (2):309–17. doi:10.22034/APJCP.2018.19.2.309. |
[15] | Shinagare AB , Balthazar P , Ip IK , Lacson R , Liu J , Ramaiya N , Khorasani R . High-Grade serous ovarian cancer: Use of machine learning to predict abdominopelvic recurrence on CT on the basis of serial cancer antigen 125 levels. J Am Coll Radiol. (2018) ;15: :1133–8. doi:10.1016/j.jacr.2018.04.008. |
[16] | Nassir M , Guan J , Luketina H , Siepmann T , Rohr I , Richter R , et al. The role of HE4 for prediction of recurrence in epithelial ovarian cancer patients—results from the OVCAD study. Tumor Biol. (2016) ;37: :3009–16. doi:10.1007/s13277-015-4031-9. |
[17] | Urban N , Thorpe J , Karlan BY , McIntosh MW , Palomares MR , Daly MB , et al. Interpretation of single and serial measures of HE4 and CA125 in asymptomatic women at high risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. (2012) ;21: :2087–94. doi:10.1158/1055-9965.EPI-12-0616. |
[18] | Santoveña A , Fariña JB , Llabrés M , Zhu Y , Dannies P . Pharmacokinetics analysis of sustained release HGH biodegradable implantable tablets using a mouse model of human ovarian cancer. Int J Pharmaceutics. (2010) ;388: :175–80. doi:10.1016/j.ijpharm.2009.12.054. |
[19] | Garsed DW , Alsop K , Fereday S , Emmanuel C , Kennedy CJ , Etemadmoghadam D , et al. Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. Clin Cancer Res. (2018) ;24: :569–80. doi:10.1158/1078-0432.CCR-17-1621. |
[20] | Granato T , Porpora MG , Longo F , Angeloni A , Manganaro L , Anastasi E . HE4 in the differential diagnosis of ovarian masses. Clinica Chimica Acta. (2015) ;446: :147–55. doi:10.1016/j.cca.2015.03.047. |
[21] | Petrillo M , Marchetti C , De Leo R , Musella A , Capoluongo E , Paris I , et al. BRCA mutational status, initial disease presentation, and clinical outcome in high-grade serous advanced ovarian cancer: A multicenter study. Am J Obstetrics Gynecol. (2017) ;217: :334.e1–334.e9. doi:10.1016/j.ajog.2017.05.036. |
[22] | Manganaro L , Michienzi S , Vinci V , Falzarano R , Saldari M , Granato T , et al. Serum HE4 levels combined with CE CT imaging improve the management of monitoring women affected by epithelial ovarian cancer. Oncol Reports. (2013) ;30: :2481–7. doi:10.3892/or.2013.2682. |
[23] | Manganaro L , Gigli S , Antonelli A , Saldari M , Tomao F , Marchetti C , et al. Imaging strategy in recurrent ovarian cancer: A practical review. Abdom Radiol. (2019) ;44: :1091–102. doi:10.1007/s00261-018-1677-y. |
[24] | Gourley C , Michie CO , Roxburgh P , Yap TA , Harden S , Paul J , et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: An extension of the ovarian BRCAness phenotype. JCO. (2010) ;28: :2505–11. doi:10.1200/JCO.2009.25.1082. |
[25] | Bolton KL . Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. (2012) ;307: :382. doi:10.1001/jama.2012.20. |
[26] | Capoluongo E , Scambia G , Nabholtz J-M . Main implications related to the switch to BRCA1/2 Tumor testing in ovarian cancer patients: A proposal of a consensus. Oncotarget. (2018) ;9: :19463–8. doi:10.18632/oncotarget.24728. |
[27] | Ledermann JA . PARP inhibitors in ovarian cancer. Ann Oncol. (2016) ;27: :i40–4. doi:10.1093/annonc/mdw094. |
[28] | Rizzo S , Del Grande M , Manganaro L , Papadia A , Del Grande F . Imaging before cytoreductive surgery in advanced ovarian cancer patients. Int J Gynecol Cancer. (2020) ;30: :133–8. doi:10.1136/ijgc-2019-000819. |
[29] | Cook SA , Tinker AV . PARP inhibitors and the evolving landscape of ovarian cancer management: A review. BioDrugs. (2019) ;33: :255–73. doi:10.1007/s40259-019-00347-4. |
[30] | del Campo JM , Matulonis UA , Malander S , Provencher D , Mahner S , Follana P , et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. JCO. (2019) ;37: :2968–73. doi:10.1200/JCO.18.02238. |
[31] | Kim SI , Lee M , Kim HS , Chung HH , Kim J-W , Park NH , Song Y-S . Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J Ovarian Res. (2019) ;12: :40. doi:10.1186/s13048-019-0511-7. |
[32] | Kim SR , Malcolmson J , Li X , Bernardini MQ , Hogen L , May T . The correlation between BRCA status and surgical cytoreduction in high-grade serous ovarian carcinoma. Gynecologic Oncol. (2021) ;162: :702–6. doi:10.1016/j.ygyno.2021.07.010. |
[33] | Liu W , Wang Z , Ma J , Hou Y , Zhao J , Dong B , et al. Elevated serum level of CA125 Is a biomarker that can be used to alter prognosis determined by BRCA mutation and family history in ovarian cancer. Genetic Test Mol Biomarkers. (2017) ;21: :547–54. doi:10.1089/gtmb.2017.0104. |
[34] | Badgwell D , Bast RC Jr . Early detection of ovarian cancer. Disease Markers. (2007) ;23: :397–410. doi:10.1155/2007/309382. |
[35] | Rosen DG , Wang L , Atkinson JN , Yu Y , Lu KH , Diamandis EP , et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecologic Oncol. (2005) ;99: :267–77. doi:10.1016/j.ygyno.2005.06.040. |
[36] | Bast RC , Badgwell D , Lu Z , Marquez R , Rosen D , Liu J , et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. (2005) ;15: :274–81. doi:10.1111/j.1525-1438.2005.00441.x. |
[37] | Medeiros LR , Rosa DD , da Rosa MI , Bozzetti MC . Accuracy of CA 125 in the diagnosis of ovarian tumors: A quantitative systematic review. Eur J Obstetrics Gynecol Reproductive Biol. (2009) ;142: :99–105. doi:10.1016/j.ejogrb.2008.08.011. |
[38] | Moore RG , Miller MC , Steinhoff MM , Skates SJ , Lu KH , Lambert-Messerlian G , Bast RC . Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am J Obstetrics Gynecol. (2012) ;206: :351.e1–351.e8. doi:10.1016/j.ajog.2011.12.029. |
[39] | Anastasi E , Manganaro L , Granato T , Benedetti Panici P , Frati L , Porpora MG . Is CA72-4 a useful biomarker in differential diagnosis between ovarian endometrioma and epithelial ovarian cancer? Disease Markers. (2013) ;35: :331–5. doi:10.1155/2013/984641. |
[40] | Oaknin A , Guarch R , Barretina P , Hardisson D , González-Martín A , Matías-Guiu X , et al. Recommendations for biomarker testing in epithelial ovarian cancer: A national consensus statement by the spanish society of pathology and the spanish society of medical oncology. Clin Transl Oncol. (2018) ;20: :274–85. doi:10.1007/s12094-017-1719-x. |
[41] | Zhong Q , Peng H-L , Zhao X , Zhang L , Hwang W-T . Effects of BRCA1 - and BRCA2 -related mutations on ovarian and breast cancer survival: A meta-analysis. Clin Cancer Res. (2015) ;21: :211–20. doi:10.1158/1078-0432.CCR-14-1816. |
[42] | Yang W-L , Lu Z , Bast RC . The role of biomarkers in the management of epithelial ovarian cancer. Expert Rev Mol Diagnostics. (2017) ;17: :577–91. doi:10.1080/14737159.2017.1326820. |
[43] | Sölétormos G , Duffy MJ , Othman Abu Hassan S , Verheijen RHM , Tholander B , Bast RC , et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: Updated guidelines from the european group on tumor markers. Int J Gynecol Cancer. (2016) ;26: :43–51. doi:10.1097/IGC.0000000000000586. |
[44] | Skates SJ , Greene MH , Buys SS , Mai PL , Brown P , Piedmonte M , et al. Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent CA125 testing in women at increased familial risk – combined results from two screening trials. Clin Cancer Res. (2017) ;23: :3628–37. doi:10.1158/1078-0432.CCR-15-2750. |
[45] | Plotti F , Terranova C , Guzzo F , De Cicco Nardone C , Luvero D , Bartolone M , et al. Role of BRCA mutation and HE4 in predicting chemotherapy response in ovarian cancer: A retrospective pilot study. Biomedicines. (2021) ;9: :55. doi:10.3390/biomedicines9010055. |
[46] | Veltman J , Mann R , Kok T , Obdeijn IM , Hoogerbrugge N , Blickman JG , Boetes C . Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur Radiol. (2008) ;18: :931–8. doi:10.1007/s00330-008-0851-y. |
[47] | Kaas R , Kroger R , Peterse JL , Hart AAM , Muller SH . The correlation of mammographic-and histologic patterns of breast cancers in BRCA1 Gene mutation carriers, compared to age-matched sporadic controls. Eur Radiol. (2006) ;16: :2842–8. doi:10.1007/s00330-006-0385-0. |
[48] | Gallotta V , Conte C , D’Indinosante M , Capoluongo E , Minucci A , De Rose AM , et al. Prognostic factors value of germline and somatic brca in patients undergoing surgery for recurrent ovarian cancer with liver metastases. Eur J Surgical Oncol. (2019) ;45: :2096–102. doi:10.1016/j.ejso.2019.06.023. |
[49] | Gallotta V , Bruno M , Conte C , Giudice M , Davià F , Moro F , et al. Salvage lymphadenectomy in recurrent ovarian cancer patients: Analysis of clinical outcome and BRCA1/2 gene mutational status. Eur J Surgical Oncol. (2020) ;46: :1327–33. doi:10.1016/j.ejso.2020.01.035. |




