Annona muricata silver nanoparticles exhibit strong anticancer activities against cervical and prostate adenocarcinomas through regulation of CASP9 and the CXCL1/CXCR2 genes axis
Abstract
BACKGROUND:
Green synthesized nanoparticles have been earmarked for use in nanomedicine including for the development of better anticancer drugs.
OBJECTIVE:
The aim of this study was to undertake biochemical evaluation of anticancer activities of green synthesized silver nanoparticles (AgNPs) from ethanolic extracts of fruits (AgNPs-F) and leaves (AgNPs-L) of Annona muricata.
METHODS:
Previously synthesized silver nanoparticles were used for the study. The effects of the AgNPs and 5-Fluorouracil were studied on PC3, HeLa and PNT1A cells. The resazurin, migration and colonogenic assays as well as qRT-PCR were employed.
RESULTS:
The AgNPs-F displayed significant antiproliferative effects against HeLa cells with an IC50 of 38.58μg/ml and PC3 cells with an IC50 of 48.17μg/ml but selectively spared normal PNT1A cells (selectivity index of 7.8), in comparison with first line drug 5FU and AgNPs-L whose selectivity index were 3.56 and 2.26 respectively. The migration assay revealed potential inhibition of the metastatic activity of the cells by the AgNPs-F while the colonogenic assay indicated the permanent effect of the AgNPs-F on the cancer cells yet being reversible on the normal cells in contrast with 5FU and AgNPs-L. CASP9 was significantly over expressed in all HeLa cells treated with the AgNPs-F (1.53-fold), AgNPs-L (1.52-fold) and 5FU (4.30-fold). CXCL1 was under expressed in HeLa cells treated with AgNPs-F (0.69-fold) and AgNPs-L (0.58-fold) and over expressed in cells treated with 5FU (4.95-fold), but the difference was not statistically significant. CXCR2 was significantly over expressed in HeLa cells treated with 5FU (8.66-fold) and AgNPs-F (1.12-fold) but under expressed in cells treated with AgNPs-L (0.76-fold).
CONCLUSIONS:
Here we show that biosynthesized AgNPs especially AgNPs-F can be used in the development of novel and better anticancer drugs. The mechanism of action of the AgNPs involves activation of the intrinsic apoptosis pathway through upregulation of CASP9 and concerted down regulation of the CXCL1/ CXCR2 gene axis.
1Abbreviations
AgNPs | Silver Nanoparticles |
AgNPs-F | Silver Nanoparticles from fruits of Annona muricata |
AgNPs-L | Silver Nanoparticles from leaves of Annona muricata |
A570 | Absorbance at 570 nm |
A600 | Absorbance at 600 nm |
5FU | 5-Fluorouracil |
CC50 | Cytotoxic concentration required to kill 50% of the normal cells |
DMEM | Dulbecco’s modified Eagle medium |
DMSO | Dimethyl Sulfoxide |
FCS | Fetal Calf Serum |
IC50 | Inhibitory concentration required to kill 50% of the cancer cells |
RPMI | Roswell Park Memorial Institute |
SD | Standard Deviation |
1Introduction
Cancer is reported to rank second among the top causes of death worldwide and was responsible for 8.8 million deaths in 2015, a figure expected to rise if no immediate interventions are put in place [1]. Cervical cancer remains the top cause of morbidity and mortality in women diagnosed with cancer in Eastern Africa while prostate cancer is the most prevalent cancer type in men within the region [2, 3]. Currently, there is a limited choice of treatments for these cancers [2– 6], and therefore the need to continue searching for more effective treatment regimens.
Among the hallmarks of human cancers is the intrinsic or acquired resistance to apoptosis. Cancer cells resistance to apoptosis is therefore a critical rationale behind treatment failure [7, 8]. CASP9 gene encodes Caspase 9 enzyme which can undergo autoproteolytic digestion and activation by the apoptosome activating factor 1 which is critical in the earliest steps in the caspase activation cascade of the intrinsic pathway [9, 10].
Chemokines are a group of low-molecular-weight chemotactic cytokines that participate in various cellular processes such as embryogenesis, angiogenic activity, leukocyte migration and tumor growth and metastasis [11–13]. CXCLs/CXCR2 signaling is important in both cancer and different inflammatory diseases. Because the increased expression of CXCR2 and neutrophil/monocyte migration is critical in chronic inflammation and cancer, the need to focus on using CXCR2 antagonists in cancer and related diseases drugs development remains eminent [12, 13]. CXCL1 gene as well as its receptor CXCR2 gene have been found to be highly associated with tumorigenesis, angiogenesis, and metastasis [11, 12].
In recent times, traditional medicine has taken an important place especially in developing countries where limited health services are available [14, 15]. Annona muricata of the Annonaceae family is one plant that has a myriad of reported medicinal values and therapeutic potential [15–24]. Annona muricata is known as Soursop (English), Graviola (Portuguese), Guanábana (Latin American Spanish), Omusitafeli / Ekitafeli (Uganda), and other local indigenous names as has been enlisted [25, 26]. Various medicinal uses have been reported across the globe ranging from the use of leaves, bark, roots, fruits and seeds of Annona muricata [15, 16, 27– 36, 17, 37– 43, 18– 24]. Despite the wide spread use of herbal products by the majority of sick people, it has been reported that most of the biologically active constituents of plant extracts, such as flavonoids, tannins, and terpenoids, are highly soluble in water, but have low absorption, because they are unable to cross the lipid membranes of the cells, have excessively high molecular size, or are poorly absorbed, resulting in loss of bioavailability and efficacy. Therefore some extracts despite having very good activity in vitro which are not reproducible in vivo, are not used clinically because of these obstacles [44]. It has therefore been widely proposed to combine herbal medicine with nanotechnology, because nanosystems can deliver the bioactive components at a sufficient concentration during the entire treatment period, directing them to the desired sites of action, and hence potentiating the action of the compounds, an aspect that conventional herbal treatments do not meet [44, 45]. To improve efficacy and bioavailability of plant derived medicinal products, green nanobiotechnology has been widely advocated for because of the various beneficial properties of nanosystems in delivery of bioactive components to target sites which conventional herbal treatments cannot meet [44, 45]. In line with the above, this study employed previously green synthesized AgNPs from ethanolic extracts of fruits and leaves of Annona muricata [46– 48] to study their anticancer activities on different cancer cell lines. It specifically involved exploring the in vitro anticancer activities of the AgNPs as well as studying their effects on expression of Caspase 9 (CASP9), C-X-C motif chemokine ligand 1(CXCL1) and C-X-C motif chemokine receptor 2 (CXCR2) genes in cervical and prostate cancer cell lines.
2Materials and methods
2.1Chemicals and reagents
RPMI1640, DMEM, Non-Essential Amino Acids, Penicillin/Streptomycin, Resazurin, and Trypsin-EDTA were procured from Solarbio (China Phosphate buffered saline (PBS), 5FU, FCS, and Dimethyl Sulfoxide (DMSO) were procured from Sigma Aldrich (Germany).
2.2Silver nanoparticles
Previously prepared and characterized AgNPs from ethanolic extracts of fruits (AgNPs-F) and leaves (AgNPs-L) of Annona muricata were used for the study [46– 48]. AgNPs-F used had an absorption maximum at 427 nm and were stable under different pH, Temperature and storage conditions. The AgNPs-F had an average crystalline size of 60.12 nm, a polydispersity index of 0.1235 and were spherical in nature. The functional groups responsible for the formation of the AgNPs included; Alkanes and alkyls, aldehydes and esters, nitro groups, alcohol groups, amines, amides, alkenes, acids and alkyl halides [46, 47]. On the other hand, AgNPs-L used had an absorption maximum at 429 nm and were stable under different pH, Temperature and storage conditions. The AgNPs-L had an average crystalline size of 87.36 nm, a polydispersity index of 0.16 and were spherical in nature. The functional groups responsible for the formation of the AgNPs included; Alkanes and alkyls, aldehydes and esters, nitro groups, alcohol groups, amines, amides, alkenes, acids and alkyl halides [47].
2.3Cell culture
Three cell lines PC3, HeLa and PNT1A were used in the study. The PC-3 and HeLa cells were Prostate and Cervical adenocarcinomas respectively, while PNT1A cells were normal immortalized prostate cells. The cells were a gift from Dr Tim Forshew, University College London, UK.
Cells were grown in appropriate media containing L-Glutamine and supplemented with 1% Penicillin/Streptomycin, 1% Non-essential amino acids and 10% fetal calf serum (FCS). PC3 and PNT1A cells were propagated in RPMI 1640 while HeLa cells were propagated in DMEM. The cells were kept in a humified Carbon dioxide incubator (5% CO2 and 95% humidity) at 37°C. Cells were passaged and used for the assays during the exponential phase at about 60– 75% confluence.
2.4Effects on cell viability using the resazurin assay
The effects of the AgNPs on the viability of the cells was determined using the Resazurin assay as previously described [49– 52]. Exponentially growing cells were harvested, washed, counted using a haemocytometer and seeded in 96 well plates containing 0.5×104 Cells/well and incubated with 100 uL per well culture media and allowed to attach overnight. Seeding media was then removed. The attached cultured cells were then treated by adding of 100μL of the treatments at concentrations of 200, 100, 50, 25, and 12.5μg/mL (in culture media). DMSO alone in media was used as the solvent control blank (DMSO = 0.5% v/v). 5-FU was used as a reference positive control drug for cancer. The treated cells were then incubated in a humified CO2 incubator at 37°C. 24 Hours later, 20μl of 0.15 mg/ml in PBS resazurin solution was added to each of the wells and then incubated for 4 more hours.
The plates were then removed from the incubator and the absorbance signal was quickly measured at 570/600 nm (excitation/emission wavelengths), using a microplate reader (Infinite M1000, Tecan). Viable cells change the resazurin from blue (oxidized) to red (reduced) forms. The percentage cell viability was determined using the formula: % Viability = (Net absorbance of treated samples/Net absorbance of blank)×100. The effect of the samples on the growth of the cells were then expressed in form of graphs of percentage cell viability against logarithm of concentration. Fifty percent of inhibitory concentration (IC50) of each of the treatments were calculated from the growth inhibition curves. Selectivity index for each of the treatments was calculated.
All subsequent studies were undertaken on only HeLa and PNT1A cells, representing the cancerous and normal cell types respectively.
2.5Cell migration assay
The effect of the AgNPs on cell migration were analyzed by use of the 2-dimensional cell culture wound closure assay as previously described [53– 55]. Cells in the logarithmic phase of growth were harvested and about 5.0×104 cells/well were plated in 12 well plates and appropriate media added, with routine monitoring and change until the cells reached 95– 100% confluence. In a sterile environment, a 100μl pipette tip was used to press firmly against the top of the tissue culture plate and swiftly made a vertical wound down through the cell monolayer in each well. The media and cell debris were then carefully aspirated. 2 ml of culture media containing the different treatments at their predetermined IC50 values (AgNPsF-IC50, AgNPsF-IC50, 5FU- IC50, and Blank – vehicle in media,) were then slowly added against the well walls, one column per treatment. For PNT1A cells, the treatments were at their predetermined CC50.
Following the generation and inspection of the wound, as well as addition of the treatments, initial pictures were taken (Time 0) using an inverted microscope. The cell culture plates were then incubated. At several time points (6, 24, and 48 Hrs), the plates were removed from the incubator and placed under an inverted microscope to take snapshot pictures and to check for wound closure. Wound closure was then analyzed and clearly presented as time series image captions against time. The treatments had different effects on the cell lines wound closure ability thus their effect on migration, metastasis and generally the regulation of cell motility.
2.6The colony formation assay
A colonogenic assay was conducted to determine the ability of the cells to proliferate indefinitely and form colonies (effect on the replicative potential) upon drug removal [56, 57]. Cells in the logarithmic growth phase were harvested and seeded at 100-fold dilutions (1000 cells/well) from the normal plating density (1X 105 cells/well) in 6 well plates, allowed to attach and then treated with appropriate volumes of the AgNPs and controls at their predetermined IC50 values and incubated at 37°C in the growth chamber for 24 h. For PNT1A cells, the treatments were at their predetermined CC50 values.
The cells in each well were then washed with PBS 3 times (to remove the treatments) and replaced with regular culture medium. The cells were then incubated, with routine media replenishment until colonies had fully recovered and formed in the wells with the blank. At the end of the experiment, surviving colonies were stained with 0.4% crystal violet (Sigma) in 50% methanol, and the number of colonies (each consisting of at least 50 cells) were then counted in an inverted microscope [56, 57]. Results were presented as mean number of colonies per treatment. At least 3 replicates per treatment were done.
2.7RNA extraction
Exponentially growing cells at a confluence of about 70% in T75 flasks were exposed to the IC50 of the different treatments for 24 hours. Total RNA was extracted from each of the samples using the DirectZol kit (Zymo Research, USA) according to manufacturer’s instructions. Briefly, cells were prepared in lysis buffer, and then purified by directly adding them to the Zymo-Spin™ II Column. This was spun and washed after which the RNA was eluted. The purity, quality, quantity and integrity of the extracted RNA was determined by resolution using a 1% agarose gel electrophoresis and nanodrop spectrophotometry (Nanodrop 2000C Spectrophotometer – Thermo Scientific).
2.8cDNA synthesis
Approximately 100 ng/μl of RNA was used for cDNA synthesis using FIREScript RT cDNA synthesis kit (Solis BioDyne, Estonia) according to manufacturer’s instructions. A 20μl reaction volume contained 10μl of template RNA (100 ng/μl), 1μl oligo (dT) primer (100μM), 0.5μl dNTP mix (20 mM), 2μl 10×RT reaction buffer with DTT, 1μl FIREScript RT, 0.5μl RNase inhibitor (40 U/μl) and 5μl nuclease free water. Using SimpliAmp™ thermal cycler (Applied Biosystems, USA), the reverse transcription process was carried out at 50°C for 60 minutes followed by enzyme inactivation at 85°C for 5 minutes. The synthesized cDNA was assessed for quality and quantity using a nanodrop (Nanodrop 2000C Spectrophotometer – Thermo Scientific).
2.9Primers design for genes of interest
Genes assayed included CASP9, CXCL1 and CXCR2. GAPDH was used as a house keeper to set baseline. Primers for use for qPCR were designed using NCBI’s Primer Blast tool. The reference sequence for each of the Homo sapiens gene were retrieved from GenBank and the FASTA sequences format used to design the primers. The primers were set to have a PCR product in the size range between 75 – 250 bps, % GC content of between 40 – 55%, as well as self-complementarity of not more than 2. The selected primer sequences for each of the genes were run on dry PCR using the Sequence Manipulation Suite Bioinformatics tools (SMS Bioinformatics) in order to confirm the products size and the sequences of the PCR products.
The studied genes, their corresponding primer sets, annealing temperatures the expected PCR product sizes as well as are as their NCBI GenBank Reference sequences are outlined in Table 1. The primers were manufactured by Macrogen Inc. South Korea.
Table 1
Primers for qRT-PCR
| Gene | Sequence | Annealing Temp. / ° C | PCR product size / bp | NCBI Ref Seq. | |
| GAPDH | F | GCTCCCACCTTTCTCATCCA | 61 | 139 | NC_000012.12 |
| R | TACTCCCCACATCACCCCTCTA | ||||
| CASP9 | F | TAAGCAGGAGATGAACAAAGGAAGA | 61 | 172 | NC_000001.11 |
| R | GAAATGGGGAGACAAGGTGAGA | ||||
| CXCLI | F | CAGTAGGACAAACAGCAACAGGT | 61 | 90 | NC_000004.12 |
| R | TCTTTTAGGAATGGGGGTGGGG | ||||
| CXCR2 | F | GCCACTCCAATAACAGCAGGT | 61 | 131 | NC_000002.12 |
| R | GCTTCTACACTTCATCACCCC | ||||
2.10qRT-PCR analysis and relative gene expression
Quantitative real-time polymerase chain reaction was used to assess the effect of the treatments on the expression of the selected genes. The qRT-PCR reaction mix were set up with EvaGreen® qPCR Mix (Solis-BioDyne, Estonia) according to manufacturer’s protocol. A 20μl reaction comprised of 4μl of the Master Mix, 0.5μl of each of forward and reverse primer set (10μM), 5μl cDNA (50 ng/μl) and 10μl nuclease-free water. Each reaction was conducted in triplicates. The qRT-PCR was carried out in a Light Cycler® 96 instrument (Roche Diagnostics GmbH, Germany) with the following cycling program: 95°C for 720 s followed by 50 cycles of 95°C for 15 s, 61°C (annealing) for 20 seconds and 72°C for 20 seconds. The above program was used for all the primers.
The threshold cycle (Ct) was calculated. The melting curve was examined for each reaction to confirm that only desired PCR products were amplified and to rule out possibility of primer-dimers contributing to the amplification signal. To confirm that only one single PCR amplicon of expected product size was produced, a 4 % agarose gel electrophoresis was run.
Relative quantification of specific mRNA levels was performed using the comparative 2–ΔΔCt method [58]. GAPDH was used as the reference gene against which the expression values were normalized. The expression levels were presented as n-fold differences relative to the controls/ calibrator.
2.11Statistical analysis
The data points represent the average of at least 3 independent experiments. The data was presented using Microsoft Office, Excel software and expressed as mean±SD. Statistical package for social scientists, IBM version 21 was used by employing the ANOVA technique to observe the significance between the groups. All analyses were conducted at p < 0.05.
2.12Ethics approval
The study was cleared by the PAUSTI board of examiners, registration number MB400-0007/17, Uganda National Council for Science and Technology (NS 43ES) as well as the Jomo Kenyatta University of Agriculture and Technology Institutional Ethics Review Committee (Ref. no: JKU/2/4/896B).
3Results
3.1Resazurin metabolic assay cytotoxicity results
There was strong registered antiproliferative activity of the tested drugs on the different cell lines as shown in Table 2. Graphs for computations of the IC50s and CC50s are available as supplementary material. Additionally, it is evident that there was some cytotoxic activity of all the AgNPs tested and the 5FU on the normal cells. The AgNPs-L had the highest cytotoxic activity compared to the AgNPs-F and 5FU. In contrast, AgNPs-F and the standard anticancer drug 5FU had a lower cytotoxic activity on the normal cells, implying their potential high selectivity for cancer cells compared to normal cells. The AgNPs-L may not be very selective, but this has to be confirmed from the selectivity index studies. It is very critical to note that selectivity is a very key factor in cancer drugs development.
Table 2
Summary of IC50 values of the different treatments corresponding to the cell lines used
| Cell line | IC50 (μg/ml) / CC50 (μg/ml) | ||
| AgNPs-F | AgNPs-L | 5FU | |
| HeLa | 38.58 | 57.63 | 10.38 |
| PC3 | 48.17 | 47.58 | 235.9 |
| PNT1A | 375.68 | 112.29 | 840.37 |
3.2Selectivity index of the AgNPs
From the results in Table 3, it was revealed that AgNPs-F had the highest selectivity index of 7.8. AgNPs-L and 5FU had very low selectivity index, and thus their higher toxicity to non-target cells.
Table 3
Selectivity index of the AgNPs and 5FU
| Cell line | IC50 (μg/ml) / CC50 (μg/ml) | ||
| AgNPs-F | AgNPs-L | 5FU | |
| PC3 | 48.17 | 47.68 | 235.9 |
| PNT1A | 375.68 | 112.29 | 840.37 |
| Selectivity Index (SI) = (CC50 / IC50) | 7.80 | 2.36 | 3.56 |
CC50 – Cytotoxic concentration required to kill 50% of the normal cells; and IC50 – Inhibitory concentration required to kill 50% of the cancer cells.
3.3Migration assay
The arrows denote boundaries of the wound regions at both ends of the monolayer with the gap in between the arrows indicating the wound. A0, A6, A24, and A48 in Table 4 represent the effects of the blank on the wound healing of HeLa cells. As shown, there was a significant increase in wound closure with time. Within 48 hours from the start of the experiment, almost the entire wound had been closed and a monolayer generally re-established. B0, B6, B24 and B48 represent the effects of the AgNPs-F on the wound closure. There was no closure of the wound observed throughout the treatment period. on the contrary, the wound/zone of clearance increased in size probably due to the effect on the treatment on the cells over time. This must have been as a result of the dying of the cells coupled with the inhibition of the pathway for production of proteins responsible for the adhesion as well as motility.
Table 4
Table showing the wound healing activity on HeLa cell line
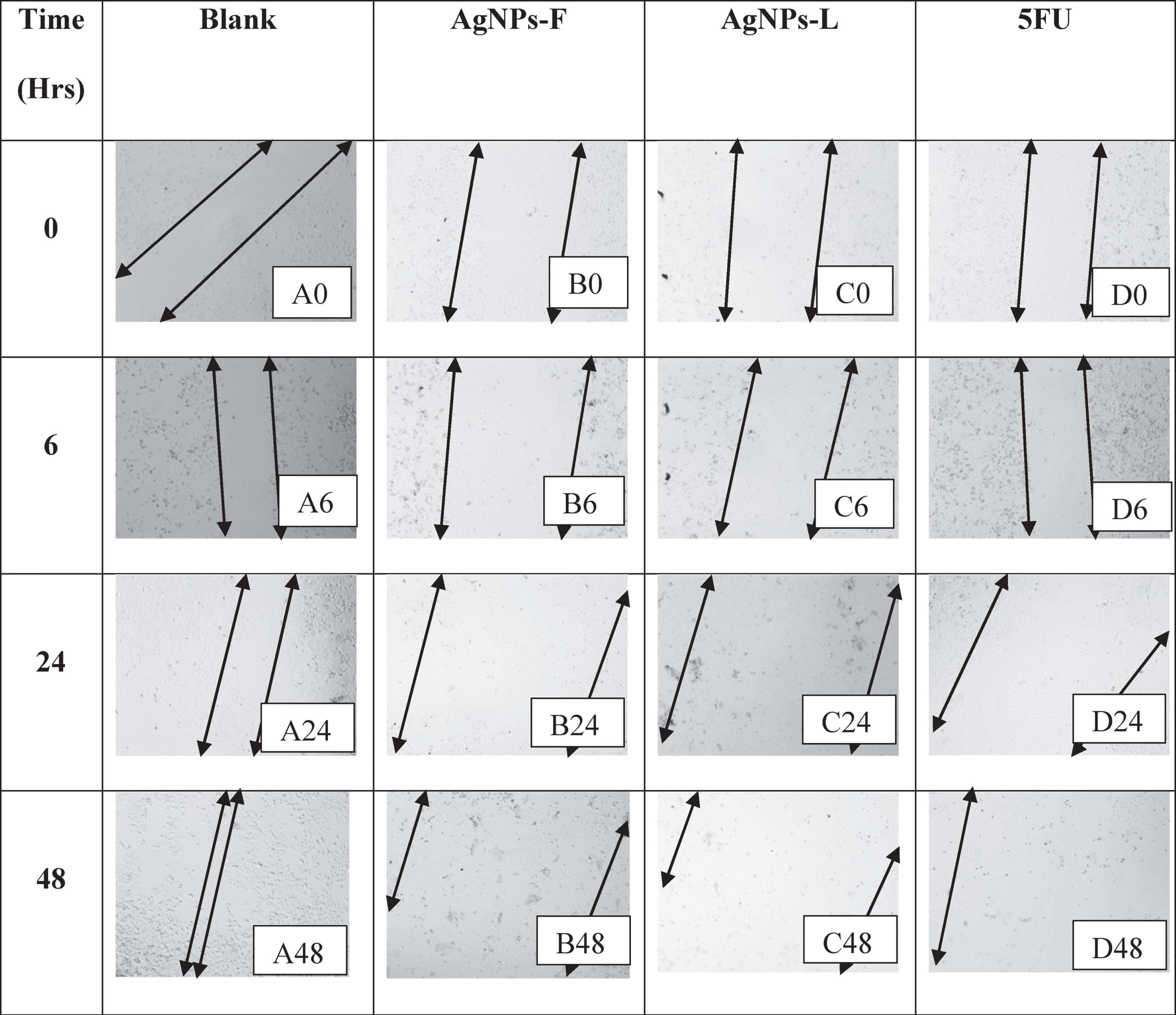 |
Similarly, the effects of AgNPs-L (represented by images C0, C6, C24 and C48) as well as that of 5FU (represented by images D0, D6, D24, and D48), inhibited the wound closure process and initiated increased cell clearance and loss of adherence and motility as explained for AgNPs-F above. This loss of ability to adhere and migrate as well as increased cell death is of great importance as it helps interfere with the process of metastasis in the cancerous cells and thus preventing further spread of the tumors.
The arrows denote boundaries of the wound regions at both ends of the monolayer, with the gap in between the arrows indicating the wound. P0, P6, P24, and P48 in Table 5 represent the effects of the blank on the wound healing of PNT1A normal cells at the CC50 values. As shown, there was an initiation in wound closure with time as shown by the cells’ movement towards the centre of the wound. Within 48 hours from the start of the experiment, there was a greater advance towards the re-establishment of monolayer, though the speed was not as fast. Probably due to the slow growth properties of the cells. On the other hand, Q0, Q6, Q24 and Q48 represent the effects of the AgNPs-F on the wound closure. There was no closure of the wound observed throughout the treatment period. on the contrary, the wound/zone of clearance remained static probably due to the effect on the treatment on the cells over time. There was no wound closure as well as no increase in the zone of clearance. This must have been as a result the inhibition of the pathway for production of proteins responsible for the adhesion as well as motility. This is an indication that even at CC50 values, the AgNPs-F have a lower cytotoxic effect on normal cells, as evidenced by the maintenance of the monolayer at uncleared zones.
Table 5
Table showing the wound healing activity on PNT1A cell line at the CC50 values
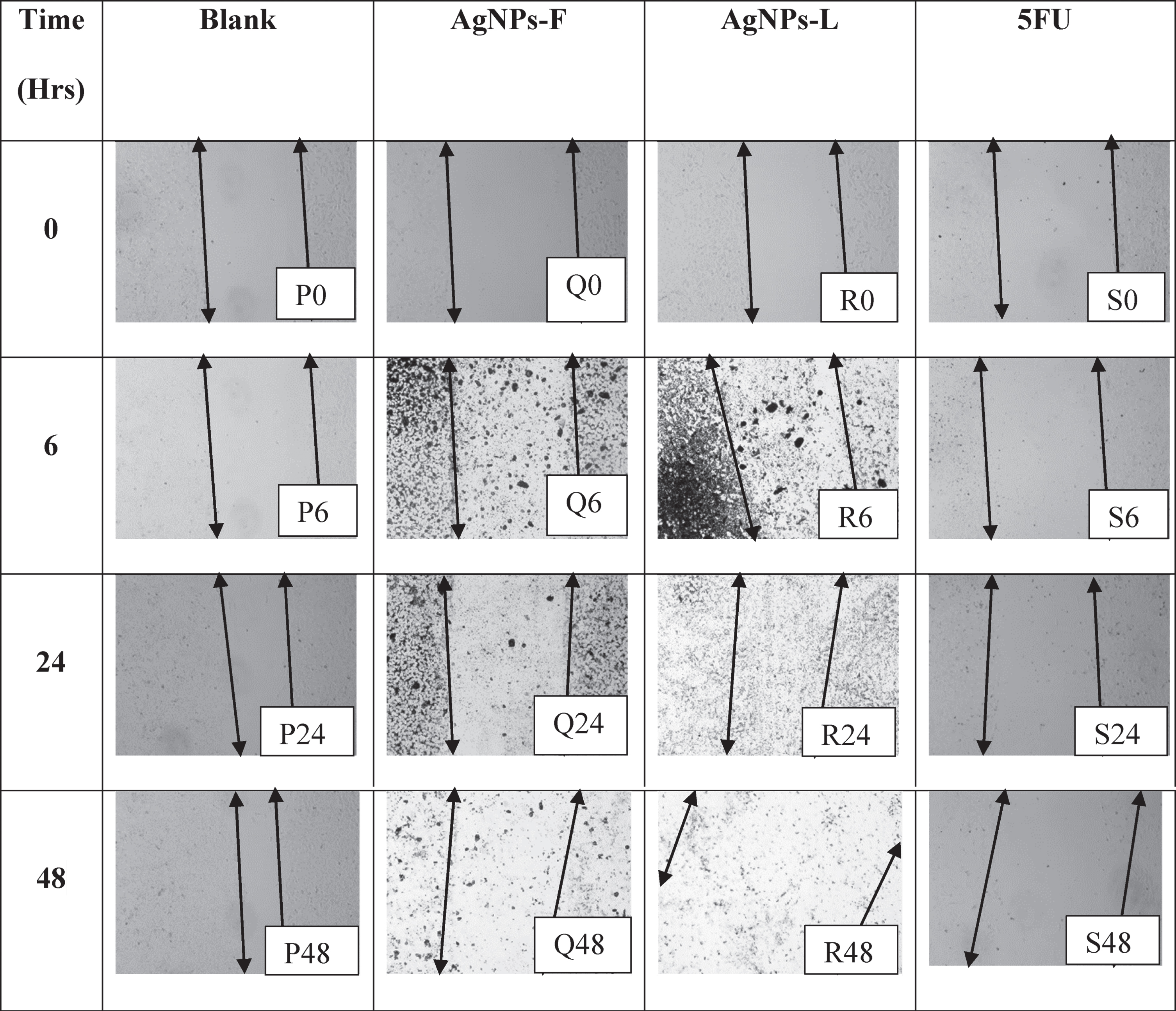 |
Conversely, the effects of AgNPs-L (represented by images R0, R6, R24 and R48) showed no wound closure coupled with an increase in the size of the zone of clearance. This could be due to the double effect of the toxic nature of the particles on the cells as well as their effects on the inhibition of the pathways responsible for the production of proteins critical in motility. The effects of 5FU (represented by images S0, S6, S24, and S48), show that they inhibited the wound closure process and initiated increased cell clearance and loss of adherence and motility as explained for AgNPs-L above. This loss of ability to adhere and migrate as well as increased cell death is of great importance as it helps shows that the 5FU and AgNPs-L could have adverse non-selective effects on the normal cells in the body, even if they are highly cytotoxic to the cancer cells. Selective cytotoxicity is very key in cancer drugs development.
3.4Colony formation assay
Figure 1 shows the results for colony formation and survival assay. It is evident that the AgNPs-F had the highest effect on the colony survival of the HeLa cells at 0% recovery post treatment. These were closely followed by 5FU (0.5%) and AgNPs-L (4.27%). Similarly, the treatments which had the lowest effect on the PNT1A normal cells were the AgNPs-F at 40.81% recovery. The 5FU and AgNPs-L were highly toxic to the normal cells as implied by their very low recovery rates of 20.66% and 6.81% respectively. These results are further in line with the earlier observation in relation to the selectivity index of the treatments where AgNPs-F (7.8) had the highest selectivity index followed by 5FU (3.56) and AgNPs-L (2.36) respectively.
Fig.1
Comparative colony formation assay results.
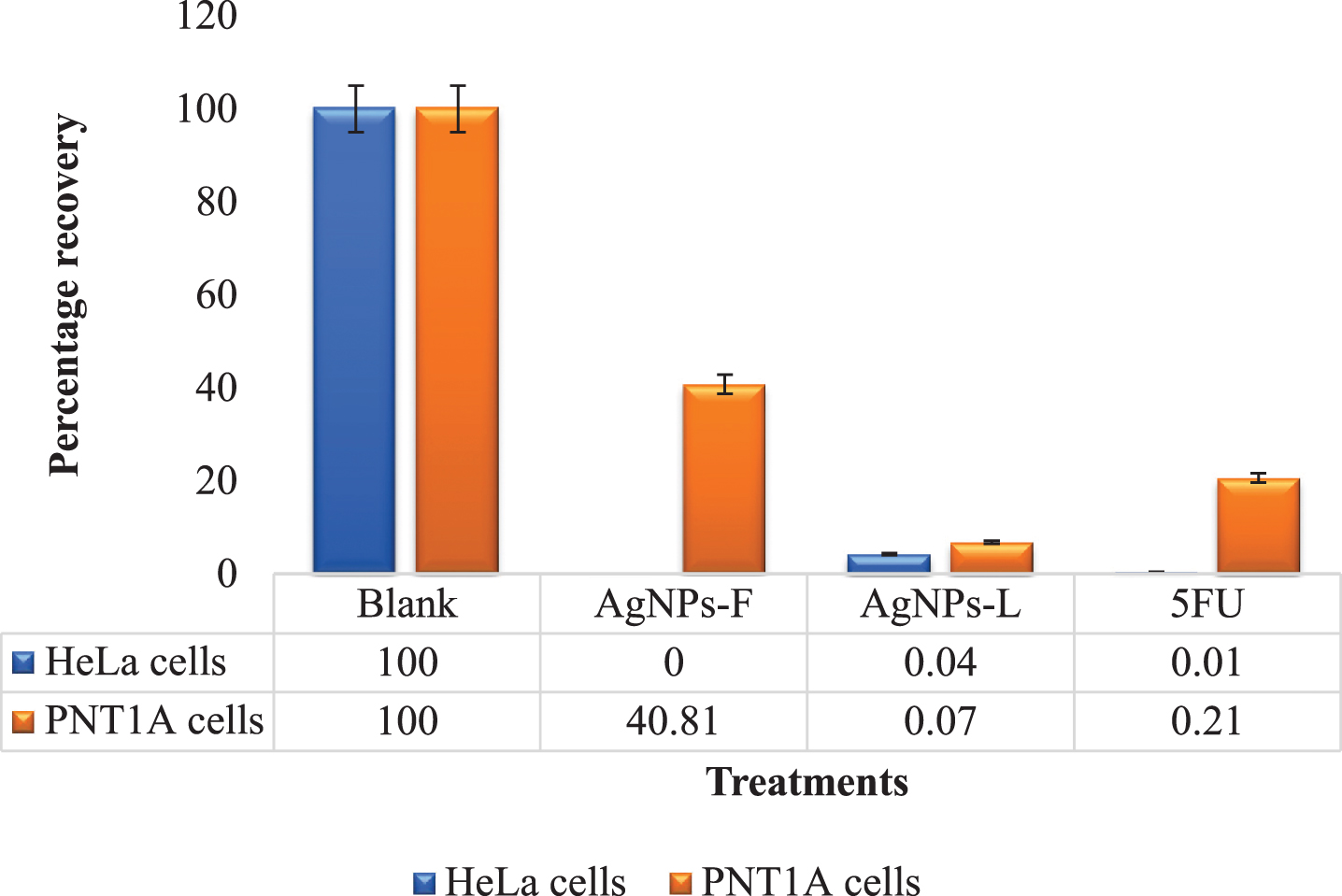
Figure 2 shows the relative gene expression in HeLa cells in response to the different treatments. Letters on top of each box indicate mean separations according to Tukey’s HSD test (≤0.05). Means followed by the same letter are not significantly different. At P = 0.03, CASP9 was significantly up regulated in HeLa cells subjected to all the 3 treatments of AgNPs-F (1.53-fold), AgNPs-L (1.52-fold) and 5FU (4.30-fold). CXCL1 was down regulated in both AgNPs-F (0.69-fold) and AgNPs-L (0.58-fold) treated HeLa cells and up regulated in 5FU (4.95-fold) treated cells. The up regulation and down regulation was however not statistically significant at P = 0.130. At P < 0.001, CXCR2 was significantly down regulated in AgNPs-L (0.64-fold) treated HeLa cells and significantly up regulated in both AgNPs-F (1.12-fold) and 5FU (8.66-fold) treated cells. The up regulation in AgNPs-F and down regulation of AgNPs-L treated cells was not statistically significantly different from the control. The up regulation of CXCR2 in 5FU treated cells was significantly different from the control.
Fig.2
Relative gene expression in HeLa cells in response to the different treatments.
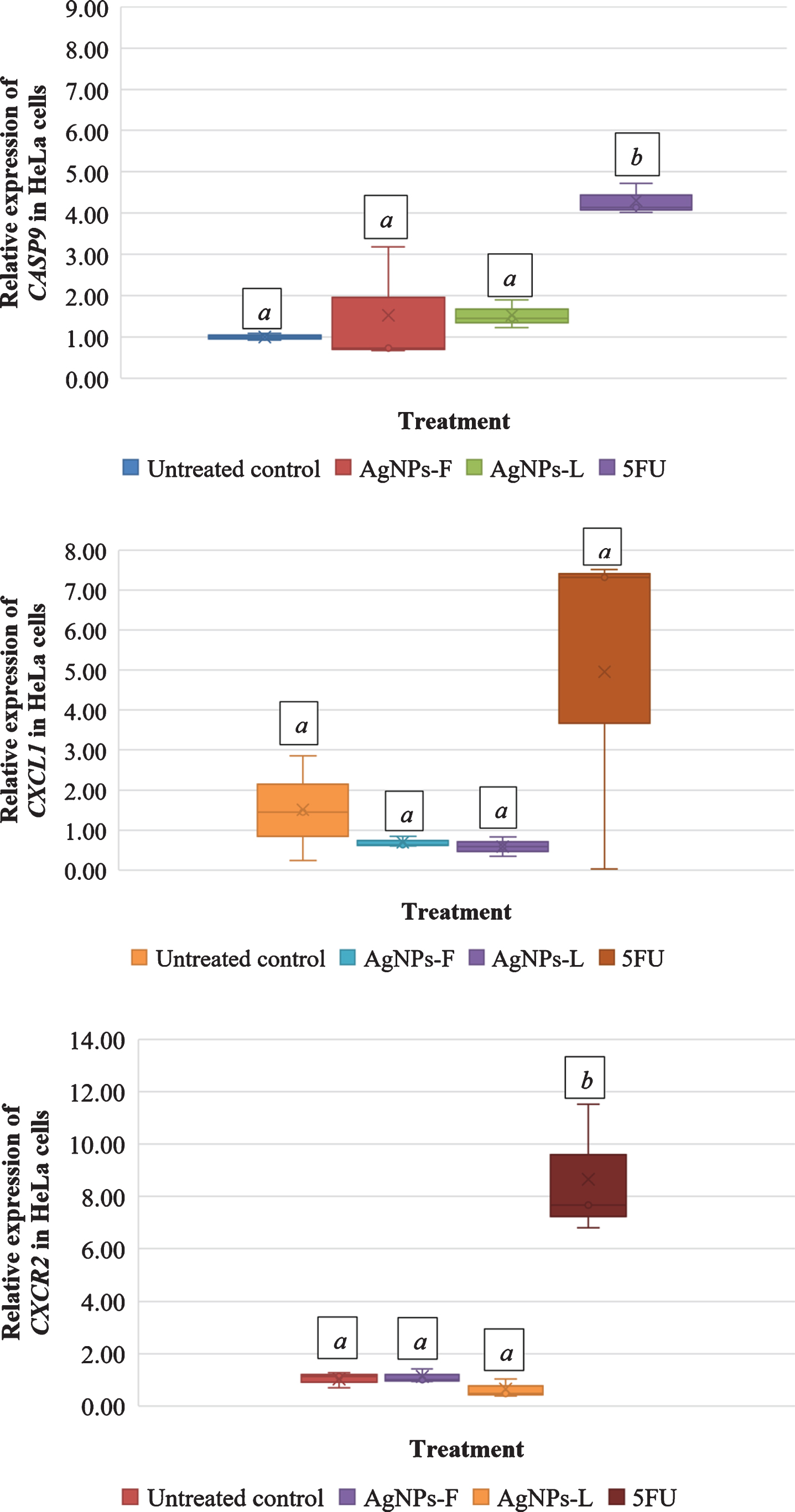
Figure 3 shows the relative gene expression in PNT1A cells exposed to different treatments. Letters on top of each box indicate mean separations according to Tukey’s HSD test (≤0.05). Means followed by the same letter are not significantly different. At P < 0.001, exposure of the cells to AgNPs-F, AgNPs-L and 5FU resulted into a significant up regulation of CASP9 at 1.37-fold, 16.78-fold and 1.36-fold respectively. However, the differences in the expression of CAPS9 in cells exposed to AgNPs-F and 5FU was not significant in relation to the control. Expressions in AgNPs-L exposed cells was significantly different from the control.
Fig.3
Relative gene expression in PNT1A cells exposed to different treatments.
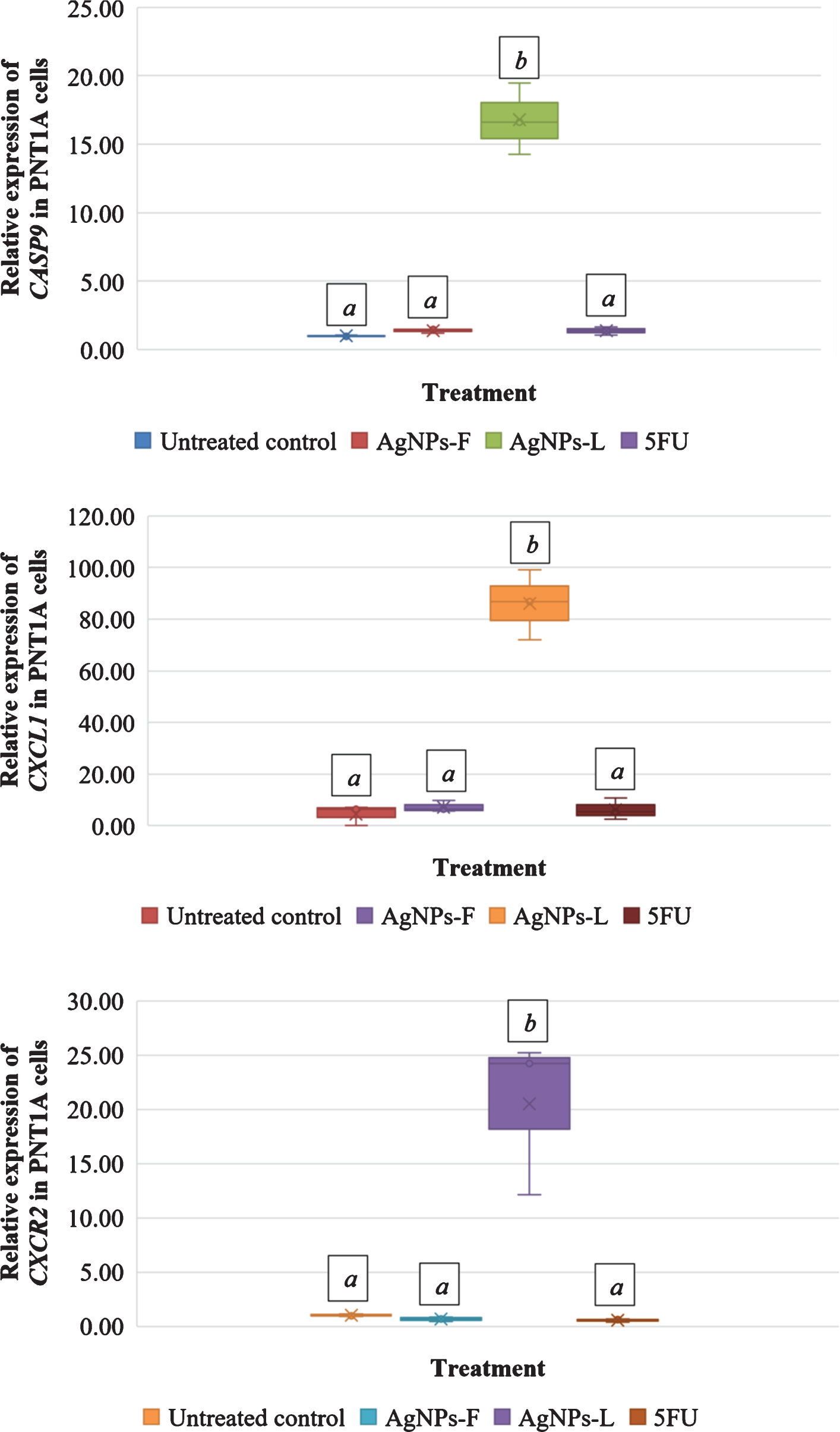
At P < 0.001, CXCL1 was significantly up regulated in PNT1A cells for all treatments of AgNPs-F (7.17-fold), AgNPs-L (85.96-fold) and 5FU (6.25-fold). There was an unusually very high up regulation of CXCR2 in cells exposed to AgNPs-L. At P < 0.001, CXCR2 was significantly down regulated in PNT1A cells exposed to both AgNPs-F (0.66-fold) and 5FU (0.54-fold), but was significantly up regulated in cells exposed to AgNPs-L (20.53-fold) just as was observed with CXCL1. There was no significant difference between the down regulation of CXCR2 in the AgNPs-F and 5FU treated cells relative to the control. There was a significant up regulation of CXCR2 in the cells exposed to AgNPs-L relative to the control.
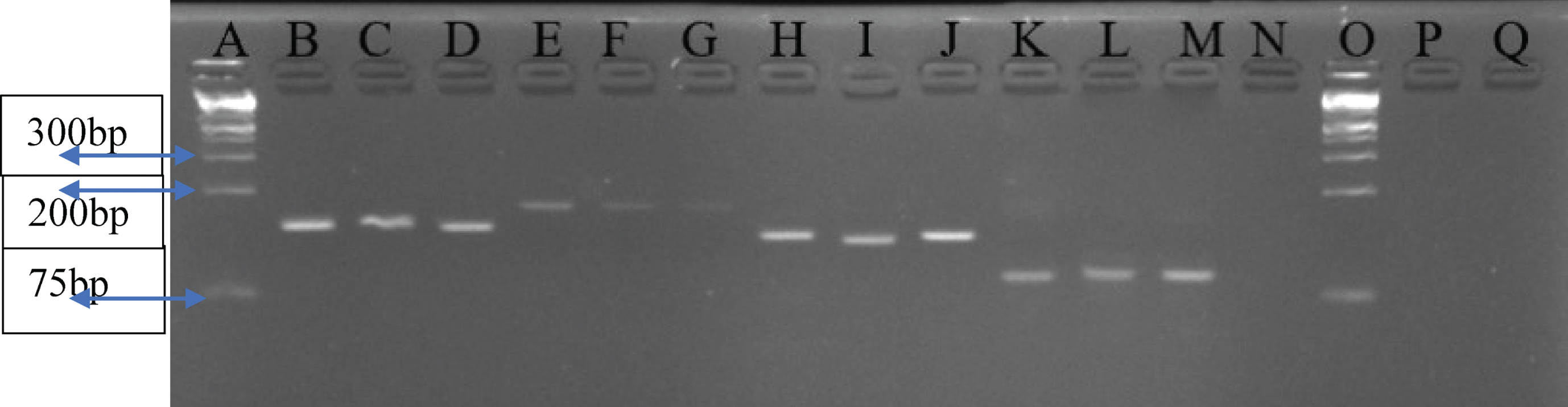
3.5qRT-PCR products electrophoresis results for selected amplicons
Figure 4 shows the qRT-PCR products electrophoresis results for selected amplicons.
Fig.4
A 4% Agarose gel analysis for selected amplicons of qRT-PCR products per gene. Lane A and O – 1 kb plus Ladder; Lanes B, C, D – GAPDH (139 kb); Lanes E, F, G – CASP9 (172 bp); Lanes H, I, J – CXCR2 (131 bp); Lanes K, L, M – CXCL1 (90 bp); N – Negative control.
4Discussion
The current study shows that both the AgNPs and 5FU had strong cytotoxic activities against HeLa cells with IC50 values of 38.58, 57.63 and 10.38μg/ml for AgNPs-F, AgNPs-L and 5FU respectively. Similarly, treatment of PC3 cells with the AgNPs and 5FU resulted in strong activities by the AgNPs with IC50 values of 48.17 and 47.68μg/ml for AgNPs-F and AgNPs-L respectively compared to 5FU with an IC50 of 235.9μg/ml. Whereas they did not use AgNPs in their studies, these results are in line with earlier studies that showed that Annona muricata extracts had strong cytotoxic activities against different cell lines [15, 26, 59]. The results are also in resonance with those from a study that used green synthesized AgNPs from Agrimoniae herba extracts against a human lung carcinoma cell line [60]. These results support the fact that AgNPs from ethanolic extracts of Annona muricata could be strong anticancer agents.
The AgNPs-F had a higher selectivity index compared to the standard drug 5FU. This implies that the AgNPs-F are safer to be used than the 5FU. Selectivity of drugs has been outlined as a critical hallmarks of any successful treatment regimen. From the current study with a selectivity index of 7.8, we do ascertain that the AgNPs-F would provide safer drugs. A higher activity on the cancerous cells while sparing the normal cells is very important. Comparatively, AgNPs-L had a much lower selectivity index than standard drug 5FU. From the current results therefore, it is evident that AgNPs-L are much more toxic than the 5FU, though within the same selectivity index range.
Metastasis is the main cause of cancer complications with 90% of deaths from solid tumors being ascribed to metastatic effects [61]. The need for novel agents that avoid metastatic spreading is therefore imperative for development of new antimetastatic drugs. The results of the migration assay in the HeLa cells indicate that the blank did not have any inhibitory activity of the migration of the cells to re-establish the mono layer. This is expected as there wasn’t any treatment added to these cells. In comparison, the marked inhibitory activity of the AgNPs and 5FU on the migration of the cells as evidenced by the failure for the cells to cover the wound as well as evident increase in the size of the wound and zone of clearance indicated that they have an effect on genes responsible for motility, invasion, inflammation, chemoattraction among others. When the ability of the cells to re-establish communication and contact is inhibited, it indicates that they can no longer be in position to move and thus directly impact on their metastatic ability [61]. These results were further be supported by gene expression studies as reported in the subsequent sections of this thesis. When metastatic cells cannot spread, then there is hope that the treatment can be a better drug.
In regard to the migration ability of the PNT1A normal cells, whereas there was initiation of wound closure observed in the plates treated with the blank, there was inhibition of the process by the AgNPs-F, AgNPs-L as well as the 5FU. Other than the inhibition of the wound closure, there wasn’t any other effect shown by the AgNPs-F. Comparatively, the 5FU and AgNPs-L, in addition to inhibiting the wound closure process, led to a partial increase in the size of the wound or zone of clearance. This observed phenomenon is probably due to the cytotoxic effect of the standard drug 5FU and AgNPs-L on normal cells that led to their continued death. This observation is in sync with the selectivity index results described much earlier, where the AgNPs-L and 5FU had relatively very low selectivity indices. These result therefore re-affirm the importance of selectivity of the drugs on the cells. Whereas all the treatments showed relatively high CC50s, the 5FU and AgNPs-L turned out to be more cytotoxic compared to the AgNPs-F, making the AgNPs-F a better drug of choice, once utilized.
The main importance of the colony survival assay is to assess the ability of the cells to re-establish their proliferative potential following withdraw of the treatment. Many major therapies used in the cancer treatment many a time result into resurgence of the cancers after the treatments withdrawn, a key factor in treatment failure as cells revert to adaption of the original hallmarks including sustaining proliferative signaling, evasion of growth suppressors, resistance of cell death, activation of replicative immortality [8]. In the current study, relative to the treatments with the blank, insights into the colony survival of the cells were elucidated. For HeLa cells, withdraw of the treatments resulted into 0% recovery for AgNPs-F, 0.5% recovery for 5FU and 4.17% recovery for the AgNPs-L. This therefore implies that the effect of the AgNPs-F on the HeLa cell lines is more less permanent with no chance of re-establishment of the cancer upon withdraw of the treatment. Comparatively, the observed 0.5% and 4.17 % recovery in the cells treated with 5FU and AgNPs-L respectively indicated that their effects are not permanent and therefore leaves some room for surviving cells to re-establish the cancer. This is a confirmation that AgNPs-F show better anticancer activity compared to the first line drug 5FU and AgNPs-L, and therefore would be a greater alternative.
Following withdraw of the drugs in the PNT1A normal cells, a different trend was observed. Relative to the blank, withdraw of the treatments resulted into 40.81 % recovery for AgNPs-F, 20.66 % recovery for 5FU, and 6.8 % recovery for AgNPs-L. This therefore implies that the effect of the AgNPs-F on the normal cell lines is temporary and cells can easily re-adopt and survive upon withdraw of the treatment. On the other hand, the observed 20 % recovery in the cells treated with 5FU indicated that whereas its effect is not very permanent on the normal cells, there is an almost 50% chances that cells will not survive exposure to 5FU compared to when they are exposed to the AgNPs-F. The same trend observed for 5FU applies to treatments with AgNPs-L. These results therefore further confirm that AgNPs-F show better selectivity for normal body cells compared to the standard drug 5FU and AgNPs-L, and therefore would be a greater alternative.
The relative up regulation of the CASP9 in all the treated HeLa cells is a direct indication of the involvement and activation of the intrinsic apoptotic pathway machinery as one of the mechanisms of action of the treatments under study. It is worth appreciating that CASP9 is the gene for the upstream caspase for the intrinsic pathway of apoptosis [9, 10, 62– 64]. Since apoptosis is one of the mechanisms of cells death, its activation due to exposure of the cancer cells to the treatments is a great indication of the effectiveness of the AgNPs and 5FU on the cytotoxicity of the cells and thus confirm the earlier observations of the resazurin assay in measurement of cytotoxicity. These are further supported by the CASP9 expression levels where 5FU that had the lowest IC50 (10.38μg/ml) also had the highest relative expression (4.30-fold), followed by AgNPs-F (IC50 of 38.58μg/ml) at 1.53-fold and finally AgNPs-L (IC50 57.63μg/ml) had the lowest expression levels at 1.52-fold. These observations are in accordance with earlier studies which reported that re-establishment of apoptosis is very vital in overcoming the immortal nature and other hallmark of cancer cells [62, 65].
Comparatively, there was up regulation of CASP9 in the PNT1A cells exposed to the AgNPs-F and 5FU, with the individual relative expression levels of 1.37-fold and 1.36-fold respectively, but the difference was not significant from the controls, implying that the up regulation of the gene was not very significantly different from the control and therefore the apoptosis not pronounced in these treatments thus increased selectivity [65]. For AgNPs-L, the relative expression levels of CASP9 at 16.78-fold is an indication of a very pronounced apoptosis on the normal cells, and thus an affirmation of the more toxic nature and non-selectivity of the AgNPs-L on the on the normal cells. The observed high toxicity of the AgNPs-L could be attributed to the original source of the phytochemicals from the leaves which were shown to be composed of various secondary metabolites, some of which highly toxic like cardiac glycosides [15, 26], which consequently affects the green synthesis process and resultant activities [47]. The evidence presented in relation to the CASP9 regulation of apoptosis corresponds with the earlier observations in regards to the selectivity indices of the AgNPs-F, 5FU and AgNPs-L.
Down regulation of CXCL1 in HeLa cells treated with AgNPs-F and AgNPs-L is in sync with the observed effects with the migration assay, where cells could not re-establish the mono layer due to inhibition of the genes involved in migration and thus invasion of the cells to other sites. The mechanism of action therefore, as earlier on proposed, involves downregulation of CXCL1, a gene heavily related to cancer cells migration and inflammation. Inhibition of migration has been proposed as one of the most effective ways to manage the effects of metastatic tumors by localization [61, 66]. On the other hand, the up regulation of the CXCL1 in HeLa cells treated with 5FU could be due the different mechanism of action of the drug that may not solely be dependent on CXCL1. Furthermore, up regulation of CXCL1 in the HeLa cells treated with 5FU presents evidence on the tumorigenicity nature of the treatment as it increases expression of the genes responsible for the development, inflammation and invasion of the cancer cells. This observation could explain as to why many 5FU based treatment regimens may not be very effective in managing different cancers. In comparison with the synthesized AgNPs, there is clear evidence that the AgNPs are much better than 5FU in regards to down regulating one of the most critical chemokines in cancer sustenance. The difference could further be attributed to the very small size of the AgNPs which have been shown to be in the range of 55 nm – 86 nm, which can therefore directly reach target sites within the DNA and thus exert their effect, in contrast to 5FU and other antimetabolite-based treatments.
The up regulation of the CXCL1 gene in PNT1A cells exposed to the different treatments is due to the fact that being normal immortalized cells, the effects of the treatments were not expected to be at a level of inhibiting normal chemotactic and growth activities in these cells. This revelation is also in sync with the earlier studies observed during the migration assays where there was evidence of initiation for re-establishment of wound closure. Normal immortalized cells have been designed to mimic the hall marks of cancer and thus are expected to continue growth and communication just like cancerous cells. Down regulation of some of the key genes involved in the chemo-activities would directly imply high toxicity levels of the treatments under study. These reported results therefore support our earlier reported observations on selectivity in normal cells.
Down regulation and near constant expression of CXCR2 gene in HeLa cells treated AgNPs-L and AgNPs-F respectively is directly due to the reduced activity of the CXCR2 protein since its corresponding ligands such as CXCL1 was already under expressed. The concerted under and expression of the CXCR2 gene further depends on a number of other factors since it is not solely specific to the CXCL1. Nevertheless, the observed phenomenon of down regulation in the HeLa cells treated with the AgNPs helps clarify on the underlying mechanisms of action. It is therefore suggested that AgNPs exert their effect via the downregulation of the CXCL1/CXCR2 gene axis thereby inhibiting the action of the proteins profoundly associated with tumorigenesis, angiogenesis, and metastasis as previously described [11, 12, 66, 67].
5Conclusions
The study presents evidence that the biosynthesized AgNPs, especially AgNPs-F (as supported by their higher selectivity index of 7.8), can be used in the development of novel and better anticancer drugs. The mechanism of action of the AgNPs involves potential activation of the intrinsic apoptosis pathway through upregulation of CASP9 and concerted down regulation of the CXCL1/ CXCR2 genes axis. To explain the recorded differences, further studies exploring the reasoning for the difference in the anti-cancer activity between AgNPs-L and AgNPs-F need to be undertaken.
Acknowledgments
The authors would wish to thank the Pan African University for the funding that allowed the study from which this data was obtained to be carried out. They further thank Busitema University for the protected time given to YG. We further thank Prof Wallace D Bulimo, Janet Majanja, Meshack Wedagu, Rachel Achilla, Silvanos Mukunzi, Agnes Gathemia, Tiffany H Wandera, Samwel Symekher, and the entire team at the Kenya Medical Research Institute (KEMRI) Centre for Virus Research, Department of Emerging Infectious Diseases, Influenza clean cell laboratory for their selfless support rendered that ensured the success of this work to be achieved.
The study was funded by the Pan African University Doctoral grant to YG (Grant number MB400-0007/17).
Author contributions
CONCEPTION: YG, HAE, GM and FW
DATA CURATION: All authors
ANALYSIS OF DATA: All authors
PREPARATION OF THE MANUSCRIPT: All authors
REVISION FOR IMPORTANT INTELLECTUAL CONTENT: All authors
FUNDING ACQUISITION: YG, ENM, GM and FW
Conflict of interest
Part of the work reported in this manuscript has been filed for a grant of patent and utility model at the African Regional Intellectual Property Organization (ARIPO) under the title: “Synthesis of Silver Nanoparticles from Extracts of Annona muricata and Use Thereof”. ARIPO Patent Application number: AP/P/2019/011514; ARIPO Utility Model Application number: AP/U/2020/000179. The above information notwithstanding, we further declare that the patent application do not in any way affect the outcome of this manuscript submission.
Availability of data and materials
Sufficient data associated with this research and enough to draw the results and conclusions has been provided within the manuscript. However, all datasets (from cytotoxicity studies) not included in this manuscript have been previously made available and published as a data article in Data in Brief Journal (https://doi.org/10.1016/j.dib.2019.104442) [68].
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/TUB-200058.
References
[1] | World Health Organization. WHO | Cancer. World Health Organization. 2017. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 10 Jul 2017. |
[2] | Torre LA , Siegel RL , Ward EM , Jemal A . Global Cancer Incidence and Mortality Rates and Trends–An Update. Cancer Epidemiol Biomarkers Prev. (2016) ;25: :16–27. |
[3] | Wabinga HR , Nambooze S , Amulen PM , Okello C , Mbus L , Parkin DM . Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer. (2013) ;135: :432–9. |
[4] | National Cancer Institute. Types of Cancer Treatment - National Cancer Institute. 2017. https://www.cancer.gov/aboutcancer/treatment/types. Accessed 10 Jul 2017. |
[5] | Cragg G , Newman D . Antineoplastic agents from natural sources: achievements and future directions. Expert Opin Investig Drugs. (2000) ;9: :2783–97. |
[6] | Cragg G , Newman D . Plants as a source of anti-cancer and anti-HIV agents. Ann Appl Biol. (2003) ;143: :127–33. |
[7] | Fulda S . Tumor resistance to apoptosis. Int J Cancer. (2009) ;124: :511–15. |
[8] | Hanahan D , Weinberg RA . Hallmarks of cancer: The next generation. Cell. (2011) ;144: :646–74. |
[9] | Fulda S . Modulation of apoptosis by natural products for cancer therapy. Planta Med. (2010) ;76: :1075–79. |
[10] | Saelens X , Festjens N , Vande Walle L , Van Gurp M , Van Loo G , Vandenabeele P . Toxic proteins released from mitochondria in cell death. Oncogene. 23 16 REV. ISS (2004) ;2: :2861–74. |
[11] | Wang B , Hendricks DT , Wamunyokoli F , Parker MI . A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. (2006) ;66: :3071–77. |
[12] | Cheng Y , Ma lei X , Wei quan Y , Wei XW . Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta - Rev Cancer. (2019) ;1871: :289–312. |
[13] | Jaffer T , Ma D . The emerging role of chemokine receptor CXCR2 in cancer progression. Transl Cancer Res. (2016) ;5: :S616–28. |
[14] | Talib W . Anticancer and Antimicrobial Potential of Plant-Derived Natural Products. In: Rasooli I, editor. Phytochemicals-Bioactivities and impact on Health, IntechOpen. (2011), pp. 141-58. |
[15] | Gavamukulya Y , Abou-Elella F , Wamunyokoli F , El-Shemy HA . Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med. (2014) ;7: (Suppl 1):S355–63. |
[16] | Leatemia JA , Isman MB . Insecticidal activity of crude seed extracts of Annona s, Lansium domesticum and Sandoricum koetjape against lepidopteran larvae. Phytoparasitica. (2004) ;32: :30–37. |
[17] | Betancur-Galvis L , Saez J , Granados H . Antitumor and antiviral activity of Colombian medicinal plant extracts. Mem Inst Oswaldo Cruz. (1999) ;94: :531–35. |
[18] | Magan˜a MA , Gama LM , Mariaca R . The use of medicinal plants in communities Maya-Chontales of Nacajuca, Tabasco, Mexico. Polibotánica. (2010) ;29: :213–62. |
[19] | DeFilipps R , Maina S , Crepin J . Medicinal plants of the Guianas (Guyana, Surinam, French Guiana).Washington, DC: Department of Botany, National Museum of Natural History, Smithsonian Institution; 2004. |
[20] | Ssenyange C , Namulindwa A , Oyik B . Plants used to manage type II diabetes mellitus in selected districts of central Uganda. Afr Health Sci. (2015) ;15: :496–502. |
[21] | Pieme CA , Kumar SG , Dongmo MS , Moukette BM , Boyoum FF , Ngogang JY , et al. Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement Altern Med. (2014) ;14: :516. |
[22] | Ross I . Medicinal plants of the world: chemical constituents, traditional and modern medicinal uses. Second. Humana Press; (2010) . |
[23] | Samuel A , Kalusalingam A , Chellappan D , Gopinath R , Radhamani S , Husain H , et al. Ethnomedical survey of plants used by the Orang Asli in Kampung Bawong, Perak, West Malaysia. J Ethnobiol Ethnomed. (2010) ;6: :5. |
[24] | Monigatti M , Bussmann RW , Weckerle CS . Medicinal plant use in two Andean communities located at different altitudes in the Bolívar Province, Peru. J Ethnopharmacol. (2013) ;145: :450–64. |
[25] | Coria-Téllez AV , Montalvo-Gonzalez E , Yahia E , Obledo-Vázquez EN . Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem. (2018) ;11: :662–91. |
[26] | Gavamukulya Y , Wamunyokoli F , El-Shemy HA . Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac J Trop Med. (2017) ;10: :835–48. |
[27] | Badrie N , Schauss A . Soursop (Annona muricata L.): composition, nutritional value, medicinal uses, and toxicology. In: Watson R, Preedy V, editors. Bioactive Foods in Promoting Health. Oxford: Academic Press; 2010. pp. 621-43. |
[28] | Langenberger G , Prigge V , Martin K , Belonias B , Sauerborn J . Ethnobotanical knowledge of Philippine lowland farmers and its application in agroforestry. Agrofor Syst. (2009) ;76: :173–94. |
[29] | Joyeux M , Mortier F , Fleurentin J . Screening of antiradical, antilipoperoxidant and hepatoprotective effects of nine plant extracts used in Caribbean folk medicine. Phyther Res. (1995) ;9: :228–30. |
[30] | Beyra A , León MC , Iglesias E , Ferrándiz D , Herrera R , Volpato G , et al. Ethnobotanical studies on medicinal plants in the province of Camagüey (Cuba). Ann Gard. 2004;185-204. |
[31] | Kossouoh C , Moudachirou M , Adjakidje V , Chalchat J-C , Figuérédo G . Essential Oil Chemical Composition of Annona muricata L. Leaves from Benin. J Essent Oil Res. (2007) ;19: :307–09. |
[32] | Vandebroek I , Balick MJ , Ososki A , Kronenberg F , Yukes J , Wade C , et al. The importance of botellas and other plant mixtures in Dominican traditional medicine. J Ethnopharmacol. (2010) ;128: :20–41. |
[33] | Boyom FF , Fokou PVT , Yamthe LRT , Mfopa AN , Kemgne EM , Mbacham WF , et al. Potent antiplasmodial extracts from Cameroonian Annonaceae. J Ethnopharmacol. (2011) ;134: :717–24. |
[34] | Nguyen-Pouplina J , Hop T , Hung T , Tuyet , Anh Phan Christiane D , Jeremy F , Tinh HT , et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol. (2007) ;109: :417–27. |
[35] | Atawodi S . Nigerian foodstuffs with prostate cancer chemopreventive polyphenols. In: Infectious Agents and Cancer. 2011. pp. S9. |
[36] | De Souza C , Karou SD , Tchacondo T , Djikpo Tchibozo MA , Abdoul-Rahaman S , Anani K , et al. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus and hypertension in the Central Region of Togo. Pharm Biol. (2011) ;49: :1286–97. |
[37] | Hajdu Z , Hohmann J . An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J Ethnopharmacol. (2012) ;139: :838–57. |
[38] | Cijo George V , Naveen Kumar DR , Rajkumar V , Suresh PK , Ashok Kumar R . Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona Muricata Linn. in normal and immortalized human cell lines. Asian Pacific J Cancer Prev. (2012) ;13: :699–704. |
[39] | Dai Y , Hogan S , Schmelz EM , Ju YH , Canning C , Zhou K . Selective Growth Inhibition of Human Breast Cancer Cells by Graviola Fruit Extract In Vitro and In Vivo Involving Downregulation of EGFR Expression. Nutr Cancer. (2011) ;63: :795–801. |
[40] | Valencia L , Muñoz D , Robledo S , Echeverri F , Arango G , Vélez I , et al. Trypanocidal and cytotoxic activity of extracts from Colombian plants. Biomédica. (2011) ;31: :552–59. |
[41] | Nawwar M , Ayoub N , Hussein S , Hashim A . Flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Arch Pharm Res. (2012) ;35: :761–67. |
[42] | Ménan H , Banzouzi J , Hocquette A , Pélissier Y . Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J Ethnopharmacol. (2006) ;105: :131–36. |
[43] | Moghadamtousi SZ , Rouhollahi E , Karimian H . The Chemopotential Effect of Annona muricata Leaves against Azoxymethane- Induced Colonic Aberrant Crypt Foci in Rats and the Apoptotic Effect of Acetogenin Annomuricin E in HT-29 Cells: A Bioassay- Guided Approach. PLoS One. (2015) ;10: :1–28. |
[44] | Bonifácio BV , Silva PB da , Ramos MADS , Negri KMS , Bauab TM , Chorilli M . Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine. (2014) ;9: :1–15. |
[45] | Ansari SH , Islam F , Sameem M . Influence of nanotechnology on herbal drugs: A Review. J Adv Pharm Technol Res. (2012) ;3: :142–46. |
[46] | Gavamukulya Y , Maina EN , Meroka AM , Madivoli ES , El-Shemy HA , Wamunyokoli F , et al. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona muricata. J Inorg Organomet Polym Mater. (2020) ;30: :1231–42. |
[47] | Gavamukulya Y , Elshemy HA , Meroka AM , Madivoli ES , Maina EN , Wamunyokoli F , et al. Advances in green nanobiotechnology: Data for synthesis and characterization of silver nanoparticles from ethanolic extracts of fruits and leaves of Annona muricata. Data Br. (2019) ;25: :104194. |
[48] | Gavamukulya Y , Maina EN , Wamunyokoli F , Meroka AM , Madivoli ES , El-Shemy HA , et al. Synthesis and Characterization of Silver Nanoparticles from Ethanolic Extracts of Leaves of Annona muricata: A Green Nanobiotechnology Approach. Biotechnol J Int. (2019) ;23: :1–18. |
[49] | Riss TL , Moravec RA , Niles AL , Duellman S , Benink HA , Worzella TJ , et al. CellViability Assays. In: Sittampalam GS, N.P. Coussens, Brimacombe K et al., editor. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2013. pp. 785-96. |
[50] | Vega-Avila E , Pugsley MK . An Overview of Colorimetric Assay Methods Used to Assess Survival or Proliferation of Mammalian Cells. Proc West Pharmacol Soc. (2014) ;54 March 2014: :10–4. |
[51] | Borra RC , Lotufo MA , Gagioti SM , Barros F , de M , Andrade PM . A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz Oral Res. (2009) ;23: :255–62. |
[52] | Kuete V , Wabo HK , Eyong KO , Feussi MT , Wiench B , Krusche B , et al. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS One. (2011) ;6: :4–10. |
[53] | Justus CR , Leffler N , Ruiz-Echevarria M , Yang LV . In vitro Cell Migration and Invasion Assays. J Vis Exp. 2014;1-8. |
[54] | Maina EN , Morris MR , Zatyka M , Raval RR , Banks RE , Richards FM , et al. Identification of novel VHL target genes and relationship to hypoxic response pathways. Oncogene. (2005) ;24: :4549–58. |
[55] | Chamcheu JC , Rady I , Chamcheu RN , Bakar A . Graviola (Annona muricata) Exerts Anti-proliferative, Anti-clonogenic and Pro-apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. 2018; June. |
[56] | Yang C , Gundala S , Mukkavilli R , Vangala S . Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis. (2015) ;36: :656–65. |
[57] | Rafehi H , Orlowski C , Georgiadis GT , Ververis K , El-Osta A , Karagiannis TC . Clonogenic Assay: Adherent Cells. J Vis Ex. (2011) ;49: :15–17. |
[58] | Livak KJ , Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. (2001) ;25: :402–08. |
[59] | Moghadamtousi SZ , Elham RH , Mehran K , Fadaeinasab Mahmood AA , Habsah Abdul K . Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther. (2014) ;8: :2099–111. |
[60] | Qu D , Sun W , Chen Y , Zhou J , Liu C . Synthesis and in vitro antineoplastic evaluation of silver nanoparticles mediated by Agrimoniae herba extract. Int J Nanomedicine. (2014) ;9: :1871–82. |
[61] | Kramer N , Walzl A , Unger C , Rosner M , Krupitza G , Hengstschläger M , et al. In vitro cell migration and invasion assays. Mutat Res - Rev Mutat Res. (2013) ;752: :10–24. |
[62] | Wong RS . Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. (2011) ;30: :87. |
[63] | Brentnall M , Rodriguez-Menocal L , De Guevara RL , Cepero E , Boise LH . Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14. |
[64] | Li P , Zhou L , Zhao T , Liu X , Zhang P , Liu Y , et al. Caspase- Structure, mechanisms and clinical application. Oncotarget. (2017) ;8: :23996–24008. |
[65] | Talib W . Anticancer and antimicrobial potential of plant-derived natural products. Phytochem Impact Heal. 2011. |
[66] | Poeta VM , Massara M , Capucetti A , Bonecchi R . Chemokines and chemokine receptors: New targets for cancer immunotherapy. Front Immunol. (2019) ;10 MAR :1–10. |
[67] | Steele CW , Karim SA , Leach JDG , Bailey P , Upstill-Goddard R , Rishi L , et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. (2016) ;29: :832–45. |
[68] | Gavamukulya Y , Maina EN , Meroka AM , El-shemy HA , Magoma G , Wamunyokoli F . In search of new anticancer drugs: Data for cytotoxic activities of green synthesized silver nanoparticles from ethanolic extracts of fruits and leaves of Annona muricata and 5- Fluorouracil against HeLa, PC3 and PNT1A cell lines. Data Br. (2019) ;26: :104442. |




