Galectin-3: A factotum in carcinogenesis bestowing an archery for prevention
Abstract
Cancer metastasis and therapy resistance are the foremost hurdles in oncology at the moment. This review aims to pinpoint the functional aspects of a unique multifaceted glycosylated molecule in both intracellular and extracellular compartments of a cell namely galectin-3 along with its metastatic potential in different types of cancer. All materials reviewed here were collected through the search engines PubMed, Scopus, and Google scholar. Among the 15 galectins identified, the chimeric gal-3 plays an indispensable role in the differentiation, transformation, and multi-step process of tumor metastasis. It has been implicated in the molecular mechanisms that allow the cancer cells to survive in the intravascular milieu and promote tumor cell extravasation, ultimately leading to metastasis. Gal-3 has also been found to have a pivotal role in immune surveillance and pro-angiogenesis and several studies have pointed out the importance of gal-3 in establishing a resistant phenotype, particularly through the epithelial-mesenchymal transition process. Additionally, some recent findings suggest the use of gal-3 inhibitors in overcoming therapeutic resistance. All these reports suggest that the deregulation of these specific lectins at the cellular level could inhibit cancer progression and metastasis. A more systematic study of glycosylation in clinical samples along with the development of selective gal-3 antagonists inhibiting the activity of these molecules at the cellular level offers an innovative strategy for primary cancer prevention.
Introduction
Cancer reigns the foremost firing-line in modern medicine. Early detection and prompt treatment linger as the basic objective of biomedical research. Currently, keen interest is being devoted to the deterrence of this disease either by primary or secondary approaches. Recent studies emphasize the role of epigenetic alteration in addition to gene mutations in tumor development and progression. Epithelial-mesenchymal transition (EMT) has been identified as a key event during invasion and metastasis of malignant tumors, particularly epithelial-derived ones and epigenetic control has been considered to be pivotal for EMT. Post-translational glycosylation is a classic example of epigenetic alteration [1]. Several fundamental alterations occur in the glycosylation pattern of cell surface and secreted glycoproteins during the development and progression of cancers. The Association of abnormal glycosylation with cancer was first demonstrated 50 years back. Thereafter, several studies have provided evidence to establish the vital role of glycosylation during the multistep process of tumorigenesis involving proliferation, invasion, metastasis, and angiogenesis [2]. A variety of glycan-binding proteins termed lectins were sanctified for decoding the information in these sugar-codes for harmonizing different biological and physiological processes accordingly. This review focuses on the functional aspects of a unique multifaceted lectin namely galectin-3 in tumor development as well as progression and suggests the potential of this lectin for the primary prevention of cancer.
Methodology
The review has been stockpiled from the literature search using the databases such as PubMed, Scopus, and Google Scholar with glycoproteins, glycans, glycosylation, lectins, Galectins, Galectin-3 as keywords. The search was based on some inclusion criteria such as the literature found in peer-reviewed journals in the English language with our keywords in the aspects of human physiology, disease biology, carcinogenesis, chemotherapy, and major hallmarks of cancer. The case studies and clinical reports were also included if they cued on our theme of interest.
Glycoproteins as Oncomarkers
Glycosylation, the addition of carbohydrate/glycan to a non-carbohydrate structure, usually proteins or lipids in a predetermined pattern is carried out by the combined effort of a group of enzymes, organelles, and other factors. This post-translational modification has been a critical determinant of protein function [3]. Nine of the twenty amino acids are reported to be modified by carbohydrates like glucose (Glc), Galactose (Gal), N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), fucose (Fuc), mannose (Man), xylose (Xyl), glucuronic acid (GlcA), iduronic acid (IdoA), and 5-N-acetylneuraminic acid (Neu5Ac/sialic acid) [4]. The worldwide acceptance of glycoproteins and glycan antigens as tumor markers as well as the expression of cancer-associated glycans like sialyl-LewisX (SLeX), Thomsen-nouvelle antigen (Tn), and sialyl-Tn (sTn) antigen by most of the tumor types further emphasize the significance of glycoproteins in cancer. By the introduction of new analytical techniques, the exact mechanism of how glycans regulate tumor cell proliferation, invasion, metastasis, and angiogenesis has been extensively evaluated [2].
Glycans and glycoconjugates have been recognized to perform major roles in diverse biological processes. Subsequently, a slight alteration in the structure or expression of glycoconjugates and glycosylation regulating factors may significantly affect the molecular physiology of the cell, which in turn alter a wide variety of biological processes mainly regulating cell-cell and cell - ECM adhesion, cell proliferation, and immune response [5] leading to chronic diseases like cancer. Cell surface glycans that are glycosylated through specific mechanisms in the ER-Golgi complex comprise a majority of the cell membrane and secreted molecules [6]. Glycosylation often occurs via the amino-group of an Asparagine residue (for N-glycans) or the hydroxyl-group of a Serine or Threonine residue on the peptide (O-glycans) [7]. Truncated O-glycans are commonly observed in neoplastic disorders [8]. More-over neoplastic cells have the potential to secrete their glycan-binding proteins which may have an impact on cell proliferation and survival [2]. Among the > 80 human glycan-binding proteins, galectins have gained an increasing interest owing to their clinical implications.
Galectins at a glance: in cell physiology and disease
Based on the carbohydrate recognition domain (CRD), the animal lectins are classified into different families, imparted in diverse cellular events and activities [9]. Galectins are a family of animal lectins that are now reported to mediate a superfluity of cellular activities such as cell survival, adhesion, migration, differentiation, and death. Protein–glycan interactions are indispensable for biomolecular communication within and between cells. Decoding of the biological information on this inter and intracellular communication is performed, partially by galectins and C-type lectins. Galectins, a lineage of endogenous lectins distributed considerably in the kingdom Animalia, specifically make out the N-acetyl lactosamine in the glycans linked to membrane proteins and lipids. Galectins often existing both intra- and extracellularly were proposed to follow a non-classical non-Golgi/ER secretion pathway due to the lack of signal sequence [10]. Fifteen different galectins have been identified so far and all of them contain a homologous carbohydrate recognition domain (CRD) comprising of a conserved sequence of about 130 amino-acids, conferring carbohydrate specificity, especially towards β-galactose containing glycoconjugates [11].
Galectins are classified into three groups, prototype, tandem repeats, and chimeras based on their structure [12]. Proto-type galectins (human Gal-1, –2, –7, –10, –13, and –14) are homodimers containing one CRD per subunit, the chimera-type galectins (galectin-3) possesses a C-terminal CRD and an N-terminal domain-containing proline and glycine-rich repeating units, and the tandem repeat (TR) galectins containing two CRDs at their N- and C-termini within one polypeptide. Galectins are soluble factors having representative characteristics of cytoplasmic molecules. They are localized in the cell membrane, cytoplasm, nucleus, and even in extra-cellular space. Galectins, after the non-classical secretion in the cytosol, are rapidly translocated into the extracellular space, where they recognize and bind to specific ligands such as galactose containing glycoproteins and glycolipids destined for several cellular activities. Over and above the typical extracellular sugar binding-dependent pathways, galectins are now widely discussed to exhibit quite a few intracellular functions executed in carbohydrate binding-independent modes. Through the well-established endocytic pathway, galectins can rapidly be recycled into the cells where the glycan-binding proteins occupy the endocytic and recycling compartments [13–15]. Some of the intracellular activities could be inhibited by molecules interacting with the galectin carbohydrate-binding site also, such as lactose [16–18].
Galectin-3 is disclosed to engage as an endocytic adaptor performing a major role in the clathrin-independent endocytic uptake of several glycosylated membrane proteins involved in cell adhesion, spreading, and migration. As per the glycolipid-lectin hypothesis, the binding of galectin to glycosylated cargo proteins such as CD44 and α5β1 integrin, activate its oligomerization, facilitating their interaction with glycosphingolipids resulting in membrane curvature, which will lead to the generation of tubular endocytic pits from which clathrin-independent endocytic carriers are formed [19, 20]. On the other hand, it has also been observed that galectins counteract the endocytic intake by forming lattices at the plasma membrane, influencing cell signaling, migration, adhesion, etc. Balancing of these two properties has been reported to occur based on the specificity, structure, and concentration of each of the galectins themselves and their glycoprotein and glycolipid ligands [21]. Furthermore, galectins have been reported to play some role in monitoring the integrity of vesicular membranes at the interface between the cytosolic and intravesicular compartments and some of the galectins have been found to accumulate around disrupted vesicles by binding to exposed glycan structures and induce clearance of damaged organelles by selective autophagy [22–28].
Galectins are hashed out to regulate several physiological activities of cells even from the very beginning of life. During embryogenesis, Gal-1, 9, and 15 are reported to be expressed in a coordinated manner influencing several developmental activities mainly through the JNK mediated integrin-binding activity [29–32]. Gal-3 and 12 are found to play an imperative role in fetal and adult bone and cartilage development by distinct mechanisms [33–35]. Gal-3 has been declared to play a significant role in endothelial cell motility, neovascularization and neuronal differentiation also [36, 37]. Gal-1 and -7 have been impacting skin, muscle, nerve, and endothelium development [38–41].
Galectin signaling impacts hematopoiesis of all lineages. Gal-1, 3, 9, and 10 are reported to regulate proliferation, differentiation, and functional maturation of hematopoietic stem cells [42, 43]. Neutrophil life-span is also regulated by gal-1 and -3 through facilitating neutrophil phagocytosis by macrophages [44]. Gal-1, 3, 8, and 9 are reported to promote the activation and chemotaxis of neutrophils, eosinophils, monocytes, and macrophages [41, 45–47] favoring MHC II-dependent antigen presentation [48, 49]. Gal-3 fine-tunes the immunostimulatory capacity of dendritic cells, [50] while gal-9 promotes dendritic cell maturation [51], and gal-1 controls dendritic cell generation, activation, and migration [52, 53]. Various galectins were found to control the development and rearrangement of T cells [54–56]. Gal-1 modulates the lymphoid and myeloid cell compartments as well as regulates platelet activation [57].
Despite the above-mentioned circadian physiological activities, galectins also influence additional cellular pathways. By recognizing the glycoconjugates on the cell surface, extracellular matrices and intracellular vesicles, galectins play a role in apoptosis [58], cell growth and proliferation [59], inflammation, immune response, [60–62] cell adhesion [63, 64] and migration [65–67]. These functions having significant roles in various diseases, particularly cancer, emphasize the prominence of this astounding molecule in biomedical research.
Galectin – 3 and Carcinogenesis
Galectins (S-type lectin), the evolutionarily conserved family of pleiotropic molecules are discussed to be relevant in a wide range of diseases amongst which; cancer has been assigned a superior grade. The discovery of galectins and their influence on tumor initiation, progression, and metastasis are now considered to be a foremost milestone in biomedical research, especially in medical oncology. Among the different galectins described so far, galectin-3 is found to be the critical one involved in tumorigenesis of most of the carcinomas of modern concern. Our data reinforces the knowledge that galectin-3 is overexpressed in thyroid, breast, and cervical carcinomas [68–71]. Galectin-3 has been shown to have a profound influence on each of the hallmarks of cancer by disrupting the intra and extracellular circuits. It is the only chimeric galectin constituting 3 functional domains such as the N terminal domain, proline-rich collagen-α-like domain, and C-terminal carbohydrate recognition domain (CRD) [41, 72]. Galectin-3 is a∼31 kDa protein encoded by a single gene in humans named LGALS3 located in the long arm of chromosome 14 (q21-q22) [73, 74]. LGALS3 gene comprises five introns and six exons with a cache of CpG islands in the promoter and first exonic region, enabling epigenetic modulation of protein expression [75].
Galectin-3 preferentially binds to type-1 or type-2 Gal β 1 ⟶ 3 (4) GlcNAc (N-acetyl lactosamine) chain [76]. Shreds of evidence suggest that the functioning of galectin-3 is also affected by its post-translational modification such as phosphorylation and partial proteolysis [77]. Galectins are attributed to be key players in several cellular functions such as cell signaling, cell adhesion, and migration, etc. However, the execution of these functions depends on the balancing of the conflicting properties exhibited by galectins that are reported to be based on specificity, structure, and concentration of each of the galectins themselves and their glycoprotein and glycolipid ligands [21]. Reports hind that galectin functioning are not solely reliant on the molecule itself, rather the cellular partners associated with galectin-specific ligands are equally important. The topological exposition of glycans and the range of ligands in addition to the architecture and dynamics of carbohydrate-binding domain impinge on the sugar-binding characters of the molecules. The complexity and side-chain functional groups in polysaccharides encroach on the glycan-binding affinity of galectins [78]. It is reported that the binding affinity is higher towards complex glycans such as polylactosamines and branched sugars [76].
In general, galectins act by forming homodimers or multimers, either in an N-terminal dependent or C-terminal dependent fashion [79]. Ligand binding attributes along with the ability to form oligomeric lattices with different ligands resolves the biological function that relied on each galectin such as endocytosis and transduction of signals [80].
Galectin-3 displaying ubiquitous expression among different species is observed both in the intracellular and extracellular purlieu at the cellular level. It is considered a versatile molecule due to its pliability in functioning. Galectin-3 interacts with a wide collection of molecules based on their subcellular localization and brings forth a diverse set of biological activities [81–83]. Galectin-3 has been universally regarded as an intracellular protein residing in the nucleus and cytoplasm, may be secreted to the extracellular side under stressful conditions for promoting cell survival and proliferation. Under such circumstances of hypoxia or nutrient deprivation, the cell marks an up-regulation in the expression levels of galectin-3 by way of transcription factors such as HIF-1α and NF-KB [77]. Upon secretion, galectin-3 interacts with an array of cell surface glycoproteins and extracellular matrix proteins thereby oligomerizing to form lectin lattices that act as a scaffold for different cell signaling such as of K-Ras. Galectin-3 has been reported to activate and regulate both intensity and duration of K-Ras signaling, in turn organizing cellular processes such as cell proliferation and survival in different carcinomas like breast and thyroid [84–86]. Galectin-3 is considered to regulate Notch signaling involved in cell proliferation and differentiation in a manner dependent on its cellular localization. When endogenous galectins are found up-regulating the expression of Notch and its dependent targets, the secreted galectins bind with the Notch receptor, enabling its activation and nuclear translocation [87, 88].
Galectin-3 residing in the nucleus interacts with the spliceosome complex, modulating pre-mRNA splicing through protein-protein interaction [89–91]. The electrostatic potential in the intracellular compartments, also, bestows three positively charged arginine residues in the carbohydrate-binding cleft of galectin-3, enabling it to interact with negatively charged DNA and RNA molecules, modulating post-transcriptional modification and damage response for cell survival [92, 93].
The mechanism of compartmentalization and secretion of galectin-3 is less explored. It is substantiated that due to the lack of secretion signal sequence, the molecules are secreted by a non-classical pathway, independent of ER/Golgi network. Many alternative approaches for galectin-3 secretion have been proposed by recent studies such as exosome-mediated secretion [94], vesicular release [95, 96], mechanotransduction-dependent mode [97], and also a passive release of the molecules in perishing cells [77]. The nuclear translocation of galectin-3 molecules that lack the nuclear localization signal (NLS) is also an area of critical concern [98–102]. It is differently expressed in different areas of the tumor. The highly unique glycosylation signatures portrayed by different cancer cells sponsor the importance of sugars and sugar-binding lectins in disease physiology that makes the expression levels and localization of these molecules, an area of keen interest for cancer researchers [103]. Galectin-3 plays a substantial role in the orchestration of stress signals in the tumor microenvironment facilitating tumor development and progression [77].
The expression of galectin-3 is said to be regulated at different levels. Galectin-3 is overexpressed in conditions of hypoxia and nutrient deprivation mediated by HIF-1α and NF-Kβ to safeguard the cells from death. HIF-1α is also reported to be the transcriptional activator for galectin-1 favoring migration in colorectal cells under hypoxic conditions [104, 105]. HIF-1α has been reported to be important for angiogenesis and metastasis in many carcinomas such as NSCLC, breast carcinoma, etc [106]. This can be attributed to a galectin-3 mediated mechanism.
Galectin-3 in modulating tumor development and sustaining the proliferative potential
Tumorigenesis is often associated with aberrant expression of oncogenes and tumor suppressor genes. Oncoproteins like Ras, dock into the cell membrane for their activities [107]. Gal-3 binds with these proteins particularly, K–Ras activating RAF1 and phosphatidylinositol 3-kinase (PI3K) thereby regulating gene expression [84]. Gal-3 modulates cell proliferation in breast cancer by gate-keeping the conversion of N-Ras to K-Ras [85]. The role of Gal-3 in the neoplastic transformation of thyroid cells and its potential as a reliable differential diagnostic marker of follicular adenoma from follicular carcinoma and follicular variant of papillary carcinoma in fine-needle aspiration cytology was also acknowledged [108].
Gal-3 prevents the endocytosis of EGF and TGF- receptors found on tumor cells via cross-linking poly-N-acetyl lactosamine residues [109]. It regulates the kinase inhibitors p21 (WAF-1) and p27 (KIP1) leading to the up-regulation and down-regulation of G1 phase and S/G2/M phase cyclins respectively [110]. Moreover, galectin-3 can also manage gene expression in the nucleus by regulating transcription factors in association with Sp1 and CRE modules in the promoter sequences of cyclin-D [111]. Gal-3 in the nucleus interacts with the thyroid transcription factor (TTF-1) of papillary carcinoma cells thereby stimulating its transcriptional activity contributing to the proliferative potential of thyroid cells [112]. Previous reports even though confirm the authenticated action of gal-3 in cell cycling and proliferation, the paradoxical role discussed above uplifts the need for further experimental validation in this aspect.
Several types of research have pointed out the role played by galectin-3 in cell cycle and cell death, whose interplay is of great importance both in carcinogenesis and chemotherapy. The tumor suppressor proteins playing a key role in cell cycle regulation such as p16 [113] are reported to be networking with galectin expression and signaling pathway. p16 has been reported to decrease mRNA stability and thereby down-regulating its galectin-3 expression level in the cell [114]. Lin et al (2000) have uplifted the impact of galectin-3 intervened p27 signaling in the chemotherapeutic intervention. Genistein has been reported to be inducing G2/M phase arrest by up-regulating p21 alone and abolishing galectin-3 mediated p27 induction, resulting in cell cycle arrest without apoptosis [115]. On the contrary, secreted galectin-3 has been declared to provoke apoptosis by binding to cell surface receptors such as CD7 and CD29 (β1 integrin) [116].
Galectin-3 in governing immune evasion by tumor cells
Recent evidence from preclinical tumor models and clinical human trials confirm the significance of T cells in the control and destruction of neoplastic cells. The discoveries of several tumor-specific antigens detected by T cells have fired the evolution of an array of vaccines and T cell-based therapy, accentuating the imperative role of the immune system in inhibiting tumor development. The extraordinary proteins expressed in the tumor cell surface will be recognized by the immune effector cells triggering the destruction of the tumor cell and thereby inhibiting tumor progression. But the highly malignant tumor cells possess the ability to escape this rigorous immune surveillance by secreting several cytokines and chemokines [117]. Quite a lot of galectins are expressed in different immune and inflammatory cells as well as secreted from normal and tumor cells. Most of these galectins were found to modulate a range of inflammatory responses and thereby help immune evasion of neoplastic cells. Extracellular gal-3 induces T- cell apoptosis with the participation of CD7 and or CD29 [116]. It has also been found that some of the surface glycoprotein receptors in T cells, such as T cell receptor (TCR), bind to gal-3 producing clustering and downregulation of receptor activity [116]. Gal-3 binding proteins present in the T cell surface also regulate cell adhesion, growth, and apoptosis. At a glance, several previous research reports the irrefutable role of galectin-3 in conferring immune dodging mechanism to tumor cells.
Galectin-3 in guiding cell-matrix interaction
Most of the cells get anchored to the extracellular matrix (ECM) through transmembrane adhesion molecules like integrins, in which galectins play a significant role. Unlike galectins that are found in cell surface and extracellular space, integrins have extracellular, intracellular, and transmembrane domains. When added exogenously, recombinant gal-3 inhibited the extracellular matrix interaction of tumor cells in experimental models [119]. Gal-3 was also found to inhibit the adhesion of cancer cells to laminin in breast cancer, prostate, and melanoma [120]. Gal-3 expressing breast cancer cells have shown adhesion to collagen IV whereas it was not showing adhesion to fibronectin in ECM [104]. Galectin-3 also regulates cell-matrix interaction by controlling the endocytosis of integrins and CD44 in turn monitoring cell invasion and migration [20]. Cancer cell glycans are reported to be an active target for a wide array of lectins. The lectin binding affinity and signaling depend on the side-chain modifications of sugars thereby imparting an undeniable role for glycosyltransferases in tumorigenesis. The O-glycosylation has been reported to impart a role in cancer progression and metastasis on a galectin-3 mediated mechanism [103]. Altogether these reports confirm the convincing role of gal-3 in the cell to matrix interface.
Galectin-3 in bringing about tumor metastasis
During tumor progression, some of the tumor cells develop the potential to invade the surrounding tissue, intravasate into the circulating blood and lymphatic vessels through the endothelial linings, extravasate into the parenchyma further pervading in outlying organs. This invasion-metastasis cascade is associated with the modification of a series of proteins linked with cell-cell and cell-matrix adhesion and signaling pathways that regulate the cytoskeletal dynamics. As gal-3 was reported to play an undeniable role in cell-matrix interaction it is quite logical to predict its importance in tumor metastasis.
Tumor development is greatly regulated by its microenvironment. The tumor microenvironment is the collective term for host cells along with the extracellular matrix (ECM) components in the vicinity of the tumor mass. ECM forms the non-cellular part that is crucial for tumor initiation and progression. Galectin-3 that can be secreted from the cell, either actively or passively, into the tumor microenvironment, signals in tumor cell migration and angiogenesis by forming oligomeric lattices with its ligand molecules. It has been reported that the secreted galectin-3 activates ERK1/2 in a calcium-sensitive PKC-dependent mode promoting cell migration in HeLa cells. It has also been proved that this interaction involved both the CRD and N-terminal domain of galectin-3 [120]. Shetty and group (2015) have also reported a CRD domain-dependent interaction with annexin A2 residing on the plasma membrane, favoring cell migration in breast cancer [121]. Galectin-3 silencing has been found to display inhibition of invasion and metastasis in a variety of cancers such as breast carcinoma, osteosarcoma, etc via different intracellular interacting partners. In osteosarcoma and tongue cancer, galectin-3 modulates cell migration through β-catenin, Akt, Src, Lyn, and MMP activation [122, 123]. The up-regulation of metalloproteinase by galectin-3 has been reported to be executed by its interaction with the transcriptional factor AP-1 [124].
Gal-3 has been shown to enhance tumor cell migration and invasion by interacting with extracellular matrix proteins like fibronectin, elastin, collagen IV, and laminin. Gal-3 dependent integrin clustering promotes both direct and ligand-motivated activation. Exogenous enhancement of gal-3 supply to breast cancer cells stimulated fibronectin-dependent cell spreading and migration by α5β1-integrin activation [125]. The extracellular galectins produce lattices in the plasma membrane in a caveolin-dependent mechanism activating FAK and RhoA enabling cellular metastasis [126–128]. Furthermore, the phosphorylation of caveolin-1 (Cav1) in an Src kinase-dependent manner enhances RhoA activation, focal adhesion turnover, and cell migration in a gal-3 reliant synergistic way [129]. The actin reorganization evident in cell migration and fibronectin remodeling under the integrin-mediated stimulation of EGF has also been shown to be dependent on gal-3 and phosphorylated Cav1. Boscher has stated the binding of N-cadherin to extracellular gal-3 in murine breast cancer cells favoring cell migration besides the loss of intercellular adhesion, which may allow the tumor cell to escape the primary tumor site [130]. Tumor-derived gal-3 has been reported to favor tumor micro-emboli formation by increasing homotypic and heterotypic adhesion by interacting with Thomson- Friedenreich carbohydrate on cancer-associated transmembrane MUC1, besides induction of cell surface polarization exposing E-cadherin, CD44, and E-selectin ligands [128].
The cancer stem cell hypothesis suggests that tumor progression and metastasis are driven by a small population of cells that show stem cell properties, which are closely linked to epithelial-mesenchymal and mesenchymal-epithelial transition. At the time of EMT, epithelial cells go through cytoskeletal remodeling and lose their polarity, cell-cell contacts, and acquire a mesenchymal morphology [131] enabling the tumor cells to migrate and invade the basement membrane, blood vessels, and lymphatics. Several pathways that are known to regulate cancer stem cells, including canonical and non-canonical Wnt signaling or transforming growth factor beta (TGFβ) pathways are also competent for inducing EMT [132]. Many studies have demonstrated that overexpression of Gal-3 increases the migration and invasion potential of tumor cells whereas, silencing of gal-3 has been shown to reduce migration and invasion of tumor cells both in vitro and in vivo [133–135]. Extracellular gal-3 secreted by tumor cells was found to involve in the migration of endothelial cells in a dose-dependent way [135]. Wesley et al. have also reported that Galectin-3 increased the in vitro migration of BV2 microglia associated with integrin-linked-kinase signaling and induced EMT like morphology in a murine epithelial cell line GE11 via β-1 integrin that triggers galectin-3 expression through demethylation of its promoter [136]. Earlier we have observed that knockdown of galectin-3 gene increases the sensitivity of MDA-MB-231 cells to drug-induced apoptosis as well as expression of epithelial-mesenchymal transition-related genes [137]. Ilmer and the group have also reported that Gal-3 interacts with and mediates the expression of many proteins, which contribute to EMT during tumor cell migration, and invasion and depletion of galectin-3 initiate EMT in breast cancer [138]. All these observations support the key role of galectin-3 in the process of EMT.
Galectin-3 in setting off tumor embolization
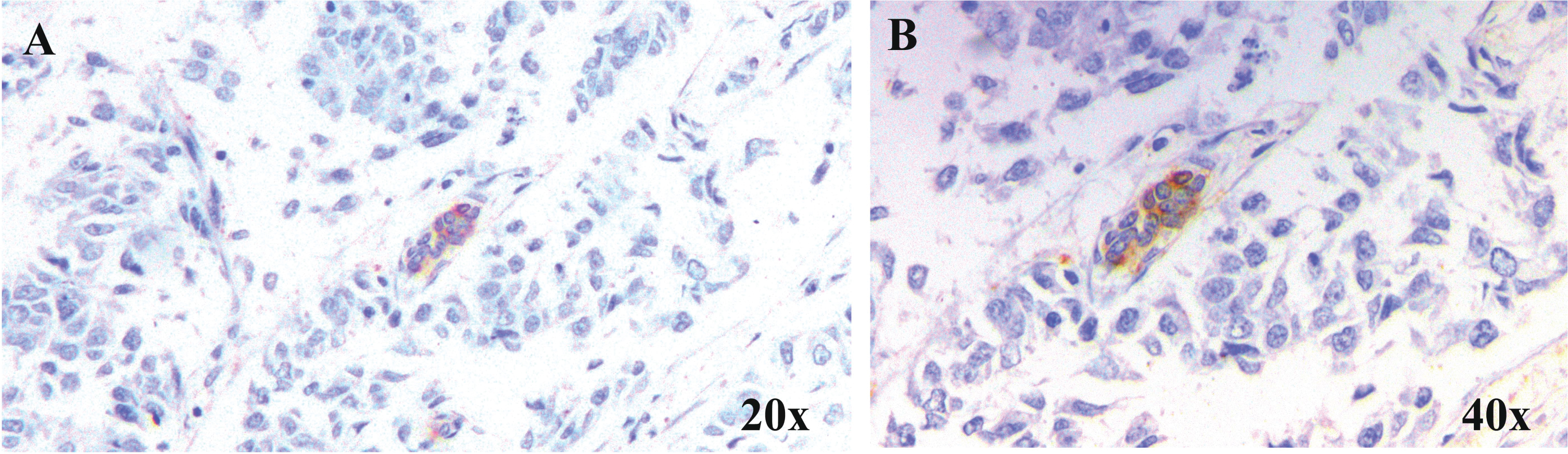
Intravasation of tumor cells into micro capillaries resulting in circulating tumor emboli which in turn extravasates at distinct sites ending up in secondary tumor formation. Tumor cells injected as aggregates were reported to colonize in the lungs of mouse models more efficiently than those given as single cell suspension [139]. Likewise, cells with high aggregation potential in vitro were found to possess high metastatic potential in vivo [140]. Though cadherins are responsible for this homotypic or heterotypic cell adhesion, soluble galectins were also revealed to be inducing the same by bridging with soluble complementary glycoconjugates. This was evident from the homotypic cell aggregation mediated by gal-3 and its strong binding partner, asialofetuin [141]. The N-glycosylated Mac-2 binding protein has been identified as a ligand for gal-3 tempted homotypic aggregation of melanoma cells in a carbohydrate-dependent way [142]. Furthermore, clustered gal-3 could be demonstrated in cell-cell contact sites in aggregated neoplastic cells [143]. All these reports reveal the significance of gal-3 in the homotypic aggregation of tumor cells churning out tumor emboli. In support of these reports, our immunohistochemical analysis of breast carcinoma samples divulge the expression of gal-3 in vascular and lymphatic tumor emboli in infiltrating duct cell carcinoma tissues (Fig. 1).
Fig. 1
Expression of Gal-3 in tumour emboli. Representative microscopic images of breast carcinoma samples at A. 20×and B. 40×showing the expression of Gal-3 in lymphatic tumour emboli. Gal-3 expression was studied in samples by immunohistochemical method and counter-staining with haematoxylin following standard protocol.
Galectin-3 in fostering angiogenesis
The avascular to vascular transition during the malignant transformation, executed through the balancing of pro-to anti-angiogenic signals is inevitable for the oncogenic feat. Both the tumor and host stromal cells are engaged in this balancing by secretion of pro-angiogenic factor [144]. In this aspect, galectin-3 plays the role of a platform for these signaling molecules on the endothelial cells and as a cofactor and amplifier of the signals triggered [135]. Neo-angiogenesis is essential for the successful growth of both primary and metastatic tumors. It is brought into action by the pro-angiogenic factors jointly secreted by the tumor as well as the stromal cells within the tumor microenvironment. A sequence of endothelial cell responses to the angiogenic poise, such as ECM wearing along with budding, and tube formation of endothelial cells is required for angiogenesis which in turn are under the strict orchestration of VEGFR, FGFR, and Notch-dependent signaling pathways [145–147]. Post-translational modification such as proteolysis in a stringent manner enables galectin-3 in soothing the epithelial-endothelial interaction web underpinning angiogenesis [148, 149].
Carbohydrate interaction has been reported to play an important role in angiogenesis, as FGF and VEGF primarily endure on the proteoglycans in the extracellular matrix, before interacting with their original receptor and most of the adhesion molecules attach to glycoconjugates on the endothelial cell surface [150]. Gal-3 was found to act as a carbohydrate-dependent chemotactic stimulus for capillary tube formation of human umbilical endothelial cells [151]. Correspondingly, it has been shown that gal-3 secreted by tumor cells increases the JAG1/Notch signaling in endothelial cells [152]. Besides its direct effect on endothelial cells, activation and migration of monocytes and macrophages occur under the spur of gal-3. Tumor-associated macrophages expressing gal-3 play a significant role in neo-angiogenesis and maturation of vessels. Overexpression of transforming growth factor-β1 (TGFβ1) has been observed in gal-3 expressing cancers, which may augment the release of VEGF by monocytes/macrophages and induce chemotaxis [152–154]. Gal-3 also targets the PKC-dependent VEGF release from platelets [155]. Consequently, the putative role of gal-3 in tumor angiogenesis is well proven and discussed from very early times. The aggressive tumor cells display functional plasticity enabling them to act as endothelial cells in tumor vessels by a process termed vasculogenic mimicry. The role of galectin-3 in this process has also been reported by many groups. However, the mechanism remains unclear [156–158].
Galectin-3 in bestowing apoptosis resistance
Attaining resistance to apoptosis enables tumor cells the capacity to evade the cell death induced by the natural killer or killer T cells, which is crucial for tumor progression. Gal-3 exhibits anti-apoptotic activity on several types of cells. Gal-3 overexpression was reported to be endowing resistance to apoptosis in lymphoma [159] and breast carcinoma [160]. Gal-3 is said to exhibit sequence homology with the established anti-apoptotic protein Bcl2. Gal-3 and Bcl2 are analogous in both the N-terminal domain, opulent in proline, glycine and alanine, [161] and C-terminal domain, possessing Asp-Trp-Gly-Arg (NWGR) motif, and any difference in the amino acid sequence of this NWGR motif will alter the anti-apoptotic potential of gal-3 [160]. Additionally, gal-3 can interact with Bcl2 protein in vitro and imitate its action by forming heterodimers [159]. Therefore, gal-3 can function as a regulator of mitochondria related apoptosis through binding to Bcl2 in various types of tumor cells. Galectin-3 has also been reported to play a key role in p53 mediated apoptosis due to the sequence similarity with Bcl-2 [162].
The Ca2+ and phospholipid binding protein, Synexin (annexin A7) is a direct binding partner of gal-3 and has been associated with its anti-apoptotic activity[163]. The interaction of gal-3 with K-Ras protein has also been discussed for its anti-apoptotic activity [164]. Even though gal-3 is not an inherent anti-apoptotic factor similar to the Bcl2 family of proteins, gal-3 gets this activity on binding with different binding partners in different cell types.
Galectin-3: A promising target for therapy
As a whole, the reports reviewed in this article substantiate the key role of gal-3 in most of the hallmarks of the tumor as well as various aspects of tumor initiation and progression. The different oncogenic activities exhibited by gal-3 are strictly based on its cellular localization and interaction with different signaling molecules. The intracellular Gal-3 once restricted to the cytoplasm of tumor cells confers apoptosis escape mechanism to the respective cell while, when secreted to the tumor microenvironment, triggers apoptosis of cancer infiltrating T cells; both of which endorse tumorigenesis. Similarly, intracellular gal-3 in tumor cells favor angiogenesis and neovascularization, while extracellular gal-3 promotes homotypic aggregation as well as tumor cell migration. Endothelium-associated gal-3 arbitrates tumor-endothelial cell interaction for docking of tumor cells, putting on metastasis. Evidence is still being added to further authenticate the multifarious regulations of gal-3 in cancer progression and metastasis (Fig. 2). Most of the cancers, particularly epithelial-derived ones have a natural history of progression, evolving from precancerous lesions like abnormal hyperplasia to dysplasia to in situ carcinoma/intraepithelial lesions and ultimately to an invasive tumor. The majority of the “Hallmarks of Cancer” is obvious in the premalignant conditions also with the opportunity for chemoprevention attempts. Gal-3 antagonists could therefore be valuable in regulating the functional role played by this molecule in tumorigenesis, invasion, metastasis, and/ restoring sensitivity to apoptosis in neoplastic cells. Substantial progress has been made in the past years to make out natural and synthetic peptides and monoclonal antibodies that specifically bind to the carbohydrate recognition domain of gal-3 [21, 165, 166]. Several small molecules have been recently found very effective in inhibiting gal-3 functional activation [167]. Furthermore, genetic modification of gal-3 resulted in inhibition of tumor growth and metastasis in orthotopic models of breast cancer [168]. Several galectin-3 antagonists have been evaluated to show potency in overcoming pathological neo-vascularisation and fibrosis and chemotherapy-associated fibrosis [169–171]. Natural ligands such as mucin, fibronectin, laminin, and IgE have been flaunted as an effective inhibitor of gal-3 [172]. Some carbohydrate analogs of natural ligands such as 1-Methyl-β-D-galactoside, 1-Methyl-β-D-lactoside, 1-Methyl-N-Acetyl lactosamine were found to inhibit gal-3 in several experimental studies. Modified citrus pectin has been found to specifically bind to the carbohydrate recognition domain of gal-3 and by this means, reducing tumor metastasis in animal models [173]. It was also found to reduce the progression of prostate cancer in phase II human trial [174]. A summary of the reports reviewed has been assembled in Table 1. Quite a few other synthetic molecules are being testified with galectin inhibiting activity which gives hope for the evolution of a specific anti-galectin-3 compound in the nearest future to prevent progression of a precancerous lesion to invasive cancer.
Fig. 2
Galectin-3 in carcinogenesis. Schematic summary of the roles of galectin-3 in different “Hallmarks of cancer”.
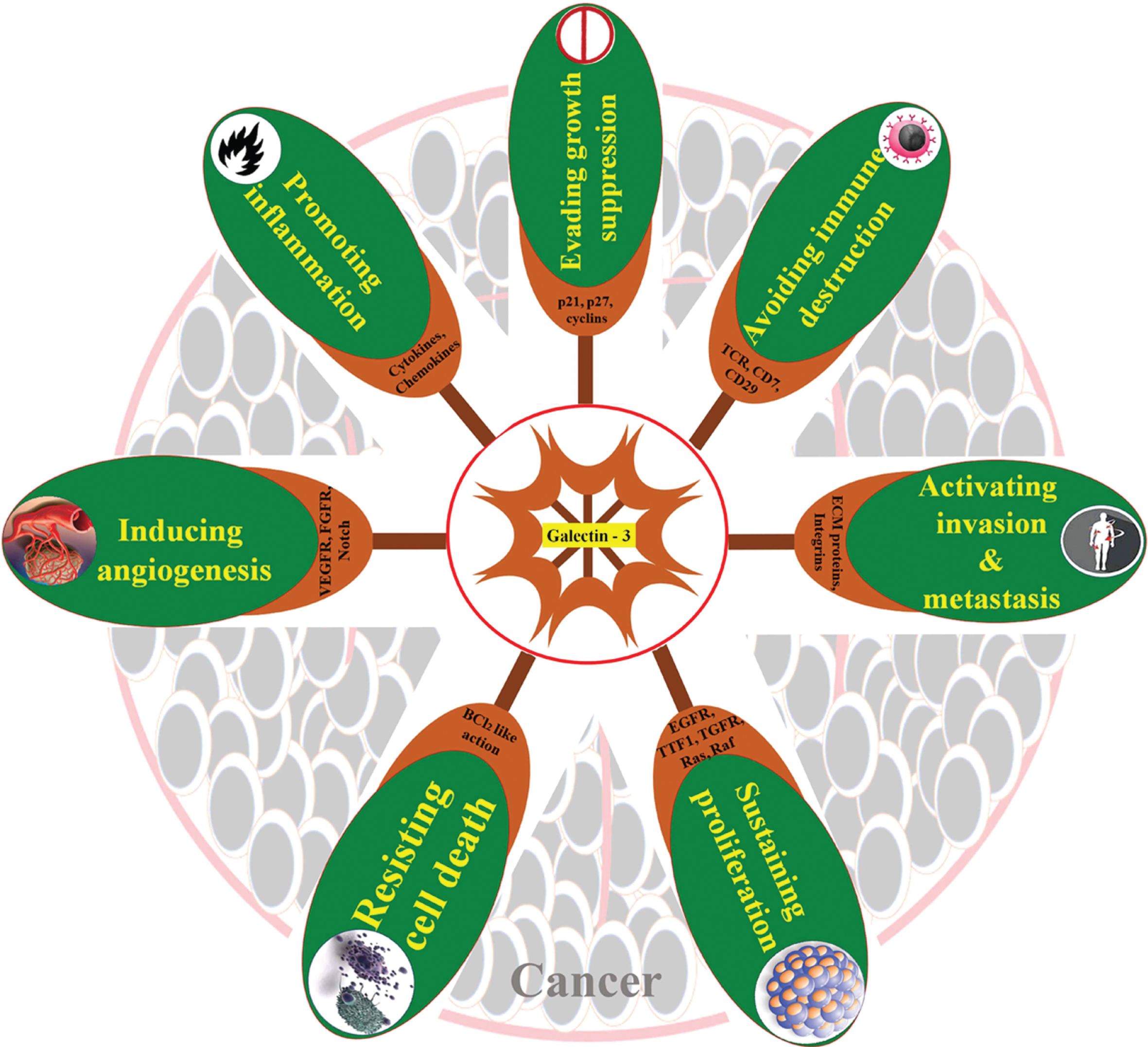
Table 1
Translation research on Galectin-3 in experimental oncology
| Sl. No. | Role investigated | Study Approach | Lesion Studied | Reference |
| 1. | Serum Gal-3 as a marker for predicting metastatic probability | Case study | Ovarian Cancer | [175] |
| 2. | Galectin-3 over expression enhances cell migration via Erk1/2 pathway | Case study, In vitro | Colon Cancer | [176] |
| 3. | Galectin-3 contributes tumor progression via Ras signalling | In vivo (Orthotropic mouse models) | Pancreatic Cancer | [177] |
| 4. | Galectin-3 contributes to tumor growth and metastasis via NFAT1 and autotaxin | In vitro, In vivo (Female Athymic BALB/c nude mice) | Melanoma | [178] |
| 5. | Intracellular levels of galectin-3 contributes carcinogenesis | Case study | Renal cell carcinoma | [179] |
| 6. | Gal-3 inhibition sensitizes cells to arsenic trioxide | In vitro | Renal cell carcinoma | [180] |
| 7. | Serum galectin-3 in tumor diagnosis | Case study | Bladder Cancer | [181] |
| 8. | Galectin-3 in prognosis of thyroid cancer | Case study | Thyroid Cancer | [182] |
| 9. | Galectin-3 antagonists inhibits carcinogenesis | Invitro, In vivo (Orthotropic, subcutaneous LLC1 model, LLC1 metastatic model, Xenograft model) | Lung Adenocarcinoma | [183] |
| 10. | Gal-3 downregulation during breast cancer progression regulates cell-associated and tumor microenvironment glycos-aminoglycans (GAGs)/ proteoglycans (PG), thus enhancing the metastatic potential of tumor cells. | In vitro, In vivo (Lgals3 + /+, Lgals3 + /- BALB/c mice) | Breast Cancer | [184] |
| 11. | Reduced Gal-3 expression results in the up-regulation of β3 integrin expression and this contribute to metastatic potential. | In vitro, In vivo (C57BL/6 mice) | Melanoma | [185] |
| 12. | Galectin-3 inhibits tumor growth by reducing tumor vasculature | In vitro, In vivo | Many cancers | [186] |
| 13. | Clinical efficacy of first-in-class galectin-3 antagonist studied | Clinical trial | Lymphocytic leukemia | [187] |
| 14. | Blocking galectin-3 expression in highly malignant human breast carcinoma MDA-MB-435 cells led to a significant suppression of tumor growth in nude mice | In vitro, In vivo (Athymic female nude mice) | Breast Cancer | [188] |
| 15. | Therapy with NH2-terminally truncated form of galectin-3 (galectin-3C) reduces metastases and tumor volumes. | In vitro, In vivo (Female athymic CD-1 nude mice) | Breast Cancer | [189] |
| 16. | Hypoxia up-regulates the expression of galectin-3, which may in turn increase tumor aggressiveness. | In vivo (NIH(s) II-nu/nu— mice) | Mammary Carcinoma | [190] |
| 17. | Targeting galectin-3 may be valuable for overcoming bone marrow microenvironment mediated protection of CML cells which may help in eliminating the problem of drug resistance | In vivo (Male NOD/SCID mice) | Leukemia | [191] |
| 18. | Lack of Galectin-3 affects tumor metastasis by modulating binding of melanoma cells onto target tissue and upregulating NK activity. | In vitro, In vivo (Gal-3 -/- mice) | Melanoma | [192] |
| 19. | Induction of in vivo angiogenesis by galectin-3-expressing BT-549cells or soluble galectin-3 further emphasizes the importance of carbohydrate-protein interactions during angiogenesis. | In vivo (Nude mice) | Breast Cancer | [135] |
| 20. | Loss of Gal-3 is associated with a mesenchymal BCSC subtype and enhanced tumorigenicity. Gal-3 levels predicts poor prognosis. | In vivo (Athymic, nu/nu, BALB/c mice) | Breast Cancer | [138] |
| 21. | Gal-3 regulates tumor angiogenesis via VEGF signalling | In vivo (C57black/6 wild-type (WT) or galectin-3 KO mice) | Melanoma | [153] |
| 22. | GCS-100 inhibit myeloma | In vitro | Myeloma | [193] |
| 23. | Modified citrus pectin inhibits metastasis | In vivo (Male Copenhagen rats) | Prostate Cancer | [194] |
| 24. | Down-regulation of galectin-3 enhances drug susceptibility | In vitro, In vivo (Female BALB/c nu/nu mice) | Pancreatic Cancer | [195] |
| 25. | Glycopeptide affinity to galectin-3 inhibits metastasis | In vitro, In vivo | Prostate Cancer | [196] |
References
[1] | Fuster M.M. and Esko J.D. , The sweet and sour of cancer: glycans as novel therapeutic targets, Nat Rev Cancer 5: ((2005) ), 526–542. |
[2] | Pinho S.S. and Reis C.A. , Glycosylation in cancer: mechanisms and clinical implications, Nat Rev Cancer 15: ((2015) ), 540–555. |
[3] | Stowell S.R. , Ju T. and Cummings R.D. , Protein glycosylation in cancer, Annu Rev Pathol 10: ((2015) ), 473–510. |
[4] | Cummings R.D. and Pierce J.M. , The challenge and promise of glycomics, Chem Biol 21: ((2014) ), 1–15. |
[5] | Chia J. , Goh G. and Bard F. , Short O-GalNAc glycans: regulation and role in tumor development and clinical perspectives, Biochimica et Biophysica Acta 1860: ((2016) ), 1623–1639. |
[6] | Cummings R.D. , The repertoire of glycan determinants in the human glycome, Mol Biosyst 5: ((2009) ), 1087–1104. |
[7] | Moremen K.W. , Tiemeyer M. and Nairn A.V. , Vertebrate protein glycosylation: diversity, synthesis and function, Nature Reviews Molecular Cell Biology 13: ((2012) ), 448–462. |
[8] | Harduin-Lepers A. , Krzewinski-Recchi M.A. , Colomb F. , et al., Sialyltransferases functions in cancers, Front Biosci (Elite Ed) 4: ((2012) ), 499–515. |
[9] | Sharon N. and Lis H. , History of lectins: from hemagglutinins to biological recognition molecules, Glycobiology 14: ((2004) ), 53R–62R. |
[10] | Pieters R.J. , Inhibition and detection of galectins, Chem BioChem 7: ((2006) ), 721–728. |
[11] | Barondes S.H. , Cooper D.N. , Gitt M.A. , et al., Galectins. Structure and function of a large family of animal lectins, J Biol Chem 269: ((1994) ), 20807–20810. |
[12] | Boscher C. , Dennis J.W. and Nabi I.R. , Glycosylation, galectins and cellular signaling, Curr Opin Cell Biol 23: ((2011) ), 383–392. |
[13] | Furtak V. , Hatcher F. and Ochieng J. , Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells, Biochem Biophys Res Commun 289: ((2001) ), 845–850. |
[14] | Stechly L. , Morelle W. , Dessein A-F. , et al., Galectin- 4-regulated delivery of glycoproteins to the brush border membrane of enterocyte like cells, Traffic 10: ((2009) ), 438–450. |
[15] | Straube T. , von Mach T. , HönigE., et al., pH-dependent recycling of galectin-3 at the apical membrane of epithelial cells, Traffic 14: ((2013) ), 1014–1027. |
[16] | Dagher S.F. , Wang J.L. and Patterson R.J. , Identification of galectin-3 as a factor in pre-mRNA splicing, Proc Natl Acad Sci USA 92: ((1995) ), 1213–1217. |
[17] | Shimura T. , Takenaka Y. , Tsutsumi S. , et al., Galectin-3, a novel binding partner of beta-catenin, Cancer Res 64: ((2004) ), 6363–6367. |
[18] | Haudek K.C. , Spronk K.J. , Voss P.G. , et al., Dynamics of galectin-3 in the nucleus and cytoplasm, Biochim Biophys Acta 1800: ((2010) ), 181–189. |
[19] | Johannes L. , Wunder C. and Shafaq-Zadah M. , Glycolipids and Lectins in Endocytic Uptake Processes, J Mol Biol ((2016) ), S0022-2836(16)30453 |
[20] | Lakshminarayan R. , Wunder C. , Becken U. , et al., Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers, Nat Cell Biol 16: ((2014) ), 595–606. |
[21] | Johannes L. , Jacob R. and Leffler H. , Galectins at a glance, J Cell Sci ((2018) ), 131. |
[22] | Paz I. , Sachse M. , Dupont N. , et al., Galectin-3, a marker for vacuole lysis by invasive pathogens, Cell Microbiol 12: ((2010) ), 530–544. |
[23] | Thurston T.L. , Wandel M.P. , von Muhlinen N. , et al., Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion, Nature 482: ((2012) ), 414–418. |
[24] | Fujita N. , Morita E. , Itoh T. , et al., Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin, J Cell Biol 203: ((2013) ), 115–128. |
[25] | Li S. , Wandel M.P. , Li F. , et al., Sterical hindrance promotes selectivity of the autophagy cargo receptor NDP52 for the danger receptor galectin-8 in antibacterial autophagy, Science signaling 6: ((2013) ), ra9. |
[26] | Maejima I. , Takahashi A. , Omori H. , et al., Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury, EMBO J 32: ((2013) ), 2336–2347. |
[27] | Aits S. , Kricker J. , Liu B. , et al., Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay, Autophagy 11: ((2015) ), 1408–1424. |
[28] | Chauhan S. , Kumar S. , Jain A. , et al., TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 Co-direct Autophagy in Endomembrane Damage Homeostasis, Dev Cell 39: (1) ((2016) ), 13–27. |
[29] | Nishioka T. , Sakumi K. , Miura T. , et al., FosB gene products trigger cell proliferation and morphological alteration with an increased expression of a novel processed form of galectin-1 in the rat 3Y1 embryo cell line, J Biochem 131: ((2002) ), 653–661. |
[30] | Dergan-Dylon S. , Salatino M. and Gabriel Rabinovich A. , Dissecting the Signal Transduction Pathways Triggered by Galectin–Glycan Interactions in Physiological and Pathological Settings, IUBMB Life 62: (1) ((2010) ), 1–13. |
[31] | Farmer J.L. , Burghardt R.C. , Jousan F.D. , et al., Galectin 15 (LGALS15) functions in trophectoderm migration and attachment, FASEB J 22: ((2008) ), 548–560. |
[32] | Tanikawa R. , Tanikawa T. , Okada Y. , et al., Interaction of galectin-9 with lipid rafts induces osteoblast proliferation through the c-Src/ERK signaling pathway, J Bone Miner Res 23: ((2008) ), 278–286. |
[33] | Kiwaki K. , Novak C.M. , Hsu D.K. , et al., Galectin-3 stimulates preadipocyte proliferation and is up-regulated in growing adipose tissue, Obesity (Silver Spring) 15: ((2007) ), 32–39. |
[34] | Ortega N. , Behonick D.J. , Colnot C. , et al., Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation, Mol Biol Cell 16: ((2005) ), 3028–3039. |
[35] | Stock M. , Schafer H. , Stricker S. , et al., Expression of galectin-3 in skeletal tissues is controlled by Runx2, J Biol Chem 278: ((2003) ), 17360–17367. |
[36] | Fukushi J. , Makagiansar I.T. and Stallcup W.B. , NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin, Mol Biol Cell 15: ((2004) ), 3580–3590. |
[37] | Kuklinski S. , Vladimirova V. , Waha A. , et al., Expression of galectin-3 in neuronally differentiating PC12 cells is regulated both via Ras/MAPK-dependent and -independent signaling pathways, J Neurochem 87: ((2003) ), 1112–1124. |
[38] | Chan J. , O’Donoghue K. , Gavina M. , et al., Galectin-1 induces skeletal muscle differentiation in human fetal mesenchymal stem cells and increases muscle regeneration, Stem Cells 24: ((2006) ), 1879–1891. |
[39] | Saegusa J. , Hsu D.K. , Liu W. , et al., Galectin-3 protects keratinocytes from UVB-induced apoptosis by enhancing AKT activation and suppressing ERK activation, J Invest Dermatol 128: ((2008) ), 2403–2411. |
[40] | Moiseeva E.P. , Williams B. , Goodall A.H. , et al., Galectin-1 interacts with beta-1 subunit of integrin, Biochem Biophys Res Commun 310: ((2003) ), 1010–1016. |
[41] | Yang R.Y. , Rabinovich G.A. and Liu F.T. , Galectins: structure, function, and therapeutic potential, Expert Rev Mol Med 10: ((2008) ), e17. |
[42] | Abedin M.J. , Kashio Y. , Seki M. , et al., Potential roles of galectins in myeloid differentiation into three different lineages, J Leukoc Biol 73: ((2003) ), 650–656. |
[43] | Fernandez G.C. , Ilarregui J.M. , Rubel C.J. , et al., Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways, Glycobiology 15: ((2005) ), 519–527. |
[44] | Stowell S.R. , Qian Y. , Karmakar S. , et al., Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion, J Immunol 180: ((2008) ), 3091–3102. |
[45] | Nishi N. , Shoji H. , Seki M. , et al., Galectin-8 modulates neutrophil function via interaction with integrin alphaM, Glycobiology 13: ((2003) ), 755–763. |
[46] | MacKinnon A.C. , Farnworth S.L. , Hodkinson P.S. , et al., Regulation of alternative macrophage activation by galectin-3, J Immunol 180: ((2008) ), 2650–2658. |
[47] | Malik R.K. , Ghurye R.R. , Lawrence-Watt D.J. , et al., Galectin-1 stimulates monocyte chemotaxis via the p44/42 MAP kinase pathway and a Pertussis toxin sensitive pathway, Glycobiology 19: ((2009) ), 1402–1407. |
[48] | Barrionuevo P. , Beigier-Bompadre M. , Ilarregui J.M. , et al., A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway, J Immunol 178: ((2007) ), 436–445. |
[49] | Sano H. , Hsu D.K. , Yu L. , et al., Human galectin-3 is a novel chemoattractant for monocytes and macrophages, J Immunol 165: ((2000) ), 2156–2164. |
[50] | Breuilh L. , Vanhoutte F. , Fontaine J. , et al., Galectin-3 modulates immune and inflammatory responses during helminthic infection: impact of galectin-3 deficiency on the functions of dendritic cells, Infect Immun 75: ((2007) ), 5148–515. |
[51] | Nagahara K. , Arikawa T. , Oomizu S. , et al., Galectin-9 increases Tim-31 dendritic cells and CD81 T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions, J Immunol 181: ((2008) ), 7660–7669. |
[52] | Fulcher J.A. , Chang M.H. , Wang S. , et al., Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and PKC signaling, J Biol Chem 284: ((2009) ), 26860–26870. |
[53] | Ilarregui J.M. , Croci D.O. , Bianco G.A. , et al., Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10, Nat Immunol 10: ((2009) ), 981–991. |
[54] | Rabinovich G.A. and Toscano M.A. , Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation, Nat Rev Immunol 9: ((2009) ), 338–352. |
[55] | Liu S.D. , Whiting C.C. , Tomassian T. , et al., Endogenous galectin-1 enforces class I-restricted TCR functional fate decisions in thymocytes, Blood 112: ((2008) ), 120–130. |
[56] | Bi S. , Earl L.A. , Jacobs L. , et al., Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways, J Biol Chem 283: ((2008) ), 12248–12258. |
[57] | Pacienza N. , Pozner R.G. , Bianco G.A. , et al., The immunoregulatory glycan-binding protein galectin-1 triggers human platelet activation, FASEB J 22: ((2008) ), 1113–1123. |
[58] | Ahmed H. and AlSadek D.M.M. , Galectin-3 as a Potential target to prevent cancer metastasis, Clin. Med. Insights Oncol 9: ((2015) ), 113–121. |
[59] | Gendronneau G. , Sidhu S.S. , Delacour D. , et al., Galectin-7 in the control of epidermal homeostasis after injury, Mol Biol Cell 19: ((2008) ), 5541–5549. |
[60] | Chen H.Y. , Weng I.C. , Hong M.H. , et al., Galectins as bacterial sensors in the host innate response, Curr Opin Microbiol 17: ((2014) ), 75–81. |
[61] | Rabinovich G.A. , van Kooyk Y. and CobbB.A., Glycobiology of immune responses, Ann N Y Acad Sci 1253: ((2012) ), 1–15. |
[62] | De Oliveira F.L. , GattoM., BassiN., et al., Galectin-3 in autoimmunity and autoimmune diseases, Exp Biol Med 240: ((2015) ), 1019–1028. |
[63] | Boscher C. , Zheng Y.Z. , Lakshminarayan R. , et al., Galectin-3 protein regulates mobility of N-cadherin and GM1 ganglioside at cell-cell junctions of mammary carcinoma cells, J Biol Chem 287: ((2012) ), 32940–32952. |
[64] | Yabuta C. , Yano F. , Fujii A. , et al., Galectin-3 enhances epithelial cell adhesion and wound healing in rat cornea, Ophthalmic Res 51: ((2014) ), 96–103. |
[65] | Fortin S. , Le Mercier M. , CambyI., et al., Galectin-1 is implicated in the protein kinase C epsilon/vimentin-controlled trafficking of integrin-beta1 in glioblastoma cells, Brain Pathol 20: ((2010) ), 39–44. |
[66] | Panjwani N. , Role of galectins in re-epithelialization of wounds, Ann Transl Med 2: ((2014) ), 89. |
[67] | Liu W. , Hsu D.K. , Chen H.Y. , et al., Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration, J Investig Dermatol 132: ((2012) ), 2828–2837. |
[68] | Shanone C.P. , Preethi T.R. and Thomas S. , et al., Galectin-3 and HBME help the differential diagnosis of thyroid malignancies in Fine Needle Aspiration Cytology, International Interdisciplinary Research Journal 3: (5) ((2013) ), 224–235. |
[69] | Sujathan K. , Somanathan T. and Remani P. , Expression of Galectin-1, Galectin-3 and T-Antigen in Breast Carcinoma Tissues and its Significance in Axillary Lymph Node Infiltration, Recent Research in Science and Technology 3: (6) ((2011) ), 85–90. |
[70] | Sujathan K. , Somanathan T. , Nair L.S. , et al., Endogenous Expression of Galectins and Tissue Binding Profile of a Galactose Specific Plant Lectin in Cervical Intra Epithelial Neoplasia: It’s Significance to Assess the Malignant Potential, Journal of Experimental Sciences 1: (8) ((2010) ). |
[71] | Sujathan K. , Somanathan T. , Nimi G.K. , et al., Down regulation of Gal-3 in primary tumor tissues of breast predict axillary lymph node metastasis, J Cancer Res and Exp Oncol 3: (4) ((2011) ), 37–49. |
[72] | Agrwal N. , Sun Q. , Wang S.Y. , et al., Carbohydrate-binding protein 35. I. Properties of the recombinant polypeptide and the individuality of the domains, J Biol Chem 268: ((1993) ), 14932–14939. |
[73] | Raimond J. , Zimonjic D.B. , Mignon C. , et al., Mapping of the galectin-3 gene (LGALS3) to human chromosome 14 at region 14q21-22, Mamm Genome 8: ((1997) ), 706–707. |
[74] | Kadrofske M.M. , Openo K.P. and Wang J.L. , The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter, Arch Biochem Biophys 349: ((1998) ), 7–20. |
[75] | Ruebel K.H. , Jin L. , Qian X. , et al., Effects of DNA methylation on galectin-3 expression in pituitary tumors, Cancer Res 65: ((2005) ), 1136–1140. |
[76] | Sato S. and Hughes R.C. , Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin, J Biol Chem 267: ((1992) ), 6983–6990. |
[77] | Cardoso A.C. , Andrade L.N. and Bustos S.O. , et al., Galectin-3 Determines Tumor Cell Adaptive Strategies in Stressed Tumor Microenvironments, Front Oncol 6: ((2016) ), 127. |
[78] | Guardia C.M. , Gauto D.F. , Di Lella S. , et al. An integrated computational analysis of the structure, dynamics, and ligand binding interactions of the human galectin network, J Chem Inf Model 51: ((2011) ), 1918–30. |
[79] | Halimi H. , Rigato A. , Byrne D. , et al., Glycan dependence of galectin-3 self-association properties, PLoS One 9: ((2014) ), e111836. doi: 10.1371/journal.pone.0111836 |
[80] | Lau K.S. , Partridge E.A. , Grigorian A. , et al., Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation, Cell 129: ((2007) ), 123–134. |
[81] | Moutsatsos I.K. , Wade M. and Schindler M. , et al., Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts, Proc Natl Acad Sci U S A 84: ((1987) ), 6452–6456. |
[82] | Gaudin J.C. , Mehul B. and Hughes R.C. , Nuclear localisation of wild type and mutant galectin-3 in transfected cells, Biol Cell 92: ((2000) ), 49–58. |
[83] | Openo K.P. , Kadrofske M.M. , Patterson R.J. , et al., Galectin-3 expression and subcellular localization in senescent human fibroblasts, Exp Cell Res 255: ((2000) ), 278–290. |
[84] | Elad-Sfadia G. , Haklai R. , Balan E. , et al., Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phospho-inositide 3-kinase activity, J Biol Chem 279: ((2004) ), 34922–34930. |
[85] | Shalom-Feuerstein R. , Cooks T. , Raz A. , et al., Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells, Cancer Res 65: ((2005) ), 7292–7300. |
[86] | Levy R. , Grafi-Cohen M. , Kraiem Z. , et al., Galectin-3 promotes chronic activation of K-Ras and differentiation block in malignant thyroid carcinomas, Mol Cancer Ther 9: ((2010) ), 2208–2219. |
[87] | Fermino M.L. , Dias F.C. , Lopes C.D. , et al., Galectin-3 negatively regulates the frequency and function of CD4(+) CD25(+) Foxp3(+) regulatory T cells and influences the course of Leishmania major infection, Eur J Immunol 43: ((2013) ), 1806–1817. |
[88] | Nakajima K. , Kho D.H. , Yanagawa T. , et al., Galectin-3 inhibits osteoblast differentiation through notch signaling, Neoplasia 16: ((2014) ), 939–949. |
[89] | Liu F.T. , Patterson R.J. and Wang J.L. , Intracellular functions of galectins, Biochim Biophys Acta 1572: ((2002) ), 263–273. |
[90] | Patterson R.J. , Haudek K.C. , Voss P.G. , et al., Examination of the role of galectins in pre-mRNA splicing, Methods Mol Biol 1207: ((2015) ), 431–449. |
[91] | Park J.W. , Voss P.G. , Grabski S. , et al., Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein, Nucleic Acids Res 29: ((2001) ), 3595–3602. |
[92] | Seetharaman J. , Kanigsberg A. , Slaaby R. , et al., X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution, J Biol Chem 273: ((1998) ), 13047–13052. |
[93] | Carvalho R.S. , Fernandes V.C. , Nepomuceno T.C. , et al., Characterization of LGALS3 (galectin-3) as a player in DNA damage response, Cancer Biol Ther 15: ((2014) ), 840–850. |
[94] | Théry C. , Boussac M. , Véron P. , et al., Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles, J Immunol 166: ((2001) ), 7309–7318. |
[95] | Sato S. , Burdett I. and Hughes R.C. , Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex, Exp Cell Res 207: ((1993) ), 8–18. |
[96] | Mehul B. and Hughes R.C. , Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion, J Cell Sci 110: (Pt 10) ((1997) ), 1169–1178. |
[97] | Baptiste T.A. , James A. , Saria M. , et al., Mechano-transduction mediated secretion and uptake of galectin-3 in breast carcinoma cells: implications in the extracellular functions of the lectin, Exp Cell Res 313: ((2007) ), 652–664. |
[98] | Davidson P.J. , Li S.Y. , Lohse A.G. , et al., Transport of galectin-3 between the nucleus and cytoplasm. I. Conditions and signals for nuclear import, Glycobiology 16: ((2006) ), 602–611. |
[99] | Li S.Y. , Davidson P.J. , Lin N.Y. , et al., Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export, Glycobiology 16: ((2006) ), 612–622. |
[100] | Nakahara S. , Oka N. , Wang Y. , et al., Characterization of the nuclear import pathways of galectin-3, Cancer Res 66: ((2006) ), 9995–10006. |
[101] | Funasaka T. , Raz A. and Nangia-Makker P. , Nuclear transport of galectin-3 and its therapeutic implications, Semin Cancer Biol 27: ((2014) ), 30–38. |
[102] | Arnoys E.J. , Ackerman C.M. and Wang J.L. , Nucleocytoplasmic shuttling of galectin-3, Methods Mol Biol 1207: ((2015) ), 465–483. |
[103] | Dimitroff C.J. , Galectin-binding O-glycosylations as Regulators of Malignancy, Cancer Res 75: (16) ((2015) ), 3195–3202. |
[104] | Hughes R.C. , Galectins as modulators of cell adhesion, Biochimie 83: ((2001) ), 667–676. |
[105] | Zhao X. , Chen T. , Xia L. , et al., Hypoxia inducible factor-1 mediates expression of galectin-1: the potential role in migration/invasion of colorectal cancer cells, Carcinogenesis 31: (8) ((2010) ), 1367–1375. |
[106] | Pezzuto A. , Perrone G. , Orlando N. , et al., A close relationship between HIF-1α expression and bone metastases in advanced NSCLC, a retrospective analysis, Oncotarget 10: (66) ((2019) ), 7071–7079. |
[107] | Barbacid M. , ras GENES, Annu Rev Biochem 56: ((1987) ), 779–827. |
[108] | Pereira S.C. , Jissa V.T. , Thomas S. , Preethi T.R. , Remani P. and Sujathan K. , Over expression of Cytosolic Galectin-3 correlates with anti-apoptotic protein Bcl-2: its significance in the differential diagnosis of follicular cell derived thyroid tumors in FNAC, International J Current Res 7: (10) ((2015) ), 21780–21788. |
[109] | Partridge E.A. , Le Roy C. , GuglielmoDi G.M., et al., Regulation of cytokine receptors by golgi N-glycan processing and endocytosis, Science 306: ((2004) ), 120–124. |
[110] | Kim H.R. , Lin H.M. , Biliran H. , et al., Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells, Cancer Res 59: ((1999) ), 4148–4154. |
[111] | Lin H.M. , Pestell R.G. , Raz A. , et al., Galectin-3 enhances cyclin D (1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells, Oncogene 21: (52) ((2002) ), 8001–8010. |
[112] | Paron I. , Scaloni A. , Pines A. , et al., Nuclear localization of Galectin-3 in transformed thyroid cells: a role in transcriptional regulation, Biochem Biophys Res Commun 302: (3) ((2003) ), 545–553. |
[113] | Pezzuto A. , D’Ascanio M. , Ricci A. , et al., Expression and role of p16 and GLUT1 in malignant diseases and lung cancer: A review, Thorac Cancer 11: (11) ((2020) ), 3060–3070. |
[114] | Sanchez-Ruderisch H. , Fischer C. , Detjen K.M. , et al., Tumor suppressor p16INK4a: Downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model, The FEBS Journal 277: ((2010) ), 3552–3563. |
[115] | Lin H-M. , Moon B-K. , Yu F. , et al., Galectin-3 mediates genistein-induced G2/M arrest and inhibits apoptosis, Carcinogenesis ((2000) ), 1941–1945. |
[116] | Fukumori T. , Takenaka Y. , Yoshii T. , et al., CD29 and CD7 Mediate Galectin-3-Induced Type II T-Cell Apoptosis, Cancer Res 63: (23) ((2003) ), 8302–8311. |
[117] | Siepl C. , Bodmer S. , Frei K. , et al., The glioblastoma-derived T cell suppressor factor/transforming growth factor beta 2 inhibits T cell growth without affecting the interaction of interleukin 2 with its receptor, Eur J Immunol 18: ((1988) ), 593–600. |
[118] | Demetriou M. , Granovsky M. , Quaggin S. , et al., Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation, Nature 409: ((2001) ), 733. |
[119] | van den Brûle F.A. , BuicuC., SobelM.E., et al., Galectin-3, a laminin binding protein, fails to modulate adhesion of human melanoma cells to laminin, Neoplasma 42: (5) ((1995) ), 215–219. |
[120] | Gao X. , Balan V. , Tai G. , et al., Galectin-3 induces cell migration via a calcium-sensitive MAPK/ERK1/2 pathway, Oncotarget 5: ((2014) ), 2077–2084. |
[121] | Shetty P. , Bargale A. , Patil B.R. , et al., Cell surface interaction of annexin A2 and galectin-3 modulates epidermal growth factor receptor signaling in Her-2 negative breast cancer cells, Mol Cell Biochem 411: ((2015) ), 221–233. |
[122] | Park G.B. , Kim D.J. , Kim Y.S. , et al., Silencing of galectin-3 represses osteosarcoma cell migration and invasion through inhibition of FAK/Src/Lyn activation and β-catenin expression and increases susceptibility to chemotherapeutic agents, Int J Oncol 46: ((2015) ), 185–194. |
[123] | Kobayashi T. , Shimura T. , Yajima T. , et al., Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of β-catenin, Int J Cancer 129: ((2011) ), 2775–2786. |
[124] | Wang Y.G. , Kim S.J. , Baek J.H. , et al., Galectin-3 increases the motility of mouse melanoma cells by regulating matrix metalloproteinase-1 expression, Exp Mol Med 44: ((2012) ), 387–393. |
[125] | Warfield P.R. , Makker P.N. , Raz A. , et al., Adhesion of human breast carcinoma to extracellular matrix proteins is modulated by galectin-3, Invasion Metastasis 17: ((1997) ), 101. |
[126] | Ruvolo P.P. , Galectin 3 as a guardian of the tumor microenvironment, Biochim Biophys Acta 1863: ((2016) ), 427–437. |
[127] | Meng F. , Joshi B. and Nabi I.R. , Galectin-3 overrides PTRF/Cavin-1 reduction of PC3 prostate cancer cell migration, PLoS One 10: ((2015) ), e0126056. |
[128] | Boscher C. and Nabi I.R. , Galectin-3- and phospho-caveolin-1-dependent outside-in integrin signaling mediates the EGF motogenic response in mammary cancer cells, Mol Biol Cell 24: ((2013) ), 2134–2145. |
[129] | Lagana A. , Goetz J. and Cheung P. , et al., Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells, Mol Cell Biol 26: (8) ((2006) ), 3181–3193. |
[130] | Shankar J. , Wiseman S. , Meng F. , et al., Co-ordinated expression of galectin-3 and caveolin-1 in thyroid cancer, J Pathol 228: (1) ((2012) ), 56–66. |
[131] | Devarajan E. , Song Y-H. , Krishnappa S. and Alt E. , Epithelial-mesenchymal transition in breast cancer lines is mediated through PDGF-D released by tissue-resident stem cells, Int J Cancer 131: ((2012) ), 1023–1031. |
[132] | Scheel C. , Eaton E.N. , LiSH-J, et al., Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast, Cell 145: ((2011) ), 926–940. |
[133] | Nangia-Makker P. , Thompson E. , Hogan C. , Ochieng J. , et al., Induction of tumorigenicity by galectin-3 in a non-tumorigenic human breast carcinoma cell line, Int J Oncol 7: ((1995) ), 1079–1087. |
[134] | Honjo Y. , Nangia-Makker P. , Inohara H. , et al., Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells, Clin Cancer Res 7: ((2001) ), 661–668. |
[135] | Nangia-Makker P. , Honjo Y. , Sarvis R. , et al., Galectin-3 induces endothelial cell morphogenesis and angiogenesis, Am J Pathol 156: ((2000) ), 899–909. |
[136] | Wesley U.V. , Vemuganti R. , Ayvaci E.R. , et al., Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling, Brain Res 1496: ((2013) ), 1–9. |
[137] | Ram J. , Santhosh Kumar T.R. and SujathanK., Galectin -3 contribute for drug resistance and EMT in TNBC, J Integr Oncol (Abstract presented on 3rd World conference on Breast and Cervical Cancer; September 24-25, 2018) (2018) ; 7. doi: 10.4172/2329-6771-C1-002 |
[138] | Ilmer M. , Mazurek N. , Gilcrease M.Z. , et al., Low expression of galectin-3 is associated with poor survival in node-positive breast cancers and mesenchymal phenotype in breast cancer stem cells, Breast Cancer Res 18: ((2016) ), 97. |
[139] | Zhao Q. , Barclay M. , Hilkens J. , et al., Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis, Mol Cancer 9: ((2010) ), 154. |
[140] | Thompson S.C. , The colony forming efficiency of single cells and cell aggregates from a spontaneous mouse mammary tumor using the lung colony assay, Br J Cancer 30: ((1974) ), 332–336. |
[141] | Raz A. , Bucana C. , McLellan W. , et al., Distribution of membrane anionic sites on B16 melanoma variants with differing lung colonization potential, Nature 284: ((1980) ), 363–364. |
[142] | Inohara H. and Raz A. , Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion, Cancer Res 55: ((1995) ), 3267–3271. |
[143] | Inohara H. , Akahani S. , Koths K. , et al., Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion, Cancer Res 56: ((1996) ), 4530–4534. |
[144] | Bergers G. and Benjamin L.E. , Tumorigenesis and the angiogenic switch, Nat Rev Cancer 3: ((2003) ), 401–410. |
[145] | Matarrese P. , Fusco O. , Tinari N. , et al., Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties, Int J Cancer 85: ((2000) ), 545. |
[146] | HanahanD and J.Folkman, Patterns and emerging mechanisms of the angio-genic switch during tumorigenesis, Cell 86: ((1996) ), 353–364. |
[147] | Bridges E. , Oon C.E. and Harris A. , Notch regulation of tumor angiogenesis, Future Oncol 7: ((2011) ), 569–588. |
[148] | Shekhar M.P. , Nangia-Makker P. , Tait L. , et al., Alterations in galec-tin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions, Am J Pathol 165: ((2004) ), 1931–1941. |
[149] | Nangia-Makker P. , Wang Y. , Raz T. , et al., Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer, Int J Cancer 127: ((2010) ), 2530–2541. |
[150] | Sakurai T. and Kudo M. , Signaling pathways governing tumor angiogenesis, Oncology 81: (Suppl 1) ((2011) ), 24–29. |
[151] | Nangia-Makker P. , Baccarini S. and Raz A. , Carbohydrate-recognition and angiogenesis, Cancer Metastasis Rev 19: ((2000) ), 51. |
[152] | Nascimento dos Santos S. , SheldonH., BernardesE.S., et al., Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation, Oncotarget 8: (30) ((2017) ), 49484–49501. |
[153] | Machado C.M. , Andrade L.N. , Teixeira V.R. , et al., Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFβ1-induced macrophages, Cancer Med 3: ((2014) ), 201–214. |
[154] | Wiseman D.M. , Polverini P.J. , Kamp D.W. , et al., Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity, Biochem Biophys Res Commun 157: ((1988) ), 793–800. |
[155] | Derynck R. , Akhurst R.J. and Balmain A. , TGF-beta signaling in tumor suppression and cancer progression, Nat Genet 29: ((2001) ), 117–129. |
[156] | Maniotis A.J. , Folberg R. , Hess A. , et al., Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry, Am J Pathol 155: ((1999) ), 739–752. |
[157] | Hendrix M.J. , Seftor E.A. , Seftor R.E. , et al., Tumor cell vascular mimicry: novel targeting opportunity in melanoma, Pharmacol Ther 159: ((2016) ), 83–92. |
[158] | Mourad-Zeidan A.A. , Melnikova V.O. , Wang H. , et al., Expression profiling of galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry, Am J Pathol 173: ((2008) ), 1839–1852. |
[159] | Etulain J. , Negrotto S. , Tribulatti M.V. , et al., Control of angiogenesis by galectins involves the release of platelet- derived proangiogenic factors, PLoS One 9: ((2014) ), e96402. |
[160] | Akahani S. , Nangia-Makker P. , Inohara H. , et al., Gal-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family, Cancer Res 57: ((1997) ), 5272–5276. |
[161] | Yang R.Y. , Hsu D.K. and Liu F.T. , Expression of galectin-3 modulates T-cell growth and apoptosis, Proc Natl Acad Sci 93: ((1996) ), 6737–6742. |
[162] | Nangia-Makker P. , Nakahara S. , Hogan V. , et al., Galectin-3 in apoptosis, a novel therapeutic target, J Bioenerg Biomembr 39: ((2007) ), 79–84. |
[163] | Yu F. , Finley R.L. , Raz A. , et al., Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation, J Biol Chem 277: ((2002) ), 15819–15827. |
[164] | Oka N. , Nakahara S. , Takenaka Y. , et al., Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells, Cancer Res 65: ((2005) ), 7546. |
[165] | Zou J. , Glinsky V.V. , Landon L.A. , et al., Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion, Carcinogenesis 26: ((2005) ), 309–318. |
[166] | Leffler H. and Barondes S.H. , Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides, J Biol Chem 261: ((1986) ), 10119–10126. |
[167] | Campo V.L. , Marchiori M.F. , Rodrigues L.C. and Dias-Baruffi M. , Synthetic glycoconjugates inhibitors of tumor-related galectin-3: an update, Glycoconj J 33: ((2016) ), 853–876. |
[168] | John C.M. , Leffler H. , Kahl-Knutsson B. , et al., Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer, Clin Cancer Res 9: ((2003) ), 2374–2383. |
[169] | Stegmayr J. , Zetterberg F. , Carlsson M.C. , et al., Extracellular and intracellular small-molecule galectin-3 inhibitors, Scientific reports 9: (1) ((2019) ), 2186. |
[170] | Chen W.S. , Cao Z. , Leffler H. , et al., Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis, Investigative ophthalmology & visual science 58: (1) ((2017) ), 9–20. |
[171] | Delaine T. , Collins P. , MacKinnon A. , et al., Galectin-3-Binding Glycomimetics that Strongly Reduce Bleomycin-Induced Lung Fibrosis and Modulate Intracellular Glycan Recognition, Chembiochem 17: (18) ((2016) ), 1759–1770. |
[172] | Knibbs R.N. , Perini F. and Goldstein I.J. , Structure of the major Concanavalin A reactive oligosaccharides of the extra cellular matrix component laminin, Biochemistry 28: ((1989) ), 6379–6382. |
[173] | Platt D. and Raz A. , Modulation of the Lung Colonization of B16-F1 Melanoma Cells by Citrus Pectin, J Natl Cancer Inst 84: ((1992) ), 438–442. |
[174] | Guess B.W. , Scholz M.C. , Strum S.B. , et al., Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer, Prostate Cancer Prostatic Dis 6: ((2003) ), 301. |
[175] | Eliaz I. , The Role of Galectin-3 as a Marker of Cancer and Inflammation in a Stage IV Ovarian Cancer Patient with Underlying Pro-Inflammatory Comorbidities, Case Rep Oncol 6: ((2013) ), 343–349. |
[176] | Wu K.L. , Huang E.Y. , Jhu E.W. , et al., Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway, J Gastroenterol 48: (3) ((2013) ), 350–359. |
[177] | Song S. , Ji B. , Ramachandran V. , et al., Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling, PLoS One 7: (8) ((2012) ), e42699. |
[178] | Braeuer R.R. , Zigler M. , Kamiya T. , et al., Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin, Cancer Res 72: (22) ((2012) ), 5757–5766. |
[179] | Straube T. , Elli A.F. , Greb C. , et al., Changes in the expression and subcellular distribution of galectin-3 in clear cell renal cell carcinoma, J Exp Clin Cancer Res 30: (1) ((2011) ), 89. |
[180] | Xu Y. , Gu X. , Gong M. , et al., Galectin-3 inhibition sensitizes human renal cell carcinoma cells to arsenic trioxide treatment, Cancer Biol Ther 14: (10) ((2013) ), 897–906. |
[181] | Sakaki M. , Oka N. , Nakanishi R. , et al., Serum level of galectin-3 in human bladder cancer, J Med Invest 55: (1-2) ((2008) ), 127–132. |
[182] | Weber K.B. , Shroyer K.R. , Heinz D.E. , et al., The use of a combination of galectin-3 and thyroid peroxidase for the diagnosis and prognosis of thyroid cancer, Am J Clin Pathol 122: (4) ((2004) ), 524–531. |
[183] | Vuong L. , Kouverianou E. , Rooney C.M. , et al., An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade, Cancer Res 79: (7) ((2019) ), 1480–1492. |
[184] | Pereira J.X. , dos Santos S.N. , PereiraT.C., et al., Galectin-3 Regulates the Expression of Tumor Glycosaminoglycans and Increases the Metastatic Potential of Breast Cancer, Journal of Oncology (2019) . |
[185] | Hayashi Y. , Jia W. , Kidoya H. , et al., Galectin-3 Inhibits Cancer Metastasis by Negatively Regulating Integrin β3 Expression, Am J of Path 184: (4) ((2019) ), 900–910. |
[186] | Linch S. , Kasiewicz M.J. , McNamara M. , et al., Galectin-3 inhibition using novel inhibitor GR-MD-02 improves survival and immune function while reducing tumor vasculature, J Immunotherapy cancer 3: ((2015) ), P306. |
[187] | Cotter F. , Smith D.A. , Boyd T.E. , et al., Single-agent activity of GCS-100, a first-in-class galectin-3 antagonist, in elderly patients with relapsed chronic lymphocytic leukemia, Journal of Clinical Oncology 27: (15) ((2009) ), 7006–7006. |
[188] | Honjo Y. , Nangia-Makker P. , Inohara H. , et al., Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells, Clinical cancer research 7: (3) ((2001) ), 661–668. |
[189] | John C.M. , Leffler H. , Kahl-Knutsson B. , et al., Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer, Clinical cancer research 9: (6) ((2003) ), 2374–2383. |
[190] | de Oliveira J.T. , RibeiroC., BarrosR., et al., Hypoxia up-regulates galectin-3 in mammary tumor progression and metastasis, PLoS One 10: (7) ((2015) ), e0134458. |
[191] | Yamamoto-Sugitani M. , Kuroda J. , Ashihara E. , et al., Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia, Proceedings of the National Academy of Sciences 108: (42) ((2011) ), 17468–17473. |
[192] | Radosavljevic G. , Jovanovic I. , Majstorovic I. , et al., Deletion of galectin-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity, Clinical & experimental metastasis 28: (5) ((2011) ), 451–462. |
[193] | Streetly M.J. , Maharaj L. , Joel S. , et al., GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death, Blood 115: (19) ((2010) ), 3939–3948. |
[194] | Pienta K.J. , Nailk H. , Akhtar A. , et al., Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin, JNCI: Journal of the National Cancer Institute 87: (5) ((1995) ), 348–353. |
[195] | Kobayashi T. , Shimura T. , Yajima T. , et al., Transient silencing of galectin-3 expression promotes both in vitro and in vivo drug-induced apoptosis of human pancreatic carcinoma cells, Clinical & experimental metastasis 28: (4) ((2011) ), 367–376. |
[196] | Guha P. , Kaptan E. , Bandyopadhyaya G. , et al., Cod glycopeptide with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis, Proceedings of the National Academy of Sciences 110: (13) ((2013) ), 5052–5057. |




