Cannabis for medicinal use in patients with rare diseases
Abstract
BACKGROUND:
Patients with Rare Diseases (RDs) present with chronic and debilitating symptoms such as pain, anxiety and epileptic seizures. Symptoms can be unresponsive to conventional treatment and may lead to a decreased Quality of Life for patients. Cannabinoids have been reported to be efficacious against chronic pain refractory to conventional analgesics, anxiety and seizures.
OBJECTIVE:
Identification of RDs for which Medicinal Cannabis (MC) can be used and identification of issues related to RDs and perceptions on the use of MC in patients with RDs.
METHODS:
Study was divided into 2 phases. Phase 1: Literature Review to identify RDs in which cannabis or cannabinoids are used Phase 2: Development, validation and dissemination of 2 questionnaires for: (i) Health Care Professionals (HCPs) and (ii) RD patients.
RESULTS:
Cannabinoids were described as possible therapeutic agents in 20 RDs. The questionnaires were completed by 101 HCPs and 38 RD patients. Thirty-three HCPs had no experience on use of MC but would consider using it in their practice for management of RDs. Most patients (n = 29) did not have experience with use of MC and 20 patients would consider using MC to treat their condition or relieve symptoms of their disease.
CONCLUSION:
The study helps identify the potential of MC use in RDs associated with chronic symptoms such as pain, muscle spasticity, seizures and anxiety.
1Background
Rare diseases (RDs) affect millions of people worldwide, are usually genetic in origin and difficult to diagnose [1–3]. Diagnosis of RDs varies between countries. In the European Union a disease is classified as rare if it affects not more than 5 per 10,000 individuals [4]. The Food and Drug Administration defines RDs as those affecting less than 200,000 patients in the United States [5].
Most patients with RDs present with chronic and debilitating symptoms that persist during their lifetime [6]. Patients with RDs such as palindromic rheumatism, complex regional pain syndrome and neurofibromatosis type 1 can experience chronic pain [7]. Patients may experience pain of different aetiology, localization, and intensity. Pain restricts patients’ physical activity, daily functioning abilities, routine tasks and sleep. Patients can experience anger, frustration, and depression, which can provoke an enhanced sensation of pain and poorer therapeutic outcomes. Chronic pain’s adverse impact is multidimensional on patients’ health-related quality of life (QoL) [8].
Patients with RDs such as complex regional pain syndrome, trigeminal neuralgia, or central pain syndrome experience chronic neuropathic pain which is often unmanageable with the use of conventional pharmacological treatment. Patients with poorly managed neuropathic pain have significantly poorer health status and elevated anxiety and depression [9, 10].
RDs such as Dravet Syndrome, Lennox-Gastaut Syndrome, Lafora Disease, and Doose syndrome are associated with epileptic seizures. Despite a significant number of antiseizure medications available, at least 30% of patients with epilepsy are unresponsive to treatment [11]. Seizures are linked to higher rates of psychological complications, such as anxiety and depression [12].
An estimated 95% of RD patients do not have an approved therapy available for their disease. Treatment of patients with RDs presents additional challenges compared to the ones presenting with more common diseases. Lack of knowledge about the RD and medical expertise leads to patients being undiagnosed or misdiagnosed. Rarity of disease significantly complicates specific medicines’ clinical development: a small group of patients in study recruitment stages, stringent ethical considerations for inclusion of vulnerable patients and lack of pre-existing knowledge on the rare condition. The development of orphan medications is not always viable for the pharmaceutical industry because of the limited number of patients, small market, lack of funding and higher production costs [13].
Cannabis-derived preparations have been used as traditional medicine in different cultures for centuries. There has been a resurgence of interest in the therapeutic properties of cannabis with the identification of main cannabinoids: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) and the discovery of the endocannabinoid system (ECS) [14].
Cannabinoids, used alone and in combination have shown efficacy in central and peripheral neuropathic pain and in muscle and joint pain [15]. Cannabinoids have been reported to be efficacious against chronic pain refractory to conventional analgesics including cancer pain [16, 17]. Cannabis used for chronic pain was also found to improve sleep quality and physical functioning of patients [18]. THC and CBD have shown efficacy in seizure prevention [19]. CBD is approved for use for treatment of two rare forms of epilepsies: Lennox-Gastaut syndrome and Dravet syndrome [20].
The aims of the study were to: (i) identify RDs for which medicinal cannabis (MC) can be used and (ii) identify issues related to RDs and perceptions on the use of MC in patients with RDs.
2Methods
The study was divided into 2 phases. Phase 1 consisted of Literature review to identify RDs in which cannabis or cannabinoids are used. Phase 2 involved the development, validation and dissemination of questionnaires for health care professionals (HCPs) and RD patients. University of Malta Faculty Research Ethics Committee (FREC) Approval was granted prior to the commencement of the study.
2.1Phase 1
Studies documenting the use of cannabis or cannabinoids in RDs were identified using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) tool [21]. Open-access peer-reviewed journal articles published in PubMed Central and MEDLINE in English between January 2020 and October 2021 were retrieved. Search terms used included: ‘rare diseases, medical marijuana’, ‘orphan disease, medical marijuana’, ‘rare disease, THC, CBD’, ‘rare disease tetrahydrocannabinol, cannabidiol.’ The type of study, type of rare disease, cannabinoid used, subjects and endpoints were compared.
2.2Phase 2
Two questionnaires were developed for: (i) HCPs (ii) RD patients. Both questionnaires were divided into 5 sections: a) demographic information b) clinical presentation of RD c) treatment of RD: d) issues related to treatment e) use of MC. Information gathered in both questionnaires included symptoms experienced by RD patients, treatment currently used to relieve symptoms experienced by RD patients, issues related to treatment for RD and experience and perceptions on use of MC.
The questionnaires were validated by six pharmacists (three working in the community, two in academia and one in regulatory sciences), a psychiatric nurse, an occupational therapist and a physician. Questionnaires were distributed electronically and by hand. The questionnaire for HCPs was distributed among healthcare associations, health centres, private medical clinics, pharmacies, and to individual healthcare professionals. The questionnaire for RD patients was disseminated via contact persons at a local RD support organisation, a pain clinic and through the local association of occupational therapists. Questionnaires of RD patients were filled in by the RD patients or their carer.
3Results
3.1Phase 1
Literature revealed 31 articles (Fig. 1) that described the use of MC as a possible therapeutic option in 20 different RDs mainly epileptic conditions and neurodegenerative diseases (Table 1).
Fig. 1
PRISMA flow diagram for literature review on Medicinal Cannabis use in Rare Diseases.
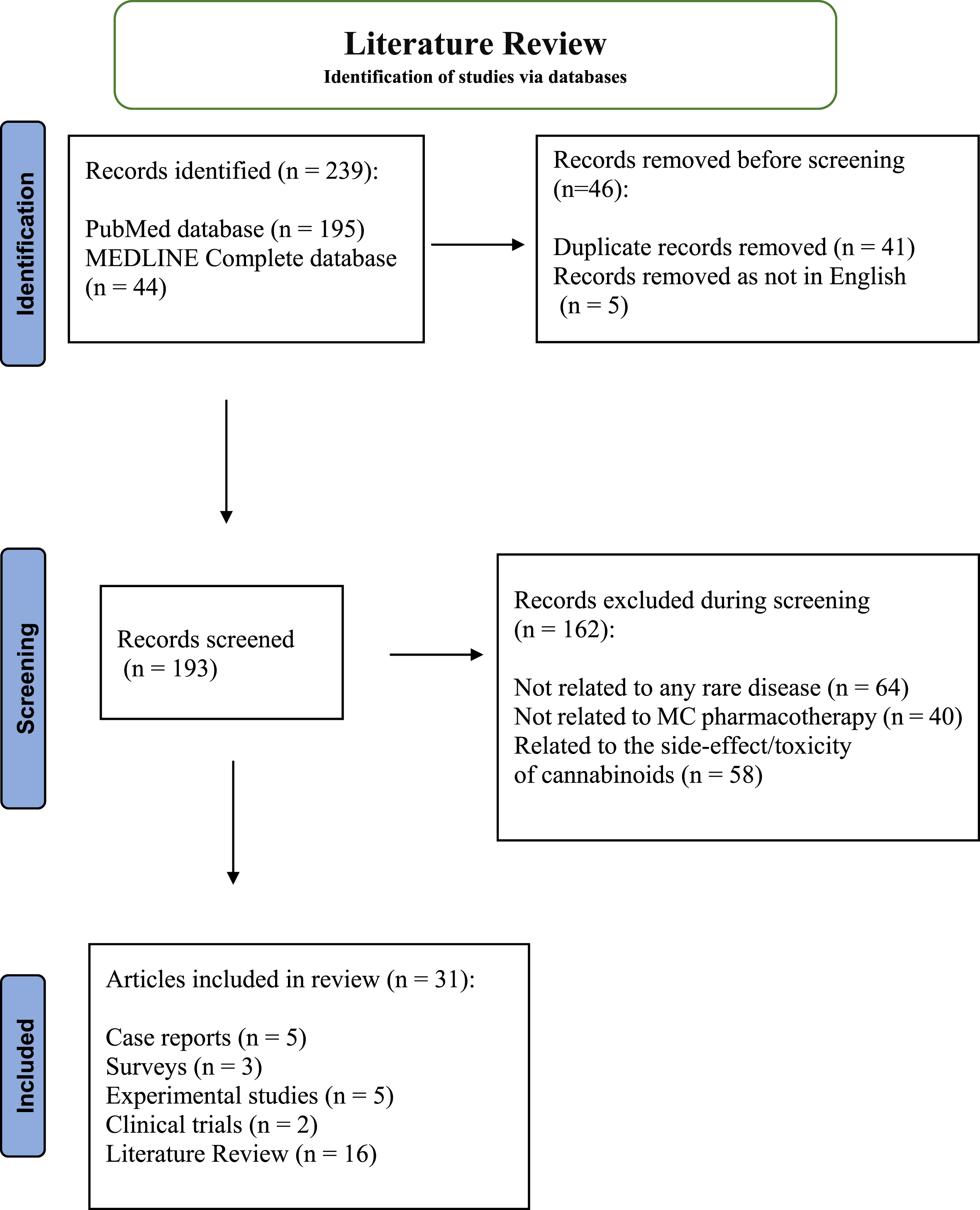
Table 1
RDs identified in literature in which MC is/can be used (N = 20)
| Rare disease |
| Autoimmune encephalitis [22] |
| Autosomal Systemic Lupus Erythematosus [23] |
| CDKL5 Deficiency Disorder [24] |
| Chronic Intestinal Pseudo-Obstruction [25] |
| Cystic Fibrosis [23] |
| Dermatomyositis [23] |
| Doose Syndrome [24], [26] |
| Dravet Syndrome [24], [26–38] |
| Epidermolysis Bullosa [39] |
| Febrile Infection-Related Epilepsy Syndrome [40] |
| Huntington disease [41], [42] |
| Lafora Disease [43] |
| Lennox-Gastaut Syndrome [24], [26], [27], [29–38] |
| Neurofibromatosis Type 1 [44] |
| Prader-Willi Syndrome [45] |
| Rett Syndrome [46], [47] |
| Systemic Sclerosis Scleroderma [23], [48] |
| Tuberous Sclerosis Complex [24], [36], [49], [50] |
| West Syndrome [51] |
| Williams-Beuren Syndrome [52] |
The most common types of studies were review studies [23, 24, 28–34, 36–38, 42, 45, 46, 51]. Studies discussed the use of cannabinoids in RDs either as the main therapeutic agent, such as in Epidermolysis Bullosa (n = 1) and Systemic Sclerosis Scleroderma (n = 1) or as an add-on therapy, such as in conditions associated with refractory epilepsy (n = 7). The most commonly used cannabinoid was CBD (n = 16).
Five case reports presented cases related to five different RDs where patients were receiving commercially available forms of MC, such as Epidiolex, Sativex or Dronabinol [25, 40, 41, 44, 52]. All patients showed improvement in presenting symptoms, including chronic sensory and motor symptoms, abdominal pain and drug-resistant seizures (Table 2).
Table 2
Results from Case Reports (n = 5)
| Rare disease | Case description | Cannabis product used | Main outcomes |
| Chronic intestinal pseudo-obstruction | Female, 19 years old with enteral feeding intolerance, presenting with abdominal pain | Dronabinol (THC) | Cessation of subocclusive episodes and gastrointestinal complaints. Improvement in QoL, appetite and food tolerance. [25] |
| Febrile infection-related epilepsy syndrome | Children (n = 7) progressing to refractory status epilepticus | Epidiolex (CBD) | Improvement in frequency and duration in focal seizures with impaired consciousness. Complete resolution of seizures in one patient. Improvements in motor, verbal and cognitive abilities. [40] |
| Juvenile and/or paediatric Huntington disease (HD) | Retrospective cohort (n = 32) patients with HD with movement disorders and polyneuropathy. | Sativex oromucosal spray (THC:CBD 1 : 1) | Marked improvement of motor symptoms in severe cases of generalised dystonia [41] |
| Neurofibromatosis type 1 (NF1) | Male, 30 years old with chronic sensory and motor symptoms | Sativex oromucosal spray (THC:CBD 1 : 1) | Improvement in QoL, walking independence and reduction in neuropathic pain and dysesthesia [44] |
| Williams-Beuren syndrome | Female, 13 years old with pharmacoresistant epilepsy, presenting with seizures of varying semiology, most of which are accompanied by cyanosis, bradycardia and desaturation | Epidiolex (CBD) | Significant reduction in seizure frequency and intensity. Improvement in cerebral activity, posture and gross motor abilities, enriched vocalisation and improved social skills [52] |
Three surveys discussed the use of MC in Epidermolysis Bullosa, in Dravet syndrome Lennox-Gastaut syndrome and Doose syndrome and in Dravet syndrome and Lennox-Gastaut syndrome. Improvement in symptoms following the use of MC was documented in the three articles [26, 27, 39].
Experimental studies described the use of cannabinoids in mice (n = 4) and zebra fish (n = 1) models. Studies reported improvement in symptoms such as pain, seizures and anxiety following the use of MC [22, 43, 47–49].
Clinical trials investigated the efficacy and safety of CBD in paediatric and adult patients who were not responsive to anti-seizure medication [35, 50]. Patients had a reduced seizure frequency following administration of CBD with somnolence and diarrhoea being the most common adverse effects (Table 3).
Table 3
Results from Clinical Trials (n = 2)
| Rare disease | Study design and population | Cannabis product used | Main outcomes |
| Dravet syndrome, Lennox-Gastaut syndrome | Open-label prospective trialPaediatric and adult patients (N = 93)12 months of treatment | Epidiolex (CBD) | 40.2% had at least a 50% reduction in seizure frequency in the first 3 monthsMost common adverse effects were somnolence (22.6%) and diarrhea (11.9%) [35] |
| Tuberous Sclerosis | Double-blind, placebo-controlled randomized clinical trial.Paediatric and adult patients (N = 224)16 weeks of treatment | Epidiolex (CBD) | Reduction in types of seizures was equal groups receiving different doses of CBD and lower in placeboMost common adverse effects were diarrhea (56%) and somnolence (26%) [50] |
3.2Phase 2
One hundred and one HCPs answered the questionnaire (Table 4). Forty-eight HCPs encountered 2-4 RD patients a year. RDs that HCPs encountered in their practice were Fabry disease (n = 4), Fragile X syndrome (n = 2), autosomal systemic lupus erythematosus (n = 2), Huntington’s disease (n = 2) and Charcot Marie tooth disease (n = 2).
Table 4
HCPs demographic information (N = 101)
| Age (years) | 20–57< |
| Gender | Female (n = 59) Male (n = 42) |
| Years of Practice | 0–2 (n = 13) |
| 3–5 (n = 20) | |
| 6–10 (n = 22) | |
| 11–15 (n = 14) | |
| >15 (n = 32) | |
| Profession | Pharmacists (n = 40) |
| General practitioners (n = 17) | |
| Occupational therapists (n = 13) | |
| Medical specialists (n = 11) | |
| Speech therapists (n = 4) | |
| Physiotherapists (n = 4) | |
| Nurse (n = 4) | |
| Other (n = 8) | |
| Area of practice | Hospital (n = 25) |
| Private clinic (n = 22) | |
| Community pharmacy (n = 29) | |
| Regulatory sciences (n = 8) | |
| Nursing home (n = 5) | |
| Public health centre (n = 5) | |
| Academia (n = 1) | |
| Other (n = 6) |
Pain (n = 51) was the most commonly encountered symptom experienced by their RD patients followed by anxiety (n = 34), muscle spasticity (n = 33) and sleep disorders (n = 20). Persistent chronic neuropathic pain (n = 31) was the most common type of pain reported by the patients of the HCPs. Patients who presented with seizures had 1-3 disabling seizures per year in most cases (n = 6) followed by 1-3 disabling seizures per month (n = 4). HCPs reported that RD patients experienced emotional and/or physical stress (n = 22).
Medication classes commonly prescribed by HCPs to RD patients included benzodiazepines (n = 69), analgesics (n = 37) and muscle relaxants (n = 35). The most common issues associated with RD patient treatment in HCPs opinion was diagnosis of the RD (n = 43), treatment financial burden (n = 27) and lack of available treatment options (n = 26).
Thirty-three HCPs had no experience on the use of MC but would consider using it in their practice. Pain was the most common symptom (n = 52) for which HCPs would prescribe cannabis, followed by muscle spasticity (n = 38), anxiety (n = 38) and sleep disorders (n = 32) (Table 5). Adverse effects that HCPs were concerned about with the use of MC were confusion (n = 30), addiction (n = 29), drowsiness (n = 23), impaired memory and concentration (n = 20) and hallucinations (n = 20).
Table 5
Indications for which HCPs would prescribe MC (N = 9)
| Indications | No. of HCPs |
| Pain | 52 |
| Muscle spasticity | 38 |
| Anxiety | 38 |
| Sleep disorders | 32 |
| Seizures | 21 |
| Nausea and/or vomiting | 19 |
| Appetite stimulation | 15 |
| Skin disorders | 8 |
| Respiratory disorders | 3 |
The RD patient questionnaire was completed by 38 respondents. The majority (n = 29) of RD patients were adults (Table 6). The most common RDs in this group of patients were Kabuki Syndrome (n = 3) and Fabry Disease (n = 2).
Table 6
RD patients demographic information (N = 38)
| Age (years) | ≥18 years (n = 9) |
| 19–40 years (n = 10) | |
| 41–50 years (n = 11) | |
| 51< (n = 8) | |
| Gender | Female (n = 21), Male (n = 17) |
Pain (n = 24) and anxiety (n = 22) were the symptoms most commonly experienced by the patients. Joint pain (n = 5) and headaches (n = 5) were the most common types of pain. RD patients reported experiencing impairment in concentration and memory (n = 12), gastro-intestinal symptoms (n = 12), weakness or dizziness (n = 10) and insomnia (n = 8).
Twenty respondents found it easy to access their medications most of the time and medicine price was not considered to be a burden by most patients (n = 21). Medication adverse effects that were reported by patients included fatigue (n = 2), nausea and vomiting (n = 1), constipation (n = 1) and insomnia (n = 1).
Most patients (n = 29) did not have experience with use of MC and 20 patients would consider using MC to treat their condition or relieve symptoms of the disease. Patients would prefer to use MC administered as oral capsules (n = 19).
The majority of patients (n = 18) were not concerned about MC-related adverse effects. Adverse effects of most concern in relation to MC use were confusion (n = 8), hallucinations (n = 7), impaired memory and concentration (n = 7) and addiction (n = 7). Other concerns related to the use of MC included cost of MC (n = 5) and social stigma (n = 5). Lack of effectiveness and accessibility of MC was not a concern of any patient.
4Conclusions
The study shed light on RDs for which MC can be used and on issues related to RDs and perceptions on the use of MC of RD patients and HCPs. MC can be considered as a possible therapeutic option for RDs where patients experience pain, seizures and anxiety. Studies included in the literature review reported positive outcomes when MC was used in the management of RDs. Case reports reported efficacy of commercially available MC products in reducing the severity of symptoms such as neuropathic pain, dystonia and seizures. Some case reports reported an improvement in the QoL of patients including improved mobility, mood and quality of sleep. Improvement in patients’ QoL can have a positive impact on their personal, social and health outcomes [53]. Although results from case reports cannot be extrapolated to the general population, they help provide an understanding of patients’ disease anamnesis and of outcomes related to MC use in specific RDs [54]. Results from surveys included in the study reported improvement in symptoms of RDs when using MC. Although surveys can help gather information from a larger number of respondents, results might not be so representative considering the rarity of the diseases and possible subjectivity when answering some of the questions. Information on dosing regimen of MC and level of compliance to treatment was not included in these studies. Results from the clinical trials reported a reduction in seizure frequency with the use of MC. Limitations of conducting clinical trials on RD patients include the small number of patients available for enrolment making interpretation of results questionable. The literature review was limited by the inclusion criteria. Including a larger number of journal articles by using different search terms, databases or a different publication time period might yield different results.
Results of the questionnaires for HCPs and RD patients indicated that pain, muscle spasticity, sleep disorders and anxiety were symptoms experienced commonly by RD patients. Although symptoms of RD patients differ according to disease, pain, fatigue and muscle spasticity are commonly reported by different RD patients [55]. Both groups of respondents had positive views about the possible use of MC for treatment of these symptoms. HCPs and RD patients were concerned about the same type of possible side effects that can occur with MC use, namely confusion, hallucinations and impaired memory or concentration. Such effects which can impact brain development and mental function have been reported with use of cannabis [56].
HCPs stated that difficulty of establishing correct diagnoses and lack of available treatment options were issues related to RDs. The European Commission stated that: ‘on average it takes five years for a patient to get a diagnosis’ and in many cases the diagnosis remains unestablished [57]. The number of drugs granted an orphan drug designation (ODD) by the FDA and European Medicines Agency declined over the last years, with a slight increase in 2020 [58]. Medicines granted ODD may still not be accessible to patients due financial constraints. On average between 30–60% of orphan medicines are reimbursed in EU countries [59, 60]. HCPs who had no experience on using MC in their practice would consider using it. Martins-Welch et al reported that HCPs strongly supported the use of MC for patients with chronic conditions [61]. There has been an increasing interest of HCPs about the therapeutic properties of MC in the last decades [62].
Although patients included in the study suffered from different RDs, they experienced similar symptoms namely pain and anxiety. Patients with chronic pain including muscle tension can commonly experience anxiety disorders [63]. Chronic stress and anxiety can lead to the development of many other stress-related symptoms such as the ones experienced by this group of patients namely difficulty in concentration and memory, gastrointestinal symptoms, weakness and insomnia which all have negative impacts on the QoL. Patients reported to be facing issues related to their RD treatment namely side-effects associated with the use of prescribed medicines such as fatigue, insomnia, nausea, vomiting and constipation.
Although the majority did not have any experience with the use of MC, RD patients were willing to use MC to relieve symptoms of their disease. Symptoms for which patients would use MC were similar to those reported by other studies: pain, anxiety, insomnia and muscle spasms [64, 65].
Patient concerns related to MC use included possible adverse effects, cost and social stigma although the majority of patients did not have these concerns. A study by Zeng et al reported that patients valued the effectiveness of MC for symptom management even when experiencing adverse effects such as impaired memory and concentration [66]. Cost of MC for use in RD would probably be less than costs of orphan medications which are usually higher in price than conventional medication [2]. Negative stigma associated with MC use might be related to a lack of knowledge about it. The role of HCPs in educating patients and the public about MC use is important to reduce sigma associated with MC [67]. Future studies could involve a greater number of respondents when it comes to the HCPs’ and RD patients’ questionnaire. Involving HCPs and RD patients who have experience on the use of MC could provide better insight and help establish a clinical rationale for MC use in RD patients. Cannabis-based medicines with different cannabinoid profiles should be clinically investigated, establishing safe and efficacious MC use for different indications in RDs. Regular use of cannabis has been associated with long-term side effects such as mood disorders, cognitive alterations, psychosis and alterations in brain function and structure [68–70]. Evaluation of negative effects associated with long-term use of cannabis in RD patients should be considered. It is also recommended to conduct pharmacoeconomic evaluations of MC use in RDs, including possible reimbursement opportunities through government medical assistance programmes.
RD patients face multiple challenges to their therapy, ranging from diagnoses to accessibility of treatment. Most RD patients’ symptoms are chronic and, in some cases, persist regardless of the therapy used. RD patients are lacking solutions for effective relief of symptoms of their disease. The study helps identify the potential of MC use in RD patients. MC can be used in RDs which are associated with chronic symptoms such as pain, muscle spasticity, seizures and anxiety. In lack of efficacious treatment options for RD patients, MC should be considered as an alternative therapy for symptom relief. Education and awareness programmes and support of the development of new effective MC treatments by establishing regulations and policies should be provided to help overcome challenges related to treatment in RDs.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
Author contributions
Jekaterina Parovincaka: Main researcher, collected and analysed data.
Janis Vella Szijj: Supervisor, drafted manuscript and corresponding author.
Anthony Serracino Inglott: Supervisor and reviewer.
Lilian M. Azzopardi: Head of Department and reviewer.
References
[1] | Lewis J. , Snydera M. and Hyatt-Knorr H. , Making 15 years of the genetic and rare diseases information centre, Translational Science of Rare Diseases 2: ((2017) ), 77–88. |
[2] | Abbas A. , Vella Szijj J. , Azzopardi L.M. and Serracino-Inglott A. , Orphan drug policies in different countries, Journal of Pharmaceutical Health Sciences Research 10: ((2019) ), 295–302. |
[3] | Schourick J. , Wack M. and Jannot A.S , Assessing rare diseases prevalence using literature quantification, The Orphanet Journal of Rare Diseases 16: ((2021) ), 139. |
[4] | European Commission, EU research on rare diseases, [cited 2022 Nov 28]. Available from: https://research-and-innovation.ec.europa.eu/research-area/health/rare-diseases_en |
[5] | Regier D.S. , Weaver J.A. , Cheng N. , Batshaw M.L. , Ottolini M. , Shy M.E. and Summar M.L. , The rare disease research scholars program: a training curriculum for clinical researchers with mixed methods evaluation study, Translational Science of Rare Diseases 6: ((2022) ), 1–11. |
[6] | Orphanet, About rare diseases, [cited 2022 Nov 28]. Available from: https://www.orpha.net/consor/cgi-bin/Education_AboutRareDiseases.php?lng=EN |
[7] | National Centre for Advancing Translational Sciences, Genetic and Rare Diseases Information Centre, [cited 2022, Nov 28]. Available from: https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases |
[8] | Hadi M.A. , McHugh G.A. and Closs S.J. , Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study, Journal of Patient Experience 6: ((2019) ), 133–141. |
[9] | Rahn E.J. and Hohmann A.G. , Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside, Neurotherapeutics 6: ((2009) ), 713–737. |
[10] | Hossain M.Z. , Hasan G.Z. and Siddiqui T.H. , Congenital bladder diverticulum causing acute urinary retention in an infant, Mymensingh Medical Journal 21: ((2012) ), 360–362. |
[11] | Fattorusso A. , Matricardi S. , Mencaroni E. , Dell’Isola G.B. , Di Cara G. , Striano P. , et al., Thepharmacoresistant epilepsy: an overview on existent and new emergent therapies, Frontiers in Neurology 12: ((2021) ), 674483. |
[12] | World Health Organisation, Epilepsy, Key facts, [cited 2022 Nov 28]. Available from: https://www.who.int/news-room/fact-sheets/detail/epilepsy |
[13] | Stoller J.K. , The challenge of rare diseases, Chest 153: ((2018) ), 1309–1314. |
[14] | Crocq M.A. , History of cannabis and the endocannabinoid system, Dialogues in Clinical Neuroscience 3: ((2022) ), 223–228. |
[15] | Xiong W. , Tanxing C. , Cheng K. , Yang F. , Chen S. , Willenbring D. , Guan Y. , et al., Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors, Journal of Experimental Medicine 209: ((2012) ), 1121–1134. |
[16] | Turgeman I. and Bar-Sela G. , Cannabis use in palliative oncology: a review of the evidence for popular indications, The Israel Medical Association Journal 19: ((2017) ), 85–88. |
[17] | Meng H. , Dai T, , Hanlon J.G. , Downar J. , Alibhai S.M. and Clarke H. , Cannabis and cannabinoids in cancer pain management, Current Opinion Support Palliative Care 14: ((2020) ), 87–93. |
[18] | Wang L. , Hong P.J. , May C. , Rehman Y. , Oparin Y. , Hong C.J. , et al., Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic reviewand meta-analysis of randomised clinical trials, British Medical Journal 374: ((2021) ). |
[19] | Devinsky O. , Cilio M.R. , Cross H. , Fernandez-Ruiz J. , French J. , Hill C. , et al., Cannabidiol: pharmacology andpotential therapeutic role in epilepsy and other neuropsychiatric disorders, Epilepsia 55: (2014), 791–802. |
[20] | García-Peñas J.J. , Gil Nagel-Rein A. , Sánchez-Carpintero R. and Villanueva-Haba V. , Cannabidiol for the treatment of Lennox-Gastautsyndrome and Dravet syndrome: experts’ recommendations for its usein clinical practice in Spain, Revista de Neurologia 73: ((2021) ), S1–S8. |
[21] | Bourton I. , Hoffmann T. , Mulrow C. , Shamseer L. , Tetzlaff J. , Akl E. , et al., The PRISMA 202 statement: an updated guideline for reporting systematic reviews, British Medical Journal 372: ((2021) ), n71. |
[22] | Sisay S. , Pryce G. , Jackson S.J. , Tanner C. , Ross R.A. , Michael G.J. , et al., Genetic background can result in a marked or minimal effect of gene knockout (GPR55 and CB2 receptor) in experimental autoimmune encephalomyelitis models of multiple sclerosis, PLoS One 8: ((2013) ), e76907. |
[23] | Burstein S.H. , Ajulemic acid: potential treatment for chronic inflammation, Pharmacology Research and Perspectives 6: ((2018) ), e00394. |
[24] | Arzimanoglou A. , Brandl U. , Cross J.H. , Gil-Nagel A. , Lagae L. , Johannsesen Landmark C. , et al., Epilepsy and cannabidiol: a guide to treatment, Epileptic Disorders 22: ((2020) ), 1–14. |
[25] | Zemrani B. , Lambe C. and Goulet O. , Cannabinoids improve gastrointestinal symptoms in a parenteral nutrition-dependent patient with chronic intestinal pseudo-obstruction, The Journal of Parenteral and Enteral Nutrition 45: ((2021) ), 427–429. |
[26] | Porter B.E. and Jacobson C. , Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy, Epilepsy and Behaviour 29: ((2013) ), 574–577. |
[27] | Press C.A. , Knupp K.G. and Chapman K.E. , Parenteral reporting of response to oral cannabis extracts for treatment of refractory epilepsy, Epilepsy and Behaviour 45: ((2015) ), 49–52. |
[28] | Strzelczyk A. and Schubert-Bast S. , Expanding the Treatment Landscape for Lennox-Gastaut Syndrome: Current and Future Strategies, CNS Drugs 35: ((2021) ), 61–83. |
[29] | Raucci U. , Pietrafusa N. , Paolino M.C. , Di Nardo G. , Villa M.P. , Pavone P. , et al., Cannabidiol treatment for refractory epilepsies in pediatrics, Frontiers in Pharmacology 11: ((2020) ), 596110. |
[30] | Singer L. , Tokish H. , Park F. , Campisi C, and Milanaik R.L. , The cannabidiol conundrum: potential benefits and risks of cannabidiol products for children, Current Opinion in Pediatrics 32: ((2020) ), 198–205. |
[31] | Gray R.A. and Whalley B.J. , The proposed mechanisms of action of CBD in epilepsy, Epileptic Disorders 22: ((2020) ), S10–S15. |
[32] | Pacher P. , Kogan N.M. and Mechoulam R. , Beyond THC and Endocannabinoids, Annual Review of Pharmacology and Toxicology 60: ((2020) ), 636–659. |
[33] | Specchio N. , Pietrafusa N. and Cross H.J. , Source of cannabinoids: what is available, what is used, and where does it come from? Epileptic Disorders 22: ((2020) ), S1–S9. |
[34] | Steriade C. , French J. and Devinsky O. , Epilepsy: key experimental therapeutics in early clinical development, Expert Opinion on Investigational Drugs 29: ((2020) ), 373–383. |
[35] | Iannone L.F. , Arena G. , Battaglia D. , Bisulli F. , Bonanni P. , Boni A. , et al., Results from an Italian expanded access program on cannabidiol treatment in highly refractory Dravet Syndrome and Lennox Gastaut Sydrome, Frontiers in Neurology 12: ((2021) ), 673135. |
[36] | Johannessen Landmark C. , Potschka H. , Auvin S. , Wilmshurst J.M. , Johannessen S. I. , Kasteleijn-Nolst D. , et al., The role of new medical treatments for the management of developmental and epileptic encephalopathies: novel concepts and results, Epilepsia 62: ((2021) ), 857–873. |
[37] | Zürcher K. , Dupont C. , Weber P. , Grunt S. , Wilhelm I. , Eigenmann D.E. , et al., Use and caregiver-reportedefficacy of medical cannabis in children and adolescents in Switzerland, European Journal of Paediatrics 181: ((2022) ), 335–347. |
[38] | Chin R.F. , Mingorance A. , Ruban-Fell B. , Newell I , Evans J , Vyas K. , et al., Treatment guidelines for rare, early-onset, treatment-resistant epileptic conditions: a literature review on Dravet Syndrome, Lennox Gastaut Syndrome and CDKL5 deficiency disorder, Frontiers in Neurology 12: ((2021) ), 734612. |
[39] | Schräder N.H. , Gorell E.S. , Stewart R.E. , Duipmans J.C. , Harris N. , Perez V.A. , et al., Cannabinoid use and effects in patients with epidermolysis bullosa: an international cross-sectional survey study, The Orphanet Journal of Rare Diseases 16: ((2021) ), 377. |
[40] | Koh S. , Wirrell E. , Vezzani A. , Nabbout R. , Muscal E. , Kaliakatsos M. , et al., Proposal to optimize evaluation and treatment of Febrile infection-related epilepsy syndrome (FIRES): A report from FIRES workshop, Epilepsia Open 6: ((2021) ), 62–72. |
[41] | Achenbach J. , Thiels C. , Lücke T and Saft C. , Clinical manifestation of juvenile and pediatric HD patients: a retrospective case series, Brain sciences 10: ((2020) ), 340. |
[42] | Pérez-Olives C. , Rivas-Santisteban R. , Lillo J. , Navarro G. and Franco R. , Recent advances in the potential of cannabinoids for neuroprotection in Alzheimer’s, Parkinson’s and Huntington’s diseases, Advances in Experimental Medicines and Biology 1264: ((2021) ), 81–92. |
[43] | Aso E. , Andrés-Benito P. , Grau-Escolano J. , Caltana L. , Brusco A. , Sanz P. , et al., Cannabidiol-enriched extract reduced the cognitive impairment but not the epileptic seizures in Lafora Disease animal model, Cannabis and Cannabinoid Research 5: ((2020) ), 150–163. |
[44] | Virgilio E. , Vecchio D. , Naldi P. and Cantello R. , Efficacy of cannabinoids on spasticity and chronic pain in a patient with co-occurrence of multiple sclerosis and neurofibromatosis Type 1, European Journal of Case Reports in Internal Medicine 8: ((2021) ), 002424. |
[45] | Carias K.V. and Wevrick R. , Preclinical testing in translational animal models of Prader-Willi Syndrome: overview and gap analysis, Molecular therapy, Methods and Clinical Development 14: ((2019) ), 344–358. |
[46] | Mouro F.M. , Miranda-Lourenco C. , Sebastião M.A. and Diógenes M.J. , From cannabinoids and neurosteroids to statins and the ketogenic diet: New therapeutic avenues in Rett Syndrome? Frontiers in Neuroscience 13: ((2019) ), 680. |
[47] | Vigli D. , Cosentino L. , Pellas M. and De Filippis B. , Chronic treatment with cannabidiolic acid (CBDA) reduces pain sensitivity in male mice and rescues the hyperalgesia in a mouse model of Rett Syndrome, Neuroscience 453: ((2021) ), 113–123. |
[48] | del Rio C. , Navarrete C. , Collado J.A. , Bellido M.L. , Gómez-Cañas M. , Pazos M.R. , et al., The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways, Scientific Reports 6: ((2016) ), 21703. |
[49] | Serra I. , Scheldeman C. , Bazelot M. , Whalley B.J. , Dallas M.L. , de Witte P.A. , et al., Cannabidiol modulates phosphorylated rpS6 signalling in a zebrafish model of Tuberous Scleorosis Complex, Behavioral Brain Research 363: ((2019) ), 135–144. |
[50] | Thiele E.A. , Bebin E.M. , Bhathal H. , Jansen F.E. , Kotulska K. , Lawson J.A. , et al., Add-on cannabidiol treatment for drug-resistant seizures in Tuberous Sclerosis Complex: a placebo-controlled randomized clinical trial, JAMA Neurology 78: ((2021) ), 285–292. |
[51] | Velísek L. and Velíšková J. , Modelling epileptic spasms during infancy: are we heading for thetreatment yet? Pharmacology and Therapeutics 212: (2020), 107578. |
[52] | Nicotera A.G. , Spanó M. , Decio A. , Valentini G. , Saia M. and Di Rosa G. , Epileptic phenotype and cannabidiolefficacy in a Williams-Beuren Syndrome patient with atypical deletion: A case report, Frontiers inNeurology 12: ((2021) ), 659543. |
[53] | Flanagan S. , Damery S. and Combes G. , The effectiveness of integrated care interventions in improving patient quality of life (QoL) for patients with chronic conditions: An overview of the systematic review evidence, Health and Quality of Life Outcomes 15: ((2017) ), 1–11. |
[54] | Ranganathan P. and Aggarwal R. , Study designs: Part 1- An overview and classification, Perspectives in Clinical Research 9: ((2018) ), 184–186. |
[55] | Mueller T. , Jerrentrup A. , Bauer M.J. , Fritsch H.W. and Schaefer J.R. , Characteristics of patients contacting a center for undiagnosed rare diseases, Orphanet Journal of Rare Diseases 11: ((2016) ), 81. |
[56] | Volkow N.D. , Swanson J.M. , Evins A.E. , DeLisi L.E. , Meier M.H. , Gonzalez R. , et al., Effects of cannabis on human behaviour including cognition, motivation and psychosis: A review, JAMA Psychiatry 73: ((2016) ), 292–297. |
[57] | D’Alessio V. , The long journey to a rare disease diagnosis, Horizon, [Cited 2022 Nov 30]. Available from: https://ec.europa.eu/research-and-innovation/en/horizon-magazine/long-journey-rare-disease-diagnosis |
[58] | Global Data Healthcare, Orphan Drugs face uphill battle in 2020 [Cited 2022 Nov 30]. Available from: https://www.pharmaceutical-technology.com/comment/orphan-drugs-2020/ |
[59] | Field M.J. and Boat T.F. , Profile of rare diseases, Rare diseases and orphan products: Accelerating research and development, National Academies Press, United States, 2010. |
[60] | Jagadeesan C.T. and Wirtz V.J. , Geographical accessibility of medicines: a systematic literature review of pharmacy mapping, Journal of Pharmacy Policy Practice 14: ((2021) ), 28. |
[61] | Martins-Welch D. , Kline M. and Modayil S. , Health providers’ perspectives on medical marijuana use, Journal of Clinical Oncology 35: ((2017) ), 235. |
[62] | Weisman J.M. and Rodriguez M. , A systematic review of medical students’ and professionals’ attitudes and knowledge regarding medical cannabis, Journal of Cannabis Research 3: ((2021) ), 1–20. |
[63] | Anxiety & Depression Association of America (ADAA). Chronic pain, [Cited 2022 Nov 30]. Available from: https://adaa.org/understanding-anxiety/related-illnesses/other-related-conditions/chronic-pain |
[64] | Kosiba J.D. , Maisto S.A. and Ditre J.W. , Patient-reported use of medical cannabis for pain, anxiety and depression symptoms: Systematic review and meta-analysis, Social Science and Medicine 233: ((2019) ), 181–192. |
[65] | Rosenthal M.S. and Pipitone R.N. , Demographics, perceptions and use of medical marijuana among patients in Florida, Medical Cannabis and Cannabinoids 4: ((2021) ), 13–20. |
[66] | Zeng L. , Wang X. , Kithulegoda N. , Agterberg S. , Shergill Y. , Esfahani M.A. , et al., Values and preferences towards medical cannabis among people with chronic pain: a mixed-methods systematic review, BMJ Open 11: ((2021) ), e050831. |
[67] | Clobes T.A. , Palmier L.A. , Gagnon L. , Klaiman C. and Arellano M. , The impact of education on attitudes toward medical cannabis, PEC Innovation 1: ((2022) ), 100009. |
[68] | Cohen K. , Weizman A. and Weinstein A. , Positive and negative effects of cannabis and cannabinoids on health, Clin Pharmacol Ther 105: (5) ((2019) ), 1139–1147. |
[69] | Battistella G. , Fornari E. , Annoni J.M. , Chtioui H. , Dao K. , Fabritius M. , et al., Long-term effects of cannabis on brain structure, Neuropsychopharmacology 39: (9) ((2014) ), 2041–2048. |
[70] | Karila L. , Roux P. , Rolland B. , Benyamina A. , Reynaud M. , Aubin H.J. , et al., Acute and long-term effects of cannabis use: a review, Curr Pharm Des 20: (25) ((2014) ), 4112–4118. |




