Efficacy and safety of long-term sirolimus use as part of multidisciplinary care in a pediatric patient with CLOVES syndrome: Case report
Abstract
BACKGROUND:
CLOVES (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, scoliosis/skeletal/spinal) syndrome is a rare and progressive genetic disorder resulting from somatic mosaicism in activating mutations in the phosphatidylinositol-4,5- bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene. PIK3CA is a cell growth master regulator where gain of function mutations give rise to abnormal activation of the PI3K-AKT- mammalian target of rapamycin (mTOR) pathway. Treatment with sirolimus, an mTOR inhibitor, may therefore be of benefit in patients with CLOVES syndrome.
OBJECTIVE:
Here we describe the efficacy and toxicity of sirolimus in a pediatric patient with progressive CLOVES syndrome.
RESULTS:
The child presented with a large and painful abdominal malformation, massive overgrowth of his feet, limb length discrepancy and genu valgum. There was dramatic clinical and radiographic improvement in the size and comfort of his abdominal mass within several months of initiating medical therapy. This, combined with orthopaedic care of his genu valgum, leg length discrepancy, and overgrowth of his feet, has allowed for significant functional gains.
CONCLUSIONS:
Multidisciplinary care is essential for comfort and functional gains in patients with CLOVES syndrome, particularly those with severe symptoms. Close monitoring while on sirolimus medical therapy combined with frequent reassessment of orthopedic needs can dramatically improve patient quality of life and outcomes.
Abbreviations Key
CLOVES | Congenital lipomatous overgrowth, vascular malformations, epidermal nevi, scoliosis/skeletal/spinal |
PIK3 | Phosphoinositide-3 kinase |
mTOR | Mammalian target of rapamycin |
PROS | PIK3CA-related overgrowth spectrum |
MRI | Magnetic resonance imaging |
mTORC1 | MTOR complex 1 |
mTORC2 | MTOR complex 2 |
Treg | T-regulatory |
1Background
Overgrowth syndromes comprise a heterogeneous group of disorders with unique clinical and molecular features. CLOVES syndrome is a rare overgrowth disorder described as a distinct entity in 2007 [1], with molecular identification in 2012 [2]. CLOVES syndrome results from a somatic, gain-of-function mutation in the PIK3CA gene, a cell growth master regulator, and is one of several other PIK3CA-related overgrowth spectrum (PROS) disorders [3, 4]. Although there are reportedly only 200 individuals worldwide living with CLOVES syndrome [5], the reported incidence rate may not reflect the true prevalence given the broad phenotypic spectrum and overlap with closely related conditions.
Clinically, individuals with CLOVES syndrome have asymmetric changes in soft tissue, bone, vascular, and internal organ systems (Table 1) and therefore require multidisciplinary care [6, 7]. The most common feature is a lipomatous mass typically on the trunk with variable amounts of lymphatic and vascular components. Capillary malformations often appear as a port-wine stain; lymphatic malformations can be microcystic or macrocystic with significant disfigurement; and venous malformations are predisposed to venous stasis and localized intravascular and consumptive coagulopathy. Affected individuals can experience chronic pain, recurrent infections, poor quality of life, and organ dysfunction with significant morbidity and mortality. Treatment is largely supportive, focused on various interventional and surgical options for palliation including debulking (with high likelihood of recurrence), sclerotherapy, and embolization.
Table 1
Clinical features of CLOVES syndrome
| Lipomatous mass | Vascular anomalies | Skeletal abnormalities | Skin changes | Other |
| Typically located on the posterolateral back/flank with infiltration into adjacent structures | Slow-flow (capillary, venous, and lymphatic) and high- flow (arteriovenous) malformations. Typically a component of the lipomatous tissue | Scoliosis Extremity length discrepancy. Wide hands and feet Sandal gap Macrodactyly Hemihypertrophy Neural tube defects. Hip dysplasia | Port-wine stain (capillary malformation) or bluish discoloration (venous malformation) Epidermal nevi | Renal anomalies (hypoplasia, agenesis, renal cysts, hydroureteroneph rosis, Wilms tumor) |
Targeted therapy preventing or slowing cellular proliferation has promise in the treatment of vascular anomalies [8, 9]. The mTOR inhibitor, sirolimus, targets the downstream pathway of cellular activation in CLOVES syndrome. mTOR is a serine/threonine kinase regulated by PI3K, acting as a master switch of numerous cellular processes including angiogenesis, lymphangiogenesis, and cell growth [10, 11]. A phase II trial evaluating safety and efficacy of sirolimus in the treatment of complicated vascular anomalies demonstrated a partial response or stable disease in 88% of patients, with limited toxicities noted [8]. Here we report our experience of initiating sirolimus in a child with progressive CLOVES syndrome, and describe an unreported toxicity associated with the use of sirolimus in patients with vascular anomalies.
2Case description
The child was diagnosed with presumed CLOVES syndrome in infancy. Prenatal ultrasounds demonstrated anomalies associated with CLOVES syndrome, noted at birth to have bilateral enlargement and malformation of his feet, a very large lipomatous and lymphatic malformation of the right lateral chest wall and back, as well as a hemangioma of the left lateral chest wall. Genetic testing on DNA isolated from a skin biopsy sample confirmed the diagnosis of CLOVES via the presence of a missense mutation in the PIK3CA gene (c.1624G>A;p.Glu542Lys). His parents and brothers are healthy and there are no other family members with vascular anomalies or similar syndromes.
Early care was provided from a multidisciplinary team including orthopedics, interventional radiology, surgery, urology, and genetics. Initial therapy involved interventional radiology guided sclerotherapy with doxycycline of the right flank mass and other lymphatic cysts at age 4 months and again at 2 years of age. Unfortunately there was not significant change to the size of the right flank lesion, though most of it became microcystic after sclerotherapy. Over time, MRI images showed lymphatic extension intra-abdominally, retroperitoneal, and into the pelvis and bilateral thighs with subsequent bilateral foot enlargement. His shoe size increased approximately a half shoe size every 6 months and required extra, extra wide shoes. With continued growth, specifically of the right flank mass contributing to daily pain, he was referred to hematology at age 3 years for additional multidisciplinary care involving initiation of medical therapy with sirolimus.
Examination at age 3 years 0 months revealed a 26 cm×16 cm right flank mass extending from the costal margin to the sacrum, a 5 cm×2 cm left thigh mass, genu valgum, left hypertrophy of his legs (right > left) with significant asymmetric overgrowth of his feet, and several port-wine stains located on the primary mass (Fig. 1A; images printed after informed consent and permission by the parents).
Fig. 1
Clinical improvement after initiation of sirolimus therapy. A) Initial examination revealed a taught, painful 26 cm×16 cm right flank mass extending from the costal margin to the sacrum and several port-wine stains. B.) There was a noticeable clinical reduction in the flank mass within 2 months of sirolimus initiation.
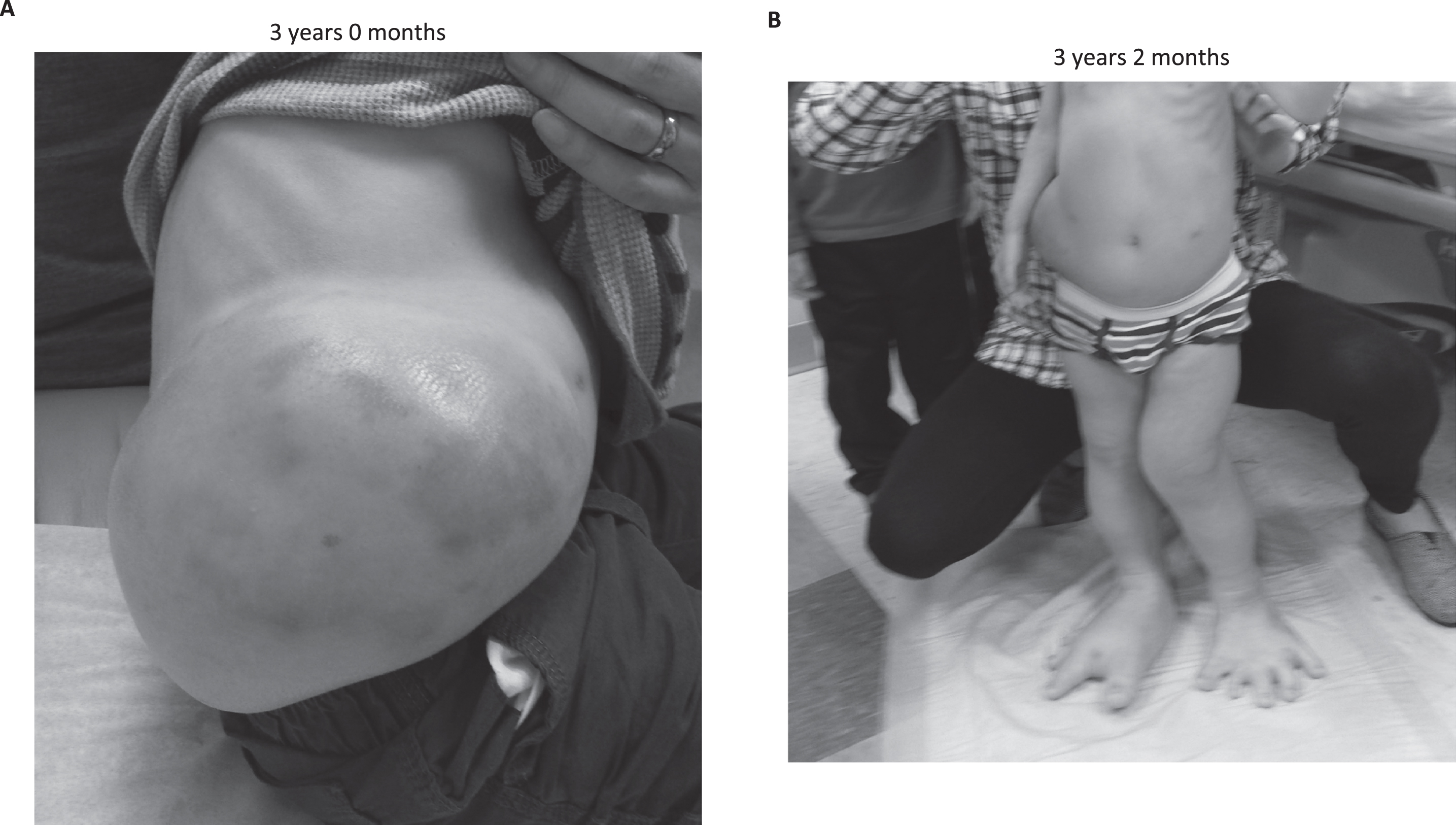
He was initiated on sirolimus (0.8 mg/m2/dose twice daily) shortly after his third birthday, with maximum dose 1.2 mg/m2/dose to maintain a target sirolimus trough of 10– 15 ng/mL (target trough decreased to 7– 10 ng/mL after one year). He was seen monthly for the first 6 months, then every 3 months for clinical evaluation and routine surveillance labs. His parents reported no side effects from sirolimus and a decrease in complaints of pain of his right flank within one month of initiating therapy. They noted obvious reduction in the size of his right flank within 2 months (Fig. 1B).
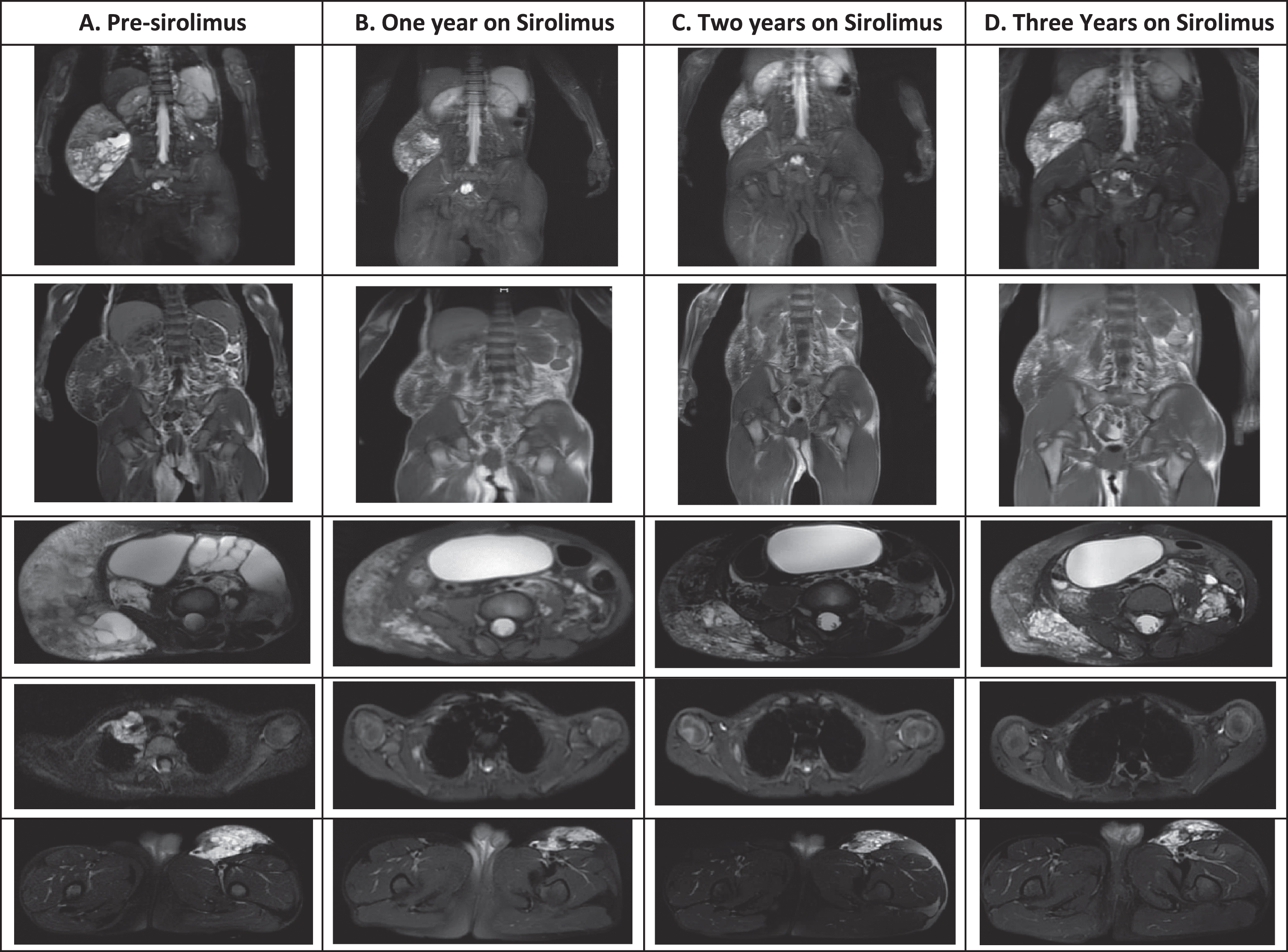
Whole body MRI was done prior to initiation of therapy (Fig. 2A) and yearly thereafter (Figs. 2 B-D). His dose was weaned to once daily (trough goal < 5 ng/mL) at 26 months of therapy when routine screening labs showed Epstein Barr virus (EBV) reactivation with a maximum detectable viral load of 159,414 copies/mL (EBV DNA reportable range: 1,000– 50,000,000 copies/mL). He denied any symptoms related to his EBV reactivation, with viral loads over the last six months on once daily dosing ranging 4,881– 27,000 copies/mL. Measurements of the lymphatic malformations did not worsen despite a lower dose of sirolimus.
Fig. 2
Radiographic changes before and after initiation of sirolimus therapy. Images from top to bottom were done with short TI inversion recovery (STIR), fat spin echo (FSE) T1, and T2 fat saturated imaging for the remaining images. A.) MRI prior to initiation of therapy demonstrated a 3.1×2.6×3.2 cm paratracheal, 5.6×15.4×13.4 cm exophytic right abdominal wall, and 9.4×6.7×3.2 cm anterior left thigh lymphatic malformations. There were multiple extensive and cystic lymphatic malformations throughout the abdomen and pelvis with extension through a defect within the left anterior pelvic wall to the left thigh, bilateral renal pelviectasis, and marked bladder extension and mass effect on the bowel secondary to the large lymphatic malformation. Age 3 years. B). Repeat MRI within one year on sirolimus therapy was no longer able to visualize the paratracheal mass, the exophytic mass decreased to 5×10.4×12.8 cm, the left thigh mass decreased to 5.4×2.1×2.4 cm, there was marked overall decrease in the cystic components, and the kidneys were normal in size and contour. Age 4 years. C-D. MRI two and three years on sirolimus therapy was stable. Age 5-6 years.
Orthopaedically, the child has required multiple surgeries to correct his deformities. At age 3 years 10 months he underwent left distal femur guided growth (hemiepiphyseostasis) to address his genu valgum, epiphyseodesis of the proximal and distal phalanges of the left III, IV,V toes and right I,II,III toes to address marked overgrowth, and hemiepiphyseodesis of the II toe middle phalanx to address angulation of the toe (Fig. 3). All toe surgeries were repeated at age 4years 8 months due to resistance of the toe physes to closure (Fig. 4). At age 6 years 1 month, the hardware was removed from the left femur and epiphyseostasis was performed of the right proximal tibia to address limb length discrepancy. As the right II toe was now of adult length, epiphyseodesis of the II toe phalanx was also performed. With these interventions, he has “grown into” his feet which have become far less disproportionate and stiff. His legs are nicely straight, and his leg length discrepancy has nearly corrected (Fig. 4A). He now has a normal gait (and run), is able to wear large but “off the shelf” shoes, and is able to keep up with his classmates.
Fig. 3
Initial clinical and radiographic orthopaedic evaluation. A-B). He presented with severe mosaic overgrowth of both feet, left genu valgum, and a mild leg length discrepancy.
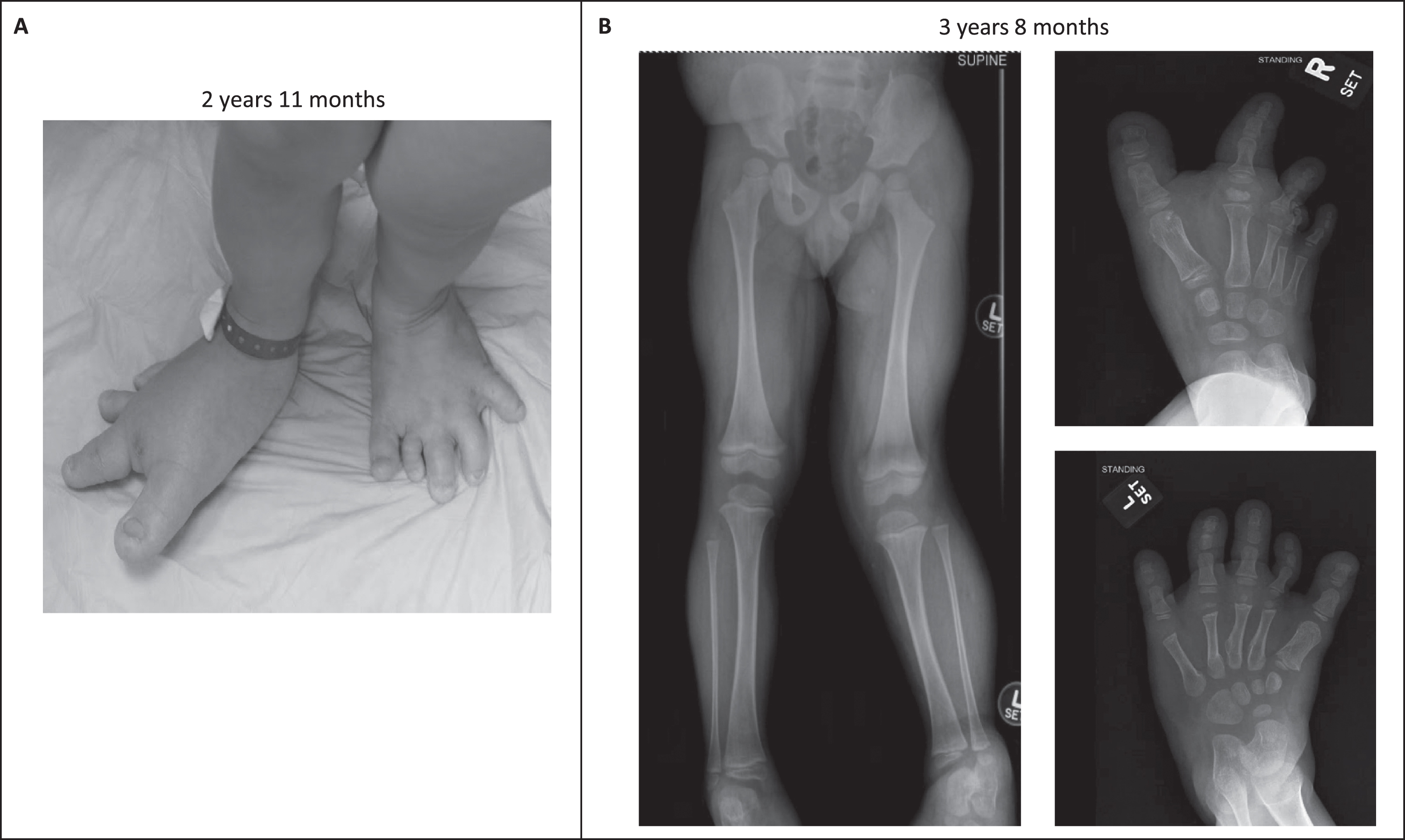
Fig. 4
Successful orthopedic intervention. A-B.) Epiphysiodesis of the proximal and distal phalanges of the right I-III and left III-V proximal phalanges, repeated once, as well as hemiepiphysiodesis medially of the right 2 toe middle phalanx, in conjunction with his sirolimus therapy has allowed him to “grow into” his feet. Hemiepiphyseostasis of the left distal medial femur has corrected his genu valgum. He now can wear regular shoes with a small lift on the left side.
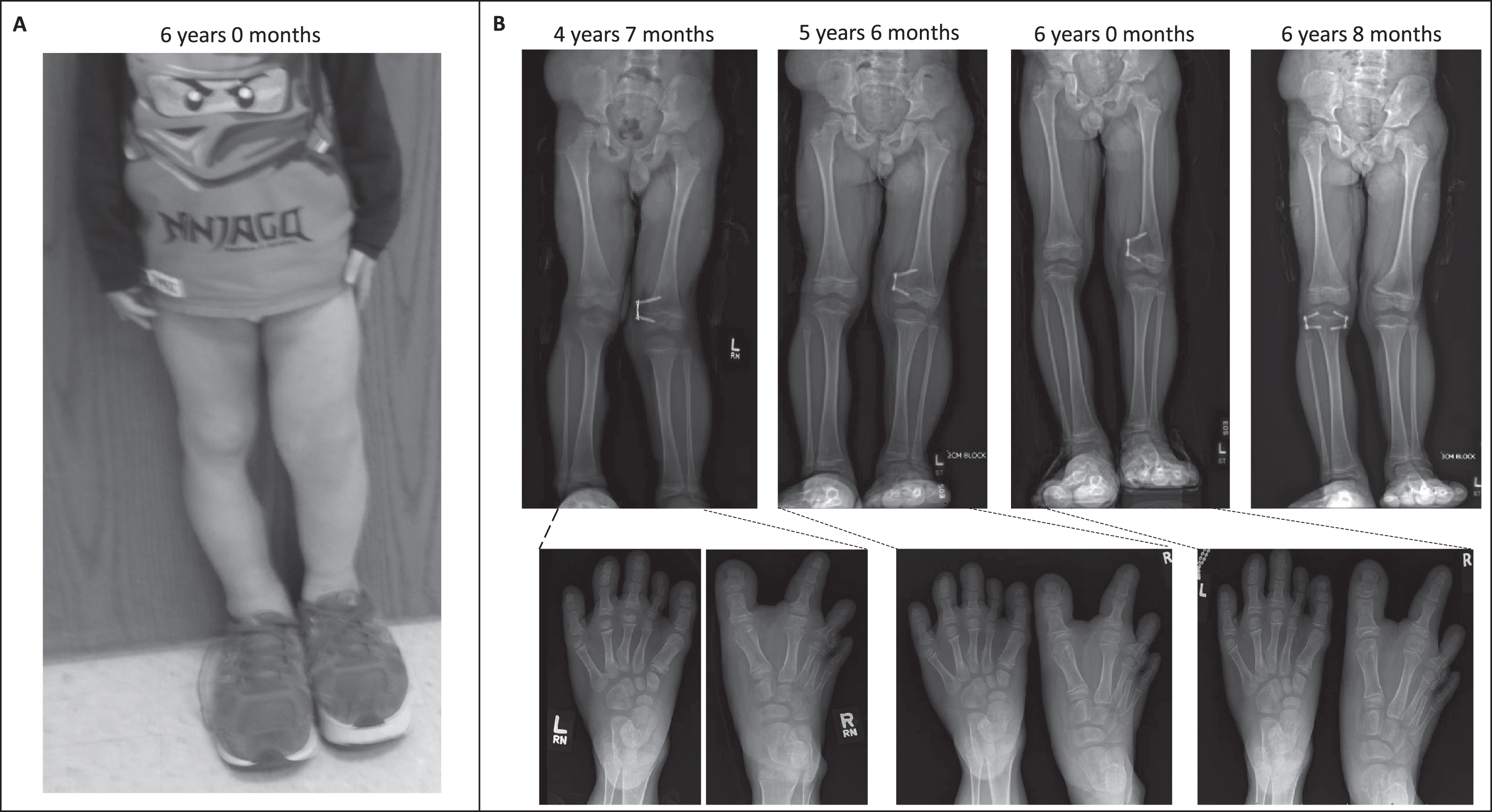
Future orthopaedic intervention will include hardware removal from the right tibia at approximately age 8 years along with possible repeat epiphyseodesis of the right II toe phalanx. Epiphyseodesis of the right I toe phalanx will be performed when that toe starts to approach the length of his father’s right II toe. He will require careful monitoring until skeletal maturity to ensure that correction of his alignment is maintained.
3Discussion
CLOVES syndrome is a genetic disorder resulting from somatic, mosaic gain-of-function mutations of the PIK3CA gene resulting in tissue overgrowth and asymmetric changes in soft tissue, bone, vascular, and internal organ systems. There are limited therapeutic options to reduce morbidity and mortality thus targeted therapy is needed. A multidisciplinary approach that involves medical therapy with sirolimus is a potentially successful additive therapy that supports standard orthopedic interventions. In this case report, the addition of sirolimus enabled marked reduction in the size of his abdominal mass and other lymphatic malformations, as well as creating less bulbous and more supple feet that were amenable to orthopedic intervention.
Inappropriate activation of the PI3K/AKT/mTOR pathway has been documented in solid tumors and overgrowth syndromes with a wide range of phenotypic variability [12]. Therefore, mTOR inhibition with sirolimus represents a targeted therapeutic strategy in individuals with PROS. Sirolimus demonstrated favorable efficacy/safety profile in children with complicated vascular malformations [8, 9]. More, recently, administration of low dose sirolimus for 26 weeks in 39 individuals with PROS was associated with a significant reduction in overgrowth. However, there was a higher rate of toxicity in this study compared to the study by Adams, et al. [13]. Our subject demonstrated rapid clinical improvement within several months of starting therapy, was stable over several years even at reduced therapeutic targeting, but demonstrated an unwanted side effect of EBV reactivation while on an off-label drug currently FDA approved for immunosuppression.
Stimuli that induce EBV reactivation are less well understood than primary EBV infection. Immunocompetent hosts control the expansion of EBV infected cells through anti-viral T cell immunity, however viral-specific T cells are inadvertently inhibited while on immunosuppressive drugs. While experimental studies suggest that sirolimus can inhibit growth of human EBV-transformed B lymphocytes [14, 15] and exert anti-tumor efficacy via anti- angiogenic properties [16], its use is potentially deleterious to EBV control due to induction of T- regulatory (Treg) cells. Inhibition of mTOR in the PI3K/AKT/mTOR pathway confers FOxP3 expression in T cells [17], a marker of specialized CD4+ Tregs that inhibit effector CD4+ and CD8+ T cells, including those controlling EBV expansion, and could be an explanation for the rise of EBV DNA viral load in our subject.
Though he has had dramatic improvement while on sirolimus, there is concern for potentially uncontrolled EBV reactivation at any time, therefore other therapies are needed. A continued multidisciplinary approach will be needed to manage his orthopaedic deformities.
Though clinical efficacy has been demonstrated, sirolimus has incomplete suppressive activity given only partial inhibition of mTOR complex 2 which provides feedback activation to AKT [18– 20]. Targeted therapy with direct PI3K or AKT inhibitors, or development of dual mTORC1 and 2 inhibitors, may be of better therapeutic promise. Recent data suggests that direct PIK3CA inhibitors are safe, and show substantial clinical improvement in patients with PROS [21]. Our patient has agreed to trial Alpelisib, a direct PIK3CA inhibitor, in place of sirolimus when approved by our institutional IRB. Until available, providers prescribing long-term sirolimus for the treatment of PROS should consider monitoring EBV viral loads to ensure risk of therapy does not outweigh the benefit.
Significant functional gains can be made in those with severe CLOVES syndrome when multidisciplinary care is provided with close monitoring and frequent reassessment. Such care and attention is essential to improve outcomes and quality of life in individuals with CLOVES syndrome.
Subject perspective
Given his young age, this individual has little memory of his painful abdominal mass, his inability to wear anything other than large sandals, or his difficulty walking. Taking his sirolimus is part of his daily routine and his parents are pleased with his improved comfort and mobility.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Images printed after informed consent and permission given in writing by the parents.
Availability of data and materials: Not applicable.
Competing interests
The authors have no competing interests to disclose.
Funding
None.
Authors’ contributions
AL, LT, and YD contributed to the care of this patient. AL wrote the manuscript, with editing from LT and YD. All authors have read and approved the manuscript.
Acknowledgments
The authors would like to thank Leslie Biesecker, Head of Clinical Genomics, Medical Genomics and Metabolic Genetics Branch/National Human Genome.
Research Institute, National Institutes of Health for support and confirmatory genetic testing in our patient.
References
[1] | Sapp J.C. , Turner J.T. , van de Kamp J.M. , van Dijk F.S. , Lowry R.B. and Biesecker L.G. , Newly delineated syndrome of congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE syndrome) in seven patients, Am J Med Genet A ((2007) ) 143A: (24), 2944–2958. |
[2] | Kurek K.C. , Luks V.L. , et al., Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome, Am J Hum Genet ((2012) ) 90: (6), 1108–1115. |
[3] | Kang H.C. , Baek S.T. , Song S. and Gleeson J.G. , Clinical and Genetic Aspects of the Segmental Overgrowth Spectrum Due to Somatic Mutations in PIK3CA, J Pediatr ((2015) ) 167: (5), 957–962. |
[4] | Martinez-Lopez A. , Blasco-Morente G. , et al., CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS), Clin Genet ((2017) ) 91: (1), 14–21. |
[5] | Vascular Anomalies Center B.C.s.H. CLOVES Syndrome [Available from: http://www.childrenshospital.org/conditions-and-treatments/conditions/c/cloves-syndrome. |
[6] | Alomari A.I. , Characterization of a distinct syndrome that associates complex truncal overgrowth, vascular, and acral anomalies: a descriptive study of 18 cases of CLOVES syndrome, Clin Dysmorphol ((2009) ) 18: (1), 1–7. |
[7] | Anderson S. and Brooks S.S. , An Extremely Rare Disorder of Somatic Mosaicism: CLOVES Syndrome, Adv Neonatal Care ((2016) ) 16: (5), 347–359. |
[8] | Adams D.M. , Trenor C.C. 3rd , et al., Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies,e, Pediatrics ((2016) ) 137: (2), 20153257–. |
[9] | Hammill A.M. , Wentzel M. , et al., Sirolimus for the treatment of complicated vascular anomalies in children, Pediatr Blood Cancer ((2011) ) 57: (6), 1018–1024. |
[10] | Huber S. , Bruns C.J. , et al., Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis, Kidney Int ((2007) ) 71: (8), 771–777. |
[11] | Salameh A. , Galvagni F. , Bardelli M. , Bussolino F. and Oliviero S. , Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways, Blood ((2005) ) 106: (10), 3423–3431. |
[12] | Akgumus G. , Chang F. and Li M.M. , Overgrowth Syndromes Caused by Somatic Variants in the Phosphatidylinositol 3-Kinase/AKT/Mammalian Target of Rapamycin Pathway, J Mol Diagn ((2017) ) 19: (4), 487–497. |
[13] | Parker V.E.R. , Keppler-Noreuil K.M. , et al., Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum, Genet Med ((2019) ) 21: (5), 1189–1198. |
[14] | Krams S.M. and Martinez O.M. , Epstein-Barr virus, rapamycin, and host immune responses, Curr Opin Organ Transplant ((2008) ) 13: (6), 563–568. |
[15] | Majewski M. , Korecka M. , et al., The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: A potential approach to prevention and treatment of posttransplant lymphoproliferative disorders, Proc Natl Acad Sci U S A ((2000) ) 97: (8), 4285–4290. |
[16] | Guba M. , von Breitenbuch P. , et al., Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor, Nat Med ((2002) ) 8: (2), 128–135. |
[17] | Haxhinasto S. , Mathis D. and Benoist C. , The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+cells, J Exp Med ((2008) ) 205: (3), 565–674. |
[18] | Neuhaus P. , Klupp J. and Langrehr J.M. , mTOR inhibitors: an overview, Liver Transpl ((2001) ) 7: (6), 473–484. |
[19] | Paplomata E. and O’Regan R. , The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers, Ther Adv Med Oncol ((2014) ) 6: (4), 154–166. |
[20] | Wiederrecht G.J. , Sabers C.J. , Brunn G.J. , Martin M.M. , Dumont F.J. and Abraham R.T. , Mechanism of action of rapamycin: new insights into the regulation of G1-phase progression in eukaryotic cells, Prog Cell Cycle Res 1: ((1995) ), 53–71. |
[21] | Venot Q. , Blanc T. , et al., Targeted therapy in patients with PIK3CA-related overgrowth syndrome, Nature ((2018) ) 558: (7711), 540–546. |




