Creating a multi-center rare disease consortium – the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR)
Abstract
Eosinophilic gastrointestinal disorders (EGIDs) affect various segments of the gastrointestinal tract. Since these disorders are rare, collaboration is essential to enroll subjects in clinical studies and study the broader population. The Rare Diseases Clinical Research Network (RDCRN), a program of the National Center for Advancing Translational Sciences (NCATS), funded the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) in 2014 to advance the field of EGIDs. CEGIR facilitates collaboration among various centers, subspecialties, patients, professional organizations and patient-advocacy groups and includes 14 clinical sites. It has successfully initiated two large multi-center clinical studies looking to refine EGID diagnoses and management. Several pilot studies are underway that focus on various aspects of EGIDs including novel therapeutic interventions, diagnostic and monitoring methods, and the role of the microbiome in pathogenesis. CEGIR currently nurtures five physician-scholars through a career training development program and has published more than 40 manuscripts since its inception. This review focuses on CEGIR’s operating model and progress and how it facilitates a framework for exchange of ideas and stimulates research and innovation. This consortium provides a model for progress on other potential clinical areas.
Eosinophilic gastrointestinal disorders (EGIDs) are a heterogeneous group of inflammatory conditions affecting various segments of the gastrointestinal tract, such as the esophagus, stomach, small intestine and colon, referred to as eosinophilic esophagitis (EoE), eosinophilic gastritis (EG), eosinophilic enteritis (EE), and eosinophilic colitis (EC), respectively. The term eosinophilic gastroenteritis (EGE) is generally reserved for EGIDs that affect the stomach and intestine and/or esophagus. The unifying feature of this group of conditions, as the name implies, is eosinophil-predominant inflammation. Under healthy conditions, eosinophils account for a small fraction of peripheral blood leukocytes and resident tissue cells. By contrast, eosinophils accumulate in large numbers in the tissues in patients with EGIDs [37, 38, 44].
Diagnosing EGIDs can be a challenge for three primary reasons. First, the pathology of EGIDs most likely extends beyond the gastrointestinal epithelium, but the depth of involvement is often difficult to ascertain due to the limited size and depth of endoscopically obtained biopsies. Second, histologic assessment of EGE and EC may be confounded by the presence of resident eosinophils in non-diseased gastrointestinal mucosa. Consensus has not been achieved on optimal cutoff values to confidently establish normal variations in eosinophil density [20, 40]. The esophagus is an exception since eosinophils are absent in the healthy esophagus, making histologic assessment of EoE less challenging [40]. Finally, increased numbers of mucosal/epithelial eosinophils are not pathognomonic for EoE, EGE, or EC, as mucosal eosinophilia can be seen in other diseases including reflux esophagitis, parasitic infestations, rheumatologic conditions, and inflammatory bowel disease [25, 45]. The current proposed diagnostic criteria used by CEGIR for EG, EGE, and EC entails histologic findings of at least twice the upper limit of normal for eosinophils per high-power field (HPF) reported in affected segments of the gastrointestinal tract [6, 26, 27].
Patient advocacy groups (PAGs) are also increasing national attention for these disorders with calls for National Institutes of Health (NIH) research funding and government support (e.g. National Eosinophil Awareness Week unanimously passed by the USA House of Representatives in 2007). A needs assessment survey conducted by CEGIR and its associated PAGs found that a significant majority of patients felt that there was not enough awareness regarding EGIDs in school and workplace (78% and 67% , respectively) [16].
Because EGIDs are rare, collaboration is essential not only to increase the number of subjects participating in clinical studies, but also to clarify whether findings from individual sites are valid in a broader population. Over the years, though the literature has slowly blossomed, investigators have mostly collaborated on a regional basis with limited intra- and inter-center communications. CEGIR, under the auspices of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Sciences (NCATS), NIH, was established in 2014 to overcome these handicaps and facilitate research and training on EGIDs. This report highlights the work of CEGIR and describes the framework needed to establish a multi-center consortium required to study the social, financial, and medical burden; diagnostic criteria; and the best treatment regimens that rare diseases such as EGIDs have.
1Structure
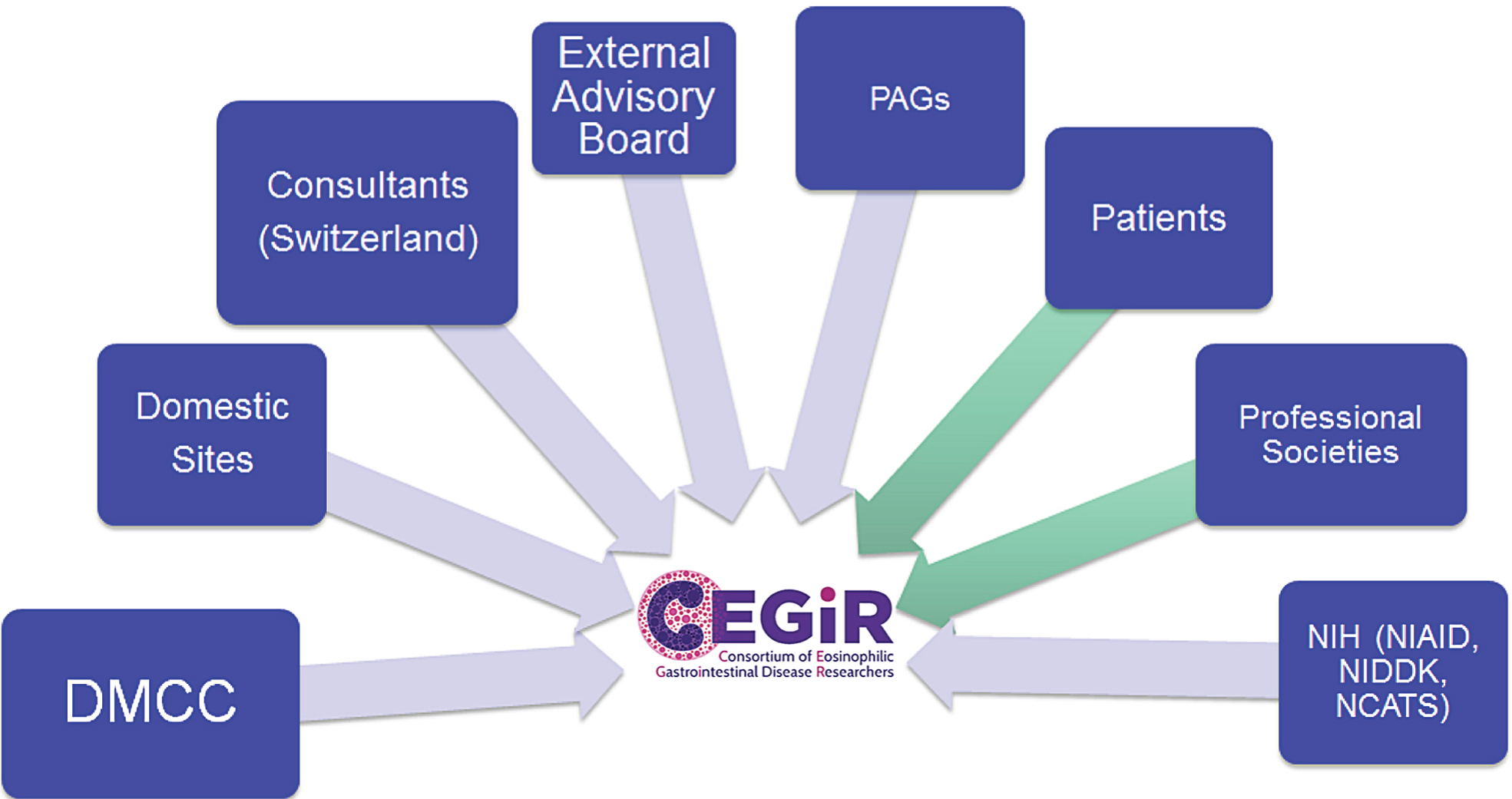
CEGIR is one of 21 multi-site consortia of RDCRN. RDCRN is an initiative established in 2003 and funded by ORDR, NCATS, and collaborating institute centers. Its main focus is to promote multi-site clinical research, foster collaborations, and generate publications to both advance science and raise awareness on rare diseases. The RDCRN has studied more than 200 rare diseases since its inception [2, 23, 30]. CEGIR comprises nine primary academic centers in California, Colorado, Illinois, Massachusetts, North Carolina, Ohio, and Pennsylvania, as well as secondary centers in Arkansas, Minnesota, New York, Tennessee, and Utah and an advisory site in Switzerland (Fig. 1). These centers were chosen based on previous collaboration, ability to integrate pediatric and adult patients into a common consortium, and access to patients with these rare diseases. Each of the primary sites has a CEGIR site-investigator. These physicians provide diverse expertise in relevant clinical specialties including gastroenterology, allergy, immunology, and pathology in both adult medicine and pediatrics. In addition to these individuals, CEGIR also includes a project scientist from NIH institute centers, the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and NCATS, as a collaborator. The mission of CEGIR is to improve the lives of individuals with EGIDs through innovative research, clinical expertise, and education via collaborations between scientists, physicians, patients, and professional organizations. Figure 2 summarizes the interacting components of CEGIR.
Fig.1
CEGIR Centers.
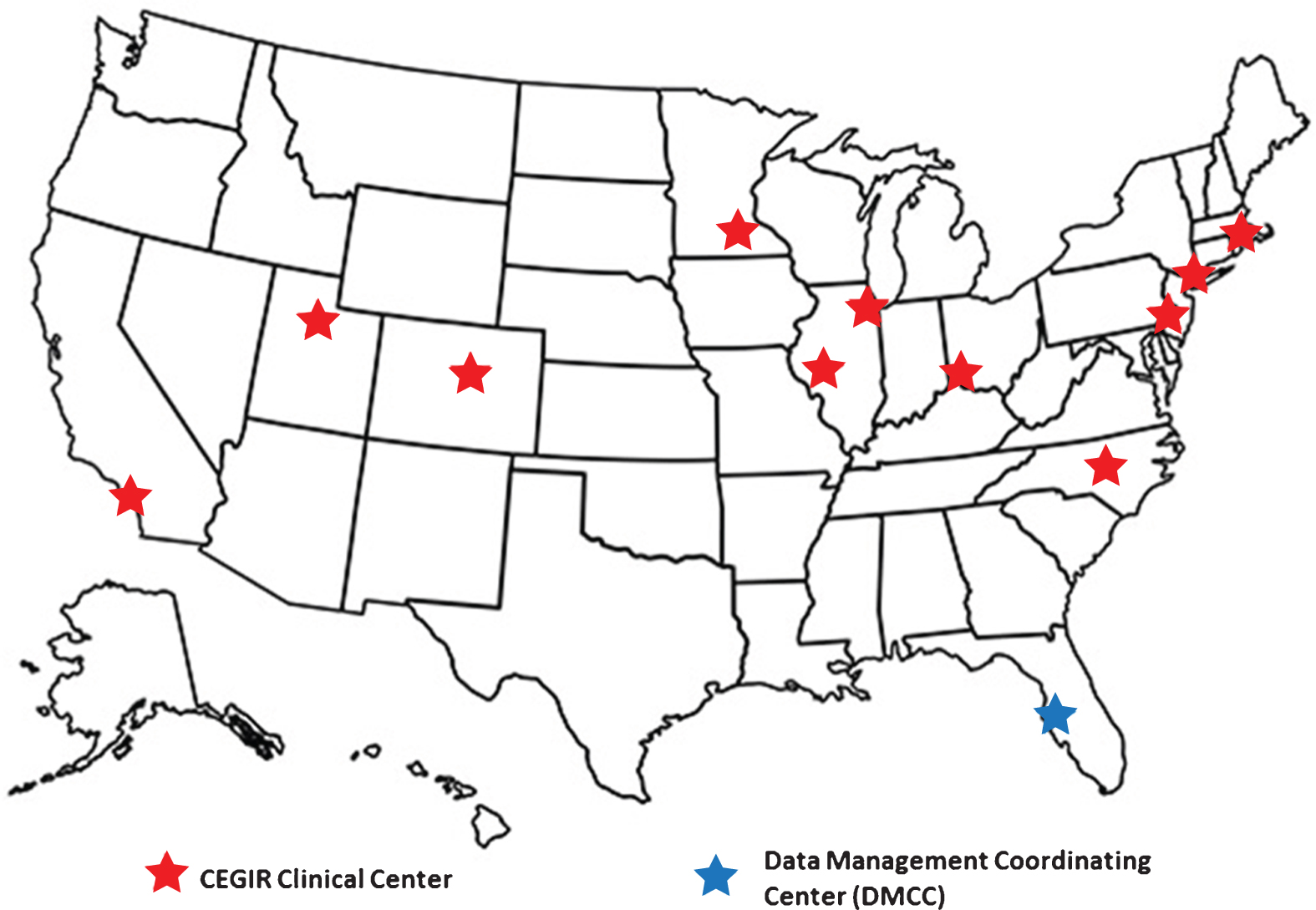
Fig.2
CEGIR Emblem: The C is in the shape of a nucleus of an eosinophil, which defines this group of disorders.
Prior to the formation of CEGIR, many of these researchers worked collaboratively as founders of The International Gastrointestinal Eosinophil Researchers (TIGERs) and co-authored numerous publications. Most notably, these investigators were involved in forming the first consensus recommendations for diagnosis and treatment of EoE in 2007 (14) and its subsequent update in 2011 (6). The RDCRN program funding and designation provided a means for collaborating investigators to increase their interaction, enroll patients into registries, test hypotheses that have been developing over the past decade, and leverage NIH networks and expertise to advance the understanding and treatment of EGIDs, ultimately improving the lives of patients with EGIDs. CEGIR is co-funded by NCATS, NIDDK, and NIAID through a cooperative agreement award.
Collaborations with PAGs as research partners is a requirement and a unique feature of the RDCRN program. CEGIR includes many PAGs as research partners, including the American Partnership for Eosinophilic Diseases (APFED, www.apfed.org), Campaign Urging Research for Eosinophilic Disease (CURED, www.cured.org), and the Eosinophilic Family Coalition (EFC, www.eoscoalition.org). These PAGs work to educate patients and support research aimed at improving treatment and cures for eosinophil-associated diseases and their complications. They have a crucial role within CEGIR in identifying and recruiting patients for clinical research. Notably, APFED and CURED have extensive patient databases through which they can regularly communicate with and identify patients who might be eligible for enrollment in CEGIR-sanctioned research initiatives. APFED and CURED also contribute financially to CEGIR.
The CEGIR Administrative Unit (Fig. 3) is spearheaded by a Principal Investigator, ProgramDirector, and the Internal CEGIR Steering Committee, which has representation from CEGIR site-investigators, PAGs, and professional medical research groups. The CEGIR Administrative Unit generates and administers policies, procedures, and communications and distributes the funds associated with CEGIR. In addition, the CEGIR External Advisory Board (EAB) oversees the overall functioning of the consortium and is composed of individuals with recognized expertise in the medical community. The CEGIR EAB assists by providing an external and objective performance review of the CEGIR initiatives in the realms of research, training, administration, individual site performance, and expansion to other medical research centers.
Fig.3
CEGIR Administrative Unit.
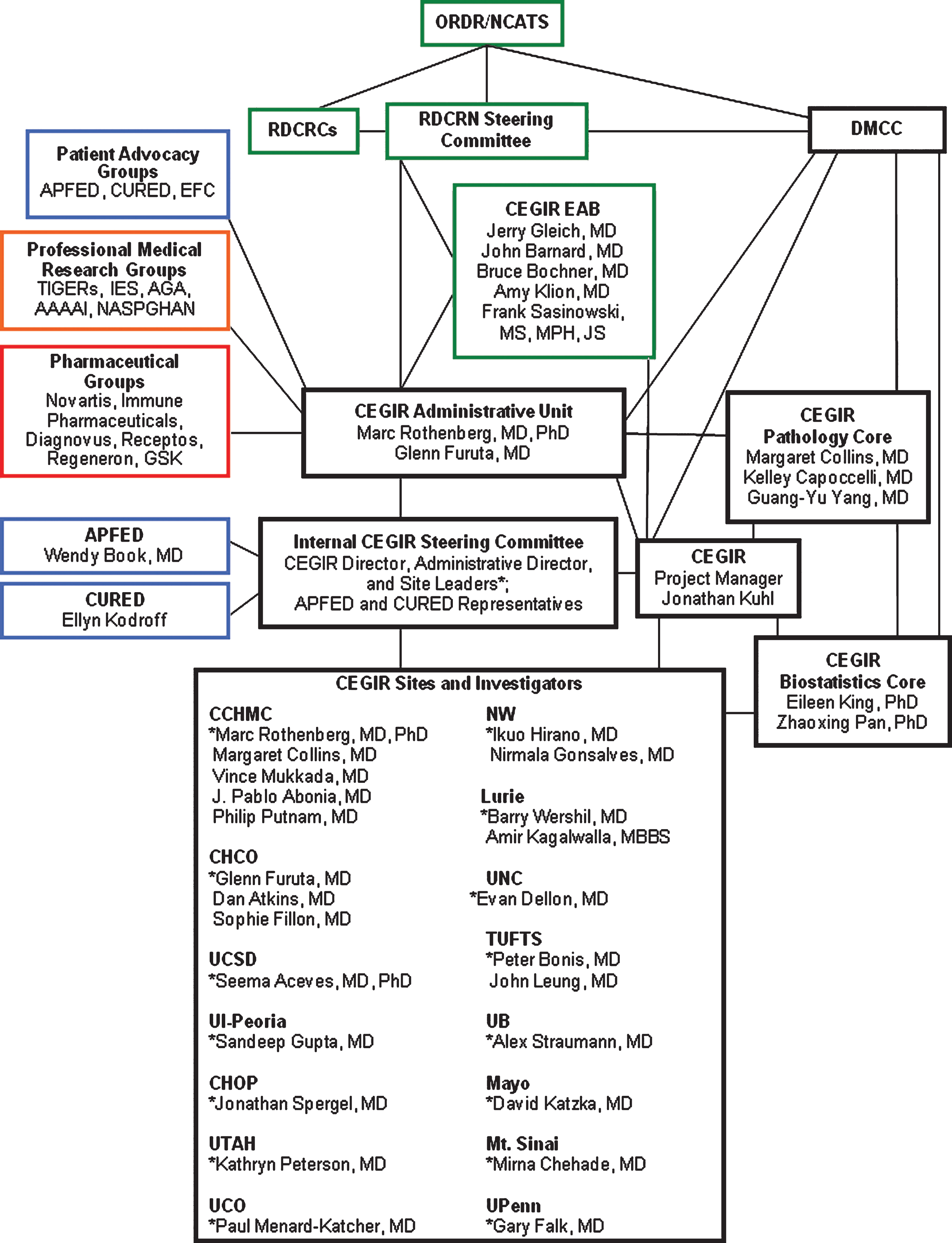
There are three main components to CEGIR: Clinical Research Projects for Observational/Longitudinal Studies (further divided into Project 1 and Project 2), Pilot/Demonstration Clinical Research Program, and Training (Career Development) Program. In addition, there is a Contact Registry (Table 1). Each of these cores has a project lead and associate project lead who both conduct studies in conjunction with the Internal CEGIR Steering Committee. They also report to the CEGIR Administrative Unit and CEGIR EAB, which then report to the RDCRN Steering Committee.
Table 1
Current projects in CEGIR
| Core | Project title | Disease | Primary Investigators | Primary Site |
| Clinical Trial | A Prospective, Multicenter Study to Compare and Validate Endoscopic, Histologic, Molecular, and Patient-Reported Outcomes in Pediatric and Adult Patients with EoE, EG and EC (OMEGA) | EoE, EG, EC | Drs. Glenn Furuta and Seema Aceves | Children’s Hospital Colorado and University of California,San Diego |
| Six versus One Food EoE Elimination Diet followed by Swallowed Glucocorticoid Trial (SOFEED) | EoE | Drs. Marc Rothenberg, Ikuo Hirano and Jonathan Spergel | Cincinnati Children’s Hospital | |
| Pilot | A preliminary open-label trial of losartan potassium in participants with EoE with or without a connective tissue disorder | EoE | Drs. Marc Rothenberg and Pablo Abonia | Cincinnati Children’s Hospital |
| Role of Microbiome in EoE, EG and EC | EoE, EG, EC | Dr. Sophie Fillon | Children’s Hospital Colorado | |
| Use of unsedated transnasal esophagoscopy to monitor dietary management of EoE | EoE | Dr. Joel Friedlander | Children’s Hospital Colorado | |
| Prospective trial of elemental diet in adults with EG | EG | Dr. Nirmala Gonsalves | Northwestern University | |
| Contact Registry | Contact Registry | Dr. Pablo Abonia | ||
| Training | Demographics, clinical characteristics, and pathology of EG and EC in a multi-site cohort | Dr. Robert Pesek | Arkansas Children’s | |
| Validation of online cohort of patients with EGIDs enrolled in CEGIR Contact Registry | Dr. Girish Hiremath | Arkansas Children’s |
Regular communications between the CEGIR Administrative Unit and other CEGIR investigators occurs on a biweekly basis, on different days and times so as to maximize participation. The CEGIR Administrative Unit also communicates with the CEGIR EAB via semiannual conference calls and by an annual written report. In addition to these regular communications, CEGIR holds an annual meeting, where all investigators, clinical research coordinators, NIH Institute Center Officer and Project Scientist collaborators, PAGs, and other stakeholders involved in CEGIR are invited to attend. The first three annual meetings have been held in conjunction with professional society meetings and have involved a growing number of broad participants, numbering over 70 individuals in the third meeting. Figure 4 shows the convened group for the most recent meeting.
Fig.4
CEGIR members at recent meeting in Atlanta on March 2017.

CEGIR complies with all applicable research ethical standards and has a central Institutional Review Board (cIRB) that can review and monitor its various projects. The formation of a cIRB allows multi-center studies to proceed more efficiently. Instead of each institution going through its own IRB reviews, this process streamlines the research administrative process through previously developed inter-institutional agreements that comply with all local IRBs. The cIRB is located at the Cincinnati Children’s Hospital Medical Center (CCHMC).
CEGIR investigators closely collaborate with the RDCRN Data Monitoring Coordinating Center (DMCC) for a variety of activities including the development of protocol electronic case report forms (CRFs). The DMCC provides monitoring instruments (e.g. compliance reports) enabling CEGIR to monitor the collection of data by protocol and ensure quality data are being obtained. Data collected through CEGIR is maintained at the DMCC, which serves as the primary data-housing center of CEGIR. There is regular communication between CEGIR and members of the DMCC. The DMCC is particularly helpful in developing templates for protocols and works closely with the medical officer (from NIAID) of CEGIR and the investigators to ensure data compliance and generate datareports.
2Clinical Research Projects for Observational/Longitudinal Studies
This component has two projects that were established to define diagnostic and clinical features of these disorders, validate clinical outcome metrics, identify biomarkers and optimize therapy.
2.1Clinical Research Project 1: Outcome Measures for Eosinophilic Gastrointestinal Diseases Across Ages (OMEGA)
This prospective, multi-center study aims to determine the best tools for diagnosing and monitoring disease characteristics. It seeks to compare and validate endoscopic, histologic, molecular, and patient-reported outcomes in both pediatric and adult patients with EoE, EG, and EC at various points of clinical care with longitudinal follow-up and biospecimen acquisition (Fig. 5).
Fig.5
Diverse biospecimens being collected and studied as part of CEGIR.
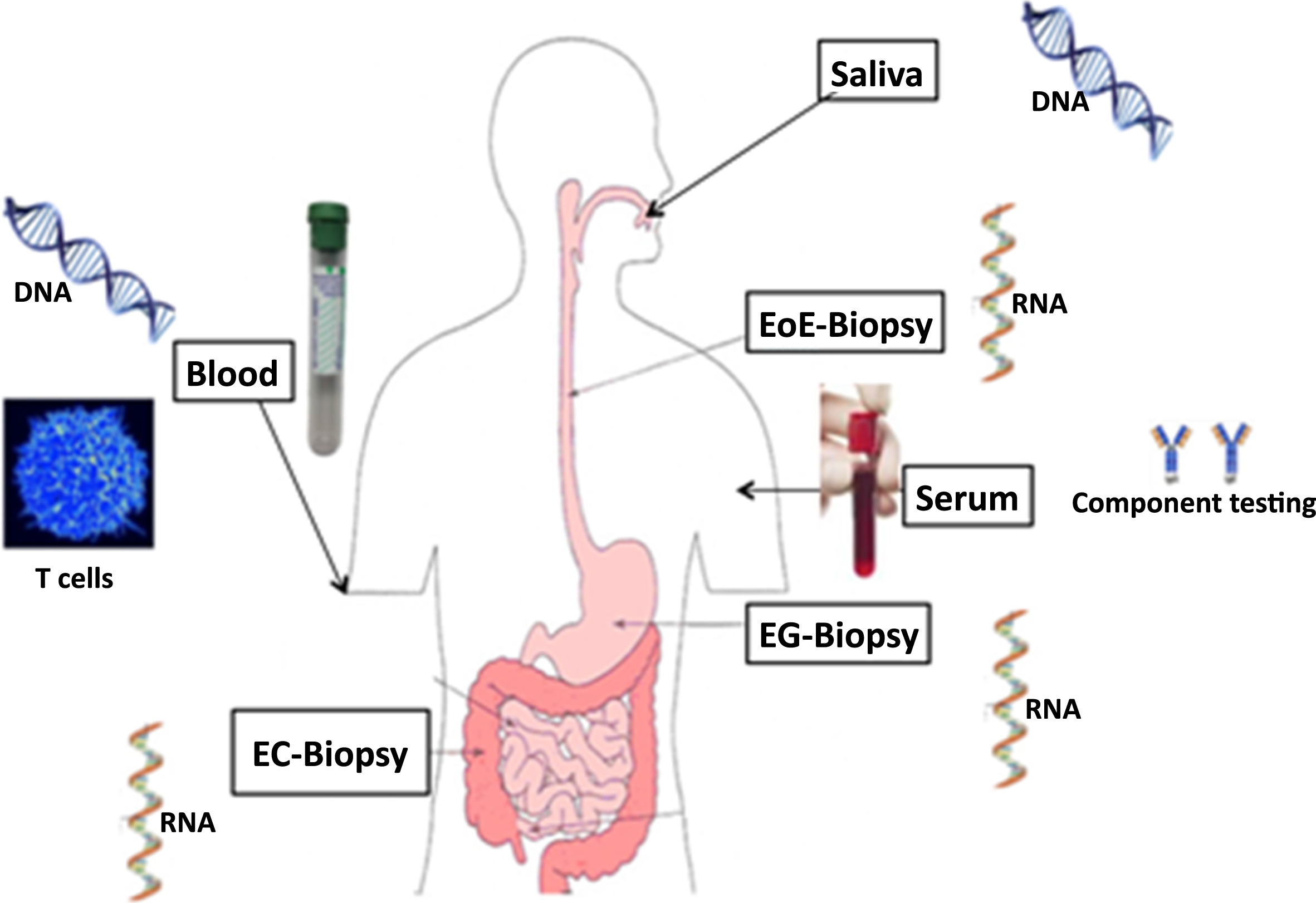
At present, tissue eosinophil counts are considered the reference standard for diagnosing and monitoring EGIDs, yet there is no consensus regarding the correlation between this parameter and clinical symptoms [15]. CEGIR investigators and others have developed some clinical outcome metrics (COM), which are instruments to objectively measure patient-reported outcomes, quality of life, and endoscopic findings [8, 28, 41]. This study hypothesizes that peak mucosal eosinophil levels correlate with COM findings. In addition, the study also tests the hypothesis that inflamed tissues of EGIDs express unique messenger RNA transcript profiles and that elucidating these molecular profiles will both advance the understanding of these enigmatic disease and provide molecular criteria for disease diagnosis and monitoring. With the large database of biopsy specimens collected through this program, researchers can seek to identify gene expression profiles in the tissues of EoE, EG, and EC to differentiate these disorders from one another and from control subject biopsies. The results of such comprehensive databases are generating clinical outcome metrics for EoE, EG, and EC and defining basic clinical, laboratory, and pathologic features of EG and EC.
At this time, more than 700 patients have been enrolled in the study throughout the USA. The study also aims to procure endoscopic biopsies at the time of enrollment and to review the first diagnostic endoscopy. Clinic visits, endoscopies, and other interventions done as part of standard of care for monitoring are captured prospectively together, with patient/family/provider-completed questionnaires to assess patient-reported outcomes such as symptoms. This project aims to provide more information on monitoring disease severity by correlating COM with clinical criteria. A biorepository composed of blood and tissue samples, including sera, plasma, DNA, and tissue RNA, is being collected; the biorepository currently contains over 4000 samples.
2.2Clinical Research Project 2: Six Food vs One Food Eosinophilic Esophagitis Elimination Diet (SOFEED)
This is a prospective, randomized trial to evaluate the effects of a diet with fewer restrictions than currently recommended for EoE. It also seeks to determine the response to swallowed steroid therapy in patients who experience diet failure. Answering this question was deemed important by the PAGs and was thus prioritized as an initial major study of CEGIR.
Dietary antigen exposure is an important driving force for the pathogenesis of EoE as this disorder is often reversible following appropriate elimination of triggering food groups [21]. Both skin test-directed and six-food elimination diet (6FED) approaches have been shown to be ∼70% effective [19, 42]. The 6FED includes removing milk, egg, wheat, soy, nuts (peanuts/tree nuts), and seafood (fish/shellfish). This study compares 1FED (avoidance of milk) versus 6FED (avoidance of all 6 aforementioned foods) in adult patients and is currently in the recruitment phase, with 75 of 126 patients enrolled. Patients are randomly assigned to one of the two diets at each participating CEGIR site. There is standardization across the various sites by providing consensus documents and links to instructional videos on diet information. Dieticians across the centers communicated and together developed these standardized documents and educational materials for patients. Patients whose disorder is in histologic remission (<15 eosinophils/HPF) at 6 weeks are deemed to have completed the study. Those whose disorder remains inflamed (≥15 eosinophils/HPF) at that time continue with the study; those who were initially in the 1FED group are reassigned to the 6FED diet, and those already on the 6FED group start swallowed glucocorticoid therapy in a standardized dose and are monitored for another 6 weeks. All patients undergo endoscopy with biopsies at the end of the study to look for endoscopic and histologic outcomes. Endpoints in this study include the percentage of patients whose disorder evidences histologic remission, distribution of patients within a histology-based scoring system (including remodeling markers), and changes in COM.
3Pilot/Demonstration Clinical Research Program
The Pilot/Demonstration Clinical Research Program supports new ideas by sponsoring pilot projects. The pilot research projects address novel areas of research pertaining to EGIDs. The projects may be collaborative among investigators at one or more CEGIR sites or with investigators outside of the CEGIR network.
The selection process aims to optimize CEGIR involvement and collaboration to enable the pursuit of potentially novel hypotheses to study these rare disorders. The Internal CEGIR Steering Committee meets every 6 months to review applications and monitor the progress of current projects. Proposals received are ranked according to specific criteria based on scientific merit, likelihood of success, ability to develop synergy with other investigators or ongoing projects in CEGIR, use of existing clinical and biological materials, novel approaches, development of biomarkers, and potential for partnership with commercial entities such as pharmaceutical companies and PAGs. These criteria concur with the goals of the NIH Taskforce on Research Needs of Eosinophil-Associated Diseases (TREAD) [10] and those identified through discussions with the Food and Drug Administration Meetings, [39] both of which detail a number of unmet needs for EGIDs. The project leaders and the Internal CEGIR Steering Committee, with the help of other CEGIR investigators, a representative of the International Eosinophil Society, and PAG representatives, meet to make the final selection(s).
Since 2014, four pilot projects have been chosen and are in various stages of development (Table 1). These projects were selected from 13 submissions and thus represent a competitive reviewprocess.
One of the pilot projects is the creation and analysis of a centralized microbiome database for EoE, EG, and EC based on tissue biopsies as well as stool samples. This unique project aims to use CEGIR’s network to collect more specimens to better understand the microbiome of patients with EGIDs and its association with treatment and prognosis. Microbiome content of stool samples has the potential to be a non-invasive biomarker for disease diagnosis and monitoring. Previous studies observed differences in the microbiome of patients with EoE, but it is unclear how this is associated with patientoutcomes [31].
Another project focuses on a preliminary, open-label trial of losartan in patients with EoE with or without a connective tissue disorder. This project is based on previous observations that TGF-β is overexpressed in EoE and that mutations in TGF-β receptors have been associated with increased risk of EoE [1, 36]. These and other findings, including studies in mice, have prompted analysis of the clinical effectiveness of losartan, as one of its effects is to lower TGF-β levels.
The third study is focused on the use of unsedated transnasal esophagoscopy (TNE) to monitor EoE disease activity [11]. Since TNE does not require the use of either moderate sedation or general anesthesia, it has the potential to lessen patient inconvenience and cost and potentially provide an opportunity for research with the ability to perform more frequent and less invasive monitoring.
Finally, a prospective trial of elemental diet therapy for adult patients with EG is being undertaken. This study will provide prospective evidence that EG is an antigen-driven disorder and will provide evidence for the clinical use of an elimination diet in the treatment of non-EoE EGIDs.
4Contact registry
In 2015 in collaboration with the RDCRN-DMCC, CEGIR launched the Patient Contact Registry, an interactive tool for patients with EGIDs. The DMCC maintains the contact registry, tracks its usage, and provides its own regulatory pathway for querying the contact registrants. The resulting centralized database allows CEGIR investigators to identify and recruit patients to new clinical studies. Upon enrollment, each patient is invited to complete a questionnaire to capture his or her disorder information. This allows collection of data on diagnosis, tests, and management from a diverse and large cohort of patients with EGIDs from all around the world. More importantly, it provides a portal to enrolled patients and families with updates on the disorders and opportunities to learn more about the various clinical trials available. Almost 1,100 patients from more than 20 countries have enrolled in the CEGIR Patient Contact Registry. This registry provides an extension of CEGIR’s network beyond the current sites and helps CEGIR achieve its goal of obtaining access to a population more representative of the affected population. CEGIR seeks to use its contact registry as a powerful platform for its EGID research. CEGIR is also conducting a validation study of the Contact Registry data through a project being conducted by one of its trainees (see Table 2).
Table 2
CEGIR Trainees
| Trainee | Mentor | Institution | Appointment | Research Interest | Other NIH funding |
| Patricia Fulkerson | Marc Rothenberg | Cincinnati Children’s Hospital | April 2015 | Eosinophil progenitors and EGIDs in pediatric patients after solid organ transplant | K08, R01 |
| Robert Pesek | Evan Dellon | Arkansas Children’s Hospital and University of Arkansas for Medical Sciences | April 2015 | Pathology of EG and EC | |
| Girish Hiremath | Pablo Abonia and Evan Dellon | Vanderbilt University | August 2015 | Assessing the needs of individuals with EGIDs through the CEGIR Contact Registry | |
| Amanda Muir | Jonathan Spergel | The Children’s Hospital of Philadelphia | August 2015 | Measurement of stretch of esophagus as potential for detection of pediatric fibrosis and stricture in EoE | K08 |
| Joshua Wechsler | Ikuo Hirano and Bruce Bochner | Northwestern University | August 2016 | The role of mast cells in EoE | K08 |
5Training (Career Development) Program
The CEGIR resources and opportunities available at each site create an opportunity to train new investigators in clinical and translational research focusing on EGIDs. After its formation in 2014, a call for applications was advertised to all fellows and junior faculty in various fields including gastroenterology, allergy/immunology, and pathology and spanning pediatrics and internal medicine specialists. The selection committee includes training section co-leads, as well as CEGIR PI and PAG representatives. The committee ranks applicants on the basis of academic background and the likelihood that candidates will continue to pursue EGID-related research after the grant’s conclusion. Since 2014, five CEGIR trainees have been selected from 31 applicants, and there are several active projects being spearheaded by these trainees (Table 2). Three of the current physician-trainees are or have been supported by NIH K08 grants.
The Training (Career Development) Program takes advantage of CEGIR resources and opportunities available at each site to create an environment that trains new investigators not only in clinical research, but also in the challenges common to the research of rare diseases through a series of lectures, webinars, and discussions. Each trainee has a variety of resources and opportunities available at each of the CEGIR sites to enhance likelihood of academic success and career development, such as RDCRN support, training-specific institutional resources, partnership with PAGs, access to 21 teaching videos/presentations prepared by CEGIR investigators and now available online (https://www.rarediseasesnetwork.org/cms/cegir/Learn-More/Educational-Videos), and the breadth and depth of the expertise of the CEGIR investigators in clinical research and mentorship.
6Pathology Core
With the large number of biopsies collected through various studies and the microbiome program, CEGIR also has a corresponding CEGIR Pathology Core that reviews the relevant slides for eosinophil density and other pathologic features. A team of pathologists provides invaluable skills, such as immunohistochemistry and electron microscopy. The CEGIR Pathology Core uses a Virtual Microscope platform to ease the protected process of collecting and transferring specimen images across the country. Whole-slide scanned images, deposited in permissions-restricted Dropbox, are provided for central pathology review. For sites that do not have slide-scanning capability, slides are sent to CCHMC and are scanned into a secure, cloud-based document CEGIR site.
7Publications Committee
The creation of the CEGIR Publications Committee has helped CEGIR publish more than 40 journal articles in the short time since its inception. This committee facilitates the timely reporting of study results through support to the primary authors. A wide variety of publications has emerged from CEGIR including review articles, [12, 14, 18, 29] editorials, and original research articles. Research published thus far has focused on a wide range of topics including disease mechanisms, [4, 5, 13, 24, 31] pathology and histology, [32, 33, 35] diagnosis, [7] and various treatment options for EGIDs including steroids, [17, 34] diet changes, [3, 43] and proton pump inhibitors [9, 22]. CEGIR and its Publications Committee will continue to report new findings, especially as the ongoing trials and projects supported by CEGIR are completed.
Summary
CEGIR, as a part of RDCRN, is a consortium created to promote collaboration among pediatric and adult academic research centers and PAGs. Importantly, the group provides a platform for collecting longitudinal data and specimens into centralized databases, thereby improving clinical management and understanding of EGIDs. Its success is predicated on synergy between multiple experts in various fields, which has translated to successful projects, more than 40 publications, and the rapid exchange of the best patient treatment methods since its formation. The PAGs have been important and core participants of CEGIR and contribute to its activities including its research agenda. The opportunities afforded by the CEGIR network, as compared with other traditional collaborative and/or grant mechanisms, are numerous as detailed in Table 3.
Table 3
CEGIR: Benefits and lessons learned
| Benefit | Comment |
| Funding | $1.2 million of annual funding is provided by NIH |
| Collaborations | Enhanced collaborations between multiple and varied stake-holders |
| Leverages other funding mechanisms | Co-funding provided by select institutions including PAGs |
| Unifies different stakeholders within the field | Brings together PAG and Researchers and NIH Institute Centers |
| Interaction with other consortia in network | Sharing of research platforms |
| Data management and coordination | RDCRN provides this service as part of the participation. The DMCC allows CEGIR to leverage a number of services not normally available to stand-alone grants. |
| Support for trainees | Trainees benefit from participating in CEGIR clinical studies, can utilize CEGIR-generated research resources, and can participate in RDCRN-wide training programs |
| Provides infrastructure upon which other studies are built | Registries provide resource for other studies |
| Partnership and engagement of expertise of NIH | U54 grant mechanism is a Cooperative Agreement and benefits from interaction with NIH experts including Medical Officer, Project Scientists, Program Officers, Data and Safety Monitoring Board (DSMB), and others |
| Develops uniform processes and consensus opinions and approaches | Examples include central pathology review and standard operating manual for common procedures such as skin testing. |
There are multiple steps that led to the creation of CEGIR. They started by identifying stakeholders such as key specialists, experts, and interest groups. The core group was composed of motivated individuals who had a common goal and were willing to collaborate. This group had frequent communication aiming to clearly define the consortium’s vision and objectives. After its initial set-up phase, the strength of the consortium was defined by its ability to evolve and provide its members with a mutually beneficial relationship. This in itself was spearheaded by a clearly defined structure allowing for both internal and external reviews at regular intervals to provide objective and continuous evaluation of the consortium’s goals, as it is important to continuously evaluate the various projects within the consortium in order to identify weaknesses, correct flaws, and build upon strengths. Besides research, the training component is essential to stimulate, promote, and nurture the next generation of investigators’ interest in the field.
Although still in its early stage, CEGIR has already enabled the study of a group of rare diseases through the creation of a collaborative environment and opportunities to participate and generate numerous research projects. The cohesiveness and productivity within this consortium, along with lessons learned as outlined in this review, is a model for other potential multi-center consortia.
Conflicts of interest
Drs. Cheng, Carpenter, Collins, Kantor, Kuhl, Bonis, Capocelli, Gopal-Srivastava, Krischer, Leung and Schoepfer have no conflict of interest to disclose.
Dr. Gupta is a consultant for Abbott. Dr. Aceves is a co-inventor of oral viscous budesonide (UCSD patented, licensed by Meritage Pharma). Dr. Chehade is a consultant for Shire and Actelion. She also receives research funding from Nutricia, Shire, and Regeneron. Dr. Dellon is a consultant for Adare, Alivio, Banner, Enumeral, Celgene/Receptos, GSK, Regeneron, and Shire. He also receives research funding from Celegene/Receptos, Meritage, Miraca, Nutricia, Regeneron, and Shire and an educational grant from Banner. Dr. Falk receives research support from Regeneron, Celgene, Shire, and Adare. Dr. Gonsalves is an author for UpToDate. Dr. Hirano is a consultant for Adare, Celgene/Receptos, Regeneron, and Shire. He has received research funding from Celegene/Receptos, Meritage, Regeneron, and Shire. Dr. Mukkada is a consultant for Shire and also receives research funding from Shire. Dr. Spergel is a consultant for DBV Technologies. He also received research grants from Aimmune Therapeutics, DBV Technologies, Shire, and Regeneron. Dr. Straumann is a consultant for Abbott, Actelion, Calypso, Falk, GSk, Merck, MSD, Novartis, Nutricia, Pfizer, Receptos-Celgene, Regeneron-Sanofi, and Tillotts. Dr. Furuta is Co-Founder of EnteroTrack and has consulted for Shire and GSK and received grant support from Nutricia. Dr. Rothenberg is a consultant for Immune Pharmaceuticals, NKT Therapeutics, Pulm One, Spoon Guru, Celgene, Shire, Astra Zeneca, and Novartis and has an equity interest in the first four companies listed and royalties from reslizumab (Teva Pharmaceuticals). Dr. Rothenberg is an inventor of several patents owned by Cincinnati Children’s.
Acknowledgments
CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is co-funded by NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED, and EFC. Dr. Glenn Furuta is supported by NIH 1K24Dk100303. Dr. Jeffrey Krischer is supported by NIH U54TR001263. The authors are grateful to Dr. Amanda Rudman-Spergel for her guidance and input.
References
[1] | Aceves S.S. et al., Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids, Allergy 65: (1) ((2010) ), 109–116. |
[2] | Batshaw M.L. , Groft S.C. and Krischer J.P. , Research into rare diseases of childhood, Jama 311: (17) ((2014) ), 1729–1730. |
[3] | Case C. et al., Diet and Stress in Pediatric Eosinophilic Esophagitis, J Pediatr Gastroenterol Nutr ((2016) ). |
[4] | Chandramouleeswaran P.M. et al., Preferential secretion of thymic stromal lymphopoietin (TSLP) by Terminally differentiated esophageal epithelial cells: Relevance to eosinophilic esophagitis (EoE), PLoS One 11: (3) ((2016) ), e0150968. |
[5] | Davis B.P. and Rothenberg M.E. , Mechanisms of disease of eosinophilic esophagitis, Annu Rev Pathol 11: ((2016) ), 365–393. |
[6] | DeBrosse C.W. et al., Quantity and distribution of eosinophils in the gastrointestinal tract of children, Pediatr Dev Pathol 9: (3) ((2006) ), 210–218. |
[7] | Dellon E.S. et al., Substantial variability in biopsy practice patterns among gastroenterologists for suspected eosinophilic gastrointestinal disorders, Clin Gastroenterol Hepatol 14: (12) ((2016) ), 1842–1844. |
[8] | Dellon E.S. et al., Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis, Aliment Pharmacol Ther 38: (6) ((2013) ), 634–642. |
[9] | Eluri S. and Dellon E.S. , Proton pump inhibitor-responsive oesophageal eosinophilia and eosinophilic oesophagitis: More similarities than differences, Curr Opin Gastroenterol 31: (4) ((2015) ), 309–315. |
[10] | Fiorentino R. et al., Cross-sector sponsorship of research in eosinophilic esophagitis: A collaborative model for rational drug development in rare diseases, J Allergy Clin Immunol 130: (3) ((2012) ), 613–616. |
[11] | Friedlander J.A. et al., Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis, Gastrointest Endosc 83: (2) ((2016) ), 299–306.e1. |
[12] | Furuta G.T. and Katzka D.A. , Eosinophilic Esophagitis, N Engl J Med 373: (17) ((2015) ), 1640–1648. |
[13] | Hill D.A. and Spergel J.M. , The immunologic mechanisms of eosinophilic esophagitis, Curr Allergy Asthma Rep 16: (2) ((2016) ), 9. |
[14] | Hirano I. , 2015 David Y. Graham Lecture: The First Two Decades Of Eosinophilic Esophagitis-From Acid Reflux To Food Allergy, Am J Gastroenterol 111: (6) ((2016) ), 770–776. |
[15] | Hirano I. , Therapeutic end points in eosinophilic esophagitis: Is elimination of esophageal eosinophils enough? Clin Gastroenterol Hepatol 10: (7) ((2012) ), 750–752. |
[16] | Hiremath G.S. et al., Analysis of unmet needs of individuals affected by eosinophilic gastrointestinal disorders: Towards health promotion and effective care, Gastroenterology 152: (5, Supplement 1) ((2017) ), S851–S852. |
[17] | Imam T. and Gupta S.K. , Topical glucocorticoid vs. diet therapy in eosinophilic esophagitis: The need for better treatment options, Expert Rev Clin Immunol 12: (8) ((2016) ), 797–799. |
[18] | Jensen E.T. and Dellon E.S. , Environmental and infectious factors in eosinophilic esophagitis, Best Pract Res Clin Gastroenterol 29: (5) ((2015) ), 721–729. |
[19] | Kagalwalla A.F. et al., Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis, Clin Gastroenterol Hepatol 4: (9) ((2006) ), 1097–1102. |
[20] | Kato M. et al., Eosinophil infiltration and degranulation in normal human tissue, Anat Rec 252: (3) ((1998) ), 418–425. |
[21] | Kelly K.J. et al., Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with an amino acid-based formula, Gastroenterology 109: (5) ((1995) ), 1503–1512. |
[22] | Kochar B. and Dellon E.S. , Management of proton pump inhibitorresponsive-esophageal eosinophilia and eosinophilic esophagitis:Controversies in treatment approaches, Expert RevGastroenterol Hepatol 9: (11) ((2015) ), 1359–1369. |
[23] | Krischer J.P. et al., The Rare Diseases Clinical Research Network’s organization and approach to observational research and health outcomes research, J Gen Intern Med 29: (Suppl 3) ((2014) ), S739–S744. |
[24] | Leung J. , Beukema K.R. and Shen A.H. , Allergic mechanisms of Eosinophilic oesophagitis, Best Pract Res Clin Gastroenterol 29: (5) ((2015) ), 709–720. |
[25] | Liacouras C.A. et al., Eosinophilic esophagitis: Updated consensus recommendations for children and adults, J Allergy Clin Immunol 128: (1) ((2011) ), 3–20.e6; quiz 21–22. |
[26] | Lowichik A. and Weinberg A.G. , A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract, Mod Pathol 9: (2) ((1996) ), 110–114. |
[27] | Lwin T. , Melton S.D. and Genta R.M. , Eosinophilic gastritis: Histopathological characterization and quantification of the normal gastric eosinophil content, Mod Pathol 24: (4) ((2011) ), 556–563. |
[28] | Martin L.J. et al., Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease, J Allergy Clin Immunol 135: (6) ((2015) ), 1519–1528.e8. |
[29] | Mehta P. and Furuta G.T. , Eosinophils in gastrointestinal disorders: Eosinophilic gastrointestinal diseases, celiac disease, inflammatory bowel diseases, and parasitic infections, Immunol Allergy Clin North Am 35: (3) ((2015) ), 413–437. |
[30] | Merkel P.A. et al., The partnership of patient advocacy groups and clinical investigators in the rare diseases clinical research network, Orphanet J Rare Dis 11: (1) ((2016) ), 66. |
[31] | Muir A.B. et al., Microbiome and its impact on gastrointestinal atopy, Allergy 71: (9) ((2016) ), 1256–1263. |
[32] | Muir A.B. et al., Eosinophilic esophagitis-associated chemical and mechanical microenvironment shapes esophageal fibroblast behavior, J Pediatr Gastroenterol Nutr 63: (2) ((2016) ), 200–209. |
[33] | Nguyen N. , Furuta G.T. and Masterson J.C. , Deeper than the epithelium: Role of matrix and fibroblasts in pediatric and adult eosinophilic esophagitis, J Pediatr Gastroenterol Nutr 63: (2) ((2016) ), 168–169. |
[34] | Nguyen N. , Furuta G.T. and Menard-Katcher C. , Sticky steroids: In search of an approved treatment for eosinophilic esophagitis, J Pediatr Gastroenterol Nutr 64: (2) ((2017) ), 172–173. |
[35] | Rajan J. et al., Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids, J Allergy Clin Immunol 137: (1) ((2016) ), 147–56.e8. |
[36] | Rawson R. et al., TGF-beta1-induced PAI-1 contributes to a profibrotic network in patients with eosinophilic esophagitis, J Allergy Clin Immunol 138: (3) ((2016) ), 791–800.e4. |
[37] | Rothenberg M.E. , Eosinophilia, N Engl J Med 338: (22) ((1998) ), 1592–1600. |
[38] | Rothenberg M.E. , Eosinophilic gastrointestinal disorders (EGID), J Allergy Clin Immunol 113: (1) ((2004) ), 11–28; quiz 29. |
[39] | Rothenberg M.E. et al., Working with the US Food and Drug Administration: Progress and timelines in understanding and treating patients with eosinophilic esophagitis, J Allergy Clin Immunol 130: (3) ((2012) ), 617–619. |
[40] | Rothenberg M.E. and Hogan S.P. , The eosinophil, Annu Rev Immunol 24: ((2006) ), 147–174. |
[41] | Schoepfer A.M. et al., Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis, Gastroenterology 147: (6) ((2014) ), 1255–1266.e21. |
[42] | Spergel J.M. et al., Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet, J Allergy Clin Immunol 130: (2) ((2012) ), 461–467.e5. |
[43] | Venter C. and Fleischer D.M. , Diets for diagnosis and management of food allergy: The role of the dietitian in eosinophilic esophagitis in adults and children, Ann Allergy Asthma Immunol 117: (5) ((2016) ), 468–471. |
[44] | Weller P.F. , The immunobiology of eosinophils, N Engl J Med 324: (16) ((1991) ), 1110–1118. |
[45] | Zuo L. and Rothenberg M.E. , Gastrointestinal eosinophilia, Immunol Allergy Clin North Am 27: (3) ((2007) ), 443–455. |



