Antigout effects and mechanisms of total flavonoids from prunus tomentosa
Abstract
BACKGROUND:
In recent years, hyperuricemia and acute gouty arthritis have become increasingly common, posing a serious threat to public health. Current treatments primarily involve Western medicines with associated toxic side effects.
OBJECTIVE:
This study aims to investigate the therapeutic effects of total flavones from Prunus tomentosa (PTTF) on a rat model of gout and explore the mechanism of PTTF’s anti-gout action through the TLR4/NF-
METHODS:
We measured serum uric acid (UA), creatinine (Cr), blood urea nitrogen (BUN), tumor necrosis factor-alpha (TNF-
RESULTS:
After PTTF treatment, all indicators improved significantly. PTTF reduced blood levels of UA, Cr, BUN, IL-1
CONCLUSIONS:
PTTF may have a therapeutic effect on animal models of hyperuricemia and acute gouty arthritis by reducing serum UA levels, improving ankle swelling, and inhibiting inflammation. The primary mechanism involves the regulation of the TLR4/NF-
1.Introduction
Hyperuricemia (HUA) is caused by deregulated purine metabolism followed by elevated blood uric acid and urate deposition [1]. Acute gouty arthritis (AGA) is an inflammatory condition caused by the formation and deposition of monosodium urate (MSU) crystals in the local joints due to uric acid (UA) exceeding its saturation level in the blood or tissue fluid [2]. An increase in serum MSU concentration is the most critical risk factor in the development of gout [3]. Gout patients also bear a high burden of related complications, such as hypertension, chronic kidney disease, obesity, diabetes, myocardial infarction, and heart failure [4]. According to the “2021 Trends in Chinese Hyperuricemia and Gout White Paper,” the overall prevalence of gout in China has been increasing year by year and trending toward a younger population. With improvements in economic conditions and living standards, risk factors for gout, such as alcohol consumption [5], high fructose beverages [6], and increased daily intake of meat and seafood [7], are also on the rise.
Currently, there is no cure for gout, and clinical management primarily involves alleviating symptoms using non-steroidal anti-inflammatory drugs [8], colchicine [9], and allopurinol [10]. However, these medications often lead to adverse effects such as diarrhea, abdominal pain, vomiting, rashes, facial edema, gastrointestinal bleeding, hepatitis, and even acute renal failure, severely affecting the physical and mental health as well as the normal life of individuals. Research has shown that flavonoids have various pharmacological effects, including antioxidant properties [11], antiviral activity, anticancer effects, anti-inflammatory actions [12], antioxidative abilities [13], protection against cerebral ischemia, anti-gastric ulcer properties, improved functional dyspepsia, inhibition of intestinal motility, gastric mucosal protection [14], anti-myocardial ischemia, anti-arrhythmia, analgesia, hepatoprotection, and lipid-lowering effects [15]. In this study, based on the various pharmacological effects of flavonoids, we aim to investigate the pharmacological effects of total flavones from Prunus tomentosa (PTTF) on hyperuricemia and AGA rat models. This research focuses on the central role of IL-1
2.Materials
2.1Experimental animals
Sixty adult male rats of SPF (Specific Pathogen Free) grade, with a body weight of (200
2.2Reagents
Total flavones from Prunus tomentosa were provided by the Pharmacology Department of Beihua University [16]. Potassium oxonate (
2.3Instruments
Model 680 Enzyme Labeling Instrument (Shanghai Yidi Electric Science Instrument Co., Ltd.); High-speed Centrifuge (Eppendorf Centrifuge 5410 R); Micropipettes (Eppendorf Research Plus, Models: 3120000062, 3120000054, 3121000023); Mixer (Experimental Instruments Co., Ltd., Haimen City, Jiangsu Province); Microscope (OLYMPUS BX53F); PV-200 Toe Volume Measuring Instrument (Chengdu Taimeng Technology Co., Ltd.); ROTANODE E7843X. Balances: United Brothers (Group) Co., Ltd. (SUZH-00000193); UVP ChemStudio Chemiluminescence Imaging System.
3.Experimental methods
3.1Solution preparation
3.1.1Preparation of hyperuricemia modeling drug
Following the method and making improvements as per relevant literature [17], 4.5 g of carboxymethylcellulose sodium (CMC-Na) was dissolved in distilled water to a total volume of 1500 ml to prepare a 0.3% CMC-Na solution [18]. Then, 15 g of adenine and 45 g of potassium oxonate were weighed and added to the prepared 0.3% CMC-Na solution, sealed, and stored at 4∘C [19].
3.1.2Preparation of monosodium urate solution (MSU)
Following the method from relevant literature [20], 1 g of monosodium urate salt was dissolved in 200 ml of boiling water containing NaOH. The solution’s pH was adjusted to 7.2 by adding hydrochloric acid. The solution was cooled and stirred at room temperature, then stored overnight at 4∘C. The precipitate was filtered from the solution, dried at low heat, and sieved through a 250
3.2Establishment of hyperuricemia rat model and drug administration
After a one-week acclimatization period, 60 rats were randomly divided into six groups: Blank group (CON), Model group (Hyperuricemia model, MOD), Positive drug group (Allopurinol/Colchicine treatment group, YO), PTTF100 mg/kgdose group (PTTF-100), PTTF 200 mg/kg-dose group (PTTF-200), and PTTF 400 mg/kg-dose group (PTTF-400) [21]. On the 8th day, the rats in all groups except the Blank group were orally administered with the prepared modeling drug (1 ml/100 g) to induce hyperuricemia. On the 15th day, the Positive drug group and PTTF treatment groups were orally administered with Allopurinol [22] (10 mg/kg) and three different doses of PTTF (100 mg/kg, 200 mg/kg, 400 mg/kg) [23] respectively. The Blank group and Model group were given an equivalent amount of distilled water. All administrations were performed daily at 10 a.m. After continuous treatment for 7 days, blood samples were collected from the eyeball to measure relevant indicators.
3.3Establishment of acute gouty arthritis model and administration method
On the 22nd day, 0.2 ml of monosodium urate was injected into the right ankle joint cavity of the rats in all groups except the Blank group. The Blank group received an equivalent amount of sterile physiological saline [24]. The Positive drug group and PTTF treatment groups were orally administered with Colchicine (1.5 mg/kg) and three different doses of PTTF, respectively. The rats’ foot swelling was observed at 4 h, 8 h, 24 h, and 48 h, and gait scoring was conducted 24 h after administration. Subsequently, rats were anesthetized with pentobarbital sodium, blood was collected from the abdominal aorta, and specimens from the ankle joint, kidneys, and feces were obtained. A portion of each group was fixed in 10% formalin, while the rest was placed in EP tubes and stored in a
3.4Parameter measurements
3.4.1Measurement of UA, Cr, and BUN
After blood collection from the eyeball [25], it was left to stand for 30 minutes, centrifuged at 3000 rpm for 10 minutes, and the supernatant was collected and frozen at
3.4.2Measurement of joint swelling
At 4 h, 8 h, 24 h, and 48 h after the injection of monosodium urate solution, the volume of the right ankle joint of the rats was measured using a toe volume measuring instrument. The degree of rat ankle joint swelling was calculated using the formula: Joint swelling degree
3.4.3Gait scoring
24 hours after modeling for acute gouty arthritis in rats, the gait of rats in each group was observed and scored as follows: Grade 0, normal walking (0 points); Grade 1, slight limping with slight bending of the lower limb, limited redness at the ankle joint (1 point); Grade 2, obvious limping with lower limb just touching the ground, significant swelling, and redness localized at the ankle joint (2 points); Grade 3, severe limping with the joint showing severe redness and swelling, the lower limb lifted off the ground, and walking on three limbs (3 points) [26].
3.4.4Measurement of IL-1β α
On the 25th day, rats in each group were anesthetized by intraperitoneal injection of pentobarbital sodium, positioned supine, the abdominal cavity was opened to expose the abdominal aorta, blood was collected, left to stand for 1 hour, centrifuged at 3000 rpm for 10 minutes, and the supernatant was collected. The measurement procedure strictly followed the instructions of the respective ELISA reagent kits, and a microplate reader was used to detect the levels of IL-1
3.4.5Histological examination
Rat ankle joint and kidney tissues, which had been fixed in 10% formalin solution, were cut into small pieces, washed with running water for 12 hours, and subjected to dehydration with a series of ethanol solutions (80%, 90%, 95%, 100%), xylene treatment, paraffin embedding, sectioning (5
3.4.6Western blot analysis of protein expression in rat ankle joints related to signaling pathways
Rat ankle joint tissues were placed on ice, thoroughly homogenized in RIPA lysis buffer, and total protein was extracted. The concentration of total protein was determined following the instructions of the BCA reagent kit. After denaturation of the protein samples, SDS-PAGE gel electrophoresis was used to separate the proteins, which were then transferred to a PVDF membrane. The membrane was blocked with 5% skim milk powder for 1 hour. Primary antibodies (NF-kb, TLR4, MyD88) were diluted to appropriate concentrations as per the instructions and incubated overnight at 4∘C. The membrane was washed three times with 1
3.5Data statistical analysis
SPSS 21.0 statistical software was used for data analysis. Results were expressed as means
4.Results
4.1Effects of PTTF on serum UA, Cr, and BUN levels in hyperuricemia rats
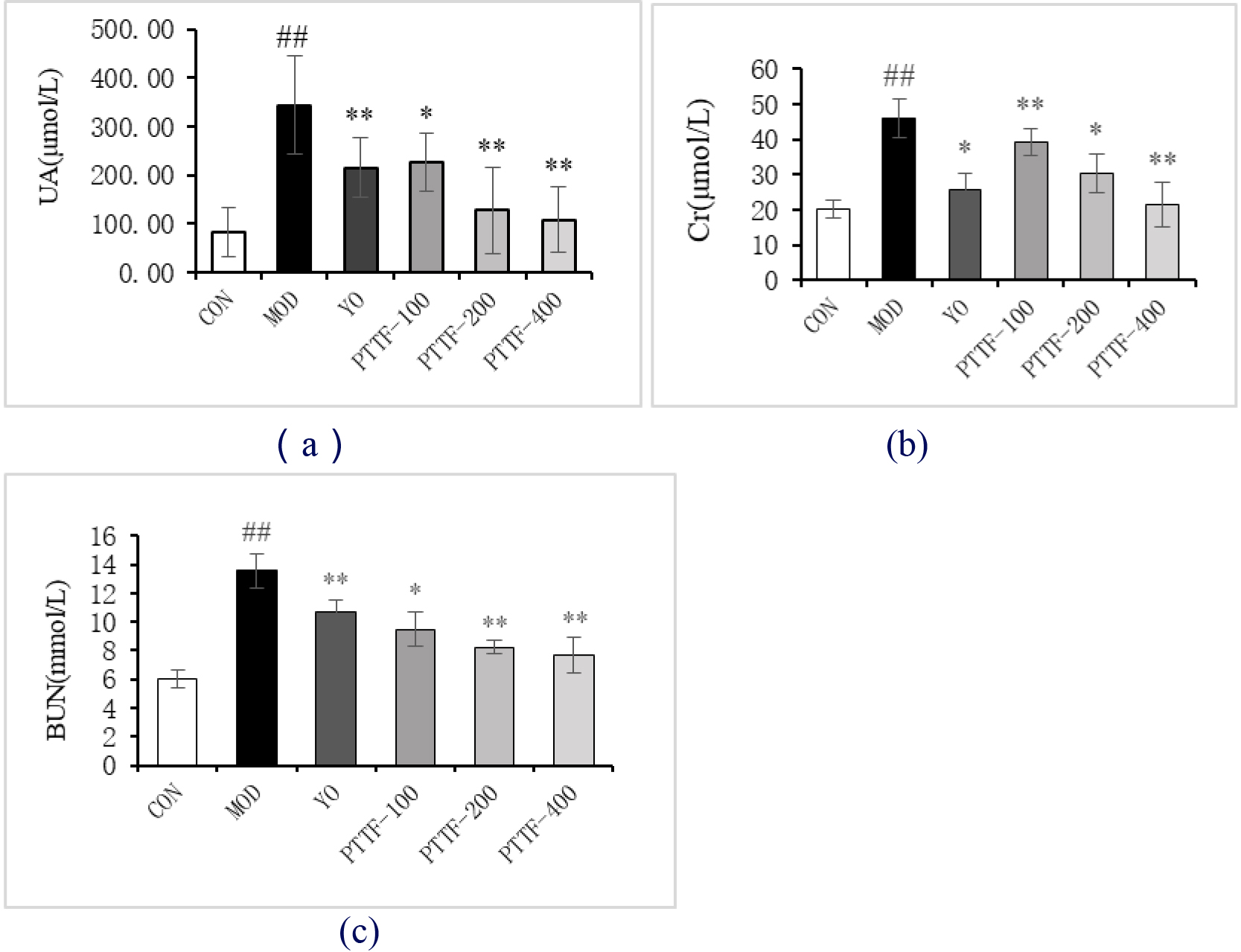
Compared to the Blank group, the Model group showed a significant increase in blood levels of UA, Cr, and BUN. In comparison to the Model group, the Treatment group exhibited a significant reduction in blood levels of UA, Cr, and BUN, and this reduction became more significant with increasing doses of PTTF. The differences were statistically significant (
Figure 1.
Effects of PTTF on serum UA, Cr, and BUN levels in hyperuricemic rats. Note: CON: Blank group; MOD: Model group; YO: Benzbromarone group; PTTF-100: Prunus Tomentosa Total Flavones 100 mg/kg-Dose group; PTTF-200: Prunus Tomentosa Total Flavones 200 mg/kg-Dose group; PTTF-400: Prunus Tomentosa Total Flavones 400 mg/kg-Dose group. Compared to the Blank group, #:
4.2Effects of PTTF on ankle joint swelling in acute gouty arthritis rats
Compared to the Blank group, the Model group showed a significant increase in foot swelling. In comparison to the Model group, the Treatment group showed a significant decrease in foot swelling, which became more significant with increasing doses of PTTF. The swelling index initially increased and then decreased, and the differences were statistically significant (
Figure 2.
Effects of PTTF on the degree of ankle joint swelling in acute gouty arthritis rats. Note: CON: Blank group; MOD: Model group; YO: Colchicine group; PTTF-100: Prunus Tomentosa Total Flavones 100 mg/kg-Dose group; PTTF-200: Prunus Tomentosa Total Flavones 200 mg/kg-Dose group; PTTF-400: Prunus Tomentosa Total Flavones 400 mg/kg-Dose group. Compared to the Blank group, #:
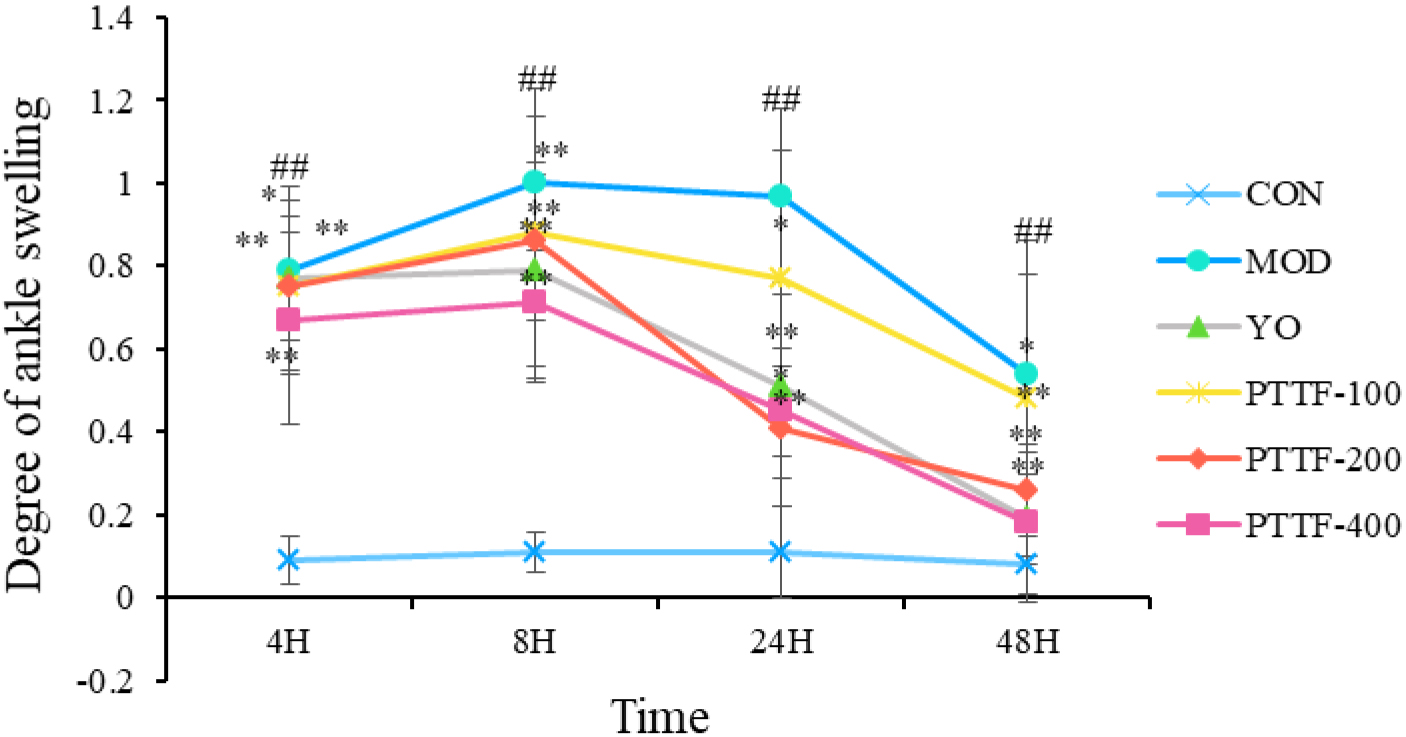
Figure 3.
Effects of PTTF on the gait of rats with acute gouty arthritis. Note: CON: Blank group; MOD: Model group; YO: Colchicine group; PTTF-100: Prunus Tomentosa Total Flavones 100 mg/kg-Dose group; PTTF-200: Prunus Tomentosa Total Flavones 200 mg/kg-Dose group; PTTF-400: Prunus Tomentosa Total Flavones 400 mg/kg-Dose group. Compared to the Blank group, #:
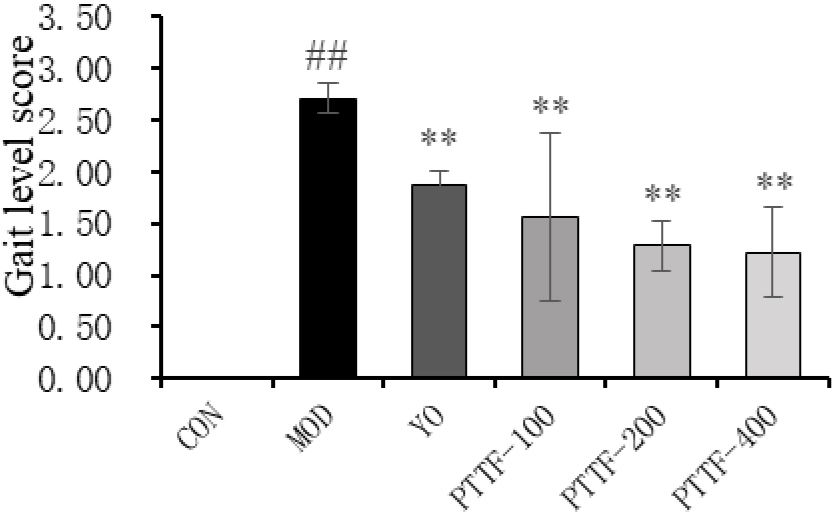
Figure 4.
The Effects of PTTF on the serum levels of IL-1
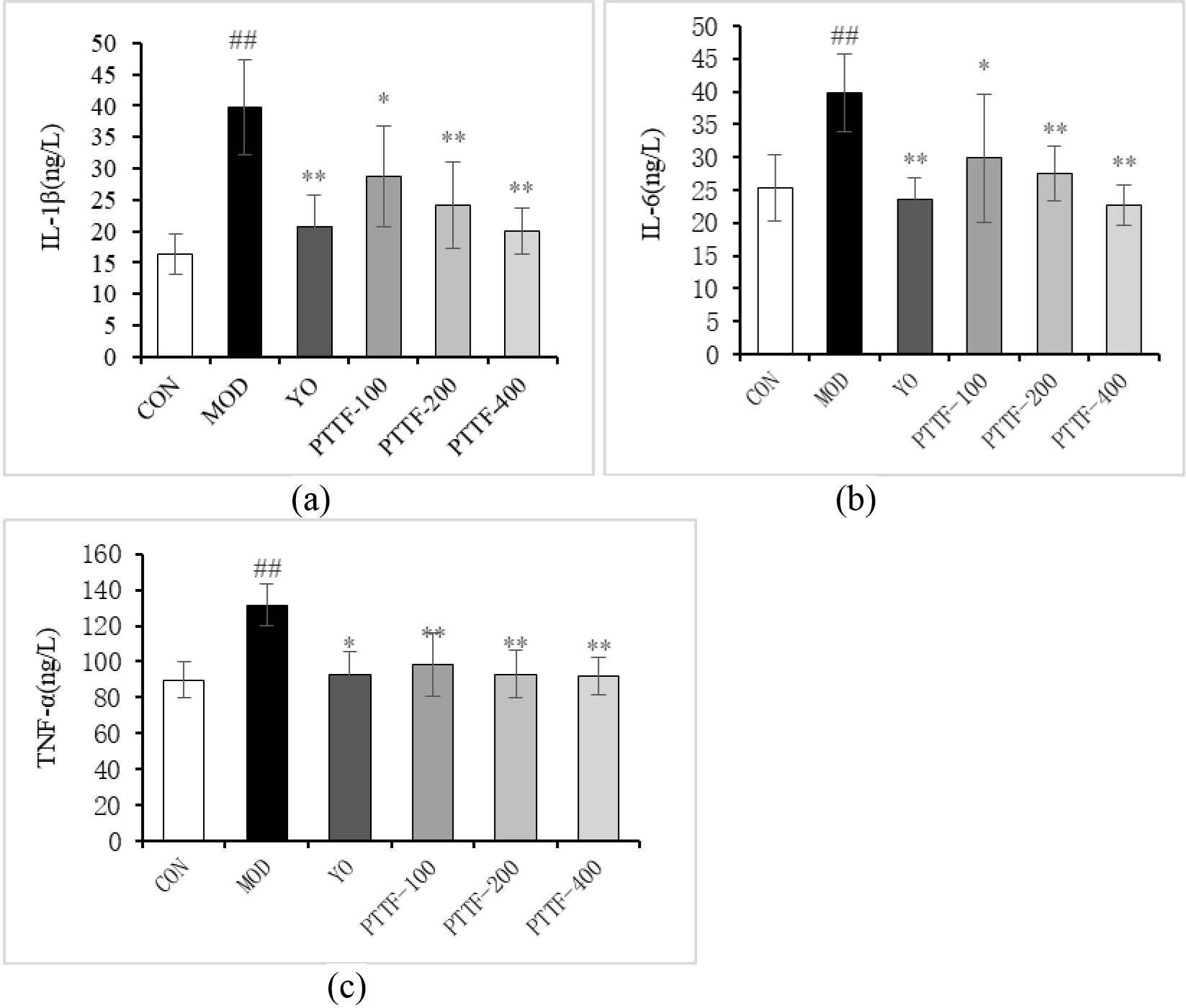
Figure 5.
The effects of PTTF on the pathological morphological changes of ankle joint tissues in gouty arthritis rats (
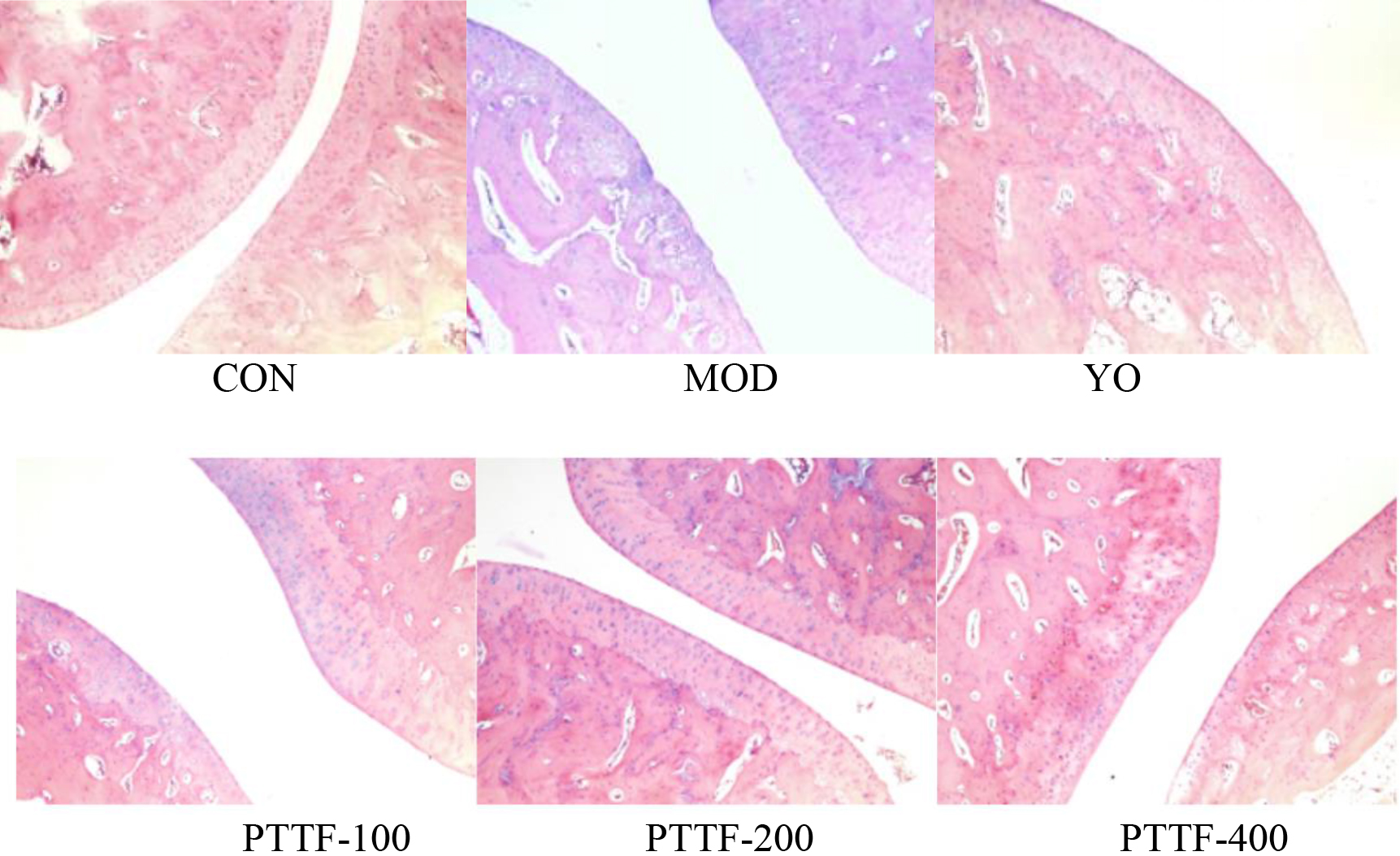
Figure 6.
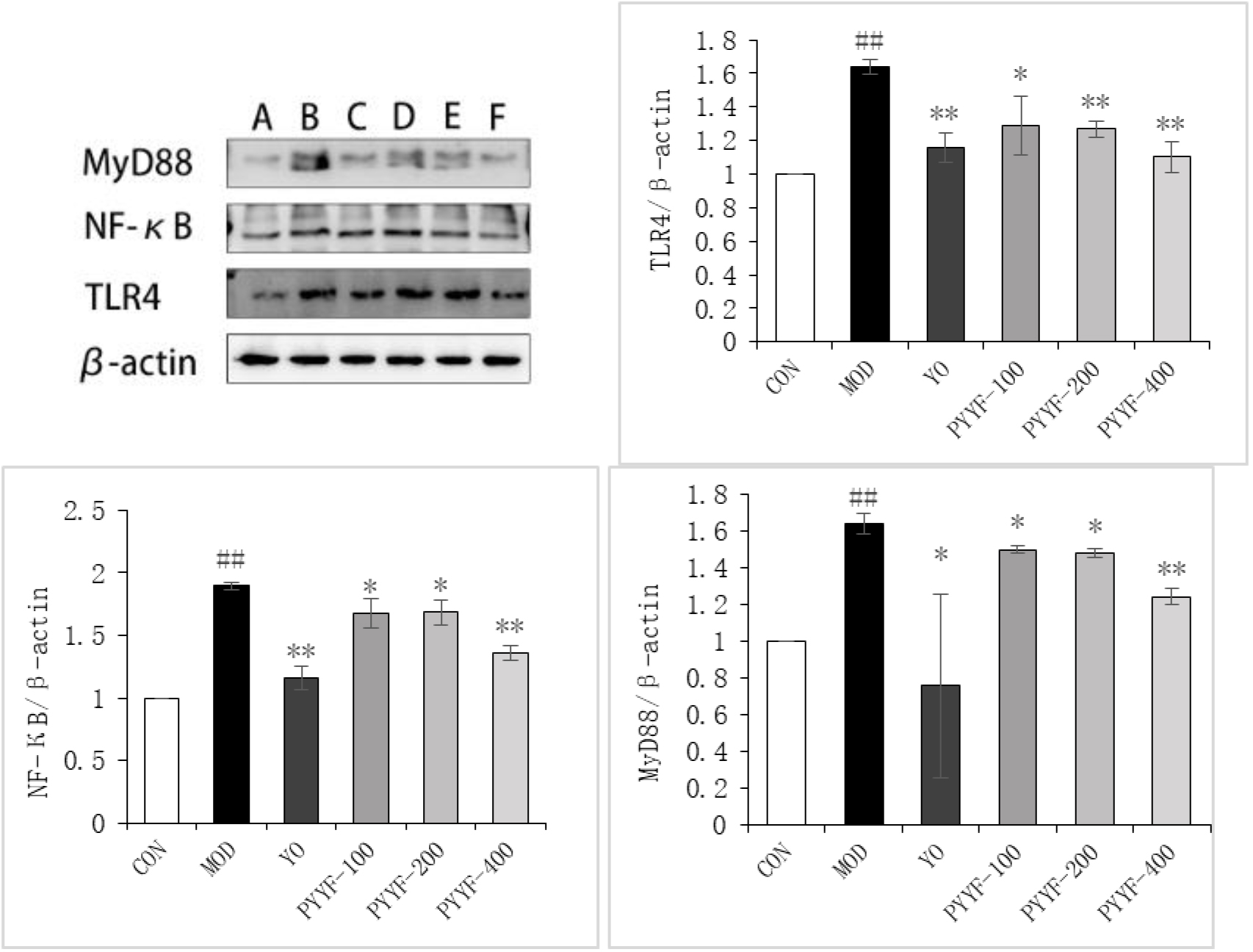
The effects of PTTF on the protein expression levels of TLR4, MyD88, and NF-
4.3Effects of PTTF on gait in acute gouty arthritis rats
Twenty-four hours after modeling, the Model group rats exhibited reduced activity, severe limping, contact or lifting of the affected limb off the ground, and three-legged walking, with severe redness and swelling of the joints. In comparison to the Model group, the Treatment groups showed significant improvements in gait, and the differences were statistically significant (
4.4Effect of PTTF on serum levels of IL-1β α
Compared with blank group, serum levels of IL-1
4.5Effect of PTTF on histopathologic morphological changes in rats
After HE staining, the morphology of synovial tissues in the ankle joint was observed under a 200x microscope. In the Blank group, no inflammatory cell infiltration was observed, and the synovial surface was intact, with clear tissue structure and no signs of vascular proliferation, congestion, or edema. In the Model group, significant inflammatory cell infiltration was observed, along with surface damage of the synovium, vascular proliferation, and edema. Compared to the Model group, the Treatment group showed varying degrees of reduction in synovial inflammation, with a gradual decrease in inflammatory cell infiltration, reduced synovial tissue proliferation, and smoother joint surfaces, approaching those of the Blank group (as shown in Fig. 5).
4.6Effects of PTTF on the expression levels of TLR4, MyD88, and NF-κ
Compared to the Blank group, the expression levels of TLR4, MyD88, and NF-
5.Discussion
A large body of literature demonstrates that dietary changes significantly increase the risk of hyperuricemia [28]. Consuming high-purine foods such as meat, seafood, and legumes in large quantities places additional strain on the kidneys, leading to hyperuricemia [29]. In this study, a rat model of hyperuricemia was successfully established by simulating high-purine intake through gastric infusion of adenine and using allopurinol as a uricase inhibitor, effectively preventing uric acid excretion and creating a hyperuricemic environment in the body. Acute gouty arthritis (AGA) is characterized by the deposition of sodium urate crystals in joint tissues and is primarily characterized by acute joint pain, redness, and fever, often being the initial symptom of gout. An AGA rat model was created by injecting sodium urate solution into the ankle joint, mimicking the acute inflammatory response characterized by joint redness and pain [30].
The kidneys are the primary organs responsible for excretion in the body, and any dysfunction in kidney function can lead to a decrease in creatinine clearance rate and filtration function, resulting in reduced uric acid excretion and an increased risk of hyperuricemia (HUA) [31]. Uric acid (UA), creatinine (Cr), and blood urea nitrogen (BUN) levels are the main indicators used to evaluate kidney filtration function. Mice in the model group showed significantly elevated levels of UA, Cr, and BUN, indicating that the combined induction of adenine and allopurinol disrupted kidney function. However, experimental results demonstrated that PTTF (Presumably the treatment being discussed) effectively restored uric acid, creatinine, and urea nitrogen levels in both blood and urine to normal levels.
The TLR4/NF-
The results of this study demonstrate that, compared to the model group, the PTTF treatment group exhibited significantly reduced levels of inflammation in synovial tissue, as indicated by decreased expression of TLR4, NF-
In conclusion, it can be inferred that PTTF has a certain therapeutic effect on hyperuricemia and acute gouty arthritis (AGA) in rats, with a more pronounced effect observed in the high-dose group. The mechanism of action appears to involve the blockade of the TLR4/NF-
Acknowledgments
The authors have no acknowledgments.
Conflict of interest
None to report.
References
[1] | Ni Q. Guidelines for the combined diagnosis and treatment of hyperuricemia and GOTU (2021-01-20). World Traditional Chinese Medicine. (2021) ; 16: (02): 183-189. |
[2] | Shekelle PG, et al. Management of gout: A systematic review in support of an american college of physicians clinical practice guideline. Annals of Internal Medicine. (2017) ; 166: (1): 37-51. doi: 10.7326/M16-0461. |
[3] | Dalbeth N, et al. Gout. Lancet (London, England). (2021) ; 397: (10287): 1843-1855. doi: 10.1016/S0140-6736(21)00569-9. |
[4] | Yokose C, et al. The role of diet in hyperuricemia and gout. Current Opinion in Rheumatology. (2021) ; 33: (2): 135-144. doi: 10.1097/BOR.0000000000000779. |
[5] | Nieradko-Iwanicka B. The role of alcohol consumption in pathogenesis of gout. Critical Reviews in Food Science and Nutrition. (2022) ; 62: (25): 7129-7137. doi: 10.1080/10408398.2021.1911928. |
[6] | Jamnik J, et al. Fructose intake and risk of gout and hyperuricemia: A systematic review and meta-analysis of prospective cohort studies. BMJ Open. 3 Oct. (2016) ; 6: (10): e013191. doi: 10.1136/bmjopen-2016-013191. |
[7] | Kakutani-Hatayama M, et al. Nonpharmacological management of gout and hyperuricemia: Hints for better lifestyle. American Journal of Lifestyle Medicine. 2 Sep. (2015) ; 11: (4): 321-329. doi: 10.1177/1559827615601973. |
[8] | van Durme CM, et al. Non-steroidal anti-inflammatory drugs for acute gout. The Cochrane Database of Systematic Reviews. 9 Dec. (2021) ; 12: (12): CD010120. doi: 10.1002/14651858.CD010120.pub3. |
[9] | Wechalekar MD, et al. The efficacy and safety of treatments for acute gout: Results from a series of systematic literature reviews including Cochrane reviews on intraarticular glucocorticoids, colchicine, nonsteroidal antiinflammatory drugs, and interleukin-1 inhibitors. The Journal of Rheumatology. Supplement (2014) ; 92: : 15-25. doi: 10.3899/jrheum.140458. |
[10] | Lee SC, et al. Allopurinol-induced severe cutaneous adverse drug reactions: An analysis of spontaneous reports in malaysia (2000–2018). Therapeutic Innovation & Regulatory Science. (2021) ; 55: (3): 514-522. doi: 10.1007/s43441-020-00245-w. |
[11] | Horng C-T, et al. Antioxidant and antifatigue activities of Polygonatum Alte-lobatum Hayata rhizomes in rats. Nutrients. 21 Nov. (2014) ; 6: (11): 5327-37. doi: 10.3390/nu6115327. |
[12] | Chen X, Wang Y, Zhu D, Zhang C. Effects of Prunus tomentosa total flavone on adjuvant-induced arthritis. Biotechnology & Biotechnological Equipment. (2018) Mar; 32: (2): 437-41. |
[13] | Faggio C, et al. Flavonoids and platelet aggregation: A brief review. European Journal of Pharmacology. (2017) ; 807: : 91-101. doi: 10.1016/j.ejphar.2017.04.009. |
[14] | Liu BH, et al. Preventive effect of anji white tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in kunming mice. Biomolecules. 4 Apr. (2019) ; 9: (4): 137. doi: 10.3390/biom9040137. |
[15] | Sun ZG, et al. Recent developments of flavonoids with various activities. Current Topics in Medicinal Chemistry. (2022) ; 22: (4): 305-329. doi: 10.2174/1568026622666220117111858. |
[16] | Shi JC, Zhao DS, Liu YY, et al. Study on anti-inflammatory activity of total flavonoids from prunus tomentosa by different preparation techniques. Journal of Beihua University: Natural Science Edition. (2017) ; (3): 345-347. |
[17] | Wang CY, Li ZL, Ye YS, et al. Study and evaluation of acute hyperuricemia animal model induced by potassium oxazinate in mice. Pharmacology and Clinics of Chinese Materia Medica. (2019) ; 35: (1): 176-180. |
[18] | Oh DR, et al. Effects of ChondroT on potassium Oxonate-induced Hyperuricemic mice: Downregulation of xanthine oxidase and urate transporter 1. BMC Complementary and Alternative Medicine. 8 Jan. (2019) ; 19: (1): 10. doi: 10.1186/s12906-018-2415-2. |
[19] | Kang L, et al. Total glucosides of herbaceous peony (Paeonia lactiflora Pall.) flower attenuate adenine- and ethambutol-induced hyperuricaemia in rats. Journal of Ethnopharmacology. (2020) ; 261: : 113054. doi: 10.1016/j.jep.2020.113054. |
[20] | Lee H-P, et al. Lemnalol attenuates mast cell activation and osteoclast activity in a gouty arthritis model. The Journal of Pharmacy and Pharmacology. (2015) ; 67: (2): 274-85. doi: 10.1111/jphp.12331. |
[21] | Bian M, Wang J, Wang Y, Nie A, Zhu C, Sun Z, et al. Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. Biomedicine & Pharmacotherapy. (2020) Nov; 110719. |
[22] | Han B, et al. Effect of Rhizoma Polygoni Cuspidati and Ramulus Cinnamomi compatibility on uric acid metabolism and urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in rats with hyperuricemia. Chinese Journal of Integrative Medicine. (2017) ; 23: (7): 535-542. doi: 10.1007/s11655-016-2649-0. |
[23] | Sato VH, Chewchinda S, Parichatikanond W, Vongsak B. In vitro and in vivo evidence of hypouricemic and anti-inflammatory activities of Maclura cochinchinensis (Lour.) Corner heartwood extract. Journal of Traditional and Complementary Medicine. (2020) Jan; 85-94. |
[24] | Han JR, et al. Zisheng Shenqi Decoction Ameliorates Monosodium Urate-Mediated Gouty Arthritis in Rats via Promotion of Autophagy through the AMPK/mTOR Signaling Pathway. Evidence-Based Complementary and Alternative Medicine: eCAM. 6 Jan. (2021) ; 2021: : 6918026. doi: 10.1155/2021/6918026. |
[25] | Han B, et al. Effect of Rhizoma Polygoni Cuspidati and Ramulus Cinnamomi compatibility on uric acid metabolism and urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in rats with hyperuricemia. Chinese Journal of Integrative Medicine. (2017) ; 23: (7): 535-542. doi: 10.1007/s11655-016-2649-0. |
[26] | Dai XJ, et al. Changes of Treg/Th17 ratio in spleen of acute gouty arthritis rat induced by MSU crystals. Inflammation. (2018) ; 41: (5): 1955-1964. doi: 10.1007/s10753-018-0839-y. |
[27] | Yan CY, et al. Celastrol ameliorates Propionibacterium acnes/LPS-induced liver damage and MSU-induced gouty arthritis via inhibiting K63 deubiquitination of NLRP3. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. (2021) ; 80: : 153398. doi: 10.1016/j.phymed.2020.153398. |
[28] | Wu J, et al. Hyperuricemia and clustering of cardiovascular risk factors in the Chinese adult population. Scientific Reports. 14 Jul. (2017) ; 7: (1): 5456. doi: 10.1038/s41598-017-05751-w. |
[29] | Han TS, et al. Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. International Journal of Obesity (2005). (2018) ; 42: (7): 1336-1344. doi: 10.1038/s41366-018-0074-5. |
[30] | Lu XH, et al. Pharmacological basis for use of madecassoside in gouty arthritis: Anti-inflammatory, anti-hyperuricemic, and NLRP3 inhibition. Immunopharmacology and Immunotoxicology. (2019) ; 41: (2): 277-284. doi: 10.1080/08923973.2019.1590721. |
[31] | Wu DS, Cao H, Yu Z, et al. Research Progress of TCM Intervention on Th17/Treg Balance in Ulcerative Colitis. Chinese Journal of Experimental Traditional Medical Formulae. (2019) ; 25: (16): 213-219. |
[32] | Zhou RY, He S, Zhu MQ, et al. Research progress on anti-infection of natural immune DNA pattern recognition receptor. Journal of China Animal Infectious Diseases. (2018) ; 26: (5): 1-9. |
[33] | Wang X, Ding D, Xue Y, Gu XF, Pang J, Zhang M, et al. Role of TLR4/NF- |
[34] | Padwal MK, et al. Comprehensive logic based analyses of Toll-like receptor 4 signal transduction pathway. PloS One. 3 Apr. (2014) ; 9: (4): e92481. doi: 10.1371/journal.pone.0092481. |
[35] | Qing YF, et al. Changes in toll-like receptor (TLR) 4-NFκB-IL1β signaling in male gout patients might be involved in the pathogenesis of primary gouty arthritis. Rheumatology International. (2014) ; 34: (2): 213-20. doi: 10.1007/s00296-013-2856-3. |
[36] | Yuan X, Fan YS, Xie GQ, et al. Based on “TLR4/NF-κB” signal pathway, the mechanism of Jiawei Simiao Pill in treating acute gouty arthritis in rats was studied. Journal of Zhejiang University of Traditional Chinese Medicine. (2017) ; 41: (1): 17-24. |
[37] | Cao YP, Zhong Q, Tang F, Yao XM, Liu Z, Zhang XD. Anethole ameliorates inflammation induced by monosodium urate in an acute gouty arthritis model via inhibiting TLRs/MyD88 pathway. Allergologia et Immunopathologia. (2022) Nov; 50: (6): 107-14. |
[38] | Liu SJ, Ding HF, Xu XC, et al. Study on serum levels of IL- 1β, IL-6, IL-18 and TNF-α in patients with acute gouty arthritis. Guide Of China Medicine. (2016) ; 14: (16): 3-5. |




