The role of splicing factor PRPF8 in breast cancer
Abstract
BACKGROUND:
Alternative splicing is a mechanism to produce different proteins with diverse functions from one gene. Many splicing factors play an important role in cancer progression. PRPF8 is a core protein component of the spliceosome complex, U4/U6-U5 tri-snRNP.
OBJECTIVE:
However, PRPF8 involved in mRNA alternative splicing are rarely included in the prognosis.
METHODS:
We found that PRPF8 was expressed in all examined cancer types. Further analyses found that PRPF8 expression was significantly different between the breast cancer and paracancerous tissues.
RESULTS:
Survival analyses showed that PRPF8-high patients had a poor prognosis, and the expression of PRPF8 is associated with distant metastasis-free survival (DMFS) and post progression survival (PPS). Gene Set Enrichment Analysis (GSEA) has revealed that PRPF8 expression is correlated with TGF-
CONCLUSIONS:
These results have revealed that PRPF8 is a significant factor for splicing in breast cancer progression.
1.Introduction
Breast cancer is a common cancer, which has a higher morbidity rate in women [1]. The clinical outcomes of breast cancer are closely linked to prognostic parameters, such as tumor size, grade, and lymph node and metastasis status. However, genes involved in mRNA alternative splicing are rarely included in the prognosis. By generating multiple mRNAs from a precursor mRNA (pre-mRNA), alternative splicing greatly diversify the genome coding capacity. Most genes are multiple-exon genes and generate more than one functional protein.
Recent studies have confirmed multiple splicing factors affect the splicing of critical breast cancer-related genes [2]. PRPF8 is a core protein component of the spliceosome complex, U4/U6-U5 tri-snRNP and contains several WD repeats, which function in protein-protein interactions. It participates in the two sequential transesterification steps of pre-mRNAs during the cut and link of pre-mRNAs. Loss of PRPF8 can lead to the death of mouse embryonic cells [3] and Drosophila cells [4]. Thus, the study aimed to investigate the significance of PRPF8 in breast cancer. Our present study, the data of Oncomine and TCGA (The Cancer Genome Atlas) were used to analyze the expression levels of PRPF8 in normal tissue and carcinomas. The clinical significance of PRPF8 in breast cancer was further explored. GSEA analysis and in vitro experiments have disclosed the possible role of PRPF8 in breast cancer.
2.Materials and methods
2.1Bioinformatic and statistical analysis
The expression of PRPF8 in different cancer types, between cancer samples and adjacent tissues, is analyzed from GEO and Oncomine database (https://www.oncomine.org/). P values less than 1
2.2Cell culture
MCF-7 and HEK-293T cells were obtained from the National Infrastructure of Cell Line Resource. MCF-7 cells were cultured in
Table 1
The shRNA sequences
| Oligo | Sequence |
|---|---|
| PRPF8-1 | TCACGTAACACATACAGGG |
| PRPF8-4 | ACAACACAAGCACAGACAG |
| Control | TTACTCTCGCCCAAGCGAG |
2.3RNA purification and quantitative reverse transcription-polymerase chain reaction
Viral packaging vectors of pMD2 (Addgene, USA) and pPAX2 (Addgene, USA) and pGIPZ (Addgene, USA) were transfected into 293T cells with Lipofectamine 2000. The shRNA sequences were listed (Table 1), for pGIPZ. After 3 days, the medium was purified with 0.45
Table 2
Primers used for quantitative PCR
| Primer | Sequence |
|---|---|
| PRPF8-qF | TGTCAGTTGCGTGTCTTCAT |
| PRPF8-qR | AGACAGTAAAACTCCCATCA |
| P21-qF | TGTCTTGTACCCTTGTGCCT |
| P21-qR | AAGATGTAGAGCGGGCCTTT |
2.4Colony formation and cell proliferation
500 MCF-7 cells per well were grown in six-well plates and maintained at 37
Figure 1.
(A) The mRNA expression of PRPF8 in different cancer cell lines. (B) The protein levels of PRPF8 are higher in breast cancer than in adjacent normal tissue. (C) PRPF8 expression in the subtypes of breast cancer.
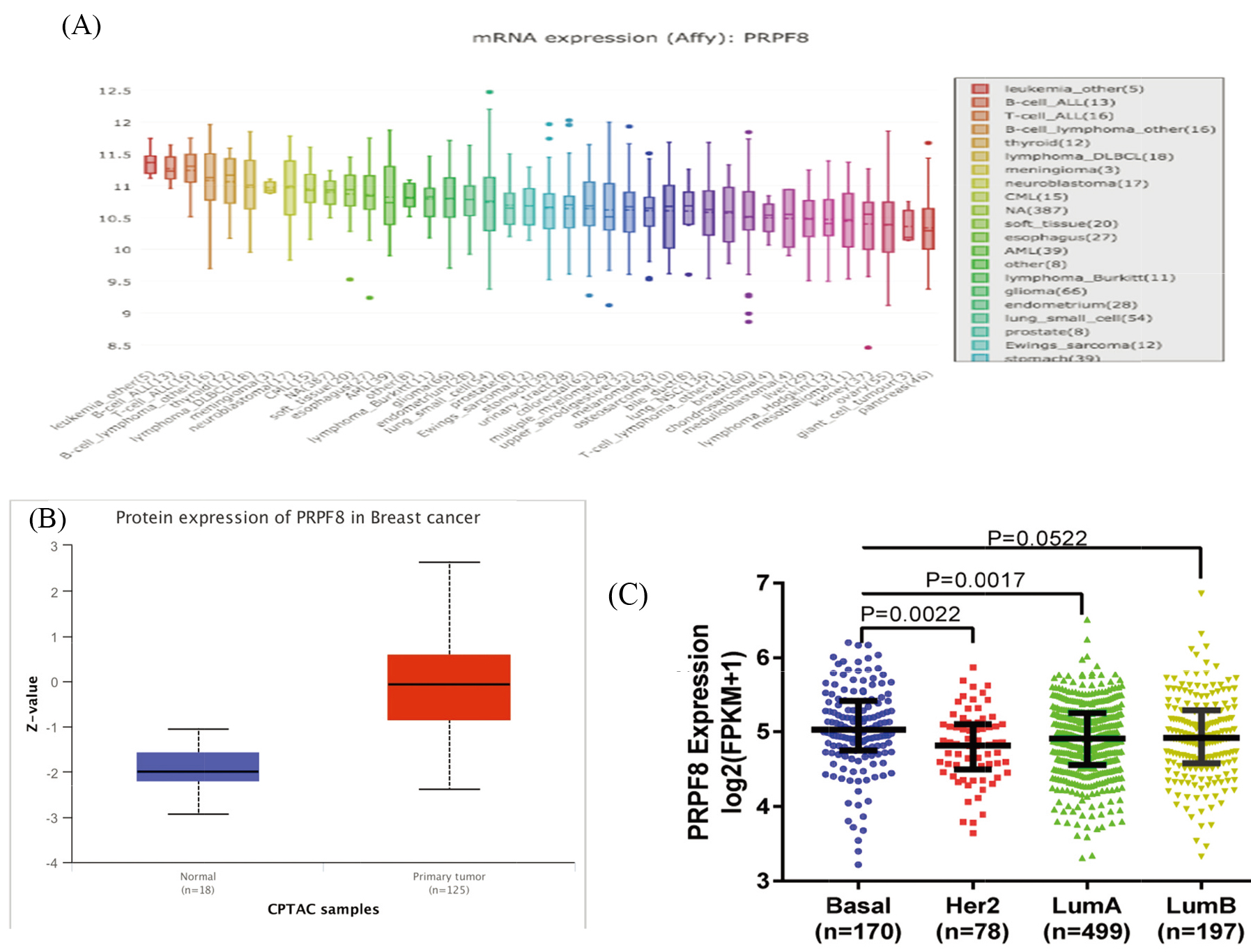
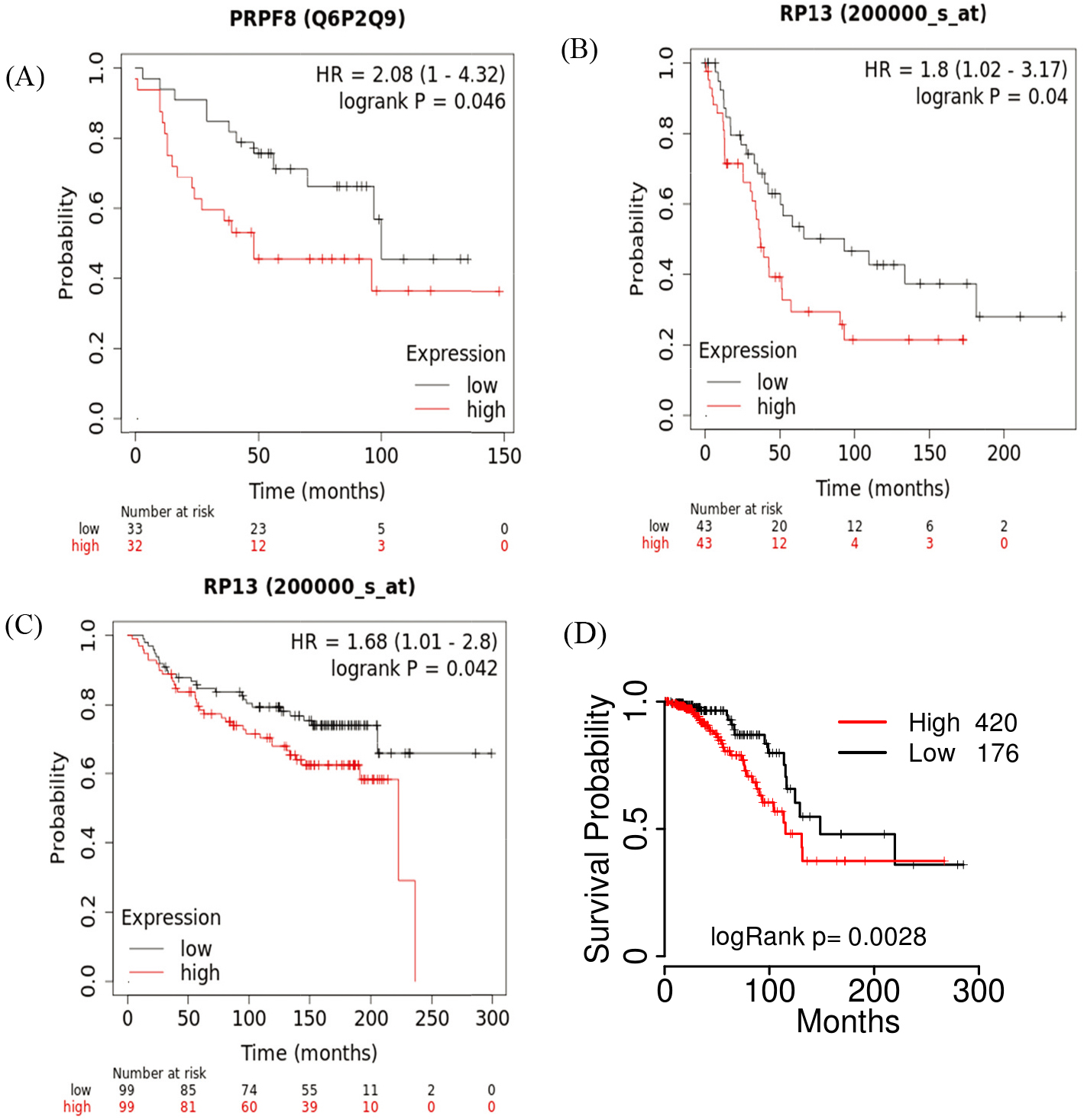
Figure 2.
(A) PRPF8 protein levels are associated with the OS of breast cancer patients in Kaplan-Meier plots. (B) The mRNA expression of PRPF8 is associated with the DMFS probabilities of breast patients. (C) The mRNA expression of PRPF8 is associated with the PPS probabilities of breast patients. (D) Prognostic analysis of PRPF8 with mRNA expression in luminal A patients.
3.Results
3.1Characterizing the expression of PRPF8 in breast cancer
There are few reports on the expression of PRPF8 in breast cancer. In this study we analyzed the expression levels of PRPF8 in various human tumors from the Oncomine database and Cancer Cell Line Encyclopedia (CCLE) (fold change of
3.2PRPF8 expression is associated with the survival of breast cancer patients
Subsequently, we investigated the association of PRPF8 expression with breast cancer patient survival. In breast cancer, the PRPF8 protein level was associated with the overall survival (OS) according to Kaplan-Meier plots database. Consistent with this result, PRPF8 mRNA expression is also associated with the OS in the Kaplan-Meier plots cohort. In the cohort of (GSE7390), we also found that PRPF8 expression was associated with the PPS (
3.3Inhibition of PRPF8 expression impaired breast cancer cell proliferation
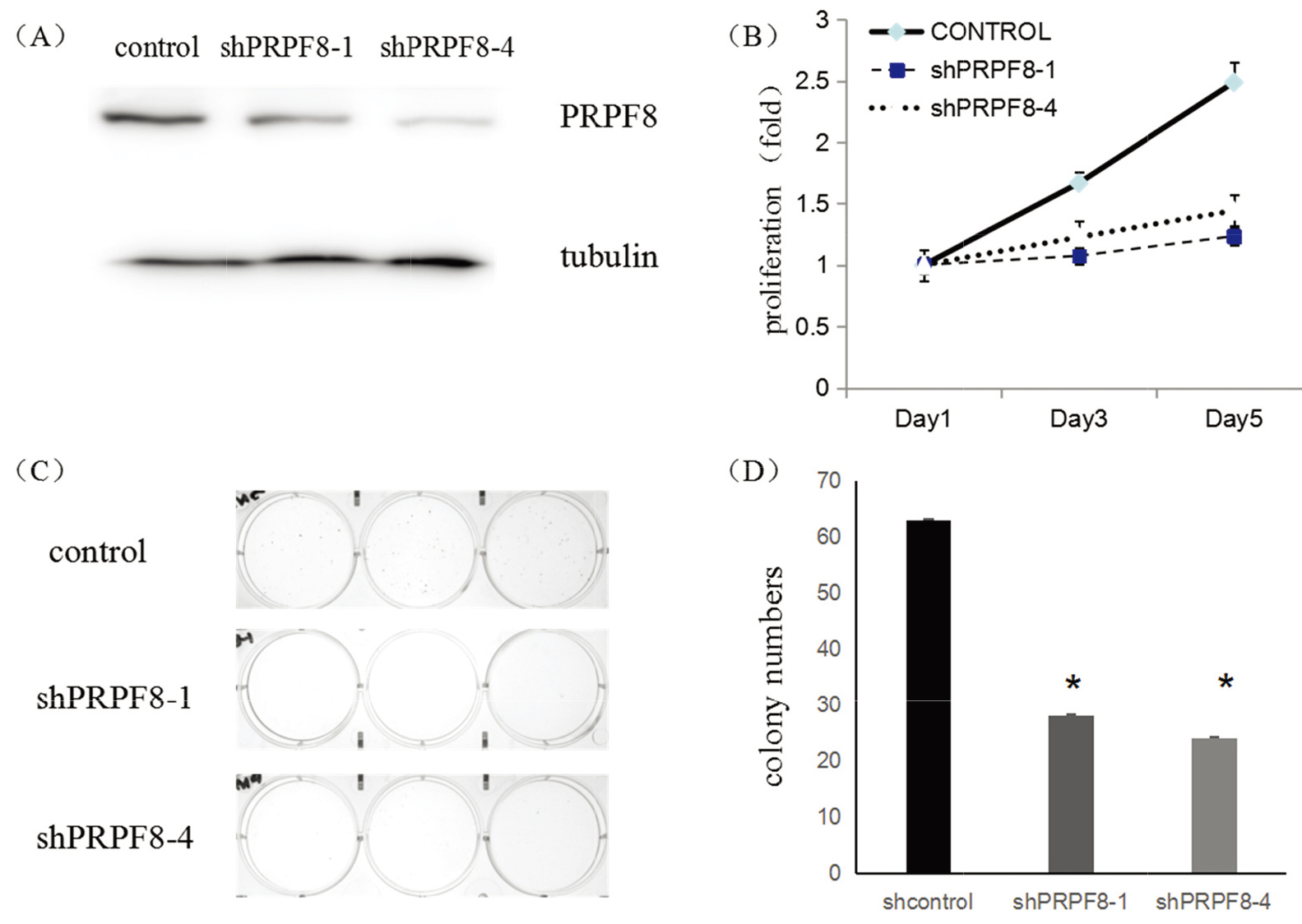
To confirm the function of the PRPF8 in breast cancer, we employed shRNAs to silence PRPF8 expression in breast cancer cells, MCF-7. Western Blot (WB) shown that both shRNAs suppressed the expression of PRPF8 (Fig. 3A). Meanwhile, inhibiting PRPF8 expression, the numbers of colonies decreased (Fig. 3C and D), and the growth curves were significantly inhibited, suggesting that PRPF8 was essential for the maintenance of the proliferation in cancer cells (Fig. 3B).
Figure 3.
(A) WB shown the inhibition of PRPF8 shRNA in MCF-7 cells. (B) MCF-7 cells growth curves by transduced with PRPF8 different shRNAs. (C) The images of MCF-7 colony formation, which were transduced with different shRNAs. (D) The numbers of colonies after MCF-7 cells were transduced with different shRNAs.
3.4PRPF8 regulates the expression of p21 in breast cancer
To further explore the functional mechanism of PRPF8 in the breast cancer cell, we used GSEA to analyze the pathways associated with PRPF8 in breast cancer. The results have shown that PRPF8 positively regulates 153 pathways and negatively regulated 31 pathways. The high expression of PRPF8 was correlated with JAK-STAT signaling pathway (ES
Figure 4.
(A) GSEA results show TGF-
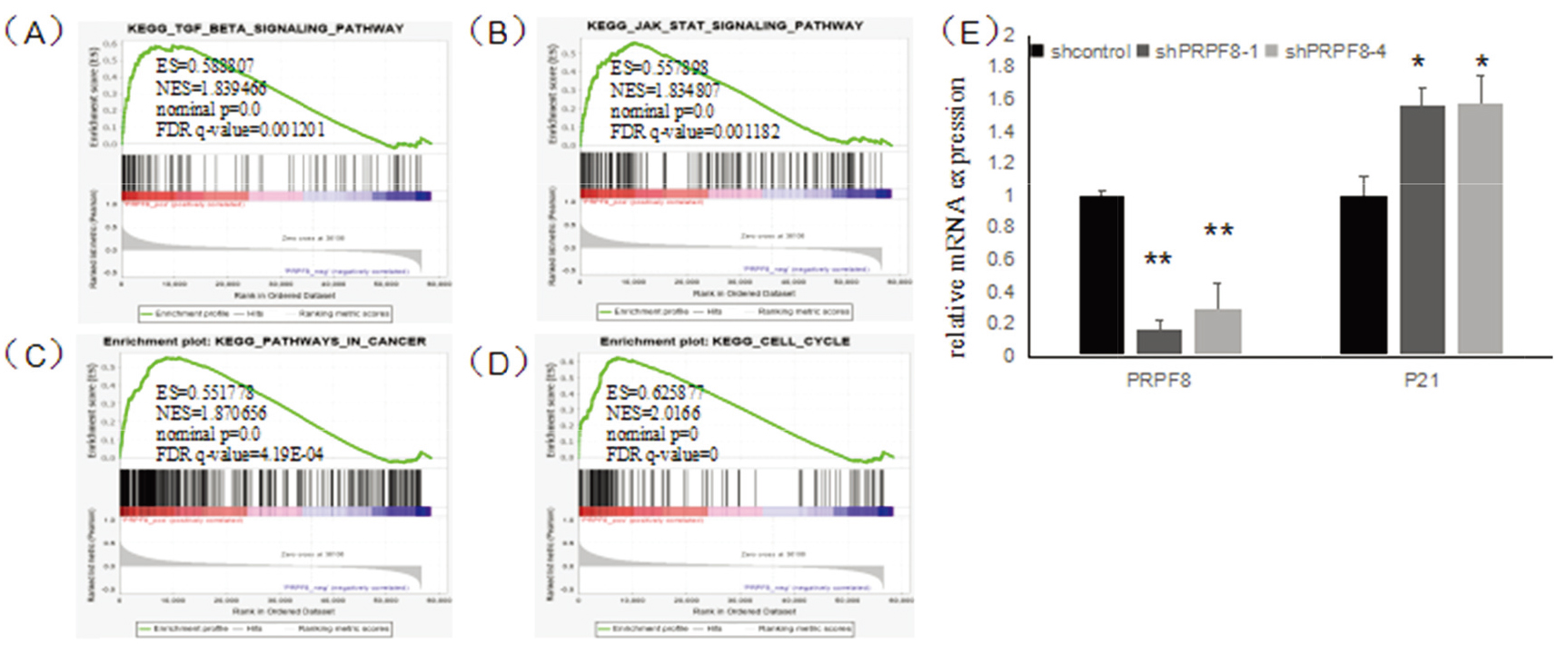
4.Discussion
In this study, we investigate the role of PRPF8 in breast cancer. PRPF8 mRNA expression is significantly elevated in breast cancer samples compared with the paracancerous tissue. PRPF8 mRNA is differentially expressed among different breast cancer molecular subtypes, and its levels were inversely correlated with the OS in breast cancer patients. Furthermore, we confirmed the role of PRPF8 in breast cancer with in vitro experiments, which have shown that silencing PRPF8 in breast cancer cells repressed cell proliferation by upregulating
Alternative splicing is one of the mechanisms to increase protein diversity [5, 6, 7, 8, 9]. Recently, with the better understanding of alternative splicing process [10, 11, 12, 13, 14, 15], it has been found that abnormal expression of splicing factors is closely related to many diseases. Many splicing factors play an important role in cancer [16, 17, 18, 19, 20, 21, 22, 23], including in breast cancer [24, 25, 26]. PRPF8 is the core component of the ribonucleoprotein (RNP) complexes in the spliceosome and participates in splice-site recognition, branch-point formation and catalysis process [27, 28, 29]. Whether PRPF8 plays a role in breast cancer is not known.
In this study, we demonstrate that PRPF8 is critical for breast cancer cell survival. Firstly, PRPF8 is elevated in breast tumors compared with the normal tissue (Fig. 1B). Second, PRPF8 was related to OS, PPS, and DMFS in breast cancer patients (Fig. 2A, B&C). More importantly, silencing of PRPF8 slowed down breast cancer cell growth and reduced the colony formation of MCF-7 cells (Fig. 3). Therefore, we found PRPF8 plays an important role in breast cancer.
5.Conclusion
Our study provides evidence that splicing factor PRPF8 is critical for breast cancer cell survival and has the potential prognostic value in breast cancer. PRPF8 may achieve its functions in breast cancer by modulating
Conflict of interest
The authors report no conflict of interest.
Funding
This work was supported by funds from the Natural Science Foundation of Heilongjiang Province (Grant Number H2017039).
References
[1] | Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates in Surg. (2017) ; 69: (3): 313-317. doi: 10.1007/s13304-017-0424-1. |
[2] | Cerasuolo A, Buonaguro L, Buonaguro FM, Tornesello ML. The Role of RNA Splicing Factors in Cancer: Regulation of Viral and Human Gene Expression in Human Papillomavirus-Related Cervical Cancer. Front Cell Dev Biol. (2020) ; 12;6: (8): e474. doi: 10.3389/fcell.2020.00474. |
[3] | Graziotto JJ, Farkas MH, Kinga B, Deramaudt BM, Zhang Q, Nandrot EF, et al. Three gene-targeted mouse models of RNA splicing factor RP show late-onset RPE and retinal degeneration. Invest Ophthalmol Vis Sci. (2011) ; 52: : 190-8. doi: 10.1167/iovs.10-5194. Print 2011 Jan. |
[4] | Rämet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RAB. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E.coli. Nature. (2002) ; 416: : 644-8. doi: 10.1038/nature735. Epub 2002 Mar 24. |
[5] | Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol. (2017) ; 18: (7): 437-451. doi: 10.1038/nrm.2017.27. Epub 2017 May 10. |
[6] | Miri DG, Regina GG, Eli E, Keren M, Rotem K, Levanon EY. Identification of recurrent regulated alternative splicing events across human solid tumors. Nucleic Acids Res. (2015) ; 43: : 5130-5144. doi: 10.1093/nar/gkv210. Epub 2015 Apr 23. |
[7] | Chen L, Tovar-Corona JM, Urrutia AO. Increased levels of noisy splicing in cancers, but not for oncogene-derived transcripts. Hum Mol Genet. (2011) ; 20: : 4422-4429. doi: 10.1093/hmg/ddr370. Epub 2011 Aug 23. |
[8] | Yoshida K, Ogawa S. Splicing factor mutations and cancer. Wiley Interdiscip Rev RNA. (2014) ; 5: (4): 445-459. doi: 10.1002/wrna.1222. Epub 2014 Feb 12. |
[9] | Biankin AV, Waddell N, Kassahn KS, Gingras MC, Gibbs RA. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. (2012) ; 491: (7424): 399-405. doi: 10.1038/nature11547. Epub 2012 Oct 24. |
[10] | Duong Nguyen TH, Galej WP, Bai XC, Savva CG, Newman AJ, Scheres SHW, et al. The architecture of the spliceosomal U4/U6. U5 tri-snRNP. Nature. (2015) ; 523: : 47-52. doi: 10.1038/nature14548. Epub 2015 Jun 24. |
[11] | Wan R, Yan C, Bai R, Wang L, Huang M, Wong CCL, Shi Y. The 3.8 A structure of the U4/U6. U5 tri-snRNP: Insights into spliceosome assembly and catalysis. Science. (2016) ; 351: : 466-475. doi: 10.1126/science. aad6466. Epub 2016 Jan 7. |
[12] | Chan S, Sridhar P, Kirchner R, Ying JL, Petrocca F. Basal-A triple negative breast cancer cells selectively rely on RNA splicing for survival. Mol Cancer Ther. (2018) ; 16: : 2849-2861. doi: 10.1158/1535-7163.MCT-17-0461. Epub 2017 Sep 6. |
[13] | Climente-González H, Porta-Pardo E, Godzik A, Eyras E. The functional impact of alternative splicing in cancer. Cell Rep. (2017) ; 20: : 2215-2226. doi: 10.1016/j.celrep.2017.08.012. |
[14] | Ner-Gaon H, Halachmi R, Savaldi-Goldstein S, Rubin E, Ophir R, Fluhr R. Intron retention is a major phenomenon in alternative splicing in Arabidopsis. Plant Journal. (2010) ; 39: (6): 877-885. doi: 10.1111/j.1365-313X.2004.02172.x. |
[15] | Teigelkamp S, Achsel T, Mundt C, Göthel SF, Lührmann R. The 20kd protein of human [U4/U6. U5] tri-snRNPs is a novel cyclophilin that forms a complex with the U4/U6-specific 60kd and 90kd proteins. RNA. (1998) ; 4: (2): 127-41. |
[16] | Dvinge H, Bradley RK. Widespread intron retention diversifies most cancer transcriptomes. Genome medicine. (2015) ; 7: : e45. doi: 10.1186/s13073-015-0168-9. eCollection 2015. |
[17] | David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. (2010) ; 24: : 2343-2364. doi: 10.1101/gad.1973010. |
[18] | CSmart A, Margolis CA, Pimentel H, He MX, Miao D, Adeegbe D, et al. Intron retention is a source of neoepitopes in cancer. Nature biotechnology. (2018) ; 8: : e16. doi: 10.1038/nbt.4239. Epub 2018 Aug 16. |
[19] | Dvinge H, Kim E, Abdel-Wahab O, Bradley RK. RNA splicing factors as oncoproteins and tumour suppressors. Nat Rev Cancer. (2016) ; 16: : 413-430. doi: 10.1038/nrc.2016.51. Epub 2016 Jun 10. |
[20] | Simon JM, Hacker KE, Singh D, Brannon AR, Parker JS, Weiser M, et al. Variation in chromatin accessibility in human kidney cancer links H3K36 methyltransferase loss with widespread RNA processing defects. Genome Res. (2014) ; 24: : 241-250. doi: 10.1101/gr.158253.113. Epub 2013 Oct 24. |
[21] | Ma PC, Kijima T, Maulik G, Fox EA, Salgia R. C-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. (2003) ; 63: : 6272-6281. |
[22] | Cancer Genome Atlas Research, N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. (2012) ; 489: : 519-525. doi: 10.1038/nature11404. Epub 2012 Sep 9. |
[23] | Cancer Genome Atlas Research, N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. (2014) ; 511: : 543-550. doi: 10.1038/nature13385. Epub 2014 Jul 9. |
[24] | Urbanski LM, Leclair N, Anczuków O. Alternative-splicing defects in cancer: splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip Rev RNA. (2018) ; 9: : e1476. doi: 10.1002/wrna.1476. Epub 2018 Apr 25. |
[25] | Venables JP, Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Koh C, et al. Identification of alternative splicing markers for breast cancer. Cancer Res. (2008) ; 68: : 9525: -9531. doi: 10.1158/0008-5472.CAN-08-1769. |
[26] | Watermann DO, Tang Y, Zur Hausen A, Jäger M, Stamm S, Stickeler E. Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer Res. (2006) ; 66: : 4774-4780. doi: 10.1158/0008-5472.CAN-04-3294. |
[27] | Grainger RJ, Beggs JD. Prp8 protein: at the heart of the spliceosome. RNA. (2005) ; 11: (5): 533-557. doi: 10.1261/rna.2220705. |
[28] | Mayerle M, Raghavan M, Ledoux S, Price A, Stepankiw N, Hadjivassiliou H, et al. Structural toggle in the RNase H domain of Prp8 helps balance splicing fidelity and catalytic efficiency. Proc Natl Acad Sci USA. (2017) ; 114: (18): 4739-4744. doi: 10.1073/pnas.1701462114. |
[29] | Kurtovic-Kozaric A, Przychodzen B, Singh J, Akonarska M, Hsi ED, Yoshida K, et al. PRPF8 defects cause missplicing in myeloid malignancies. Leukemia. (2015) ; 29: (1): 126-136. doi: 10.1038/leu.2014.144. Epub 2014 Apr 30. |
[30] | Rayala SK, Molli PR, Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs of tamoxifen sensitivity. Can Res. (2006) ; 66: (12): 5985-5988. doi: 10.1158/0008-5472.CAN-06-0978. |
[31] | Balasenthil S, Sahin AA, Barnes CJ, et al. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. The Journal of Biological Chemistry. (2004) ; 279: (2): 1422-1428. doi: 10.1074/jbc.M309937200. Epub 2003 Oct 6. |
[32] | Giles KM, Daly JM, Beveridge DJ, Thomson AM, Voon DC, Furneaux HM, et al. The 3′-untranslated region of p21WAF1 mRNA is a composite cisacting sequence bound by RNA-binding proteins from breast cancer cells, including HuR and poly (C)-binding protein. J Biol Chem. (2003) ; 278: (5): 2937-2946. doi: 10.1074/jbc.M208439200. Epub 2002 Nov 12. |
[33] | Wu BQ, Jiang Y, Zhu F, Sun DL, He XZ. Long noncoding RNA PVT1 promotes EMT and cell proliferation and migration through downregulating p21 in pancreatic Cancer cells. Technol Cancer Res Treat. (2017) ; 16: : 819-827. doi: 10.1177/1533034617700559. Epub 2017 Mar 30. |
[34] | Wang LL, Guo HH, Zhan Y, Feng CL, Huang S, Han YX, et al. Specific up-regulation of p21 by a small active RNA sequence suppresses human colorectal cancer growth. Oncotarget. (2017) ; 8: (15): 25055-25065. doi: 10.18632/oncotarget.15918. |
[35] | Shu L, Yan W, Chen X. RNPC An RNA-binding protein and a target of the p53 family, is required for maintaining the stability of the basal and stress-induced p21 transcript. Genes Dev. (2006) ; 20: (21): 2961-2972. doi: 10.1101/gad.1463306. Epub 2006 Oct 18. |
[36] | Jiang D, Wang X, Liu X, Li F. Gene delivery of cyclin dependent kinase inhibitors p21 Waf1 and p27 Kip1 suppresses proliferation of MCF-7 breast cancer cells in vitro. Breast Cancer. (2014) ; 21: : 614-623. doi: 10.1007/s12282-012-0438-y. Epub 2013 Jan 22. |
[37] | Ibnat N, Kamaruzman NI, Ashaie M, Chowdhury EH. Transfection with p21 and p53 tumor suppressor plasmids suppressed breast tumor growth in syngeneic mouse model. Gene. (2019) ; 701: : 32-40. doi: 10.1016/j.gene.2019.02.082. Epub 2019 Mar 18. |
[38] | Li CY, Suardet L, Little JB. Potential Role of WAF1/Cip1/p21 as a Mediator of TGF-β Cytoinhibitory Effect. J Biol Chem. (1995) ; 270: : 4971-4974. doi: 10.1074/jbc.270.10.4971. |
[39] | Hu W, Wang Z, Li Q, Wang J, Li L, Jiang G. Upregulation of lincRNA-p21 in thoracic aortic aneurysms is involved in the regulation of proliferation and apoptosis of vascular smooth muscle cells by activating TGF-β1 signaling pathway. J Cell Biochem. (2019) ; 120: (3): 4113-4120. doi: 10.1002/jcb.27696. Epub 2018 Oct 9. |
[40] | Diab-Assaf M, Semaan J, El-Sabban M et al. Inhibition of Proliferation and Induction of Apoptosis by Thymoquinone via Modulation of TGF Family, p53, p21 and Bcl-2α in Leukemic Cells. Anticancer Agents Med Chem. (2018) ; 18: (2): 210-215. doi: 10.2174/1871520617666170912133054. |
[41] | Li B, Mao Z, Wu Z, Wang Z. Expression of Smad4, TGF-beta and P21∼(waf1) in esophageal squamous cancer tissue and its biological significance. Oncol Lett. (2015) ; 9: (6): 2847-2853. doi: 10.3892/ol.2015.3146. Epub 2015 Apr 23. |
[42] | Tu X, Zhang Y, Zheng X, Deng J, Li H, Kang Z, et al. TGF-β induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice. Sci Rep. (2017) ; 7: (1): e2957. doi: 10.1038/s41598-017-03175-0. |
[43] | Lui AJ, Geanes ES, Ogony J, Behbod F, Marquess J, Valdez K, et al. IFITM1 suppression blocks proliferation and invasion of aromatase inhibitor-resistant breast cancer in vivo by JAK/STAT-mediated induction of p21. Cancer Lett. (2017) ; 399: : 29-43. doi: 10.1016/j.canlet.2017.04.005. Epub 2017 Apr 12. |
[44] | Bhunia AK, Piontek K, Boletta A, Liu L, Feng Q, Xu PN, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. (2002) ; 109: (2): 157-68. doi: 10.1016/s0092-8674(02)00716-x. |




