Evaluating photoneutron dose equivalents for lung cancer using PMMA phantoms undergoing 15 MV IMRT
Abstract
BACKGROUND:
According to statistics of the Ministry of Health and Welfare in 2017, the second leading cause of death in Taiwan was lung cancer.
OBJECTIVE:
Routine treatment planning does not consider photoneutron dose equivalent (PNDE) of patient induced secondary radiation resulting from primary exposure of lung cancer. However, such treatment is potentially important for improving estimates of health risks.
METHODS:
This study used 10, 30, 50, 70, and 90 kg of polymethylmethacrylate (PMMA) phantoms as patient to measure PNDE varying anatomical area during lung cancer of intensity modulated radiotherapy (IMRT) treatment. Paired thermoluminescent dosimeters (TLD-600 and 700) were calibrated using university reactor neutrons. TLDs were inserted into phantom which was closely corresponded of the represented tissues or organs.
RESULTS:
Neutron doses (ND) of organ or tissue (
CONCLUSION:
The estimated
1.Introduction
Medical electron linear accelerators (linac) operating above 10 MV produce high-energy bremsstrahlung photon, then induce neutrons via (x, yn) reactions [1, 2, 3]. These reactions occur at the accelerator target (tungsten, iron), flattening filters, shielding materials, collimator jaws (inside the linac head), and in the patient body [1, 2, 3, 4]. The linac treatment room contains a mixed radiation field produced by the induced neutrons and photons. The medical linac, Siemens Primus (Siemens AG, Munich, Germany) at the Radiation Oncology Department of Changhua Christian Hospital (CCH) which could provide energies that accelerated 10 and 15 MV voltages. The linac is designed of intensity modulated radiotherapy (IMRT). The fast neutron flux (
2.Materials and methods
2.1Polymethylmethacrylate (PMMA) phantom measurement
Lung treatment plans of IMRT have been developed, delivered to anthropomorphic Rando phantom. This was made of Radiology Support Devices, Long Beach, Canada. Treatment planes of these phantoms were reviewed by professional medical doctor (Mu-Tai Liu, MD) and senior radiotherapist (Sing-Sheng Huang) [7, 8, 9, 10, 11]. To calculate the PNDE associated with IMRT treatment, this study conducted paired TLDs measurement in Rando and PMMA phantoms. The Rando phantom was 175 cm and 73.5 kg. This phantom was comprised 35 axial segments, those contained simulated human skeleton as well as lung soft tissue. Rando is suitable for IMRT radiation measurements [4]. The phantom was sliced at 2.5 cm intervals and holes were drilled in each slice for TLD-600 insertions. Paired TLDs were loaded into each slice of phantoms as well as can use to directly assess PNDE and secondary radiation to phantoms.
This study developed five phantoms using anthropometric skeletons made of PMMA and epoxy-resin to simulate average body-weights of 90, 70, 50, 30 and 10 kg [12]. The PMMA phantom was designed as published [9]. PMMA and epoxy-resin were chosen as the neutron and photon transport properties. Density were formulated as follows: skeleton-cortical-bone, 1.486; skin, 1.105; and lung-tissue 0.296 g/cm
Table 1
Rando, polymethylmethacrylate (PMMA) phantoms’ dimension and physical properties
| Phantom | Rando | Polymethylmethacrylate (PMMA) | ||||
|---|---|---|---|---|---|---|
| Weight (kg) | 70 | 10 | 30 | 50 | 70 | 90 |
| Height (cm) | 94.5 | 50 | 78 | 84 | 93 | 112 |
| Weight (kg) | 34.5 | 6.75 | 19.0 | 31.5 | 44.1 | 57 |
| Slices | 35 | 31 | 31 | 31 | 31 | 31 |
| cm/slices | 2.5 | 1.6 | 2.3 | 2.7 | 3.0 | 3.6 |
2.2Paired TLDs
For subtracting the signal (SN) contributed by photons from total SNs of TLD-600, and 700, all neutron measurements were performed using paired TLDs approach composed with a batch of 88 high sensitivity 3.2
2.3Calibrated paired TLDs
The ND calibrations were performed at the boron neutron capture therapy (BNCT) beams of National Tsing Hua University (NTHU). Using BNCT beams are 3.10
(1)
(2)
Where
(3)
2.4Computed tomography (CT) scans and treatment planning for lung cancer
Before each treatment, numerous measurements were conducted using inhomogeneous PMMA phantoms. This study obtained archival Computed tomography (CT) scan images of the phantoms at CCH. A 3 cm
Figure 1.
Use polymethylmethacrylate (PMMA) phantoms as patient surrogates (a) outer appearances (b) from 9th to 20th scout view of 70 kg (c) 15th CT slice of 70 kg phantom.
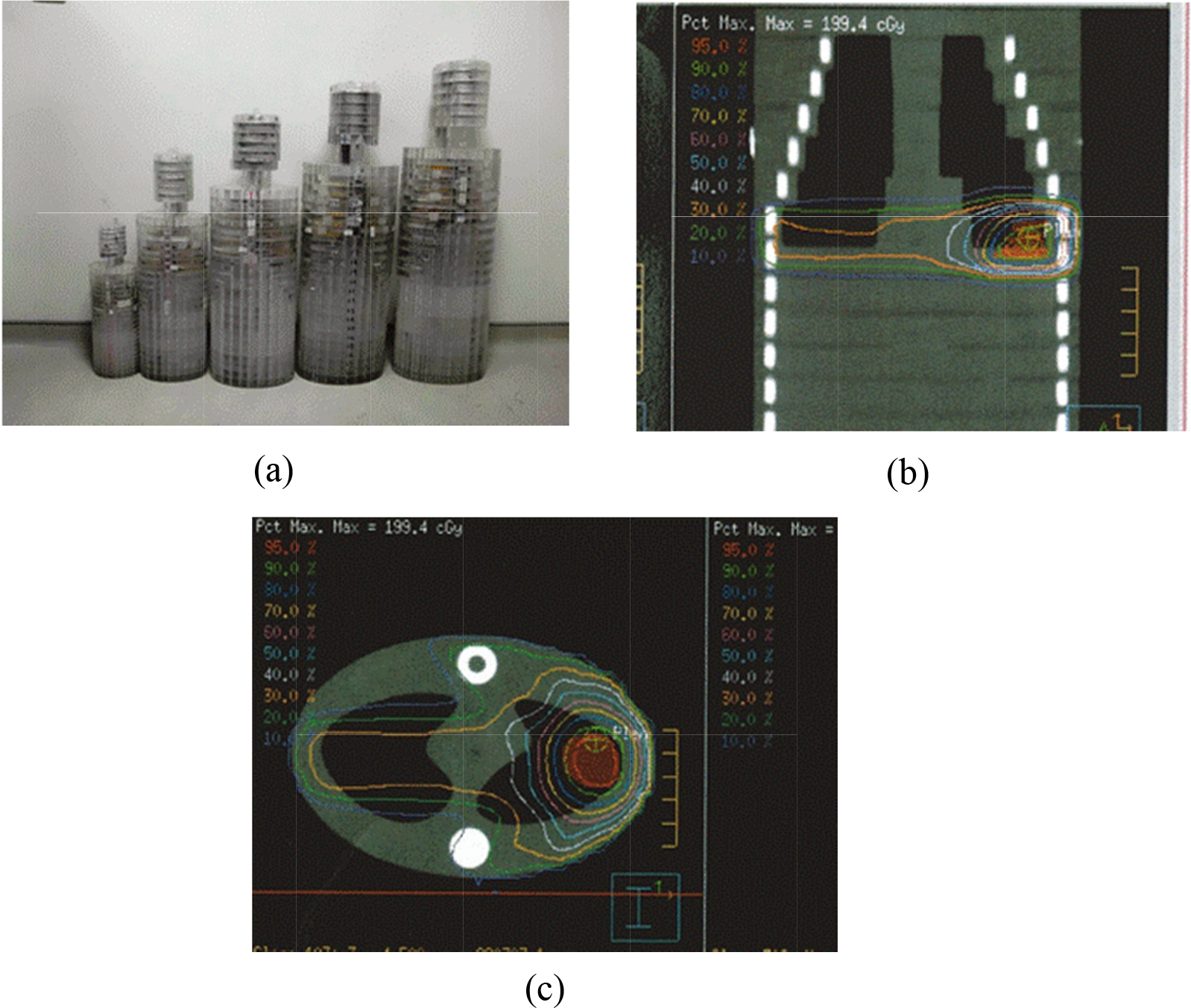
2.5Paired TLDs inserted into Rando and PMMA phantoms
Calculation points are located in the lung and tumor center in the superior portion of the torso of these phantoms of radiotherapy 15 MV linac during lung cancer treatment [1]. Forty-four groups, each containing 2 TLD-600 and 700s, were inserted into phantoms at the positions of slices 3–35 (Rando) and 3–31 (PMMA), respectively. For all measuring locations of NDs, two TLD-600s were irradiated together as well as average signal determined. Four group TLDs were attached to 15
2.6Evaluation of the PNDE
PNDE were determined for 16 critical organs and remainders recommended by ICRP 103 as published [12]. In addition, prostate of male and uterus of female selected as at particular risk of fatal secondary malignancy recommended by ICRP 103 [11, 13]. Table 2 lists the positions of 44 groups imparted within these phantoms and weighting factors (
Table 2
44 ND measurement points, and the weighting factors
| Organ | Measurement points | ICRP 103 | Practical evaluation |
|---|---|---|---|
| Brain | Brain | 0.01 | 0.01 |
| Salivary | Salivary | 0.01 | 0.01 |
| Thyroid | Thyroid | 0.04 | 0.04 |
| Red bone marrow | 0.12 | ||
| Clavicle vertebrae | 0.04 | ||
| Thoracic vertebra | 0.04 | ||
| Thighbone femur | 0.04 | ||
| Lung | Lung | 0.12 | 0.12 |
| Skin | Skin | 0.01 | 0.01 |
| Esophagus | Esophagus | 0.04 | 0.04 |
| Breast | Breast | 0.12 | 0.12 |
| Bone surface | 0.01 | ||
| Clavicle vertebrae | 0.025 | ||
| Thoracic vertebra | 0.025 | ||
| Thighbone | 0.025 | ||
| Rib | 0.025 | ||
| Liver | Liver | 0.04 | 0.04 |
| Colon | 0.12 | ||
| Ascending | 0.03 | ||
| Transverse | 0.03 | ||
| Descending | 0.03 | ||
| Sigmoid flexure | 0.03 | ||
| Stomach | Stomach | 0.12 | 0.12 |
| Bladder | Bladder | 0.04 | 0.04 |
| Gonads | Testes | 0.08 | 0.08 |
| Remainder | 0.12 | ||
| Lens | 0.017 | ||
| Herat | 0.017 | ||
| Pancreas | 0.017 | ||
| Spleen | 0.017 | ||
| Kidney | 0.017 | ||
| Small intestine | 0.017 | ||
| Prostate (male)/uterus (female) | 0.017 | ||
| Total | 1.00 | 1.000 | |
2.7Evaluating N D T
The PNDE was obtained for the position of chest PA. Meanwhile, PNDE denotes the total weighted equivalent doses of 16 organs and the “remainder” recommended by ICRP 103 as listed in Table 2, expressed as follows:
(4)
(5)
Where
3.Results
3.1Calibration of TLD-600
The response linearity of photon dose for paired TLDs was tested ranging from
3.2Photoneutron dose equivalent (PNDE)
Figure 2.
Estimate of photoneutron dose equivalent (PNDE) and skin dose per Gy (mSv/Gy) undergoing lung cancer of 15 MV IMRT treatments.
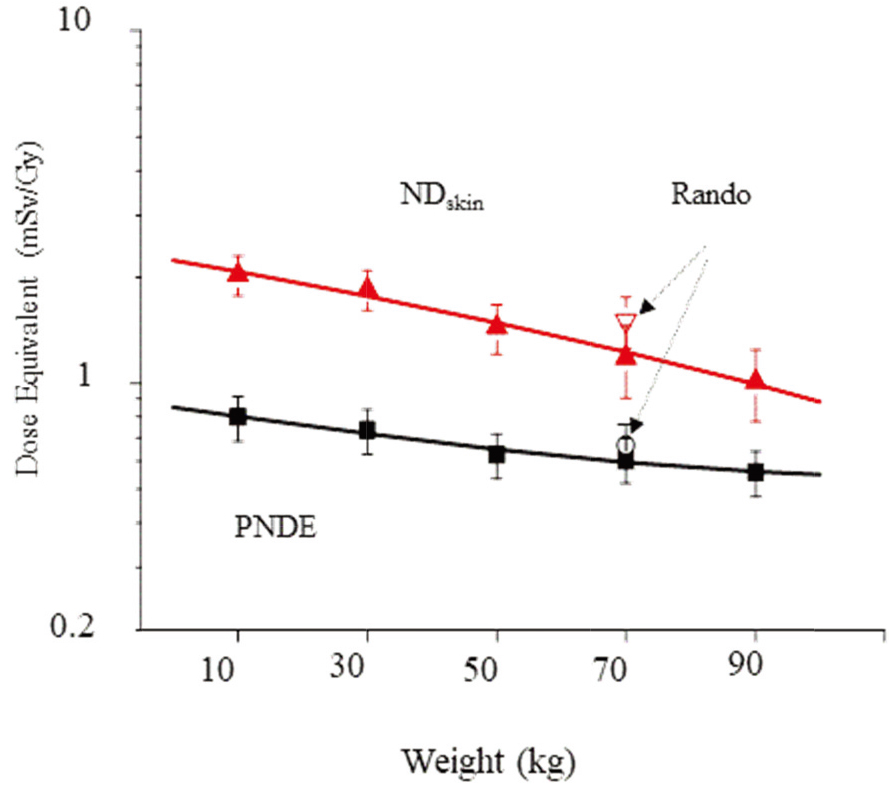
Specifically, no standardized whole-body phantoms exist for evaluating the PNDE of different weights. Thus, this investigation developed five phantoms to simulate different body-weights, from 10 to 90 kg. As illustrated in Fig. 2, PNDE at the different weights decrease abruptly with body-weight. Meanwhile, the maximum
(6)
(7)
Where PNDE and
4.Discussions
4.1Estimates of the secondary N D T
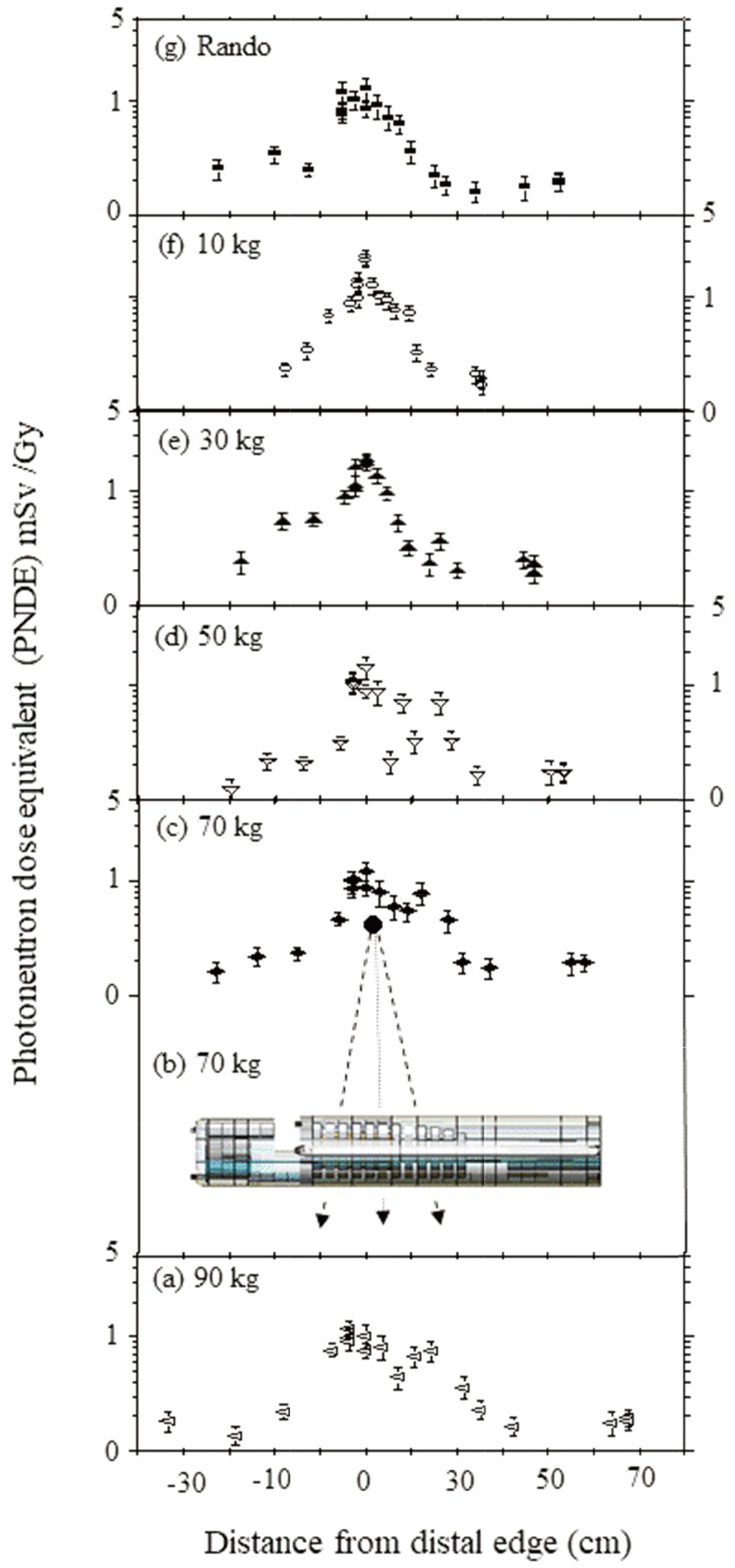
Most neutrons those originate in the gentry head, then move from the tumor center to the patients lens and gonad during IMRT. Due to the ND of the 44 measurement (Table 2), estimates were evaluated that reflected the ND to anywhere in bodies of different body-weights for lung cancer treatment. Figure 3a–g present the estimates of the total
Measured
4.2Comparison with other experimental data
This is the first to use different body-weight of PMMA to evaluate PNDE herein, and compared the results presented here under lung cancer treatment with organ absorbed ND investigated with bubble detector in water used for 15 MV x-ray linac-based radiotherapy of Siemens Primus, CCH under a 10
Table 3
PNDE (mSv/Gy) and estimated risk (%) of secondary cancer coming from a total 72 Gy for Rando and PMMA phantoms
| Phantom (kg) | PNDE (mSv/Gy) | Risk (%) |
|---|---|---|
| Rando | 0.67 | 2.85 |
| 10 | 0.80 | 8.35 |
| 30 | 0.74 | 7.73 |
| 50 | 0.63 | 2.68 |
| 70 | 0.61 | 2.59 |
| 90 | 0.56 | 2.38 |
Figure 3.
Secondary ND at distal edge of lung on the central axis varying distances during IMRT treatment with 15 MV (a) 90 kg, (b) 70 kg irradiation, (c) 70 kg, (d) 50 kg, (e) 30 kg, (f) 10 kg, (g) Rando phantoms.
4.3Risk of secondary malignancies
The risk coming from neutron-induced malignancies is evaluated based on the differences between in the effective dose of the PMMA and Rando phantoms calculated by the BEIR VII [14]. BEIR VII stated that radiotherapy patients’ risk should be approximately 5.9% per Sv for lung cancer patients given a large population spanning all ages and 14.5% per Sv for 10 year old male [4, 14]. The prescription used for phantoms was 72 Gy of the PTV during IMRT. Comparing calculated PNDE of five PMMA phantoms, Table 3 demonstrates that the risk also increases with decreasing body-weight, as well as with decreasing PNDE. This study is in good agreement with Kry et al. stated the risk following prostate radiotherapy was 2–5% for all linac [15].
5.Conclusion
This feasibility study involves the evaluated PNDE of weight-dose relationship for each patient in the lung cancer treatment based on photoneutron contamination using the paired TLDs approach. The highest
High
Acknowledgments
The authors would like to thank senior radiotherapist Sing-Sheng Huang for many fruitful discussions, and radiotherapists of CCH and the staff of NTHU for their efficient support.
Conflict of interest
None to report.
References
[1] | Hashemi SM, Hashemi-Malayeri B, Raisali G, et al. Measurement of photoneutron dose produced by wedge filters of a high energy linac using polycarbonate films. Journal of Radiation Research. (2008) ; 49: (3): 279-283. |
[2] | Chao JH, Liu WS, Chen CY. Estimation of 41Ar concentrations in the vicinity of a high-energy medical accelerator. Radiatation Measurments. (2007) ; 42: : 1538-1544. |
[3] | National Council on Radiation Protection and Measurements, ((1984) )Neutron contamination from medical accelerators, NCRP-79. Washington, DC |
[4] | Harrison RM. Introduction to dosimetry and risk estimation of second cancer induction following radiotherapy. Radiation Measurements. (2013) ; 57: : 1-8. |
[5] | Lin JP, Chua TC, Lin SY, et al. The measurement of photoneutrons in the vicinity of a Siemens Primus linear accelerator. Applied Radiation and Isotopes. (2001) ; 55: : 315-321. |
[6] | Lin JP, Liu WC, Lin CC, Investigation of photoneutron dose equivalent from high-energy photons in radiotherapy. Applied Radiation and Isotopes. (2007) ; 65: : 599-604. |
[7] | International Commission on Radiological Protection. ((1991) )Recommendation of the ICRP, ICRP Publication 60, Annals of the ICRP, No. 1–3; Pergamon Press, Oxford, UK |
[8] | Ministry of Health and Welfare, ((2019) )Statistics of General Health Welfare of 2019, available at https://dep.mohw.gov.tw/ DOS/mp-113.html |
[9] | Hsu FY, Chiu MC, Chang YL, et al. Estimation of photon and neutron dose distributions in the THOR BNCT treatment room using dual TLD method. Radiation Measurements. (2008) ; 43: (2-6): 1089-1094. |
[10] | International Commission on Radiation Units and Measurement, ((1992) )Phantoms and computational models in therapy, diagnosis and protection ICRU-48. Bethesda |
[11] | Matsumoto T. Monte Carlo simulation of depth-dose distribution in several organic models for boron neutron capture therapy. Nuclear Instruments and Methods in Physics Research, A. (2007) ; 508: : 552-557. |
[12] | Lin HC, Lai TJ, Tseng HC, et al. Radiation doses with various body weights of phantom in brain 128-slice MDCT Examination. J Radiation Research. (2019) ; 60: (4): 466-475. |
[13] | International Commission on Radiological Protection, ((2007) )Recommendations of the International Commission on Radiological Protection, ICRP-103, Pergamon Press, Oxford |
[14] | Health Risks from Exposure to Low Levels of Ionizing Radiation, ((2006) ) BEIR VII-Phase 2. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. National Research Council The National Academies Press, Washington DC. |
[15] | Kry SF, Salehpour M, Followill DS, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. International Journal of Radiation Oncology Biology Physics. (2005) ; 62: (4): 1195-1203. |




