Lumbar spinal stenosis – surgical outcome and the odds of revision-surgery: Is it all due to the surgeon?
Abstract
BACKGROUND:
Surgical decompression is the intervention of choice for lumbar spinal stenosis (LSS) when non-operative treatment has failed. Apart from acute complications such as hematoma and infections, same-level recurrent lumbar stenosis and adjacent-segment disease (ASD) are factors that can occur after index lumbar spine surgery.
OBJECTIVE:
The aim of this retrospective case series was to evaluate the outcome of surgery and the odds of necessary revisions.
METHODS:
Patients who had undergone either decompressive lumbar laminotomy or laminotomy and spinal fusion due to lumbar spinal stenosis (LSS) between 2000 and 2011 were included in this analysis. Demographic, perioperative and radiographic data were collected. Clinical outcome was evaluated using numeric rating scale (NRS), the symptom subscale of the adapted version of the german Spinal Stenosis Measure (SSM) and patient-sreported ability to walk.
RESULTS:
Within the LSS- cohort of 438 patients, 338 patients underwent decompression surgery only, while instrumentation in addition to decompression was performed in 100 cases (22.3%). 38 patients had prior spinal operations (decompression, disc herniation, fusion) either at our hospital or elsewhere. Thirty-five intraoperative complications were documented with dural tear with CSF leak being the most common (33/35; 94.3%). Postoperative complications were defined as complications that needed surgery and differentiated between immediate postoperative complications (
CONCLUSIONS:
While looking for predictors of revision surgery due to re-stenosis, instability or same/adjacent segment disease none of these were found. Within our cohort no significant differences concerning demographic, peri-operative and radiographic data of patients with or without revision wer noted. Patients, who needed revision surgery were older but slightly healthier while more likely to be male and smoking. Surprisingly, significant differences were noted regarding the distribution of intraoperative and early postoperative complications among the 6 main surgeons while these weren’t obious within the intial index group of late revisions.
1.Introduction
Neurogenic claudication (NC) due to symptomatic lumbar spinal stenosis (LSS) is a painful condition causing significant functional disability. While the cause of LSS is multifactorial, thickened ligamentum flavum (LF) accounts for up to 85% of spinal canal narrowing [1]. Any factor leading to additional narrowing of the spinal canal, such as axial loading and disc bulging, translational instability under loading conditions and functional hyperlordosis will typically worsen clinical symptoms [2]. At the same time, these dynamic phenomena are often not accurately reflected in supine cross-sectional imaging [3]. Current management options for LSS include medications, physical therapy, epidural injections, alternative medicine, and surgery. While surgical techniques already vary to a large extent [4, 5, 6, 7], unfortunately, no clear consensus has been reached to determine which patients are most likely to benefit from a concomitant lumbar fusion. On the one hand patient satisfaction following lumbar decompression alone ranges from 59 to 96% [8], on the other hand reoperation rates of up to 25% within the first 5 years are reported. The relief of leg symptoms by surgical decompression for lumbar stenosis is well supported by the literature, while the effect on back pain is more controversial. Some studies support the theory that back pain relief should not be an expected outcome of decompression and that substantial back pain may be a contraindication to decompression only [9]. Therefore, stabilization may be recommended for patients with substantial preoperative back pain even in the absence of well-accepted indications for stabilization such as spondylolisthesis, severe deformity, or sagittal malalignment [10]. Asking patients about their expectations of surgical outcome after decompression surgery the most common answers are pain relief and improved ability to walk [11, 12]. While perioperative complications such as CSF leaks or hematoma and infections might be related to surgical performance surgical failures have different components. Early surgical failures might result from insufficient decompression and preoperative lumbar instability [4, 13] with late recurrence of back or leg problems due to acquired spinal instability or adjacent level degeneration [4, 13, 14, 15, 16, 17, 18, 19, 20]. Mostly, revision surgery is required at the index or at adjacent levels. The rates for reoperation after decompression without fusion for LSS are rather consistently reported to be around 8–10% 2 to 4 years following surgery [21, 22]. Kim et al. reported a reoperation rate of 5.88% in a case series of 983 patients after decompression and fusion [23]. The metaanalysis by Goel et al. with 29000 patients reviewed fenestration surgeries and showed an average reoperation rate of 7.58%
While recent studies were able to identify particular factors, such as obesity, smoking, or fusion as decisive for the outcome of lumbar operations, the literature has not demonstrated yet whether these factors influence reoperation [17, 25, 26].
The goal of this study was to evaluate whether factors can be evaluated to predict revision surgery.
2.Methods
2.1Patient population
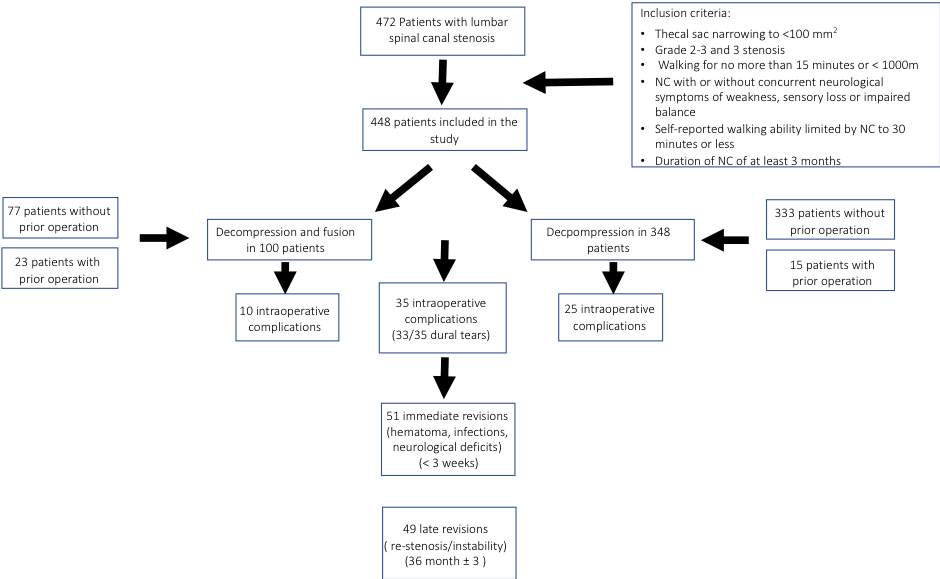
Between January 1, 2000 and December 31, 2011, 472 consecutive patients at our institution underwent spinal surgery due to symptomatic spinal canal stenosis and failure of conservative care. These patients form the basis for this retrospective study on prospectively collected data. Data extraction began after institutional approval for this study was obtained. As for demographic data, sex, age, BMI, comorbidities, smoking and history of osteoporosis were recorded. Operative and anesthesia reports as well as hospital charts were reviewed to add data on number and levels of surgery, fusion or decompression, main surgeon as well as complications, pre- and postoperative NRS (numeral rating scale), the symptom subscale of the adapted german SSM, claudication and self-reported ability to walk (in m). Patients who were able to walk for more than 30 minutes without any symptoms, who had critical stenosis of the aorta or disabling osteoarthritis in the knee or hip joint were excluded. In the end 448 patients were included in the study (Fig. 1).
Figure 1.
Inclusion criteria.
2.2Radiological evaluation
Most patients underwent MR imaging of the lumbar spine prior to indication to estimate the extent of spinal stenosis. However, we also saw patients, that were unable to undergo MRI because of claustrophobia, cardiac pacemakers, neuro-modulators or where the initial MR imaging procedure in a horizontal position did not reveal the full extent of compression. Therefore, in these cases we performed lumbar myelography which was completed by a postmyelography CT scan. Additionally, plain anteroposterior and lateral radiographs of the lumbar spine and in some cases CT scans were obtained prior to surgery to exclude congenital disorders. The degree of stenosis was defined as Grade 1 for 1/3 reduction of canal area, Grade 2 for 1/3 to 2/3 reduction of canal area, and Grade 3 for greater than 2/3 reduction of canal area [27].
2.3Indications for surgery
An indication for surgery was made in adult patients with radiologically confirmed spinal canal narrowing and neurogenic claudication. Included were patients with 1) thecal sac narrowing to less than 75 mm
Either decompressive lumbar laminotomy or laminotomy and spinal fusion was performed The decision for decompression only or decompression with fusion was made after thorough evaluation of patient’s symptoms (dominant back- vs. leg-pain), imaging (degenerative disease) and discussion with the patient.
3.Results
3.1Patient demographics
Four hundred forty-eight consecutive patients, two hundred-seventees males and two hundred thirty-one females, with lumbar spinal stenosis (LSS) who had undergone decompression surgery or decompression and spinal fusion were included in our study. 38 patients had prior spinal operations without fusion (decompression, disc herniation) either at our hospital or elsewhere. The median age at surgery was 69.9 years (range 34–89 years), while more women were operated on.
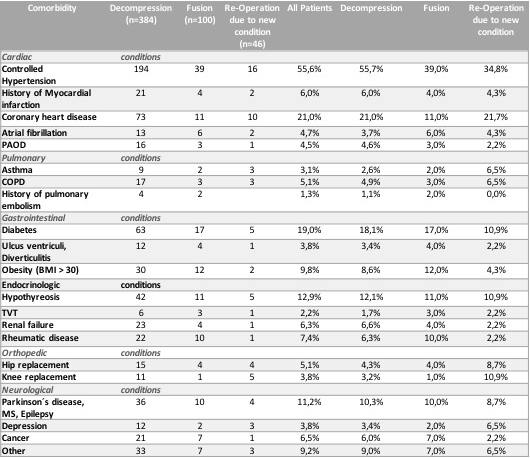
Regarding comorbidities hypertension, heart disease (heart failure, coronary heart disease) and diabetes were the most frequent followed by hypothyroidism and neurological diseases (Table 1). Almost every patient (418/438; 95%) presented with at least one comorbidity and there was a mean of 2 comorbidities per patient (range 0–8). A history of cigarette smoking was present in 12.6% of patients.
Table 1
Comorbidities
|
3.2Surgical details
Within the cohort, three hundred forty-eight patients (77.5%) received decompression only, while fusion in addition to decompression was performed in 100 cases (22,5%) (Fig. 2).
Figure 2.
Distribution decompression/fusion.
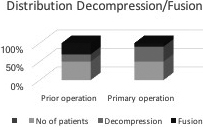
A significant difference was seen in the distribution of decompression/fusion in between the patients with primary operation as 80% (333) received decompression only with just 39% [15] of patients in the group that had prior operation. Chi
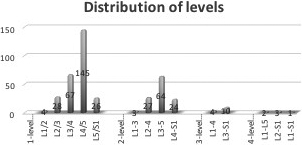
1-, 2-, 3-, 4-and 5-level surgeries were performed in 274 patients (61.0%), 130 (29.0%), 38 (8.5%), 5 (1.1%) and 2 (0.4%), respectively with a total of 686 levels treated. The most common level of surgery was L4–5 (33.0%) followed by L3–4 (16.0%), L3-5 (14.7%) and L4–S1 (6.3%) (Fig. 3).
Figure 3.
Distribution of treated levels.
The median estimated blood loss for 1-, 2-, and 3-level decompression surgeries was 50, 100, and 200 ml, respectively with highest blood loss of 2 l in a 4-level and 2.5 l in the 5 level revision surgery with fusion. In the 2 cases of severe bleeding, one operation had to be suspended.
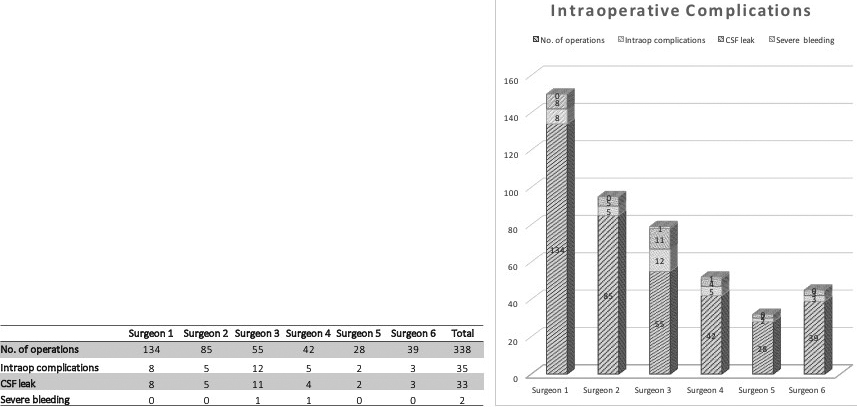
Eighty-seven percent (383/438) of the operations were performed by 6 surgeons, who were the leading spine surgeons of the Orthopedic Department over the last 10 years (range 28–134 procedures) with the remaining 13 percent (63/448) distributed between 3 more experienced (more than 15 procedures) and 5 less experienced (
Thirty-five intraoperative complications were documented with dural tear with CSF leak being the most common (33/35; 94,3%). These were far more common during decompression sugery than fusion (11/24). All dural tears were repaired intraoperatively. 4 patients needed further surgery due to complications of the dural tear, such as persistent csf leak or imminent infection.
A significant difference was seen in the distribution of intraoperative complications within the group of main-surgeons with 2 surgeons having significantly more complications. Chi
Figure 4.
Relation of intraoperative complications to main-surgeon.
Postoperative complications were defined as complications that needed further surgery and differentiated between immediate postoperative complications (
Within all patients 45 revisions (10,0%) were classified as immediate complications of the index operation with infections, neurological deficits and hematoma being the most common. Within this group only 17 patients had fusion surgery in the first place, while 28 were treated by decompression only (Fig. 5).
Figure 5.
Immediate postop complications.
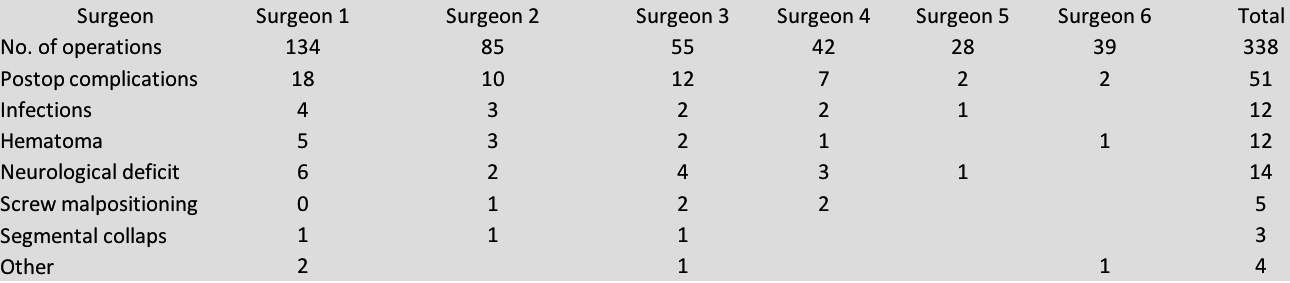
Here again, we found a significant difference between surgeons.
Revision surgery at a later date was indicated by 53 patients due adjacent level spinal stenosis, adjacent level spinal stenosis plus instability and stand-alone instability (11.8%). While 4 patients decided against surgery, 49 revision surgeries were planned. 28 were performed at the same level, 10 at the same level plus an adjacent level, and 10 were executed at index level with indications of. Therefore, the incidence of reoperation at the index level is 6.4%, while adjacent level reoperation accounted for 2.2%. Interestingly risk of re-operation seemed to be higher in male patients, but no other risk-factors were seen within our study.
The median time to revision was 36 month
No significant differences (
3.3Clinical outcome
The mean preoperative backpain according NRS was 6/10
3.4Evaluation of revision cases
All revision operations due to new conditions were evaluated by plain radiographs and/or flexion/extension films in addition to MRIs to assess potential instability caused by previous decompression. In the 8 patients diagnosed with recurrent index level spinal stenosis/adjacent level spinal stenosis plus instability the radiographs showed mild spondylolisthesis (grade I) without any signs of excessive facetectomy. None of the patients with preoperative spondylolisthesis demonstrated any increased slippage.
4.Discussion
Spinal stenosis with and without associated degenerative spondylolisthesis is a common problem, and treatment choices vary from multiple non-operative modalities to surgical interventions of variable extent. With fusion techniques becoming more and more common for spinal stenosis within the last couple of years, various studies tried to evaluate reasoning in treatment choices [8, 21, 29, 30, 31, 32, 33, 34]. These studies demonstrated benefit from surgical intervention initially but a steady decline with time and re-operation rates varying between 5 and 23% [22, 35, 36, 37, 38]. Simultaneously repeated surgery for the treatment of lumbar stenosis is associated, on the average, with lower effectiveness and greater complications as compared with initial surgery [39, 40]. This is supported by our study as patients with prior operations and patients with revision surgeries were more likely to need fusion. The presence of degenerative spondylolisthesis has often been considered to be a sign of instability, although definition of that term is still in dispute. Some studies have suggested that there may be a risk of iatrogenic slip or an increased degree of spondylolisthesis after decompression surgery in patients with degenerative spondylolisthesis or even without due to compromising segmental spinal stability by the very nature of the surgical approach, with the likelihood of excessive motion augmented when wider decompressions are performed, greater ligamentous disruption occurs, or multiple levels are included [4, 41, 42]. Indeed, postlamino- or laminectomy instability is one of the most common indications for reoperation following decompression as it was in our study. Minimally invasive surgery (MIS) techniques support, among other advantages, are supposed to preserve the posterior osseoligamentous structures, and may therefore minimize destabilization while achieving adequate decompression of the neural elements [16, 43, 44, 45, 46, 47]. In our cohort only 3 patients in the revision group developed mild progressive spondylolisthesis within 2 to 4 years after the index surgery, which is a lot lesser than described in many other studies [41].
Within the group of intraoperative complications we saw a significant difference in the incidence of incidential durotomy within the main surgeon group. With occurrence of dural tears in 7.3% (33/448) of all procedures, we did not see more CSF leaks than other groups, but a more heterogenous distribution [43, 48, 49]. While the literature reports up to 10% of incidential durotomies during decompression surgery, many multi-center studies report homogenous rates associated with age, BMI, previous surgery at the same spinal level, minimally/less invasive surgery and laminectomy [50]. Even though studies documented that durotomy did not have negative effect on long-term outcome and quality of life in patients undergoing first-time decompression surgery without fusion [51] association with a significant increase in the patient’s length of stay, and risk for re-intervention for the treatment of persisting CSF leakage is reported. Even more, evidence suggests a possible inferior outcome in terms of low back pain improvement [49]. Hence the elaborateness of the surgeon needs to be discussed in cases of inhomogenous occurrence as in our cohort.
In contrast to Aono et al. [52], we saw more immediate complications due to infections and hematoma leading to neurological deficits within the decompression group, so one has to discuss that due to the small incisions hemostasis might be more challeging. The use of post-operative drains and the type of post-operative dressing is still at the discretion of the treating surgeon with no available clinical guidelines. While Glennie et al. showed in their systematic review in 2015 that the use of a post-operative drain did not influence healing rates and had no effect secondarily on infection (odds ratio [OR] 1.33; 95% confidence interval [CI] 0.76–2.30), they were not able to establish whether surgical drains prevent hematomas causing neurologic compromise [53]. These heterogenous findings are supported by the existing literature [54, 55, 56]. Postoperative surgical complications, medical conditions and unplanned reoperations have shown to be associated with higher rates of unplanned readmission, large financial burdens and less beneficial outcome being most common in decompression procedures [57].
The overall revision rate to address recurrent symptoms at the index level or adjacent segments is reported between 10–25% [58, 59, 60]. These results correspond with our findings. In contrast to Martin [22], who observed a higher risk of reoperation in younger patients, females and patients with any comorbidities, the only specific patient demographic risk factor associated with reoperation in our study seemed to be male gender and smoking. This is at least partly supported by Sato, who identifed body mass index and disc height as independent risk factors for same segmental disease and male gender and facet degeneration as independent risk factors for adjacent segment disease [61].
Even though in our patient population history revealed at least one comorbidity in almost every patient (418/438; 95%) with a mean of 2 comorbidities per patient (range 0–8), incidence was even lower in the re-operation group. This is even more surprising as the patients were on average 3 years older than the patients in the non-revision groups.
As surprising as these findings are, they are in part supported by similar outcomes in the corresponding SPORT trial subgroup [17], who postulated that “lumbar fusion or particular patient characteristics, such as obesity, would render patients with lumbar stenosis more susceptible to reoperation at the index or adjacent levels” concluded that “The prevalence of other clinical factors suspected to affect outcome, such as obesity, smoking, or older age, was not significantly different between reoperation and non-reoperation groups”.
Even though reported successful clinical outcomes and functional improvement rates after revision spine surgery vary widely [26, 62, 63, 64], our findings are mirrored in the literature. Concurent with our study, Schlegel et al. evaluated patients who had undergone decompression alone or decompression plus extension of fusion for symptomatic ASD and observed a significant improvement in VAS scores in 70% of patients who had undergone extension of their fusion construct after decompression [65]. The same applies for Adogwa’s study showing that revision lumbar decompression and extension of fusion provides improvement in LBP, disability, and quality of life and should be considered a viable treatment option for elderly patients with persistent or recurrent back and radicular pain [64]. In a meta-analysis, Chang et al. reported a similiar overall effect odds ratio (OR) (95% CI) of major complications, number of reoperations, and clinically excellent and good rates between the two groups ( 0.70 (0.60, 0.81), 1.04 (0.90, 1.19) and 0.31 (0.06, 1.59) (
5.Conclusion
In conclusion one has to state that the odds of revision surgery due to intraoperative complications might depend predominantly on the surgeons elaborateness, while re-operation rates due to re-stenosis, adjacent level stenosis or degenerative disease after surgery can’t be predicted by evaluateing patients demographics, but seem to be enhanced by male gender, facet disease and deformity.
The limitations of this study are well recognized. It is a retrospective review with all the commensurate confounding factors and biases. Although preoperative and perioperative variables were recorded into a registry at the time of surgery, these variables were assessed post hoc at the time of the study’s initiation. Furthermore, no data on health-related quality of life instruments like SF-36 and Euroqol (EQ5D) measures can be provided, which are now prospectively collected. Still, as in other studies the main postoperative goals of patients were assessed with VAS/NRS and ability to walk. Lastly, within the observed period (10 years), techniques in managing lumbar spinal canal stenosis have changed, which might be reflected in re-operation rates of procedures performed by surgeons during the early 2000s.
Conflict of interest
None to report.
References
[1] | Arbit E, Pannullo S. Lumbar stenosis: a clinical review. Clin Orthop Relat Res. (2001) ; (384): 137-43. |
[2] | Ciricillo SF, Weinstein PR. Lumbar spinal stenosis. West J Med. (1993) ; 158: (2): 171-7. |
[3] | Schonstrom N, Willen J. Imaging lumbar spinal stenosis. Radiol Clin North Am. (2001) ; 39: (1): 31-53, v. |
[4] | Fox MW, Onofrio BM, Hanssen AD. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg. (1996) ; 85: (5): 793-802. |
[5] | diPierro CG. Gregory A. Helm, Christopher I. Shaffrey, James B. Chadduck, Scott L. Henson, Jacek M. Malik, et al. Treatment of lumbar spinal stenosis by extensive unilateral decompression and contralateral autologous bone fusion: operative technique and results. Journal of Neurosurgery. (1996) ; 84: (2): 166-73. |
[6] | Ahmet MM, Tufan C, Adem Y, Halit Ç, İsmail Y, Yunus A. Midterm outcome after a microsurgical unilateral approach for bilateral decompression of lumbar degenerative spondylolisthesis. Journal of Neurosurgery: Spine. (2012) ; 16: (1): 68-76. |
[7] | Micankova Adamova B, Vohanka S, Dusek L, Jarkovsky J, Bednarik J. Prediction of long-term clinical outcome in patients with lumbar spinal stenosis. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. (2012) ; 21: (12): 2611-9. |
[8] | Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar Spinal Stenosis: Conservative or Surgical Management: A Prospective 10-Year Study. Spine. (2000) ; 25: (11): 1424-36. |
[9] | Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for Low Back Pain: A Review of the Evidence for an American Pain Society Clinical Practice Guideline. Spine. (2009) ; 34: (10): 1094-109. |
[10] | III, Glassman SD, Mummaneni PV, Knightly JJ, Asher AL. Back pain improvement after decompression without fusion or stabilization in patients with lumbar spinal stenosis and clinically significant preoperative back pain. Journal of Neurosurgery: Spine. (2016) ; 25: (5): 596-601. |
[11] | Markman JD, Gewandter JS, Frazer ME, Pittman C, Cai X, Patel KV, et al. Evaluation of outcome measures for neurogenic claudication: A patient-centered approach. Neurology. (2015) ; 85: (14): 1250-6. |
[12] | Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. The New England Journal of Medicine. (2008) ; 358: (8): 818-25. |
[13] | Deen HG, Jr., Zimmerman RS, Lyons MK, Wharen RE, Jr., Reimer R. Analysis of early failures after lumbar decompressive laminectomy for spinal stenosis. Mayo Clin Proc. (1995) ; 70: (1): 33-6. |
[14] | Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg. (1992) ; 77: (5): 669-76. |
[15] | Thornes E, Ikonomou N, Grotle M. Prognosis of surgical treatment for degenerative lumbar spinal stenosis: a prospective cohort study of clinical outcomes and health-related quality of life across gender and age groups. Open Orthop J. (2011) ; 5: : 372-8. |
[16] | Papavero L, Thiel M, Fritzsche E, Kunze C, Westphal M, Kothe R. Lumbar spinal stenosis: prognostic factors for bilateral microsurgical decompression using a unilateral approach. Neurosurgery. (2009) ; 65: (6 Suppl): 182-7; discussion 7. |
[17] | Radcliff K, Curry P, Hilibrand A, Kepler C, Lurie J, Zhao W, et al. Risk for Adjacent Segment and Same Segment Reoperation After Surgery for Lumbar Stenosis: A subgroup analysis of the Spine Patient Outcomes Research Trial (SPORT). Spine. (2013) ; 38: (7): 531-9. |
[18] | Owoicho A, Ricardo KC, Katherine K, Isaac K, Carlos AB, Ziya L. Gokaslan, et al. Revision lumbar surgery in elderly patients with symptomatic pseudarthrosis, adjacent-segment disease, or same-level recurrent stenosis. Part 1. Two-year outcomes and clinical efficacy. Journal of Neurosurgery: Spine. (2013) ; 18: (2): 139-46. |
[19] | Kazuhiro H, Ko K, Haruka S, Toshiaki H. Biomechanical evaluation of destabilization following minimally invasive decompression for lumbar spinal canal stenosis. Journal of Neurosurgery: Spine. (2013) ; 18: (5): 504-10. |
[20] | Mendenhall SK, Parker SL, Adogwa O, Shau DN, Cheng J, Aaronson O, et al. Long-term outcomes after revision neural decompression and fusion for same-level recurrent lumbar stenosis: defining the effectiveness of surgery. J Spinal Disord Tech. (2014) ; 27: (7): 353-7. |
[21] | Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, et al. Surgical Versus Nonoperative Treatment for Lumbar Spinal Stenosis Four-Year Results of the Spine Patient Outcomes Research Trial. Spine. (2010) ; 35: (14): 1329-38. |
[22] | Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Are Lumbar Spine Reoperation Rates Falling With Greater Use of Fusion Surgery and New Surgical Technology? Spine. (2007) ; 32: (19): 2119-26. |
[23] | Kim SK, Park SW, Lim BC, Lee SC. Comparison of Reoperation after Fusion and after Decompression for Degenerative Lumbar Spinal Stenosis: A Single-Center Experience of 987 Cases. J Neurol Surg A Cent Eur Neurosurg. (2020) ; 81: (5): 392-8. |
[24] | Goel SA, Modi HN. Reoperations Following Lumbar Spinal Canal Stenosis. Indian J Orthop. (2018) ; 52: (6): 578-83. |
[25] | Athiviraham A, Wali ZA, Yen D. Predictive factors influencing clinical outcome with operative management of lumbar spinal stenosis. Spine J. (2011) ; 11: (7): 613-7. |
[26] | Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine (Phila Pa 1976); (1999) ; 24: (21): 2229-33. |
[27] | Lurie JD, Tosteson AN, Tosteson TD, Carragee E, Carrino JA, Kaiser J, et al. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine (Phila Pa 1976). (2008) ; 33: (14): 1605-10. |
[28] | Slätis P, Malmivaara A, Heliövaara M, Sainio P, Herno A, Kankare J, et al. Long-term results of surgery for lumbar spinal stenosis: a randomised controlled trial. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. (2011) ; 20: (7): 1174-81. |
[29] | Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database of Systematic Reviews. (2016) (1). |
[30] | Inoue G, Miyagi M, Takaso M. Surgical and nonsurgical treatments for lumbar spinal stenosis. European Journal of Orthopaedic Surgery & Traumatology. (2016) ; 26: (7): 695-704. |
[31] | Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-Term Outcomes of Surgical and Nonsurgical Management of Lumbar Spinal Stenosis: 8 to 10 Year Results from the Maine Lumbar Spine Study. Spine. (2005) ; 30: (8): 936-43. |
[32] | Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Blood E, Hanscom B, et al. Surgical versus Nonsurgical Therapy for Lumbar Spinal Stenosis. New England Journal of Medicine. (2008) ; 358: (8): 794-810. |
[33] | Yavin D, Casha S, Wiebe S, Feasby TE, Clark C, Isaacs A, et al. Lumbar Fusion for Degenerative Disease: A Systematic Review and Meta-Analysis. Neurosurgery. (2017) ; 80: (5): 701-15. |
[34] | Ulrich N, Burgstaller JM, Pichierri G, Wertli MM, Farshad M, Porchet F, Steurer J, Held U. Decompression Surgery Alone Versus Decompression Plus Fusion in Symptomatic Lumbar Spinal Stenosis: A Swiss Prospective Multi-center Cohort Study with 3 Years of Follow-up. Spine. (2017) Jan(13). |
[35] | Jansson K-Å, Németh G, Granath F, Blomqvist P. Spinal stenosis re-operation rate in Sweden is 11% at 10 years – A national analysis of 9,664 operations. European Spine Journal. (2005) ; 14: (7): 659-63. |
[36] | Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. Journal of neurosurgery. (1992) ; 77: (5): 669-76. |
[37] | Gelalis I, Arnaoutoglou C, Christoforou G, Lykissas MG, Batsilas I, Xenakis T. Prospective analysis of surgical outcomes in patients undergoing decompressive laminectomy and posterior instrumentation for degenerative lumbar spinal stenosis. Acta Orthop Traumatol Turc. (2010) ; 44: (3): 235-40. |
[38] | Aizawa T, Ozawa H, Kusakabe T, Tanaka Y, Sekiguchi A, Hashimoto K, et al. Reoperation rates after fenestration for lumbar spinal canal stenosis: a 20-year period survival function method analysis. European Spine Journal. (2015) ; 24: (2): 381-7. |
[39] | Deyo RA, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Revision Surgery Following Operations for Lumbar Stenosis. The Journal of Bone and Joint Surgery American Volume. (2011) ; 93: (21): 1979-86. |
[40] | Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. (2010) ; 303: (13): 1259-65. |
[41] | Guha D, Heary RF, Shamji MF. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurgical Focus. (2015) ; 39: (4): E9. |
[42] | Lee KK, Teo EC, Qiu TX, Yang K. Effect of facetectomy on lumbar spinal stability under sagittal plane loadings. Spine. (2004) ; 29: (15): 1624-31. |
[43] | Alimi M, Hofstetter CP, Pyo SY, Paulo D, Härtl R. Minimally invasive laminectomy for lumbar spinal stenosis in patients with and without preoperative spondylolisthesis: clinical outcome and reoperation rates. Journal of Neurosurgery: Spine. (2015) ; 22: (4): 339-52. |
[44] | Claudius T, Dimitris Z, Olaf L, Hansjörg B, Christiane P-S, Johannes W, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. Journal of Neurosurgery: Spine. (2005) ; 3: (2): 129-41. |
[45] | Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. (2002) ; 51: (suppl_2): S2-146-S2-54. |
[46] | Parikh K, Tomasino A, Knopman J, Boockvar J, Härtl R. Operative results and learning curve: microscope-assisted tubular microsurgery for 1-and 2-level discectomies and laminectomies. (2008) . |
[47] | Matthias HM, Nicola N, Martin M, Marcos ST. Lumbar spinal stenosis in elderly patients: is a unilateral microsurgical approach sufficient for decompression? Journal of Neurosurgery: Spine. (2011) ; 14: (3): 305-12. |
[48] | Strömqvist F, Jönsson B, Strömqvist B, Swedish Society of Spinal S. Dural lesions in decompression for lumbar spinal stenosis: incidence, risk factors and effect on outcome. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. (2012) ; 21: (5): 825-8. |
[49] | Kothe R, Quante M, Engler N, Heider F, Kneißl J, Pirchner S, et al. The effect of incidental dural lesions on outcome after decompression surgery for lumbar spinal stenosis: results of a multi-center study with 800 patients. Eur Spine J. (2017) ; 26: (10): 2504-11. |
[50] | Herren C, Sobottke R, Mannion AF, Zweig T, Munting E, Otten P, et al. Incidental durotomy in decompression for lumbar spinal stenosis: incidence, risk factors and effect on outcomes in the Spine Tango registry. Eur Spine J. (2017) ; 26: (10): 2483-95. |
[51] | Ulrich NH, Burgstaller JM, Brunner F, Porchet F, Farshad M, Pichierri G, et al. The impact of incidental durotomy on the outcome of decompression surgery in degenerative lumbar spinal canal stenosis: analysis of the Lumbar Spinal Outcome Study (LSOS) data–a Swiss prospective multi-center cohort study. BMC Musculoskeletal Disorders. (2016) ; 17: : 170. |
[52] | Hiroyuki A, Tetsuo O, Noboru H, Hidekazu T, Kenta A, Takeshi F, et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. Journal of Neurosurgery: Spine. (2011) ; 15: (2): 202-5. |
[53] | Andrew Glennie R, Dea N, Street JT. Dressings and drains in posterior spine surgery and their effect on wound complications. Journal of Clinical ï¼®euroscience: Official Journal of the Neurosurgical Society of Australasia. (2015) ; 22: (7): 1081-7. |
[54] | von Eckardstein KL, Dohmes JE, Rohde V. Use of closed suction devices and other drains in spinal surgery: results of an online, Germany-wide questionnaire. Eur Spine J. (2016) ; 25: (3): 708-15. |
[55] | Chandratreya A, Giannikas K, Livesley P. To drain or not drain: literature versus practice. Journal of the Royal College of Surgeons of Edinburgh. (1998) ; 43: (6): 404-6. |
[56] | Parker MJ, Roberts C. Closed suction surgical wound drainage after orthopaedic surgery. The Cochrane database of systematic reviews. (2001) (4): Cd001825. |
[57] | Bobby DK. Timothy RS, Seokchun L, George RC, John YSK. Predictors of unplanned readmission in patients undergoing lumbar decompression: multi-institutional analysis of 7016 patients. Journal of Neurosurgery: Spine. (2014) ; 20: (6): 606-16. |
[58] | Brodke DS, Annis P, Lawrence BD, Woodbury AM, Daubs MD. Reoperation and revision rates of 3 surgical treatment methods for lumbar stenosis associated with degenerative scoliosis and spondylolisthesis. Spine (Phila Pa 1976). (2013) ; 38: (26): 2287-94. |
[59] | Kelleher MO, Timlin M, Persaud O, Rampersaud YR. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine (Phila Pa 1976). (2010) ; 35: (19): E981-7. |
[60] | Schar RT, Kiebach S, Raabe A, Ulrich CT. Reoperation Rate After Microsurgical Uni- or Bilateral Laminotomy for Lumbar Spinal Stenosis with and Without Low-Grade Spondylolisthesis: What do Preoperative Radiographic Parameters Tell Us? Spine (Phila Pa 1976). (2018) . |
[61] | Sato S, Yagi M, Machida M, Yasuda A, Konomi T, Miyake A, et al. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J. (2015) ; 15: (7): 1536-44. |
[62] | Kim SS, Michelsen CB. Revision surgery for failed back surgery syndrome. Spine (Phila Pa 1976). (1992) ; 17: (8): 957-60. |
[63] | Cassinelli EH, Eubanks J, Vogt M, Furey C, Yoo J, Bohlman HH. Risk factors for the development of perioperative complications in elderly patients undergoing lumbar decompression and arthrodesis for spinal stenosis: an analysis of 166 patients. Spine (Phila Pa 1976). (2007) ; 32: (2): 230-5. |
[64] | Adogwa O, Carr RK, Kudyba K, Karikari I, Bagley CA, Gokaslan ZL, et al. Revision lumbar surgery in elderly patients with symptomatic pseudarthrosis, adjacent-segment disease, or same-level recurrent stenosis. Part 1. Two-year outcomes and clinical efficacy: clinical article. J Neurosurg Spine. (2013) ; 18: (2): 139-46. |
[65] | Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine (Phila Pa 1976). (1996) ; 21: (8): 970-81. |
[66] | Chang W, Yuwen P, Zhu Y, Wei N, Feng C, Zhang Y, et al. Effectiveness of decompression alone versus decompression plus fusion for lumbar spinal stenosis: a systematic review and meta-analysis. Arch Orthop Trauma Surg. (2017) ; 137: (5): 637-50. |
[67] | Austevoll IM, Gjestad R, Brox JI, Solberg TK, Storheim K, Rekeland F, et al. The effectiveness of decompression alone compared with additional fusion for lumbar spinal stenosis with degenerative spondylolisthesis: a pragmatic comparative non-inferiority observational study from the Norwegian Registry for Spine Surgery. Eur Spine J. (2017) ; 26: (2): 404-13. |




