H2S improves doxorubicin-induced myocardial fibrosis by inhibiting oxidative stress and apoptosis via Keap1-Nrf2
Abstract
OBJECTIVE:
We waimed to investigate whether H
METHODS:
Sprague-Dawley (SD) rats were randomly divided into four groups: normal control group (Control); DOX model group (DOX); H
RESULTS:
Myocardial injury and myocardial cell apoptosis were significantly increased, the H
CONCLUSIONS:
These results suggest that H
1.Introduction
Doxorubicin is the first-choice anti-tumor chemotherapy drug for various malignant tumors (e.g., acute leukemia, lymphoma, sarcoma, and breast cancer) as effectively improving patients’ survival rate. Yet doxorubicin may also lead to cardiomyopathy and congestive heart failure (CHF) in a dose-dependent manner. As statistics suggest, nearly 10% of patients would develop cardiomyopathy after being treated with doxorubicin. The use of doxorubicin will lead to obvious cardiotoxicity, to a certain degree, as well as limiting its clinical use and curative benefits. Furthermore, the clinical manifestations of doxorubicin-induced cardiomyopathy in the animal model are very similar to those of dilated cardiomyopathy (DCM) in human beings as well. Thus, it has become the most commonly used and most classic animal model in DCM-related studies [1, 2]. As a type of heterogeneous cardiomyopathy characterized by systolic dysfunction in the left ventricle or both ventricles, DCM is considered the leading cause of sudden cardiac death and cardiac failure [3, 4]. The pathogeneses of DCM and doxorubicin-induced cardiomyopathy still need further studies. Yet it has been found that myocardial fibrosis and the resulting myocardial remodeling are the key links in the occurrence and development of cardiomyopathy and cardiac failure. Accordingly, it is of great scientific significance and necessary to discuss the internal mechanism of myocardial fibrosis induced by doxorubicin.
The clinical pathological change of doxorubicin-induced myocardial fibrosis is very similar to DCM. It has now been found that oxidative stress and cell apoptosis are the major mechanisms that induce myocardial injury and myocardial remodeling. Oxidative stress means that excessive generation of reactive oxygen species (ROS) causes damage to tissue cells [5]. ROS may trigger an inflammatory response and lead to cell apoptosis. Also, it may lead to lipid peroxidation, thereby up-regulating malondialdehyde (MDA) expression. The current study has suggested that oxidative stress, inflammatory response, and cell apoptosis are closely correlated with the mechanism of doxorubicin-induced myocardial fibrosis and DCM [6, 7, 8].
A Keap1-Nrf2 signaling pathway is a signaling pathway that has been found resistant to oxidative damage in and outside the organism recently. It is critical for anti-oxidative stress, anti-apoptosis, anti-inflammatory response, anti-tumor, and anti-atherosclerosis [9, 10, 11]. Hydrogen sulfide (H
This study aimed to preliminarily discuss the potential mechanism of doxorubicin-induced myocardial damage and myocardial fibrosis and to investigate the effect of H
2.Experiment material
2.1Experiments with animals
Forty male adult SD rats, weighing 200
2.2Main experimental reagents
Doxorubicin hydrochloride was purchased from Meilun Biotech Co., Ltd., China; TGF-
3.Experiment methods
3.1Establishing the animal models and grouping
After 1 week of adaptive feeding at a constant temperature, 40 rats were randomly stratified into Control group, DOX group, DOX+H
3.2Echocardiographic detection
After 8 weeks, all rats were examined using the ultrasound instrument probe to measure the left ventricular long-axis view of the sternum at the level of the long-axis papillary muscles. We analyzed and measured the parameters of three consecutive cardiac cycles. The average value was calculated and recorded: left ventricular end-diastolic Internal diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), left ventricular short-axis shortening rate (LVFS).
3.3Calculating cardiac body mass index
All rats were weighed before sacrifice. All animals were anesthetized with chloral hydrate (350 mg/kg). The heart was washed out with saline and then washed with filter paper. Next, the heart weight was weighed. The heart weight index (heart weight/body weight, HW/BW, mg/g) was calculated.
3.4Masson staining
We observed the deposition of collagen in the myocardial tissue of each group. Samples of heart tissue fixed with formaldehyde were collected, then impregnated with 70% and 90% alcohol until the water in the tissue was removed, then transparent with xylene for about 1 hour, and then paraffin was added around the myocardial tissue until the tissue was completely immersed, cooled and embedded for 0.5 hour. Then the wax block was sliced under a slicer, the thickness of the slice was about 4
3.5Transmission electron microscopy observation of myocardial fibers and ultrastructure
2.5% glutaraldehyde fixed heart tissue was fixed, acetone was dehydrated, pure acetone
3.6Detecting the target protein expressions via Western blotting
The left ventricle myocardium was taken from each group, and the protein was extracted and quantified under BCA colorimetry. The protein sample was boiled at 99
3.7Detection of H2
All procedures for H
3.8Statistical analysis the experimental data were statistically analyzed with SPSS18.0 software
The observed index values were expressed as mean
4.Experimental results
4.1Rat body weight (BW), heart weight (HW), and heart weight to body weight ratio (HW/BW)
The BW, HW and HW/BW of each group 8 weeks later are shown below. BW of DOX group was declined, and HW/BW was increased, showing a statistically significant difference (
Table 1
Body weight, heart weight and ratio of heart weight and body weight in each group (mean
| Groups | Number | BW (g) | HW (mg) | HW/BW (mg/g) |
|---|---|---|---|---|
| Control | 10 | 354.17 | 1050.00 | 2.96 |
| DOX | 6 | 265.00 | 1041.67 | 3.93 |
| DOX+H | 7 | 310.00 | 1002.71 | 3.23 |
| H | 10 | 353.33 | 1028.17 | 2.91 |
Note:
4.2Comparisons of ECG results between different groups
As the results suggested, LVEDD and LVSED of DOX group were obviously increased, and EF and FS were substantially decreased compared with those of Control group; LVEDD and LVSED of DOX+H
Table 2
Comparison of the echocardiographic parameters in each group (mean
| Group | LVEDD (mm) | LVESD (mm) | EF (%) | FS (%) |
|---|---|---|---|---|
| Control | 5.18 | 2.65 | 85.18 | 49.03 |
| DOX | 6.83 | 4.80 | 63.57 | 30.03 |
| DOX+H | 5.84 | 3.73 | 73.07 | 35.93 |
| H | 5.30 | 2.90 | 83.08 | 46.03 |
Note:
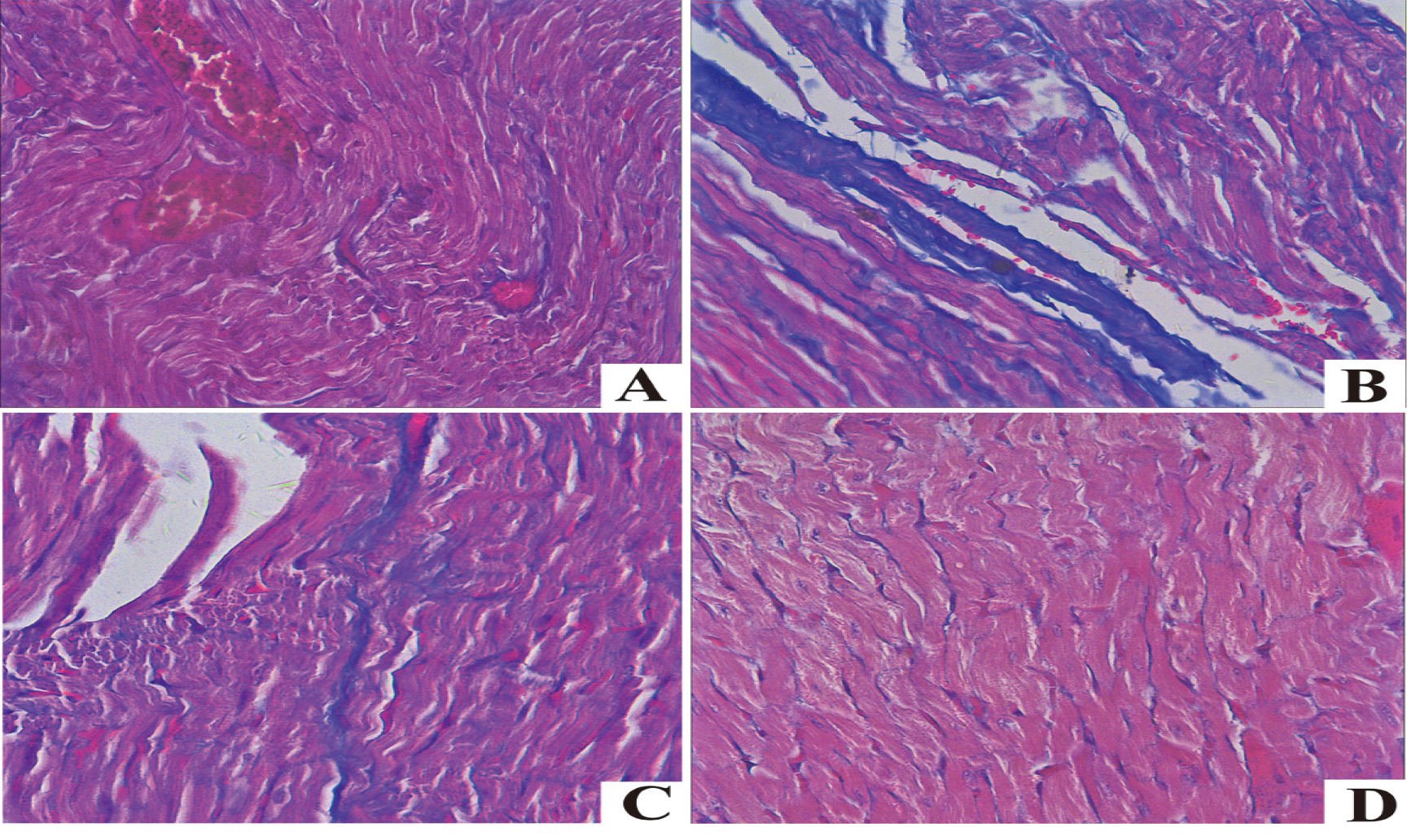
4.3Masson staining results of rat myocardial tissue of different groups
In Masson staining, collagen fiber and muscle fiber were blue and red, respectively. The myocardial cells of the DOX group were misarranged, blue collagen fiber in myocardial interstitium was remarkably increased, and there was obvious myocardial fibrosis compared with those of the Control group. The myocardial cells of DOX+H
Figure 1.
Masson staining of myocardial tissues in each group. A. Control; B. DOX; C. DOX+H
4.4Observation results of rat myocardial tissue of different groups under TEM
TEM results are shown in the Figure. Myocardial fibers of the Control group and H
Figure 2.
Electron microscopic observation of mitochondrial swelling and vacuole formation in the selected area of the DOX group. H
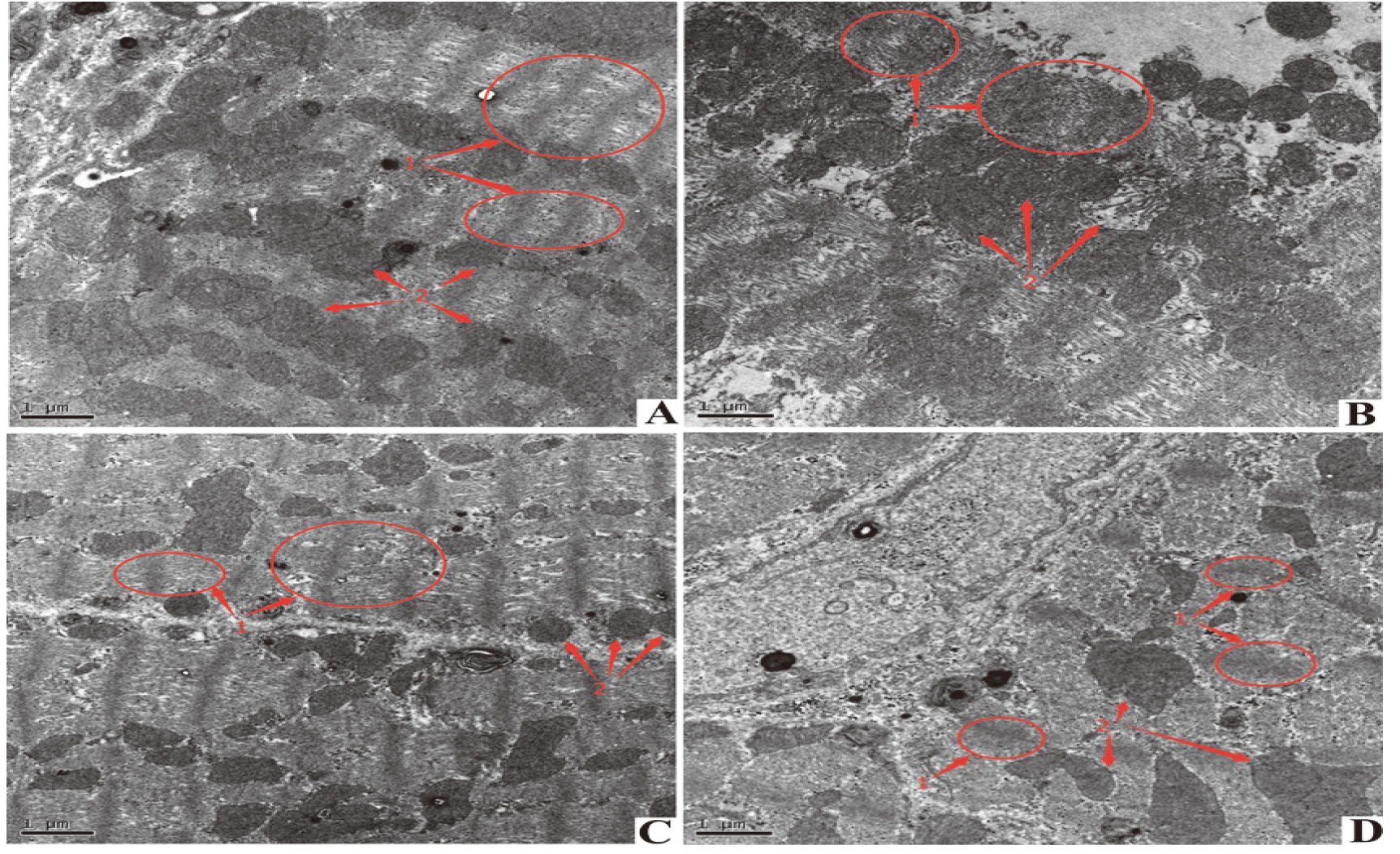
The H
Figure 3.
Quantification of myocardial H
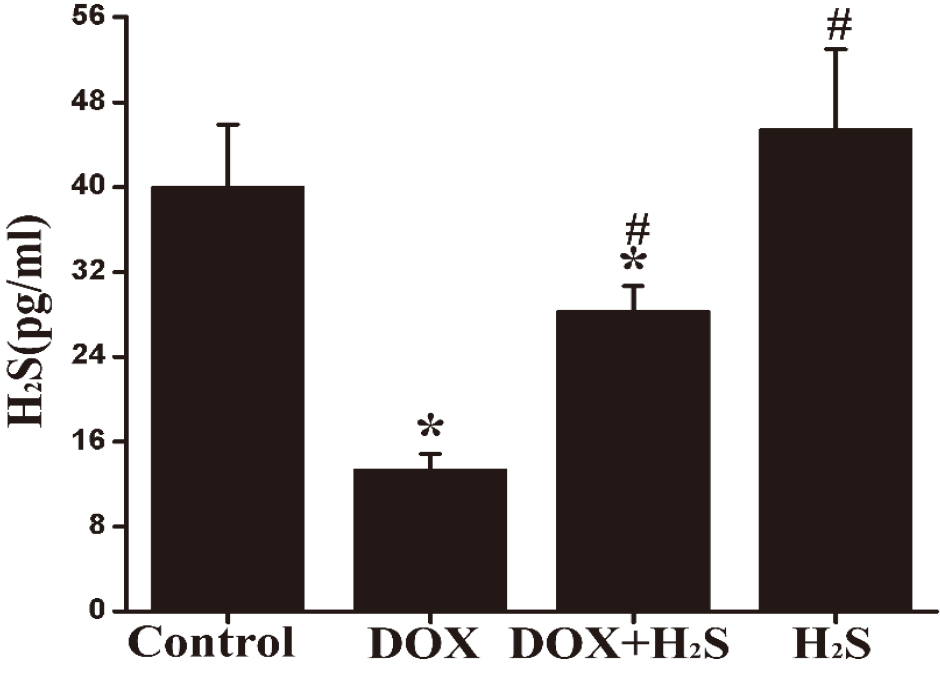
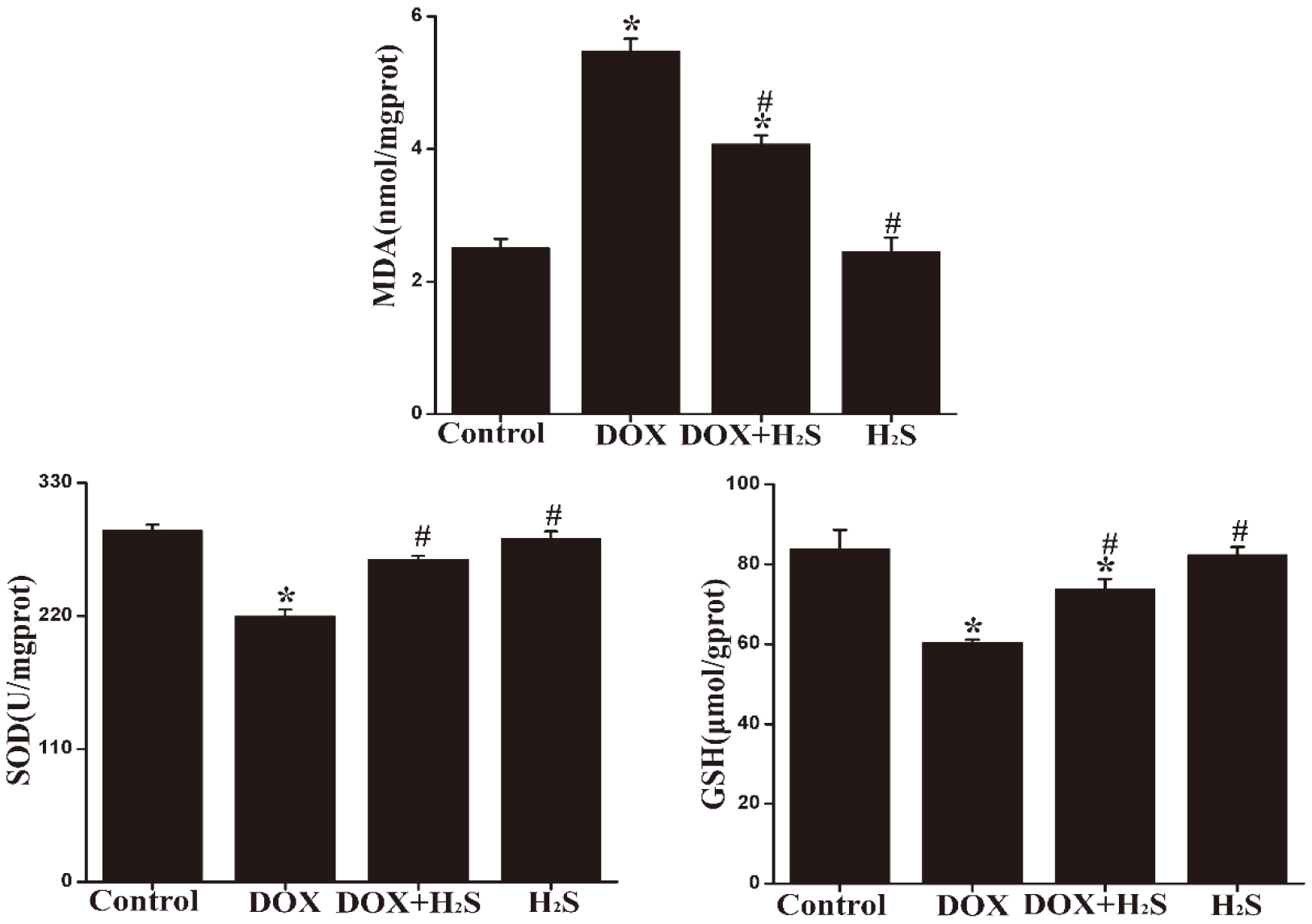
4.5Expression of MDA, SOD, and GSH in rat myocardial tissue of different groups
MDA expression in myocardial tissue was up-regulated in the DOX group, and SOD and GSH expression was significantly down-regulated compared with those of the Control group, showing statistically significant differences (
Figure 4.
The expression levels of MDA, SOD and GSH in myocardial tissues from each group.
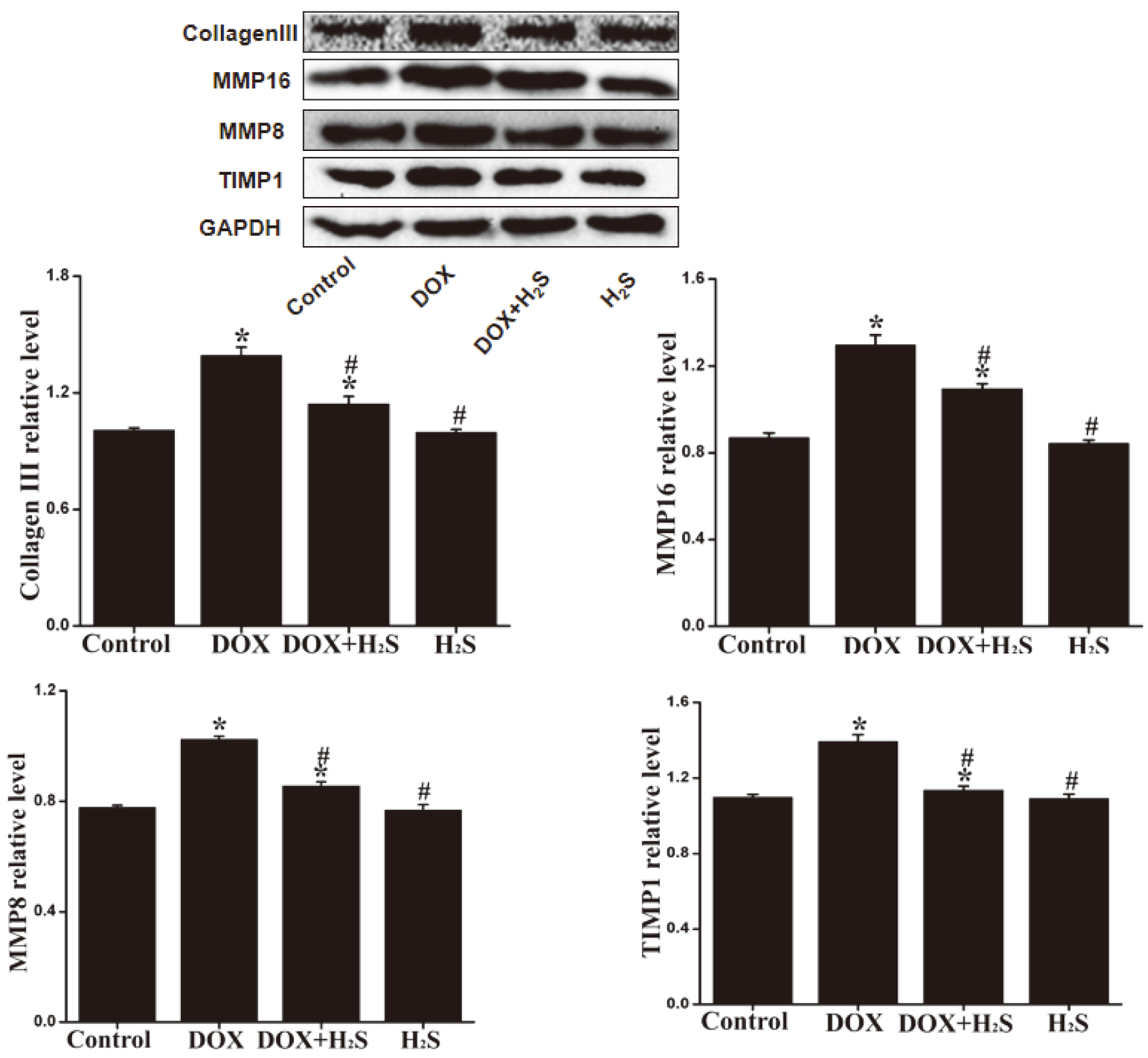
4.6Expression levels of Collagen III, MMP-8, MMP-16, and TIMP-1 in rat myocardial tissue of different groups
The expression levels of Collagen III, MMP-8, MMP-16 and TIMP-1 in rat myocardial tissue of DOX group were significantly down-regulated (
Figure 5.
The expression levels of Collagen III, MMP-8, MMP-16 and TIMP-1 in rat myocardial tissue of different groups.
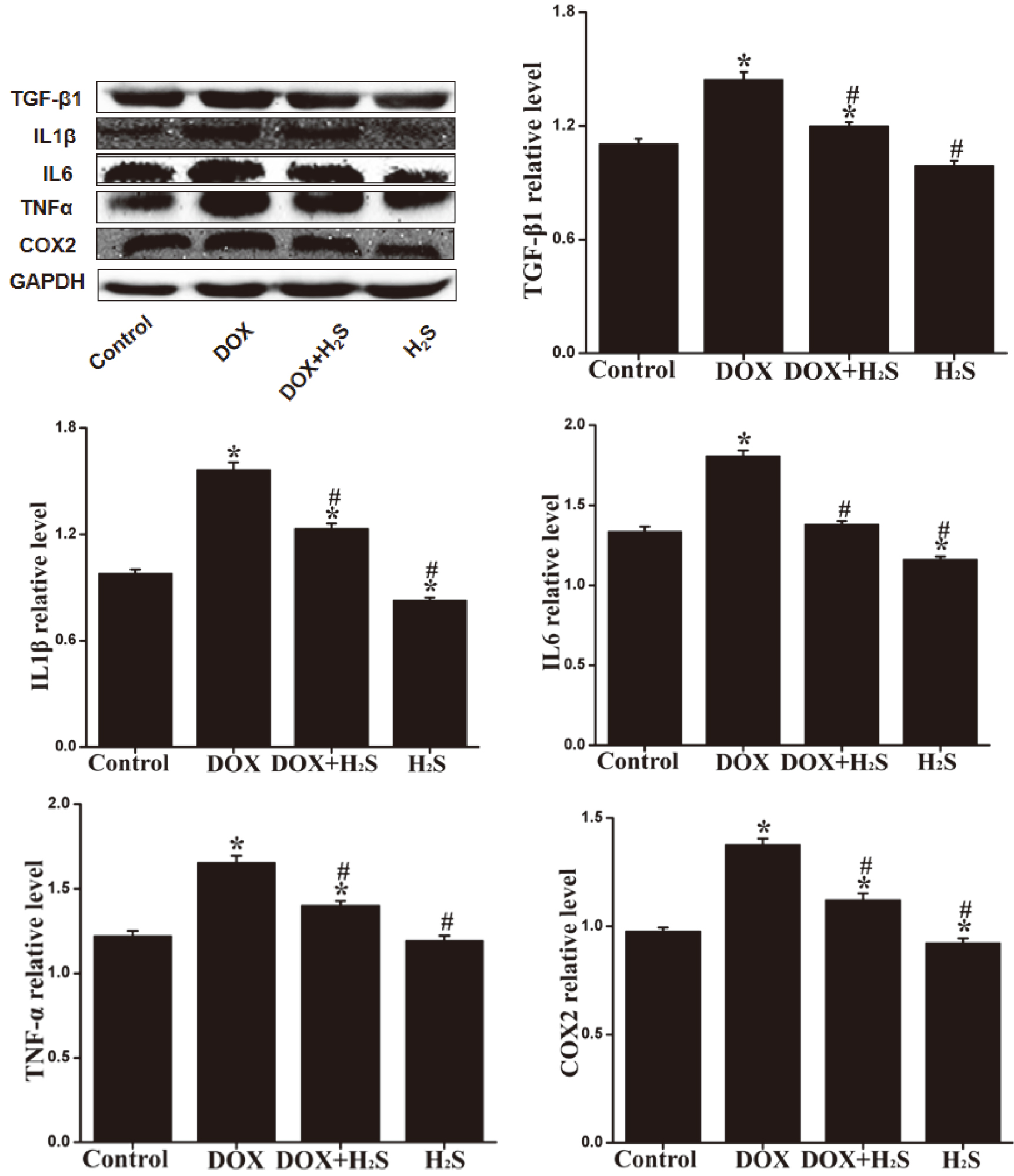
4.7Expression levels of TNF-α β β
The expression levels of caspase-3, caspase-9, Bax, TNF-
Figure 6.
Expression of TNF-
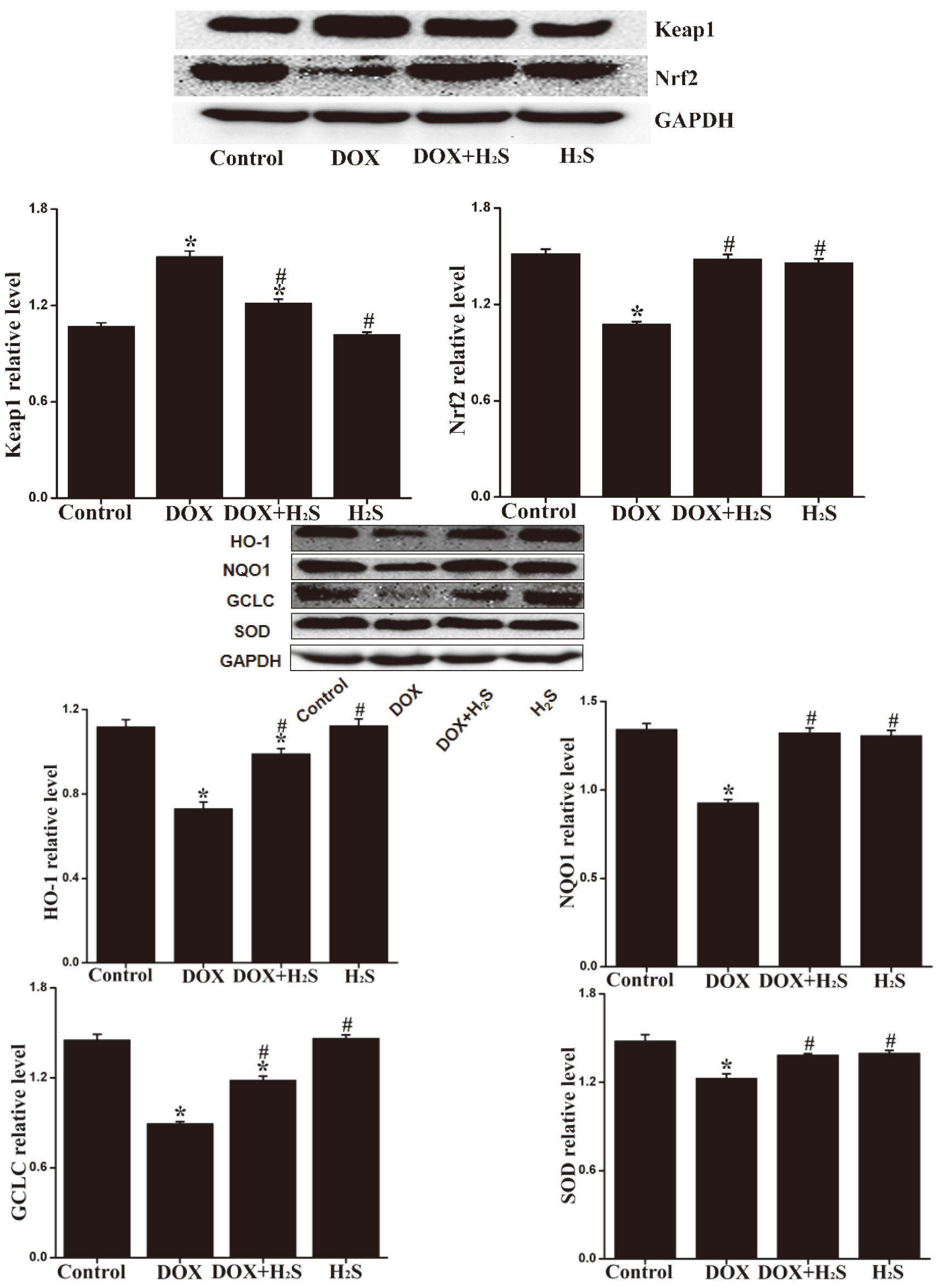
4.8Expression levels of Keap1, Nrf2, SOD, NQO1, HO-1, and GCLC in rat myocardial tissue of different groups
The expression levels of Keap1 in rat myocardial tissue of DOX group were up-regulated (
Figure 7.
Expression of Keap1, Nrf2, SOD, NQO1, HO-1, and GCLC proteins in myocardial tissue of each group.
5.Discussion
Myocardial fibrosis is a vital pathological change in the occurrence and development of DCM and doxorubicin-induced cardiomyopathy. It is a key link in myocardial remodeling following myocardial injury as well. Myocardial fibrosis is the result of the imbalance between the synthesis and degradation of extracellular matrix (ECM), resulting in the extensive deposition of ECM in the myocardial interstitium. Matrix metalloproteinases (MMPs) are the major enzymes degrading extracellular matrix components, while tissue inhibitor of metalloproteinases (TIMPs) serves as the endogenous specific inhibitor of MMPs. The interaction between MMPs and TIMPs is critical for maintaining the dynamic equilibrium of ECM. Thus, the formation of myocardial fibrosis is closely associated with MMPs/TIMPs interaction [17, 18]. Also, the inflammatory reaction is involved in the occurrence mechanism of myocardial fibrosis, and in particular, TGF-
Oxidative stress is a significant mechanism in the occurrence and development of cardiomyopathy causing myocardial damage. Oxidative stress usually suggested a serious imbalance between the generation of ROS and the elimination of ROS in the antioxidant defense system in the body, causing substantial accumulation of ROS that may lead to lipid peroxidation and up-regulate the expression levels of malondialdehyde (MDA), 4-hydroxynonenal (4-HNE) and other RNS metabolites. As a result, the heart is very sensitive to oxidative damage [23, 24]. Some antioxidant enzymes (e.g., superoxide dismutase (SOD) and glutathione (GSH) in the body) can reduce or eliminate ROS, being critical for preventing oxidative stress damage. Doxorubicin is a clinically commonly-used anthraquinone broad-spectrum anti-tumor drug. A large number of literature have suggested that doxorubicin can significantly up-regulate the level of oxidative stress in myocardial tissue and lead to myocardial apoptosis [25]. In this study, we found that the DOX group induced by doxorubicin had obvious myocardial fibrosis, the level of oxidative stress was up-regulated, the expression levels of MDA and 4-HNE were up-regulated, and those of SOD, NQO1, HO-1, GCLC, and other antioxidants were significantly down-regulated. Existing studies have verified that ROS can also lead to apoptosis as a major factor in inducing oxidative stress in the body. Apoptosis has a significant impact on the occurrence and development of myocardial fibrosis. The Caspases cascade pathways in myocardial tissue will be activated separately with the action of various pro-apoptotic factors, thereby inducing apoptosis. Bax has been found as a common pro-apoptotic protein, while Bcl-2 has been proven to block caspase activation and inhibit apoptosis. In this study, it is being suggested that the level of apoptosis in myocardial tissue induced by doxorubicin was up-regulated significantly with the level of oxidative stress, Caspases and Bax were up-regulated, and Bcl-2 was down-regulated, suggesting that apoptosis is also involved in the mechanism of doxorubicin-induced myocardial fibrosis.
Keap1/Nrf2 signaling pathway is an important antioxidant stress pathway in the body. Numerous studies have suggested that the Keap1/Nrf2 signaling pathway is critical for anti-oxidative stress, anti-inflammation, anti-apoptosis, anti-cell proliferation, and protection of myocardial cells in the body [26]. In the Keap1/Nrf2 signaling pathway, Keap1 primarily modulates the transcriptional activity of Nrf2 and negatively regulates the function of Nrf2 [27]. When the body is damaged by oxidative stress, inflammatory reaction, etc., the activation of the Keap1/Nrf2 signaling pathway in the body may start transcript downstream phase II detoxifying enzymes, antioxidant enzymes, etc., including catalase (CAT), superoxide (SOD), glutathione S-transferase (GST), oxidoreductase (NQO1) and heme oxygenase (HO-1) [28], to alleviate cell damage caused by active oxygen and electrophilic substances, keep the cells in a stable state, and be critical for anti-oxidative stress, anti-apoptosis, and anti-inflammatory. A study made by Siyin et al. revealed that Nrf2 deficiency aggravates doxorubicin-induced cardiotoxicity and cardiac dysfunction [29]. In this study, we found that doxorubicin-induced the remarkable up-regulation of Keap1 and inhibition of Nrf2 expression, as well as the down-regulation of the downstream phase II detoxifying enzymes and antioxidant enzymes of Keap1/Nrf2 signaling pathway, e.g., NQO1, HO-1, and GCLC. This suggested that the Keap1/Nrf2 signaling pathway may be involved in the mechanism of doxorubicin-induced myocardial fibrosis, oxidative stress, and apoptosis.
As a colorless, smelly rotten-egg gas, H
In conclusion, the findings of the present study suggest that H
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81202830) and the Hunan Provincial Innovation Foundation For Postgraduates (Grant No. CX20200973).
Conflict of interest
None to report.
Availability of data and materials
All data generated or analyzed during the study period is included in this article.
References
[1] | Nithipongvanitch R, Ittarat W, Cole MP, et al. Mitochondrial and nuclear p53 localization in cardiomyocytes: redox modulation by doxorubicin (Adriamycin). Antioxid Redox Signal. (2007) ; 9: : 1001-1008. |
[2] | Gu R, Bai J, Ling L, et al. Increased expression of integrin-linked kinase improves cardiac function and decreases mortality in dilated cardiomyopathy model of rats. PLoS ONE. (2012) ; 7: : e31279. |
[3] | Luk A, Ahn E, Soor GS, Butany J. Dilated cardiomyopathy: a review. J Clin Pathol. (2009) ; 62: : 219-225. |
[4] | Lakdawala NK, Winterfield JR, Funke BH. Dilated cardiomyopathy. Circulation Arrhythmia & Electrophysiology. (2013) ; 6: : 228-37. |
[5] | Wang J, Xue Z, Lin J, et al. Proline improves cardiac remodeling following myocardial infarction and attenuates cardiomyocyte apoptosis via redox regulation. Biochem Pharmacol. (2020) ; 178: : 114065. |
[6] | Zare MFR, Rakhshan K, Aboutaleb N, et al. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. (2019) ; 232: : 116623. |
[7] | Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. (2005) ; 38: : 73-79. |
[8] | Fang L, Ellims AH, Beale AL, et al. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res. (2017) ; 9: : 5063-5073. |
[9] | Choi WS, Jeong JW, Kim SO, et al. Anti-inflammatory potential of peat moss extracts in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int J Mol Med. (2014) ; 34: : 1101-1109. |
[10] | Guo S, Cheng X, Lim JH, et al. Control of antioxidative response by the tumor suppressor protein PML through regulating Nrf2 activity. Mol Biol Cell. (2014) ; 25: : 2485-2498. |
[11] | Sandra V, Anne S, Michael J. Parnhamet al: Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. (2017) ; 18: : 2772. |
[12] | Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide-mediated cytoprotection. Antioxidants & Redox Signaling. (2010) ; 12: : 1203-1217. |
[13] | Liu MH, Lin XL, Yuan C, et al. Hydrogen sulfide attenuates doxorubicin-induced cardiotoxicity by inhibiting the expression of peroxiredoxin III in H9c2 cells. Mol Med Rep. (2016) ; 13: : 367-372. |
[14] | Wen SY, Tsai CY, Pai PY, et al. Diallyl trisulfide suppressesdoxorubicin-induced cardiomyocyte apoptosis by inhibiting MAPK/NF-κB signaling through attenuation of ROS generation. Environ Toxicol. (2018) ; 33: : 93-103. |
[15] | Renu K, Abilash VG, Tirupathi PP, et al. Molecular mechanism of doxorubicin-induced cardiomyopathy-AnUpdate. European Journal of Pharmacology. (2017) ; 818: (2018): 241-253. |
[16] | Xia Y, Chen Z, Chen A, et al. LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. Journal of Molecular & Cellular Cardiology. (2017) ; 108: : 138-148. |
[17] | Li Y, Li L, Zeng O, Liu JM, Yang J. H2S improves renal fibrosis in STZ-induced diabetic rats by ameliorating TGF-β1 expression. Renal Failure. (2017) ; 39: : 265-272. |
[18] | Ma J, Ma S-Y, Ding C-H. Curcumin reduces cardiac fibrosis by inhibiting myofibroblast differentiation and decreasing transforming growth factor β1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinase 1. Chinese Journal of Integrative Medicine. (2017) ; 23: : 362-369. |
[19] | Seeland U, Haeuseler C, Hinrichs R, et al. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. (2002) ; 32: : 295-303. |
[20] | Ayinapudi K, Samson R, Le Jemtel TH, et al. Weight reduction for obesity-induced heart failure with preserved ejection fraction. Curr Hypertens Rep. (2020) ; 22: (8): 47. |
[21] | Fang L, Ellims AH, Beale AL, et al. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res. (2017) ; 9: : 5063-5073. |
[22] | Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. (2007) ; 102: : 500-507. |
[23] | Deepa M. Gopal, flora sam: new and emerging biomarkers in left ventricular systolic dysfunction – insight into dilated cardiomyopathy. J Cardiovasc Transl Res. (2003) ; 6: : 516-527. |
[24] | Herbet M, Korga A, Gawrońska-Grzywacz M, et al. Chronic variable stress is responsible for lipid and DNA oxidative disorders and activation of oxidative stress response genes in the brain of rats. Oxidative Medicine & Cellular Longevity. (2017) ; 2017: : 7313090. |
[25] | Octavia Y, Tocchetti CG, Gabrielson KL, et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. (2012) ; 52: : 1213-1225. |
[26] | Syed Minhaj Uddin Ahmed, Lin Luo, Akhileshwar Namani: Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta. (2016) ; 1863: : 585-597. |
[27] | McMahon M, Itoh K, Yamamoto M, et al. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. The Journal of Biological Chemistry. (2003) ; 278: : 1592-21600. |
[28] | Xiao L, Liang S, Ge L, et al. 4, 5-di-O-caffeoylquinic acid methyl ester isolated from Lonicera japonica Thunb. targets the Keap1/Nrf2 pathway to attenuate H2O2-induced liver oxidative damage in HepG2 cells. Phytomedicine. (2020) ; 70: : 153219. |
[29] | Li S, Wang W, Niu T. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid Med Cell Longev. (2014) ; 2014: : 748524. |
[30] | Pan L-L, Qin M, Liu X-H, et al. The role of hydrogen sulfide on cardiovascular homeostasis: an overview with update on immunomodulation. Front Pharmacol. (2017) ; 8: : 686. |
[31] | Liu M, Li Y, Liang B, et al. Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway. Int J Mol Med. (2018) ; 41: : 1867-1876. |




