Gefitinib-coated balloon inhibits the excessive hyperplasia of intima after vascular injuries through PI3K/AKT pathway
Abstract
OBJECTIVE:
To explore the effect of gefitinib-coated balloon suppressive action on the excessive hyperplasia of intima after balloon injury of common carotid artery in rats and on the PI3K/AKT signal pathway.
METHODS:
MTT method and the expression of Bcl-2 and Caspase-3 proteins were detected in vitro; Adult SD rats were randomly split into 5 groups, namely sham group, model group, low-dosage gefitinib-coated balloon group, high-dosage gefitinib-coated balloon group, and paclitaxel-coated balloon group. The intimal proliferation of arteries, PCNA, P-AKT and PI3K protein expression, the cell apoptosis, expression of MMP9, TGF
RESULTS:
At the same time and concentration, Gefitinib suppressed the proliferation of smooth muscle cell more significantly than paclitaxel. Bcl-2 and Caspase-3 in vascular smooth muscle and endothelial cells (VSMC, EC) were significantly down-regulated and up-regulated after the cells were treated with gefitinib and paclitaxel. In gefitinib- and paclitaxel-coated balloon groups, significant up-regulations were found in the area of lumen. Furthermore, the expression of PCNA suggested that all coated balloons could suppress the excessive proliferation of smooth muscle cells in the hyperplastic intima compared with the control group. In gefitinib- and paclitaxel-coated balloon group, the expression of PI3K/AKT was significantly down-regulated. The use of drug-coated balloons mitigated the cell apoptosis in TUNEL. The expressions of MMP9, TGF
CONCLUSIONS:
Gefitinib-coated balloons were able to suppress the excessive proliferation in the common carotid arterial intima of rats more effectively than the paclitaxel-coated ones. The underlying mechanism may cover the PI3K/AKT signal pathway.
1.Introduction
The migration and the proliferation of vascular smooth muscle cells (VSMCs) are the critical mechanisms, e.g. the restenosis after percutaneous coronary intervention (PCI). However, drug-coated balloon (DCB) can prevent the formation of restenosis effectively inside the nude stent by suppressing the intimal hyperplasia, which widens its application in vascular intervention. Rapamycin and paclitaxel are two types of non-selective cytotoxic drugs used for drug-coated stents and balloons for their ability to suppress the excessive proliferation of SMCs and mitigate the intimal repair [1]. Accordingly, cytotoxic drugs exhibiting more accurate selectivity on SMCs are the optimal choice for the new coating drugs.
Gefitinib, the 3
2.Basic experimental reagents and methods
2.1Reagents and animals
Hematoxylin, and TUNEL kit (Wellbio company, China). Chloral hydrate (Shanghai Shan Pu chemical company, China). Two-Step Kit (Golden Bridge Company, China). HRP Goat anti-rat IgG, BCL
2.2Cell cultures
The newly purchased rat smooth muscle cells (Number: RAT-iCell-c004) and endothelial cells (Number: RAT-iCell-c003) were suspended in DMEM medium (containing 20% fetal bovine serum, 100 IU/ml penicillin and 100 g/ml streptomycin) and then cultured in an incubator containing 5% CO2 at 37
2.3Cytotoxicity assays
Bioassays were performed to determine the number of viable cells in proliferation or cytotoxicity tests by MTT, namely a colorimetric method. The MTT tetrazolium compounds were bio-reduced by cells into a colored formazan product, which is soluble in tissue culture medium and correlated with the cell number. Smooth muscle cells and endothelial cells were cultured using the above method and triturated into cell suspension (3
2.4Measurement of the expressions of Bcl-2 and Caspase-3 protein by western blotting
One umol/L gefitinib and paclitaxel were used to treat VSMC and EC for 48 h, respectively, another group of VSMC and EC no reagent added (control group), and total cell protein was extracted with RIPA kit. Bradford protein analysis kit was employed to measure the concentration of the extracted protein. The extracted protein was placed in EP tube and quantified by BCA colorimetry. After undertaking gel preparation (separation and concentration), comb placement, sample addition and electrophoresis, the protein was transferred to the PVDF membrane by the wet transfer method, and then sealed with the sealing liquid for 1 h. The primary antibodies of GAPDH (dilution of 1:1000), Bcl-2, Caspase-3 (dilution of 1:500) were incubated at 37
2.5Establishment of the animal model
The experiment of this study strictly followed the rules of animal use and protection of the Experimental Animal Research Institute of South China University (Hengyang, China). To ensure the diet access, a total of 50 male SD rats of weight from 300 to 330 g were placed into cages (1 rat/cage) for 1 week of feeding, in which the temperature was set as 24
2.6Preparation of drug-coated balloons
Gefitinib and paclitaxel were dissolved in DMSO to prepare the stock solutions (20 mmol/L), which were then kept at
2.7Detecting the pathological changes in the common carotid artery via H&E staining
After rats were euthanized, samples of their bilateral arteries were collected by being fixed in formaldehyde, dehydrated, cleared, embedded with paraffin and stained with hematoxylin-eosin following conventional methods. Balsam was dropped on the cleared section and sealed using cover glass. Subsequently, section was placed under the microscope (100
2.8Detecting the protein expressions of PCNA, PI3K, and P-AKT through immunohistochemistry
Dewaxed and rehydrated sections prepared as mentioned above underwent overnight incubation at 4
2.9Detecting cell apoptosis in the hyperplastic intima via TUNEL staining
TUNEL staining was performed on dewaxed section samples accordingly with the manufacturer’s instructions. Subsequently, sections were sealed and placed under the microscope to be observed. Images were loaded to the Image-Pro Plus software to be analyzed. For each sample, 5 visions under the microscope were used to calculate the positive expression rate, and the average was taken as the result. The positive rate was equal to the quantity of cell nuclei with positive expression under a single vision divided by the total number of cell nuclei under this vision.
2.10Determining the relative expression of MMP9, TGFβ
Total RNA was extracted from rat vascular tissue and then served as the template for cDNA reverse transcription. The mentioned operations were performed strictly following the instructions. RT-qPCR quantification was performed by SYBR Green method. Each primer sequence and product size are listed in Table 1. Gel electrophoresis was performed for the amplification products, and photos were captured. The solubility curve suggested that the PCR reaction product was separate double-stranded DNA. With GAPDH as the internal reference, the relative expressions of MMP9, TGF
Table 1
Each primer sequence and product size of MMP9, TGF
| Gene | Primer sequence | Product length/bp |
|---|---|---|
| GAPDH | Forward ACAGCAACAGGGTGGTGGAC | 252 |
| Reverse TTTGAGGGTGCAGCGAACTT | ||
| MMP9 | Forward CCCCGAGACCTGAAAACCTCCAAC | 189 |
| Reverse GGCCTTTAGTGTCTCGCTGTCCA | ||
| TGF | Forward CCGCTTCTGCTCCCACTCCC | 101 |
| Reverse CGTTTCACCAGCTCCATGTCGAT | ||
| IL6 | Forward TCACTATGAGGTCTACTCGG | 141 |
| Reverse CATATTGCCAGTTCTTCGTA |
2.11Statistical analysis
Statistical analysis of the mentioned data was conducted using the SPSS 18.0 statistical software package. Measurement data are denoted as mean
3.Results
3.1Cytotoxic effects of gefitinib on smooth muscle cells and endothelial cells
Figure 1 shows that the proliferation of endothelial and smooth muscle cells was significantly suppressed by gefitinib and paclitaxel, whereas at the same time and the same concentration (
Figure 1.
Comparison of gifitinib and paclitaxel effect on inhibition rate of cell proliferation. Note: As compared to paclitaxel inhibition to VSMC,
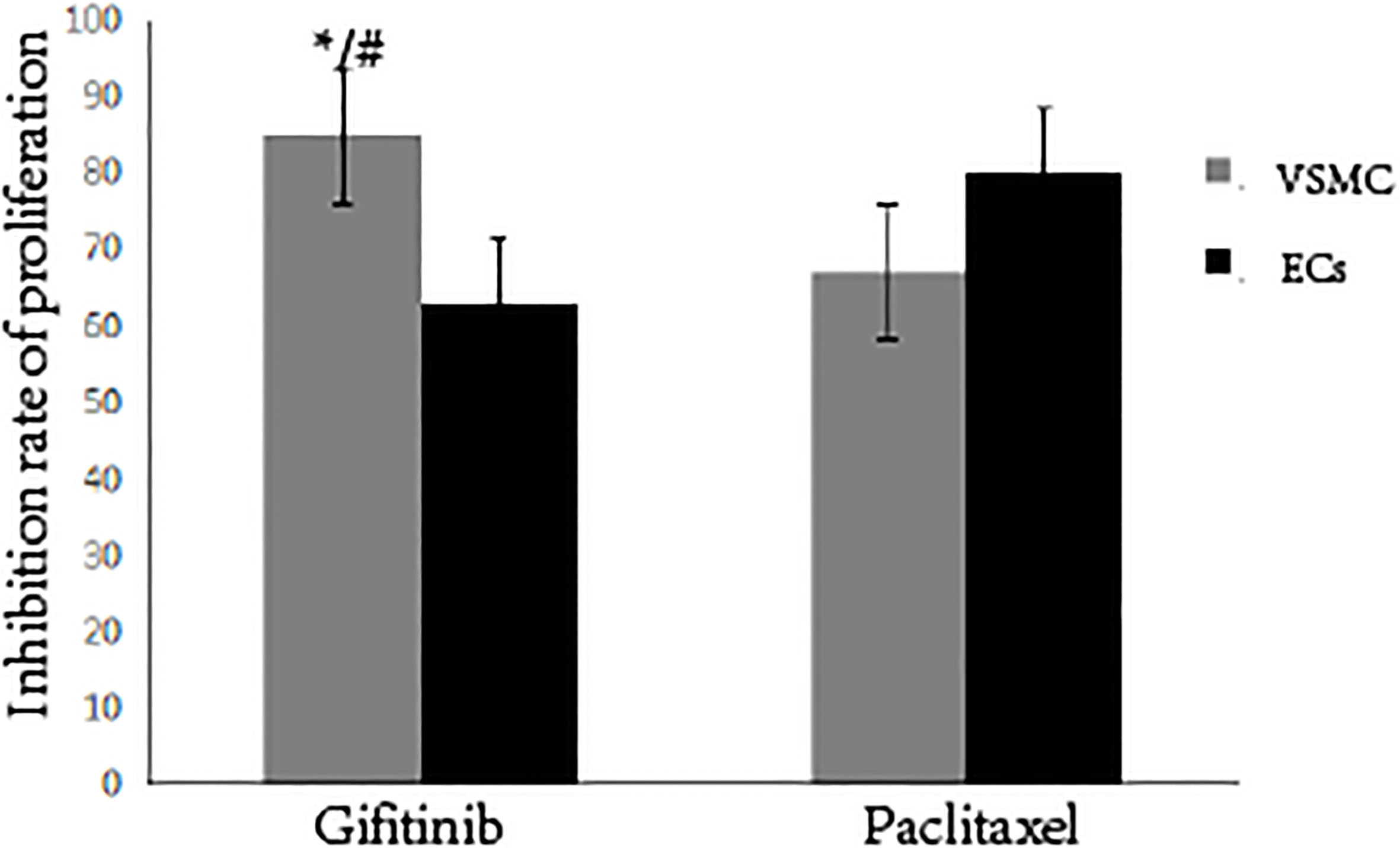
Figure 2.
The expression of Bcl2 and Caspase-3 in different cells. Note: Compare with control group,
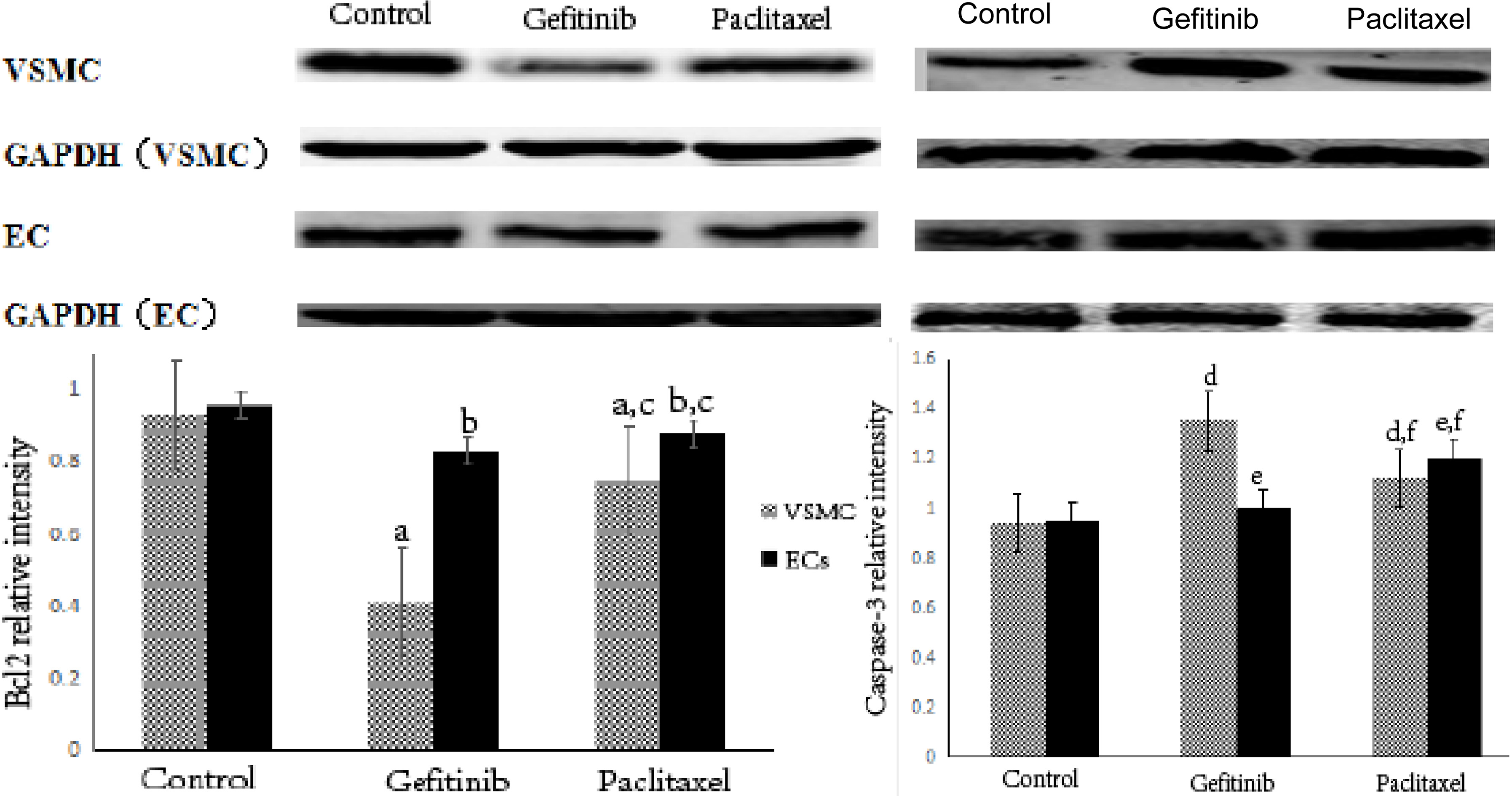
3.2Expression of Bcl-2 and Caspase-3 proteins after being treated with two types of cell drugs
As shown in the Fig. 2, for VSMC, the expression of Bcl-2 protein in the gefitinib group and the paclitaxel group was significantly down-regulated, while the expression of Caspase-3 protein was significantly up-regulated compared with the Control group (
3.3Detecting the pathological changes in common carotid artery via H&E staining
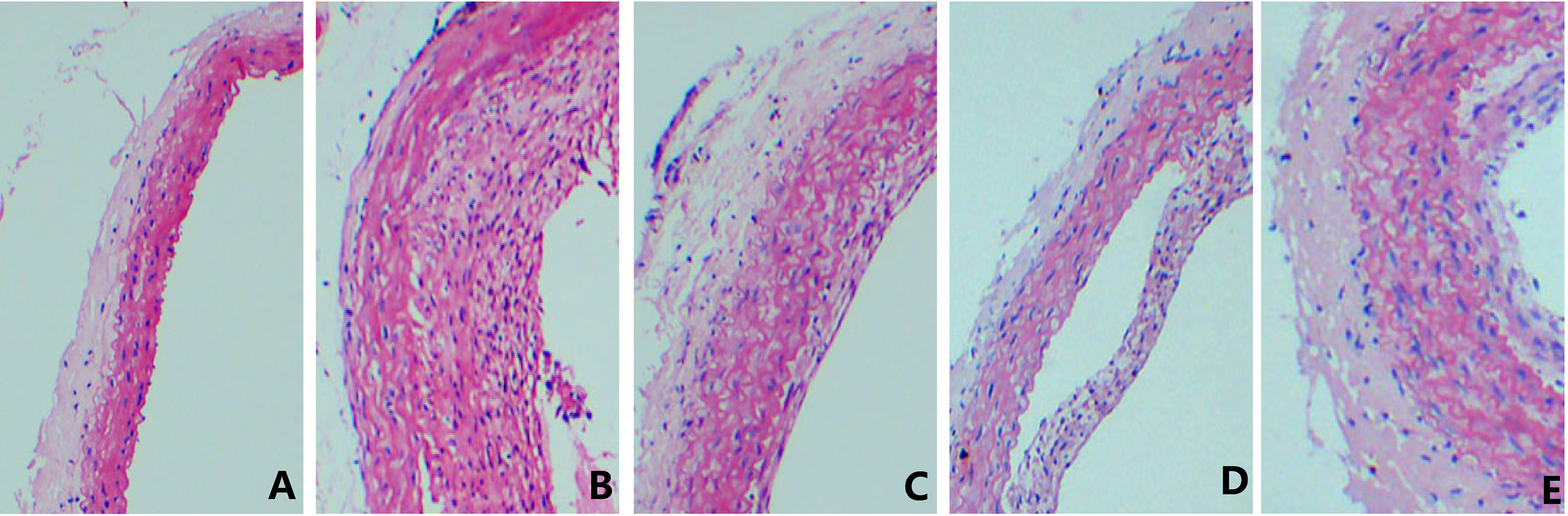
Figure 3 suggests that a significant increase in the thickness of common carotid artery and obvious stenosis in lumen were identified in the control group rats compared with the sham operation group; compared with the model group, alleviations were found in the hyperplasia of intima in common carotid arteries of the rats in the low- and high-dosage gefitinib-coated balloon groups and the paclitaxel-coated balloon group, and stenosis in lumen was also significantly improved, especially in the high-dosage gefitinib-coated balloon group. However, no statistically significant difference was found in comparison of intimal hyperplasia between the low-dosage gefitinib- and the paclitaxel-coated balloon groups.
Figure 3.
The influence on vascular lumen area. Note: A: Sham operation group; B: Model group; C: Low-dosage gefitinib group; D: High-dosage gefitinib group; E: Paclitaxel group.
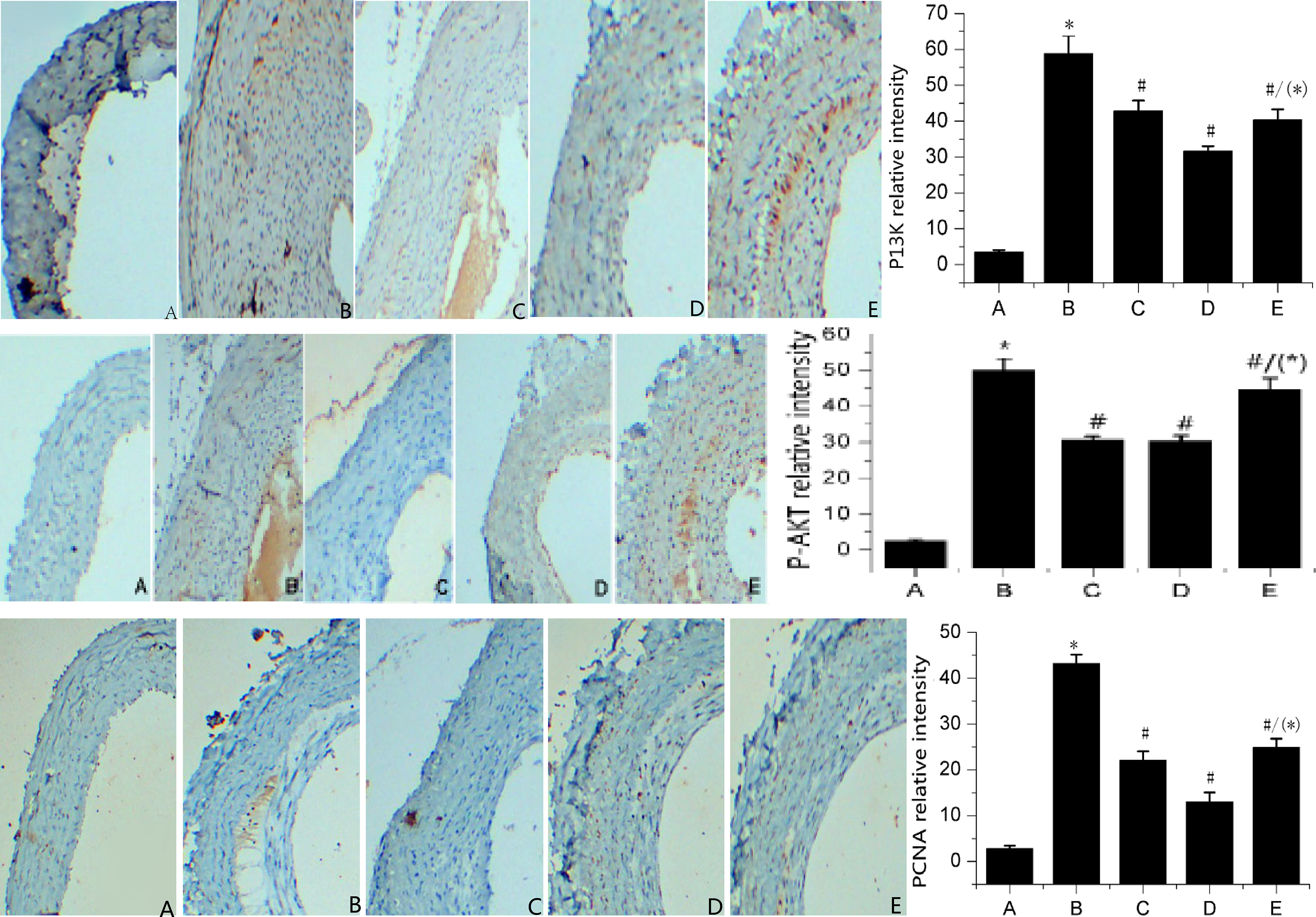
3.4Detecting the protein expression of PCNA, P-AKT, and PI3K via immunohistochemistry
Figure 4 shows that compared with the sham operation group, the protein expression of PCNA, P-AKT, and PI3K in the hyperplastic intima in common carotid arteries of the model group rats was significantly augmented (
Figure 4.
Protein expressions of PI3K, P-AKT, and PCNA for investigated groups. Note: A: Sham operation group; B: Model group; C: Low-dosage gefitinib group; D: High-dosage gefitinib group; E: Paclitaxel group; Comparison with sham operation group yields
3.5Detecting cell apoptosis in the hyperplastic intima via TUNEL staining
Figure 5 indicates a significant increase in the apoptotic cells in hyperplastic intima of common carotid artery of the model group rats, compared with the sham operation group ones (
Figure 5.
Growth of apoptotic cells in the investigated groups. Note: A: Sham operation group; B: Model group; C: Low-dosage gefitinib group; D: High-dosage gefitinib group; E: Paclitaxel group; Comparison with sham operation group yields
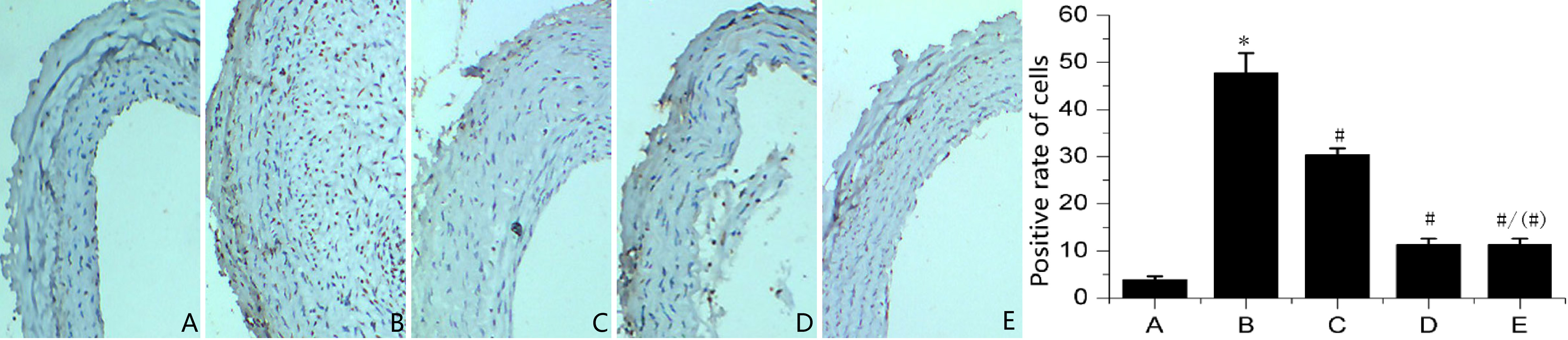
3.6Determining the relative expression of MMP9, TGFβ
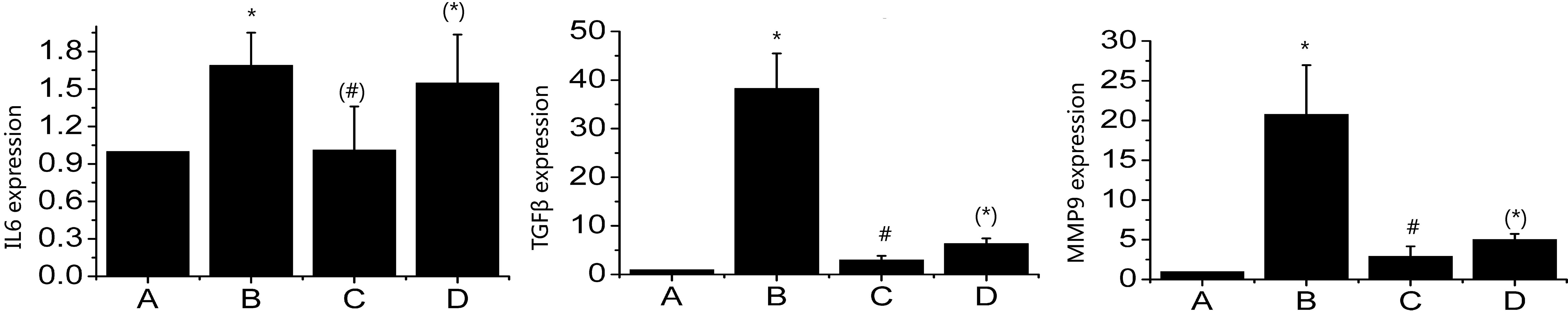
Figure 6 suggests that compared with the sham operation group, the expression of MMP9, TGF
Figure 6.
Expression levels of MMP9, TGF
4.Discussion
Percutaneous transluminal coronary angioplasty (PTCA) has become one of the regular vascular intervention treatment methods, yet the incidence rate of restenosis in 6 months after implantation of nude stent, reaching 10 to 20%, makes it the basic postoperative complication after intervention therapy [16]. The pathogenesis of restenosis after stent implantation is primarily correlated with the migration of VSMCs to intima and the continuous proliferation of VSMCs. At present, to reduce the incidence rate of stenosis after PCI, drug-coated stents (DCS) and DCB have been widely applied clinically, and the latter gained various achievements in prophylaxis and treatment of restenosis. Numerous studies reported that DCB, a novel instrument used for the intervention treatment for coronary heart disease, have exhibited promising safety and high effectiveness in prophylaxis of restenosis [17, 18, 19]. The major principle of DCB is that the drugs used for their coating should be rapidly released and absorbed by vascular wall, thereby rapidly suppressing the excessive proliferation of intima after vascular injuries [20]. However, paclitaxel or rapamycin, used as the drugs of DCS or DCB coatings, not only suppresses the excessive proliferation of intima after vascular injuries, but also hinders the migration and repair of endothelial cells, thereby severely affecting the endothelialization of stent after PCI and inducing the in-stent thrombosis or restenosis. Thus, cytotoxic drugs with a more accurate selectivity on SMCs will be the best choice for new generation of coating drugs.
Gefitinib is a selective inhibitor on EGFR-TKIs. Some researchers reported that proliferative SMCs and endothelial cells can express more EGFR than those in the resting state, In the in-vitro experiment of this study, we also found that gefitinib inhibits the proliferation of SMCs more effectively than paclitaxel but exhibits a relatively weak cytotoxic effect on endothelial cells, with a selective cytotoxic effect being also revealed in the endothelium and SMCs. Accordingly, the results achieved strongly suggest that gefitinib, a DCB coating drug, can effectively suppress the excessive SMC proliferation and intimal hyperplasia, while reducing the injuries to endothelial cells. However, the available in-vivo experimental results are insufficient to clarify whether gefitinib can effectively suppress the excessive SMC proliferation and intimal hyperplasia. In this study, gefitinib-coated DCB were used to observe the suppressive effect on excessive hyperplasia of intima after the common carotid artery of rats was injured by these DCB. The results showed that high- or low-dosage gefitinib-coated balloons can sufficiently suppress the excessive hyperplasia of intima after the common carotid artery of rats was injured with significant alleviations in stenosis of lumen in contrast to the sham group. Compared with the paclitaxel-coated DCB, high-dosage gefitinib-coated ones exhibit a stronger suppressive effect on the excessive proliferation of intima. These in-vivo experimental results revealed that gefitinib, a DCB coating drug, can effectively suppress the excessive hyperplasia of intima after the common carotid artery of rats was injured, and, thus, it can be recommended as a new-generation coating drug, while gefitinib-coated balloons, with a promising application, can be used in the new techniques for prophylaxis and treatment of restenosis after PCI.
Gefitinib, a member of EGFR-TKI family, is capable of regulating cell proliferation, differentiation and apoptosis by modulating the activation of the EGFR signal pathway [21]. The PI3K-AKT signal pathway is a vital part of the EGFR downstream signal pathway, and PI3K-AKT signal pathway plays critical roles in regulating the cell cycle, cell growth, proliferation and apoptosis according to previous studies [22, 23, 24]. PCNA, as an auxiliary protein of DNA polymerase, participates in the DNA synthesis and serves as a specific indicator of cell proliferation [25]. In this study, it has been found that, compared with the sham group, PCNA protein expression was significantly increased in the hyperplastic intima in the common carotid artery of rats in the control group, suggesting that rapid proliferation occurs in SMCs in the hyperplastic intima after intimal injuries. However, compared with the control group, dramatic decreases were found in the protein expressions of PCNA in the hyperplastic intima of the rats in the low- and high-dosage gefitinib-coated balloon groups and the paclitaxel-coated balloon group, indicating that gefitinib- and paclitaxel-coated balloons can suppress the proliferation of SMCs in the hyperplastic intima, whereas the suppressive effect in the high-dosage group is more obvious. Moreover, apoptosis is vital for the occurrence and development of many cardiovascular diseases. When the body is long subject to the action of various pro-apoptotic factors, Caspases cascade pathways in tissues can be activated in sequence, thereby inducing apoptosis. Bcl-2 has been proved to block the activation of caspase and suppress apoptosis [26]. Western blot results of the in vitro experiment here showed that the expression of Bcl-2 protein of the two groups was and the expression of caspase-3 protein were significantly down-regulated and significantly up-regulated, respectively, after 1umol/L gefitinib and paclitaxel were applied to VSMC and EC for 48 h, respectively. For the two types of cells, the variation of gray ratio was more obvious in the gefitinib group. This further revealed that gefitinib and paclitaxel had suppressive effects on both VSMC and EC and both promoted apoptosis. However, gefitinib had significantly stronger suppressive effects on VSMC than paclitaxel, while its inhibitory effects on EC were weaker than those of paclitaxel. Accordingly, it is further proved that gefitinib has a strong suppressive effect on VSMC and a weak suppressive effect on EC. In this experiment, the variations of apoptosis in hyperplastic endometrium were also observed in vivo. The results showed that apoptosis was found in the hyperplastic endometrium of the model group. This result is also similar to our in vitro experiment results and the results observed by other scholars, which may be related to the rapid increase of cell density caused by the excessive proliferation of smooth muscle cells in the hyperplastic endometrium, thereby suppressing cell contact and the induced apoptosis. It has been reported that gefitinib can suppress the PI3K-AKT signaling pathway by regulating the phosphorylation of mammalian target of rapamycin (mTOR) [27]. In this study, P-AKT and PI3K protein expressions were significantly up-regulated in the intima of carotid artery hyperplasia in the model group compared with those of the sham group. However, P-AKT’s and PI3K’s expressions were significantly down-regulated in the hyperplasia intima of rats in the low and high dose gefitinib balloon group and paclitaxel balloon group, which is consistent with the PI3K-AKT signaling pathway expression that we have previously studied in vitro. The results suggested that the mechanism of gefitinib and paclitaxel coated balloon suppressing endometrial hyperplasia was primarily associated with the suppression of smooth muscle cell hyperplasia and the promotion of apoptosis, and the specific suppressing mechanism of smooth muscle cell proliferation may have a relation to the suppression of the PI3K/AKT pathway. However, in-vivo experiments suggested that the proliferation of smooth muscle cells in the hyperplastic endometrium was suppressed after drug balloon intervention, and the cell contact suppression and apoptosis induced by it were significantly reduced. Thus, the primary mechanism is still considered to inhibit cell proliferation. TGF-
This study helps us understanding the suppressive effect of gefitinib on VSMC proliferation and its possible mechanism and provides a theoretical basis for the screening of stent-coated drugs in patients with coronary heart disease.
5.Conclusion
The results of this study prove that gefitinib-coated balloons suppress the excessive proliferation of SMC through regulating the PI3K-AKT signal pathway to block the intimal hyperplasia after injuries caused by balloon with a similar effect to the paclitaxel-coated balloons. However, the expression of the PI3K/AKT pathway in-vivo which detected by western blotting method requires further experimentation. Furthermore, endothelial protection and more effective safety evaluation need further studies, and this is also our next research direction.
Acknowledgments
The authors gratefully acknowledge the financial supports of the National Natural Science Foundation of China (Grant nos. 81270181 and 1202830).
Conflict of interest
None to report.
References
[1] | O’Donnell NG, Asbury AJ. Sirolimus- and taxol-eluting stents differ towards intimal hyperplasia and re-endothelialization. Journal of Invasive Cardiology, (2005) , 17: : 497-502. |
[2] | Zhixiong W, Jian L, Fang L, et al. Gefitinib Can More Specifically Inhibit Smooth Muscle Cell Proliferation Compared with Paclitaxel as A New Stent Coating Material. In: Electrical and Control Engineering & Materials Science and Manufacturing: The Proceedings of Joint Conferences of the 6th (ICECE2015) and the 4th (ICMSM2015). |
[3] | Vahidnezhad H, Youssefian L, Uitto J. Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses: diagnostic implications and treatment opportunities. J Investigative Dermatology, (2015) , 136: (1): 15-23. |
[4] | Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. American Journal of Cancer Research, (2015) , 5: : 1602-1609. |
[5] | Wu H, Fan F, Liu Z, Shen C, Wang A, Lu Y. Norcantharidin combined with EGFR-TKIs overcomes HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via inhibition of Met/PI3k/Akt pathway. Cancer Chemotherapy and Pharmacology, (2015) , 76: : 307-315. |
[6] | Yip PY. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR) signaling pathway in non-small cell lung cancer. Translationallung Cancer Research, (2015) , 4: : 165-176. |
[7] | Kim GD. Hesperetin inhibits vascular formation by suppressing of the PI3K/AKT, ERK, and p38 MAPK signaling pathways. Preventive Nutrition and Food Science, (2014) , 19: : 299-306. |
[8] | Li Y, Fu S, Chen H, Feng Q, Gao Y, Xue H. Inhibition of endothelial Slit2/Robo1 signaling by thalidomide restrains angiogenesis by blocking the PI3K/Akt pathway. Digestive Diseases and Sciences, (2014) , 59: : 2958-2966. |
[9] | Tetsu O, Phuchareon J, Eisele DW, Hangauer MJ, Mccormick F. AKT inactivation causes persistent drug tolerance to EGFR inhibitors. Pharmacological Research, (2015) , 102: : 132-137. |
[10] | Kunter I, Erdal E, Nart D, Yilmaz F, Karademir S, Sagol O. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncology Reports, (2014) , 31: : 573-80. |
[11] | Segarra M, Garcíamartínez A, Snchez M, Hernndezrodríguez J, Lozano E, Grau JM. Gelatinase expression and proteolytic activity in giant-cell arteritis. Annals of the Rheumatic Diseases, (2007) , 66: : 1429-35. |
[12] | Zabini D, Granton E, Hu Y, Miranda MZ, Weichelt U, Breuils BS. Loss of SMAD3 promotes vascular remodeling in pulmonary arterial hypertension via MRTF disinhibition. American Journal of Respiratory & Critical Care Medicine, (2017) , 1. |
[13] | Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respiratory Research, (2009) , 10: : 6. |
[14] | Normanno N, Gullick WJ. Epidermal growth factor receptor tyrosine kinase inhibitors and bone metastases: different mechanisms of action for a novel therapeutic application? Endocrine-related Cancer, (2006) , 13: : 3. |
[15] | Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR. Inhibition of PDGF, TGF-β, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. Journal of Hepatology, (2011) , 55: : 612. |
[16] | Meraj PM, Jauhar R, Singh A. Bare metal stents versus drug eluting stents: where do we stand in 2015. Curr Treat Options Cardiovasc Med, (2015) , 17: : 393. |
[17] | Scheller B, Speck U, Abramjuk C, Bernhardt U, Böhm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation, (2004) , 110: : 810-814. |
[18] | Xu B, Gao R, Wang J, Yang Y, Chen S, Liu B. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the PEPCAD China ISR trial. JACC Cardiovasc Interv, (2014) , 7: : 204-211. |
[19] | Gao L, Chen YD. Application of drug-coated balloon in coronary artery intervention: Challenges and opportunities. J Geriatr Cardiol, (2016) , 13: : 906-913. |
[20] | Cortese B, Bertoletti A. Paclitaxel coated balloons for coronary artery interventions: a comprehensive review of preclinical and clinical data. Int J Cardiol, (2012) , 161: : 4-12. |
[21] | Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys, (2004) , 59: : 21-26. |
[22] | Li L, Wang S, Zheng F. Chinese herbal medicine Fuzheng Kang-Ai decoction sensitized the effect of gefitinib on inhibition of human lung cancer cells through inactivating PI3-K/Akt -mediated suppressing MUC1 expression. Ethnopharmacol, (2016) , 194: : 918-929. |
[23] | Zhou G, Zhang F, Guo Y, Huang J, Xie Y, Yue S. MiR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibits cell migration via targeting ZEB1. Biomed Pharmacother, (2017) , 85: : 113-119. |
[24] | Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer, (2013) , 14: : 322-32. |
[25] | Takada M, Tanaka H, Yamada T, Ito O, Kogushi M, Yanagimachi M. Antibody to thrombin receptor inhibits neointimal smooth muscle cell accumulation without causing inhibition of platelet aggregation or altering hemostatic parameters after angioplasty in rat. Circ Res, (1998) , 82: : 980-987. |
[26] | Quinn L. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO (European Molecular Biology Organization) Journal, (2003) , 22: (14): 3568-3579. |
[27] | Moore JC, Sumerel JL, Schnackenberg BJ, Nichols JA, Wikramanayake A, Wessel GM. Cyclin D and cdk4 are required for normal development beyond the blastula stage in sea urchin embryos. Mol Cell Biol, (2002) , 22: : 4863-75. |
[28] | Hasegawa A, Sato K, Shirai R, Watanabe R, Yamamoto K, Watanabe K. Vasoprotective effects of urocortin 1 against atherosclerosis in vitro and in vivo. Plos One, (2014) , 9: : e110866. |
[29] | Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration the role of ERK1/2, PI3K, and matrix metalloproteinases. Circulation Research, (2004) , 95: : 364-71. |




