The coexistence of a patent foramen ovale and obstructive sleep apnea may increase the risk of wake-up stroke in young adults
Abstract
BACKGROUND:
Patent foramen ovale (PFO) and obstructive sleep apnea (OSA) are independent risk factors for young conscious stroke which may also be concomitant symptoms with it. But there is no sufficient attention on these phenomena.
OBJECTIVE:
To investigate the relationship between PFO, OSA and young stroke, and to look for proper treatment.
METHODS:
Three patients with young conscious stroke were reported, each of them was combined with PFO and OSA. All patients were diagnosed as wake-up stroke (WUS). Contrast-enhanced transcranial doppler ultrasound (c-TCD) and polysomnography (PSG) test were used for auxiliary diagnosis.
RESULTS:
Right-to-left shunts and moderate to severe sleep apnea were observed. Increased body mass index (BMI), hemoglobin (HGB) and hematocrit (HCT) index were also observed. After continuous positive airway pressure (CPAP) therapy, the number of microbubbles was reduced in one patient.
CONCLUSIONS:
These suggest that coexistence of PFO and OSA may associate with a greater risk of youth stroke. Decrease risk of stroke might occur if treating with CPAP in patients with OSA.
1.Introduction
According to recent studies millions of people have suffered a stroke resulting in a stroke-related death and disability per year, producing an extremely high burden. Wake-up stroke (WUS), defined as a stroke symptom under an awakened situation that was not present prior to falling asleep, affects about 20% of patients with stroke [1]. Significant evidence demonstrated a rising stroke incidence in young adults [2]. There are multiple etiological mechanisms underlying youth stroke. Classical vascular risk factors in the young could be acceptable reasons [3]. Patent foramen ovale (PFO) is a common congenital heart malformation with atrial septal abnormalities in adult. Like the function of a valve, it can cause right-to-left shunting (RLS). Indicated as one of the most important underlying causes in young adults with stroke, PFO was detected in 50% of the patients with cryptogenic stroke [4]. Affecting up to 24% of men and 9% of women is obstructive sleep apnea (OSA), a common disorder [5]. Resulting in increased right atrial pressure, it is characterized by intermittent hypoxia occurring during sleep, which induces hypoxic pulmonary vasoconstriction. Increasing the risk of stroke and cardiovascular morbidity, which may also provide the nidus for systemic embolization, OSA confers an increased incidence of hypertension, nocturnal arrhythmias and hematological disturbances. Thus PFO is usually asymptomatic and OSA is frequently undiagnosed. This result then helps to explain the fact that the association between both of these pathologies is not well known and little studied.
In order to investigate the risk of coexistence of a PFO and OSA in youth stroke, we reported these 3 young WUS patients coexisted with symptomatic OSA and PFO. In the present study, a written informed consent was obtained from each patient.
2.Materials and methods
2.1Contrast-enhanced transcranial Doppler ultrasound (c-TCD)
Using a Multi-DopX4 TCD detector (DWL, Sipplingen, Germany; EME, Companion III , Germany) with bilateral middle cerebral artery (MCA) monitoring, contrast-enhanced TCD examinations were performed. In the supine position, patients had an 18-gauge needle inserted into the cubital vein. Using 9 mL of saline solution, 1 mL air and a drop of the patient’s blood, contrast agent was prepared which was vigorously mixed between two 10 mL syringes via a three-way stopcock. The contrast agent, after 30 mixing cycles, was rapidly injected as a bolus. Then all patients underwent in three different positions: supine, left lateral decubitus and upright sitting. Testing was conducted during normal breathing and subsequently with Valsalva maneuver (VM). Within 20 seconds after VM, the microbubbles (MBs) were recorded. An MB was thus defined as an audible and visible click, chirp or whistle with a short duration and a high-intensity signal within the doppler flow spectrum. The classification, according to the number of MBs, was as follows: none (0 MB, negative result); mild (1–10 MBs); moderate (11–25 MBs); and large (
2.2Polysomnography (PSG) measurement
Conducted using standard digital polysomnographic evaluation with an Alice 5 Diagnostic Sleep system (Philips Respironics Inc., Murrysville, PA, USA) was an overnight PSG. Recorded was apnea-hypopnea index (AHI), mean oxygen saturation (MSaO), low Oxygen saturation (LSaO) and thoracic and abdominal movement. Diagnosis and severity of OSA, according to the AASM criteria, was determined by AHI. Defined as an AHI of
2.3Case presentations
2.3.1Case 1
Table 1
General clinical data and test results in 3 patients
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Gender | Male | Male | Male |
| Age | 29 | 33 | 44 |
| Medical history |
|
|
|
| Personal history |
|
|
|
| Family history |
|
|
|
| Blood pressure |
|
|
|
| Hemoglobin |
|
|
|
| Erythrocyte ratio |
|
|
|
| Blood glucose |
|
|
|
| Uric acid |
|
|
|
| Low-density lipoprotein |
|
|
|
| Abnormalliver function |
|
|
|
| Homocysteine |
|
|
|
| Immune index |
|
|
|
Table 2
Embolus signal characteristics of c-TCD in 3 patients
| Calm breath | Valsalva maneuver | ||||
|---|---|---|---|---|---|
| TM | Quantity | TM | Quantity | Number of emboli after the action | |
| Case 1 | 4th cardiac cycle | 1 | 6th cardiac cycle | Rain curtains | 2 |
| Case 2 | 9th cardiac cycle | 2 | 8th cardiac cycle | Rain curtains | 1 |
| Case 3 | 0 | 4th cardiac cycle | Rain curtains | 4 | |
TM
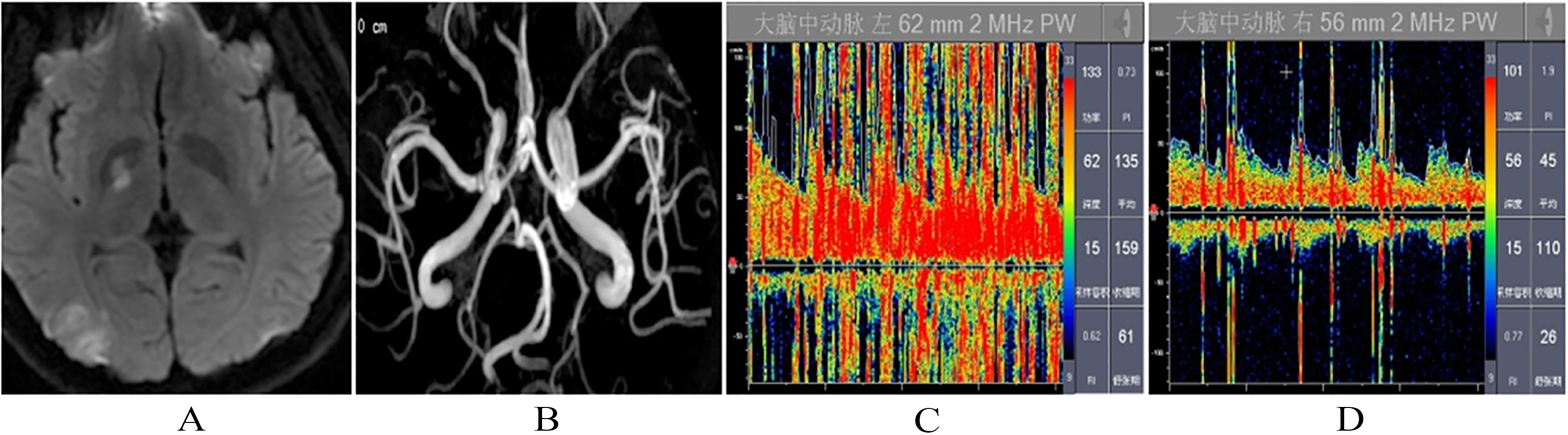
When he woke up in the morning, a 29-year-old man, with a history of hypertension and headache, noted the right limb numbness. Although he had a past history of hypertension and headache, the patient had no special family history. His body weight was 110 kg, while his height was 172 cm. His general physical examination was normal and blood pressure was 150/100 mmHg. He was both alert and oriented. Even though his speech was dysarthric, it was correctly responsive to verbal commands. With bilaterally horizontal nystagmus, adduction of both eyes was preserved. Deviating to left was the tongue. Found with extensor plantar response on left lower extremity, was mild weakness of the left upper limb extensors and left lower limb flexors. Furthermore, his pain sensation of left upper and lower extremities was lost. Hyperintensity of the right basal ganglia and right occipital lobe using fluid-attenuated inversion recovery (FLAIR) sequences were revealed by brain magnetic resonance imaging (MRI) (Fig. 1A). Performed 48 hours later, Magnetic resonance angiography (MRA) of neck and intracranial arteries exhibited normal (Fig. 1B). Showing no abnormal finding was dynamic ECG, vascular ultrasound of the neck and echocardiography. The following examinations were negative: anti-neutrophil cytoplasmic antibodies, anticardiolipin antibodies, antinuclear antibodies, ENA spectrum, antithrombin, homocysteine. Blood routine examination showed hemoglobin 170 g/L, hematocrit 50.8%; Uric acid 505 umol/L; alanine transaminase (ALT) 93 U/L, glutamic oxalaceticaminopherase (GOT) 52 U/L, low density lipoprotein cholesterol (LDL) 4.06 mmol/L (Table 1). In the state of calm breathing by c-TCD test, there is no embolus signal. Detected in the fourth cardiac cycle after Valsalva action were a large number of microembolic signals (rain curtain shape, Fig. 1C). Disappearing after the end of Valsalva action was the embolism rain (Table 2). TEE examination showed PFO (Fig. 1D). Sleep stage test found normal sleep latency through whole night PSG examination. Breathing and related events: In the night sleep, more than 80% of the time in the supine position and AHI is 93.5 times/hour, of which 836 is documented as obstructive apnea (up to 64.5 seconds). The lowest and average oxygen saturation were 29% and 78%, respectively; more related to respiratory abnormalities is the micro-arousal index which measured 91.3 times per hour; Accounting for 11.0% of sleep time was snoring time. These results were in line with diagnostic criteria for severe “obstructive sleep apnea hypopnea syndrome” (Table 3). The patient was given antiplatelet treatment causing the neurological impairment recovered gradually. Examination of c-TCD showed the embolic signal was significantly reduced and classified as mild after CPAP treatment at home for 3 months.
Figure 1.
The main observation results in patient case 1: (A) MRI of the right basal ganglia and right occipital lobe. (B) Magnetic resonance angiography (MRA) of neck and intracranial arteries. (C) c-TCD test in the fourth cardiac cycle after Valsalva action. (D) TEE examination.
2.3.2Case 2
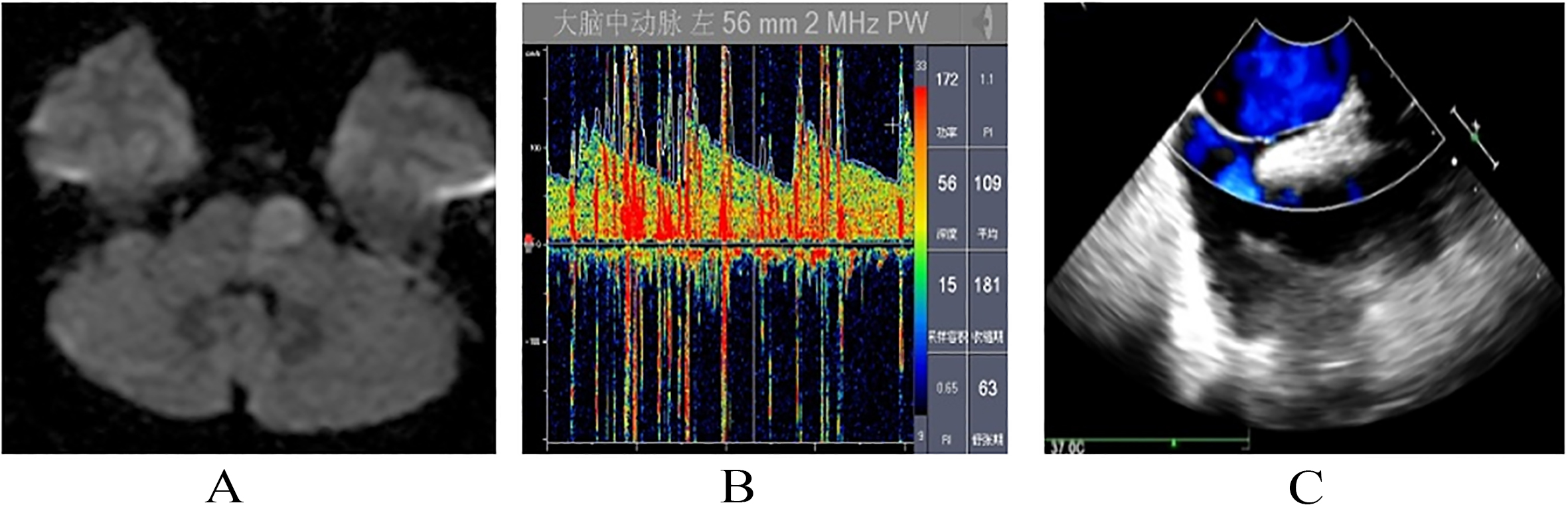
When waking in the early morning (3:30 am), a 33-year-old male presented with persistent right limb weakness and verbal confusion. There was no record of special past history or family history. He smoked 2–5 cigarettes per day and had been drinking for 10 years. His height was 170 cm and weight 81 kg, while his blood pressure was 155/110 mmHg. The subject was alert and conscious and his speech was dysarthric with right central facial weakness. Found with extensor plantar response on right lower extremity was mild weakness of the right upper limb extensors and right lower limb flexors. Abnormal long signal of the left pontine was showed in brain MRI by FLAIR sequences (Fig. 2A). Conducting Computed Tomography Angiography (CTA) of neck and intracranial arteries revealed limited narrow on the right anterior cerebral artery A2 segment and the left anterior cerebral artery segment A3 and limited moderate stenosis on the initial segment of the right vertebral artery (Fig. 2B). Echocardiography on dynamic ECG, color Doppler ultrasound of the neck and echocardiography were found to be normal. Measurements of hemoglobin 170 g/L, hematocrit 46.1%, ALT 48 U/L, GOT 278 U/L, blood glucose 7.6 mmol/L, homocysteine 17.2 umol/L, triglyceride 10.5 mmol/L, LDL 3.38 mmol/L. Results of the examination of anti-neutrophil cytoplasmic antibodies, anticardiolipin antibodies, antinuclear antibodies, ENA spectrum and antithrombin III were all normal (Table 1). Two emboli were detected during 9th cardiac cycle when the patient breathed quietly by c-TCD test. During the 8th cardiac cycle (rain curtain) after Valsalva action, a large number of embolus signals were revealed (Fig. 2C). After Valsalva action, embolism rain disappeared (Table 2). TEE examination is not tolerated by patients. Sleep stage test reviewed normal sleep latency after whole night PSG examination. The lowest and average oxygen saturation was 72% and 93%, respectively. Snoring time accounted for 38.0% of sleep time. In summary, the diagnosis for the patient was “obstructive sleep apnea syndrome” (Table 3).
Table 3
Results of polysomnography monitoring in 3 patients
| Monitoring items | Case 1 | Case 2 | Case 3 | |||
|---|---|---|---|---|---|---|
| Total sleep time (min) | 591 | .5 | 629 | .5 | 579 | .5 |
| NREM I (%) | 71 | .6 | 25 | .5 | 65 | .4 |
| NREM II (%) | 10 | .0 | 49 | .6 | 7 | .4 |
| NREM III (%) | 0 | .0 | 0 | .8 | 0 | .4 |
| REM (%) | 10 | .1 | 18 | .3 | 11 | .9 |
| Apnea hypopnea index (AHI) | 93 | .5 | 46 | .1 | 102 | .4 |
| The longest period of apnea (s) | 64 | .5 | 77 | .5 | 46 | .0 |
| Minimum oxygen saturation (%) | 29 | .0 | 72 | .0 | 32 | .0 |
| Average oxygen saturation (%) | 78 | .0 | 93 | .0 | 95 | .0 |
| BMI (kg/m | 37 | .2 | 28 | .0 | 28 | .1 |
| Epworth Sleepiness Scale | 17 | .0 | 10 | .0 | 15 | .0 |
REM
Figure 2.
The main observation results in patient case 2: (A) Brain MRI of left pontine by FLAIR sequences. (B) Conducting Computed Tomography Angiography (CTA) of neck and intracranial arteries. (C) c-TCD test in the 8th cardiac cycle after Valsalva action.
2.3.3Case 3
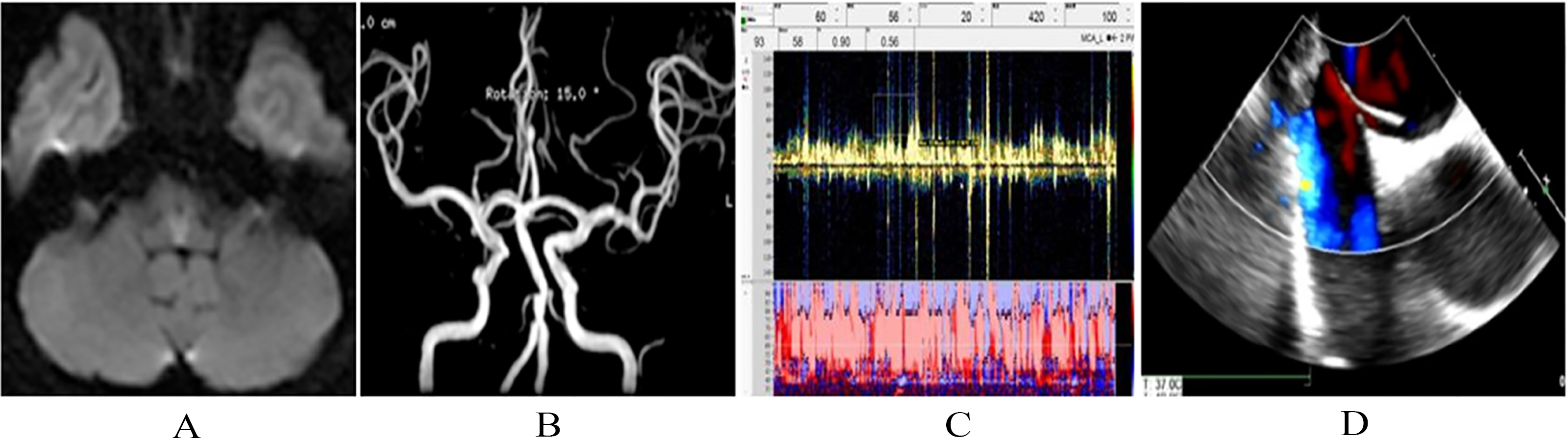
A 44-year-old male, presenting with sudden numbness, weakness throughout whole body and pain sensibility, at admission at 4:30 am, could not walk. His height was 174 cm and weight was 85 kg, and his blood pressure was 140/100 mmHg. Limb muscle strength 4
Figure 3.
The main observation results in patient case 3: (A) Brain MRI of bilateral medulla. (B) MRA of basal artery. (C) c-TCD test in the 4th cardiac cycle after Valsalva action. (D) TEE examination.
3.Discussion
Three cases of WUS in young men with coexistence of PFO and OSA were the focus of the present study. The risk of stroke in men has been shown to increase with OSA [7]. The study of awake stroke and non-awake stroke showed that the incidence of WUS was higher in males than in females. Interestingly, men with stroke were younger than women in the general OSA population [8, 9]. Frequent severe hypoxemia and poor tolerance to hypoxia in men than in women at night, especially in young men, might explain the possibility of high incidence of WUS [10]. In this study the three cases were male. With longer duration, significant autonomic nerve involvement, fluctuations in blood pressure, OSA hypoxia were very severe during REM. As well, efforts to breathe caused abnormal chest pressure, which increased the number of emboli from right to left shunt in PFO, thereby increasing the probability of stroke. Associated with a greater incidence and a greater degree in WUS is OSA [8]. Until this study, no large-scale correlation study between PFO and awakened stroke has been published.
The micro-emboli from the venous system, under a normal situation, were filtered by a huge capillary network after entering the pulmonary circulation rather than entering the left cardiac cavity. In PFO case, emboli in venous system such as fat, gas, thrombus emboli, serotonin, parasites, bacterial emboli, reflux to the right atrium and then arrive in the left atrium through the patent foramen ovale. PFO case then reaches to systemic circulation to become abnormal emboli. That the right atrial pressure exceeds the left atrium, a necessary condition for PFO, is more easily provided by patients with OSA. PFO and OSA coexistence significantly increased the risk of stroke. Young patients with apoplectic stroke, and c-TCD and PGS examination combined with PFO and OSA were the subjects reported in this paper. Highly consistent, these patients had common disease characteristics, gender, morbidity, obesity, high hemoglobinemia and high blood pressure. Possible risk factors for PFO/OSA and stroke are these clinical features.
Whether PFO occlusion or CPAP, or after antiplatelet therapy or anticoagulation after stroke, there is no clear specification for the treatment of patients with OSA combined with PFO. Pinet reported a case of severe OSA in with PFO was treated by CPAP for one week. Drastically decreasing from 19% to only 6% was the right-to-left shunt [11]. Reports indicated that PFO interventional occlusion significantly improved nocturnal hypoxia accompanied with OSA [12, 13, 14]. Individual cases are reported limited to these studies. Reports indicate that, compare with drug therapy, anticoagulation is superior to antiplatelet [15]. A new guideline for treatment of recurrent stroke with PFO was recently published by the American Academy of Neurolog. The guideline published clearly states transcatheter percutaneous closure of the PFO for the treatment of patients with unexplained ischemic stroke is not recommended because stroke does not have an additional benefit compared to surgical complications. The guideline emphasizes that anticoagulant drugs are not superior to antiplatelet drugs compared with drugs that can significantly reduce stroke recurrence for stroke prevention. While the efficacy of novel anticoagulants is being further studied, the usage of antiplatelet agents is recommended [16]. Following the new guideline the prognosis of these patients were improved after we carried out antiplatelet therapy. Examination of c-TCD of patients who were given the CPAP treatment showed that PFO right to left shunt were significantly reduced.
4.Conclusions
Patients with unexplained WUS, especially overweight young male patients with erythrocytosis, should participate in periodic FPO and OSA screening. It is more probable to find a coexistence of a PFO, OSA and WUS. Having great clinical significance is early diagnosis for treatment options and disease recurrence assessment and the establishment of secondary prevention program for the subsequent stroke.
Acknowledgments
This work was supported by grants from Shandong Provincial Natural Science Foundation of China (ZR2019MH090), the Colleges and Universities Technology Plan Item Foundation of Shandong Province (J17KA139) and the Projects of Medical and Health Technology Development Program of Shandong Province (2016WS0607, 2017WSA10002).
Conflict of interest
None to report.
References
[1] | Thomalla G, Gerloff C. Treatment Concepts for Wake-Up Stroke and Stroke With Unknown Time of Symptom Onset, Stroke. (2015) ; 46: (9): 2707-13. |
[2] | Bejot Y, Delpont B, Giroud M. Rising Stroke Incidence in Young Adults: More Epidemiological Evidence, More Questions to Be Answered, Journal of the American Heart Association. (2016) ; 5: (5). |
[3] | de los Rios F, et al., Trends in Substance Abuse Preceding Stroke Among Young Adults A Population-Based Study, Stroke. (2012) ; 43: (12): 3179-3183. |
[4] | Ali Ebrahimi H, Hamzeaie Moghadam A, Aredestani E. Evaluation of patent foramen ovale in young adults with cryptogenic stroke, ARYA Atheroscler. (2011) ; 7: (2): 74-7. |
[5] | Wimms A, et al., Obstructive Sleep Apnea in Women: Specific Issues and Interventions, Biomed Res Int. (2016) ; 2016: : 1764837. |
[6] | Collop NA, et al., Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients, Journal of Clinical Sleep Medicine. (2007) ; 3: (7): 737-+. |
[7] | Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study, Am J Respir Crit Care Med. (2010) ; 182: (2): 269-277. |
[8] | Koo BB, et al., Observational Study of Obstructive Sleep Apnea in Wake-Up Stroke: The SLEEP TIGHT Study, Cerebrovasc Dis. (2016) ; 41: (5-6): 233-41. |
[9] | Koo BB, et al., Rapid eye movement-related sleep-disordered breathing: influence of age and gender, Chest. (2008) ; 134: (6): 1156-61. |
[10] | Philip P, et al., Prevalence and correlates of nocturnal desaturations in a sample of elderly people, J Sleep Res. (1997) ; 6: (4): 264-71. |
[11] | Pinet C, Orehek J. CPAP suppression of awake right-to-left shunting through patent foramen ovale in a patient with obstructive sleep apnoea, Thorax. (2005) ; 60: (10): 880-881. |
[12] | Agnoletti G, et al., Obstructive sleep apnoea and patent foramen ovale: Successful treatment of symptoms by percutaneous foramen ovale closure, J Interv Cardiol. (2005) ; 18: (5): 393-5. |
[13] | Silver B, Greenbaum A, McCarthy S. Improvement in sleep apnea associated with closure of a patent foramen ovale, J Clin Sleep Med. (2007) ; 3: (3): 295-6. |
[14] | White JM, Veale AG, Ruygrok PN. Patent foramen ovale closure in the treatment of obstructive sleep apnea, J Invasive Cardiol. (2013) ; 25: (8): E169-71. |
[15] | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L. CLOSURE I Investigators, Closure or medical therapy for cryptogenic stroke with patent foramen ovale, N Engl J Med. (2012) ; 366: (11): 991-9. |
[16] | Messe SR, et al., Practice advisory: Recurrent stroke with patent foramen ovale (update of practice parameter): Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology, Neurology. (2016) ; 87: (8): 815-21. |




