Prospects for Stem Cell-Based Regenerative Therapies in India
Abstract
Stem cells offer a promising therapeutic strategy to not only treat several incurable diseases but also regenerate damaged tissues. The current global boom in the field of stem cell and regenerative therapies had led to India becoming a global hotspot for stem cell-based therapies. In this review, we assess the current status of stem cell therapy trials in India and show that the bone marrow-derived stem cells, like mesenchymal stem/stromal cells (MSCs), are predominantly used. Phase 1 and 2 clinical trials have also used MSCs to alleviate symptoms of severe novel coronavirus infections. Recent breakthroughs in gene editing technologies, combined with stem cell therapy, can be effectively harnessed to devise large-scale and affordable treatments for haematological diseases that are highly prevalent in India, like beta-thalassemia and sickle cell diseases. Innovations in stem cell therapy in India can make treatments more affordable to address the needs of in-country patients.
ABBREVIATIONS
NA | Data not available |
MSC | Mesenchymal stem/stromal cells |
MNC | Mononuclear cells |
HSC | Hematopoietic stem cells |
Ad-MSC | Adipose derived-MSC |
INTRODUCTION
The contribution of stem cells to modern medicine is immense as they can not only replace damaged cells or tissues but can also help create relevant disease models for developing novel therapies. For e.g., by growing patient-derived induced pluripotent stem cells (iPSC) and generating organoids, scientists have generated models mimicking cystic fibrosis and Alagille syndrome [1, 2]. Stem cell therapy has shown promising results in preclinical and clinical trials for several diseases including, Parkinson’s disease, diabetes mellitus, Crohn’s disease, and various hematological disorders [3–6]. Nevertheless, many hurdles remain, and considerable efforts are needed to address some of the key issues in stem cell therapy like immune rejection, genetic instability of stem cells and ethical issues concerning the use of embryonic stem cells for therapy [7–9].
A growing the number of chronic diseases like diabetes, sickle cell anaemia, thalassemia, and myocardial infarction in countries like India require multiple novel treatment strategies, including stem cell-based regenerative therapies that need to be affordable and accessible to most Indians. In this review, we assess the current scenario of stem cell-based therapies in India and the conditions targeted based on data available in the public domain. We found that bone marrow-derived stem cells, such as mesenchymal stem/stromal cells (MSC) or Hematopoietic stem cells (HSCs), are predominantly used as cell sources, and that the use of other stem cells, like embryonic stem cells (ESC) or iPSCs, is negligible. As India continues to show progress in information technology and biotechnological research, it is also likely to show similar a trend and bring innovation to stem cell-based therapies that are efficient, scalable, and affordable.
CLASSIFICATION OF STEM CELLS AND THEIR CHARACTERISTICS: INDIAN PERSPECTIVE
Stem cells are, by definition, undifferentiated cells that have the ability to self-renew and differentiate into all cell types of the organism. Stem cells can be isolated from the early stages of embryos, such as from the blastocyst, or from certain adult tissues, like the bone marrow, adipose tissue, or skin. Importantly, their differentiation potential depends on their origin, and readers are requested to refer to extensive reviews on stem cells [8, 10]. Briefly, totipotent cells such as zygotic cells can differentiate into all the three germ layers, namely the ectoderm, the mesoderm, and the endoderm, and also into extra embryonic tissues like the placenta. Stem cells derived from blastocysts in the inner cell mass (i.e., 5-6 days post fertilization) are called human embryonic stem cells (hESCs), are pluripotent, and can differentiate into all 3 germ layers but not into extra-embryonic tissues (Fig. 1) [8]. In India, multiple hESC lines have been established from embryos deemed not suitable for in vitro fertilization. They reflect the genetic diversity of the Indian population and are available through stem cell repositories. For example, BJNhem20 is a hESC line, developed by the Jawaharlal Nehru Center for Advanced Scientific Research (JNCASR), Bangalore, has been submitted to the UK stem cell bank (UKSCB R-08-021), and is listed in the European Pluripotent Stem Cell registry and the National Institutes of Health (NIH) Human Embryonic Stem Cell Registry (NIH Approval No. NIHhESC-10-0084) [11, 12]. Several other well-characterized hESC lines from India include BJNhem19, KIND1, KIND2, and Relicell hES1-4, which have been developed by various publicly funded labs and private companies [11, 13, 14].
Fig. 1
Stem cell types and characterization: Totipotent stem cells can form embryonic and extra embryonic tissue, and zygotic cells are the best example of such totipotent cells. Totipotent cells of the zygote develop into a blastocyst, and the cells of the inner cell mass can be isolated and cultured as embryonic stem cells (ESC), which are pluripotent (i.e., can form embryonic cells but not extra embryonic cell types). Multipotent stem cells like MSCs and HSCs can give rise to multiple cell types, and MSCs can be found in the developing fetus, umbilical cord, and in adult tissues like the bone marrow. Terminally differentiated cells can be reprogrammed to become pluripotent cells by exposing them to certain signals called the reprogramming factors, and such cells are called induced pluripotent stem cells (iPSC).
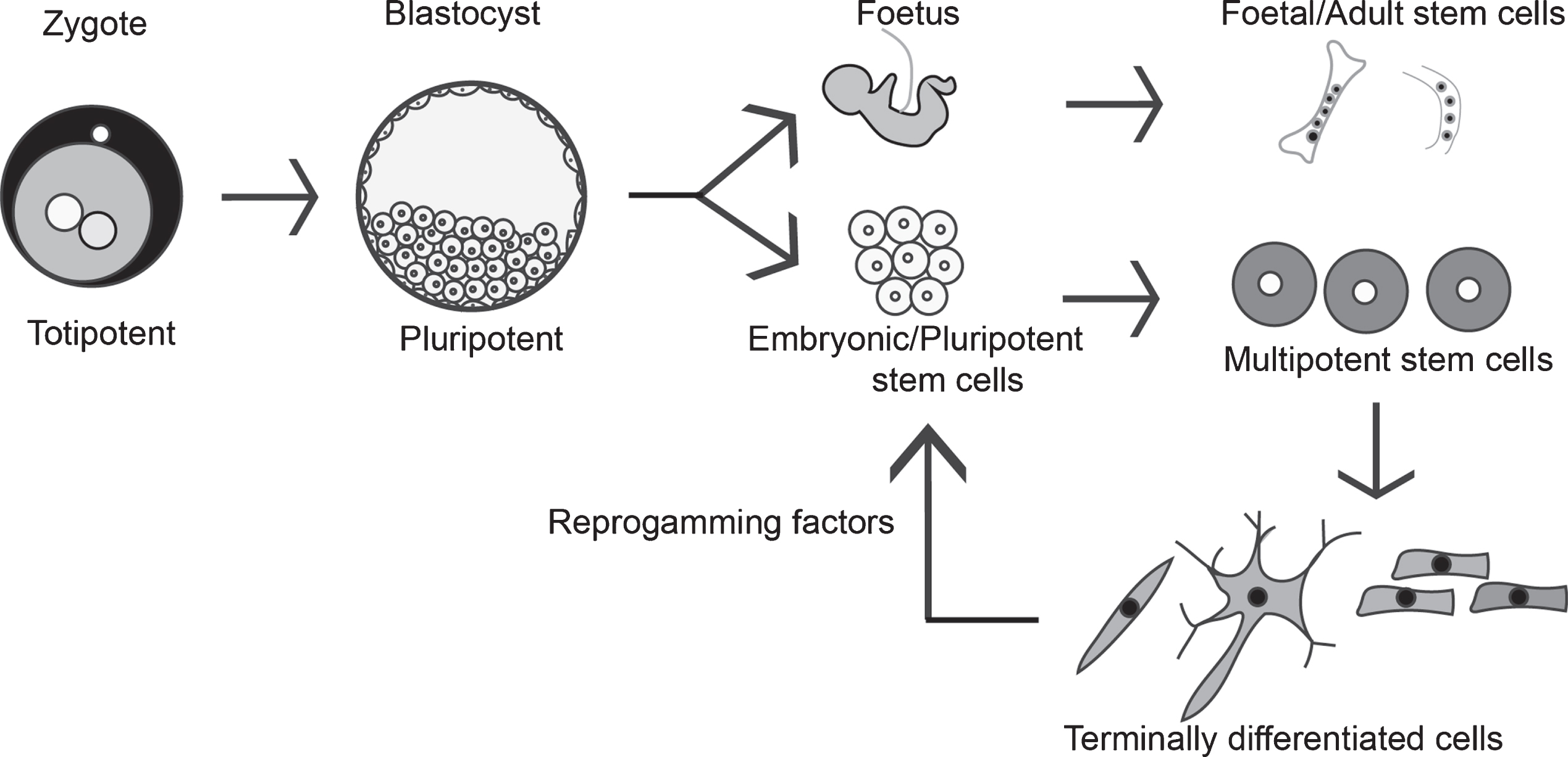
Pluripotency can also be induced under certain conditions, e.g., by treating somatic cells with a combination of induction factors like OCT3/4, Sox2, Klf4a, and c-Myc, and such cells are called iPSCs. These inducing factors are also referred to as Yamanaka factors or reprogramming factors and are named after the author of the seminal paper that identified these factors (Fig. 1) [15]. In India, several iPSC lines from healthy individuals (example, NCCSi005A and NCCSi006A), and patient-derived cell lines have been established to study familial Alzheimer’s disease (example, NCBSi001-A, NCBSi002-A) from a well-characterized dementia cohort in the Indian population [16, 17]. A biorepository has been established as a resource in India (https://www.ncbs.res.in/adbs/bio-repository) to store iPSC lines covering five major mental illness, namely, addiction, dementia, obsessive compulsive disorder, bipolar disorder, and schizophrenia, along with cognate control cell lines. Further, multi-institutional collaborations between stem cell labs in India and the Centre for iPS Cell Research and Applications (CiRA), Kyoto, Japan, have been established for iPSC technology and knowledge dissemination.
Stem cells are not restricted to embryos—they are also found in the developing foetus and in adult organs where their functions are to maintain tissue homeostasis and repair damage tissues. Multipotent stem cells, such as HSC, are found in adult bone marrow and can differentiate into all cell types of the blood lineage, during which unipotent or bipotent progenitor intermediates are generated. Multipotent stem cells are also found in other tissues like the gut, where intestinal stem cells located at the base of the crypts in the adult intestinal epithelium can give raise to enterocytes, goblet cells, enteroendocrine, and Paneth cells upon differentiation [8, 18, 10].
Mesenchymal stem cells, also known as mesenchymal stromal cells (hereafter abbreviated as MSCs), are a heterogenous mixture of multipotent stem cells that can differentiate into several lineages, including adipose tissue, osteoblasts, and muscles. MSCs are present in wide variety of tissues like bone marrow, adipose tissue, cord tissue, and dental pulp [19]. Multiple publically funded labs and biotech companies in India have generated allogenic MSCs to treat various disease conditions [20, 21]. In contrast to multipotent stem cells, unipotent stem cells have a restricted ability to differentiate, i.e., they can only give rise to one particular cell type. For example, the cornea acts as a protective barrier against infectious agents and Limbal Epithelial Stem Cells (LESC) are found in the limbal region of the cornea where their primary role is to maintain normal tissue homeostasis and to replace damaged cells [22]. Such unipotent stem cells are also present in various other tissues, for example, spermatogonial stem cells present in the testis that can only make sperm. In tissues like the bone marrow, skin, or the gut, there is a high turnover of cells (with shorter lifespan) and the stem cells reside in a “stem cell niche”, which is a micro-environment that favours their self-renewal and facilitates constant supply of differentiated cells to their respective tissues. In certain tissues like the brain, the stem cells are limited in number and they contribute to the cell types with low turnover/longer lifespan [23].
STEM CELL THERAPY
Stem cell-based therapies offer great potential as novel therapeutic agents to restore and regenerate damaged or injured tissues. Currently, various types of stem cells, including embryonic and iPSCs, and diverse adult stem cells, are being used to treat various diseases across the globe. HSCs have been extensively used over the last 60 years in the form of bone marrow transplants to treat various hematological cancers and blood disorders; thus, HSCs are the most used stem cell type in the clinic. In the 1960s, Tim and McCulloch defined two hallmarks of HSCs, namely, (i) they can self-renew, and (ii) they can give rise to all types of blood cells [24], and thus opened the field of stem cell biology. The ease of isolating HSCs from various sources, like bone marrow, peripheral blood, and cord blood, among others, makes them an excellent stem cell type for transplantation and for treating various blood disorders. Currently, more than 50,000 bone marrow transplants are being performed annually worldwide and such transplants are the defined standard of care for several hematological disorders [25].
After HSCs, MSCs are the most widely used and preferred cell type for therapy due to ease of isolation from various tissues. Further, several preclinical studies have demonstrated the anti-inflammatory and immune modulatory role of MSCs, leading to them being more frequently used in transplantation studies. Over 1000 clinical trials using MSCs to treat various disease conditions have been registered at ‘clinicaltrials.gov’ (see recent review by Levy et al., 2020 [26]). However, several MSC-based clinical trials have not been able to reach their primary endpoint and have showed low-to-negligible effects in alleviating the targeted condition.
APPROVED MSC-BASED STEM CELL THERAPY PRODUCTS
Despite several clinical trials with MSCs not resulting in desired outcomes, multiple MSC-based stem cell products have been approved for clinical use. Alofisel®, a therapeutic product developed by TiGenix/Takeda, is based on adipose-derived MSCs (AT-MSCs) and is used to treat complex perianal fistulas in Crohn’s disease (CD). In the trials, patients were injected with 120 million allogenic AT-MSC in a single dose and saline was used as placebo control (NCT01541579). As administration of Alofisel® showed significant remission in CD patients, it was granted clearance as a novel therapeutic agent by the European Medicines Agency (EMA) in 2018 and became an allogenic stem cell medical therapeutic product that is authorized and has been provided orphan medical product designation [27]. Although the mechanism of action of Alofisel® is not completely understood, data from preclinical studies show an increase in anti-inflammatory cytokines and a decrease in pro-inflammatory cytokines through the induction of indoleamine 2,3 dioxygenase (IDO) [28]. Apart from Alofisel®, several other MSC-based therapies have been approved in various countries such as Prochymal® (bone marrow-derived MSC for graft-versus-host disease (GvHD, approved in Canada and New Zealand), Temcell® HS (bone marrow-derived MSC for GvHD, Japan), Cartistem® (Umbilical cord-derived MSCs for knee articular cartilage defects, South Korea), Cellgram® (bone marrow-derived MSC for acute myocardial infarction, South Korea), and Stemirac® (bone marrow-derived MSC for spinal cord injury, Japan) [26].
In India, Stempeucel®, a human bone marrow-derived MSC-based therapy was approved in 2016 as treatment for critical limb ischemia and is the only stem cell therapy product currently approved in India [26]. In future, it is expected that several other MSC-based cell therapy products will be available in India as, currently, Stempeutics is conducting multiple clinical trials, in various phases, to treat atherosclerosis, osteoarthritis, diabetic foot ulcers, and perianal fistula due to Crohn’s disease. Other private companies in India like Advancells, Reelabs, Regenexx, and several others offer stem cell-based therapies for variety of diseases like leukaemia, thalassemia, sickle cell anaemia, several hematological cancers, orthopedic and eye disorders, among others. Regrettably, details about clinical outcome(s), treatment parameters like number of cells infused, and infusion mode and frequency, are not available in the public domain or in peer reviewed publications.
Stem cell therapy for COVID-19
Clinical trials of MSC-based therapy for treating COVID-19 have been approved by the USFDA and more than sixty studies are registered at clinicaltrial.gov. Severely affected patients who needed ventilators or those who developed Acute Respiratory Distress Syndrome (ARDS) were treated with these MSCs [29]. ARDS is the leading cause of death associated with COVID-19 disease wherein excessive production of proinflammatory mediators results in a cytokine storm and subsequent multiple organ failure. In a phase 1/2a randomized trial from Miami University involving 24 subjects, umbilical cord-derived MSCs (100 million cells/infusion) were administered twice, with a 72-hour gap between infusions. MSC administration led to a significant reduction in proinflammatory cytokine levels and improved survival rates in patients with COVID-19-associated ARDS [30]. Additionally, a few other studies from China have confirmed the beneficial effects of MSC-based cell therapy in alleviating severe complications due to COVID-19 [31, 32]. Various biotech and pharmaceutical companies like Mesoblast and Nestcell have been granted regulatory approval for MSC-based therapy for COVID-19 in the US and in other countries. In parallel, other cell types like natural killer cells, dendritic cells, and cord blood-derived mononuclear cells have been tested as potential therapy options against COVID-19 [33]. Interestingly, several ongoing clinical trials are exploring the use of MSC-derived exosomes to treat COVID-19 as these cell-free exosomes are considered safer than MSC themselves due to the absence of actual cells [34].
In India, Reelabs Pvt. Ltd, have received approval to conduct randomized placebo-controlled phase 1 trials to treat COVID-19 with MSCs obtained from the placenta and the umbilical cord (CTRI/2020/08/027043). This study will involve 20 patients, 10 in each group, such that patients will be provided 100 million or 200 million MSCs, as per their group allocation. Two doses of cells (on days 1 and 4) will be administered, and the safety and efficacy of MSC administration, effect on oxygenation index, multi organ function, and progression to severe ARDS will be studied. Bangalore-based Stempeutics Research Private limited, along with the East European partner Educell, aim to attempt cell-based therapy to treat COVID-19-associated ARDS in India and in Europe.
STEM CELL THERAPY IN INDIA
A pioneering study to successfully accomplish ocular surface regeneration using corneal limbal progenitor cells was conducted in India in 2003 [35]. Simple Limbal Epithelial Transplantation (SLET), a novel therapeutic strategy, was established by Sangwan et al, where limbal tissue harvested from six donor eyes were used for autografting into eyes with unilateral stem cell deficiency following ocular burns. Limbal tissue was extracted from the healthy donor eye (2×2 mm tissue), chopped into tiny pieces (8–10 pieces), and secured on the recipient eye over a human Amniotic membrane (hAM) graft, which is an in vivo cultivation method mimicking in vitro petri dish expansion. Limbal epithelial cells grew multi-directionally on the explant and generated a confluent stratified sheet of epithelium on the corneal surface by 2 weeks where the hAM became the new basement membrane for the regenerated cornea. Importantly, in this autografting procedure, all six patients showed improved vision, and none developed any complications in the donor eye, thus making it an effective technique in treating unilateral limbal stem cell deficiency following ocular burns [36]. Compared to Cultivated Limbal Epithelial transplantation (CLET), an autologous stem cell expansion method culturing stem cells on fibrin scaffold in vitro [37], SLET showed better anatomical and functional success [38]. Further, Holoclar®, a limbal stem cell-based therapeutic product to repair damaged cornea, is the first stem cell-based product approved by the European Medicines Agency. The therapeutic potential of Limbus-derived mesenchymal/stromal cells (LMSCs), along with other sources of MSC, have been used to treat various corneal stromal pathologies through pre-clinical and early clinical studies. For example LMSCs (derived from cadaveric corneal scleral rims) transplanted into mouse corneas prevented scar formation [39]. Further, a pilot clinical trial examined the safety and efficacy of LMSCs in corneal stromal regeneration in superficial corneal pathologies like scars, ulcers and burns (NCT02948023).
While limbal stem cell-based therapy to treat corneal blindness in India represents a success story, similar advancements in stem cell therapy for other diseases remain limited. We performed a systematic database search of clinicaltrials.gov and Clinical Trials Registry India (CTRI) using the words “stem cells” to identify stem cell therapy trials from India that are recruiting, ongoing, or have been completed. Our aim was to compile all reported stem cell trials to evaluate these studies based on cell origin and cell type used, phase of the study, and disease/condition targeted.
We found 29 studies in total, of which 18 have been completed and the remaining 11 are in the recruiting stages (as of January 2021; Supplementary Table 1). Bone marrow-derived stem cells like MSCs, HSCs, or a combination of both were the predominantly used sources (Fig. 2). Bone marrow-derived cells were most frequently chosen because they can be obtained from non-controversial sources, and exhibit both ease of use and evidence of anti-inflammatory function. Autologous cell transplants were more common (19 /29 studies) than allogenic transplants (9/29 studies). A total of 22 conditions were targeted for stem cell-based therapy and type 2 diabetes was the most common condition (n = 4), followed by critical limb ischemia (n = 3), liver cirrhosis (n = 2), and spinal cord injury (n = 2). We found only 1 trial each for several other diseases (Fig. 2).
Fig. 2
Status of stem cell therapy in India: Ongoing and completed studies/trials of stem cell therapies were database searched at ‘clinicaltrials.gov’ and ‘cell therapy registry India (CTRI)’. (A) The source of the cells used, i.e., “cell origin”, are listed and their percentage among the 29 studies is shown. Bone marrow-derived stem cells predominate. (B) Autologous stem cell transplants are more common than allogenic transplants (C) Type 2 diabetes was treated in 4 studies while critical limb ischemia was targeted in 3 studies; several other diseases were treated in single studies. (D) Majority of the trials of stem cell therapies are Phase 2 studies (16 out of 29), with 5 studies in phase 3, and 3 studies each in phase 1 and 4.
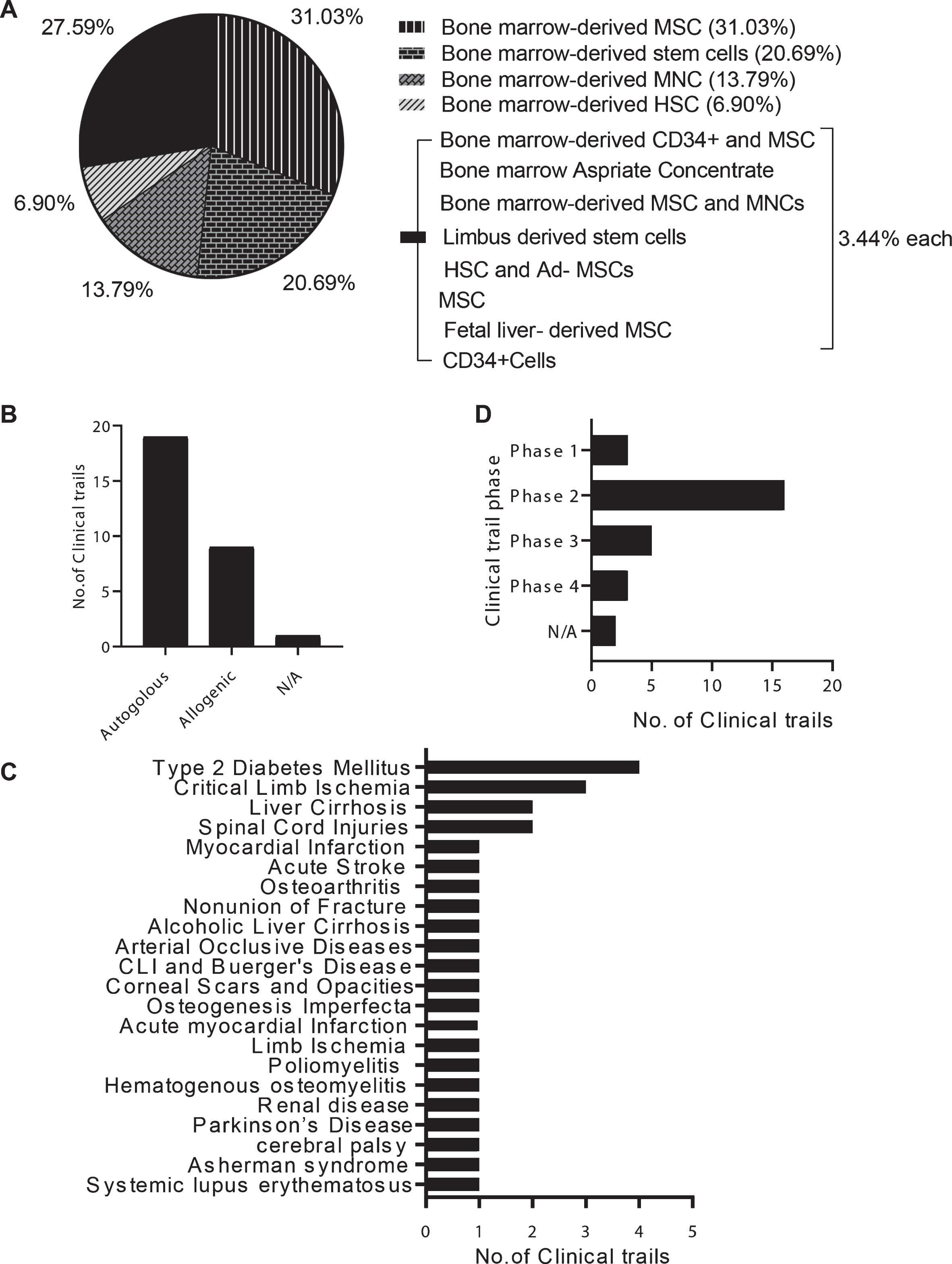
Of the 18 studies that have been completed, only 10 (10/18; 55%) have posted results while no data is available for the others. Regardless of trial outcome, researchers should disclose the results (either positive or negative) as this will help the research community learn important lessons and design better clinical trials in the future. Importantly, the reported results show that it is safe to administer bone marrow-derived stem cells without any adverse effects and that treatment resulted in moderate-to-no improvement for certain disease conditions (Supplementary Table 2). Multiple reasons are attributed to such moderate or suboptimal outcomes and they include potential immune rejection by monocytes within 24 h after injection resulting in poor graft survival, inadequate efficacy data from animal model studies, and a poor understanding of the underlying mechanism(s) of action. Preclinical studies have used various administration strategies, stem cell homing strategies, or bioengineered encapsulation methods to protect MSCs, which show better survival and function in host tissue [40, 41]. Thus, the trend in India is restricted to bone marrow-derived stem cells as the primary source and other stem cell sources like pluripotent stem cells are rarely used (1 out of 47 trials with iPSCs, globally) [42].
STEM CELL BANKING
Umbilical cord blood (UCB) is collected and stored as the new-born is delivered and it is a potential source of hematopoietic stem and progenitor cells; these stem cells are cryopreserved in private or public stem cell banks. Cord blood-derived stem cells serve as an alternate source to bone marrow transplantation that benefit patients with hematological disorders.
The first umbilical cord blood transfusion to treat Fanconi anaemia was successfully conducted in 1988 and the recipient survived for over 25 years with complete hematological and immunological donor cell reconstitution [43]. Globally, by 2013, more than 30,000 hematopoietic stem cell transplantations using cord blood-derived stem cells have been performed and over 600,000 cord blood samples have been stored [(44)]. A survey of 281 clinical trials for advanced cell therapy that were registered between 2005 to 2015 showed that perinatal tissue-derived cells, mostly MSCs from cord blood, were predominantly used for hematopoietic disorders, followed by various neurological conditions [45].
In India, only umbilical cord blood is banked, and this is the primary source of preserved stem cells. Several private companies are involved in the cryopreservation of cord blood-derived stem cells. In such private banks, stem cells are preserved for use by a particular individual, e.g., for autologous transplantation. In contrast, public cord blood banks serve as a repository of HLA-matched donor samples that are made available to anyone in need. Jeevan, the only public cord blood bank in Chennai, India, has cryopreserved cord blood stem cells from about 6400 donors; unfortunately, it is not acquiring new samples due to paucity of funds. Therefore, public cord blood banking facilities with HLA typing of samples need to be funded by the government to address the growing needs for stem cell transplantation. Interestingly, in Japan, HLA-typed iPSC stock cells that match major HLA loci in the Japanese population are being created and, in principle, these HLA-matched iPSCs could be readily used for any type of regenerative therapy [46]. Such banks of iPSCs that match the diverse Indian population will be a valuable resource for regenerative therapies in India.
Recently, MSCs cells have been identified in dental pulp, human exfoliated deciduous teeth, periodontal ligament, molars, and incisors, and these cells are also a potential stem cell source that can be banked [47]. Further, MSCs from dental tissues offer various advantages, in that they are a natural and non-invasive source of stem cells that can not only be used for dental applications but also for other stem cell-based therapies where MSCs are used. Long-term cryopreservation of dental stem cells is becoming a popular stem cell banking strategy that appears to be similar to cord blood-derived stem cell banking, i.e., MSCs are isolated from dental tissue, expanded by in vitro culture, and cryopreserved for long term storage. Several public- or private-funded, FDA-approved stem cell banks that store cells derived from dental tissues are present in the USA, like BioEDEN, StemSave, National Dental Pulp Library. Similarly, dental stem cell banks are present in countries like Japan, Norway, and India [48].
REGULATORY AFFAIRS FOR CELL THERAPY IN INDIA
Several countries like China, Mexico, Panama, and Thailand are preferred destinations for “stem cell tourism”, which involves procedures with no relevant regulation or meaningful scientific oversight. Similarly, unproven stem cell therapies are practiced in hundreds of clinics in India even though there are strict guidelines and regulations against fraudulent advertisements and clinical practice [49]. In the US, multiple business and clinics are engaged in ‘direct-to-consumer’ marketing of unproven stem cell therapies [50].
In India, the “National Stem Cell Guidelines” is the regulatory document prepared by the Indian Council of Medical research (ICMR) and Department of Biotechnology (DBT). It was first published in 2007 and later revised in 2013 and in 2017 (http://dbtindia.gov.in/sites/default/files/National_Guidelines_StemCellResearch-2017.pdf). However, neither departments have jurisdiction over violators who are liable to be punished. In India, laboratories involved in stem cell research and or those involved in clinical trials need to be approved by the Institutional Committee for Stem Cell Research (IC-SCR) and by the National Apex Committee for Stem Cell Research and Therapy (NAC-SCRT). Further, these stem cell laboratories must also be certified for Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP). For clinical trials, IC-SCR and NAC-SCRT approval and trial approval by the Cell Biology-Based Therapeutic Drug Evaluation Committee (CBBTDEC), are essential. The hospital and the clinical trial site must obtain IEC and IC-SCR approvals and the trials must be registered at the clinical trials registry of India (CTRI). A data safety monitoring board must be appointed, and its members must have the independence to monitor any serious outcome(s) from the trial.
POTENTIAL INNOVATIONS IN STEM CELL THERAPY
Stem cell therapy is associated with high costs across the globe; for example, Prochymal® (a bone marrow derived allogeneic MSC treatment for GvHD or Provenge® (an autologous cell therapy of dendritic cells from metastatic forms of prostate cancer), cost anywhere between US $100,000 and US $200,000 [51]. According to the International Society for Stem cell Therapy, a single dose of cell therapy can cost about € 30,000 to € 40,000 [52], which is an exorbitant cost, especially for a country like India. Mass production of allogenic stem cells like MSCs and improving methods for graft survival can serve to bring down the cost.
Recent advances in gene editing technologies like CRISPR, and the recent demonstration of CRISPR-edited BCL11A gene in autologous HSCs, have opened possibilities to autologously treat thalassemia using a patient’s own cells [6]. Similarly, short hairpin-based interference of the BCL11A gene in an erythroid-specific manner has been shown to be effective and beneficial in patients with sickle cell anaemia [53]. These advances in gene editing and transcriptional silencing can help patients on a large scale by using autologous HSCs. In India, currently, 97 transplant centres report to the Indian Society for Blood and Marrow Transplantation with the database showing that 19,000 transplants have been conducted to treat various disorders; however, the waiting list to undergo HSC transplantation is long in several centres [54]. Bringing down the cost, increasing availability of transplantation centres, and enabling HLA-matched donor banks will improve these current trends. One such initiative would be the re-establishment of public cord blood banking facilities with HLA typing of samples that is funded by the government to address the growing need for HSC transplantation in patients with thalassemia, sickle cell anaemia, and hematological cancers. Additionally, banks of matched-iPSCs that reflect the diverse Indian population will also be a valuable resource for regenerative therapies in India.
PROSPECTS FOR STEM CELL THERAPY IN INDIA
Our assessment of clinical trials in India clearly showed that a majority of the studies were restricted to bone marrow-derived cells and that only a few diseases were targeted. India has over 100,000 patients suffering from beta thalassemia and about 150,000 with sickle cell anaemia, and tremendous opportunities exist for treating several hematological disorders with HSC transplantation. However, such treatments are unaffordable to many due to high process-associated costs such as stem cell banks, HLA typing, etc. For example, only 20–30 % of aplastic anaemia patients get the current standard of care i.e., HSC transplantation and immunosuppression [55]. Innovative approaches are required to reduce the cost of cryopreserving HSCs, retrieving them, HLA typing, and ensuring availability of transplantation facilities across the country. Relevant changes must occur at multiple levels and include (1) fostering a high quality research environment at graduate and postgraduate levels; (2) encouraging ideas and building entrepreneurship as spin offs from graduate to doctorate levels; (3) infrastructure and knowledge base to develop research and clinical grade products within the country such as cell culture reagents, enzymes and medical devises, as currently all these items are imported which results in increased cost and waiting time; (4) training medical professionals to enhance their expertise and improving availability of transplantation specialists across the country; (5) foster closer relations between scientists and clinicians to understand available opportunities to innovate and address local issues; (6) developing transplant programs in government-funded hospitals that can make treatment more affordable for many patients. On a brighter note, research articles on stem cells from India have increased over the years and several stem cell lines, including human embryonic stem cell lines, have been derived in India [56]. As most stem cell research is conducted in government-funded Institutes and universities, students at the undergraduate level onwards should be exposed to relevant scientific methods and research environment to help establish strong R &D capabilities.
CONCLUDING REMARKS
Stem cell therapy can be a game changer in addressing the growing numbers of chronic diseases, especially in countries like India that have a large population size, genetic diversity, and a complex social structure. Chronic diseases are projected to account for 53% of all deaths in India, with diabetes and heart disorders predicted to be major contributors. Stem cell therapy has the potential to offer transformative treatments for several incurable diseases, but the cost of such remedies is currently not scalable for wide use; thus, many biotech companies have focused on making stem cell therapy more affordable. For example, Eyestem a Bangalore-based biotech company is aiming to bring cell-based therapy to treat Dry Age-related Macular degeneration (Dry AMD) by providing Retinal Pigment Epithelium cells. In India, an estimated 15 to 45 million people suffer from Dry AMD, and blurred vision in the earlier stages can progress to complete blindness [57, 58]. Increasing research funding after the COVID-19 pandemic and encouraging a ‘start-up’ milieu for biotech industries will lead to a paradigm shift in stem cell-based therapy from developing economies like India. Nurturing and developing R & D expertise, by strengthening the education system with research focus will also open opportunities to bring cutting-edge therapies to clinics in India.
ACKNOWLEDGMENTS
We are thankful to Dr. Vasuprada Iyengar for language and content editing.
FUNDING
No funding was received to assist the preparation of this manuscript. The authors declare no financial interest. D.B, R.P and G.K are employees of Vowels Lifesciences Private Limited, a healthcare company associated with Cord blood banking and teaching activities.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR’S CONTRIBUTION
D.B analysed and prepared graphs on the clinical trials, R.P contributed on the regulatory aspects of stem cell therapy and banking, G.K conceived the idea and wrote the manuscript.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/STJ-210002.
STEMJOURNAL OPEN REVIEW
The evaluations from peer reviewers for this article are freely available and can be found as supplementary material here: https://dx.doi.org/10.3233/STJ-210002.
REFERENCES
[1] | Dekkers JF , Wiegerinck CL , de Jonge HR , Bronsveld I , Janssens HM , de Winter-de Groot KM , et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. (2013) ;19: (7):939–45. |
[2] | Huch M , Gehart H , van Boxtel R , Hamer K , Blokzijl F , Verstegen MMA , et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell. (2015) ;160: (1-2):299–312. |
[3] | Schweitzer JS , Song B , Herrington TM , Park T-Y , Lee N , Ko S , et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson–s Disease. N Engl J Med [Internet]. 2020 May 13 [cited 2021 Apr 30]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa1915872 |
[4] | Rezania A , Bruin JE , Arora P , Rubin A , Batushansky I , Asadi A , et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. (2014) ;32: (11):1121–33. |
[5] | Panés J , García-Olmo D , Assche GV , Colombel JF , Reinisch W , Baumgart DC , et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. The Lancet. (2016) ;388: (10051):1281–90. |
[6] | Frangoul H , Altshuler D , Cappellini MD , Chen Y-S , Domm J , Eustace BK , et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med. . (2021) ;384: (3):252–60. |
[7] | Aly RM . Current state of stem cell-based therapies: an overview. Stem Cell Investig [Internet]. (2020) May 15 [cited 2021 Jan 16];7: (0). Available from: https://sci.amegroups.com/article/view/42512 |
[8] | Kolios G , Moodley Y . Introduction to Stem Cells and Regenerative Medicine. Respiration. (2013) ;85: (1):3–10. |
[9] | Master Z , Crowley AP , Smith C , Wigle D , Terzic A , Sharp RR . Stem cell preservation for regenerative therapies: ethical and governance considerations for the health care sector. Npj Regen Med. (2020) ;5: (1):1–6. |
[10] | Zakrzewski W , Dobrzyński M , Szymonowicz M , Rybak Z . Stem cells: past, present, and future. Stem Cell Res Ther. (2019) ;10: (1):68. |
[11] | Inamdar MS , Venu P , Srinivas MS , Rao K , VijayRaghavan K . Derivation and characterization of two sibling human embryonic stem cell lines from discarded grade III embryos. Stem Cells Dev. (2009) ;18: (3):423–33. |
[12] | Shetty R , Inamdar MS . Derivation of Human Embryonic Stem Cell Lines from Poor Quality Embryos. In: Turksen K, editor. Human Embryonic Stem Cells Handbook [Internet]. Totowa, NJ: Humana Press; 2012 [cited 2021 May 1]. pp. 151-61. (Methods in Molecular Biology). Available from: https://doi.org/10.1007/978-1-61779-794-1_9 |
[13] | Nagvenkar P , Pethe P , Pawani H , Telang J , Kumar N , Hinduja I , et al. Evaluating differentiation propensity of in-house derived human embryonic stem cell lines KIND-1 and KIND-2. In Vitro Cell Dev Biol Anim.. (2011) ;47: (5-6):406–19. |
[14] | Mandal A , Bhowmik S , Patki A , Viswanathan C , Majumdar AS . Derivation, characterization, and gene expression profile of two new human ES cell lines from India. Stem Cell Res. (2010) ;5: (3):173–87. |
[15] | Takahashi K , Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. (2006) ;126: (4):663–76. |
[16] | Khan M , Zende S , Vaidyanath A , Avatade R , Shiras A . Generation of two induced pluripotent stem cell lines NCCSi005A and NCCSi006A from CD4+T cells of healthy individuals of Indian origin. Stem Cell Res.. (2019) ;39: :101506. |
[17] | Najar AH , Sneha KM , Ashok A , Babu S , Subramaniam AG , Kannan R , et al. Derivation of iPSC lines from two patients with familial Alzheimer’s disease from India. Stem Cell Res. (2019) ;34: :101370. |
[18] | Barker N , Wetering M van de , Clevers H . The intestinal stem cell. Genes Dev. (2008) ;22: (14):1856–64. |
[19] | Pittenger MF , Discher DE , Péault BM , Phinney DG , Hare JM , Caplan AI . Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med.. (2019) ;4: (1):1–15. |
[20] | Gupta PK , Krishna M , Chullikana A , Desai S , Murugesan R , Dutta S , et al. Administration of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells in Critical Limb Ischemia Due to Buerger’s Disease: Phase II Study Report Suggests Clinical Efficacy. Stem Cells Transl Med. (2017) ;6: (3):689–99. |
[21] | Chullikana A , Majumdar AS , Gottipamula S , Krishnamurthy S , Kumar AS , Prakash VS , et al. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. (2015) ;17: (3):250–61. |
[22] | Nowell CS , Radtke F . Corneal epithelial stem cells and their niche at a glance. J Cell Sci. (2017) ;130: (6):1021–5. |
[23] | Seim I , Ma S , Gladyshev VN . Gene expression signatures of human cell and tissue longevity. NPJ Aging Mech Dis. (2016) ;2: :16014. |
[24] | Becker AJ , McCulloch EA , Till JE . Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. (1963) ;197: :452–4. |
[25] | Aljurf M , Weisdorf D , Alfraih F , Szer J , Müller C , Confer D , et al. “Worldwide Network for Blood & Marrow Transplantation (WBMT) special article, challenges facing emerging alternate donor registries. ” Bone Marrow Transplant. (2019) ;54: (8):1179–88. |
[26] | Levy O , Kuai R , Siren EMJ , Bhere D , Milton Y , Nissar N , et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. (2020) ;6: (30):eaba6884. |
[27] | Detela G , Lodge A . EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation. Mol Ther Methods Clin Dev.. (2019) ;13: :205–32. |
[28] | Panés J , García-Olmo D , Van Assche G , Colombel JF , Reinisch W , Baumgart DC , et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet Lond Engl. (2016) ;388: (10051):1281–90. |
[29] | Durand N , Mallea J , Zubair AC . Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. Npj Regen Med. (2020) ;5: (1):1–9. |
[30] | Lanzoni G , Linetsky E , Correa D , Messinger Cayetano S , Alvarez RA , Kouroupis D , et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021 Jan 5; |
[31] | Leng Z , Zhu R , Hou W , Feng Y , Yang Y , Han Q , et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. (2020) ;11: (2):216–28. |
[32] | Liang B , Chen J , Li T , Wu H , Yang W , Li Y , et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine (Baltimore) [Internet]. (2020) Jul 31 [cited 2021 Jan 18];99: (31). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7402800/ |
[33] | Khoury M , Cuenca J , Cruz FF , Figueroa FE , Rocco PRM , Weiss DJ . Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. (2020) :55: (6). |
[34] | Sengupta V , Sengupta S , Lazo A , Woods P , Nolan A , Bremer N . Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. (2020) ;29: (12):747–54. |
[35] | Sangwan VS , Vemuganti GK , Singh S , Balasubramanian D . Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci Rep. (2003) ;23: (4):169–74. |
[36] | Sangwan VS , Basu S , MacNeil S , Balasubramanian D . Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. (2012) ;96: (7):931–4. |
[37] | Pellegrini G , Traverso CE , Franzi AT , Zingirian M , Cancedda R , Luca MD . Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. The Lancet. (1997) ;349: (9057):990–3. |
[38] | Shanbhag SS , Nikpoor N , Donthineni PR , Singh V , Chodosh J , Basu S . Autologous limbal stem cell transplantation: a systematic review of clinical outcomes with different surgical techniques. Br J Ophthalmol. (2020) ;104: (2):247–53. |
[39] | Basu S , Hertsenberg AJ , Funderburgh ML , Burrow MK , Mann MM , Du Y , et al. Human limbal biopsy–derived stromal stem cells prevent corneal scarring. Sci Transl Med. (2014) ;6: (266):266ra172–266ra172. |
[40] | Levit RD , Landázuri N , Phelps EA , Brown ME , García AJ , Davis ME , et al. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. (2013) ;2: (5):e000367. |
[41] | Moll G , Ankrum JA , Kamhieh-Milz J , Bieback K , Ringdén O , Volk H-D , et al. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med.. (2019) ;25: (2):149–63. |
[42] | Deinsberger J , Reisinger D , Weber B . Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis. Npj Regen Med. (2020) ;5: (1):1–13. |
[43] | Auerbach AD , Liu Q , Ghosh R , Pollack MS , Douglas GW , Broxmeyer HE . Prenatal identification of potential donors for umbilical cord blood transplantation for Fanconi anemia. Transfusion (Paris). (1990) ;30: (8):682–7. |
[44] | Ballen KK , Gluckman E , Broxmeyer HE . Umbilical cord blood transplantation: the first 25 years and beyond. Blood. (2013) ;122: (4):491–8. |
[45] | Couto PS , Bersenev A , Verter F . The first decade of advanced cell therapy clinical trials using perinatal cells (2005–2015). Regen Med. (2017) ;12: (8):953–68. |
[46] | Umekage M , Sato Y , Takasu N . Overview: an iPS cell stock at CiRA. Inflamm Regen. (2019) ;39: (1):17. |
[47] | Ledesma-Martínez E , Mendoza-Núñez VM , Santiago-Osorio E . Mesenchymal Stem Cells Derived from Dental Pulp: A Review [Internet]. Vol. 2016, Stem Cells International. Hindawi; 2015 [cited 2021 Feb 2]. pp. e4709572. Available from: https://www.hindawi.com/journals/sci/2016/4709572/ |
[48] | Bates KD , Gallicchio VS , Vs G , Kd B . Dental Stem Cell Banking and Applications of Dental Stem Cells for Regenerative Medicine: A Literature Review. 2020;17. |
[49] | Tiwari SS , Desai PN . Unproven Stem Cell Therapies in India: Regulatory Challenges and Proposed Paths Forward. Cell Stem Cell. (2018) ;23: (5):649–52. |
[50] | Turner L . The US Direct-to-Consumer Marketplace for Autologous Stem Cell Interventions. Perspect Biol Med. (2018) ;61: (1):7–24. |
[51] | Soria-Juan B , Escacena N , Capilla-González V , Aguilera Y , Llanos L , Tejedo JR , et al. Cost-Effective, Safe, and Personalized Cell Therapy for Critical Limb Ischemia in Type 2 Diabetes Mellitus. Front Immunol [Internet]. 2019 Jun 4 [cited 2021 Jan 23];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6558400/ |
[52] | Hernandez I , Prasad V , Gellad WF . Total Costs of Chimeric Antigen Receptor T-Cell Immunotherapy. JAMA Oncol. (2018) ;4: (7):994–6. |
[53] | Esrick EB , Lehmann LE , Biffi A , Achebe M , Brendel C , Ciuculescu MF , et al. Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. N Engl J Med [Internet]. 2020 Dec 5 [cited 2021 Jan 23]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa2029392 |
[54] | Saikia TK . Blood and bone marrow transplantation in India: Past, present, and future. Indian J Med Paediatr Oncol. . (2020) ;41: (3):308. |
[55] | Kulkarni U , George B . Access to hematopoietic stem-cell transplantation in India. J Postgrad Med. (2019) ;65: (1):1–4. |
[56] | Lander B , Thorsteinsdóttir H , Singer PA , Daar AS . Harnessing Stem Cells for Health Needs in India. Cell Stem Cell. (2008) ;3: (1):11–5. |
[57] | Srinivasan S , Swaminathan G , Kulothungan V , Ganesan S , Sharma T , Raman R . Age-related macular degeneration in a South Indian population, with and without diabetes. Eye. (2017) ;31: (8):1176–83. |
[58] | Woo JH , Sanjay S , Au Eong K-G . The epidemiology of age-related macular degeneration in the Indian subcontinent. Acta Ophthalmol (Copenh). (2009) ;87: (3):262–9. |




