Role of ingested amino acids/protein in the promotion of resistance exercise-training adaptations in aging: analysis of meta-analyses
Abstract
A current lack of consensus exists regarding the effect of protein supplementation during resistance exercise on the phenotypic adaptation in aging adults. Thus, we critically assessed the collection of meta-analytic evidence to provide clarity regarding the differences between meta-analyses examining the combined effectiveness of chronic exposure to PRO/AA supplementation and resistance exercise to enhance the adaptive response. Thirteen meta-analyses, with relatively similar titles, presented different results on the topic. This divergence is mainly due to an inconsistent study selection process resulting in distinct study populations and varied types of protein-focused nutritional interventions and not RCT study quality. The methods applied to extract and estimate effects from RCTs with incorrectly formatted data for meta-analyses likely provide an additional reason for divergent results. PRO/AA supplements (when combined with resistance exercise training) produced a positive, albeit minor effect on the promotion of whole-body lean mass growth, yet a minimal and inconsistent effect on muscle mass, muscle strength, or functional capacity. The lack of an effect was skewed in studies with a higher proportion of obese and overweight participants and somewhat less noticeable in those containing sarcopenic and frail older adults, who would have the greatest need for an intervention to enhance muscle mass. Researchers are encouraged to provide the change scores mean and standard deviations for all their outcomes by group or even making the data sets available to improve future meta-analyses and advance the field.
Abbreviations
1RM | one-repetition maximum |
AA | amino acids |
ALM | greaterthan appendicular lean mass |
BMI | body mass index |
β-HMB | β-Hydroxy β-Methyl butyrate |
CI | confidence interval |
Corr | correlation coefficient |
CSA | cross-sectional area |
FFM | fat-free mass |
FM | fat mass |
kg | kilograms |
MD | mean difference |
MPS | muscle protein synthesis |
mTORC1 | mechanistic target of rapamycin complex 1 |
MVC | maximum voluntary contraction |
PEDro scale | Physiotherapy Evidence Database |
PRO | protein |
RCT | randomized controlled trials |
RE | RE |
RET | resistance exercise training |
SDs | standard deviations |
SEMs | standard error of the mean |
SMD | standardized mean difference |
SPPB | short physical performance battery |
TUG | timed up and go test |
y | greaterthan years |
1Introduction
Human aging is characterized by the gradual loss of muscle mass and strength [1, 2], accelerated in periods of inactivity and illness [3, 4]. As these decrements compound, they contribute to loss of vital lean mass and functional strength, resulting in limitations of functional independence, development of disability, [5] and a greater susceptibility to poor health. These emerging concerns present a mounting health care burden. Thus, strategies to maintain musculoskeletal health, delay entry into the disability threshold, and prolong quality of life are of prime importance for the booming aging population. Readily available, inexpensive, and modifiable lifestyle modifications such as exercise and nutrition are commonly thought to be effective strategies in this regard. In particular, resistance exercise, when performed over a period of time, clearly yields many systemic health benefits, in particular, increased muscle mass, strength, and functional independence [6]. Many recent reports have promoted the combined effectiveness of resistance exercise and protein and/or amino acid supplementation (PRO/AA) compared to these interventions in isolation (resistance exercise or protein nutrition) to maximize these health outcomes and help mitigate the cost of the looming health care crisis. Over two decades ago the estimated economic burden of sarcopenia was ∼$20 billion [7]. The costs have increased as persons with sarcopenia are more expensive to care for [8] and more likely to be hospitalized and readmitted [9] There have been less systematic reviews or meta-analyses that describe the phenotypic result or even the clinical relevance of PRO supplementation during RET in older adults - until the past decade. Unfortunately, the consensus from these meta-analyses have been contradictory and unclear, making interpretation and application for the healthcare professional challenging. Therefore, our goal in this review is to critically assess the collection of meta-analytic evidence to provide clarity regarding the differences between meta-analyses examining the combined effectiveness of chronic exposure to PRO/AA supplementation and resistance exercise to enhance the adaptive response.
2Methods
We have set out to provide a critical examination of the meta-analytic evidence characterizing the physiological and phenotypic response of human muscle adaptation and to determine whether giving PRO/AA in close proximity to resistance exercise enhances this effect. The evidence was collected by database searches of reviews and hand-searching author lists on the topic. Population: analysis of older adults (> 40y) as a main analysis, or a subgroup analysis. Intervention: Resistance exercise training and protein and/or amino acid nutrition with and without other nutrients. Resistance exercise training is defined as non-continuous dynamic exercise with defined sets and repetitions to contract against a posing force to generate external resistance with intent to increase muscle size and strength. Comparison: A comparison of the above intervention vs. resistance exercise training plus placebo or resistance exercise alone. All comparisons must include resistance exercise training (> 4 weeks in duration). Outcomes: Include a measure of body composition, muscle mass/size, strength, physical function testing. Physical function testing included, but was not limited to short physical performance battery (SPPB), gait speed, timed up and go (TUG), walk capacity and/or chair rise testing. Type of study design: Meta-analyses studies of Randomized Clinical Controlled Trials (RCTs). Search methods for identification of studies: We searched MEDLINE (including in-process and other non-indexed citations), Biomedical Reference Collection: Pubmed, CINAHL, Web of Science, SPORTDiscus, and reference lists of articles (in June 2021). Several studies were not retrieved via standard methods. The most fruitful method of discovering studies were 1) manually searching for articles published by researchers who are well-known to work in the area of muscle protein metabolism/exercise training, 2) using the reference lists of all retrieved articles to identify potentially missing sources, and 3) using key papers on the topic to select PubMed’s “similar” and “cited by” search options combined with the “meta-analysis” filter. As such, we comprehensively and critically assessed all the RCTs (see tables), to our knowledge, regarding the meta-analytic assessment of chronic exposure to RET and PRO/AA supplementation in older adults.
3Results and discussion
Thirteen meta-analyses were discovered that met our criteria. Eight other studies were excluded because they could not address our research question concerning older adults described in the methods. Hudson et al. [10], Bergia et al. [11], Valenzuela et al. [12], and Messina et al. [13] did not have a subgroup analysis for older adults alone, restricted to our topic and question. Hita-Contreras et al. 2018 [14] did not have a comparison of resistance exercise versus resistance exercise plus supplementation. Cheng et al. [15] and Liao et al. [16] were too broad with their exercise type by including aerobic exercise and rehabilitation from injury. Since Colonetti et al. [17] included only one study and Thomas et al. [18] were excluded for having too few studies (<2) included in the qualitative analysis.
The titles, main outcomes examined, and the number of participants per outcome from these meta-analyses on the topic are summarized in Table 1 and the descriptive characteristics of these meta-analyses are in Table 2. Table 1 also shows the number of participants per meta-analysis for the pooled estimate of each outcome they examined. The number of subjects pooled for analysis has not linearly increased over the years, suggesting these analyses were more distinct than what most of their titles would suggest. All but two studies examined fat-free/lean mass [27, 33] for this indirect estimation of muscle mass. All but two meta-analyses [19, 20] examined a measure of muscle strength. Physical function was examined in six studies [20–25]. Direct measures of muscle mass and size were only examined in three meta-analyses [22, 24, 26]. Myofiber size changes, for older adults only, were only reported in one meta-analysis [27]. The titles of these studies suggest very similar goals upon first glance, but examination of the methods and characteristics of included studies for these meta-analytic studies reveals several distinctions. The standout areas include differences in inclusion and exclusion criteria, participant population, and analytical approach, specifically heterogeneity of pooled outcomes and approach to estimate incomplete data.
Table 1
The number of older adult participants analyzed per outcome in meta-analyses examining specific adaptations to the chronic effect of resistance exercise training with protein and/or amino acid nutrition and their titles
| N size per outcome | Title of meta-analysis | |||||||
| Author&Year | FFM | FM | 1RM Strength | MVC Strength | Physical Function | Muscle Size | Myofiber CSA | |
| Cermak 2012 | 215 | 215 | 81 | – | – | – | 97 | Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis |
| Finger 2016 | 462 | – | 462 | – | – | 462? | – | Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis |
| Gomes-Neto 2017 | – | – | 239 | 98 | 0– | – | – | Whey protein supplementation in association with resistance training on additional muscle strength gain in older adults: A meta-analysis |
| Liao 2017 | 802 | 306 | 647 | – | ∼500,100,200,400 | 242 | – | Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis |
| Luo 2017 | 252 | 213 | – | – | 180–207 | – | – | Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis |
| Morton 2017 | 483 | 199 | 285 | 294 | – | – | 290 | A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults |
| Haaf 2018 | 896 | – | 896 | – | 600–744 | 149 | – | Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: a systematic review and meta-analysis |
| Hidayat 2018 | 502 | 454 | – | – | – | – | – | Effects of Milk Proteins Supplementation in Older Adults Undergoing Resistance Training: A Meta-Analysis of Randomized Control Trials |
| Hou 2019 | 789 | 789 | 978 | – | 977 | – | – | Effect of Protein Supplementation Combined with Resistance Training on Muscle Mass, Strength and Function in the Elderly: A Systematic Review and Meta-Analysis |
| Li 2019 | 431 | 290 | 732 | – | – | – | – | Effect of whey protein supplementation during resistance training sessions on body mass and muscular strength: a meta-analysis |
| Liao 2019 | 599 | 599 | 61 | – | 645 | – | – | The Role of Muscle Mass Gain Following Protein Supplementation Plus Exercise Therapy in Older Adults with Sarcopenia and Frailty Risks: A Systematic Review and Meta-Regression Analysis of Randomized Trials |
| Labata-Lezaun 2020 | – | – | UL: 137; LL: 589 | – | 111–212 | – | – | Effectiveness of Protein Supplementation Combined with Resistance Training on Muscle Strength and Physical Performance in Elderly: A Systematic Review and Meta-Analysis |
| O’Bryan 2020 | 430 | – | 280 | – | – | – | – | Do multi-ingredient protein supplements augment resistance training-induced gains in skeletal muscle mass and strength? A systematic review and meta-analysis of 35 trials |
Data are sample sizes. 1RM Strength, one-repetition maximum; FM, fat mass; FFM, fat-free mass; LL, lower limb; Myofiber CSA, myofiber cross-sectional area; N, sample size; UL, upper limb.
Table 2
Brief description of meta-analyses for effects of protein supplementation plus exercise on outcomes in older adults
| Participant characteristics | Intervention design | |||||||
| RET | Treatment | Control | ||||||
| Study (Author, Year, Reference) | Population | BMI/Fat | Age | Duration (wk) | Frequency (d/wk) | Methods | Actually used | Compare |
| Cermak 2012 | All | <30 | 50+ (62±6) | Varied, 6+ | 2+, AE | PRO, PRO diet, exclude other nutrients | Dairy, egg PRO with Vit D and Ca++ | RET-A or RET-P |
| Haaf 2018 | Healthy, no ER | ∼25–32 | 50+ | 4+ (6–78) | 3+ (3–7) | Any PRO or PRO diet, no restrictions | WH, Cas, EAA, Dietary PRO. Milk PRO (8–63 g/day) | RET-A or RET-P |
| Morton 2017 | Healthy, no ER | – | 45+ (67±7) | 6+ (10–24) | 2+ (2–3) | PRO, PRO diet, exclude other anabolic nutrients | Lean red meat, Cas, WH, PRO+CHO, Milk (15–63 g/day) | RET-A or RET-P |
| Gomes-Neto 2017 | No chronic disease | – | 60+ 68–78 | 12+ (12–24) | 3 | WH Only | WH only (20–40 g/day) | RET-A or RET-P |
| Li 2019 | All | ∼26 | 40+ | 6+ (12–24) | 2–3,5 (2–5) | WH or WH+ CHO only | WH (20–40 g/day), some with CHO | RET-A or RET-P |
| Labata-Lezaun 2020 | Mixed, Healthy vs Sarco | 22–31 | 60+ | 10+ | 2–4 | PRO, exclude other nutrients | Cas, Col, WH, Leu (10–40 g/day) | RET-A or RET-P |
| O’Bryan 2020 | Mixed | 24–30 | 45+ (48–83) | 6+ (16–52) | 2–5 | PRO + MNT | PRO Multinutrient | RET-A or RET-P |
| Finger 2015 | All | – | 60+ (61–79) | 12+ | 2–3 | PRO or AA sup or Diet, exclude other nutrients including CHO &FAT | ∼20.7g daily WH, milk PRO, EAA (6–40 g/day) | RET-A or RET-P |
| Hidayat 2018 | All | 24–33 | 60+ (60–80) | 12+ (12–72) | 2–3,5 | Milk proteins | PRO+ Multinutrient (CHO, Cr, HMB, Leu, calcium, vit D and/or PUFAs) | RET-P or low-protein diet |
| Liao 2017 | Mixed | >25, >27–38% | 60+ (60–85) | 8+ (12–24) | 2–3,7 | PRO or PRO diet, no restrictions | WH, EAA, Cas, soy, milk PRO, HMB, creatine, bovine colostrum (10–35 g/day) | RET-A or RET-P |
| Hou 2019 | Mixed | 18–33 | 50+ | 10+ (10–72) | 2–4 | PRO, PRO diet, exclude other nutrients | EAA, Leu, Col, meat, Cas, milk (10–45 g/day) | RET-A or RET-P |
| Luo 2017 | Sarco | ∼18–26 | 65+ (65–80) | 12+ (12–16) | 2–3,5,AE | PRO or PRO diet, no restrictions | WH (20–40 g/day), EAA (6–17 g/day), AAs, Leu, WHs, &vitamin D | RET-A |
| Liao 2019 | Sarco/frail | ∼24–28 | 60+ (64–89) | 8+ (3–36) | 2–3,5–7,AE | PRO or PRO diet, no restrictions | WH, milk PRO, EAA, Cas, &soy alone or with nutrients (Cr, AA) (3–40 g/day) | RET-A or RET-P |
Data are presented as WMD or SMD with 95% confidence interval in parentheses.1-RM LP, one-repetition maximum leg press; 5CRT, five-chair-rise-test; AA, amino acids; AE, aerobic exercise; ALM, appendicular lean mass; BMI, body mass index; Ca++, calcium; Cas, casein; CHO, carbohydrates; Col, collagen; CR, chair rise; CSA, cross-sectional area; d, days; EAA, essential amino acids; ER, energy restriction; FFM, fat-free mass; FM, fat mass; g, grams; GS, gait speed; HG, hand grip; HMB, hydroxymethylbutyrate; KE, knee extension; LBM, lean body mass; Leu, leucine; LL, lower limbs; LM, lean mass; LP, leg press; MD, mean difference; Mixed, mix of all muscle proteins; MNT, multi-nutrient; Myofiber CSA, myofiber cross-sectional area; PA, physical activity; PRO, protein; PROS, protein supplementation; PUFAs, polyunsaturated fatty acids; RET, resistance exercise training; RET-A, resistance exercise training alone; RET-P, resistance exercise training with placebo; Sarco, sarcopenic; SMD, standardized mean difference; SPPB, short physical performance battery; UL, upper limbs; Vit D, vitamin D; WH, whey; wk, week.
Unfortunately, an area of confusion is that these meta-analyses have different results but very similar titles. Mostly all these meta-analyses provide the general title of “effects of protein supplementation on…etc., yet a few distinctly specify whey protein [19, 28], milk protein only [29], “multi-ingredient protein” [30] or more generally, “nutrient” [25] in their titles. Those studies not restricted to whey or milk proteins took a few different and somewhat unclear approaches as described below. A few studies were entitled “protein supplementation” and actually defined criteria in their excluding protein supplementation with other “potentially hypertrophic agents” or “supplements known to induce muscle hypertrophy” [21, 26, 27, 31]. This very broad classification was normally a very short list with one to three items mentioned. The only consistent item mentioned was creatine, which was mentioned six times [21, 26–28, 30, 31] - what other items authors chose to exclude under that umbrella term remain unknown. Another study was more strict and even mentioned exclusion of added carbohydrates and fat, yet it included egg and milk-based supplementation/diet, which includes those macronutrients [26]. A protein-containing multi-ingredient meta-analysis accepted all forms of supplementation that contained protein, but specifically excluded androgenic agents [30]. One study excluded “other hypertrophic” (creatine and β-HMB) and androgenic agents [31]. Some studies marketing “protein supplementation” in the title could have been more specific to reflect their use of any protein-enriched supplement, whether it was multi-nutrient or not [22–24]. One study with “nutritional supplementation” in the title was less broad in actuality as it only examined “protein-containing nutritional supplementation” [25].
4Effect on fat-free mass/ lean mass
A statistically significant effect of PRO/AA during RET in older adults on total fat-free/lean mass was found in eight meta-analyses (17,21–23,26,27,29,30) with mean difference (MD) effects of 0.23–0.81 kg or about 0.23 to 5.78 as standardized mean difference (SMD) (Fig. 1 and Table 2). Whereas three meta-analyses did not find an effect on fat-free mass (FFM) [24, 28, 31] a few meta-analyses did not examine this metric [19, 20]. A few studies collected data on regional lean mass (appendicular, arm, leg), and a significant effect (0.39 kg MD; 0.3–0.4 SMD) was found in two studies [21, 22] with borderline insignificance in a third [23]. However, these latter studies were primarily enriched with sarcopenic and frail participants.
Fig. 1
Meta-analysis-calculated effects of protein supplementation and resistance exercise training compared to resistance exercise training with or without placebo in older adults for the outcomes of tissue composition, strength and physical function. Panels A, C and E show the mean difference (MD) of Protein vs Placebo for tissue composition (A), Strength (C) and physical function (C). Panels B, D and F show the standardized mean difference (MD) of Protein vs Placebo for tissue composition (B), Strength (D) and physical function (F). Data are MD or SMD with 95% CI as extracted from each meta-analysis. Effect favoring protein is to the right and the effect favoring placebo is to the left on the x-axis.
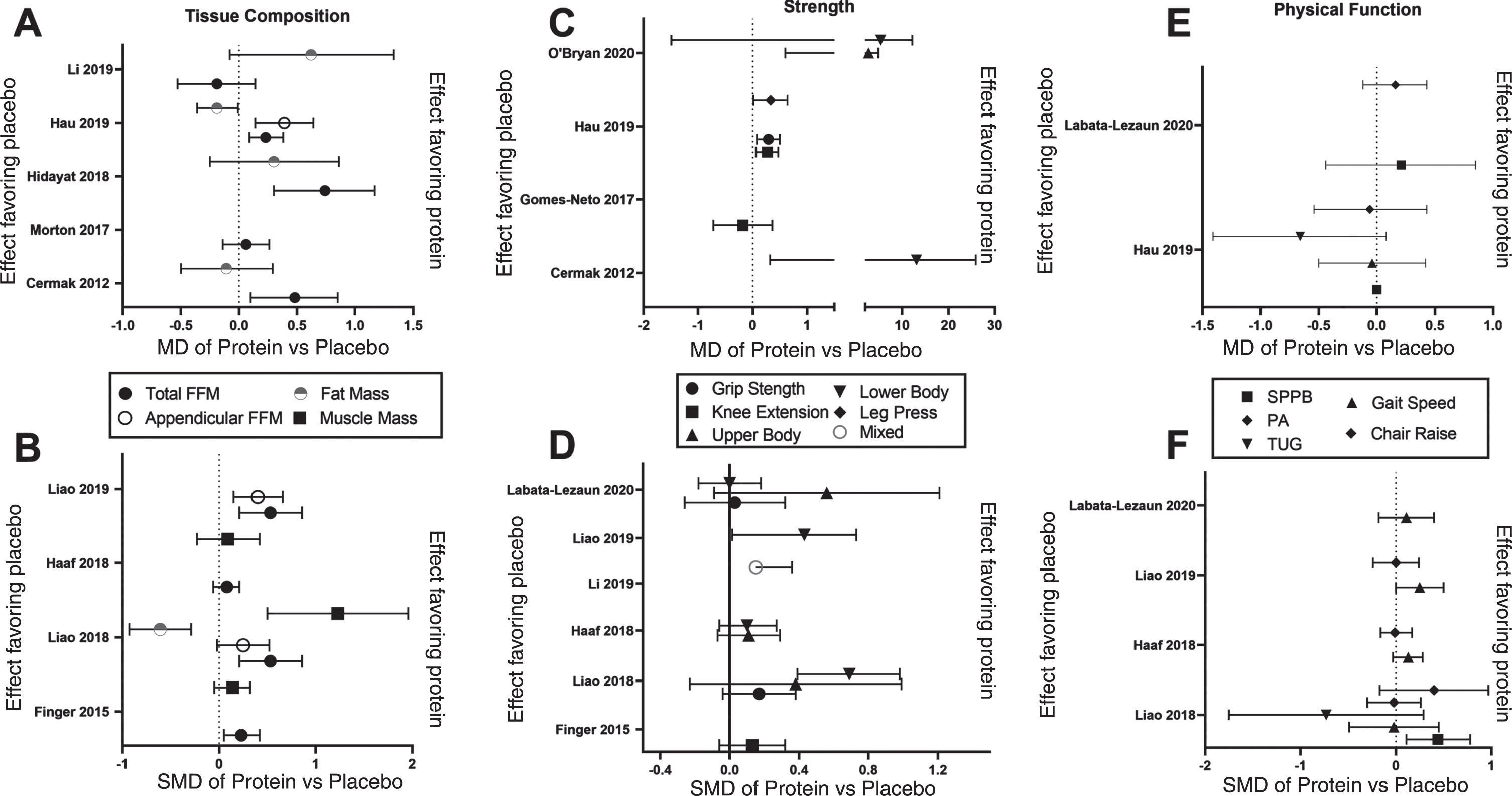
5Effect on fat mass
A statistically significant effect of PRO/AA during RET in older adults on total fat-free/lean mass was only found in two [21, 22] but not four [25, 27–29] other meta-analyses The MD effect magnitude ranged from –0.19 to 0.62 kg and SMD from –0.61 to –2.5.
6Effect on strength
There was a wide variety of strength measures utilized in the RCTs, so the meta-analyses often categorized them as upper or lower body exercises and which required use the SMD metric as the pooled estimate. Six of the 12 meta-analyses that examined muscle strength found statistically significant effects of PRO/AA during RET in older adults on at least one of the strength outcomes they assessed [21–23, 25, 27, 30], whereas the remaining found no effects in any strength outcomes [19, 20, 24, 26, 28, 31]. These studies demonstrating some effect were focused on multi-nutrient protein containing supplements [22, 23, 25, 30] or contained an enrichment of sarcopenic/frail participants [21–23, 25] with most of the effects found in the leg [21–23, 25, 27], but one of four studies revealed an effect in the upper body [30]. Type of strength assessment and frequency of use by chosen RCTs is another potential reason for divergence in results. For example: Cermak et al. [27] used leg press muscle strength from three studies, Finger et al. [26] used knee extension 1RM from ten studies. Other studies conducted multiple analyses for each individual exercise test [19, 21, 25, 26], sub-classified them as all leg/lower limb [20–24, 27, 30) or upper limb 20, 22, 24, 30], or pooled them all together [28].
7Effect on muscle mass and myofiber size
A statistically significant effect of PRO/AA during RET in older adults on muscle mass/volume/size was found in one (SMD: 1.23) [22] but not the others (SMD: 0.09–0.14) [24, 26] meta-analyses. The likely reason for this difference is that those analyses that found an effect included a high enrichment of sarcopenic participants [22, 25]. Additionally, further analysis of one study [25] revealed that its pooled estimate of “muscle mass” was only a bioelectrical impedance derived estimate and not a direct measure of muscle mass. Few studies have reported direct assessments of muscle mass/size like the expensive gold standard MRI [32]. It should be noted that Finger et al. included both myofiber CSA and whole muscle size measures into their pooled estimate of muscle size [26]. Only one meta-analysis investigated the effect of PRO/AA during RET in older adults specifically on myofiber cross-sectional area [27], and it demonstrated no effect in either slow or fast twitch myofibers.
8Effect on physical function
Three meta-analyses found no effect on any physical function outcome [20, 20, 24]. The remaining meta-analyses had several physical function outcomes yet only one statistically significant effect of PRO/AA during RET in older adults of those outcome of physical function [22, 23, 25]. Thus, many physical function outcomes in those studies were not significant, even though they were enriched with sarcopenic and frail patients who would arguably demonstrate the greatest improvement as they would have the most to gain. Liao et al. 2017 only found a significant effect with the short physical performance battery (SPPB), but not gait speed, physical activity, timed up and go (TUG), or chair rise protocols [22]. Luo et al. 2017 only investigated usual walk speed 0.57 (0.19, 0.95) [25] and Liao et al. 2019 only found a significant effect with walk capacity, but not chair rise tests [23].
9Relevance of these pooled estimates
These meta-analyses have collectively demonstrated that the effects of protein supplementation on enhancing the adaptations of resistance exercise training in older adults are not easily apparent, and it is more difficult to observe significance for outcomes of physical function, strength, muscle mass, and fat mass compared to fat-free mass. Although protein supplementation in older adults does not often improve strength or physical function beyond the improvements seen with resistance exercise training alone, some increase in lean body mass may occur. Enhancement could provide potential for fatigue resistance [33], a greater postabsorptive glucose disposal, or the presence of a greater amino acid reservoir acting as a buffer against the acute periods of sickness, injury, or disuse common with aging [34].
One could argue that most studies showing no effect for PRO were underpowered for their outcome of interest. Although some RCTs do report how they determine sample size goals, most calculations are centered on whole body FFM as the primary outcome. Future studies are advised to describe this and to be clearer in reporting variability and mean of the change values of their outcomes such that effect size/sample size estimations can be calculated for more specific outcomes. To determine whether studies are appropriately powered to show a chronic effect of PRO/AA supplementation during RET, we extracted the mean effects and standard deviations to calculate the sample size needed to determine a significant effect of PRO/AA supplementation during RET (Supplemental Table 1) in these meta-analyses. These effect sizes are very small, and the sample sizes needed to determine an effect of PRO/AA are very large. As suggested by others [31], this further cements the understanding that the vast majority of the benefits on strength and other outcomes come from the resistance exercise training modality itself, with very little contribution from the protein supplementation. The absence of effects and the low effect sizes could be attributable to the heterogeneity of individual responses to RE such as body type [35] and other factors [36–38].
10Selection of randomized control’s trials and their overlap in meta-analyses
Within the past decade, many new studies have contributed to the literature. We extracted all of the current randomized control trials used in these meta-analytic reviews to examine presence or absence of overlap for use. As the years progressed, one would assume that the same studies would be included year after year with the addition of newly published studies, but that was not the clear case. The Supplemental Connectivity Map and Supplemental Table 2 highlight a prominent reason for the discrepancy of results between meta-analyses in that each meta-analysis had a diverse selection of studies. We discovered many studies not included in any of these meta-analyses. A few RCTs were consistently included [39–43], but sparsely, as they were typically in less than 50% of the meta-analyses. Each particular RCT was utilized in a meta-analysis on average 2.7 times. 25 RCT’s were used once, 9 RCT’s were used twice, 11 RCT’s were used three times, 2 RCTs were used five times, 3 RCTs were used six times, and 4 RCTs were used seven times. 23 RCT’s were also cited by meta-analyses, but not used in quantitative analysis in some meta-analyses. In reality, only 7 studies [39–45] demonstrated a consistent pattern (used in about half the studies) for inclusion in meta-analyses and as such, they have a more consistent weight across the literature in influencing the results of these meta-analyses.
An online interactive connectivity map was also made to help visualize the connection of each RCT to each meta-analysis by outcome (fat-free mass, fat mass, muscle mass, strength, and physical function (Supplemental online material). Supplemental Table 3 also highlights that these meta-analyses also only cited less than 50% of previous meta-analyses, again indicating a lack of cohesion in the literature or divergence in topics not readily apparent from initial inspection of the article titles and abstracts. However, this may be a result of insufficient space for citations and or publishing lag.
We examined if study quality (Fig. 2 and Table 3) was a potential determining factor on study exclusion into these meta-analyses as it often should be. Typical RCT methodological quality metrics assess items like random allocation, concealed allocation, similarity at baseline, subject blinding, therapist blinding, assessor blinding, follow up for at least one key outcome, intention-to-treat analysis, between-group statistical comparisons, and point and variability measures for at least one key outcome. Each meta-analysis, except two [21, 27], reported a methodology quality metric and score for each individual RCT it used in its meta-analysis. Although most meta-analyses utilized the PEDro scale (0–10) to assess study quality, not all did. We converted those on a different scale to a 10-point scale for comparison purposes so that methodological quality approaches could be better assessed, to a degree, across and within meta-analyses (Table 3 and Fig. 2). As with the PEDro approach, methodological quality was considered high, ≥7 points; medium, 4–6 points; low, ≤3 points. Therefore, we report a summary of the RCT methodology quality scores provided by each meta-analysis.
Table 3
Methodological Quality Score for the RCT’s used in Meta Analyses, and the Coefficient of Variation (CV) for that score when used across multiple meta-analyses
| Quality Score | CV | ||
| N | Mean | Mean | |
| All | 134 | 7.80 (7.54–8.06) | |
| Single use | 22 | 6.72 (5.69–7.76) | |
| 2 | 7 | 7.74 (6.85–8.64) | 18.50 (9.81–27.20) |
| 3 | 10 | 8.14 (7.40–8.89) | 11.82 (4.66–18.97) |
| 4 | 4 | 7.50 (5.02–9.99) | 17.71 (8.39–27.02) |
| 5 | 3 | 7.79 (5.62–9.95) | 17.46 (12.44–22.47) |
| 6 | 4 | 7.86 (5.67–10.05) | 18.51 (12.69–24.34) |
| 7 | 5 | 8.11 (7.03–9.19) | 14.71 (5.01–24.41) |
| 2+ | 33 | 7.84 (7.46–8.22) | 15.29 (12.36–18.21) |
All methodological quality tools were scaled 0–10. Data are Mean and 95% Confidence Intervals.
Fig.2
Average quality score from the randomized controlled trials included in the meta-analyses. Data are mean with 95% confidence intervals.
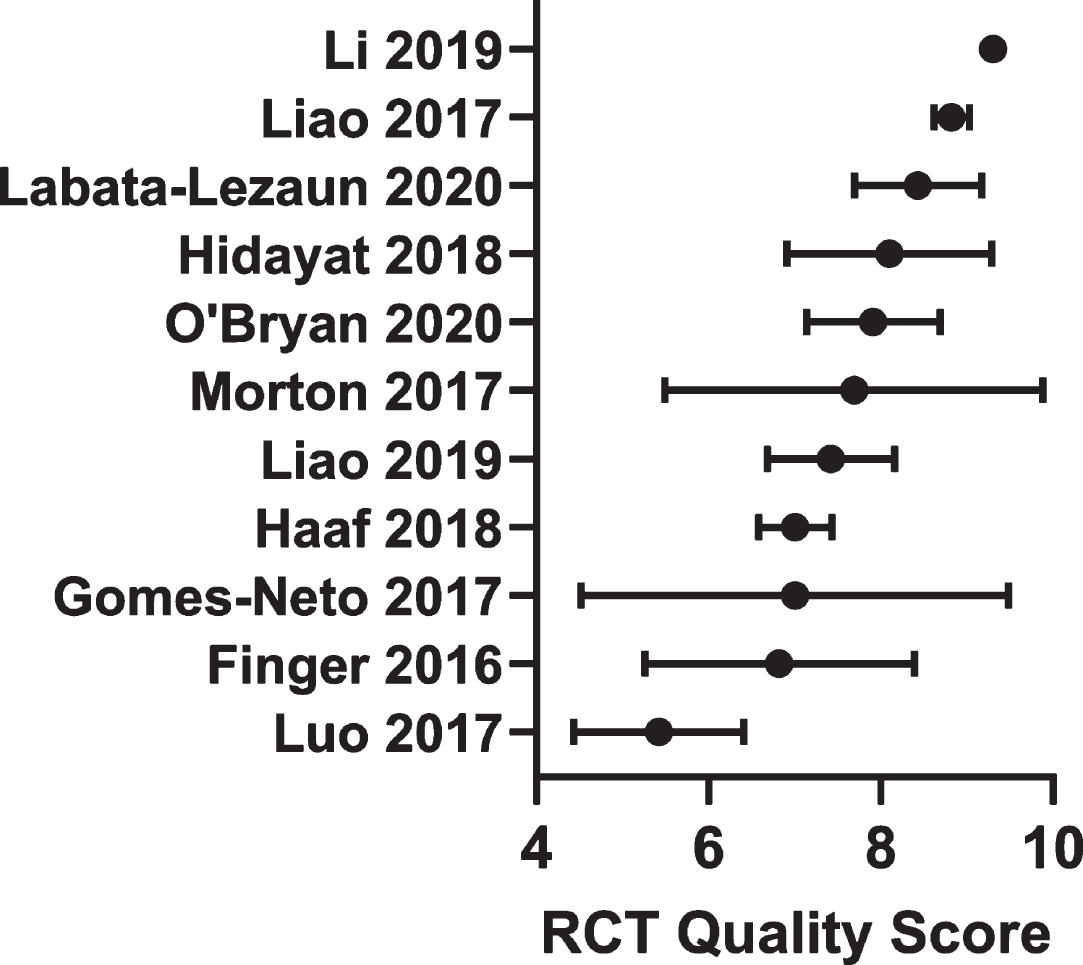
We averaged the RCT study quality scores from each meta-analysis with lower and upper 95% confidence intervals (Fig. 2). These scores reflect the quality of the RCTs and/or the nature of the approach (conservative or not) taken by the respective authors of the meta-analyses when making these assessments. All but two studies scored a high (≥7 points) methodological quality in their collected RCTs. One was borderline medium/high at 7.69 (5.49–9.88 95% CI) [26] while another was noticeably composed of medium quality RCTs or was more conservative with its assessment at 5.42 (4.43–6.41 95% CI) [25]. One meta-analysis conducted subgroup analysis demonstrating that those RCTs with a PEDro score less than or greater than 7 tended to show different outcome responses [23] suggesting that study quality is a relevant variable.
All the 134 RCT’s used in these analyses resulted in an average quality score of 7.80 (7.54–8.06 95% CI) to be considered high quality. Studies that were used only once scored 6.72 (5.69–7.76 95% CI), which was slightly less than those utilized more than once in a meta-analysis at 7.84 (7.46–8.22 95% CI), but not significantly so. Those multi-use studies had a variation coefficient of 15.3% (12.4–18.2 95% CI) when comparing scores across meta-analyses. This suggests that although methodological quality may have been used as a criterion for exclusion from some meta analyses, it was a minor one, and there are likely other more prominent reasons for the infrequent use of many of these RCTs across these meta analyses.
11Inclusion and exclusion criteria
Differences between these meta-analyses’ RCT selections are likely due to the inclusion and exclusion criteria for included RCTs (Table 2 and Supplemental Table 4). Age was one criterion with selection cut points of >40 to >65 years of age, with the majority of meta-analyses picking 50–60y as the cutpoint, but as low as 40y in some cases. Length of resistance training was also a selection criterion, with cutoff points of 4+ to 12+ weeks. Weekly frequency of RET sessions was normally limited to RCT’s with two or more sessions per week, with one study allowing a minimum of 1 session per week [21].
11.1Differences in population
The selection of RCTs and their inherent participant populations found in the selected RCT’s is a likely strong contributor to the divergent conclusions in the literature. When examining the meta-analyses that included an enrichment of greater than 25% frail and sarcopenic older adults, seven of eight meta-analyses demonstrated a positive effect on at least one outcome with protein supplementation during resistance training (Table 4). Indeed, as the enrichment of frail and sarcopenic adults increases (moving from left to right in Table 4), a trend for more significant effects are found in these meta-analyses, specifically for hospitalized or institutionalized older adults [23, 25]. The increasing number or reports that RET + AA/Pro supplementation actually have a greater impact advanced age/frailty presents an interesting juxtaposition between with the concept that anabolic resistance increases with age. We know the concept of anabolic resistance is more complex than just increasing anabolic resistance with every year of aging [46]. The frail older adult has the greatest need as they are likely the most nutrient/protein deficient and physically inactive. Although speculative it is common knowledge that those who benefit most with any nutritional supplementation are those who are deficient in that nutrient and those who have the least physical activity reap the most rapid health benefits by engaging in physical exercise. So, within the aging population, they may have the most to gain when they start RET. Indeed, meta-analyses with data pooled from younger and older adults has shown an attenuation of the effect of protein supplementation with age [28, 31] To examine this age and anabolic resistance relationship, within the older adult age group further a large collection of RCTs with a heterogenous population of older adults across age with varied health conditions would be needed for a meta-regression. Unfortunately, most meta-analyses that did look at age as a continuous covariable had a homogenous population of older adults [31] and those that did have a varied population of older adults did not conduct a meta-regression age as a continuous covariable.
Table 4
Outcomes effective for lean mass, muscle strength, and physical function and percent of each participant category in each meta-analysis
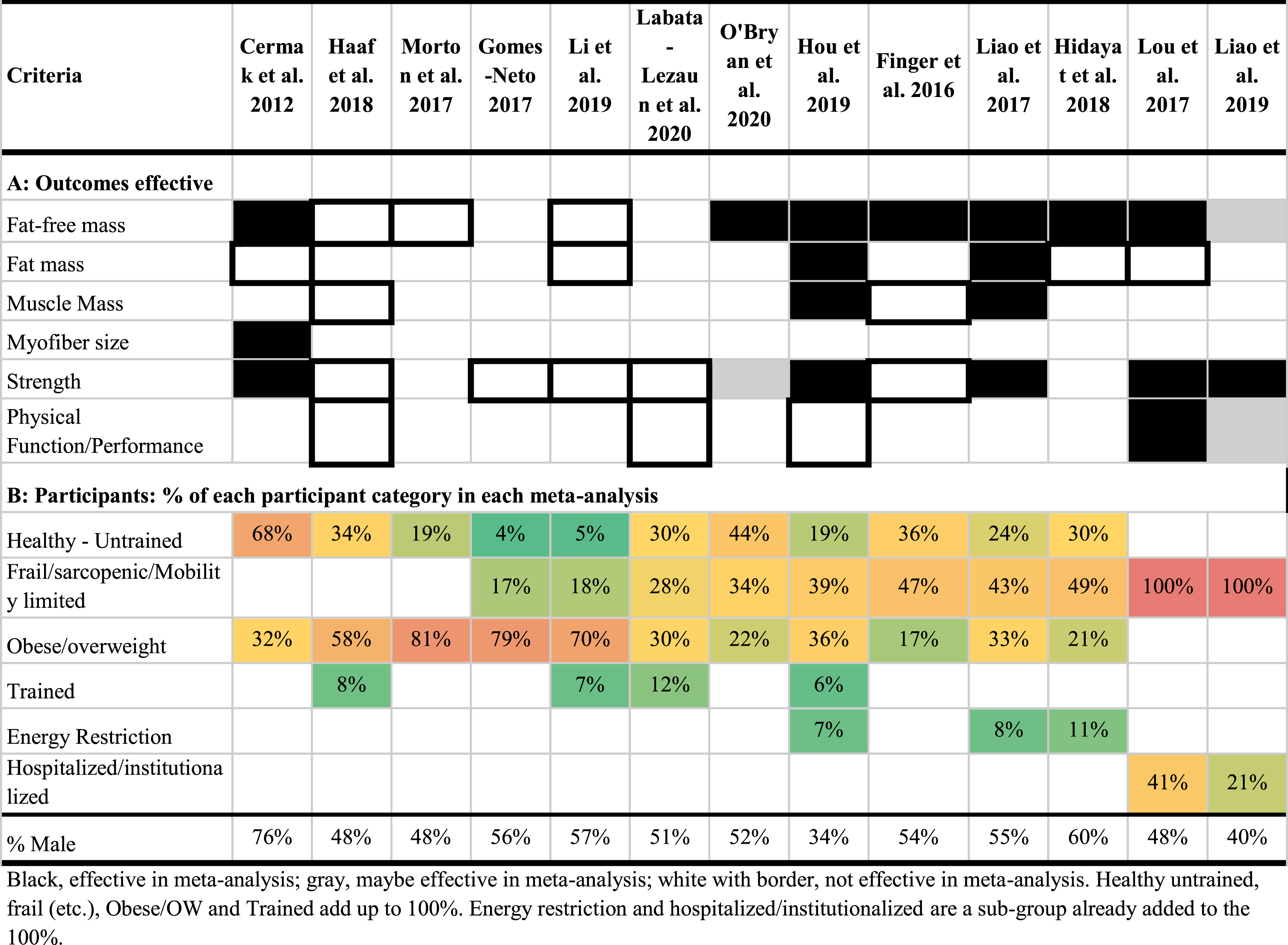 |
Conversely, meta-analyses with a greater enrichment of overweight and obese participants appear to demonstrate no effect [19, 24, 28, 31]. This would be reflective of anabolic resistance with excess weight [47], which can be common to older adults. However, Liao et al. 2019 presented conflicting findings with its subgroup analysis of older adults greater than or less than a BMI of 30. They reported an equivalent lean mass gain, body mass and body fat loss, and lower body strength and no changes in upper body strength [22]. These minor improvements in obese participants in Liao et al. 2017 may be more reflective of the presence of a cocktail of other nutrients and anabolic agents (creatine) included in the supplementation other than protein and or inclusion of an energy restriction trials [48]. As recently suggested by O’Bryan et al., in 2020, a protein-containing multi-nutrient intervention with RET may be slightly more effective (but not statistically so) than just protein alone with RET in older adults [30]. Regardless, when examining the pattern across these meta-analyses it becomes apparent that the effect of protein supplementation to enhance the adaptations to RET are attenuated in overweight and obese older adults who are less likely to be deficient in protein and more physically inactive.
Most meta-analyses had an equal proportion of men and women with about 48–60% male (Table 5). Although one meta-analysis was 76% male participants [20], another was 34% male participants [27] they both demonstrated positive effects on their chosen outcomes. This is reflective of other meta-analysis subgroup examinations showing minimal sex-differences [22, 23, 29], with the single exception of lean mass in one early study [22]. Thus, there appears to be a similar response (or lack thereof) in both males and females.
11.2Differences by length of intervention
Longer RCT RET duration may [49–51] or may not [52] impact strength and muscle mass gain [50]. Muscle cross sectional area increases of ∼15% seem to plateau at about 3 months of training in the general population [53], but specific long term >3 months’ time course of muscle hypertrophy in older adults specifically is unclear. Therefore, we looked to see if length of intervention had any patterns with positive outcomes on whether or not a meta-analysis was effective in increasing fat-free mass, muscle strength, and/or function. The intervention lengths of studies within the meta-analyses lasted anywhere from 3 to 104 weeks with most lasting from 12 to 72 weeks (Fig. 3A). There was no clear pattern, from examination at the level of these meta-analyses, to suggest that length of intervention may impact effectiveness of resistance training and protein supplementation for older adults. Because the majority of studies are ∼12 to 16 weeks in length, there is little spread in this data, making it difficult to assess the relationship of study duration on these outcomes as shown in previous meta-regressions [28]. As longer RCTs (>20 weeks) are included future meta-regression may be able to provide more insight in this regard.
Fig.3
Volcano plot of intervention length (A) and daily protein dose (B) in meta-analyses examining the effects of protein supplementation and resistance exercise training compared to resistance exercise training with or without placebo in older adults for the outcomes of tissue composition, strength and physical function. Data are individual RCTs included in each meta-analysis. The suggested optimal dose of protein is highlighted as a dotted line on the x-axis in panel B.
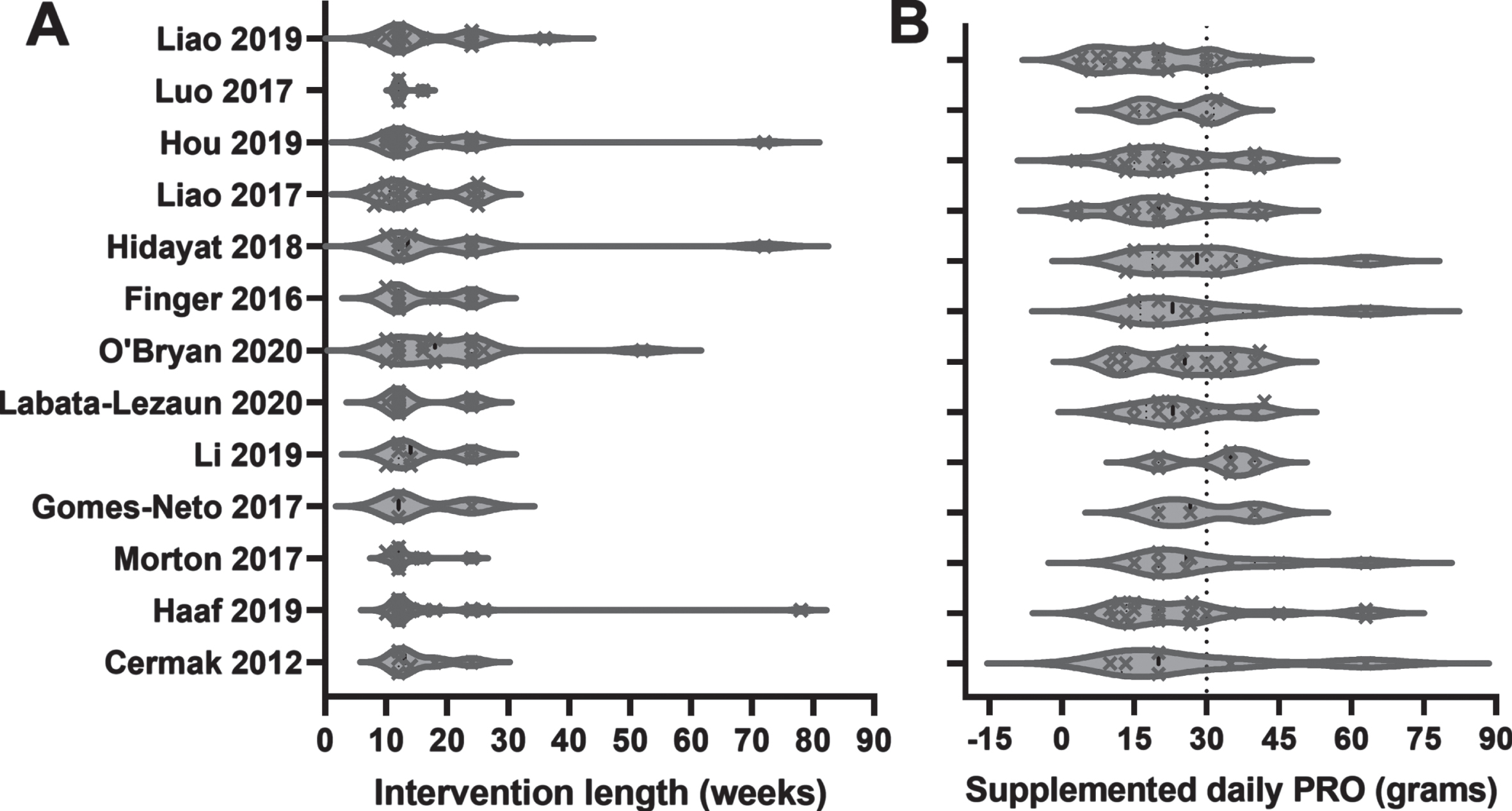
Previous studies have conducted subgroup analyses to determine if length of the RET intervention may optimize the effect of protein supplementation. One study found no difference between RCTs < or >12 weeks in duration with a direct statistical assessment [29]. One study [22] defined short a <12 weeks, medium as 12–24 weeks and long as >24 weeks and suggested greater enhancement of LM and physical function with medium and long interventions vs short interventions. The issue is that they defined sub-groups to examine the effect of protein supplementation and demonstrate statistical effects within, but not between short, medium or long sub-groups although their conclusions would suggest otherwise. Further meta-analyses are encouraged to design hypotheses with appropriate analyses to test for the ideal training duration to evaluate a protein and exercise interaction.
11.3Differences by nutritional intervention
Another area of study design variation is possibly the impact of type of protein nutritional intervention included in these meta-analyses. Supplemental Table 5 shows the different types of nutritional interventions in the meta-analyses and whether or not the meta-analysis was considered effective. The figure also highlights the inclusion and exclusion criteria authors (Supplemental Table 4) used to define which nutritional supplements were allowed and then also what types of nutritional supplements were actually included. All of the possible combinations of protein containing nutritional supplements could have included, whey protein, milk protein, egg protein, flesh protein, plant protein, amino acids, β-HMB and additional macronutrients, micronutrients, creatine and other androgenic agents. A few RCTs were giving their interventions in states of energy restriction (Supplemental Table 5). Most studies were rather broad in their inclusion and exclusion criteria for the nutritional supplement leaving much up to interpretation. Often meta-analyses did not follow their own criteria mentioned in their methods. With the exception of Haaf et al. [24] most of the meta-analyses that were generally non-effective [19, 20, 26, 28, 31] did not include many studies with non-protein components. Whereas, the studies that generally showed more consistent effects with protein supplementation [21–23, 29, 30] did include a high enrichment of studies that contained non-protein nutrients - half of which included fat and carbohydrate. The exceptions were two studies that had a comparatively low number of RCTs [25, 27] and had frail participants [25]. Also, most of the ineffective meta-analyses did not specify any specific types of protein supplementation used. Many of the effective meta-analyses specified they used many different types of protein, vitamins, minerals, and other nutritional agents. Including a variety of different protein sources, EAA, vitamins, minerals, CHO or fat, creatine, etc. can have a positive outcome on increasing fat-free mass, muscle strength, and/or function. This idea was recently examined specifically in a meta-analysis where they determined that multi-nutrient protein-containing supplements were effective in demonstrating effects on outcomes during resistance exercise training compared to placebo [30]. However, they could not distinguish a statistical effect of multi-nutrient protein containing supplements compared to protein only supplements with the existing literature available to them. Thus, protein supplements in isolation may not be as effective as protein supplements with other nutrients included.
While the included ingredients of a protein supplement may have had an effect on increasing fat-free mass, muscle strength, and/or function, the dose of protein supplementation may also influence the response. Several meta-analyses have hypothesized that protein dose is a potential contributing factor that needs to be optimized in future studies to enhance the effect of protein supplementation during resistance exercise training [24, 26, 31]. Indeed, in a detailed analysis Morton et. al. did not find that postexercise dose influenced the results, however that was likely because the majority of the RCTs in said analysis gave a sub-optimal dose (<20 g) of protein and there were insufficient studies giving an optimal dose of closer to about 30 g [31]. In Fig. 3B we show the dose given by each RCT in each of the meta-analyses and we demonstrate that the majority of these studies are enriched with a sub-optimal (<30 g) protein dose. More clear conclusions can be drawn regarding the need for higher protein doses in older participants during resistance exercise training once more RCTs testing a higher dose are included in future meta-analyses. Yet, we need to be careful of continually suggesting higher protein doses, especially when considering sedentary conditions. The common belief that more is better is dangerous. A key variable regarding the effect of supplemental protein is engagement in the growth process with muscle contraction. Yet, supplemental and additional protein in healthy older adults eating sufficient protein does not provide additional benefits. For indeed, excess stimulation of anabolic pathways with protein nutrition through the BCAAs does lead to increased cardiometabolic disease risk [54]. More than enough is not better than enough.
12Differences in analytical approach
Other distinctions between meta-analyses are much less apparent and “hidden” in the methodological approaches. These factors are only apparent to the statistician and/or those who regularly conduct meta-analytic research, thus a small proportion of the population. Yet, these factors have a tremendous impact on the outcomes and results so they should be better appreciated by those in the field.
12.1Differences in the effect outcomes
Some meta-analyses limit the data from included studies to absolute changes in the outcome (kilograms for lean mass or weight lifted) when there were not distinct units or different measurement tools for an outcome. In the case of varied units or approaches, standardized mean difference (SMD) was utilized to portray the differences between groups. In some cases, meta-analyses excluded studies or outcomes that would not allow for reporting of MD [19, 21, 27, 29–31], or they reported MDs for some outcomes, but SMDs for others [20, 28]. The remaining meta-analyses reported SMD exclusively [22–26]. The use of SMD has the potential to be less sensitive in regards to MD for detecting differences in means relative to the SDs in each study [26]. This may explain a small proportion of the differences between meta-analyses.
12.2Differences in heterogeneity
Although many meta-analyses excluded certain RCT’s to obtain a more homogenous set of pooled estimates, this was often not possible due to many differences between RCTs making them too unique. Lack of uniform direction and magnitude responses (i.e. heterogeneity) will often introduce too much variability to determine the effect of a pooled estimate. Typically, significant heterogeneity could indicate the need for subgroup analysis. Most meta-analyses tested statistical heterogeneity of their outcomes with the use of I2 and/or X2 statistics, but the cutoff for determination of heterogeneity was not uniform. Higher heterogeneity was defined in four studies as I2 values >50% [21, 22, 24, 30], in four studies as I2 values >75% [20, 26, 27, 29], as a p < 0.05 cut-off [22, 31] or not defined [19, 25, 28]. In most cases, presence of low heterogeneity allowed for fixed-effects statistical modeling, and high heterogeneity indicated use of random-effects statistical modeling to determine the pooled estimates. Examination of the heterogeneity approaches does not easily appear to impact the results of these studies at face value, but other analytical approaches to these meta-analyses may.
12.3Differences in estimation of incomplete data during data extraction procedures
In order to conduct a meta-analysis on this type of continuous data in the context of protein supplementation during resistance training, the mean change score from baseline of both the treatment group and the placebo/control group outcomes of interest is needed in addition to the standard deviation of those outcome change scores from baseline for each group. Although some authors of RCTs do report some of their data in this format, it is unfortunately uncommon practice. The authors of RCTs may report the data in this way via graphical format, but even then, one needs to “eyeball” the data to guess the exact values needed for extraction, which leads to inherent error and is by nature, imprecise. However, some tools like webplotdigitizer can reduce that inherent error, but not eliminate it. There are several methods applying statistics to cleverly extract the correct information, but some are less precise than others [55] and several assumptions are needed. More complex is the lack of reporting of change scores and standard deviations. It is more common to report the point estimate means at Pre and Post for both the treatment group and the placebo/control group with their respective measure of variability (SDs or SEMs). In this case, one can still calculate the change score for each group by the arithmetic difference between pre and post. However, this data does not give indication of the variability of the changes. This problem is “dealt with” by imputing a correlation coefficient (Corr) to estimate the missing change score SD through several ways. This is not an optimal approach, but it is often needed.
According to the Cochrane Handbook, there are three approaches to this issue. “Corr may be calculated from another study in the meta-analysis, imputed from elsewhere, or hypothesized based on reasoned argument”. Discussion of this method and the many assumptions and rules or its use can be found in the Cochrane Handbook [55] in more detail. Corr can be calculated by using other studies in the meta-analysis that have that outcome and do report the data in the correct format needed. This is done by using the change score SDs from those studies providing the correctly formatted data to generate a correlation coefficient that is used to estimate the change score in studies that do not report it. These assumptions and limitations are almost never discussed in these meta-analyses, yet the choice and approach to input missing data has tremendous impact on the results of meta-analysis results. Using a value with a higher SD change score will result in a lower weighted study with a larger confidence interval for MD analysis. Using a larger SD change score value for SMD analysis will skew towards non-significance [55]. Brief points to summarize are this 1) each group (protein and placebo should have its own Corr value). Unfortunately, the need for this type of imputation is common, and all the meta-analyses provided would face this issue. Yet only 7 of the 13 meta-analyses reported or mentioned the Corr value, whereas the remainder did not [20, 25, 26, 28, 30]. Four studies applied one Corr value for all outcomes with 0.5 used for [24, 29], 0.95 for [21], and 0.98 for [22]. The following studies used specific Corr values for certain outcomes, which is likely a more appropriate approach given that variability is different for different metrics (e.g. FFM vs physical function). Also, they report different Corr values, which were calculated for the various outcomes, justifying their precise use in this context. Liao et al. 2019 used 0.98 for FFM, 0.92 for strength, and 0.8 for physical function outcomes [23]. Morton et al. used 0.98 for FFM and FM, 0.99 for muscle mass, 0.75 for myofiber size, 0.69 for maximum voluntary contraction (MVC), and 0.84 for 1-RM outcomes [31]. Cemak et al. used 0.98 for FFM and FM, and then for strength, used 0.7 for the protein group and 0.9 for the placebo group in addition to group and fiber type-specific effects for myofiber size [27].
Altogether, these results indicate that higher Corr values of ∼0.98 for tissue composition and generally lower Corr values for strength, muscle mass, and physical function are needed. Some authors indicated published work demonstrating that application of Corr values used in other studies is acceptable practice [56]. Yet, the credibility is considered less [56], especially since the above-mentioned studies illustrate differences in the Corr values across studies, outcomes, and even the comparison groups. Another concern when applying a Corr value from other meta-analyses is that dissimilarities in the design (participant population, outcomes, intervention, etc.) are highly likely to impact the variability of the outcomes’ responses and invalidate their application.
Only one of these meta-analyses acknowledged the impact that the Corr value has on these results in its discussion, and the impact is not trivial. Haaf et al. discussed the absence of a protein effect may come from the fact that it used a 0.5 Corr value compared to other studies that applied a 0.98 value. They did a sensitivity analysis to demonstrate that when substituted in the 0.98 value, there was a significant protein effect [24]. This may be a contributing factor why the FFM outcome typically is more common to demonstrate a positive effect of protein supplementation compared to other outcomes. The response of this outcome is also less variable than other outcomes.
12.4Problems with extraction measures of outcome variability
Another issue could arise in the process of extracting the data is the misreporting/mistaking the standard errors as standard deviations by the RCT authors and/or the authors of the meta-analysis. Additional complication arises when the SEM is given, but the sample size for that specific outcome is not, prohibiting the calculation of SD needed. This may be an issue when certain outcomes of interest have a different number of participants as a result of the common logistical difficulties of clinical research. These different sample sizes by outcome may not be easily observable.
The choice of the Corr value and other imputation and data extraction methods have a tremendous impact on how the interventional effect of a RCT is calculated in these meta-analyses. For example, the effect of protein supplementation on fat-free mass in the Tieland et al. 2012 RCT [45] was significant at 0.79 (0.27,1.132) in Liao et al. 2017, and at 1.60 (0.64, 2.56) in Hidayat et al. 2018. Yet, it was insignificant at 0.23 (–0.27, 0.73) in Finger et al. 2017 and 0.49 (–0.05,1.04) in Huo et al. 2019. This really serves to highlight how the combined effect of the choice of pooled studies and the method used to input missing change score SDs can impact the results to explain divergence in the literature.
Authors would be encouraged to make their data sets publicly available or at least report change scores and the variability of the change scores in their published work for ALL their data, even as supplemental data, so that these issues can be avoided and the field can glean more precise, accurate meta-analytic results.
13Summary and conclusion
Several reports have demonstrated that when contraction and sufficient protein are acutely combined, the age-related anabolic resistance diminishes. Thus, it would seem intuitive that supplementing protein during RET would be a more effective strategy to enhance muscle size, strength, and physical function. Several meta-analyses sought to answer this question and although they shared remarkably similar titles, they had pronounced differences in 1) results, 2) type of protein-focused nutritional intervention, 3) data extraction approach, and 4) RCT study selection/participants used. However, the compiled evidence from these meta-analyses clearly indicates that the phenotypic response to RET and protein-focused nutrition is very similar to that of resistance training alone, suggesting that chronic application diminishes this beneficial effect of combined resistance exercise and protein-focused nutrition. The lack of an effect is most apparent and difficult to discern with muscle strength, muscle mass, fat mass and physical function, but somewhat more common, although still minimal with fat-free mass. When considering the other factors in these meta-analyses such as: participant population, quality score, sample size, differences in protein intake with supplementation or between supplement groups, diet quality, protein dosing, or distribution of protein at each meal, the current findings are no surprise. The lack of an effect is clearly noticeable in analyses containing enrichment of overweight and obese participants. However, in sarcopenic and frail older adults (or those on a poor diet), optimizing the total amount, and quality protein throughout the day may be useful in enhancing muscle growth and function in response to RET. Further, RCTs need to be conducted to fill the gaps in the literature and to provide complete data sets in the correct format for meta-analyses such that the field is able to progress.
Authors’ contributions
P.T.R. formulated the review topic; P.T.R., A.K.D., J.M.M., A.M.B., C.N.K. and C.E.P conducted the literature review, data extraction, and formatting; P.T.R., A.K.D., J.M.M., A.M.B., and C.N.K. reviewed the manuscript; P.T.R., A.K.D. and J.M.M. wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgments
This article was supported by a College of Education Health and Society Summer Research grant and the Miami University Committee for Faculty Research. We would like to thank Anh Nguyen, Arushi Verma, Alison Riley, and the Center for Data Analytics at Miami University for the creation of the connectivity map.
Funding
The authors report no funding.
Conflict of interest
The authors have no conflicts of interest.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NHA-220183.
References
[1] | Delmonico MJ , Harris TB , Visser M , Park SW , Conroy MB , Velasquez-Mieyer P , et al., Longitudinal study of muscle strength, quality, and adipose tissue infiltration, Am J Clin Nutr, (2009) ;90: (6):1579–85. |
[2] | Doherty TJ , The influence of aging and sex on skeletal muscle mass and strength, Curr Opin Clin Nutr Metab Care(2001) ;4: (6):503–8. |
[3] | English KL , Paddon-Jones D , Protecting muscle mass and function in older adults during bed rest, Current opinion in clinical nutrition and metabolic care(2010) ;13: (1):34–9. |
[4] | Trombetti A , Reid KF , Hars M , Herrmann FR , Pasha E , Phillips EM , et al., Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life, Osteoporos Int(2016) ;27: (2):463–71. |
[5] | den Ouden MEM , Schuurmans MJ , Arts IEMA , van der Schouw YT , Physical performance characteristics related to disability in older persons: A systematic review, Maturitas(2011) ;69: (3):208–19. |
[6] | American College of Sports Medicine Position Stand, Exercise and physical activity for older adults, Med Sci Sports Exerc(1998) ;30: (6):992–1008. |
[7] | Janssen I , Shepard DS , Katzmarzyk PT , Roubenoff R , The healthcare costs of sarcopenia in the United States, J Am Geriatr Soc(2004) ;52: (1):80–5. |
[8] | Steffl M , Sima J , Shiells K , Holmerova I , The increase in health care costs associated with muscle weakness in older people without long-term illnesses in the Czech Republic: Results from the Survey of Health, Ageing and Retirement in Europe (SHARE), Clin Interv Aging(2017) ;12: :2003–7. |
[9] | Álvarez-Bustos A , Rodríguez-Sánchez B , Carnicero-Carreño JA , Sepúlveda-Loyola W , Garcia-Garcia FJ , Rodríguez-Mañas L , Healthcare cost expenditures associatedto frailty and sarcopenia, BMC Geriatrics(2022) ;22: (1):747. |
[10] | Hudson JL , Wang Y , Bergia Iii RE , Campbell WW , Protein intake greater than the RDA differentially influences whole-body lean mass responses to purposeful catabolic and anabolic stressors: A systematic review and meta-analysis, Adv Nutr(2020) ;11: (3):548–58. |
[11] | Bergia RE , Hudson JL , Campbell WW , Effect of whey protein supplementation on body composition changes in women: A systematic review and meta-analysis, Nutr Rev(2018) ;76: (7):539–51. |
[12] | Valenzuela PL , Mata F , Morales JS , Castillo-García A , Lucia A , Does beef protein supplementation improve body composition andexercise performance? A systematic review and meta-analysis ofrandomized controlled trials, Nutrients(2019) ;11: (6):E1429. |
[13] | Messina M , Lynch H , Dickinson JM , Reed KE , No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise, Int J Sport Nutr Exerc Metab(2018) ;28: (6):674–85. |
[14] | Hita-Contreras F , Bueno-Notivol J , Martínez-Amat A , Cruz-Díaz D , Hernandez AV , Pérez-López FR , Effect ofexercise alone or combined with dietary supplements onanthropometric and physical performance measures incommunity-dwelling elderly people with sarcopenic obesity: Ameta-analysis of randomized controlled trials, Maturitas(2018) ;116: :24–35. |
[15] | Cheng H , Kong J , Underwood C , Petocz P , Hirani V , Dawson B , et al., Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions, Br J Nutr(2018) ;119: (5):527–42. |
[16] | Liao CD , Wu YT , Tsauo JY , Chen PR , Tu YK , Chen HC , et al., Effects of protein supplementation combined with exercise training on muscle mass and function in older adults with lower-extremity osteoarthritis: A systematic review and meta-analysis of randomized trials, Nutrients(2020) ;12: (8):E2422. |
[17] | Colonetti T , Grande AJ , Milton K , Foster C , Alexandre MCM , Uggioni MLR , et al., Effects of whey protein supplement in the elderly submitted to resistance training: Systematic review and meta-analysis, Int J Food Sci Nutr(2017) ;68: (3):257–64. |
[18] | Thomas DK , Quinn MA , Saunders DH , Greig CA , Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: A systematic review, J Am Med Dir Assoc(2016) ;17: (10):1–959.e9. |
[19] | Gomes-Neto M , Braga da Silva TLT , Carvalho VO , Whey protein supplementation in association with resistance training on additional muscle strength gain in older adults: A meta-analysis, Science & Sports(2017) ;32: (4):214–20. |
[20] | Labata-Lezaun N , Llurda-Almuzara L , López-de-Celis C , Rodríguez-Sanz J , González-Rueda V , Hidalgo-García C , et al., Effectiveness of protein supplementation combined withresistance training on muscle strength and physical performance inelderly: A systematic review and meta-analysis, Nutrients(2020) ;12: (9):E2607. |
[21] | Hou L , Lei Y , Li X , Huo C , Jia X , Yang J , et al., Effect of protein supplementation combined with resistance training on muscle mass, strength and function in the elderly: A systematic review and meta-analysis, J Nutr Health Aging(2019) ;23: (5):451–8. |
[22] | Liao CD , Tsauo JY , Wu YT , Cheng CP , Chen HC , Huang YC , et al., Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis, Am J Clin Nutr(2017) ;106: (4):1078–91. |
[23] | Liao CD , Chen HC , Huang SW , Liou TH , The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials, Nutrients(2019) ;11: (8):E1713. |
[24] | Ten Haaf DSM , Nuijten MAH , Maessen MFH , Horstman AMH , Eijsvogels TMH , Hopman MTE , Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis, Am J Clin Nutr(2018) ;108: (5):1043–59. |
[25] | Luo D , Lin Z , Li S , Liu SJ , Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis, International Journal of Nursing Sciences(2017) ;4: (4):389–401. |
[26] | Finger D , Goltz FR , Umpierre D , Meyer E , Rosa LHT , Schneider CD , Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis, Sports Med(2015) ;45: (2):245–55. |
[27] | Cermak NM , Res PT , de Groot LCPGM , Saris WHM , van Loon LJC , Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis, Am J Clin Nutr(2012) ;96: (6):1454–64. |
[28] | Li M , Liu F , Effect of whey protein supplementation during resistance training sessions on body mass and muscular strength: A meta-analysis, Food Funct(2019) ;10: (5):2766–73. |
[29] | Hidayat K , Chen GC , Wang Y , Zhang Z , Dai X , Szeto IMY , et al., Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials, J Nutr Health Aging(2018) ;22: (2):237–45. |
[30] | O’Bryan KR , Doering TM , Morton RW , Coffey VG , Phillips SM , Cox GR , Do multi-ingredient protein supplements augment resistance training-induced gains in skeletal muscle mass and strength? A systematic review and meta-analysis of 35 trials, Br J Sports Med(2020) ;54: (10):573–81. |
[31] | Morton RW , Murphy KT , McKellar SR , Schoenfeld BJ , Henselmans M , Helms E , et al., A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults, Br J Sports Med(2018) ;52: (6):376–84. |
[32] | Sizoo D , de Heide LJM , Emous M , van Zutphen T , Navis G , van Beek AP , Measuring muscle mass and strength in obesity: A review of various methods, Obes Surg(2021) ;31: (1):384–93. |
[33] | Gryson C , Ratel S , Rance M , Penando S , Bonhomme C , Le Ruyet P , et al., Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants, Journal of the American Medical Directors Association(2014) ;15: (12):1–958 e9. |
[34] | Wolfe RR , The underappreciated role of muscle in health and disease, The American Journal of Clinical Nutrition(2006) ;84: (3):475–82. |
[35] | Van Etten LM , Verstappen FT , Westerterp KR , Effect of body build on weight-training-induced adaptations in body composition and muscular strength, Medicine and Science in Sports and Exercise(1994) ;26: (4):515–21. |
[36] | Davidsen PK , Gallagher IJ , Hartman JW , Tarnopolsky MA , Dela F , Helge JW , et al., High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol. 2010. |
[37] | Pescatello LS , Kostek MA , Gordish-Dressman H , Thompson PD , Seip RL , Price TB , et al., ACE ID genotype and the muscle strength and size response to unilateral resistance training, Med Sci Sports Exerc(2006) ;38: (6):1074–81. |
[38] | Hubal MJ , Gordish-Dressman H , Thompson PD , Price TB , Hoffman EP , Angelopoulos TJ , et al., Variability in muscle size and strength gain after unilateral resistance training, Medicine and Science in Sports and Exercise(2005) ;37: (6):964–72. |
[39] | Verdijk LB , Jonkers RA , Gleeson BG , Beelen M , Meijer K , Savelberg HH , et al., Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men, The American Journal of Clinical Nutrition(2009) ;89: (2):608–16. |
[40] | Arnarson A , Gudny Geirsdottir O , Ramel A , Briem K , Jonsson PV , Thorsdottir I , Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: Double blind, randomised controlled trial, European Journal of Clinical Nutrition(2013) ;67: (8):821–6. |
[41] | Leenders M , Verdijk LB , Van der Hoeven L , Van Kranenburg J , Nilwik R , Wodzig WK , et al., Protein supplementation during resistance-type exercise training in the elderly, Medicine and Science in Sports and Exercise(2013) ;45: (3):542–52. |
[42] | Candow DG , Chilibeck PD , Facci M , Abeysekara S , Zello GA , Protein supplementation before and after resistance training in older men, European Journal of Applied Physiology(2006) ;97: (5):548–56. |
[43] | Kukuljan S , Nowson CA , Sanders K , Daly RM , Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: An 18-mo randomized controlled trial, J Appl Physiol(2009) ;107: (6):1864–73. |
[44] | Chale A , Cloutier GJ , Hau C , Phillips EM , Dallal GE , Fielding RA , Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults, The Journals of Gerontology Series A, Biological Sciences and Medical sciences(2013) ;68: (6):682–90. |
[45] | Tieland M , Dirks ML , van der Zwaluw s N , Verdijk LB , van de Rest O , de Groot LC , et al., Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial, Journal of the American Medical Directors Association(2012) ;13: (8):713–9. |
[46] | Paulussen KJM , McKenna CF , Beals JW , Wilund KR , Salvador AF , Burd NA , Anabolic resistance of muscle protein turnover comes in various shapes and sizes. Frontiers in Nutrition [Internet]. 2021[cited 2022 Oct 26];8. Available from: https://www.frontiersin.org/articles/10.3389/fnut.2021.615849. |
[47] | Beals JW , Burd NA , Moore DR , van Vliet S , Obesity alters the muscle protein synthetic response to nutrition and exercise, Front Nutr(2019) ;6: , 87. |
[48] | Verreijen AM , Verlaan S , Engberink MF , Swinkels S , de Vogel-van den Bosch J , Weijs PJM , A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial, Am J Clin Nutr(2015) ;101: (2):279–86. |
[49] | Silva NL , Oliveira RB , Fleck SJ , Leon ACMP , Farinatti P , Influence of strength training variables on strength gains in adults over 55 years-old: A meta-analysis of dose-response relationships, J Sci Med Sport(2014) ;17: (3):337–44. |
[50] | Chen N , He X , Feng Y , Ainsworth BE , Liu Y , Effects of resistance training in healthy older people with sarcopenia: A systematic review and meta-analysis of randomized controlled trials, Eur Rev Aging Phys Act(2021) ;18: (1):23. |
[51] | Borde R , Hortobágyi T , Granacher U , Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis, Sports Med(2015) ;45: (12):1693–720. |
[52] | Liu CJ , Latham NK , Progressive resistance strength training for improving physical function in older adults, TheCochrane database of systematic reviews(2009) ;(3):CD002759. |
[53] | Wernbom M , Augustsson J , Thomeé R , The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans, Sports Med(2007) ;37: (3):225–64. |
[54] | de la O V , Zazpe I , Ruiz-Canela M , Effect of branched-chain amino acid supplementation, dietary intake and circulating levels in cardiometabolic diseases: An updated review, Curr Opin Clin Nutr Metab Care(2020) ;23: (1):35–50. |
[55] | Chapter 6: Choosing effect measures and computing estimates of effect [Internet]. [cited 2022 Jan 14].Available from: https://training.cochrane.org/handbook/current/chapter-06. |
[56] | Furukawa TA , Barbui C , Cipriani A , Brambilla P , Watanabe N , Imputing missing standard deviations in meta-analyses can provide accurate results, Journal of Clinical Epidemiology(2006) ;59: (1):7–10. |




