Effect of FLAVAnols on bone turnover markers in type 2 diabetes mellitus–post hoc analysis from a 3-month randomized placebo-controlled trial
Abstract
BACKGROUND:
Individuals with type 2 diabetes mellitus (T2DM) have increased fracture risk with high bone mineral density, possibly related to advanced glycation end products (AGEs) accumulation in bone. Flavanol supplementation in postmenopausal women reduced AGEs formation and decreased bone resorption markers. However, to date, these effects have not been investigated in T2DM.
OBJECTIVE:
We used a post hoc secondary analysis to determine the effect of monomeric and oligomeric flavanols supplementation on bone turnover markers (BTMs) in individuals with T2DM.
METHODS:
Eighty-three individuals with T2DM, aged 40–85 years, with microalbuminuria were enrolled from 4 trial centers in Rotterdam, the Netherlands, into a randomized, double-blind, placebo-controlled trial with renal vascular health as the primary outcome. Participants were randomized (1:1) to receive either a placebo or 200 mg of monomeric and oligomeric flavanols as intervention for three months. Serum alkaline phosphatase (ALP), type I collagen crosslinked beta C-telopeptide (β-CTx), and type I procollagen-N-propeptide (P1NP) were measured at baseline and three months. ANCOVA was performed on rank transformed BTMs at three months as the outcome, adjusting for baseline BTMs, group, age, sex, and BMI.
RESULTS:
Baseline characteristics did not differ between the two arms. The adjusted mean change in BTMs at three months was not different between the placebo vs. intervention arm: ALP –0.059 (–0.262–0.145) vs. 0.060 (–0.135–0.356), p = 0.41; β-CTx 0.013 (–0.205–0.231) vs. 0.100 (–0.109–0.310), p = 0.53 and P1NP 0.091 (–0.080–0.262) vs. 0.030 (–0.134–0.195), p = 0.61. There was no significant within-group change in BTMs after three months in both study arms.
CONCLUSION:
Supplementation with daily 200 mg of flavanols during three months, on top of usual care in individuals with T2DM, did not result in changes in BTMs compared to placebo. Future studies are needed to show whether long-term supplementation in higher dosages may positively affect BTMs in individuals with T2DM.
1Introduction
Individuals with type 2 diabetes mellitus (T2DM) have a high risk of fracture, despite having normal or high bone mineral density (BMD) [4, 5]. The pathogenesis underlying the higher fracture risk in this population is multifactorial. Two important mechanisms are considered to be involved in low bone quality: low bone turnover due to bone cell dysfunction [6] and collagen stiffness and microarchitecture changes due to accumulation of advanced glycation end-products (AGEs) [7]. A standardized diagnostic and therapeutic approach to deal with low bone quality in T2DM is lacking. Therefore, novel therapeutic and dietary approaches are urgently required to prevent the increased fracture risk in the rapidly growing group with T2DM.
Dietary flavonoids are naturally present in fruits, vegetables, and beverages, including coffee, tea, and wine. Flavonoids are divided into six main subclasses according to their chemical structure: flavones, isoflavones, flavanones, flavonols, flavanols, and anthocyanins [3]. Grape seeds are rich in flavanols and their consumption has been associated with improved bone strength in multiple epidemiological and animal studies [8, 9]. In a British cohort of 3160 twin women, daily flavonoid intake was positively associated with spine BMD. The largest effect was observed for anthocyanins [10]. Total flavonoid intake was associated with higher BMD in 3000 Scottish women between 45–54 years of age [11]. A positive association was observed between dietary flavonoids intake and BMD in 2239 Chinese women but not in men [12]. Grape seeds and individual flavonoids, namely quercetin [13] and kampferol [14] have been shown to improve bone biomechanical properties in rats and ovariectomized mice [15]. Together, these findings point to a possible role of different flavonoids in enhancing bone strength.
Despite preclinical evidence, intervention trials evaluating the effect of dietary flavanol supplementation on bone health in T2DM have not been performed. However, short-term intervention trials with flavanols in individuals with T2DM have shown promising results in domains other than bone such as improvement in insulin sensitivity, cardiovascular health and neurocognition [16–18]. Regarding bone, biochemical markers of bone turnover are released during bone formation and resorption and can serve as a proxy of the rate of bone remodeling [19, 20]. Changes in bone turnover markers (BTMs), therefore, can be seen earlier than changes in bone mineral density during short-term intervention studies [21]. The extent and direction of changes in BTMs by flavanol supplementation could hypothetically be affected by baseline bone turnover. For instance, in postmenopausal women with high bone turnover–flavanols have been reported to slow down the bone turnover (mainly resorption) possibly by decreasing oxidative stress and pro-inflammatory cytokines [22–24]. Conversely, in T2DM subjects with a tendency towards low bone turnover [6]–flavanols might lead to an upsurge in bone turnover (formation) through their antioxidant properties [25] and by reducing formation of advanced glycation end products (AGEs). Thus, we intended to ascertain whether flavanols supplementation affects bone metabolism in subjects with T2DM.
Here, we present the results of a post hoc analysis of the FLAVA trial [26], where we investigated the effects of daily 200 mg monomeric and oligomeric flavanols supplementation vs. placebo for three months on changes in BTMs in middle-aged and elderly subjects with T2DM of both sexes.
2Materials and methods
2.1Design
The FLAVA trial is a randomized, double-blind, placebo-controlled multicenter trial. The trial was registered in the Netherlands Trial Register, NTR4669, on 7 July 2014 [27]. The primary end point of this trial was the effect of daily 200 mg monomeric and oligomeric flavanols on renal endothelial function in subjects with T2DM with albuminuria compared to placebo [26]. We report the effect of these flavanols on serum bone metabolism markers compared to placebo after three months supplementation. More detailed information on the methods of the FLAVA trial has been reported previously [26].
2.2Participants
Individuals with T2DM were enrolled between December 2014 and April 2019 from four different diabetes care units in the Rotterdam region, The Netherlands. Inclusion criteria were the presence of T2DM, age between 40–85 years and micro-albuminuria. Exclusion criteria were diabetes mellitus other than type 2, consumption of a diet or supplement providing ≥25 mg per day of flavanols during one month prior to inclusion, acute or chronic organ failure, organ transplant, current chemo- or radiotherapy, presence of cancer, use of anticoagulants and pregnancy or lactation. Written informed consent was provided by all participants.
2.3Intervention
Included participants were randomized in a 1:1 ratio to the intervention or the placebo group. Both the participants, the investigator and the medical staff were blinded to the allocation during the whole period of data collection. During the post hoc analysis, the investigators remained blinded for the sample assessment and data analysis. The intervention group received a powdered extract from grape seeds (Vitis vinifera) containing 200 mg monomeric and oligomeric flavanols (Masqueliers’ OPC, I.N.C. Agency B.V. Loosdrecht, The Netherlands). The control group received a powdered placebo similar to the research product in packaging, taste and color. Detailed composition of the intervention and placebo can be found in supplementary Table 2. Participants received one box containing the sachets supply needed for the three-month trial. They were instructed to use one sachet daily for three months and consume it dissolved in tap water. Study visits were planned at baseline, six weeks and three months from the start of the intervention. Each participant was contacted through telephone between the first and second study visit to promote compliance and monitor side effects.
Participants continued to receive their usual diabetes care from their treating physician or specialist during the study period. They were asked to report any change in medication use and dosage or other medical interventions. Participants were instructed to keep their lifestyle unchanged during the study period. Other than the research product containing ≥25 mg monomeric and oligomeric flavanols, there were no dietary restrictions.
2.4Measurements
2.4.1Demographic and clinical measurements
Standardized questionnaires were used to collect demographic and lifestyle-related variables. Anthropometric parameters were collected at baseline and three months. Food frequency questionnaires were used to estimate the daily amount of flavanols consumed with the diet. This amount was calculated based on the Phenol-Explorer database version 3.6 (http://www.phenol-explorer.eu) [28]. To measure the compliance and possible adverse effects, self-reported questionnaires were used at six weeks and three months.
2.4.2Biochemical measurements
Venous blood samples were collected in EDTA and heparin tubes at baseline and at 3 months. Blood samples were centrifuged at 4000 g for 10 minutes at 4°C and plasma was isolated and stored at –80°C until analysis. Other parameters like fasting glucose, serum creatinine and glycated hemoglobin were determined by standard clinical chemistry assays. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation formula [29].
All bone markers were assessed in heparinized samples. Total Alkaline phosphatase (ALP) measurements were performed according to the IFCC reference procedure (Roche, Mannheim, Germany). Inter-assay variation coefficients were respectively 5.0% % and 3.0% for low (28 U/l) and high (302 U/l) ALP levels, respectively. P1NP levels were measured using the chemiluminescence technology based immunoassay IDS-iSYS Intact PiNP (IDS, Boldon, UK). Inter-assay variation coefficients were respectively 5.3%, 4.5% and 4.5% for low (32μg/L), normal (132μg/L) and high (231μg/L) P1NP levels, respectively.
Plasma β-CTX was measured using an electrochemiluminescent immunoassay (Roche, Mannheim, Germany). Inter-assay variation coefficients were respectively 4.1% % and 3.3% for low (0.29μg/L) and high (0.66μg/L) β-CTX levels, respectively.
2.5Statistical analysis and Power calculation
The sample size calculation for the FLAVA trial was based on the primary outcome, which was the between-group difference in Albumin Excretion Rate (AER) during three months of intervention, as compared to placebo. For 80% power with a two-sided alpha set at 0.05 in order to detect a 20% improvement in the primary outcome, at least 48 patients were required in each study group. For this secondary analysis using bone turnover markers (BTMs), we did not perform a post hoc power analysis [1, 2].
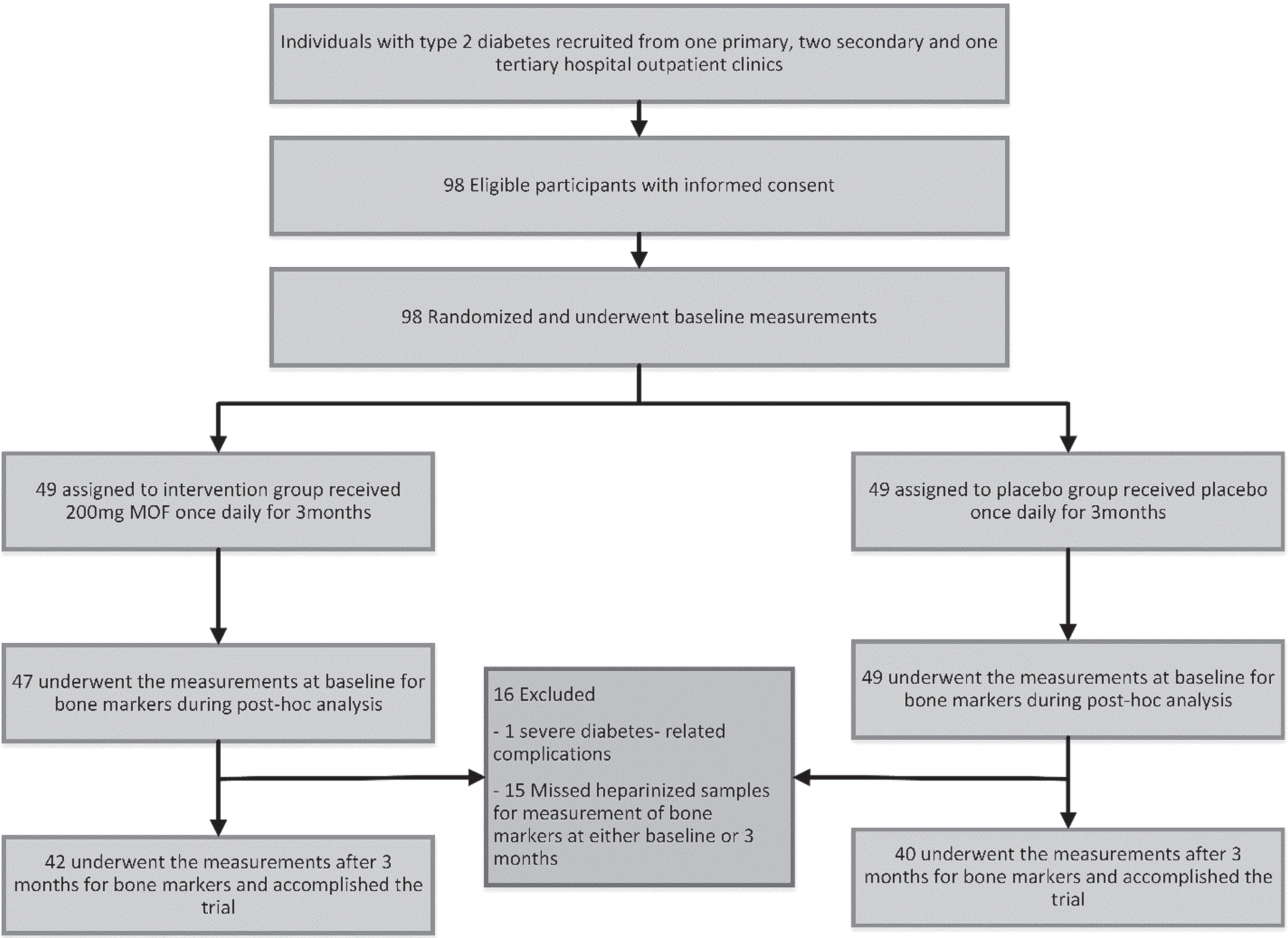
One patient was excluded from the analysis after randomization, because of abrupt progressive diabetes-related complications and subsequent major changes in the anti-diabetic and anti-hypertensive medication shortly after inclusion. Due to the post hoc nature of this current study, 15 participants were additionally excluded from the analysis because they missed heparinized blood samples at either baseline or at 3 months for measurement of bone markers (Fig. 1 flowchart of the inclusion).
Fig. 1
Study flow-chart for post hoc assessment of bone metabolism in T2DM subjects after 3-months of monomeric and oligomeric flavanols (MOFs) supplementation.
All analyses were performed according to the intention-to-treat principle. Continuous data are expressed as mean±standard deviation or median (interquartile range) depending on the distribution of normality, while categorical data are expressed as number (%). Group differences were analyzed with Mann Whitney test for independent samples and with Wilcoxon signed rank test for related samples. We graphically examined the distribution of BTMs. Because the distribution of BTMs is highly skewed at large values, we rank transformed the BTMs based on Blom’s transformation. Based on pre- and post-treatment design, ANCOVA was performed on rank transformed bone turnover marker values at three months adjusting for baseline values of the bone markers, age, sex, group and BMI. Linear regression analysis was performed between baseline flavanols intake and baseline values of bone markers in our subjects with T2DM. We performed one unadjusted model and one model adjusted for age, sex and BMI. All statistical analysis was performed using IBM SPSS statistics 25 (version 25.0).
Table 1
Baseline characteristics of participants from the FLAVA trail
| Total | Intervention | Placebo | p-value* | |
| Number of participants | 82 | 42 | 40 | |
| Age, years | 63.5±9.2 | 62.34±10.2 | 64.1±8.1 | 0.60 |
| Females (%) | 37.3 | 39.5 | 35.0 | 0.67 |
| BMI, kg/m2 | 30.8±6.2 | 30.4±6.0 | 31.2±6.4 | 0.59 |
| Waist/hip ratio | 0.98±0.07 | 0.98±0.07 | 0.99±0.08 | 0.39 |
| Mean arterial pressure, mm Hg | 98.1±11.7 | 97.7±13.1 | 98.6±10.1 | 0.65 |
| Duration of diabetes, years | 16.05±8.7 | 16.3±8.8 | 15.8±8.7 | 0.80 |
| HbA1c, mmol/mol | 63 (56–73.5) | 64 (59.5–78.5) | 63 (55–72) | 0.49 |
| eGFR, ml/min/1.73 m2 | 78.9±18.7 | 83.5±18.3 | 73.3±19.8 | 0.22 |
| Total Alkaline phosphatase, U/L | 78 (64–105) | 80 (64–106) | 78 (67–101) | 0.67 |
| P1NP, μg/L | 36 (29–47) | 36 (31–47) | 34.5 (19–47.5) | 0.48 |
| β–CTx, μg/L | 0.18 (0.12–0.27) | 0.18 (0.13–0.26) | 0.17 (0.12–0.27) | 0.59 |
| Microvascular complication ≥2 | 48.2% | 51.2% | 45.0% | 0.57 |
| Physical activity ≥30 min/day (%) | ||||
| 0 days/week | 10.4 | 11.1 | 9.7 | 0.33 |
| 1–4 days/week | 32.8 | 25.0 | 41.9 | |
| 5–7 days/week | 56.7 | 63.9 | 48.4 | |
| Smoking status (%) | ||||
| Never | 76 | 77 | 75 | 0.52 |
| Past | 19 | 20 | 17 | |
| Current | 5 | 3 | 8 | |
| Composite vascular complications (%) | 50.6 | 53.5 | 47.5 | 0.59 |
| CVD | 10.8 | 11.6 | 10.0 | 0.81 |
| Retinopathy | 28.8 | 30.3 | 27.3 | 0.79 |
| Polyneuropathy | 31.8 | 21.2 | 42.4 | 0.11 |
| Daily dietary flavanols intake, mg/day | 75.3(37–220) | 70.7(28–189) | 101.7(37–242) | 0.32 |
| Medications (%) | ||||
| Insulin | 73 | 72 | 74 | 0.82 |
| Metformin | 66 | 60.5 | 72 | 0.53 |
| RAS-I | 75 | 76 | 74 | 0.58 |
| Lipid lowering | 92 | 86 | 97 | 0.09 |
Data are expressed as mean±SD, median (IQR) and percentage (%). T2DM, type 2 diabetes mellitus; BMI, body mass index; MAP, mean arterial blood pressure; eGFR, estimated glomerular filtration rate; HbA1C, glycosylated hemoglobin; β-CTx, type I collagen crosslinked beta C-telopeptide; P1NP, type I procollagen-N-propeptide; RAS-I: renin-angiotensin system inhibitor.
3Results
3.1Baseline characteristics
Eighty-two patients were included in this post hoc analysis. Figure 1 shows the flow chart of inclusion in this analysis. Table 1 shows the baseline characteristics. Of the included participants, 38.1% were females, mean BMI was 30.6±6.0 kg/m2, mean age 63.0±9.5 years, mean duration of diabetes was 15.7±8.6 years and median (interquartile range) HbA1c was 63 (18) mmol/L. Around 50% of the included participants had vascular complications. The median baseline flavanols consumption through habitual dietary intake was 77.4 (IQR 43.5–223) mg/day. There were no significant differences in the baseline characteristics between the placebo and intervention arms. Also, no significant difference between the study arms was observed at baseline in serum BTMs (all p > 0.05). Median BTMs in our individuals with T2DM were in the low normal range when compared to the thresholds used in the clinical practice [30].
3.2Within-group changes in bone markers after the 3-month intervention
Within-group changes in bone markers in the intervention group were not statistically significant different when baseline values were compared with values at three months ([ALP 80 (56–140) vs. 80 (47–152), p = 0.78], [P1NP 36 (16–110) vs. 38 (16–112), p = 0.69] and [β-CTx 0.18 (0.08–0.58) vs. 0.19 (0.07–0.71), p = 0.89]). ALP, P1NP and β-CTx also did not show a change in the placebo group when baseline values were compared with values at three months (p = 0.34, p = 0.37 and p = 0.74, respectively (Supplementary Table 1).
3.3Between-group differences in bone markers after the 3-month intervention
Unadjusted plasma ALP, P1NP and β-CTx levels at baseline were similar in the placebo and intervention arm. After three months of treatment, ALP, P1NP and β-CTx were not different between both arms (see Table 2).
Table 2
Comparisons of change in the bone turnover markers (ALP, β-CTx and P1NP) in placebo and intervention arm at 3 months from baseline
| Intervention arm (n = 42) | Placebo arm (n = 40) | **p-value | |
| Adjusted mean β (95% CI or SE) | Adjusted mean β (95% CI or SE) | ||
| Alkaline Phosphatase* | |||
| Unadjusted | 0.051 (–0.135–0.237) | –0.048 (–0.239–0.142) | 0.46 |
| Model 1 | 0.060 (–0.135–0.356) | –0.059 (–0.262–0.145) | 0.41 |
| P1NP* | |||
| Unadjusted | 0.014 (–0.151–0.178) | 0.027 (–0.142–0.195) | 0.91 |
| Model 1 | 0.030 (–0.136–0.195) | 0.092 (–0.080–0.264) | 0.61 |
| β-CTx * | |||
| Unadjusted | 0.103 (–0.105–0.311) | –0.037 (–0.251–0.176) | 0.35 |
| Model 1 | 0.104 (–0.105–0.314) | 0.009 (–0.209–0.227) | 0.53 |
*ANCOVA was performed on rank-transformed bone turnover marker values at 3 months using Blom’s formula. **P-value is based on a statistical comparison of adjusted means (estimated marginal means) obtained from ANCOVA coefficients. Unadjusted: ALP, P1NP or β-CTx at baseline and group (intervention/placebo). Model 1: Adjusted for sex, age and BMI.
After adjustment for baseline values, the between group changes of mean ALP, P1NP and β-CTx were not significant between the placebo arm and intervention arm [Rank transformed data for ALP: β= –0.05 (95% CI –0.24–0.14) vs. 0.05(–0.14–0.24), p = 0.46], [Rank transformed data for P1NP: β= 0.03 (–0.14–0.19) vs. 0.01 (–0.15–0.18), p = 0.91] and [Rank transformed data for β-CTx: β= –0.04 (–0.25–0.17) vs. 0.10 (–0.11–0.31), p = 0.35]. Additional adjustment for age, sex and body mass index (BMI) did not change these results (Table 2). Also, additional adjustment for baseline use of flavanols and HbA1c for all three bone turnover markers did not change these results (data not shown).
3.4Baseline dietary flavanol intake and baseline values of bone markers
Although there was no difference in the baseline intake of flavanols between the placebo and intervention arm, we cross-sectionally analyzed the association between the bone markers and the baseline dietary flavanol intake. There was no association between the baseline intake of flavanols and baseline values of ALP (log-transformed data: β= 0.05, p = 0.69), P1NP (β= –0.05, p = 0.70) and β-CTx (β= –0.01, p = 0.93) in all included participants irrespective of whom received treatment. Additional adjustment for age, sex and BMI did not change the results for all three bone markers (Table 3).
Table 3
Linear regression analysis between the baseline flavanol intake and baseline values of bone markers for total, placebo and intervention group
| Total | Intervention | Placebo | |
| Unstandardized | Unstandardized | Unstandardized | |
| coefficients (95% CI) | coefficients (95% CI) | coefficients (95% CI) | |
| Alkaline Phosphatase | |||
| Unadjusted | 0.018 (–0.151–0.188) | –0.045 (–0.249–0.233) | 0.028 (–0.230–0.286) |
| Model 1 | 0.017 (–0.155–0.188) | –0.019 (–0.260–0.221) | 0.030 (–0.233–0.293) |
| Model 2 | 0.007 (–0.159–0.172) | –0.012 (–0.244–0.221) | 0.008 (–0.249–0.265) |
| P1NP | |||
| Unadjusted | –0.097 (–0.244–0.049) | –0.174 (–0.375–0.027) | –0.035 (–0.260–0.189) |
| Model 1 | –0.093 (–0.241–0.055) | –0.168 (–0.372–0.037) | –0.032 (–0.263–0.199) |
| Model 2 | –0.090 (–0.238–0.058) | –0.168 (–0.376–0.040) | –0.020 (–0.251–0.212) |
| β-CTx | |||
| Unadjusted | –0.039 (–0.294–0.115) | –0.183 (–0.420–0.055) | 0.086 (–0.124–0.296) |
| Model 1 | –0.032 (–0.185–0.120) | –0.172 (–0.409–0.065) | 0.091 (–0.115–0.296) |
| Model 2 | –0.025 (–0.174–0.124) | –0.176 (–0.415–0.063) | 0.107 (–0.093–0.308) |
Model 1: log_baseline dietary flavanol + age + sex. Model 2: Model 1 + BMI.
Higher baseline flavanols intake did not show any relationship to baseline ALP, P1NP and β-CTx values in either placebo or intervention arm in both unadjusted or models adjusted for age, sex and BMI. (Table 3).
4Discussion
In this post hoc analysis from an RCT in participants with T2DM, we did not observe a change in bone turnover markers after three months of 200 mg monomeric and oligomeric flavanols supplementation compared to placebo.
Bone health in individuals with T2DM is reduced by multiple mechanisms, including abnormal micro-architecture, accumulation of advanced glycation end-products and chronic oxidative and inflammatory stress affecting the functioning of bone cells [31]. Longer duration of T2DM (> 5 years) and poor glycemic control have been associated with uncoupling of bone turnover process with both decreased bone formation and resorption, but an intact mineralization [6, 32]. Table 4 shows a comparison of trials evaluating the effect of polyphenol supplementation on skeletal health in T2DM patients and those at high risk of T2DM (pre-diabetes). While we found no effect on BTMs, a recent RCT in 192 patients with T2DM and low BMD reported an improvement in whole- body BMD after six months of 500 mg resveratrol, a different polyphenol found in grapes’ skin [33]. Moreover, 3-months of 66 mg isoflavone supplementation in T2DM men with hypogonadism resulted in a decline in β-CTx without a change in P1NP, suggesting a net reduction in bone resorption [34]. In addition to hypogonadism, T2DM participants in these trials had a much shorter duration of diabetes (mean 7.5/8 vs. 15 years) and better glycemic control (HbA1c 53/58 vs. 63 mmol/mol) compared to our participants. This could partially explain the lack of improvement of BTMs in our study, as those with a longer duration of T2DM and a worse glycemic control may be in a more advanced stage of diabetic bone disease and possibly benefit less from dietary interventions. Future studies with flavanols in people with T2DM should consider comparing the effects based on duration and control of diabetes. Furthermore, bioavailability differs between different classes of polyphenols (500 mg resveratrol or 200 mg flavanols) based on various factors as dietary composition, gut microbiota, metabolic conversion, medication use, etc. [35, 36] and a one-to-one comparison of different polyphenols is challenging.
Table 4
Randomized controlled trials in human subjects with diabetes or at high risk of diabetes to evaluate the effect of polyphenols on bone health
| First author, year | Study population and design | Duration of diabetes and glycemic control | Intervention (mg per day) | Results (BMD and bone markers) |
| Type 2 Diabetics T2DM | ||||
| Ornstrup et al. [42] | 16-weeks RCT, 66 obese men with Metabolic syndrome, mean age 49.3 years | Not applicable | 1. Resveratol (RSV) 2×500 mg 2. RSV 2×75 mg 3. Placebo | ↑ BALP in RSV1000 (+16%), RSV500 vs. placebo ↑ trabecular vBMD at the LS (+2.6%) in RSV1000 ↔ Areal BMD or cortical BMD at hip or spine OPG ↔, P1NP ↔, CTX ↔, NTX ↔ |
| Poulsen et al. [43] | 24-weeks randomized, controlled trial, 24 obese non-diabetic men, mean age 32 years in placebo and 45 years in RSV group | Not applicable | 1. RSV 3×500 mg 2. Placebo | ↑ BALP in RSV1500 (+15%) vs. placebo, also in ALP OC ↔, P1NP ↔, CTX ↔ Calcium ↔, PTH ↔ |
| Bo et al. [33] | 6-month double-blind, RCT, 192 out-patient T2DM mean age 65 years | Diabetes duration mean (SD) 8.0 (10.0) years; glycated hemoglobin 6.9±1.0 % (∼52 mmol/mol) | 1. RSV 1×500 mg 2. RSV 1×40 mg 3. Placebo | ↓TB-BMD and BMC in placebo and RSV40 (no changes in RSV500). ↔ LS- and Hip BMD. Subgroup analysis: ↑ BMD in RSV500 arm in those with lower Calcium and 25-OH vitamin D |
| Sathyapalan et al. [34] | 3-month RCT, 200 men with T2DM and hypogonadism, mean age 52 years | Diabetes duration median (interquartile range) 7.5 (4–9) years; glycated hemoglobin 57 (52–64) mmol/mol | 1. 15 g soy protein + 66 mg isoflavone (SPI) 2. 15 g soy protein (SP) | ↓ β-CTx in SPI vs. SP (–15%) ↔ P1NP in SPI vs. SP Note: Reduction in β-CTx is linearly correlated with reduction in HbA1c |
| Waqas et al. [This study] 2021 | 3-month RCT, 82 out-patient T2DM mean age 63 years | Diabetes duration median (interquartile range) 15.7 (8.4) years; glycated hemoglobin 66.3 (13.4) mmol/mol | 1. MOFs 1×200 mg 2. Placebo | ALP ↔, P1NP ↔, β-CTx ↔ Subgroup analysis: ↑ALP in women in MOF200 arm, but not in men |
RCT, randomized controlled trial; T2DM, type 2 diabetes; RSV, Resveratol; SPI, soy protein isoflavone; MOFs, Monomeric and oligomeric flavanols; BALP, bone alkaline phosphatase; vBMD, volumetric bone mineral density; BMC, bone mineral content; TB, total body; PTH, parathyroid hormone.
In vitro and animal studies have shown osteoprotective effects of green tea extracts (GTE), rich in flavanols. An increase in BMD and improvement in bone microarchitecture was seen in 14-month-old female ovariectomized rats provided with GTE-rich drinking water for 16 weeks [37, 38]. In vitro studies have shown that GTE enhance osteoblastogenesis with increasing differentiation, proliferation and mineralization of stem cell derived osteoblasts, and attenuate osteoclastogenesis by enhancing osteoclast apoptosis. GTE decreased the RANKL/OPG ratio and the production of pro-inflammatory cytokines, and as a result, osteoclastogenesis [39, 40]. We presumed that monomeric and oligomeric flavanols from grape seeds in patients with T2DM would reduce oxidative and inflammatory stress and reduce AGEs accumulation in bone, leading to an increase in bone turnover. However, in our study, we did not find an association between baseline MOFs intake and baseline BTMs. Habitual intake of procyanidins, catechins and anthocyanins (MOFs) in epidemiological studies in Scottish, English and Chinese peri- and postmenopausal women has been associated with higher BMD values [10–12]. Likewise, randomized controlled trials in postmenopausal osteopenic women supplementing flavonoids (a broader class including flavanols) for 6–12 months reported an increase in bone-specific ALP/TRAP ratio, reduction in P1NP, and β-CTx [22, 24], suggesting a net decrease in bone resorption. These studies consisted of postmenopausal (osteopenic) women, who might had higher bone turnover at baseline than our subjects with T2DM. This makes comparing the results between these studies difficult.
This trial has several strengths. Strengths are the large group of individuals with T2DM and a state-of-the-art double-blind randomized study design. There are several limitations as well. All analyses using BTMs are post hoc and were not planned prospectively as a part of the initial study protocol. We did not perform a power calculation during this secondary analysis, as this is advised against in methodological literature [2]. However, we cannot rule out the possibility that the trial might be underpowered to detect associations of changes in BTMs with flavanols supplementation. The duration of flavanols supplementation was relatively short. One can argue that a longer intervention time would be needed in order to produce a significant change in BMD. However, for short-term intervention studies, such as ours, BTMs have shown earlier changes than BMD [41]. Participants had a long duration of T2DM (mean 15.5 years) and might have tenacious bone AGEs and suppressed bone turnover compared to subjects having T2DM for a shorter period or in a pre-diabetes stage. Lastly, we did not measure some risk factors that might influence the bone metabolism such as serum calcium, vitamin D, and PTH at baseline although the possibility that they differ in placebo and intervention arm was theoretically low due to randomization.
In conclusion, three months of monomeric and oligomeric flavanols supplementation in individuals with T2DM failed to show any change in bone metabolism as measured with bone turnover markers, hypothetically due to the advanced stage of disease of our participants. Future trials evaluating the influence of flavanols on bone metabolism in people with T2DM should have a longer duration and consider the effect of diabetes control and severity of the disease on bone health.
Funding statement
The Jaap Schouten Foundation, Rotterdam, The Netherlands, kindly provided funding for investigating the role of Advanced Glycation End Products in musculoskeletal health and participation in this trial. The funding source had no role in the study design, conducting the experiments, data analysis, interpretation, writing of the report, or decision to submit the article for publication.
Conflict of interest
All authors involved in this trial declare that they have no competing interests.
Acknowledgments
This study has been presented at the annual congress of European calcified tissue society (ECTS) 2021 (abstract published in Bone) and American Society of Bone and Mineral research (ASBMR) 2021.
References
[1] | Gaskill BN , Garner JP . Power to the people: Power, negative results and sample size, J Am Assoc Lab Anim Sci (2020) ;59: (1):9–16. |
[2] | Dziak JJ , Dierker LC , Abar B . The interpretation of statistical power after the data have been gathered, Curr Psychol (2020) ;39: (3):870–7. |
[3] | Amiot MJ , Riva C , Vinet A . Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review, Obes Rev (2016) ;17: (7):573–86. |
[4] | Oei L , Zillikens MC , Dehghan A , Buitendijk GH , Castano-Betancourt MC , Estrada K ,et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study, Diabetes Care (2013) ;36: (6):1619–28. |
[5] | Ma L , Oei L , Jiang L , Estrada K , Chen H , Wang Z , et al. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies, Eur J Epidemiol (2012) ;27: (5):319–32. |
[6] | Hygum K , Starup-Linde J , Harsløf T , Vestergaard P , Langdahl BL . MECHANISMS IN ENDOCRINOLOGY: Diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis, Eur J Endocrinol (2017) ;176: (3):R137–R57. |
[7] | Yamamoto M , Sugimoto T . Advanced glycation end products, diabetes, and bone strength, Curr Osteoporos Rep (2016) ;14: (6):320–6. |
[8] | Kwak SC , Cheon YH , Lee CH , Jun HY , Yoon KH , Lee MS , et al. Grape seed proanthocyanidin extract prevents bone loss via regulation of osteoclast differentiation, apoptosis, and proliferation, Nutrients. (2020) ;12: (10). |
[9] | Tenkumo T , Aobulikasimu A , Asou Y , Shirato M , Shishido S , Kanno T , et al. Proanthocyanidin-rich grape seed extract improves bone loss, bone healing, and implant osseointegration in ovariectomized animals, Sci Rep (2020) ;10: (1):8812. |
[10] | Welch A , MacGregor A , Jennings A , Fairweather-Tait S , Spector T , Cassidy A . Habitual flavonoid intakes are positively associated with bone mineral density in women, J Bone Miner Res (2012) ;27: (9):1872–8. |
[11] | Hardcastle AC , Aucott L , Fraser WD , Reid DM , Macdonald HM . Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women, Eur J Clin Nutr (2011) ;65: (3):378–85. |
[12] | Zhang ZQ , He LP , Liu YH , Liu J , Su YX , Chen YM . Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men, Osteoporos Int (2014) ;25: (10):2417–25. |
[13] | Siddiqui JA , Sharan K , Swarnkar G , Rawat P , Kumar M , Manickavasagam L , et al. Quercetin-6-C-β-D-glucopyranoside isolated from Ulmus wallichiana planchon is more potent than quercetin in inhibiting osteoclastogenesis and mitigating ovariectomy-induced bone loss in rats, Menopause. (2011) ;18: (2):198–207. |
[14] | Trivedi R , Kumar S , Kumar A , Siddiqui JA , Swarnkar G , Gupta V ,et al. Kaempferol has osteogenic effect in ovariectomized adult Sprague-Dawley rats, Mol Cell Endocrinol (2008) ;289: (1-2):85–93. |
[15] | Hohman EE , Weaver CM . A grape-enriched diet increases bone calcium retention and cortical bone properties in ovariectomized rats, J Nutr (2015) ;145: (2):253–9. |
[16] | Kar P , Laight D , Rooprai HK , Shaw KM , Cummings M . Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity, Diabet Med (2009) ;26: (5):526–31. |
[17] | Decroix L , van Schuerbeek P , Tonoli C , van Cutsem J , Soares DD , Heyman E , et al. The effect of acute cocoa flavanol intake on the BOLD response and cognitive function in type 1 diabetes: A randomized, placebo-controlled, double-blinded cross-over pilot study, Psychopharmacology (Berl). (2019) ;236: (12):3421–8. |
[18] | Hokayem M , Blond E , Vidal H , Lambert K , Meugnier E , Feillet-Coudray C , et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients, Diabetes Care (2013) ;36: (6):1454–61. |
[19] | Eastell R , Szulc P . Use of bone turnover markers in postmenopausal osteoporosis, Lancet Diabetes Endocrinol. (2017) ;5: (11):908–23. |
[20] | Vasikaran SD . Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis, Crit Rev Clin Lab Sci (2008) ;45: (2):221–58. |
[21] | Bauer DC , Black DM , Bouxsein ML , Lui LY , Cauley JA , de Papp AE , et al. Treatment-related changes in bone turnover and fracture risk reduction in clinical trials of anti-resorptive drugs: A meta-regression, J Bone Miner Res (2018) ;33: (4):634–42. |
[22] | Sathyapalan T , Aye M , Rigby AS , Fraser WD , Thatcher NJ , Kilpatrick ES , et al. Soy reduces bone turnover markers in women during early menopause: A randomized controlled trial, J Bone Miner Res (2017) ;32: (1):157–64. |
[23] | Panahande SB , Maghbooli Z , Hossein-Nezhad A , Qorbani M , Moeini-Nodeh S , Haghi-Aminjan H , et al. Effects of French maritime pine bark extract (Oligopin®) supplementation on bone remodeling markers in postmenopausal osteopenic women: A randomized clinical trial, Phytother Res (2019) ;33: (4):1233–40. |
[24] | Shen CL , Chyu MC , Yeh JK , Zhang Y , Pence BC , Felton CK , et al. Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: A 6-month randomized placebo-controlled trial, Osteoporos Int. (2012) ;23: (5):1541–52. |
[25] | Hussain T , Tan B , Murtaza G , Liu G , Rahu N , Saleem Kalhoro M , et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy, Pharmacol Res (2020) ;152: , 104629. |
[26] | Rashid M , Verhoeven AJM , Mulder MT , Timman R , Ozcan B , van Beek-Nieuwland Y , et al. The effect of monomeric and oligomeric FLAVAnols in patients with type 2 diabetes and microalbuminuria (FLAVA-trial): A double-blind randomized controlled trial, Clin Nutr (2021) ;40: (11):5587–94. |
[27] | Rashid M , Verhoeven AJM , Mulder MT , Timman R , van Beek-Nieuwland Y , Athumani AA , et al. Use of monomeric and oligomeric flavanols in the dietary management of patients with type 2 diabetes mellitus and microalbuminuria (FLAVA trial): Study protocol for a randomized controlled trial, Trials (2018) ;19: (1):379. |
[28] | Rothwell JA , Perez-Jimenez J , Neveu V , Medina-Remon A , M’Hiri N , Garcia-Lobato P , et al.Phenol-Explorer 3, A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) (2013) ;0: , bat070. |
[29] | MacIsaac RJ , Ekinci EI , Premaratne E , Lu ZX , Seah JM , Li Y , et al. The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation does not improve the underestimation of Glomerular Filtration Rate (GFR) in people with diabetes and preserved renal function, BMC Nephrol (2015) ;16: , 198. |
[30] | Bauer D , Krege J , Lane N , Leary E , Libanati C , Miller P , et al. National Bone Health Alliance Bone Turnover Marker Project: Current practices and the need for US harmonization, standardization, and common reference ranges, Osteoporos Int (2012) ;23: (10):2425–33. |
[31] | Moseley KF . Type 2 diabetes and bone fractures review, Curr Opin Endocrinol Diabetes Obes (2012) ;19: (2):128–35. |
[32] | Kulkarni SV , Meenatchi S , Reeta R , Ramesh R , Srinivasan AR , Lenin C . Association of glycemic status with bone turnover markers in type 2 diabetes mellitus, Int J Appl Basic Med Res (2017) ;7: (4):247–51. |
[33] | Bo S , Gambino R , Ponzo V , Cioffi I , Goitre I , Evangelista A ,et al. Effects of resveratrol on bone health in type 2 diabetic patients, A double-blind randomized-controlled trial. Nutr Diabetes (2018) ;8: (1):51. |
[34] | Sathyapalan T , Aye M , Rigby AS , Fraser WD , Kilpatrick ES , Atkin SL . Effect of soy on bone turn-over markers in men with type 2 diabetes and hypogonadism - a randomised controlled study, Sci Rep (2017) ;7: (1):15366. |
[35] | Hollands WJ , Philo M , Perez-Moral N , Needs PW , Savva GM , Kroon PA . Monomeric flavanols are more efficient substrates for gut microbiota conversion to hydroxyphenyl-γ-valerolactone metabolites than oligomeric procyanidins: A randomized, placebo-controlled human intervention trial, Mol Nutr Food Res (2020) ;64: (10):e1901135. |
[36] | Andreu-Fernández V , Almeida Toledano L , Pizarro N , Navarro-Tapia E , Gómez-Roig MD , de la Torre R , et al. Bioavailability of epigallocatechin gallate administered with different nutritional strategies in healthy volunteers, Antioxidants (Basel) (2020) ;9: (5). |
[37] | Shen CL , Wang P , Guerrieri J , Yeh JK , Wang JS . Protective effect of green tea polyphenols on bone loss in middle-aged female rats, Osteoporos Int (2008) ;19: (7):979–90. |
[38] | Shen CL , Yeh JK , Stoecker BJ , Chyu MC , Wang JS . Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats, Bone (2009) ;44: (4):684–90. |
[39] | Shen CL , Chyu MC , Wang JS . Tea and bone health: Steps forward in translational nutrition, Am J Clin Nutr (1694) ;98: (6 Suppl):S-9S. |
[40] | Huang HT , Cheng TL , Lin SY , Ho CJ , Chyu JY , Yang RS , et al. Osteoprotective roles of green tea catechins, Antioxidants (Basel) (2020) ;9: (11). |
[41] | Poulsen MW , Bak MJ , Andersen JM , Monosík R , Giraudi-Futin AC , Holst JJ , et al. Effect of dietary advanced glycation end products on postprandial appetite, inflammation, and endothelial activation in healthy overweight individuals, Eur J Nutr (2014) ;53: (2):661–72. |
[42] | Ornstrup MJ , Harslof T , Kjaer TN , Langdahl BL , Pedersen SB . Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: A randomized placebo-controlled trial, J Clin Endocrinol Metab. (2014) ;99: (12):4720–9. |
[43] | Poulsen MM , Ornstrup MJ , Harslof T , Jessen N , Langdahl B , Richelsen B , et al. Short-term resveratrol supplementation stimulates serum levels of bone-specific alkaline phosphatase in obese non-diabetic men, Journal of Functional Foods (2014) ;6: (6):305–10. |




