Impact of an intensive lifestyle program on low attenuation plaque and myocardial perfusion in coronary heart disease: A randomised clinical trial protocol
Abstract
IMPORTANCE:
The evidence that maintaining a healthy body weight in conjunction with healthier eating patterns, exercise training, and reduced stress can improve clinical outcomes in patients with atherosclerotic cardiovascular disease is substantial. However, little is known about the magnitude and temporal effects of a comprehensive lifestyle treatment on coronary artery anatomy, myocardial inflammation, and fibrosis in people affected by coronary heart disease.
OBJECTIVE:
To conduct a randomised clinical trial to determine the impact of a 12-month intense lifestyle intervention delivered via an mHealth platform (in the form of a mobile App) versus standard clinical care on low attenuation plaque volume and structure, stress myocardial perfusion, and diastolic function.
DESIGN:
A single centre, parallel-group, randomised controlled trial. The co-primary endpoints are: 1-Low Attenuation Plaque (LAP) volume (mm3) using coronary computed tomography angiography (CCTA) at 12 months, and 2-Adenosine stress myocardial blood flow (stress MBF, mL/min/g) using cardiovascular magnetic resonance imaging (MRI) at 12 months. Other key measurements include liver steatosis by MRI, subclinical abnormalities detected by advanced electrocardiography, arterial stiffness, endothelial function, genomic, metabolomic, and gut microbiome-related adaptations to these structural changes. An intention-to-treat principle will be used for all analyses.
SETTING:
Participants will be recruited from a large academic cardiology office practice (Central Sydney Cardiology) and Royal Prince Alfred Hospital (RPAH) Departments of Cardiology and Radiology. All clinical investigations will be undertaken within the Charles Perkins Centre-RPAH clinic.
PARTICIPANTS:
Individuals (n = 150) with stable coronary heart disease who have low attenuation plaque based on a CCTA within the past 3 months, will be randomised to a lifestyle intervention program comprising a 5:2 pesco-vegetarian diet, exercise training, and mindfulness-based stress reduction (n = 75) or usual care (n = 75).
DISCUSSION:
This trial will represent the single most detailed and integrated analysis of the effects of a comprehensive lifestyle intervention targeting multiple metabolic pathways, delivered via a customized mHealth App on smart devices, on coronary macro- and microcirculation, heart physiology, and cardiometabolic risk. It will provide a new framework for allowing clinicians and individuals to optimise metabolic health for the prevention and management of atherosclerotic cardiovascular diseases that is epidemic in modern society.
Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR). ACTRN12620001151921. https://www.anzctr.org.au/ACTRN12620001151921.aspx
1Introduction
Cardiovascular disease (CVD) is becoming the primary cause of morbidity, disability and premature death not only in Western countries but also in the developing world [1]. Lifetime risk of a first CVD event at age 50 is ∼65% in men [2], and is increasingly affecting younger women and men, especially in some ethnic groups that for genetic and epigenetic reasons poorly tolerate the caloric surplus and the build-up of excess abdominal fat [3]. Some accumulation of cellular and tissue damage within the cardiovascular system is an inevitable part of advancing age. However, persistent exposure to traditional and emerging risk factors (including insulin resistance, triglyceride-rich lipoproteins, trimethylamine N-oxide, and accelerated clonal haematopoiesis) associated with unhealthy lifestyle behaviours play a substantial role in the initiation and development of CVD [4, 5] (Fig. 1). Approximately 25% of patients who develop a myocardial infarction have extensive coronary atherosclerosis in the absence of any traditional modifiable cardiovascular risk factors [6].
Fig. 1
Classical and novel modifiable risk factors for cardiovascular disease.
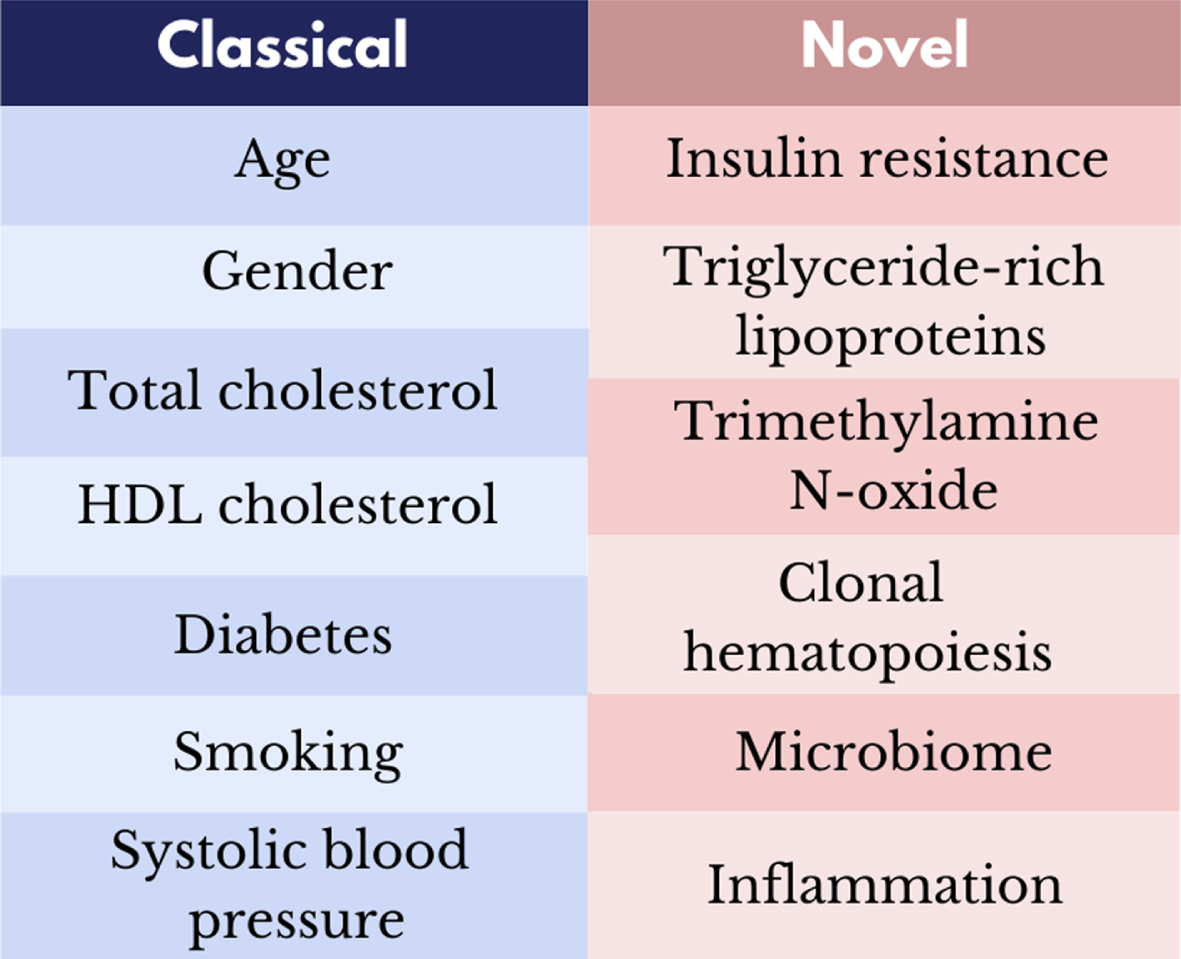
A growing body of data from animal, epidemiological and clinical studies have demonstrated that calorie excess, poor nutrition, physical inactivity, sleep disturbances, smoking, and mental stress by acting on multiple metabolic and molecular pathways play major roles in the pathogenesis of many cardiometabolic diseases [4]. Findings from the CALERIE phase 1 and 2 trials have shown that calorie restriction without malnutrition even in non-obese individuals can reduce inflammation and oxidative stress, and improve left ventricular diastolic function and multiple cardiometabolic risk factors at supraphysiological levels [7, 8]. Fifty-year-old participants of the Framingham Study with cardiovascular risk factors similar to those achieved during CALERIE-2 [8] and in people practicing long-term calorie restriction [9] had a 5% lifetime risk of developing CVD, whereas the risk increased to 69% in those with 2 or more abnormal cardiometabolic risk factors [10].
Data from nutritional secondary prevention trials in patients with hypertension [11], type 2 diabetes [8], diabetic nephropathy [12], fatty liver disease [13], and high cardio-metabolic risk [14] also showed spectacular improvements in metabolic outcomes and a reduction in major cardiovascular events. The Lyon and Indo-Mediterranean Diet Heart trials have demonstrated a striking protective effect of a Mediterranean-style diet against coronary recurrence rate and sudden cardiac death in those who already suffered from a prior myocardial infarction [12, 15]. Modifications of meal timing, diet quality and the gut microbiome [4, 16] together with regular physical activity [17–19], improved sleep patterns [20, 21] and a reduction in mental stress [3, 22, 23] have also been shown to improve cardiovascular outcomes in those at risk.
1.2Current challenges
Despite substantial evidence supporting lifestyle behaviour change, there are three major challenges to address. Firstly, there is a need for more mechanistic studies to elucidate the impact of lifestyle on macro- and microvascular structure and function, and advancements in imaging techniques will facilitate this. Coronary computed tomography angiography (CCTA) technology has advanced significantly over recent years and can now identify the volume and structure of low attenuation non-calcified plaques (LAP). LAP is characterised by inflammation, microcalcification, a thin fibrous cap and large lipid-rich necrotic core [24], and in a recent study was the strongest predictor of subsequent myocardial infarction compared to classical risk predictors in individuals with stable Coronary heart disease (CHD). Indeed, those with LAP burden >4% were almost 5 times more likely to have subsequent myocardial infarction [25]. Early evidence suggests that lifestyle intervention may yield LAP changes observable as early as 12 months [26], however there is a dearth of evidence in this area.
Quantification of myocardial blood flow during hyperaemic stress is now possible via automated in-line perfusion mapping by cardiovascular magnetic resonance imaging (MRI). Non-invasive myocardial and hemodynamic phenotyping by comprehensive and state-of-the-art cardiac stress MRI can provide quantitative characterisation not only of myocardial blood flow measured during adenosine stress, but also of the volumes and function of heart chambers, left ventricular hypertrophy and diastolic dysfunction, focal and global myocardial inflammation, myocardial infarction or non-ischemic scarring, diffuse myocardial fibrosis, coronary microvascular function including coronary spasm measured with the cold pressor test, and pulmonary congestion at rest and at peak supine ergometer exercise stress [27, 28].
Secondly, current lifestyle interventions are limited in their ability to be scaled up and rolled out across the clinical setting in an affordable way. However, over the past decade there has been an exponential rise in smartphone and wearable use [29], and this type of digital platform may address some of these barriers. Thirdly, the greatest limitation to initiating, engaging and sustaining effective lifestyle change, is adherence. Digital health approaches using an ecosystem which uses a smart phone to deliver these programs have been shown to have superior effectiveness. Smartphone delivery of cardiac rehabilitation was delivered much more effectively than traditional face-to-face cardiac rehabilitation, with enhanced engagement (94% vs. 68% p < 0.05) and program completion (80% vs. 47%, p < 0.05) being significantly higher with digital health programs delivered by smartphone [30]. Some advantages of smartphone technology are that it can be initiated rapidly, updated easily, and delivered at anytime from anywhere.
1.3Aims of LIVEPLUS
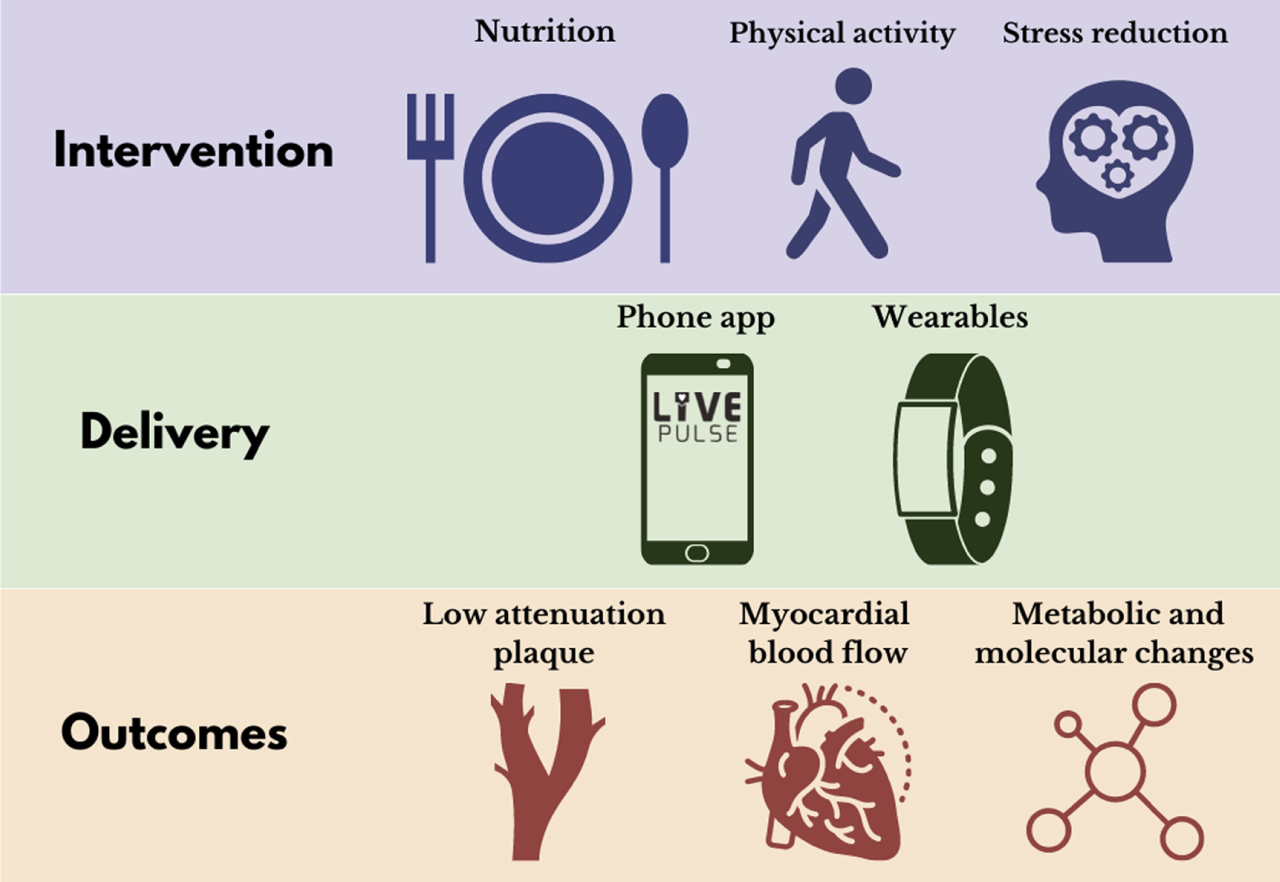
The primary aim of the LIVEPLUS study is to assess the effectiveness of a 12-month intensive lifestyle intervention which combines a 5:2 pesco-vegetarian diet, physical activity and stress reduction training, on macrovascular coronary disease (CCTA assessed LAP volume) and microvascular disease (MRI measured stress myocardial perfusion). Secondary aims are to identify changes in other cardiovascular and metabolic/molecular adaptations to this lifestyle intervention. At the centre of the LIVEPLUS study program is digital health ecosystem delivered via a smartphone app (Fig. 2) [31]. Such a digital solution, if proven to be effective, is readily scalable and translatable for incorporation into clinical practice across diverse health care systems at a cost these systems can sustain.
Fig. 2
LIVEPLUS randomised clinical trial.
2Methods
2.1Study design
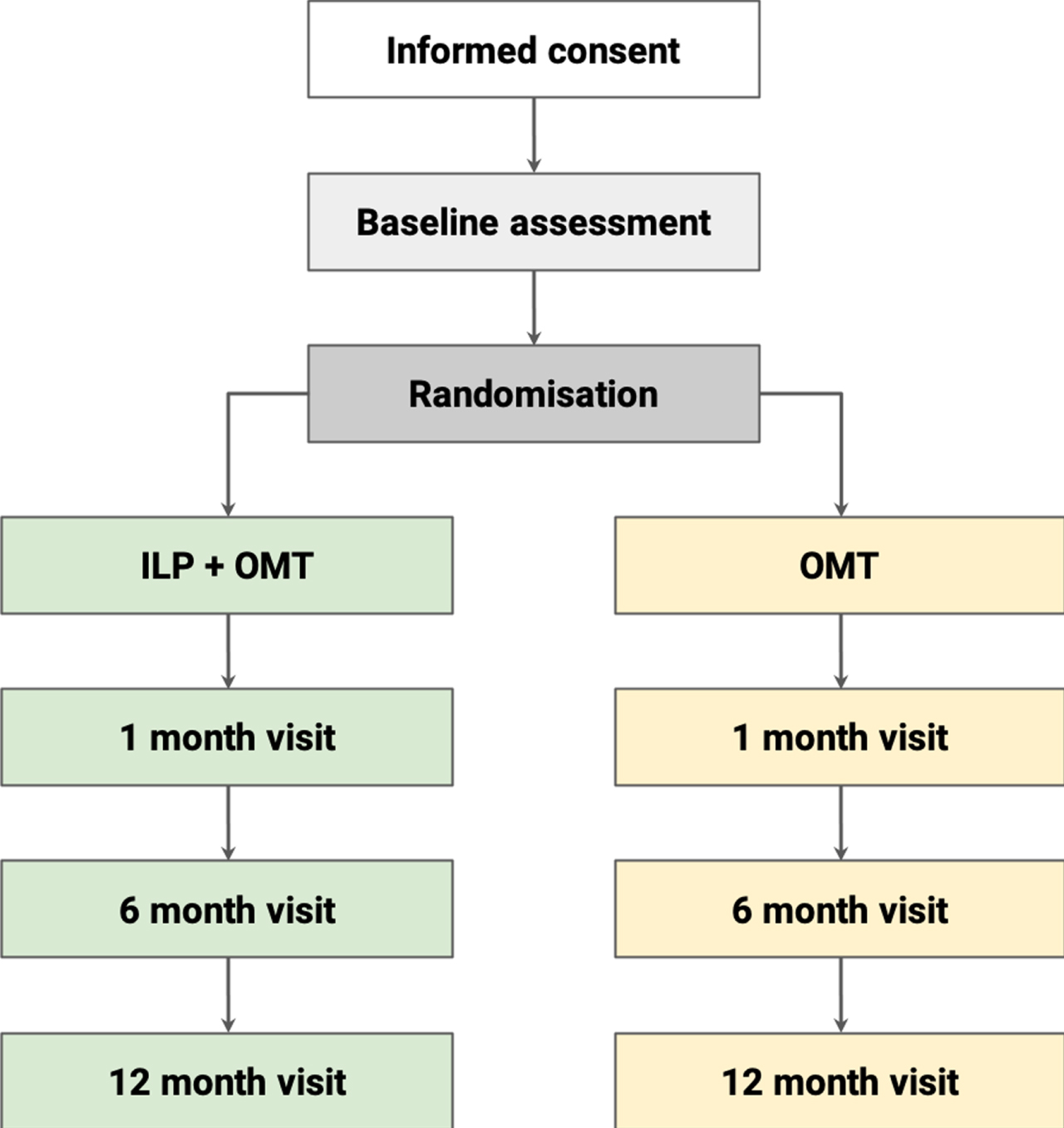
LIVEPLUS is a parallel-group, randomised controlled trial (RCT) with a total sample of 150 participants. A 1:1 allocation ratio will be applied to either the intervention group (ILP + OMT) or control group (OMT). Participants in both arms will be followed up over a period of 12 months. A comprehensive set of outcome assessments will be performed prior to initiating the intervention, with follow-up assessments at month 1, 6 and 12 (Fig. 3). This study has been approved by the Sydney Local Health District Ethics committee (2020/ETH01273). Study visits will take place at the Charles Perkins Centre-Royal Prince Alfred Hospital (CPC-RPA) clinic, Royal Prince Alfred Hospital Department of Radiology, and the North Shore Radiology and Nuclear Medicine (NSRNM) facility, Sydney.
Fig. 3
Study flow. ILP, intensive lifestyle program; OMT, optimal medical therapy.
2.2Eligibility criteria
Inclusion criteria are; (1) Presence of LAP on CCTA; (2) 18–80 years of age; (3) BMI >22.0 kg/m2; (4) Able to provide full informed consent; (5) Considered capable of undergoing all study assessments and adhering to the rigors of the ILP intervention. Exclusion criteria are (1) Non-MRI-compatible implanted devices; (2) Estimated glomerular filtration rate (eGFR) <30 mL/kg/1.73 m2; (3) Inability to exercise via supine ergometer; (4) Contraindications for adenosine or glyceryl trinitrate; (5) Previous severe allergic reaction to iodinated contrast media; (6) Pregnancy or breastfeeding; and (7) History of any chronic disease process that could interfere with the interpretation of results.
2.3Recruitment
Participants who have undergone a clinically indicated CCTA within the past 3 months will be recruited if they have quantifiable LAP (plaque within –30 to 150 HU range). At least two experienced CCTA reporters will evaluate the coronary tree for the presence of such plaque. In the general cardiology outpatient population, we expect approximately 20–40% of patients to have LAP. The predominant source of referral for clinically indicated CCTA at RPA for this study will be from a single large academic clinical practice (Central Sydney Cardiology, RPAH Medical Centre) and from patients who have attended the RPA Radiology Department for a clinically indicated CCTA.
2.4Pre-screening and consent
Following a pre-screening telephone call, an initial video call will take place with eligible, potential participants. During this video call, the research team will deliver a short presentation to the participant to explain the rationale, implications and constraints of the protocol, known side effects and any risks involved in taking part. There will be an opportunity for participants to ask questions and discuss any details of the study. Willing participants will be invited to the CPC-RPA clinic to obtain electronic informed consent through REDCap. Any participant judged by the research team to not possess the capacity for fully informed consent will be excluded from the study, as per the study exclusion criteria.
2.5Baseline visits
Following consent, participants will be enrolled into the study and undergo a number of clinical baseline assessments. The exact timing of these assessments will be flexible (e.g., due to participant or equipment availability) and can be spread over a number of days, however a potential schedule is shown in Table 1.
Table 1
Schedule of visits and testing procedures at the CPC-RPA clinic. The MRI scan will take place at the North Shore Radiology and Nuclear Medicine facility
| Baseline assessment | 1 month | 6 month | 12 month | |||||||
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Notes | These visits are flexible and tests can be moved around. | These visits are flexible and tests can be moved around but all should be undertaken within 1 week of end-intervention | ||||||||
| Informed consent | 1 | |||||||||
| Full body examination (including BP, weight, waist/hip cm, vital signs) | 1 | 1 | 1 | 1 | 1 | |||||
| Medical and family history questionnaires | 1 | |||||||||
| OGTT | 1 | 1 | ||||||||
| Psychosocial questionnaires | 1 | 1 | ||||||||
| Physical activity assessment (Fitbit &7-Day PAR) | 1 | 1 | ||||||||
| Food diary assessment | 1 | 1 | 1 | 1 | ||||||
| Sleep assessment | ||||||||||
| (questionnaires and home sleep study) | 1 | 1 | ||||||||
| Urine and Faeces Sample | 1 | 1 | 1 | 1 | ||||||
| MRI | 1 | 1 | ||||||||
| Randomisation | 1 | |||||||||
| Fasting blood sample (Biochemistry and Haematology) | 1 | 1 | 1 | |||||||
| DXA | 1 | 1 | ||||||||
| Carotid intima-media thickness | 1 | 1 | 1 | 1 | ||||||
| Vascular endothelial function | 1 | 1 | 1 | 1 | ||||||
| Autonomic function | 1 | 1 | 1 | 1 | ||||||
| Pulse Wave Velocity | 1 | 1 | 1 | |||||||
| 6 minute walk test | 1 | 1 | ||||||||
| CCTA | 1 | |||||||||
BP, blood pressure; CCTA, coronary computed tomography angiography; DXA, Dual-energy X-ray absorptiometry; OGTT, oral glucose tolerance test; MRI, magnetic resonance imaging; PAR, physical activity recall.
2.6Randomisation and blinding
Enrolled participants will be randomised using random permuted blocks with randomly selected block sizes, via a web-based randomisation system in REDCap, and stratified by age, sex, and BMI. The allocation sequence was generated using blocked size of 2, 4, 6, and 8 from the R package, blockrand. An uneven block was included in the middle of the sequence to guard against guessing the group assignment [33]. Investigators and participants will be blinded to the group allocation until all baseline measures have been completed, as the REDCap support statistician is the only individual with access to the upcoming treatment allocations within the randomisation list. After the final baseline measurement, the research co-ordinator will use REDCap to randomise the participant, revealing the group assignment to the study team and participant simultaneously. Due to the nature of the intervention, investigators and participants cannot be blinded during the study, however those performing CCTA, MRI and statistical analysis will be blinded to group allocation.
2.7Follow-up visits
Table 1 provides an overview of the follow up visits and testing procedures which are planned for months 1, 6, and 12.
2.8Intervention
The ILP+OMT group will continue to receive standard of care treatment, as well as three main lifestyle intervention components over the 12-month study period. These will target nutrition, physical activity and stress reduction. In the context of each target behaviour, participants will be asked to use the LIVEPULSE app (see section 2.10.1. LIVEPULSE phone app for details) to read 12 education modules (1 per month), and complete multichoice questions at the end of each module to assess learning and provide automatic feedback. The content of the 12 education modules (Table 2) is informed by evidence-based, peer-reviewed research carefully rewritten into lay language, making them accessible and engaging, particularly to those who are new to such lifestyle practices. Participants will also be given online coaching sessions with a dietitian, physical activity coach, and stress reduction coach (Table 3). Each coaching session will be tailored to an individual’s progress and needs, using evidence-informed behaviour change techniques [34]. Each coach will receive training in delivery of specific behaviour change techniques to effectively promote change in relation to each target lifestyle behaviour (i.e. nutrition, physical activity and stress reduction). The training will be delivered by a UK-registered practitioner health psychologist with expertise in health behaviour change, and will include use of behaviour change counselling skills as well as behaviour change techniques. Additional online coaching calls may be scheduled to improve adherence to the intervention if considered necessary by the study team.
Table 2
Education content for diet, physical activity and stress reduction components for the intervention group
| Month | Diet | Physical activity | Stress reduction |
| 1 | Introduction of Nutritional Intervention | Importance of physical activity | Introduction |
| 2 | Pesco-vegetarian diet | FITT principles and safety | Simple Awareness |
| 3 | Intermittent fasting | Physical activity and the heart | Attention and the Brain |
| 4 | Healthy eating | Physical activity and the blood vessels | Dealing with Thoughts |
| 5 | Dietary measurements | Physical activity and obesity | Biological Basis of Stress: Responding vs. Reacting |
| 6 | Fats | Physical activity and mental health | Dealing with Difficult Emotions of Physical Pain |
| 7 | Carbohydrates | Physical activity and glucose metabolism | Mindfulness and Communication |
| 8 | Protein | Physical activity and fat metabolism | Self-Compassion Cultivation |
| 9 | Meal planning | Physical activity and skeletal muscle | Social Connectedness |
| 10 | Nutritional labelling | Physical activity and inflammation | A Closer Look at Happiness |
| 11 | Dietary patterns and cardiovascular health | Physical activity and brain health | On Relationships |
| 12 | Review | Physical activity and cardiorespiratory fitness | Conclusion |
FITT: frequency, intensity, time, type.
Table 3
Planned schedule of coaching calls, which can be adapted according to participant preference
| Month 1 | Month 2 | Month 3 | Months 4–12 | ||||
| Week 1 | Week 3 | Week 5 | Week 7 | Week 9 | Week 11 | Monthly | |
| Dietitian | 1 hour | 30 min | 30 min | 30 min | 30 min | 30 min | 30 min |
| Exercise physiologist | 1 hour | 30 min | 30 min | 30 min | 30 min | 30 min | 30 min |
| Mindfulness coach | 1 hour | 30 min | 30 min | 30 min | 30 min | 30 min | 30 min |
2.8.1Nutrition
Several interventional studies have clearly shown that individuals who consume diets rich in fish and nutrient-dense minimally processed plant foods have a lower risk of developing CHD than people who consume energy dense low-fibre Western diets high in saturated and trans fatty acids, animal protein and salt [35–37]. In the LIVEPLUS trial, participants will be asked to follow a 5:2 pesco-vegetarian diet for 12 months. This diet involves a combination of a 5:2 diet and a pesco-vegetarian diet. The aim of a pesco-vegetarian diet will be to substitute meat with fish and processed and refined foods with a range of minimally processed plant-based foods. Red meats, including beef, pork, lamb and other red meat products, and poultry including chicken, duck, goose, and other poultry products are prohibited with this diet. In addition, individual participants will be asked to practice a 5:2 diet, which consists of fasting on 2 non-consecutive days of the week with energy restriction of around 2000 kJ per day for women and around 2500 kJ for men.
During the first 4 weeks of the intervention, participants will receive fresh ingredients and recipes from Marley Spoon (a commercial meal delivery service that provides pre-portioned ingredients and recipes to designated addresses for meal preparation), which provides lunch and dinner for 5 days per week to assist with their preparation of a pesco-vegetarian diet. The menu selected by the participants will be sent to the study dietitian and the nutritionist of Marley Spoon to ensure it complies with a pesco-vegetarian diet. If participants select meal plans with meat, it will be altered to a pesco-vegetarian diet before delivery. Participants will be required to prepare their breakfasts each day and light meals on fasting days, and the study dietitian will provide the recipes for these meals through the LIVEPULSE mobile app. After the first 4 weeks, they will meet with the study dietitian to design their individualised weekly meal plans and menus to facilitate transition into preparing their own food. The goal is to improve diet quality but also to achieve at least 7-8% weight loss, as we have found this is vital to improve inflammatory, metabolic and cardiac markers [38]. Strategies to implement dietary changes, meal planning, healthy recipes and relevant nutritional information for cardiovascular health will be discussed during dietitian appointments. Along with the education modules, other materials related to the 5:2 pesco-vegetarian diet (e.g. recipes for a pesco-vegetarian diet, recipes for fasting days, food sources that are rich in iron) will also be delivered via the LIVEPULSE mobile app.
2.9Physical activity
There is strong evidence that reducing sedentary behaviour and increasing overall physical activity level is associated with reduced cardiovascular events and mortality [39]. Participants will be given an individualised home-based programme which will be based on the guidelines from the American College of Cardiology Foundation/American Heart Association to manage individuals with stable ischemic heart disease [40]. The principles we will advise include; 1) to perform 30 to 60 minutes of moderate-intensity aerobic physical activity, such as brisk walking, at least 5 days and preferably 7 days per week; 2) increase daily lifestyle physical activity (e.g. walking breaks at work, gardening, household work); and 3) perform resistance training at least 2 days per week. We do not have one specific training programme for all individuals, but rather these general principles will be individualised based on baseline activity levels and progress throughout the year. To measure baseline activity, participants will wear a Fitbit Inspire 2 physical activity tracker for 7 continuous days and will complete a PAR questionnaire. Fitbit Inspire 2 is equipped with 3-axis accelerometer and optical heart-rate tracker, however it lacks GPS receiver for accurate distance data. The Fitbit device will enable the researchers to access minute by minute step count, heart rate and heart rate variability for all participants, as well as information about duration and timing of sleep, and participants will be asked to wear it for 12 consecutive months. The goal for all participants will be to reach at least 150 minutes of moderate-intensity aerobic physical activity per week within the first 3 months. If participants respond well during the first 3 months of the physical activity intervention, the following 9 months (month 4–12) will aim to vary the prescription in terms of mode, duration, and intensity. Alongside the education modules, the team have also developed a number of exercise circuit videos aimed at a range of intensities to suit a broad range of fitness and confidence levels, which will be available for viewing anytime via the LIVEPULSE app.
2.10Stress reduction
Chronic stress is an independent risk factor for atherosclerosis [41]. Body-inclusive stress reduction techniques, such as slow breathing [22, 23], gentle yoga [42], and mindfulness [43], have been shown to improve hypertension and autonomic (cardiovagal) function, as well as mental health and quality of life. In the LIVEPLUS trial, the 12-month stress reduction program will draw techniques from Mindfulness Based Stress Reduction (MBSR) and will be supplemented with additional slow and pressurized breathing techniques, gentle yoga, and concepts from positive psychology on evidence-based tips for cultivating happiness and social connectedness. MBSR is a specific mindfulness approach that integrates various mindfulness meditation techniques, as well as gentle hatha yoga, and has been shown clinically and supported by systematic reviews to be beneficial for cardiovascular and mental health [44]. Each month, participants will be introduced to new stress reduction educational content through the app, as well as asked to practice a new breathing (for about 5–10 minutes, 6 days per week) and mindfulness technique (about 20 minutes, once per week). Breathing and yoga videos have been created by our research team to guide participants through their practice. Example breathing and mindfulness practices include slow breathing at six breaths per minute, alternate nostril breathing, sitting meditation, and mindful yoga.
2.10.1LIVEPULSE mobile app
LivePulse is a health data collection, management and intervention tool consisting of a mobile app for participants and a web dashboard for the research team to use. The app allows participants to create personalised goals, receive reminders, access educational materials, record and share their health data (weight, exercise, diets etc.) with the research team. The app is publicly available both on the Apple App Store and on the Google Play Store in Australia. The underlying technology of the app has been implemented in similar apps created by the developer, Vulsen, for other research studies, some examples include: Success CKD [45], and My Home Hemo [46]. Key features of the app include;
1-Education: Each component of the ILP (nutrition, physical activity and stress reduction) has 12 online modules, and each will be released on a monthly basis for participants to read and interact with. Interactive components include multi-choice questions [26] (Table 2). Video content will be updated throughout the study and will include expert interviews, exercise sessions, breathing techniques and yoga sequences.
2-Monitoring: Participants themselves and members of the research team will be able to monitor progress through a number of app features, including weekly entry of body weight, upload of food images and logging mindfulness practice. The app will also extract data on physical activity and sleep from the Fitbit device, and in response to participant progress, the app has a facility for research team members to send short messages and reminders to participants, individually and as a group [47, 48]. The app includes a multi subject weight change simulator to predict whether participants are on track with reaching their dietary goals as evidenced by their weight loss trajectory. The algorithm is modelled from the participant’s entry of age, height, gender, and regular updated body weight inputs, as well as our input of their target calorie deficit required to achieve the desired percentage weight loss of 7–8% after 1 year. If a participant falls outside of their error region at any point throughout the study, they will be given the opportunity to receive enhanced support.
3-Goal setting: Participants will be encouraged to set short- and long-term behavioural and outcome goals (e.g., dietary, physical activity and weight), which will be discussed and adapted over the duration of the intervention with the research team coaches [48].
4-Social networks: Participants will be encouraged to connect with other users of the app using the group messaging function to facilitate social support.
5-Just-in-time adaptive intervention (JITAI): Intervention arm participants will receive personalised messages prompting physical activity using JITAI. JITAIs aim is to optimise delivery of intervention components in terms of the right time and location for patients. Micro-randomised trials (MRT) offer a way to optimise such interventions by enabling modelling of causal effects and time-varying effect moderation for individual intervention components within a JITAI [49]. For this reason, we will perform an MRT within the intervention arm.
2.11Control
OMT group participants will continue to receive standard of care treatment but will also be given a Fitbit device, and offered video-call appointments with a study dietitian every 3 months. In addition, they will be instructed to follow the American Heart Association (AHA) Diet and Lifestyle Recommendations to include physical activity [50] and stress reduction. The study dietitian will summarise the content on healthy lifestyles from the AHA website into 8 modules of educational materials. These materials will be delivered to participants each month via email for the first 8 months of the study. The content will cover general healthy eating, healthy meal planning and preparation, and tips to increase vegetable intake. They will also be offered the stress reduction programme at the end of the study.
3Outcomes
3.1Primary
3.1.1Low-attenuation plaque (LAP) volume (mm3)
LAP volume will be measured at baseline as part of a clinically indicated CCTA and at 12 months for the purpose of this study. CCTA will be performed at Royal Prince Alfred Hospital on a Siemens SOMATOM Force 2×192 slice Dual Source scanner and will take approximately 10 minutes, with a total visit duration of approximately 30 minutes. Coronary images will be transferred to a workstation with the use of plaque- analysis software. This software will produce colour-coded maps overlaid with differentiated plaque categories by Hounsfield Unit (HU) values, and those with LAP (–30 to 150 HU) will be invited into the study. LAP volume has been consistently shown to be the best marker of instability and strongest prognostic predictor of a future adverse CV event. CCTA readers will be blinded to group allocation.
3.1.2Stress myocardial blood flow (MBF, ml/min/g)
Stress MBF will be measured at baseline and at 12 months follow-up during an MRI examination at North Shore Radiology and Nuclear Medicine (NSRNM). The duration of the research MRI examination will be approximately 1.5 hours, with a total visit duration of approximately 2 hours.
The cardiac MRI examination will include the assessment of LV diastolic function at rest and exercise, as well as myocardial perfusion at rest and during different types of stress.
Graded exercise testing will be performed using a supine ergometer while the participant is lying supine in the MRI scanner. The work rate will be increased gradually with a total exercise duration of approximately 8–12 minutes [51]. Assessment of diastolic function at stress will be performed immediately after supine ergometry.
The study utilises the same intravenous gadolinium-based contrast agent that is used clinically to assess myocardial perfusion. The cardiac MRI myocardial perfusion images will be acquired at rest, and during the following stress protocols.
(i) Adenosine stress
Intravenous adenosine infusion will be administered at 140μg/kg/min via a peripheral intravenous cannula. This is the same protocol as is used at invasive coronary physiological assessment.
(ii) Cold-pressor testing (CPT)
CPT will be carried out, as previously described, by immersing the hands or feet of the participant in ice water measuring at approximately 0–4°C for a duration of 4 minutes. Continuous blood pressure and heart rate will be recorded at one minute intervals throughout the scan, thus about 10–20 minutes before (depending on how long it takes to finish the initial scans) and during CPT [52].
(iii) Glyceryl Trinitrate (GTN)
Exogenous nitrate is utilised to vasodilate the coronary arteries and assess for endothelial dysfunction. 300 mcg sublingual GTN will be given just prior to acquisition of cardiac MRI images.
3.2Secondary outcomes
The secondary outcomes to be conducted are listed below. For detailed information on each one, please see the supplement.
• Perivascular Adipose Tissue
• Myocardial inflammation
• Diastolic dysfunction
• Pulmonary congestion during exercise
• Secondary plaque characteristics, including noncalcified plaque volume, dense calcified plaque volume and total atheroma volume (TAV, mm3)
• Liver fat fraction (%) by MRI
• Flow-mediated dilatation (FMD) (%)
• Carotid intima-media thickness (cIMT) (mm)
• Pulse wave velocity (PWV) (m/s) and Augmentation Index (AI) (%)
• Heart rate variability (HRV) during wakefulness and sleep: Low Frequency (LF) and High Frequency (HF) (ms2)
• Heart age by resting electrocardiography
• Sleep parameters (total sleep time, Rapid Eye Movement (REM) and Non-REM (NREM) durations, respiratory disturbances, and oxygen saturation)
3.3Exploratory
There are a number of exploratory outcomes, which are listed below. For detailed information on each one, please see the supplement.
• Body composition
∘ Weight (kg)
∘ Height (cm)
∘ Waist/Hip ratio (cm)
∘ Dual-energy X-ray absorptiometry (DEXA) body composition
• Intermediate risk factors that are predictive of developing atherosclerosis
∘ Glucose (mmol/L) and Insulin (pmol/L)
∘ Blood pressure (mmHg) including both central and peripheral pressures
∘ Blood biomarkers
• Urinary levels of F2-isoprostane levels
• Serum hormones, proteomics, lipidomics, metabolomics, genetic and epigenetic profiling of white blood cells
• 6-minute walk test
• Dietary intake (total daily kJ, macronutrient and micronutrient intake (%))
• Time spent in moderate physical activity
• Psychometrics
3.4Safety reporting
Adverse Events (AE) and Serious Adverse Events (SAE) will be recorded and reported throughout the study to monitor and ensure participant safety. The following information will be reported: description, data of onset and end date, severity, assessment of relatedness to trial intervention or device, and action taken. A team of physicians, including cardiologists, will use their clinical judgement to decide whether an AE is of sufficient severity to remove the participant from the study. SAE will be reported to the primary investigator within 24 hours of the study team becoming aware of the event. The full course of the SAE, including any therapy given, will be reported to the study sponsor. All significant safety issues will be reported to the local Human Research Ethics Committee and Research Governance Officers.
3.5Sample size calculation
This is a randomised two-arm study design with 2 continuous co-primary endpoints repeatedly measured at baseline and after 12-month follow-up. A total of 150 participants (75 in each arm) will provide 90% power to demonstrate the efficacy of either outcome. The sample size is based on a 2-by-2 repeated measures design using each of the co-primary outcomes (PASS 13 Power Analysis and Sample Size Software (2014)). The conservative Bonferroni correction was applied to control the type I error rate due to multiplicity test. The significance level (alpha) is 0.025 using a two-sided, two-sample t-test. The assumptions specific to each co-primary outcome are defined below:
For Low-attenuation plaque volume, an absolute mean difference between groups of ≥9.0 mm3 at 12-month is expected to declare the efficacy of the intervention (ILP + OMT) over the control (OMT). The control group mean (standard deviation) is 39.0 (12) mm3 and 32.3 (15) mm3 at baseline and after 12-month follow-up respectively [53]. A correlation of between measurement pairs of 0.50 is assumed.
For Myocardial blood flow, an absolute mean difference between groups of ≥0.4 ml/min/g at 12-month is expected to declare the efficacy of the intervention (ILP + OMT) over the control (OMT). The control group mean (standard deviation) is 2.71 (0.61) mL/min/g and 2.55 (0.57) mL/min/g at baseline and after 12-month follow-up respectively [54]. A correlation of between measurement pairs of 0.50 is assumed. The overall sample size allows for a 20% attrition rate. Identifying eligible participants, enrolling them into the study, and maintaining their commitment to this intensive intervention over a period of 12 months can be challenging. However, the enrolment of 150 participants over a period of 34–36 months is thought to be feasible with the resources available based on previous research.
3.6Statistical analysis plan
This is the first detailed investigation of effects of ILP on coronary CT coronary plaque modification and myocardial blood flow over an extended period of time in human participants. Efficacy analyses will be conducted on an ’intention to treat’ basis. The primary analysis will be performed using Analysis of Covariance (ANCOVA), adjusted for baseline values [55]. Group comparisons will be two tailed with a nominal 2.5% significance level to adjust for the multiplicity testing using Bonferroni method. Subgroup analyses will be conducted to assess differences in intervention effects across the pre-specified gender and age subgroups. Tests of intervention effect modification will be performed by fitting intervention group and the relevant subgroup main effects and interaction into the models adjusted for baseline scores. Interpretation of evidence of heterogeneity of intervention effects among subgroups will remain exploratory (hypothesis generating) given the study is not powered to test subgroups.
Many of the secondary endpoints are exploratory in nature and will need to be confirmed by follow-up studies. Type-II error, i.e., failing to detect a significant effect when it exists is important to this study for all secondary and exploratory outcomes. Thus, all tests of significance for between-group comparisons will be performed at the p = 0.05 level of significance. The primary analysis strategy will utilise Intention-to-Treat principles. LIVEPLUS is also interested in mechanistic questions concerning the effect of ILP, and to address these issues, the Marginal Structural Model (MSM) of Robins and colleagues [56] will be applied. All major secondary outcomes are observed repeatedly at well-defined time points over participant follow-up, so that statistical methods for longitudinal and repeated measures analysis will be applied. Subgroup analyses will be tested by evaluating the treatment by subgroup interaction. Withdrawal from the intervention, drop-out from the study, or death (if any) will be analysed using the standard techniques for survival data.
3.7Trial status
Recruitment began Feb 2022.
4Conclusions
LIVEPLUS will help us to identify how a mechanism-based comprehensive lifestyle intervention can affect the major and minor vessels in the heart, perivascular fat, cardiac structure and function, arterial stiffness, endothelial function, and liver steatosis. The sophisticated clinical, metabolic and molecular (multi-omic) phenotyping to nutritional, physical activity and cognitive inputs will provide a unique platform for cybernetic/systems modelling responses in patients affected by coronary heart disease. The data generated will also populate complex systems-based models and provide insights that drive the discovery of new predictive biomarkers of disease. Additionally, the smartphone app is an exciting digital health and educational tool, which can empower individuals to engage with, and sustain, the lifestyle changes they need as individuals to reduce their future risk of adverse cardiovascular outcomes and to track the changes they achieve in a personalised way in near real time. Such a personalised digital health solution, if proven to be effective, is ultimately translational and scalable to use in clinical practice and as an educational tool for health care professionals.
Author contributions
SC drafted the manuscript and integrated additional text by co-authors. CMK managed manuscript revisions by co-authors. LF conceived the idea for the study and is the senior author of the manuscript. All authors contributed meaningfully to the design of this study, drafted sections of the manuscript based on their respective expertise, provided editorial assistance with early drafts, and approved the final version of the submitted manuscript.
Conflict of Interest
Luigi Fontana is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Protocol version
v4.0.
Funding statement
This study will be supported by grants from Australian Youth and Health Foundation and the Australian NHMRC Investigator Grant (APP1177797).
The Australian Health and Youth Foundation.
Supplementary data
Supplementary data to this article can be found online at https://dx.doi.org/10.3233/NHA-210146
References
[1] | World Health Organization. The top 10 causes of death. 2014. Available at: https://www.who.int/news-room/factsheets/detail/the-top-10-causes-of-death. Accessed: 23rd August 2021>. |
[2] | Stenling A , Haggstrom C , Norberg M , Norstrom F Lifetime risk predictions for cardiovascular diseases: Competing risks analyses on a population-based cohort in Sweden, Atherosclerosis (2020) ;312: :90–8. |
[3] | Despres JP , Lemieux I , Abdominal obesity and metabolic syndrome, Nature (2006) ;444: :881–7. |
[4] | Fontana L , Interventions to promote cardiometabolic health and slow cardiovascular ageing, Nat Rev Cardiol (2018) ;15: (9):566–77. |
[5] | Libby P , The changing landscape of atherosclerosis, Nature (2021) ;592: (7855):524–33. |
[6] | Mazhar J , Figtree G , Vernon ST , Galougahi KK , Carlo J , Nissen SE , Nicholls SJ , Progression of coronary atherosclerosis in patients without standard modifiable risk factors, Am J Prev Cardiol (2020) ;4: :100116. |
[7] | Most J , Tosti V , Redman LM , Fontana L , Calorie restriction in humans: An update, Ageing Res Rev (2017) ;39: :36–45. |
[8] | Lean MEJ , Leslie WS , Barnes AC , Brosnahan N , Thom G , McCombie L , Peters C , Zhyzhneuskaya S , Al-Mrabeh A , Hollingsworth KG , Rodrigues AM , Rehackova L , Adamson AJ , Sniehotta FF , Mathers JC , Ross HM , McIlvenna Y , Stefanetti R , Trenell M , Welsh P , Kean S , Ford I , McConnachie A , Sattar N , Taylor R , Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial, The Lancet (2018) ;1012: (3910)541–51. |
[9] | Fontana L , Meyer TE , Klein S , Holloszy JO , Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans, Proc Natl Acad Sci U S A (2004) ;101: (17):6659–63. |
[10] | Lloyd-Jones DM , Leip EP , Larson MG , D’Agostino RB , Beiser A , Wilson PW , Wolf PA , Levy D , Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age, Circulation (2006) ;113: (6):791–8. |
[11] | Sacks FM , Svetkey LP , Vollmer WM , Appel LJ , Bray GA , Harsha D , Obarzanek E , Conlin PR , Miller ER , 3rd , Simons-Morton DG , Karanja N , Lin PH ,Group DA-SCR, Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet, DASH-Sodium Collaborative Research GrouN Engl J Med (2001) ;344: (1):3–10. |
[12] | Ruggenenti P , Abbate M , Ruggiero B , Rota S , Trillini M , Aparicio C , Parvanova A , Petrov Iliev I , Pisanu G , Perna A , Russo A , Diadei O , Martinetti D , Cannata A , Carrara F , Ferrari S , Stucchi N , Remuzzi G , Fontana L , Group CRSOS, Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: A randomized controlled trial, Diabetes (2017) ;66: (1):75–86. |
[13] | Franco M , Bilal U , Ordunez P , Benet M , Morejon A , Caballero B , Kennelly JF , Cooper RS , Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba -Repeated cross sectional surveys and ecological comparison of secular trends, BMJ (2013) ;346: :f1515. |
[14] | Estruch R , Ros E , Salas-Salvado J , Covas MI , Corella D , Aros F , Gomez-Gracia E , Ruiz-Gutierrez V , Fiol M , Lapetra J , Lamuela-Raventos RM , Serra-Majem L , Pinto X , Basora J , Munoz MA , Sorli JV , Martinez JA , Martinez-Gonzalez MA , Investigators PS , Primary prevention of cardiovascular disease with a Mediterranean diet, N Engl J Med (2013) ;368: (14):1279–90. |
[15] | Tosti V , Bertozzi B , Fontana L , Health benefits of the mediterranean diet: Metabolic and molecular mechanisms, J Gerontol A Biol Sci Med Sci (2018) ;73: (3):318–26. |
[16] | Heianza Y , Ma W , Manson JE , Rexrode KM , Qi L . Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies, J Am Heart Assoc (2017) ;6: (7). |
[17] | Riordan MM , Weiss EP , Meyer TE , Ehsani AA , Racette SB , Villareal DT , Fontana L , Holloszy JO , Kovacs SJ , The effects of caloric restriction- and exercise-induced weight loss on left ventricular diastolic function, Am J Physiol Heart Circ Physiol (2008) ;294: (3):H1174–82. |
[18] | Fontana L , Villareal DT , Weiss EP , Racette SB , Steger-May K , Klein S , Holloszy JO , Washington University School of Medicine CG, Calorie restriction or exercise: Effects on coronary heart disease risk factors, A randomized, controlled trial. Am J Physiol Endocrinol Metab (2007) ;293: (1):E197–202. |
[19] | Stewart RAH , Held C , Hadziosmanovic N , Armstrong PW , Cannon CP , Granger CB , Hagstrom E , Hochman JS , Koenig W , Lonn E , Nicolau JC , Steg PG , Vedin O , Wallentin L , White HD , Investigators S , Physical activity and mortality in patients with stable coronary heart disease, J Am Coll Cardiol (2017) ;70: (14):1689–700. |
[20] | St-Onge MP , Zuraikat FM , Reciprocal roles of sleep and diet in cardiovascular health: A review of recent evidence and a potential mechanism, Curr Atheroscler Rep (2019) ;21: (3):11;. |
[21] | Khan SU , Duran CA , Rahman H , Lekkala M , Saleem MA , Kaluski E , A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea, Eur Heart J (2018) ;39: (24):2291–7. |
[22] | Gerbarg PL , Brown RP , Streeter CC , Katzman M , Vermani M , Breath practices for survivor and caregiver stress, depression, and post-traumatic stress disorder: Connection, Co-regulation, compassion, OBM Integrative and Complementary Medicine (2019) ;4: (3):1. |
[23] | Bernardi L , Porta C , Spicuzza L , Bellwon J , Spadacini G , Frey AW , Yeung LY , Sanderson JE , Pedretti R , Tramarin R , Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure, Circulation (2002) ;105: (2):143–5. |
[24] | Stone GW , Maehara A , Lansky AJ , de Bruyne B , Cristea E , Mintz GS , Mehran R , McPherson J , Farhat N , Marso SP , Parise H , Templin B , White R , Zhang Z , Serruys PW , Investigators P , A prospective natural-history study of coronary atherosclerosis, N Engl J Med (2011) ;364: (3):226–35. |
[25] | Williams MC , Kwiecinski J , Doris M , McElhinney P , D’Souza MS , Cadet S , Adamson PD , Moss AJ , Alam S , Hunter A , Shah ASV , Mills NL , Pawade T , Wang C , Weir McCall J , Bonnici-Mallia M , Murrills C , Roditi G , van Beek EJR , Shaw LJ , Nicol ED , Berman DS , Slomka PJ , Newby DE , Dweck MR , Dey D , Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: Results from the multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART), Circulation (2020) ;141: (18):1452–62. |
[26] | Roth GA , Mensah GA , Johnson CO , Addolorato G , Ammirati E , Baddour LM , Barengo NC , Beaton AZ , Benjamin EJ , Benziger CP , Bonny A , Brauer M , Brodmann M , Cahill TJ , Carapetis J , Catapano AL , Chugh SS , Cooper LT , Coresh J , Criqui M , DeCleene N , Eagle KA , Emmons-Bell S , Feigin VL , Fernandez-Sola J , Fowkes G , Gakidou E , Grundy SM , He FJ , Howard G , Hu F , Inker L , Karthikeyan G , Kassebaum N , Koroshetz W , Lavie C , Lloyd-Jones D , Lu HS , Mirijello A , Temesgen AM , Mokdad A , Moran AE , Muntner P , Narula J , Neal B , Ntsekhe M , Moraes de Oliveira G , Otto C , Owolabi M , Pratt M , Rajagopalan S , Reitsma M , Ribeiro ALP , Rigotti N , Rodgers A , Sable C , Shakil S , Sliwa-Hahnle K , Stark B , Sundstrom J , Timpel P , Tleyjeh IM , Valgimigli M , Vos T , Whelton PK , Yacoub M , Zuhlke L , Murray C , Fuster V , Group G-N-JGBoCDW, Globalburden of cardiovascular diseases and risk factors, -Update from the GBD study, J Am Coll Cardiol (2020) ;76: (25):2982–3021. |
[27] | Messroghli DR , Moon JC , Ferreira VM , Grosse-Wortmann L , He T , Kellman P , Mascherbauer J , Nezafat R , Salerno M , Schelbert EB , Taylor AJ , Thompson R , Ugander M , van Heeswijk RB , Friedrich MG , Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI), J Cardiovasc Magn Reson (2017) ;19: (1):75. |
[28] | Ramos JG , Fyrdahl A , Wieslander B , Thalen S , Reiter G , Reiter U , Jin N , Maret E , Eriksson M , Caidahl K , Sorensson P , Sigfridsson A , Ugander M , Comprehensive cardiovascular magnetic resonance diastolic dysfunction grading shows very good agreement compared with echocardiography, JACC Cardiovasc Imaging (2020) ;13: (12):2530–42. |
[29] | Widmer RJ , Collins NM , Collins CS , West CP , Lerman LO , Lerman A , Digital health interventions for the prevention of cardiovascular disease: A systematic review and meta-analysis, Mayo Clin Proc (2015) ;90: (4):469–80. |
[30] | Varnfield M , Karunanithi M , Lee CK , Honeyman E , Arnold D , Ding H , Smith C , Walters DL , Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: Results from a randomised controlled trial, Heart (2014) ;100: (22):1770–9. |
[31] | Cassidy S , Hunyor I , Wilcox I , Fontana L Changing the conversation from ’chronic disease‘ to ’chronic health‘. Eur Heart J. 2021. |
[32] | Snow G blockrand: Randomization for Block Random Clinical Trials. R package version 1.5. https://CRAN.R-project.org/package=blockrand. 2020. |
[33] | Schulz KF , Grimes DA , Unequal group sizes in randomised trials: Guarding against guessing, The Lancet (2002) ;359: (9310)966–70. |
[34] | Michie S , Richardson M , Johnston M , Abraham C , Francis J , Hardeman W , Eccles MP , Cane J , Wood CE , The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions, Ann Behav Med (2013) ;46: (1):81–95. |
[35] | Singh RB , Dubnov G , Niaz MA , Ghosh S , Singh R , Rastogi SS , Manor O , Pella D , Berry EM , Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): A randomised single-blind trial, The Lancet (2002) ;360: (9344)1455–61. |
[36] | de Lorgeril M , Salen P , Martin JL , Monjaud I , Delaye J , Mamelle N , Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study, Circulation (1999) ;99: (6):779–85. |
[37] | Estruch R , Ros E , Salas-Salvado J , Covas MI , Corella D , Aros F , Gomez-Gracia E , Ruiz-Gutierrez V , Fiol M , Lapetra J , Lamuela-Raventos RM , Serra-Majem L , Pinto X , Basora J , Munoz MA , Sorli JV , Martinez JA , Fito M , Gea A , Hernan MA , Martinez-Gonzalez MA , Investigators PS , Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts, N Engl J Med (2018) ;378: (25):e34. |
[38] | Kraus WE , Bhapkar M , Huffman KM , Pieper CF , Krupa Das S , Redman LM , Villareal DT , Rochon J , Roberts SB , Ravussin E , Holloszy JO , Fontana L , 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial, The Lancet Diabetes & Endocrinology (2019) ;7: (9):673–83. |
[39] | Winzer EB , Woitek F , Linke A , Physical activity in the prevention and treatment of coronary artery disease, J Am Heart Assoc (2018) ;7: (4). |
[40] | Fihn SD , Gardin JM , Abrams J , Berra K , Blankenship JC , Dallas AP , Douglas PS , Foody JM , Gerber TC , Hinderliter AL , King SB , 3rd , Kligfield PD , Krumholz HM , Kwong RY , Lim MJ , Linderbaum JA , Mack MJ , Munger MA , Prager RL , Sabik JF , Shaw LJ , Sikkema JD , Smith CR Jr , Smith SC Jr , Spertus JA , Williams SV , Anderson JL , AmericanCollege of Cardiology Foundation/American Heart Association Task Fcollab. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons, Circulation (2012) ;126: (25):e354–471. |
[41] | Yao BC , Meng LB , Hao ML , Zhang YM , Gong T , Guo ZG , Chronic stress: A critical risk factor for atherosclerosis, J Int Med Res (2019) ;47: (4):1429–40. |
[42] | Bussing A , Michalsen A , Khalsa SB , Telles S , Sherman KJ , Effects of yoga on mental and physical health: A short summary of reviews, Evid Based Complement Alternat Med (2012) ;2012: :165410. |
[43] | Reive C , The biological measurements of mindfulness-based stress reduction: A systematic review, Explore (NY) (2019) ;15: (4):295–307. |
[44] | Praissman S . Mindfulness-based stress reduction: A literature review and clinician’s guide, J Am Acad Nurse Pract (2008) ;20: (4):212–6. |
[45] | Muscat DM , Lambert K , Shepherd H , McCaffery KJ , Zwi S , Liu N , Sud K , Saunders J , O’Lone E , Kim J , Robbins A , Webster AC , Supporting patients to be involved in decisions about their health and care: Development of a best practice health literacy App for Australian adults living with Chronic Kidney Disease, Health Promot J Austr (2021) ;32: (Suppl 1):115–27. |
[46] | Huang R , Liu N , Nicdao MA , Mikaheal M , Baldacchino T , Albeos A , Petoumenos K , Sud K , Kim J , Emotion sharing in remote patient monitoring of patients with chronic kidney disease, J Am Med Inform Assoc (2020) ;27: (2):185–93. |
[47] | Shcherbina A , Hershman SG , Lazzeroni L , King AC , O’Sullivan JW , Hekler E , Moayedi Y , Pavlovic A , Waggott D , Sharma A , Yeung A , Christle JW , Wheeler MT , McConnell MV , Harrington RA , Ashley EA , The effect of digital physical activityinterventions on daily step count: A randomised controlled crossoversubstudy of the MyHeart Counts Cardiovascular Health Study, TheLancet Digital Health (2019) ;1: (7):e344–e52. |
[48] | Samdal GB , Eide GE , Barth T , Williams G , Meland E , Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses, Int J Behav Nutr Phys Act (2017) ;14: (1):42 |
[49] | Klasnja P , Hekler EB , Shiffman S , Boruvka A , Almirall D , Tewari A , Murphy SA , Microrandomized trials: An experimental design for developing just-in-time adaptive interventions, Health Psychol (2015) ;34S: :1220–8. |
[50] | The American Heart Associaton Diet and Lifestyle Recommendations. 2021. Available at: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations. Accessed: 1 September 2021. |
[51] | Mezzani A , Cardiopulmonary exercise testing: Basics of methodology and measurements, Ann Am Thorac Soc (2017) ;14: (Supplement_1):S3–11. |
[52] | Hines EA , Brown GE , The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects, American Heart Journal (1936) ;11: (1):1–9. |
[53] | Vaidya K , Arnott C , Martinez GJ , Ng B , McCormack S , Sullivan DR , Celermajer DS , Patel S , Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: A CT coronary angiography study, JACC Cardiovasc Imaging (2018) ;11: (2 Pt 2):305–16. |
[54] | Brown LAE , Onciul SC , Broadbent DA , Johnson K , Fent GJ , Foley JRJ , Garg P , Chew PG , Knott K , Dall’Armellina E , Swoboda PP , Xue H , Greenwood JP , Moon JC , Kellman P , Plein S , Fully automated, inline quantification of myocardial blood flow with cardiovascular magnetic resonance: Repeatability of measurements in healthy subjects, J Cardiovasc Magn Reson (2018) ;20: (1):48. |
[55] | European Medicines Agency Committee for Medicinal Products for Human Use. Adjustment for baseline covariates in clinical trials | European Medicines Agency. 2015. |
[56] | Robins JM , Hernan MA , Brumback B , Marginal structural models and causal inference in epidemiology, Epidemiology (2000) ;11: (5):550–60. |




