Sidr Kashmiry honey and its fractions induced apoptosis in hepatocellular carcinoma in vitro
Abstract
Positive evidence for anticancer activities of honey is growing and the mechanism on how honey has anticancer characteristics is an area of great interest. Honey has been studied in various cancer cell lines for its ability to induce apoptosis, with several mechanisms of action being suggested. This study aims to evaluate the apoptotic activity of the non cytotoxic Sidr Kashmiry honey and its residue of successive fractionation as well as the cytotoxic chloroform-methanol and ethyl acetate fractions against hepatocellular carcinoma cells in vitro. Apoptosis was assessed by DNA fragmentation, diphenyl amine assay, and ultrastructure investigation. Micronuclei test was carried out to assess genotoxicity. Crude honey, residue, ethyl acetate and chloroform-methanol induced apoptosis, however, the residue and ethyl acetate caused high genotoxicity. Non cytotoxic Sidr Kashmiry honey and its cytotoxic chloroform-methanol fraction could be a powerful pro-apoptotic and non-genotoxic anticancer agent.
Abbreviations
DPA diphenyl amine
HepG2 hepatocellular carcinoma cell line
HMF 5-hyhydroxymethylfurfural
MN micronuclei
RPMI1640 Roswell Park Memorial Institute medium
TEM transmission electron microscope
TCA trichloroacetic acid
1Introduction
Apoptosis is a programmed cell death which maintains the healthy survival/death balance in normal human cells. Defect in apoptosis may cause cancer, while enhanced apoptosis may cause degenerative diseases [1]. Since cytotoxic anticancer treatments are not specific for cancer cells, cancer patients suffer from adverse side effects. Targeted therapies that recognize specific targets for cancer cell improve the efficacy of the treatment and reduce side effects [2].
For cancer treatment, there is a pressing need to develop cytotoxic and non-cytotoxic apoptosis-inducing anticancer agents that have the potential to be developed into effective targeted cancer therapies [3–5]. Non-cytotoxic agents have the advantage that they can minimize the side effects and are intended for long-term use with continuous daily administration unlike cytotoxic agents which are administered in short-term phases [6].
Programmed cell death, apoptosis, is categorized into three phases: an induction phase, effector phase and degradation phase. The last degradation phase comprises nuclear and cytoplasmic events includes chromatin and nuclear condensation, cell shrinkage, DNA fragmentation, and membrane blebbing. The cell is finally destined into fragmented apoptotic bodies which are phagocytosed by macrophages or other surrounding cells [7].
Non-genotoxic anticancer drug considered a great challenge for researchers. Genotoxicity of anticancer drugs to normal cells is one of the most serious problems of chemotherapy due to the possibility of inducing secondary malignancies. Although a precise definition of “genotoxicity” is elusive, there is no doubt that DNA damage plays an important role in most mechanisms underlying the action of anticancer drugs interacting with DNA [8]. The changes in the genetic material of an organism can be detected at specific levels by using various genotoxicity assay system like chromosomal aberration, sister chromatid exchange, micronucleus assay and comet assay [9].
Honey is a collection of nectar from many plants processed by honey bees. This natural product is well known for its high nutritional and prophylactic medicinal value. It contains various vitamins, minerals and amino acids as well as glucose and fructose and is popular as a natural food [10]. In addition, honey contains many polyphenols which known with its antioxidant properties [11]. It is well known that it exerts direct nutrient effect on regeneration of tissue because of its contents specially the large quantities of readily assimilable sugars [12].
Apitherapy, is a form of folk medicine for treating diseases including cancer [13]. Honey potentiates the antitumor activity of chemotherapeutic drugs such as 5-fluorouracil and cyclophosphamide [7, 14]. Studies exhibiting anticancer and chemoprevention effects of honey range from tissue cultures [11, 15, 16] and animal models [7, 14, 17] to clinical trials [7]. In oncology, honey has been used as a barrier against the implantation of tumors in laparoscopic oncological surgical procedures. In the same field, the intake of honey reduces chemotherapy related neutropenia fever by alleviating pancytopenia [18]. Generally, there has been a renaissance in the usage of honey as a medicine, however the mechanism of action of several of its properties remains obscure and needs further investigation.
This study aims to figure out the apoptotic activity of the non cytotoxic crude Sidr Kashmiry honey and the residual fraction as well as its cytotoxic chloroform-methanol and ethyl acetate fractions against hepatocellular carcinoma (HepG2) cells in vitro.
2Materials and methods
Sidr Kashmiry honey was purchased from the Kingdom of Saudi Arabia markets and tested for adulteration.
2.1Honey adulteration tests
2.1.1Diastase activity
Diastase activity was measured according to Tosi et al. [19]. Briefly, honey solution (20% in acetate buffer containing 0.5 M NaCl solution was mixed with 2% soluble starch solution at 40°C for 15 minutes, diluted iodine solution was used to stop the reaction. The relative color intensity was determined at 575 nm using Shimadzu UV-2401 spectrophotometer. Diastase activity was calculated using the following equation and expressed in units per gram of honey. Diastase activity (units / gram) = 60/t×0.1/0.01×1/2 = 300/t Where (t) is the number of minutes (t) required to reach 50% transmittance value.
2.1.25-hydroxymethylfurfural (HMF) content
Honey is considered adulterated when the value of 5-hyhydroxymethylfurfural (HMF)>15 mg/100 g honey. HMF were extracted with ether and then dissolved in distilled water then filtered through Whatman No.1 paper. Ethanol was added to the aqueous solution of the honey extract (1:1/v:v). Resorcinol solution was added and mixed thoroughly. The color intensity was measured at 490 nm. Estimation of HMF concentration in honey extract was done using the molecular extinction coefficient of pure HMF at 285 nm which equals 16.5 using the following equation: Concentrations (μ mole) = Absorbance/Extinction coefficient [20].
2.1.3Detection of commercial glucose
Commercial glucose was detected according to Muli et al. (2007). Honey sample was diluted with distilled water (1:1 w/v), absolute ethanol were added then shacked and centrifuged. The precipitate was dissolved in distilled water. Ascending paper chromatography was used using isoamyl alcohol/pyridine/water (7:7:6) as a developing solvent. The chromatogram was dried, then dipped in aniline-diphenylamine chromogenic reagent. The chromatogram was heated at 85–95°C for 5–8 minutes until the blue color of the control glucose spot developed. A honey sample containing 5% of commercial glucose shows a series of blue maltodextrin spots of low Rf. The natural honey dextrin spot is distinctly brown or gray not blue.
2.2Honey extraction
Sider Kashmiry honey was successively fractionated by ethyl acetate, and chloroform-methanol (6:4), respectively.
2.3Cell culture
Hepatocellular carcinoma (HepG2) cell line was supplied by Naval American Research Unit, Egypt (NAmRU). Cells were propagated in complete RPMI1640 medium. Cells were treated with honey or one of its fractions using the effective concentration (EC) = 100 and 10 μg/ ml respectively for 72 h according to our previous studies [20].
2.4DNA fragmentation assay
DNA fragmentation pattern (DNA ladder) was carried out by agarose gel electrophoresis. Cells treated were suspended in lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 10 mM EDTA) and incubated for 2 h. After that the samples were loaded onto a 1.5% agarose gel containing ethidium bromide, electrophoresed in Tris acetate/EDTA buffer for 2 h at 50 V, and then photographed under UV illumination [21].
2.5Diphenyl amine reaction assay
Treated hepatocellular carcinoma (HepG2) cells were collected then lysed using lysis buffer. Trichloroacetic acid (TCA 25%) was added and incubated at 4°C for 24 h. Cells were centrifuged and the pellet was suspended in 5% TCA then incubaed at 83°C for 20 min. Diphenyl amine (DPA) solution was added and incubated at room temperature for 24 h. The proportion of fragmented DNA was calculated from absorbance reading at 600 nm [22].
2.6Micronuclei (MN) assay
For the micronucleus test, treated cells were harvested with a hypotonic solution (KCl 0.075 M) and fixed twice with methanol: acetic acid (3:1). Thereafter, cell suspension was smeared on slides and air-dried. Staining was performed with Giemsa stain at 5% in Sorensen Buffer (v/v). Slides were washed in tap water and allowed to dry at room temperature overnight. Visual scoring for micronuclei (MN) was analyzed and the MN index (MNI) was calculated by dividing the number of mono-nucleated cells on the total number of cells [23].
2.7Ultrastructure assay
Treated HepG2 cells were prepared for ultrastructure investigations [24] and harvested then washed twice with PBS and fixed in 3% glutaraldehyde 25 mM sodium phosphate. Analysis was then performed with Transmis-sion Electron Microscope (JEM-2000EX; JEOL Co; Japan).
2.8Statistical analysis
Statistical analysis was done using SPSS (version 16). Results of micronucleus, DNA fragmentation and diphenyl amine tests were performed by analysis of variance (ANOVA) followed by multiple post-hoc comparisons made by Tukey analysis, where P < 0.05 was considered to indicate a statistically significant difference.
3Results
3.1Honey exhibited DNA fragmentation pattern in HepG2 cells
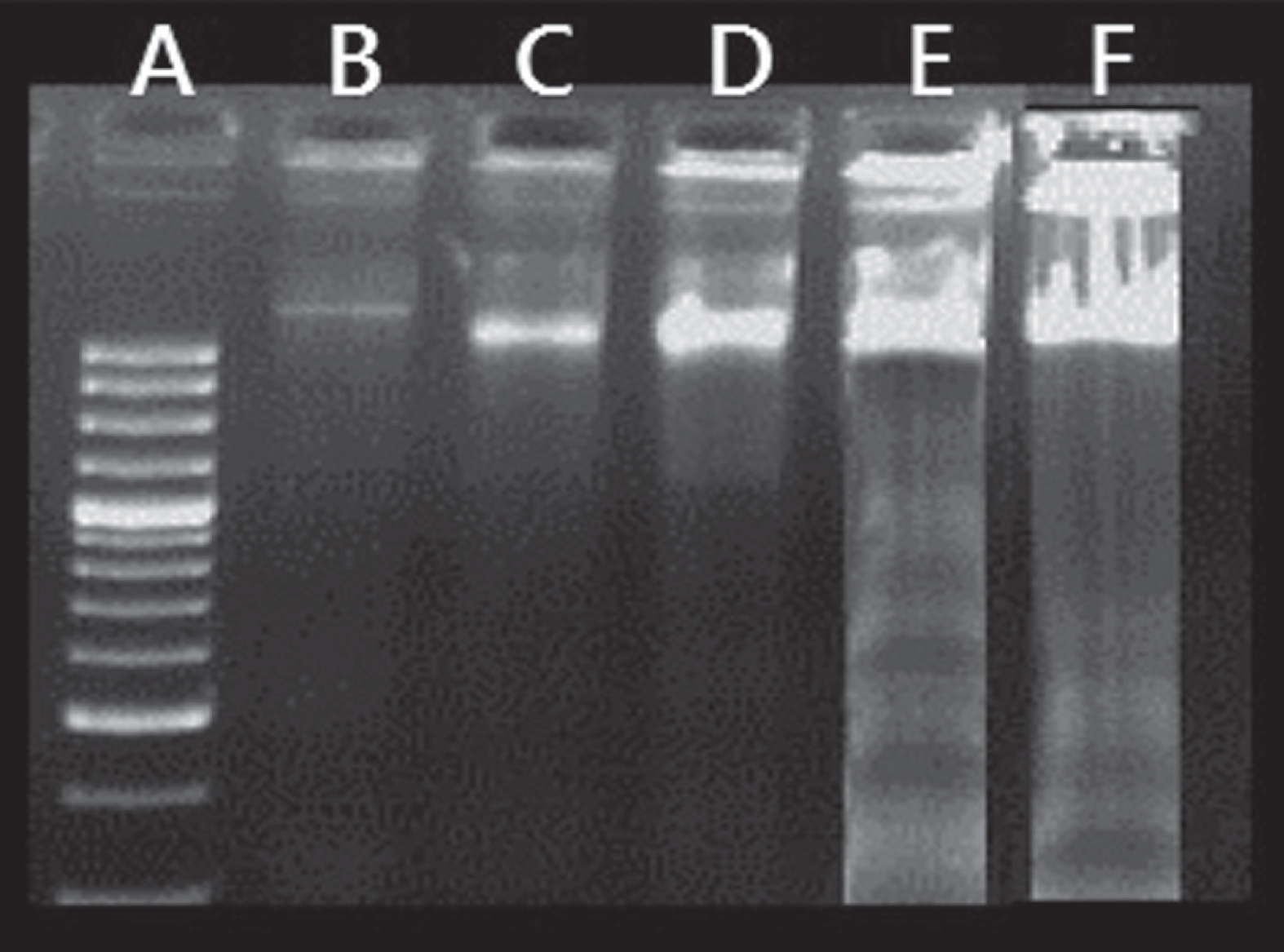
HepG2 cells treated with honey or its residual fraction displayed DNA fragmentation, while honey fractions exhibited less DNA fragmentation (Fig. 1). Results using quantitative estimation of DNA fragmentation revealed that honey and its residual fraction significantly (P < 0.05) increased percentage of DNA fragmentation at (58±4.5% and 54±4.2% respectively) (Fig. 1E and F respectively) compared to control (30±4.4%) (Fig. 1B). Chloroform-methanol and ethyl acetate extracts showed a slight significant increase (P < 0.05) of percentage of DNA fragmentation to 35±4.4%, and 38±4.9%, respectively (Fig. 1C and D respectively).
Fig.1
Agarose-gel-electrophoretic patterns showing DNA fragmentation of HepG2 cells. Lane (A): Represents the molecular marker. Lane (B): Control HepG2 cells. Lane (C) HepG2 cells treated with chloroform-methanol fraction. Lane (D): HepG2 cells treated with ethyl acetate fraction. Lane (E): HepG2 cells treated with honey. Lane (F): HepG2 cells treated with residue.
3.2Honey induced electron microscopic morphological changes
Transmission electron microscopy was used to demonstrate the changes of internal cellular structure after exposure to honey, residue, ethyl acetate and chloroform- methanol factions (Fig. 2C, D, E, F respectively). Control HepG2 cells appeared with intact nuclear membranes and normal cytoplasm. The nuclei were large, irregular in shape and nuclear indentations were observed. The nucleolus was compact and dense in appearance (Fig. 2B).
Fig.2
Transmission electron micrograph of HepG2 cell showing A: Marker. B: Control cells. C: Cells treated with honey. D: Cells treated with residue. E: Cells treated with ethyl acetate fraction. F: chloroform-methanol extract treated HepG2 cell. Large and irregular nucleus (N) nucleolus (Nu). Mitochondria with transverse cristae (M) Autophagic vacuoles (Av) osmophilic lysosomes (Ly).
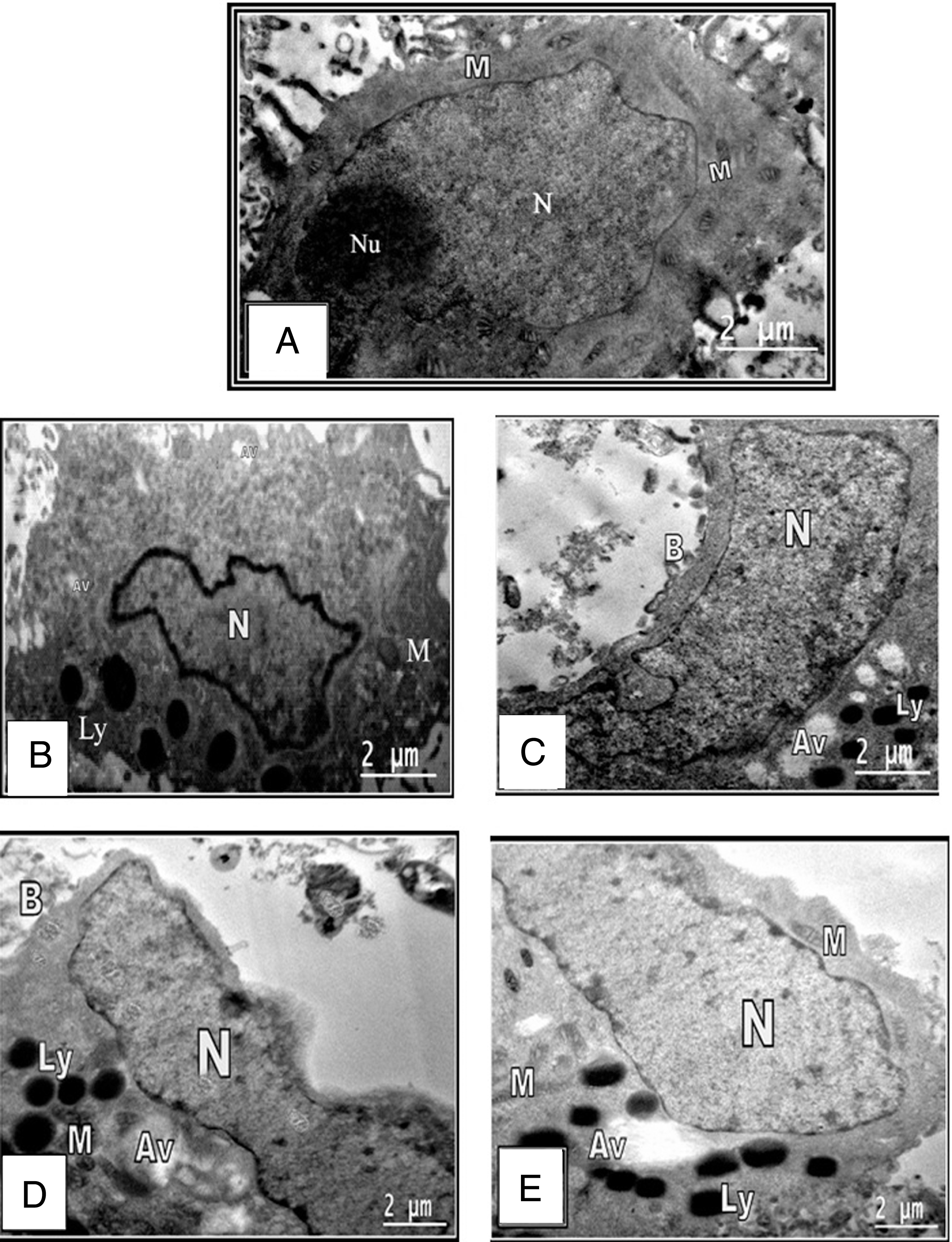
Treatment of HepG2 cells with honey or residual part revealed several dystrophic alterations in cytoplasm, nucleus, cell organelles and membrane blebbing. The nucleus revealed condensed granulated chromatin, numerous presumably autophagic vacuoles and apoptotic bodies. The cytoplasm became vacuolated together with opacity of the cytoplasmic organelles. Mitochondria appeared as dense bodies with a dark matrix and translucent cristae (Fig. 2C and D). The late signs of apoptosis were observed in honey treated HepG2 cells as the nuclei were completely vanished and appearance of apoptotic bodies (Fig. 2C).
The treatment with chloroform-methanol or ethyl acetate fractions resulted in shrinkage of cells and opacity of cytoplasmic organelles. Nuclei were also shrunken and accompanied by condensed chromatin which dispersed in the nucleus as small granules (Fig. 2F).
3.3Micronuclei frequency
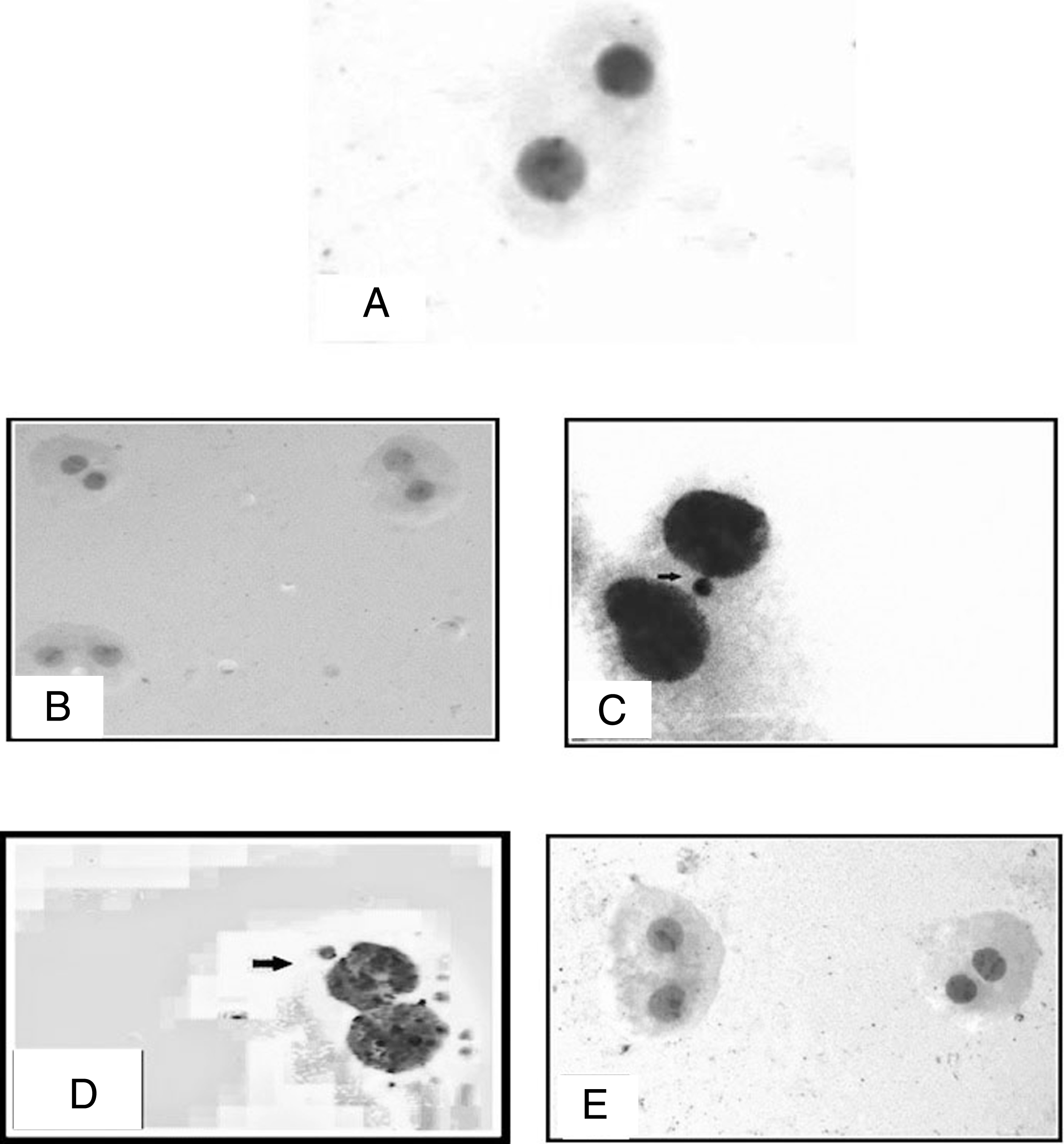
Treatment with honey did not change the frequency of micronuclei significantly (Fig. 3B) in HepG2 cells where it was 55.0±1.7 compared to control (60±0.6 at P > 0.05) (Fig. 3A), however residue increased the micronuclei frequency (Fig. 3C). Ethyl acetate extract exhibited a significant increase in the frequency of micronuclei to 80.0±1.0 (Fig. 3D) while chloroform-methanol fraction reduced the frequency of micronuclei to 24.0±0.9 (Fig. 3E).
Fig.3
Photomicrographs of HepG2 cells stained by Geimsa stain A: control HepG2 cells B: Cells treated with honey C: Cells treated with residue showing binucleated cell containing micronucleus (arrow) D: Cells treated with ethyl acetate fraction showing binucleated cell containing micronuclei (thick arrow) E: Cells treated with Chloroform- methanol fraction showing multiple binucleated cells without micronuclei. The micronucleus has a similar appearance and intensity to the main nuclei (10×40).
4Discussion
Honeys from diverse floral sources have demonstrated potential anticancer activity [25–27]. It potentially inhibits the development of cancer by blocking the three main stages of carcinogenesis: initiation, proliferation, and progression via several mechanisms including the induction of apoptosis [28]. Although honey has been referred to as an apoptotic inducer through DNA fragmentation [29], the mechanism of its therapeutic activity has not been completely understood. According to the literature, honey may stimulate or inhibit tumor cell growth, many literatures stated the two opposite effects [30, 31]. They stated that several types of honey inhibits proliferation and induce apoptosis, besides other anticancer activities [7, 24, 30, 32, 33]. On the other side, thyme and pine honey exerted little reduction of cell viability of MCF-7 cells at high concentration, while some types of honey enhanced the viability of tumor cells [20, 28, 32, 33]. These dual effects of honey are mostly likely due to their high contents of phenolic compounds such as kaempferol and quercetin which known with their dual actions, both inhibitory and stimulatory. Regardless the effect of honey on cell proliferation (induction or inhibition), other antitumor activities were observed including induction of apoptosis [31]. It is well known that the bioactivities of honey seems not to be due to sugar content alone, but is also due to other chemical minor constituents as phenolic acids and flavonoids [30, 34].
The aim of this work is to illustrate the apoptotic activity of the non-cytotoxic crude Sidr Kashimry honey and the cytotoxic ethyl acetate and chloroform-methanol fractions as well as the non cytotoxic residual fraction in HepG2 cells in vitro regardless their effect on cell growth.
In this regard, we previously found that the crude Sidr Kashimry honey and its residue were non cytotoxic with a slight increase in cell number of HepG2 cells [20]. The growth promoting effect may be attributed to their fructose and glucose oligosaccharides as well as quercetin content. On the other side, sugar free ethyl acetate and chloroform-methanol fractions were cytotoxic [20, 32, 33, 35]. In this study, we found that the non cytotoxic Sidr Kashimry honey and residual fraction, which contain most of the sugars content, induced apoptosis through qualitative and quantitative estimation of DNA fragmentation and electron microscope investigations. Honey has the advantage that it is not genotoxic, whereas residue considered as genotoxic agent. Therefore, honey act as an apoptotic inducer regardless its growth induction activity. These results concomitant with the augmentary effect of fir honey on MCF-7 cell proliferation which exerted a stimulatory effect it may be related to its nutrient (glucose, amino acids, minerals), hydrogen peroxide, and phenolic constituent content, especially quercetin, which has been reported to exert biphasic effects on cancer cells [32]. The cytotoxic ethyl acetate and chloroform-methanol fractions induced apoptosis with weak induction of DNA fragmentation which concomitant with the fact that apoptosis could be induced without the DNA fragmentation [35].
In conclusion, the study proved that crude Sider Kashmiry honey considered a powerful proapoptotic agent regardless its proliferation induction activity. Its cytotoxic ethyl acetate and chloroform-methanol fractions could also induced apoptosis. Further studies should be done to illustrate this point.
Conflict of interest
None.
Acknowledgments
This work was supported by the National Research Center-Egypt [grant number 10160003].
References
[1] | Raza H , John A , Shafarin J . Potentiation of LPS-induced apoptotic cell death in human hepatoma HepG2 cells by aspirin via ROS and mitochondrial dysfunction: Protection by N-acetyl cysteine. PLoS One. (2016) ;11: :e0159750. |
[2] | Maione P , Rossi A , Airoma G , Ferrara C , Castaldo V , Gridelli C . The role of targeted therapy in non-small cell lung cancer. Crit Rev Oncol Hematol. (2004) ;51: :29–44. |
[3] | Essack M , Bajic VB , Archer JA . Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment. Mar Drugs. (2011) ;9: :1580–606. |
[4] | Fulda S . How to target apoptosis signaling pathways for the treatment of pediatric cancers. Front Oncol. (2013) ;3: :22. |
[5] | Zhao GX , Xu LH , Pan H , Lin QR , Huang MY , Cai JY , et al. The BH3-mimetic gossypol and noncytotoxic doses of valproic acid induce apoptosis by suppressing cyclin-A2/Akt/FOXO3a signaling. Oncotarget. (2015) ;6: :38952–66. |
[6] | Narang AS , Desai DS . Anticancer drug development unique aspects of pharmaceutical development. Pharmaceutical Perspectives of Cancer Therapeutics. (2009) ;49–92. |
[7] | Ahmed S , Othman NH . Honey as a potential natural anticancer agent: A review of its mechanisms. Evid Based Complement Alternat Med. (2013) ;829070. |
[8] | Blasiak J , Gloc E , Warszawski M . A comparison of the in vitro genotoxicity of anticancer drugs idarubicin and mitoxantrone. Acta Biochim Pol. (2002) ;49: :145–55. |
[9] | Celik A , Ekinci SY , Guler G , Yildirim S . In vitro genotoxicity of fipronil sister chromatid exchange, cytokinesis block micronucleus test, and comet assay. DNA Cell Biol. (2014) ;33: :148–54. |
[10] | de Vere N , Jones LE , Gilmore T , Moscrop J , Lowe A , Smith D , et al. Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Scientific Reports. (2017) ;7: :42838. |
[11] | Alvarez-Suarez JM , Tulipani S , Diaz D , Estevez Y , Romandini S , Giampieri F , et al. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem Toxicol. (2010) ;48: :2490–9. |
[12] | Vijaya KK , Nishteswar K . Wound healing activity of honey: A pilot study. Ayu. (2012) ;33: :374–7. |
[13] | Trumbeckaite S , Dauksiene J , Bernatoniene J , Janulis V . Knowledge, attitudes, and usage of apitherapy for disease prevention and treatment among undergraduate pharmacy students in Lithuania. Evid Based Complement Alternat Med. (2015) ;2015: :172502. |
[14] | Gribel NV , Pashinskii VG . [The antitumor properties of honey]. Vopr Onkol. (1990) ;36: :704–9. |
[15] | Fauzi AN , Norazmi MN , Yaacob NS . Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem Toxicol. (2011) ;49: :871–8. |
[16] | Ghashm AA , Othman NH , Khattak MN , Ismail NM , Saini R . Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement Altern Med. (2010) ;10: :49. |
[17] | Orsolic N , Knezevic A , Sver L , Terzic S , Hackenberger BK , Basic I . Influence of honey bee products on transplantable murine tumours. Vet Comp Oncol. (2003) ;1: :216–26. |
[18] | Bardy J , Slevin NJ , Mais KL , Molassiotis A . A systematic review of honey uses and its potential value within oncology care. J Clin Nurs. (2008) ;17: :2604–23. |
[19] | Tosi E , Martinet R , Ortega M , Lucero H , Ré E . Honey diastase activity modified by heating. Food Chemistry. (2008) ;106: :883–7. |
[20] | Amer M , H HR , Baumy A , Kiwan H . Antitumor effect of bee honey extracts on hepatoma cells in vitro. 35th Annual Conference Cooperation among Scholars in Egypt & Abroad. (2008) : 117. |
[21] | Rahbar Saadat Y , Saeidi N , Zununi Vahed S , Barzegari A , Barar J . An update to DNA ladder assay for apoptosis detection. Bioimpacts. (2015) ;5: :25–8. |
[22] | Gercel-Taylor C . Diphenylamine assay of DNA fragmentation for chemosensitivity testing. Methods Mol Med. (2005) ;111: :79–82. |
[23] | Doherty AT . The in vitro micronucleus assay. Methods Mol Biol. (2012) ;817: :121–41. |
[24] | Miao Q , Bi LL , Li X , Miao S , Zhang J , Zhang S , et al. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: Roles of apoptosis and autophagy. Int J Mol Sci. (2013) ;14: :1370–82. |
[25] | Fernandez-Cabezudo MJ , El-Kharrag R , Torab F , Bashir G , George JA , El-Taji H , et al. Intravenous administration of manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS One. (2013) ;8: :e55993. |
[26] | Spilioti E , Jaakkola M , Tolonen T , Lipponen M , Virtanen V , Chinou I , et al. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS One. (2014) ;9: :e94860. |
[27] | Canini. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. International Journal of Oncology. (2010) ;37. |
[28] | Erejuwa OO , Sulaiman SA , Ab Wahab MS . Honey: A novel antioxidant. Molecules. (2012) ;17: :4400–23. |
[29] | Miguel MG , Antunes MD , Faleiro ML . Honey as a Complementary Medicine. Integr Med Insights. (2017) ;12: , 1178633717702869. |
[30] | Alvarez-Suarez JM , Tulipani S , Romandini S , Bertoli E , Battino M . Contribution of honey in nutrition and human health: A review. Mediterranean Journal of Nutrition and Metabolism. (2009) ;3: :15–23. |
[31] | Erejuwa OO , Sulaiman SA , Wahab MS . Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules. (2014) ;19: :2497–522. |
[32] | Porcza LM , Simms C , Chopra M . Honey and cancer: Current status and future directions. Diseases. (2016) ;4. |
[33] | Al-Jadi A-M , Kanyan Enchang F , Mohd Yusoff K . The effect of Malaysian honey and its major components on the proliferation of cultured fibroblasts. Turkish Journal of Medical Sciences. (2014) ;44: :733–40. |
[34] | Martos I , Ferreres F , Yao L , D’Arcy B , Caffin N , Tomas-Barberan FA . Flavonoids in monospecific eucalyptus honeys from Australia. J Agric Food Chem. (2000) ;48: :4744–8. |
[35] | Liegler TJ , Hyun W , Yen TS , Stites DP . Detection and quantification of live, apoptotic, and necrotic human peripheral lymphocytes by single-laser flow cytometry. Clin Diagn Lab Immunol. (1995) ;2: :369–76. |




