Effect of Artocarpus altilis fruit based-diet on liver and kidney function indices on alloxan-induced diabetic rats
Abstract
BACKGROUND: The prevalence of diabetes mellitus continues to increase alarmingly in spite of years of intensive research. The need to explore alternative therapy such dietary and phytotherapy has been gaining attention in the management/treatment of diabetes mellitus.
OBJECTIVE: The present research work was carried out to investigate the biochemical indices in the liver and kidney of alloxan-induced diabetic rats fed with Artocarpus altilis fruit based-diet.
METHODS: Diabetes was induced by a single intraperitoneal injection of alloxan (150 mg/kg) in albino rats. A total of thirty two albino rats (Rattus norvegicus) were grouped into four; control (non-diabetic rat), diabetic untreated rats, diabetic rats administered with metformin daily and diabetic rats fed with Artocarpus altilis fruit based-diet groups. Biochemical indices in the serum, liver and kidney were determined.
RESULTS: The results shows significant (P < 0.05) decrease in alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubin, urea, creatinine and lipid peroxidation of diabetic fed with Artocarpus altilis fruit based-diet. Also diabetic rats fed with Artocarpus altilis fruit based-diet demonstrated significant increase (p < 0.05) in albumin and total protein concentrations as well as antioxidant enzymes activities when compared with diabetic untreated rats and diabetic rats administered with metformin daily.
CONCLUSION: The results obtained in this study demonstrated that consumption of Artocarpus altilis fruit based-diet may ameliorate the liver and kidney function indices of alloxan-induced diabetic rats.
1Introduction
Diabetes is a chronic condition associated with abnormally high levels of sugar in the blood that result from defects in insulin secretion, or action, or both [1]. It is characterized by hyperglycemia, glucosuria and several micro-vascular and macro-vascular complications [2, 3]. The term “diabetes”, when used alone, generally refers to diabetes mellitus and not a rare, unrelated disease called diabetes insipidus.
Diabetes develops when the pancreas fails to produce sufficient quantities of insulin [4]. Insulin deficiency in turn leads to chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism [5]. The incidence of diabetes has reached an epidemic proportion worldwide. It is estimated that about 2.8% of the world’s population suffer from diabetes, according to World Health Organisation [6]. This rapid increase in the incidence of the disease is associated with rapidly changing lifestyle and environmental factors [7].
Diabetes mellitus was classified based on whether or not the patient is insulin dependent/ independent. Insulin dependent diabetes mellitus is also called Type I diabetes mellitus; it is usually characterized clinically by abrupt onset of symptoms and dependence on injected insulin to sustain life. Insulin independent diabetes mellitus or Type II diabetes mellitus is the second subclass of diabetes, frequently present with minimal or no symptoms referable to the metabolic aberrations of diabetes [5]. The high blood glucose level that accompanies type I diabetes mellitus and Type II diabetes mellitus can cause serious health complications including ketoacidosis, kidney failure, heart disease, stroke, blindness etc.
There is yet no effective cure for diabetes. The currently available drugs as well as insulin used in managing disease are associated with several undesirable side effects [8]. Also their high cost has led to search for plants and diets with normoglycemic properties in the management of diabetes [9, 10]. The use of herbal medicine and diets are widespread throughout the world. Many medicinal plants have been confirmed to have hypoglycaemic properties. The hypoglycaemic properties of plants used in management of diabetes are reported to be due to their content of flavonoids, glycosides, alkaloids, plant polysaccharides and other bioactive compounds [11]. Some of them include Pterocarpus massupum (Indian Kino), Allium sativium (Garlic), Carica papaya (unripe fruit), Trigonella foerum (Fenugreek), Aloe barbadensis miller etc. [12].
Artocarpus altilis has been believed to be endowed with medicinal usefulness. It is an herbaceous perennial plant of the moraceae family and it is grown widely in tropical, pacific, Caribbean and African countries. Artocarpus altilis is a good source of antioxidants, carotenoids, fiber, copper, thiamine, niacin, calcium, iron and magnesium [13]. It has been shown that senescence leaves were used to prepare tea to reduce blood pressure, and it is also thought to control diabetes, treat liver diseases and fevers and has antioxidant and antitumor effects [13]. Although, the anti-diabetic effectiveness of Artocarpus altilis fruit is acclaimed traditionally, there is no proven scientific evidence to ascertain this. It is therefore imperative to investigate the effect of Artocarpus altilis fruit based-diet on liver and kidney function indices of alloxan-induced diabetic rats.
2Materials and methods
2.1Sources of materials
2.1.1Plant material and authentication
Artocarpus altilis fruits (breadfruit (Fig. 1) were purchased at Ikere market, Ikere- Ekiti, Ekiti State, Nigeria. Authentication and identification of the fruit was carried out at the Department of Plant Science, Ekiti State University, Ado-Ekiti, Ekiti State.
2.1.2Chemicals
Albumin, bilirubin, total protein, urea, creatinine and all enzymes assay kits used were produced from Erba Diagnostics Mannheim, Germany. Alloxan and all other chemicals used were obtained from Sigma.
2.1.3Experimental animals
A total of thirty two (32) Albino rats (Rattus norvegicus) with an average weight of 120-180 g were obtained from the animal house of the Department of Biochemistry, Afe Babalola University, Ado-Ekiti, Ekiti State.
2.1.4Induction of diabetes
A single dose of 150 mg/kg (intraperitonial) of alloxan monohydrate was dissolved in normal saline (0.9%) and injected into the albino rats. The confirmations were carried out at 48 hours. Rats with blood glucose level ≥250 mg/dl were selected for this study.
2.1.5Processing of the Artocarpus altilis fruit
Artocarpus altilis fruit (breadfruit) was peeled, to remove the seed. The fruit flesh was washed with distilled water and oven-dried at 60°C until its dried (to obtain a constant weight), thereafter the fruit flesh was pulverized into powder using an electric blender. This was used for various analyses.
2.1.6Animal grouping
The animals were grouped into four (4):
1 Group A: Control (rats) fed with yam flour based-diet.
2 Group B: Diabetic untreated rats fed with yam flour based-diet.
3 Group C: Diabetic rats fed with yam flour based-diet administered metformin daily.
4 Group D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
2.1.7Formulation of experimental diets
The composition of the experimental diets (g/100 g) is presented in Table 1. Each component of the experimental diets were weighed, thoroughly mixed and packed into airtight containers.
2.1.8Collection and treatment of blood samples
After three weeks of feeding, the animals were anaesthetized with diethyl ether and sacrificed by simply incising the jugular vein, the blood samples were collected into plain sample bottles.
2.1.9Preparation of serum
The blood samples were allowed to stand at room temperature for 30 minutes to form clot after which it was centrifuged at 1000 g (gravity) for 15 minutes. After centrifugation, the clot forms sediment at the bottom of the centrifuge and the supernatant which is the serum was collected using a Pasteur’s pipette. The serum, thus obtained were appropriately labeled and stored in a freezer at –5°C until required for further analysis.
2.2Preparation of tissue homogenates
2.2.1Isolation and homogenization of tissues
After sacrifice, the rats were dissected in order to isolate the tissues of interest (liver and kidney). The isolated tissues were cleansed with cotton wool to remove blood stains, weighed and immediately stored in ice cold 0.25 M sucrose solution. The liver and kidney was cut with a clean scalpel, then subjected to homogenization using Teflon homogenizer in ice-cold 0.25 M sucrose solution (1 : 5 w/v). The homogenates were stored in the freezer until required for further analysis.
2.2.2Enzyme assay and measurement of serum metabolites
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by the method described by Reitman and Frankel [14]. Alkaline phosphatase (ALP) activity was assayed by para-nitrophenyl phosphate (PNPP) method of Wright et al. [15]. Serum albumin was assayed as described by Doumas et al. [16]. Serum protein concentration was determined using Biuret reagent as described by Gornall et al. [17]. Serum bilirubin was determined by the method of Sherlock [18]. Serum creatinine was determined by Bartels and Bohmer [19] method. Serum urea concentration was assayed as described by Fawcett and Scott [20]. Lipid peroxidation was determined by measuring the thiobarbituric acid reactive substances (TBARS) present in the test sample which is produced during lipid peroxidation according to the method of Varshney and Kale [21], the malondialdehyde (MDA) level was calculated according to the method of Adam-Vizi and Seregi [22]. Catalase (CAT) activity was determined as described by Sinha [23]. Superoxide dismutase (SOD) activity was determined by the method described by Misra and Fridovich [24] and gluthathione peroxidase (GPx) was carried out according to the method describe by Rotruck et al. [25].
2.2.3Histopathological studies
Tissues (liver and kidney) were fixed in 10% formalin. They were dehydrated in ascending grades of ethanol, cleared in xylene, and processed to paraffin blocks, sectioned (5 μm thick) and stained with Hematoxyline and Eosin stain. They were examined using light microscopy for demonstration of tissues pathological changes including atrophy, cell destruction and necrosis [26].
2.2.4Statistical analysis
All data were expressed as the mean of seven replicates ± standard error of mean (S.E.M). Statistical evaluation of data was performed by SPSS version 16, using one way analysis of variance (ANOVA), followed by Dunett’s posthoc test for multiple comparism. Values were considered statistically significant at p < 0.05 (confidence level = 95%) [27].
3Results
Tables 2, 3 and 4 show the activities of ALT, AST and ALP respectively in the tissues and serum of the experimental rats. There was significant increase (p < 0.05) in the diabetic untreated rats fed with yam flour based-diet (group B) when compared with the control rats (group A), diabetic rats fed with yam flour based-diet and administered metformin (group C), and diabetic rats fed with Artocarpus altilis fruit based-diet (group D). However, the values obtained for group A, C and D were not significantly different (p > 0.05) from each other.
Table 5 shows the serum concentrations of albumin, total protein, globulin and bilirubin in the experimental rats. Albumin, total protein and globulin shows a significant decrease (p < 0.05) in the diabetic untreated rats fed with yam flour based-diet (group B) when compared with the control rats (group A). However there was a significant increase (p < 0.05) in diabetic rats fed with yam flour based-diet and administered with metformin (group C) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D) when compared with the control rats (group A).
In addition, Table 5 also shows a significant increase (p < 0.05) in total and conjugated bilirubin in the diabetic untreated rats fed with yam flour based-diet (Group B) when compared with the control rats (group A) diabetic rats fed with yam flour based-diet administered with metformin (group C) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D) while group A, C and D were not significantly different (p > 0.05) from one another.
Table 6 shows the serum concentrations of creatinine and urea in the experimental rats. There was a significant increase (p < 0.05) in diabetic untreated rats fed with yam flour based-diet (group B) when compared with the control rats (group A), whereas there was a significant decrease in diabetic rats fed with yam flour based-diets administered with metformin (group C) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D) when compared to diabetic untreated rats fed with yam flour based-diet (group B).
Table 7 shows lipid peroxidation in the tissue homogenates of the experimental rats. There was a significant increase (p < 0.05) in the diabetic untreated rats fed with yam flour based-diet (group B) when compared with the control rats (group A), however there were no significant difference between control rats (group A), diabetic rats fed with yam flour based-diet and administered with metformin (group C) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D) of the liver. Meanwhile the kidney shows a significant increase (p < 0.05) in diabetic untreated rats fed with yam flour based-diet (group B) and diabetic rats fed with yam flour based-diet and administered with metformin (group C) when compared with the control rats (group A) but no significant difference between control rats (group A) and diabetic rats fed with Artocarpus altilis fruit based-diet(group D).
Furthermore, Table 8 revealed no significant difference (p > 0.05) in CAT, SOD and GPx in the liver and kidney of control rats (group A) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D). But there was a significant decrease (p < 0.05) in the diabetic untreated rats fed with yam flour based-diet (group B) when compared with the control rats (group A), diabetic rats fed with yam flour based-diet and administered metformin (group C) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D) in CAT, SOD and GPx. While there was a significant decrease (p < 0.05) in diabetic rats fed with yam flour based-diet and administered with metformin (group C) when compared with control rats (group A) and diabetic rats fed with Artocarpus altilis fruit based-diet (group D).
3.1Histological changes of Artocarpus altilis fruit based-diet in liver and kidney of alloxan-induced diabetic rats
Histopathological studies of the liver and kidney of control rats showed normal histology (Figs. 2a/3a). For the diabetic untreated rats fed with yam flour based-diet, portal congestion, periportal cellular infiltration and vacuolar degeneration of hepatocytes was observed (Figs. 2b/3b). The diabetic rats fed with yam flour based-diet and administered with metformin also showed almost normal liver histology (Figs. 2c/3c) while the diabetic rats fed with Artocarpus altilis fruit based-diet showed normal liver histology (Figs. 2d/3d).
4Discussion
The measurement of the activities of various enzymes in the tissues and body fluids plays a significant role in disease investigation and diagnosis [28]. This enzymes activities in the tissues are often used as ‘marker’ to ascertain early toxic effects of administered foreign compounds (like alloxan) to experimental animals [29]. Tissue enzyme assay can also indicate tissue cellular damage long before structural damage can be picked up by conventional histological techniques [30].
ALT and AST are cytosolic enzymes involved in the transfer of amino group from α-amino to α-keto acids. They are also involved in the biochemical regulation of intracellular amino acid pool. These enzymes are highly concentrated in the liver and kidney and are only found in the serum in significant quantities when the cell membrane becomes leaky and even completely ruptured [31, 32] indicating tissue injury or organ dysfunction.
ALT as a liver cytosol enzyme is more specific to the liver, a rise only occurs in liver diseases [33]. The liver also contains considerable amount of AST, where it is associated with liver parenchyma cells. AST is important in the diagnosis of heart and liver damage caused by heart attack, drug toxicity, or infection although, it is not as specific as liver marker enzyme as ALT. The increase in the serum AST and ALT as indicated in alloxan-induced diabetes produced an alteration in hepatic function, and this may be due to the leakage of the named enzymes from the liver cytosol into the blood stream [34] while a general decrease in ALT and AST may perhaps suggests that the experimental diet confer protection on the liver tissues against injury, damage or disease, which are often the direct cause of elevation of the enzymes in the blood stream [35].
This study shows that diabetic rats fed with Artocarpus Altilis fruit based-diet decreased serum and tissue homogenates activities of ALT and AST (as shown in Tables 2 and 3 respectively). Artocarpus altilis fruit is a good source of antioxidants, carotenoids, fiber, copper, thiamine, niacin, calcium, iron and magnesium. It is also believed to be useful in managing liver diseases, fevers etc. [36]. Therefore this reduction can be attributed to the protective effect of Artocarpus altilis fruit.
Also, an increased activity of ALP (membrane bound enzyme) is a well known diagnostic indicator of liver injury and it is considered the bio-markers for liver functions. At the end of this study, diabetic rats fed with Artocarpus altilis fruit based-diet were observed to have a significant decrease in the ALP activities (Table 4). This may indicate hepatoprotective and renoprotective abilities of the diet. Meanwhile elevated ALP activities in the diabetic untreated rats is an indication of pathological processes which could be liver impairment or kidney dysfunction as described by Bogin et al. [37]. The fact that the enzyme activities were maintained in the serum and tissue homogenates of the diabetic rats fed with Artocarpus altilis fruit based-diet implies that the compounded diet has no membrane labializing effect on these organs.
Total protein, albumin and globulin are plasma proteins (Table 5) that measure synthetic function of the liver. They help in maintaining blood osmotic pressure. Hypoproteinaemia is the deficiency of protein in the plasma, partly due to excessive excretion or muscle wasting. The induction of alloxan in Wistar rats has displayed this effect, as illustrated by the significant decrease in the total serum proteins. This was reversed by consumption Artocarpus altilis fruit based-diet by the diabetic rats.
Albumin which is manufactured by the liver can be used to assess the health status of liver. It is the major protein present within the blood. Low serum albumin has also been associated with low protein intake. Albumin serves in the maintenance of osmotic pressure of the blood and body fluids, and transport of inorganic anions, fatty acids, and drugs [38]. Therefore, decrease in serum albumin level would affect the metabolism of these substances that are transported by it [39]. Any effect that negatively affects albumin content would be expected to have a deleterious impact on total plasma proteins as in massive hepatic necrosis, chronic cirrhosis and other disorders with significant destruction or replacement of liver cells. In this study there was an observable depletion of albumin in the diabetic untreated rats fed with yam flour based-diet when compared to the control rats; this is in agreement with hypoalbuminemia observed in diabetes [40]. Hypoalbuminemia is a common problem in diabetic animals and is generally attributed to the presence of diabetic nephropathy. However diabetic rats fed with yam flour based-diet administered with metformin and diabetic rats fed with Artocarpus altilis fruit based-diet showed a significant (p < 0.05) increase in the concentration level of albumin (Table 5).
Also, this study shows a significant decrease (p < 0.05) of globulin in the Artocarpus altilis diabetic untreated rats. This may due to decrease in total protein and albumin levels in diabetic rat. Previous studies have reported that induction of alloxan have a negative effect on the serum total proteins, albumin and globulin leading to a decrease in their concentration [41]. Whereas, consumption of Artocarpus altilis fruit based-diet by diabetic rats tend to normalize the decrease in serum total protein, albumin and globulin levles.
Bilirubin is a waste product, which is the principle pigment in bile. It is derived from the breakdown of haemoglobin. After several degradation steps, the free bilirubin becomes bound by albumin and is transported through the blood to the liver. It is removed from the blood by the liver; hence it is a good indicator of liver function. A low concentration of bilirubin is found in normal plasma hence elevated bilirubin concentration in the blood is either by increased production of bilirubin or decreased liver uptake (as a result of liver disease) or due to an abnormally high peripheral breakdown of haemoglobin, termed haemolysis. Cheesebrough [42] reported that a rise in the concentration of serum bilirubin indicate or suggests liver damage since the liver serves as an excretory unit rather than a distributing unit for bilirubin. Total billirubin and conjugated billirubin are formed through breakdown of red blood cells by hepatocytes and used to access extent of hepatocellular damage [43].
In this study, it was observed that diabetic rats fed with Artocarpus altilis fruit based-diet demonstrated significant reduction in the levels of total and conjugated bilirubin (Table 6). It has been reported that when a toxicant is induced it leads to hyperbilirubinaemia (high level of bilirubin) which is often the first and sometimes the only manifestation of a liver disease [44] characterized with diabetes mellitus.
Serum creatinine and urea are useful clinical tool in assessing renal function. They are established markers of glomerular filtration rate (GFR). Though creatinine is a more sensitive index of kidney function compared to urea level. This is because creatinine fulfils most of the requirements for a perfect filtration marker [45]. Creatinine is also helpful in recognizing when there is an acute drop in kidney function, thus creatinine is used in monitoring disease progression [46].
With respect to kidney function, serum creatinine and urea elevation are evidence of acute kidney dysfunction [47]. Researcher also posited that high urea levels in diabetes could be attributed to a fall in the filtering capacity of the kidney thus leading to accumulation of waste products within the system [48]. The result of this work (Table 6) suggests that Artocarpus altilis fruit based-diet may have little or no nephrotoxic substances. The significant increase observed in the serum creatinine and urea levels of diabetic untreated rats might due to increase synthesis from the damaged pancreatic cells caused by reactive oxygen species generated by alloxan induction.
Lipid peroxidation is a characteristic of diabetes. The increase of free radicals in diabetic condition is suggested to be due to increase lipid peroxidation [49] and damage of antioxidant defence system as a result of increased production and/ or decreased activities of antioxidant enzymes [50]. Elevated lipid peroxide is commonly based on concentration of thiobarbituric acid reactive substances (TBARS) and decreased activity of antioxidant molecules in diabetic rats could probably be associated with oxidative stress and decreased antioxidant defence potential [51].
In the present study, as shown in Table 7, Artocarpus altilis fruit based-diet was found to reduce levels of lipid peroxidative damage in diabetic rats. This may be attributed to antioxidative potential of the diet. The increase in lipid peroxidation was observed in alloxan-induced diabetic groups due to the generation of free radicals [52].
It is well known that SOD, CAT and GPx (Table 8) play an important role as protective enzymes against free radical formation in tissue [53]. SOD protects tissues against oxygen free radicals by converting the superoxide radical into hydrogen peroxide and molecular oxygen, while catalase catalyses the detoxification of hydrogen peroxide, preventing damage to cell membranes and other biological structures. Consumption of antioxidants rich diet might stimulate cell survival by strengthening the defence systems and also exerting scavenging effects on the generated free radicals thereby protecting the cells [59]. This may be the reason behind significant increase in all the antioxidant enzymes activities of diabetic rats fed on Artocarpus altilis fruit based-diet. The superoxide radicals produced during diabetes mellitus damage the essential components of extracellular matrix, lipoproteins and DNA. The activity of the SOD decreased significantly in the liver of alloxan-induced diabetic animals due to increased oxidative stress.
The histopathology evaluation of the liver and kidney shows that the liver of the diabetic untreated rats fed with yam flour based-diet was markedly damaged, probably due to oxidative damage by alloxan induction while diabetic rats fed on Artocarpus altilis fruit based-diet demonstrated normal liver and kidney structure, which compared favourably with normal control. The protective effect of the diet as observed in the result may be linked to its antioxidants.
5Conclusion
From the results of this study, it can be deduced that diabetic rats fed with Artocarpus altilis fruit based-diet produces heptoprotective and renoprotective effects. It did not have any adverse effects on the liver and kidney functions and protected the organs against oxidative stress by altering the increased levels of lipid peroxidation and enhanced activities of CAT, SOD and GPx. Therefore, Artocarpus altilis based-diet may be useful in the management of diabetes mellitus.
Ethical Approval
All applicable Afe Babalola University guidelines for the care and use of animals were properly followed (ABUAD/034).
Funding
This research work was self sponsor.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to use this opportunity to appreciate the technologists in Chemical Sciences Laboratory for their support during this experiment.
References
[1] | William S Jr . medicinenet.com/diabetesmellitus/article.htm; (2013) . |
[2] | Brownlee M . Biochemistry and molecular cell biology of diabetic complications. Nature. (2001) ;414: :813–820. |
[3] | Virella Lopes MF , Virella G . The role of immune and inflammatory processes in the development of macrovascular disease in diabetes. Fron Biosci. (2003) ;8: :750–768. |
[4] | Diabetes Research and Wellness Foundation; (2007) . |
[5] | Kumar PJ , Clark M . Textbook of Clinical Medicine. London: Saunders; (2002) . |
[6] | Wild S , Roglic G , Green A , Sicree R , King H . Global prevalence of diabetes: estimates for 2000 and projections for 2030. Diabetes Care. (2004) ;27: (5):1047–1053. |
[7] | Adesokan AA , Akanji MA , Adewara GS . Evaluation of hypoglycaemic efficacy of aqueous seed extract of aframomum melegueta in alloxan-induced diabetic rats. Sierra Leone Journal of Biomedical Research. (2010) ;2: :91–94. |
[8] | Ajiboye BO , Ojo OA . Effect of Aqueous Leaf Extract of Senecio biafrae on Hyperglycaemic and Haematological parameters of Alloxan-induced Diabetic rats Journal of Pharmacology. (2014) ;3: :163–169. |
[9] | Calixto JB . Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicine (Phytotherapeuticagents). Braz J Med Biol Res. (2000) ;33: :179–189. |
[10] | WHO. WHO news: Traditional medicine strategy launched. Bull. World Health Organization. (2002) ;80: :610–610. |
[11] | Iweala EEJ , Oludare FD . Hypoglycemic effect, biochemical and histological changes of spondias mombin linn and parinari polyandra benth seeds ethanolic extracts in alloxan-induced diabetic rats. Journal of Pharmacology and Toxicology. (2011) ;6: (2):101–112. |
[12] | Adesokan AA , Akanji MA , Aderibigbe A . Serum glucose and lipid levels in alloxan-induced diabetic rats following oral administration of aloe barbadensis miller juice extract. Trop J Health Sci. (2006) ;13: :11–14. |
[13] | Deivanai S , Subhash JB . Breadfruit (Artocarpus altilis Fosh) – an underutilized and neglected fruit plant species. Middle-East Journal of Scientific Research. (2010) ;6: :418–428. |
[14] | Reitman S , Frankel S . A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. (1957) ;28: :56–63. |
[15] | Wright PJ , Plummer DT , Leathwood PT . Enzyme in rat urine. Alkaline phosphatase. Enzymologia. (1972) ;42: :317–327. |
[16] | Doumas BT , Watson WA , Biggs HC . Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chem Acta. (1971) ;31: :87–96. |
[17] | Gornall AG , Bardawill CJ , David MM . Determination of serum proteins by means of the biuret reagent. J Clin Med. (1949) ;177: :51–66. |
[18] | Sherlock S . Liver disease (determination of total and direct bilirubin, colorimetric method). Churchill, London. (1951) ; p. 204. |
[19] | Bartels H , Bohmer MHC . Serum creatinine determination without protein precipitation. Clinica Chemica Acta. (1972) ;37: :193–197. |
[20] | Fawcett JK , Scott JE . A rapid and precise method for the determination of urea. American Journal of Clinical Pathology. (1960) ;13: :156–159. |
[21] | Varshey R , Kale RK . Effect of calmodulin lipid peroxidation in microsome. Int J Radiat Biol. (1990) ;58: :733–743. |
[22] | Adam-Vizi V , Seregi A . Receptor independent stimulatory effect of noradrenaline on Na, K-ATPase in rat brain homogenate. Role of lipid peroxidation. Biochemical Pharmacology. (1982) ;34: :2231–2236. |
[23] | Sinha KA . Colorimetric assay of catalase. Anal Biochem. (1971) ;47: :389–394. |
[24] | Misra HP , Fridovich I . The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem. (1972) ;247: :3170–3175. |
[25] | Rotruck JT , Pope AL , Ganther HE , Swanson AB , Hafeman DG , Hoekstra WG . Selenium: Biochemical role as a component of glutathione peroxidase. Science. (1973) ;179: :588–590. |
[26] | Drury RAB , Wallington EA . Tissue histology In: Carleton’s Histological Technique 4th ed. Oxford University Press, New York. (1973) ; p. 58. |
[27] | Zar JH . Biostatistical analysis. Prentice-Hall Inc, USA. (1984) ; pp. 620. |
[28] | Fishman WH . Acid phosphatase activity. Journal of Biological Chemistry. (2006) 89–92. |
[29] | Adesokan AA , Akanji MA . Effect of administration of aqueous extract of Enantia chlorantha on the activities of some enzymes in the small intestine of rats. Nig J Biochem Mol Biol. (2004) ;18: :103–105. |
[30] | Szasz G . A kinetic photometric method for serum gammaglutamyl trans peptidase. Clinical Chemistry. (1969) ;22: :124–136. |
[31] | Cotran RS , Kumar V , Robbins SL . Robbins pathologic basis of disease. 4th ed. Philadelphia: Saunders, (1989) , p. 817 . |
[32] | Ngaha EO . Renal effects of potassium dichromate in the rat: composition of urinary excretion with corresponding tissue pattern. Gen Pharmacol. (1981) ;12: :291–358. |
[33] | Poli G , Albano E , Dianzani M . The role of lipid peroxidation in liver damage. Chem Phys Lipids. (1987) ;45: :117–142. |
[34] | Navarro P , Durrens P , Aigle M . Protein-protein interaction between the gene products of Saccharomyces cerevisiae. Biochim Biophys Acta. (1343) 187–192. |
[35] | Sanjiv C . The liver book. A comprehensive guide to diagnosis, treatment and recovery. Atria jimcafe company; (2002) . |
[36] | Deivanai S , Subhash JB . Artocarpus Altilis fruit (Artocarpus altilis Fosb.) – An underutilized and neglected fruit plant species. . Middle-East J Sci Res. (2010) ;6: (5):418–28. |
[37] | Bogin E , Marom M , Levi Y . Changes in serum, liver and kidneys of cisplatin-treated rats: Effects of antioxidants. Eur J Chem Clin Biochem. (1994) ;32: :843–851. |
[38] | Brunt EM , Janney CG , Di-Bisceglie AM , Neuschwander-Tetri BA , Di-Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. (1999) 2467–2474. |
[39] | Pasternak CA . An introduction to Human Biochemistry. (1979) ;18–25. |
[40] | Porte D , Halter JB . Textbook of endocrinology, edited by Williams R H. (W.B. Saunders Co., Philadelphia). (1981) ; pp. 716–843. |
[41] | Sumana G , Suryawanshi SA . Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rat. Indian J Exp Biol. (2001) ;39: :748–759. |
[42] | Cheesbrough M . Medical Laboratory Manual for Tropical Countries. Vol. 1: . EESB, Cambridge, UK, 2nd edition; (1992) . |
[43] | Paliwal A , Gurjar RK , Sharma HM . Analysis of liver enzymes in albino rat under stress of l-cyhalothrin and nuvan toxicity; (2009) . |
[44] | Nuhu AA , Aliyu R . Effects of cassia occidentalis aqueous leaf extract on biochemical markers of tissue damage in rats. Trop J Pharm Res. (2008) ;7: (4):1137–1142. |
[45] | Perrone RD , Madias NE , Levey AS . Serum creatinine as index of renal function. Clin Chem. (1992) ;38: :1933–1953. |
[46] | Mitch WE , Walser M . Nutrition Therapy of Uremic Patients. In; The Kidney, Brenner B.M. And Rector F.C. (Eds). Saunders Philadelphia. (1986) ; pp. 1759–1790. |
[47] | Saumya RP , Satyaranjan M , Sabuj S , Prasana KP . Nephroprotective effect of Bauhinia variegata (Linn). whole stem extract against cisplatin-induced nephropathy in rats. Indian J Pharmacol. (2011) ;43: (2):200–202. |
[48] | Idonije BO , Oloruntoba F , Olarewaju MO . Plasma glucose, creatinine and urea levels in Type 2 Diabetic patients attending a Nigerian teaching hospital. Research Journal of Medical Sciences. (2011) ;5: (1):1–3. |
[49] | Baynes J . Role of oxidative stress in the development of complications in diabetes. Diabetes. (1991) ;40: :405–412. |
[50] | Moussa SA . Oxidative Stress in Diabetes Mellitus. Romanian J Biophys. (2008) ; 18: (3), 225–236. Bucharest. |
[51] | Rosa EVC , Valgas C , Souza-Sierra MM , Correa AXR , Radetski CM . Biomass growth, micronucleus induction, and antioxidant stress enzyme responses in Vicia faba exposed tocadmium in solution. Environ Toxicol Chem. (2003) ;22: (3):645–649. |
[52] | Burade KB , Kuchekar BS . Antidiabetic activity of Madhunashimi in alloxan induced diabetes mellitus. J cell tissue Res. (2011) ;11: :2515–2520. |
[53] | Maruthupandian A , Mohan VR . GC-MS analysis of ethanolic extract of Wattakaka volubilis (L.f.) Stapf. Leaf. International Journal of Phytomedicine. (2011) ;3: :59–62. |
[54] | Dallatu MK , Anoya PO , Bilbis LS , Mojiminiyi FBO . Antioxidant macronutrient potentials in strengthening the antioxidant defence in alloxan induced diabetic rats. Nig J Pharm Sci. (2009) ;8: :89–94. |
[55] | Mitch WE . Measuring the Rate of Progression of Renal Insufficiency In: Contemporary Issues of Nephrology Progressive Nature of Renal Disease, Mitch W.E., Brenner B.M. and Stein J.H., (Eds). Vol. 14: , Churchill livingstone, New York; (1986) ; pp. 167–187. |
Figures and Tables
Fig.1
Breadfruits (Artocarpus altilis fruit).

Fig.2
Changes in histology of Alloxan-Induced Diabetic Rats livers. Arrows: shows portal congestion, periportal cellular infiltration, and vacuolar degeneration of hepatocytes.
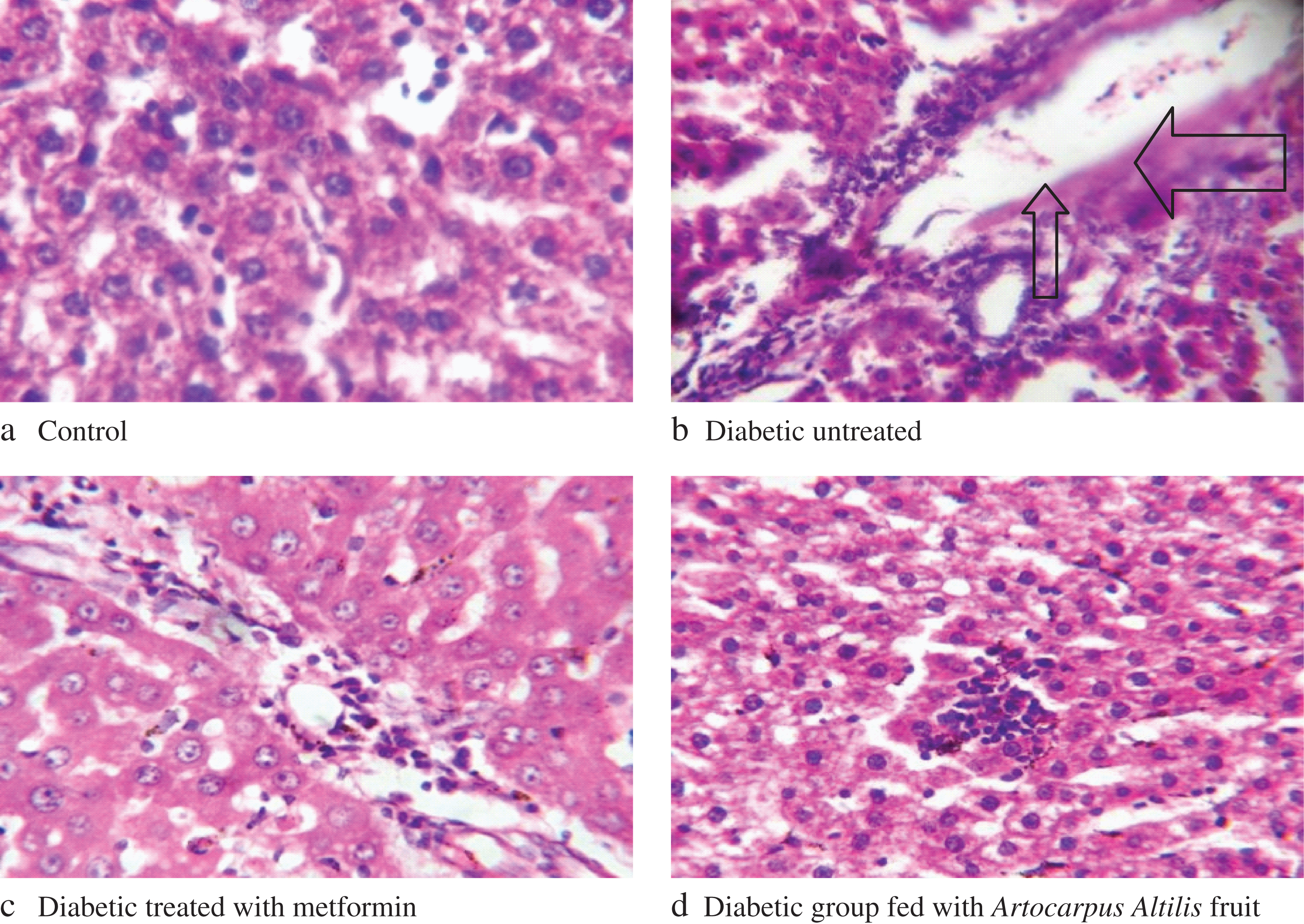
Fig.3
Changes in histology of Alloxan-Induced Diabetic Rats kidneys. Arrows: shows portal congestion, periportal cellular infiltration, and vacuolar degeneration of nephrocytes.
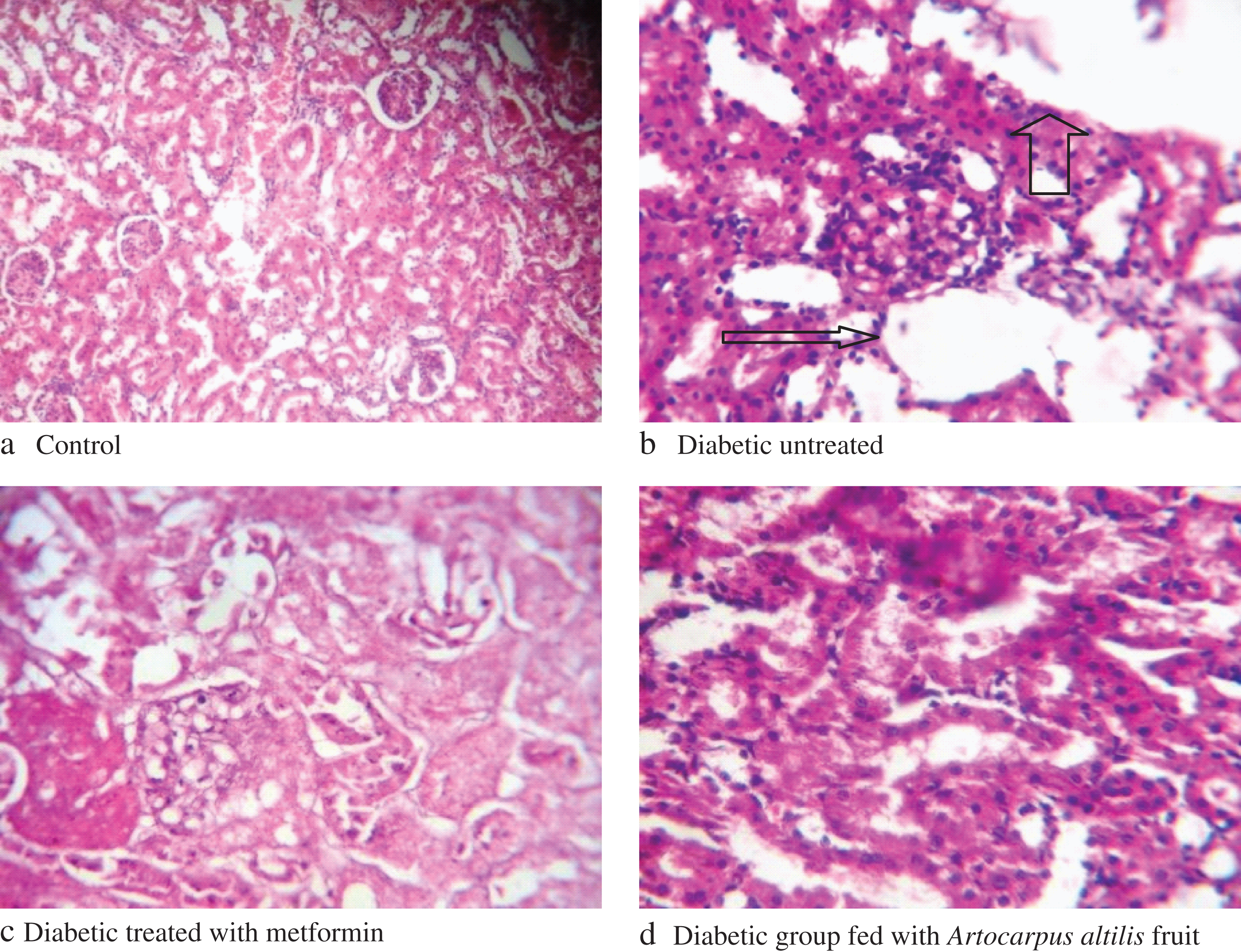
Table 1
Composition (g/100 g) of the Formulated Diets
| Ingredients | Control | Group 1 | Group 2 | Group 3 |
| Artocarpus altilis fruit | – | – | – | 57.6 |
| Yam flour | 57.6 | 57.6 | 57.6 | – |
| Cellulose | 6 | 6 | 6 | 6 |
| Soya beans | 25 | 25 | 25 | 25 |
| Soya beans oil | 6 | 6 | 6 | 6 |
| *Vit/Minerals | 5 | 5 | 5 | 5 |
| D-methonine | 0.4 | 0.4 | 0.4 | 0.4 |
*Vitamin/ Mineral mix: Vitamin A 4,000,000 i.u; Vitamin D3, 800,000 i.u; Tocopherois, 400 i.u; Vitamin K3 800 mg, Folacin, 200 mg; Thiamine, 600 mg; Riboflavin 1,800 mg; Niacin, 6000 mg; Calcium pathothenate, 4 mg; Biotin, 8 mg; Manganese, 30,000 mg, Zinc, 20,000 mg; Iron, 8,000 mg; Choline chloride 80,000 mg; Copper, 2,000 mg; Iodine, 480 mg; Cobalt, 80 mg; Selenium, 40 mg; BHT, 25,00 mg.
Table 2
Effect of Artocarpus altilis Fruit Based-diet on ALT Activity (U/L) in Serum and Tissues Homogenates of Alloxan-Induced Diabetic Rats
| Groups | Serum | Liver | Kidney |
| A | 23.12 ± 0.12a | 200.10 ± 0.10a | 60.10 ± 0.01a |
| B | 38.40 ± 0.05b | 600.00 ± 2.00b | 100.00 ± 1.10b |
| C | 23.40 ± 0.50a | 204.00 ± 0.05a | 64.10 ± 1.10a |
| D | 23.20 ± 0.10a | 203.00 ± 0.12a | 62.10 ± 1.00a |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
Table 3
Effect of Artocarpus altilis Based-diet on AST Activity (U/L) in Serum and Tissues Homogenates of Alloxan-Induced Diabetic Rats
| Groups | Serum | Liver | Kidney |
| A | 13.84 ± 1.45a | 100.11 ± 1.10a | 40.21 ± 0.05a |
| B | 27.21 ± 0.01b | 400.14 ± 0.11b | 84.21 ± 0.10b |
| C | 14.02 ± 1.64a | 108.00 ± 0.20a | 44.10 ± 0.01a |
| D | 14.28 ± 1.12a | 104.20 ± 0.50a | 42.98 ± 0.10a |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
Table 4
Effect of Artocarpus altilis Fruit Based-diet on ALP Activity (UI) in Serum and Tissues Homogenate of Alloxan-Induced Diabetic Rats
| Groups | Serum | Liver | Kidney |
| A | 100.66 ± 1.01a | 430.00 ± 2.01a | 826.00 ± 2.01a |
| B | 240.08 ± 0.10b | 602.00 ± 1.34b | 928.00 ± 1.20b |
| C | 104.01 ± 1.10a | 426.00 ± 3.10a | 821.00 ± 1.20a |
| D | 102.02 ± 0.10a | 428.00 ± 2.14a | 824.00 ± 2.00a |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
Table 5
Effect of Artocarpus altilis Fruit Based-diet on Albumin, Total Protein, Globulin, Total and Conjugated Bilirubin in serum of Alloxan-Induced Diabetic Rats
| Groups | Albumin(g/l) | Total protein(g/dl) | Globulin(g/l) | Total Bilirubin(mg/dl) | Conjugated |
| Bilirubin(mg/dl) | |||||
| A | 26.40 ± 0.02a | 54.62 ± 1.14a | 28.22 ± 0.05a | 14.80 ± 0.06a | 4.20 ± 1.01a |
| B | 10.50 ± 0.05d | 21.30 ± 1.10d | 10.80 ± 0.03d | 28.75 ± 2.40b | 12.16 ± 2.30c |
| C | 28.20 ± 1.00b | 73.20 ± 4.10b | 45.00 ± 0.06b | 15.40 ± 1.80a | 5.84 ± 1.40b |
| D | 32.40 ± 1.03c | 96.40 ± 3.10c | 64.00 ± 2.10c | 14.95 ± 1.20a | 5.28 ± 1.00b |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
Table 6
Effect of Artocarpus altilis fruit Based-diet on Creatinine and Urea in Serum of Alloxan-Induced Diabetic Rats
| Groups | Creatinine (mg/dl) | Urea (mg/dl) |
| A | 45.10 ± 2.00a | 12.03 ± 1.10a |
| B | 60.40 ± 2.50d | 28.00 ± 0.13d |
| C | 48.40 ± 0.05c | 19.04 ± 2.01c |
| D | 42.40 ± 2.40b | 14.10 ± 1.40b |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
Table 7
Effect of Artocarpus altilis Fruit Based-diet on Lipid Peroxidation (MDA) (×10–5 nmol/ml) of Alloxan-Induced Diabetic Rats
| Groups | Liver | Kidney |
| A | 1.08 ± 1.10a | 0.06 ± 1.50a |
| B | 4.89 ± 2.10c | 1.63 ± 2.00c |
| C | 1.10 ± 2.01a | 0.12 ± 1.40b |
| D | 1.06 ± 1.50a | 0.08 ± 1.00a |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.
Table 8
Effect of Artocarpus altilis Fruit Based-diet on antioxidant enzymes in Liver and Kidney of Alloxan-Induced Diabetic Rats
| Groups | CAT (unit/mg protein) | SOD (m/mg protein) | GPx (m/ml) | |||
| Liver | Kidney | Liver | Kidney | Liver | Kidney | |
| A | 342 ± 1.10a | 186 ± 2.10a | 588.41 ± 3.00a | 246.10 ± 1.64a | 429.01 ± 2.00a | 146.00 ± 1.20a |
| B | 142 ± 1.20c | 84 ± 1.01c | 238.00 ± 2.10c | 108.20 ± 2.00c | 120.40 ± 2.50c | 94.20 ± 2.00c |
| C | 268 ± 2.10b | 142 ± 3.10b | 568.00 ± 2.00b | 242.01 ± 1.50b | 401.10 ± 2.00b | 142.00 ± 2.00b |
| D | 340 ± 5.00a | 182 ± 4.00a | 584.00 ± 4.20a | 248.00 ± 2.50a | 426.00 ± 3.00a | 144.01 ± 2.00a |
Each value is a mean of seven determination ± SEM. Values with different superscripts along the column are significantly different (p < 0.05). A: Control (rats) fed with yam flour based-diet. B: Diabetic untreated rats fed with yam flour based-diet. C: Diabetic rats fed with yam flour based-diet and administered metformin daily. D: Diabetic rats fed with Artocarpus altilis fruit based-diet.




