Perspectives on Neoadjuvant Clinical Trial Participation for Patients with Kidney Cancer: A Survey-Based Examination
Abstract
Background:
Kidney cancer is amongst the deadliest genitourinary malignancies. Neoadjuvant systemic therapy has the potential to improve survival and overall outcomes in select patients. Enrolling patients in trials of neoadjuvant treatment for kidney cancer is challenging, which limits neoadjuvant treatment development.
Objective:
This study aims to develop a better understanding of the barriers patients face in kidney cancer clinical trial participation, with a particular focus on neoadjuvant trials for renal cell carcinoma.
Methods:
From 2022–2023, we recruited participants with a history of kidney cancer through a Qualtrics survey that was sent to the Kidney Cancer Association (KCA) and Kidney Cancer Cure (KCCure) mailing lists and social media pages. Patient responses on demographics, clinical information, and perspectives were evaluated.
Results:
Ninety-four individuals completed the survey. Eighty-one percent of respondents reported not participating in clinical trials due to not being informed about potential applicable trials. Importantly, many (76%) respondents reported that prevention of cancer return was a highly important reason to participate in clinical trials. Most respondents reported a willingness to undergo a kidney biopsy (59%), and/or additional appointments (58%) and surgery delays.
Conclusions:
Increased patient awareness about clinical trials with the potential to delay cancer recurrence may increase patient participation in clinical trials. Clinical trial design, including additional appointments or interventions and/or minor surgery delays are not major barriers to trial participation.
INTRODUCTION
Kidney cancer is one of the deadliest urinary tract malignancies [1]. Research is therefore critical to identify potential novel treatment strategies. Neoadjuvant therapy has been demonstrated in multiple cancer types to improve cancer-specific and overall survival, and improve patients’ quality of life [2, 3]. This benefit is especially apparent for patients who have cancer types in which adjuvant therapy also has a role [3]. This treatment paradigm has the potential to decrease recurrence for patients with locally advanced kidney cancer, but more clinical trials are required to effectively optimize treatment protocols.
However, while some groups have been successful, for many, enrolling patients for trials on neoadjuvant treatment for locally advanced kidney cancer has historically been challenging, and retention of patients on these trials poses additional difficulties [4–6]. One prior study on neoadjuvant therapy in locally advanced clear cell renal cell carcinoma undergoing nephrectomy had to close the trial early due to slow patient accrual [7]. The current literature does not address challenges to recruitment and retention for kidney cancer trials, which is critical for designing clinical trials to measure the efficacy of neoadjuvant therapy.
We therefore performed a survey of patients with a history of kidney cancer to better understand barriers for kidney cancer trial participation, with a particular focus on neoadjuvant trials for renal cell carcinoma. By better understanding the historic challenges from patients’ perspectives, we can improve trial design and recruitment efforts.
METHODS
Participant recruitment
The Kidney Cancer Association (KCA) and Kidney Cancer Cure (KCCure) maintain mailing lists of patients with a history of kidney cancer, family members of patients with kidney cancer and others with an interest in kidney cancer. Participants were recruited through these mailing lists via email, in addition to social media posts from KCA’s account. The mailing lists mostly include individuals in North America, with a much smaller international component. All participants consented in English to study participation, which was obtained by the participant’s voluntary response to the emailed survey. Survey data were collected from May 2022 to May 2023. All survey responses were confirmed to have come from unique IP addresses to control for duplicate responses, but a kidney cancer diagnosis was not confirmed by the research team. This study was deemed exempt by the Institutional Review Board of Duke University (Pro00110499). The data is presented in accordance with STROBE guidelines.
Study design and measures
The study team developed the survey questions; we based these questions on an expert panel discussion of challenges of neoadjuvant trials presented and discussed at the annual International Kidney Cancer Symposium in November 2021. Patient advocates then reviewed and provided input on the study content and questions prior to posting the survey on Qualtrics, a secure online data collection platform [8].
The survey (Appendix A) consisted of a total of 22 single- and multiple-select and open-ended questions. Information collected included the following: patient demographics (gender, race, ethnicity, age at the time of diagnosis), cancer type and stage, cancer course and treatment, prior clinical trial participation, reason(s) for participating in clinical trials, and factors that increase likelihood of participating in clinical trials. Participants were eligible for the study if they had ever received a diagnosis of kidney cancer, including metastatic.
Statistical analysis
All data were analyzed using R version 4.1.2 (The R Foundation for Statistical Computing). Participant demographics and clinical characteristics were summarized using N (%) for categorical data and continuous variables were represented as median (interquartile range/IQR). Differences were tested using a two-proportion z test, where appropriate. All figures and tables were generated based on count data from the survey results. Missing data was counted as “unknown”.
RESULTS
A total of 123 participants opened the study link, of whom 94 met the inclusion criteria. Table 1 demonstrates participant demographics. The majority of participants were women (66%, n = 62), white (84%, n = 79), and non-Hispanic or Latino (72%, n = 68). The most common age at the time of diagnosis was 50–59 (31%, n = 29), followed by 40–49 (27%, n = 25). The timing of cancer diagnoses ranged from 2000 to 2022, with the median time between kidney cancer diagnoses and survey response being 17 months (IQR 6–57 months). The vast majority of participants stated that they had localized cancer at the time of diagnosis (80%, n = 75) and most participants reported a diagnosis of clear cell renal carcinoma (64%, n = 60). Many participants reported having their kidney partially or completely removed by surgery (95%, n = 89), and few participants reported a recurrence of their kidney cancer (15%, n = 14).
Table 1
Participant demographics and clinical characteristics of kidney cancer in a study on views on kidney cancer trial participation
| Characteristic | N = 94 |
| Months between diagnosis and survey – median (IQR) | 17 (6–57) |
| Age at time of diagnosis | |
| 30–39 | 14 (15%) |
| 40–49 | 25 (27%) |
| 50–59 | 29 (31%) |
| 60–69 | 20 (21%) |
| 70–79 | 4 (4.3%) |
| Unknown | 2 (2.1%) |
| Gender | |
| Female | 62 (66%) |
| Male | 30 (32%) |
| Unknown | 2 (2.1%) |
| Race | |
| White | 79 (84%) |
| Black or African American | 4 (4.3%) |
| Asian | 3 (3.2%) |
| Mixed Race | 3 (3.2%) |
| Other | 2 (2.1%) |
| Prefer not to say | 1 (1.1%) |
| Unknown | 2 (2.1%) |
| Ethnicity | |
| Hispanic or Latino | 5 (5.3%) |
| Not Hispanic or Latino | 68 (72%) |
| Mixed ethnicity | 3 (3.2%) |
| Other | 11 (12%) |
| Prefer not to answer | 2 (2.1%) |
| Unknown | 5 (5.3%) |
| Clinical stage at the time of diagnosis | |
| Localized | 75 (80%) |
| Lymphatic spread | 5 (5.3%) |
| Metastatic | 3 (3.2%) |
| Other | 7 (7.4%) |
| Unknown | 4 (4.3%) |
| Cancer type | |
| Clear cell renal cell carcinoma | 60 (64%) |
| Papillary renal cell carcinoma | 8 (8.5%) |
| Chromophobe renal cell carcinoma | 6 (6.4%) |
| Sarcomatoid renal cell carcinoma | 3 (3.2%) |
| Translocation renal cell carcinoma | 3 (3.2%) |
| Rhabdoid renal cell carcinoma | 1 (1.1%) |
| Other | 6 (6.4%) |
| Don’t know | 2 (2.1%) |
| No biopsy or tumor removed in order to define pathologic tumor type | 5 (5.3%) |
| Unknown | 0 (0%) |
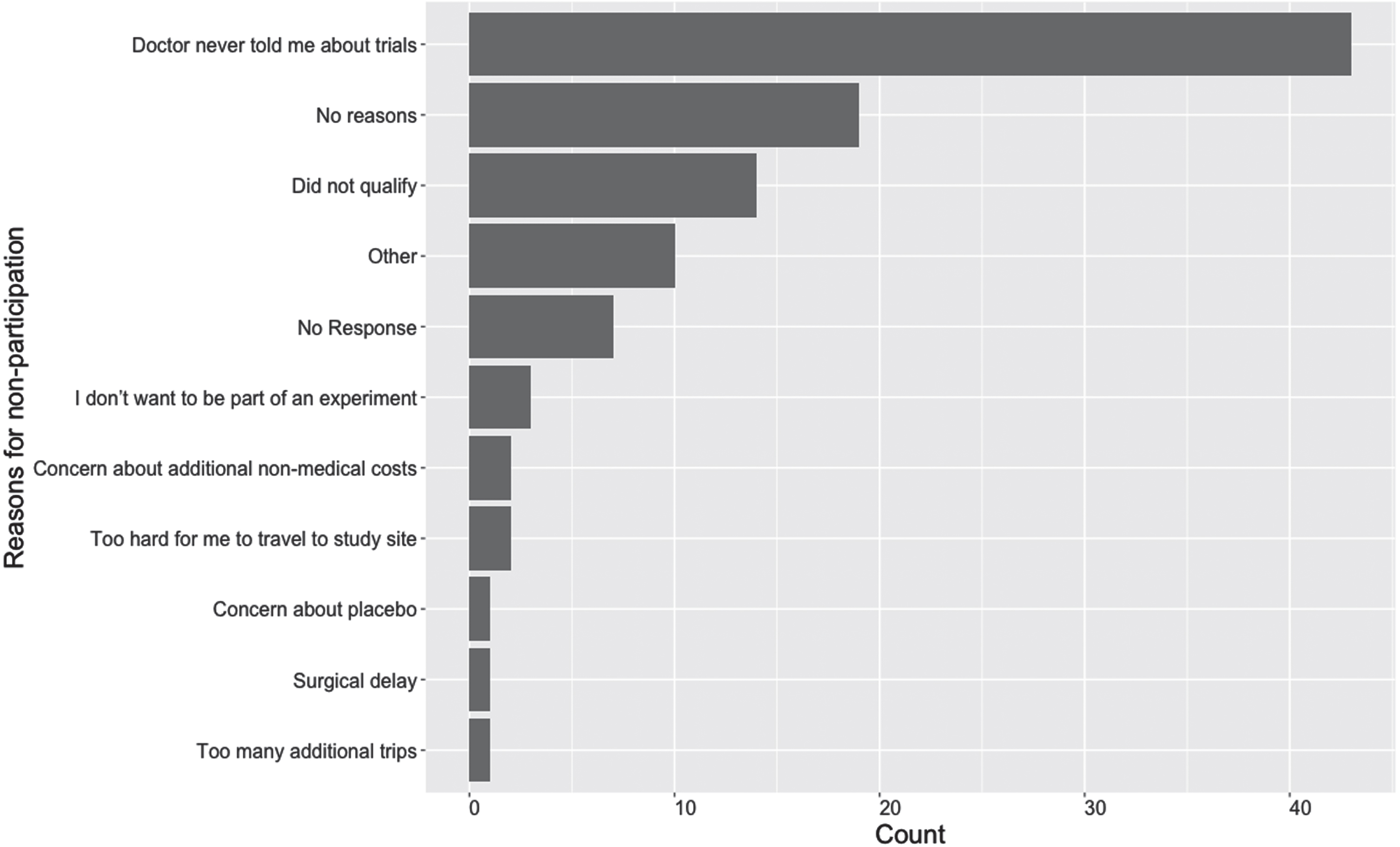
Most participants reported that they had never participated in a clinical trial (81%, n = 76). Figure 1 shows the reasons for non-participation in clinical trials. The most common reason that survey participants reported not participating in a clinical trial was because they were not informed about clinical trials by their physicians (38%, n = 36); this was followed by “no specific reason,” (15%, n = 14) and being told that they did not qualify for a study at the time (13%, n = 12).
Fig. 1
Reasons for prior non-participation in clinical trials (Participants could accept all that apply) in a study on views on kidney cancer trial participation.
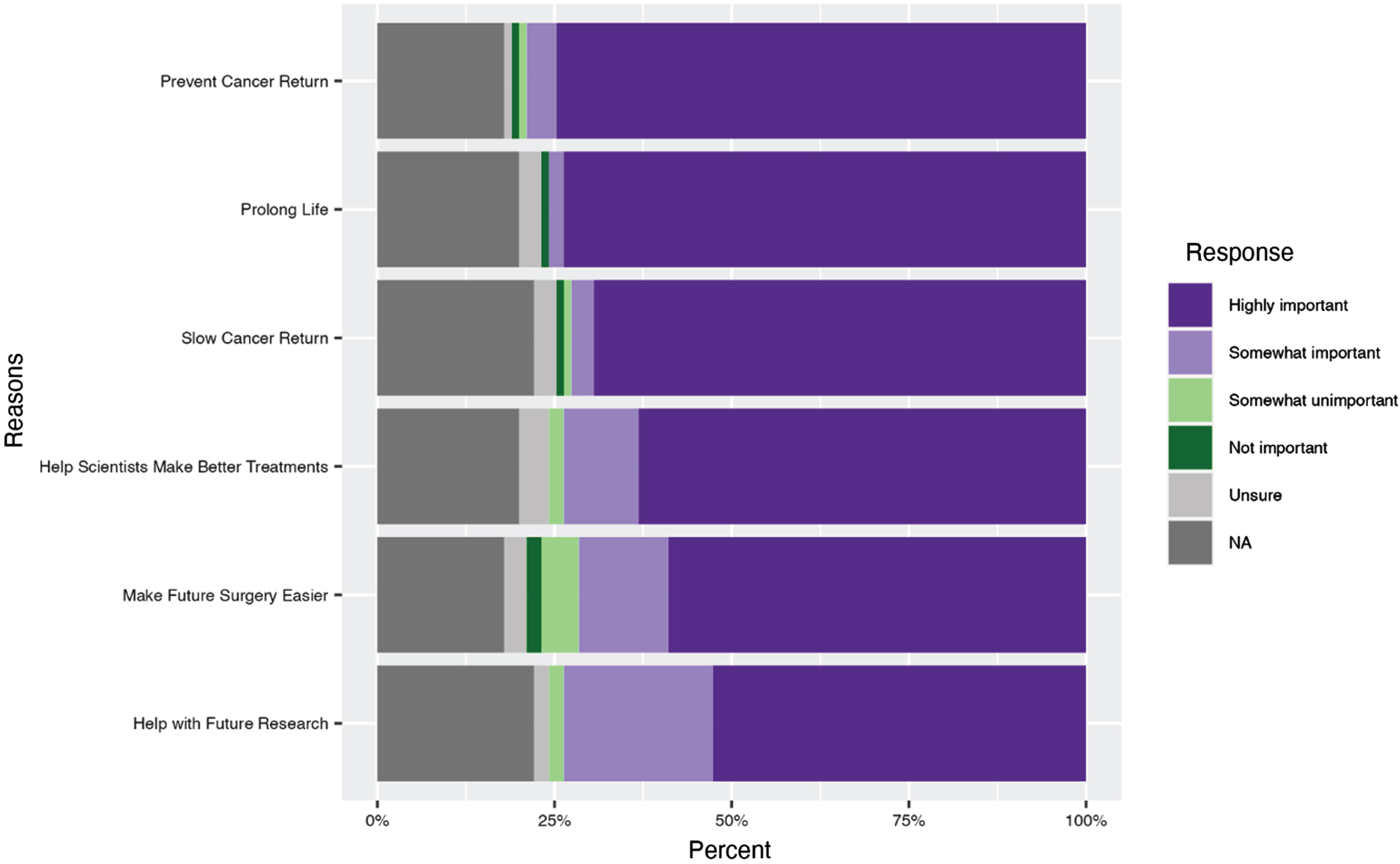
Participants rated the importance of listed criteria when deciding whether to participate in a clinical trial. The majority of survey participants reported high importance for the clinical trial to have the potential to help prevent the return of their own cancer (76%, n = 71) (Fig. 2). Other criteria reported as being highly important included that the study would help the respondent to live longer (74%, n = 70) and help slow the return of their cancer (70%, n = 66). Also important to a slightly lesser extent to survey participants were that the clinical trials help make their surgery easier (60%, n = 56 reported this criterion as highly important) and that they help with treatment for future participants (53%, n = 50 reported this criterion as highly important).
Fig. 2
Reported reasons to participate in a clinical trial in a study on views on kidney cancer trial participation.
There were several facilitators to clinical trial participation. Importantly, 37% of participants stated that they would not consider participating in a clinical trial where they could potentially be treated with a placebo (37%, n = 35) while 18% (n = 17) expressed hesitancy about a clinical trial with a placebo. Similarly, 67% (n = 63) of participants reported that treatment with a new or proven drug was important (highly important or somewhat important). Additional facilitators to trial participation included surgery not being delayed > 2 weeks (34%, n = 32 reporting highly important) or delayed > 4 weeks (38%, n = 36 reporting highly important) (p = 0.57, Appendix B). Only 15% (n = 14) would still participate in a clinical trial that led to a delay (any length of time) in surgery while 27% (n = 25) would not consider a clinical trial that involved any delay in surgery. Additionally, 33% (n = 31) of participants said that it was highly important for extra expenses such as childcare, parking, and time off from work to be covered (Fig. 3). To help increase participation in clinical trials, 84% (n = 63) of participants would want to talk directly with their doctor, 60% (n = 45) would want to talk directly to someone on the research study team, and 51% (n = 38) would want read printouts online.
Fig. 3
Facilitators to clinical trial participation in a study on views on kidney cancer trial participation.
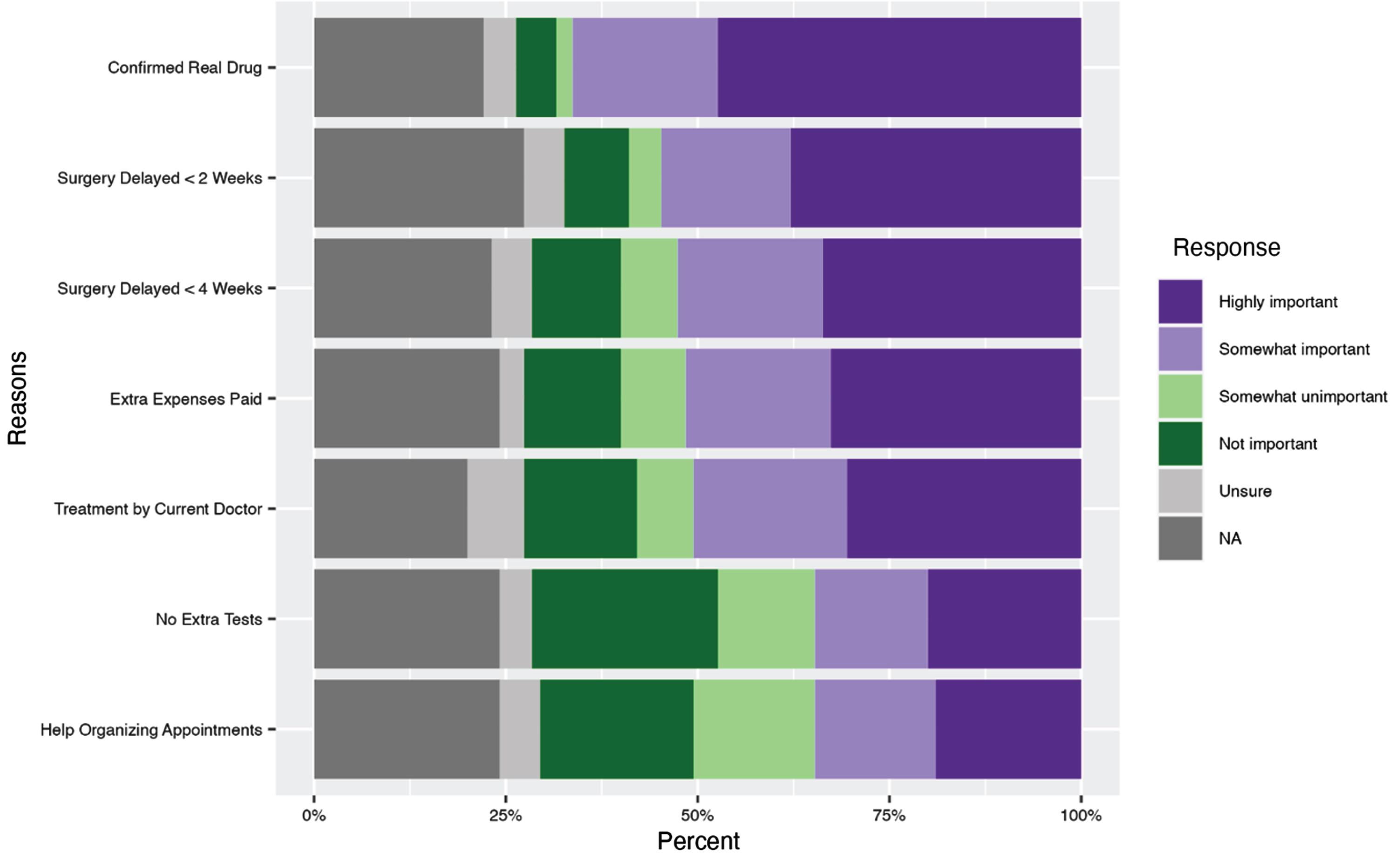
Factors that were reported by survey participants as being less important included the following: having assistance in organizing scheduled appts (19%, n = 18 reported this factor as highly important) and not having additional tests performed (20%, n = 19 reported this factor as highly important). Very few (4.3%, n = 4) participants reported that having to go to additional appointment(s) would prevent them from participating in a clinical trial. Contrary to some previously described expectations, thirty-nine percent of participants (n = 37) stated that they would participate in a clinical trial that required a kidney biopsy, and 20% (n = 19) reported that they would consider participating if a kidney biopsy was required, totaling to 59% of participants willing to undergo a kidney biopsy. About one-third of participants reported that they would travel greater than 50 miles for an appointment to participate in a clinical trial (36%, n = 34) while an additional 22% (n = 21) of participants would consider participating in a clinical trial that required that amount of travel, totaling to 58% of participants willing to travel.
For two of the questions asking about reasons to participate in a clinical trial and facilitators that would make participation easier, participants were able to rate options from highly important to not important (Appendix A). In a sensitivity analysis of the ranking of the percent of participants who considered factors “highly important” versus “important” (highly important and somewhat important combined), there was no difference in the top two factors that were considered the most important (Appendix B).
DISCUSSION
In this study, we used survey data from participants with kidney cancer to examine barriers to clinical trial participation, especially focused on trials examining neoadjuvant systemic therapy for advanced localized kidney cancer. We determined that many survey participants have never participated in a clinical study, with a primary reason of non-participation being that he/she/they had not been informed of any trials. Additionally, prolonged survival was not the most important factor for clinical participation, with participants reporting that delayed return of cancer was a highly important factor. Factors that were lower barriers to participation included additional doctor’s appointments or procedures/tests, additional interventions including a kidney biopsy, and assistance scheduling appointments. Collectively, our findings suggest that there are many areas on which to intervene to improve clinical trial participation.
Our finding of a high proportion of participants never participating in a clinical trial is consistent with prior literature [9]. The most common patient-reported reason for non-participation in this study was not being informed about clinical trials. Not being informed about a clinical trial and/or lack of trial awareness could reflect a physician not informing patients of clinical trials, a clinician not being aware of relevant clinical trials, and/or lack of trial availability at a clinical location where an individual is receiving care. This is especially important to keep in mind for our sample, which consisted of 80% participants who were initially diagnosed with localized disease, for which neoadjuvant kidney cancer trials are generally not available. Despite less than 5% national participation in clinical trials [10], we found most participants were open to participating in clinical trials; therefore, a lack of available trials combined with a lack of patient awareness is a key issue. A gap in clinicians informing participants about clinical trials and/or a lack of patient awareness of clinical trial availability among participants is also consistent with prior findings of little to no differences in willingness to participate in clinical trials across race and ethnicity, despite disparities in actual clinical trial enrollment [11]. Here we show that from the patient’s perspective, not being informed of clinical trials (whether available or not available) was a key barrier.
Our data are also consistent with the current literature which suggests that patients are hesitant to participate in clinical trials in which there may be no added benefit, even though trials involving placebos are still considered ethical as long as not receiving treatment isn’t causing undue harm. [12, 13]. The fact that over one-third of our cohort would never consider participation in a clinical trial involving a placebo elucidates a potential area of improvement in clinical trial recruitment. To increase patient participation in a clinical trial, patients should be informed that placebos are only used in cases where a placebo is not considered worse than the standard of care, like when standard treatment is no drug at all.
Our findings of significant facilitators to clinical trial participation include lack of placebo and extra study expenses paid. Similar to our findings, a prior study demonstrated that cost was an important consideration for clinical trial participation [14]. While additional help organizing appointments or lack of extra tests were not highly cited facilitators of trial participation, this may be highly dependent on individual patient characteristics. For example, patients with multi-morbidity increased healthcare system interactions, time lost, polypharmacy, and difficulty arranging appointments/transportation may be burdensome and contribute to reduced trial participation [15].
Finally, other prior research reports that many participants who were already undergoing a clinically indicated kidney biopsy would agree to additional passes for research. However, only one-fifth of participants in one particular study would agree to a kidney biopsy for research if it was not already clinically indicated [16]. This contradicts our finding that about 59% participants would still consider a clinical trial that requires a kidney biopsy. Part of this difference may be explained by the way that the kidney biopsy was described in the respective studies and by the fact that our study did not specify whether the biopsy would be clinically indicated or not. Having the majority of participants being open to a kidney biopsy for research is a surprising result, which reflects that participants may be willing to inconvenience themselves to a certain extent to participate in a clinical trial. This is further supported by the finding that only 34% of participants reported that it was highly important to not have their surgery delayed by greater than two 2 weeks. This highlights that participants may have other motivating factors, such as the potential to delay recurrence, that increase their interest in clinical trial participation.
Our findings of the most common highly important reasons for participating in clinical trials of preventing cancer return, prolonging life, and slowing cancer return are also consistent with prior research suggesting that the biggest positive indicator for clinical trial participation is personal gain [17]. While personal gain predominated, however, the majority of participants also endorsed altruistic goals (helping scientists to make better treatments, make future surgeries easier, and help with future research). This is particularly relevant in the context of participants being less concerned about other barriers such as a kidney biopsy, driving to appointments, and delayed surgery.
Interestingly, prolonged survival was not the main driver of interest in clinical trial participation; prevention of cancer return was also a major factor. In fact, our data implies that patients may be more concerned with delaying cancer recurrence than improving overall survival. This has particular relevance for clinical trial design and meaningful endpoints; namely, delay to cancer recurrence, even if it does not lead to overall improvements in survival may be a meaningful endpoint. Furthermore, trialists may have to be less concerned with aiming to prove improvements in overall survival in order to recruit study p. This is in accordance with prior work, with one survey-based study showing that participants were more psychosocially impacted by disease-related anxiety and fear of recurrence over fear of dying when managing their renal cell carcinoma. [18] Another study surveying patients with kidney cancer showed that participants were most interested in therapy with the highest chance of eliminating all evidence of disease [19].
Our study has several limitations. First, a large proportion of participants in the study have localized kidney cancer, for whom neoadjuvant treatments are currently not the standard of care, this could contribute to individuals not being made aware of clinical trials. Furthermore, responses could be different when a patient has a > 90% chance of cure with surgery alone, when compared to a patient with stage 3 cancer, which has a recurrence rate of around 50% [20]. Additionally, participants may have been treated in a center where clinical trials were not available. In spite of this, however, the survey was phrased to ask about barriers to trial participation, if a trial was available. Second, the demographics of our respondent population presented were notably younger with more female representation when compared to Center for disease control (CDC) prevalence data [21]. Notably, 4% of our participants were over 70 years old while the CDC reported a figure of about 27%, indicating a lack of participation from older patients in our study. Similarly, 66% of our survey participants identified as female whereas about 37% of the patients in the CDC database were female [21]. These findings, however, are consistent with current literature on survey data, which report measured differences in survey participation based on age and sex [22]. Furthermore, our study includes few minority participants, which is also consistent with current clinical trial participation [23]. Given that this paper aimed to initiate discussions about patient interest in neoadjuvant clinical trials for kidney cancer, these findings are still useful for gathering initial data about patient motivators and obstacles for participation. Third, our study likely includes few participants outside the United States. Trial participation outside of the Unites States has increased dramatically and there are likely differing levels of clinical trial awareness and hesitancy [24]. A concerted effort by the United Kingdom’s government to increase clinical trial awareness greatly increased participation [25]. Fourth, our study potentially under- or over- estimates some of the barriers and facilitators to study participation as our sample was derived from mailings from cancer research and support groups and that the questionnaires were circulated online, which requires stable internet connection. However, our sample still reflect experiences and desires of many patients with kidney cancer. Fifth, when survey participants were asked about treatment with a placebo, they were not specifically asked about being treated with a placebo for a clinical trial where the standard of care is no drug treatment. However, our survey does bring up relevant patient concerns about receiving quality care, which can still help inform providers on how to explain how the use of a placebo in a clinical trial does not mean that they are receiving less than the standard of care. Finally, our study did not confirm that survey participants had a diagnosis of kidney cancer. However, the survey questions were worded in a manner with relevance only to individuals with a kidney cancer diagnosis.
These limitations notwithstanding, our findings have significant implications for cancer research networks and support groups, clinicians and scientists in taking actionable steps to improve trial design and increase clinical trial participation. For cancer research networks and support groups, given the lack of patient awareness of clinical trial eligibility, increasing clinician awareness of current open clinical trials and encouraging clinicians to discuss these trials with their patients will be critical. This could be implemented in the form of a nationally available cheat sheet of renal cell carcinoma trials per stage that urologists can reference in clinic. For clinicians, as many patients are amenable to trial participation, but are unaware of trials and would prefer to speak with their own clinician about participation, it is also important for clinicians to be aware of and discuss applicable clinical trials to increase enrollment. Furthermore, engaging patient community involvement with clinical trial participation may prove beneficial. Additionally, clinicians should better educate patients on placebo trials noting that they are not necessarily offering treatments below the standard of care. For scientists, as many patients are amenable to trial participation, but would prefer to speak with their own clinician about participation, expanding locations has the potential to increase enrollment. It may also be helpful to design neoadjuvant clinical trials with delayed recurrence of disease as opposed to prolonged overall survival. Furthermore, allocating funding for additional trial expenses may prove beneficial. Scientists could also design improved informational packets about the study and the proposed benefits of neoadjuvant treatment to appeal to patients’ hopes for personal treatment gain from clinical trials. It is critical to outline the potential benefits of the clinical trial while ensuring that the patients’ expectations for their personal gain is managed prior to participation. Finally, given the factors that were not considered burdensome, clinical trials should allow for a wider radius of interest and include kidney biopsies if relevant.
In the future, it will be important to expand the survey population, such as surveying in clinics, the community and offering the study in multiple languages. A particular emphasis should be placed on how to recruit and maintain minority patient participation [23]. Discussions with clinical or research teams on how they approach trial participation with patients, and also prior participants of phase II neoadjuvant clinical trials would provide additional insights. By better understanding patient reported barriers to clinical trial participation, we can improve participation and potentially reduce disparities in participation.
ACKNOWLEDGMENTS
The authors would like to thank the KCA and KCCure for helping to recruit participants and for advising on the survey instrument.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
KN participated in performance, interpretation of data, and writing the article. SM participated conception, performance, and writing of the article. IF participated conception, performance, and writing of the article. NH participated conception, performance, and writing of the article. JK participated conception, performance, and writing of the article. PL participated conception, performance, and writing of the article. BS participated conception, performance, and writing of the article. DM participated conception, performance, and writing of the article. LP participated conception, performance, and writing of the article. VM participated conception, performance, and writing of the article. TZ participated in conception, performance, interpretation of data, and writing the article. DK participated in conception, performance, interpretation of data, and writing the article.
CONFLICT OF INTEREST
We disclose that Primo Lara, Jr., a co-author on this paper, is one of the editors of Journal Kidney Cancer. For fairness and transparency purposes, Dr. Primo Lara, Jr. recused him/herself from any editorial decisions related to this submission. Naomi B. Haas and Tian Zhang are Editorial Board Members of this journal, but were not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review. We have no other disclosures.
DATA AVAILABILITY
The data supporting the findings in the paper are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] A copy of the survey filled out by participants is included in the supplementary material.
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-240009.
REFERENCES
[1] | Ferlay J , Soerjomataram I , Dikshit R , Eser S , Mathers C , Rebelo M , et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. (2015) ;136: (5):E359–86. |
[2] | Kasi A , Abbasi S , Handa S , Al-Rajabi R , Saeed A , Baranda J , Sun W . Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer. JAMA Network Open. (2020) ;3: (12):e2030097. |
[3] | Patel SP , Othus M , Chen Y , Wright GP , Yost KJ , Hyngstrom JR , et al. Neoadjuvant–Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. New England Journal of Medicine. (2023) ;388: (9):813–23. |
[4] | Sariaya B . Enhancing Patient Centered Care in Systemic Therapy and Clinical Trials. In: (Chair) C-HLaES, editor. Virtual: International Kidney Cancer Symposium. [Symposium]; 2021, November 5-6. |
[5] | Kadam RA , Borde SU , Madas SA , Salvi SS , Limaye SS . Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res. (2016) ;7: (3):137–43. |
[6] | Stewart GD , Welsh SJ , Ursprung S , Gallagher FA , Jones JO , Shields J , et al. A Phase II study of neoadjuvant axitinib for reducing the extent of venous tumour thrombus in clear cell renal cell cancer with venous invasion (NAXIVA). British Journal of Cancer. (2022) ;127: (6):1051–60. |
[7] | Carlo MI , Attalla K , Mazaheri Y , Gupta S , Yildirim O , Murray SJ , et al. Phase II Study of Neoadjuvant Nivolumab in Patients with Locally Advanced Clear Cell Renal Cell Carcinoma Undergoing Nephrectomy. Eur Urol. (2022) ;81: (6):570–3. |
[8] | Johnson L . Exploring factors associated with pregnant women’s experiences of material hardship during COVID-19: a cross-sectional Qualtrics survey in the United States. BMC Pregnancy and Childbirth. (2021) ;21: (1). |
[9] | Unger JM , Hershman DL , Till C , Minasian LM , Osarogiagbon RU , Fleury ME , Vaidya R . “When Offered to Participate”: A Systematic Review and Meta-Analysis of Patient Agreement to Participate in Cancer Clinical Trials. JNCI: Journal of the National Cancer Institute. (2020) ;113: (3):244–57. |
[10] | Unger JM , Fleury M . Nationally representative estimates of the participation of cancer patients in clinical research studies according to the commission on cancer. Journal of Clinical Oncology. (2021) ;39: (28_suppl):74. |
[11] | Byrne MM , Tannenbaum SL , Glück S , Hurley J , Antoni M . Participation in Cancer Clinical Trials: Why Are Patients Not Participating? Medical Decision Making. (2013) ;34: (1):116–26. |
[12] | Mills EJ , Seely D , Rachlis B , Griffith L , Wu P , Wilson K , et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. (2006) ;7: (2):141–8. |
[13] | Placebo-Controlled Trials and Active-Control Trials in the Evaluation of New Treatments. Part 1: Ethical and Scientific Issues. Annals of Internal Medicine. (2000) ;133: (6):455–63. |
[14] | Williams CP , Geiger AM , Norton WE , De Moor JS , Everson NS . Influence of Cost-Related Considerations on Clinical Trial Participation: Results from the 2020 Health Information National Trends Survey (HINTS). Journal of General Internal Medicine. (2023) ;38: (5):1200–6. |
[15] | Rosbach M , Andersen JS . Patient-experienced burden of treatment in patients with multimorbidity – A systematic review of qualitative data. PLOS ONE. (2017) ;12: (6):e0179916. |
[16] | Moledina DG , Cheung B , Kukova L , Luciano RL , Peixoto AJ , Wilson FP , et al. A Survey of Patient Attitudes Toward Participation in Biopsy-Based Kidney Research. Kidney International Reports. (2018) ;3: (2):412–6. |
[17] | Wright JR , Whelan TJ , Schiff S , Dubois S , Crooks D , Haines PT , et al. Why Cancer Patients Enter Randomized Clinical Trials: Exploring the Factors That Influence Their Decision. Journal of Clinical Oncology. (2004) ;22: (21):4312–8. |
[18] | Giles R , Maskens D , Bick R , Martinez R , Packer M , Heng D , et al. Patient-reported Experience of Diagnosis, Management, and Burden of Renal Cell Carcinomas: Results from a Global Patient Survey in 43 Countries. Eur Urol Open Sci. (2022) ;37: :3–6. |
[19] | KCCure. 2019 Kidney Cancer Patient Survey kccure.org [Available from: https://kccure.org/kidney-cancer-patient-survey-2019-treatment-preferences-patient-reported-outcomes/. |
[20] | Janzen NK , Kim HL , Figlin RA , Belldegrun AS . Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. (2003) ;30: (4):843–52. |
[21] | Prevention CfDC. United States Cancer Statistics: Data Visualizations: U.S. Department of Health & Human Services; 2020 [Available from: https://gis.cdc.gov/Cancer/USCS/#/NationalPrevalence/. |
[22] | Smith W . Does Gender Influence Online Survey Participation? A Record-Linkage Analysis of University Faculty Online Survey Response Behavior. Online Submission. (2008) . |
[23] | Hamel LM , Penner LA , Albrecht TL , Heath E , Gwede CK , Eggly S . Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control. (2016) ;23: (4):327–37. |
[24] | (WHO) WHO. Number of clinical trials by year, country, WHO region and income group (1999–2022) 2023 [Available from: https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/number-of-clinical-trials-by-year-country-who-region-and-income-group. |
[25] | Sinha G . United Kingdom Becomes the Cancer Clinical Trials Recruitment Capital of the World. JNCI: Journal of the National Cancer Institute. (2007) ;99: (6):420–2. |




