Comparison of Oncological and Functional Outcomes of Radical Versus Partial Nephrectomy for cT1b Renal Cell Carcinoma: A Two-Centre, Matched Analysis
Abstract
BACKGROUND:
It remains unclear which patients with cT1b renal cell carcinoma (RCC) benefit most from partial nephrectomy (PN) versus radical nephrectomy (RN) considering oncological outcomes and renal function.
OBJECTIVE:
To compare oncological and functional outcomes of RN with PN for cT1b RCC.
METHODS:
This is a retrospective analysis of patients who underwent RN or PN for cT1b between 2010 and 2022 (n = 241). Patients were grouped by RN or PN and matched by age, sex, Charlson Comorbidity Index, BMI, PADUA score, RENAL score, ASA score, and preoperative kidney function (eGFR) using propensity score matching. The 10-year overall survival (OS), 10-year cancer-specific survival (CSS), and 10-year recurrence-free survival (RFS) were compared. Change in eGFR from baseline to 5-year follow-up was assessed.
RESULTS:
After matching, 100 patients remained in each group for analysis. The 10-year OS, CSS, and RFS rates were similar between groups. For patients classified as low risk, the PN group displayed a higher recurrence rate compared to RN (7 vs. 0, p = 0.01). Patients who underwent RN had worse 1-year postoperative eGFR than PN (RN: 57 [44–65], PN: 73 [60–87], p < 0.001). RN was more likely to induce new-onset chronic kidney disease (CKD) stage ≥3b compared to PN (p < 0.001). Complication rate after PN was significantly higher (p = 0.003).
CONCLUSION:
10-year survival rates were similar, despite more recurrences in the PN group. Our data shows that post-surgical renal function is superior for PN. Nevertheless, RN is a reliable treatment option when preservation of renal function is not a priority.
INTRODUCTION
Renal cell carcinoma (RCC) represents approximately 3% of all malignancies, with nearly 138,611 new cases in Europe in 2020 [1, 2]. Up to 25% of these cases are staged cT1b, with surgical treatment options consisting of partial nephrectomy (PN) or radical nephrectomy (RN) [3, 4]. It remains unclear which patients with cT1b tumours benefit most from PN versus RN considering oncological outcomes and kidney function.
According to European Association of Urology (EAU) guidelines, PN is advised for cT1b tumours when technically feasible, in order to preserve maximum kidney function. PN is recognized to be a more complex and challenging surgery than RN, resulting in a higher postoperative complication rate [5, 6]. Thus, it is important to weigh potential risk factors and perioperative morbidities associated with PN during treatment planning. Moreover, a positive surgical margin (PSM) is discovered in 4–7% of patients after PN, which is correlated with (local) recurrence [7–9]. The occurrence of PSM after RN is rare [10]. To date, there is one randomized controlled trial (RCT) comparing RN with PN which included only tumours ≤5 cm. A lower incidence of renal dysfunction was observed after PN compared to RN [11, 12]. Survival analysis demonstrated comparable cancer-specific survival (CSS) rates for PN vs. RN. Meta-analyses show conflicting results regarding survival rates for PN vs. RN, thus it is difficult to draw definitive conclusions regarding the oncological outcomes of these procedures [13, 14]. Moreover, robust data is still missing regarding kidney function reduction after RN or PN for cT1b tumours. This study aims to compare oncological, operative and functional outcomes for cT1b tumours treated by RN or PN.
METHODS
This study was approved by the review board of both institutions. Data was retrieved from a retrospective database consisting of patients who underwent PN or RN due to clinical suspicion for RCC between 2010 and 2022. Patients with clinical suspicion for RCC staged cT1bN0/xM0/x, who were ≥18 years of age at the time of surgery and underwent elective PN or RN were included. All data was collected retrospectively by reviewing electronic patient medical records and importing data into Castor EDC. Exclusion criteria are listed in Fig. 1. Patient characteristics included age, gender, American Society of Anaesthesiologists (ASA) score, Charlson Comorbidity Index age-adjusted (CCI-A), body mass index (BMI), pre-operative serum creatinine (sCr) and eGFR according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [15]. All patients received preoperative contrast-enhanced CT or MRI for tumour size, TNM classification and nephrometry scores (RENAL score, PADUA score) [16, 17]. Selection for PN or RN was done based on EAU guidelines and patient characteristics. Follow-up check-ups were done depending on the risk group, as reported in the EAU guidelines. The following risk groups were used: low, intermediate and high risk. For patients with clear-cell RCC this was done according to the Leibovich score. For remaining patients the following classification was used: low risk: pT1a-b/pNx-0 M0 and histological grade 1 or 2; intermediate risk: pT1b pNx-0 and/or histological grade 3-4; high risk: pT1-pT4 with any histological grade or pT any, pN1 cM0 with any histological grade [18]. Follow-up data including sCr, eGFR, (local) recurrence status and radiological results were retrieved from these check-ups. Local recurrence was defined as RCC recurrence in the operated kidney or renal fossa. In case of doubt regarding PSM based on the pathology report, the slides were re-examined by the pathologist.
Fig. 1
Selection of the study population.
Primary outcomes
Survival analyses included overall survival (OS), cancer-specific survival (CSS) and recurrence-free survival (RFS). OS was defined as the time from surgery to death from any cause, CSS was defined as the time from surgery to death related to RCC and RFS was defined as the time from surgery to biopsy-proven (local) recurrence or metastasis. Renal function was evaluated postoperatively based on sCr and eGFR. Postoperative eGFR was followed over time during follow-up. Difference in eGFR and sCr was defined absolutely and relatively compared to the pre-operative kidney function. Patients were classified in CKD stages pre-and postoperatively for comparison [19]. Patients with benign tumours were not included in survival analyses.
Secondary outcomes
Secondary outcomes included surgical approach, ischemia time, surgical time, estimated blood loss (EBL) and pathology results. Additionally, length of hospital stay (LOS), complication rate (according to Clavien-Dindo classification), surgical margin status and readmission within 30 days were evaluated [20].
Matching
A propensity score was generated including age, sex, BMI, RENAL-score, PADUA-score, ASA-classification, CCI-A and preoperative eGFR to reduce differences due to selection bias and confounding. We employed the nearest neighbour method with no replacement and a maximum caliper-width of 0.2 for the propensity scores to match.
Statistical analysis
All statistical analyses were done with the cohort after matching. A paired T-test was used for parametric data and the Pearson Chi-squared test and Wilcoxon Signed Ranks test were used for non-parametric data (Fisher’s exact test for values <5). Survival analyses were conducted using Kaplan-Meier estimates with a paired logrank test. Additionally, univariate and multivariate Cox regression was conducted to determine predictors of OS, CSS, and RFS. For multivariate Cox regression the backward elimination method was used. Mixed models were applied to analyse repeated measures of eGFR and sCr during follow-up. Statistical significance was indicated with a P < 0.05. All statistical analyses were carried out using STATA 17.0 software.
3RESULTS
A total of 386 patients with a cT1b tumour underwent RN or PN during the study period, of whom 145 were excluded because they did not meet the inclusion criteria. Propensity scores were generated for the remaining 241 patients with cT1b tumours. After matching there were 100 patients in each treatment group (Fig. 1).
3.1Patient and tumour characteristics of the matched cohort
Both treatment groups were similar in age, BMI, gender, ASA and CCI-A score. Preoperative eGFR was similar between the two groups: RN 81 (69–92) and PN 79 (71 –94) (p = 0.82), as shown in Table 1. A difference in both nephrometry scores was observed between the two groups: RENAL, RN 10 (9–10) and PN 9 (8–10) (p < 0.001) and PADUA, RN 11 (10–12) and PN 9 (9–10) (p = 0.003), respectively.
Table 1
Patient characteristics of the matched cohorts
| RN | PN | p-value | |
| No. of patients | 100 | 100 | |
| Gender | 0.10 | ||
| Male n (%) | 40 (40) | 29 (29) | |
| Female n (%) | 60 (60) | 71 (71) | |
| Age (years) mean (SD) | 60.7 (12.2) | 60.0 (10.2) | 0.67 |
| BMI (kg/m2) median (IQR) | 27 (24–31) | 28 (26–31) | 0.10 |
| CCI-A score median (IQR) | 2 (1–4) | 2 (1–4) | 0.47 |
| ASA score n (%) | 0.38 | ||
| 1 | 19 (19) | 21 (21) | |
| 2 | 56 (56) | 62 (62) | |
| 3 | 25 (25) | 17 (17) | |
| Preoperative creatinine (mg/dl) median (IQR) | 81 (69–92) | 79 (71–94) | 0.82 |
| Preoperative eGFR (ml/min/1.73 m2) median (IQR) | 83 (69–95) | 84 (72–95) | 0.73 |
| Preoperative CKD3a (%) | 11 (11) | 5 (5) | 0.12 |
| Preoperative≥CKD3b (%) | 3 (3) | 8 (8) | 0.21 |
| Tumour size (mm) median (IQR) | 55 (48–60) | 49 (45–55) | <0.001 |
| RENAL-score median (IQR) | 10 (9–10) | 9 (8–10) | <0.001 |
| PADUA-score median (IQR) | 11 (10–12) | 10 (9–11) | 0.003 |
CCI-A: Charlson Comorbidity Index (age adjusted), CKD: chronic kidney disease.
3.2Surgical and pathological outcomes
Both groups were comparable concerning pathologic RCC subtype, ISUP grade, risk group, and readmission rate within 30 days, though there were 4 readmissions among patients who underwent PN, compared to none in the RN group. Postoperative complications, graded according to Clavien-Dindo (CD), showed 26 patients with a CD complication grade I (12% in RN; 14% in PN), 34 patients with a CD complication grade II (10% in RN, 24% in PN) and 10 patients with CD complication grade III (2% in RN, 8% in PN) (p = 0.003). Two patients in the PN group had a complication grade IV. There was significantly more pathological upstaging in the RN group (12%) than in the PN group (3%, [p < 0.03]). The median EBL was significantly higher for patients who underwent PN (150 ml vs. 50 ml, p < 0.001). Six percent of patients in the PN group had a PSM (Table 2) of which one showed recurrence of disease.
Table 2
Surgical and pathological outcomes of the matched cohort
| RN | PN | p-value | |
| Approach n (%) | <0.001 | ||
| Open | 7 (7) | 24 (24) | |
| Laparoscopic | 53 (53) | 11 (11) | |
| Robot-assisted | 40 (40) | 65 (65) | |
| Surgical time (min) median (IQR) | 162 (131–197) | 203 (170–252) | <0.001 |
| Estimated blood loss (ml) median (IQR) | 50 (25–100) | 150 (67.5–300) | <0.001 |
| Length of stay (days) median (IQR) | 4 (3–5) | 4 (4–6) | 0.04 |
| Complications (Clavien-Dindo Score) n (%) | 0.003 | ||
| I | 12 (12) | 14 (14) | |
| II | 10 (10) | 24 (24) | |
| III | 2 (2) | 8 (8) | |
| IV | 0 | 2 (2) | |
| V | 0 | 0 | |
| Readmission within 30 days n (%) | 0 | 4 (4) | 0.05 |
| ISUP n (%) | 0.30 | ||
| Grade 1 | 15 (15) | 14 (14) | |
| Grade 2 | 43 (43) | 45 (45) | |
| Grade 3 | 30 (30) | 24 (24) | |
| Grade 4 | 4 (4) | 1 (1) | |
| Histology n (%) | 0.31 | ||
| Clear-cell RCC | 75 (75) | 63 (63) | |
| Papillary RCC | 10 (10) | 18 (18) | |
| Chromophobe RCC | 4 (4) | 11 (11) | |
| Other* | 6 (6) | 4 (4) | |
| Benign lesions | 5 (5) | 4 (4) | |
| <pT1b n (%) | 12 (12) | 15 (15) | 0.54 |
| >pT1b n (%) | 12 (12) | 3 (3) | 0.03 |
| pT2 | 3 (3) | 1 (1) | |
| pT3 | 9 (9) | 2 (2) | |
| Positive surgical margin n (%) | 0 | 6 (6) | 0.03 |
| Risk group** n (%) | 0.30 | ||
| Low | 47 (49) | 58 (61) | |
| Intermediate | 33 (33) | 26 (27) | |
| High | 7 (7) | 5 (5) | |
| Follow-up time (months) median (IQR) | 38 (17–66) | 42 (14–74) | 0.82 |
| Number of deaths during 10-yr follow-up | 7 (7.3) | 9 (9.4) | 0.82 |
*Other includes: combination, inconclusive results and other RCC subtypes. **Risk groups according to EAU guidelines. Risk groups for clear-cell RCC are according to the Leibovich score. For other RCC subtypes the risk groups are based on pathological TNM stage and nuclear grade. Only RCC cases included in oncological analyses.
3.3Survival outcomes
The median follow-up was similar in both groups (RN 38 [17–66]; PN 42 months [14–74>]). During the 10-year follow-up period seven patients died in the RN group versus 9 patients in the PN group. Of the deaths within the RN group, three were RCC-related, three were of other causes and one death was unknown. Among the patients who underwent PN, two deaths were RCC related, four were non-RCC related and in three cases the cause of death was unknown. There were no significant differences observed in survival rates between the two cohorts regarding RFS, OS and CSS (Fig. 2A–C). Cox univariate and multivariate regression analyses showed high risk group (Hazard rate [HR] = 7.8, p = 0.005) as a predictor of OS. Postoperative eGFR or CKD group was not predictive of OS. Intermediate (HR 3.2, p = 0.018) and high risk groups (HR 5.5, p = 0.013) were both predictors of RFS (Supplementary tables 1a–c). Local recurrence occurred only in the PN group (8% vs. 0%), as shown in Table 3.
Fig. 2
A–C: Kaplan-Meier estimates of recurrence-free survival (adjusted for risk group) (A), overall survival (B), cancer-specific survival (C).
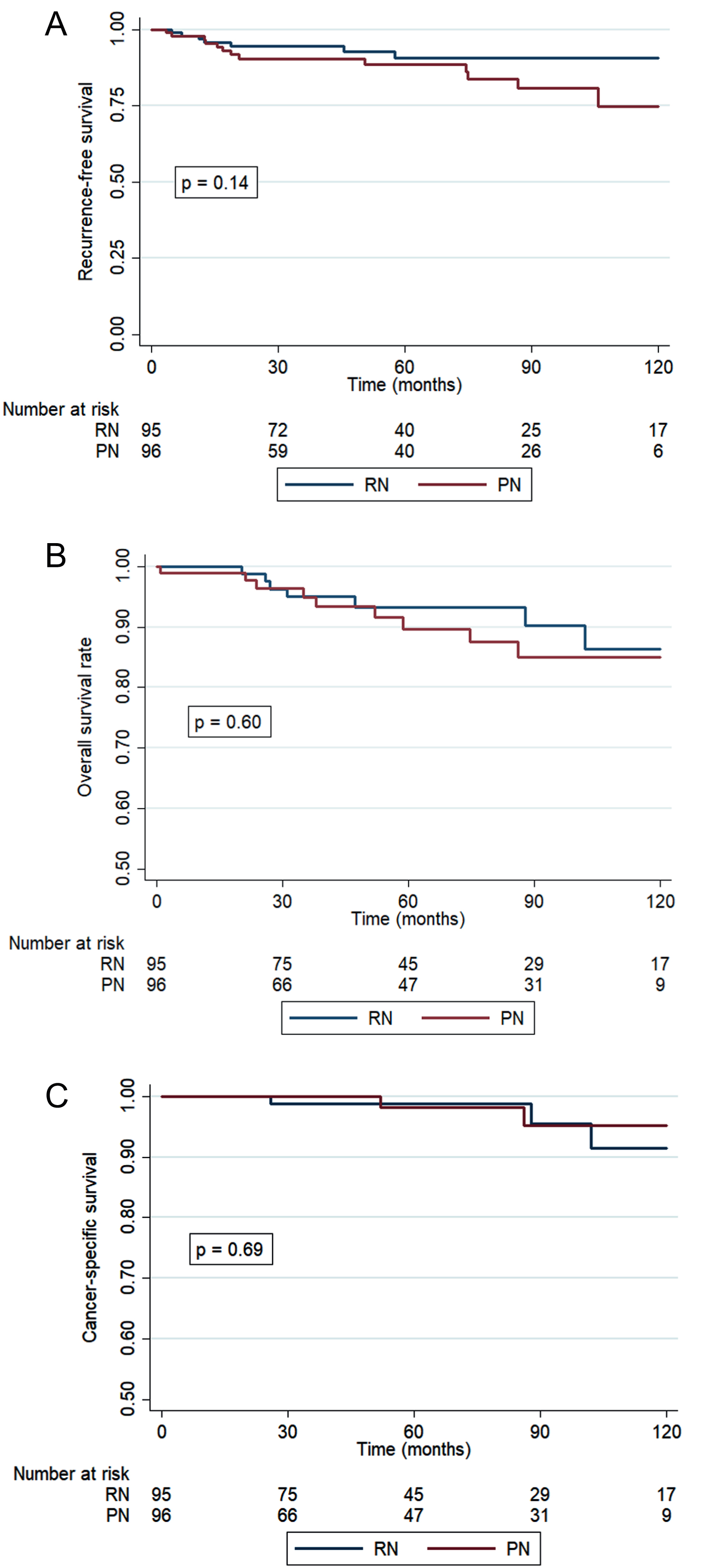
Table 3
Recurrences (%) after RN and PN
| Risk group n (% within risk group) | RN | PN | p-value |
| Low | 0 (0) | 7 (12) | 0.01 |
| Intermediate | 5 (15) | 5 (19) | 0.68 |
| High | 2 (29) | 1 (20) | 0.83 |
| Total n (%) | 7 (8) | 13 (14) | 0.10 |
| Progression type n (L(ow), M(edium), H(igh) risk group) | 0.01 | ||
| Local recurrence | 0 | 8 (6 L, 1 M, 1 H) | |
| Contralateral kidney | 1 (0 L, 1 M, 0 H) | 2 (0 L, 2 M, 0 H) | |
| Metastasis | 6 (0 L, 4 M, 2 H) | 3 (1 L, 2 M, 0 H) |
Risk groups according to EAU guidelines. Risk groups for clear-cell RCC are based on the Leibovich score. For other RCC subtypes the risk groups are based on pathological TNM stage and nuclear grade.
3.4Kidney function outcomes
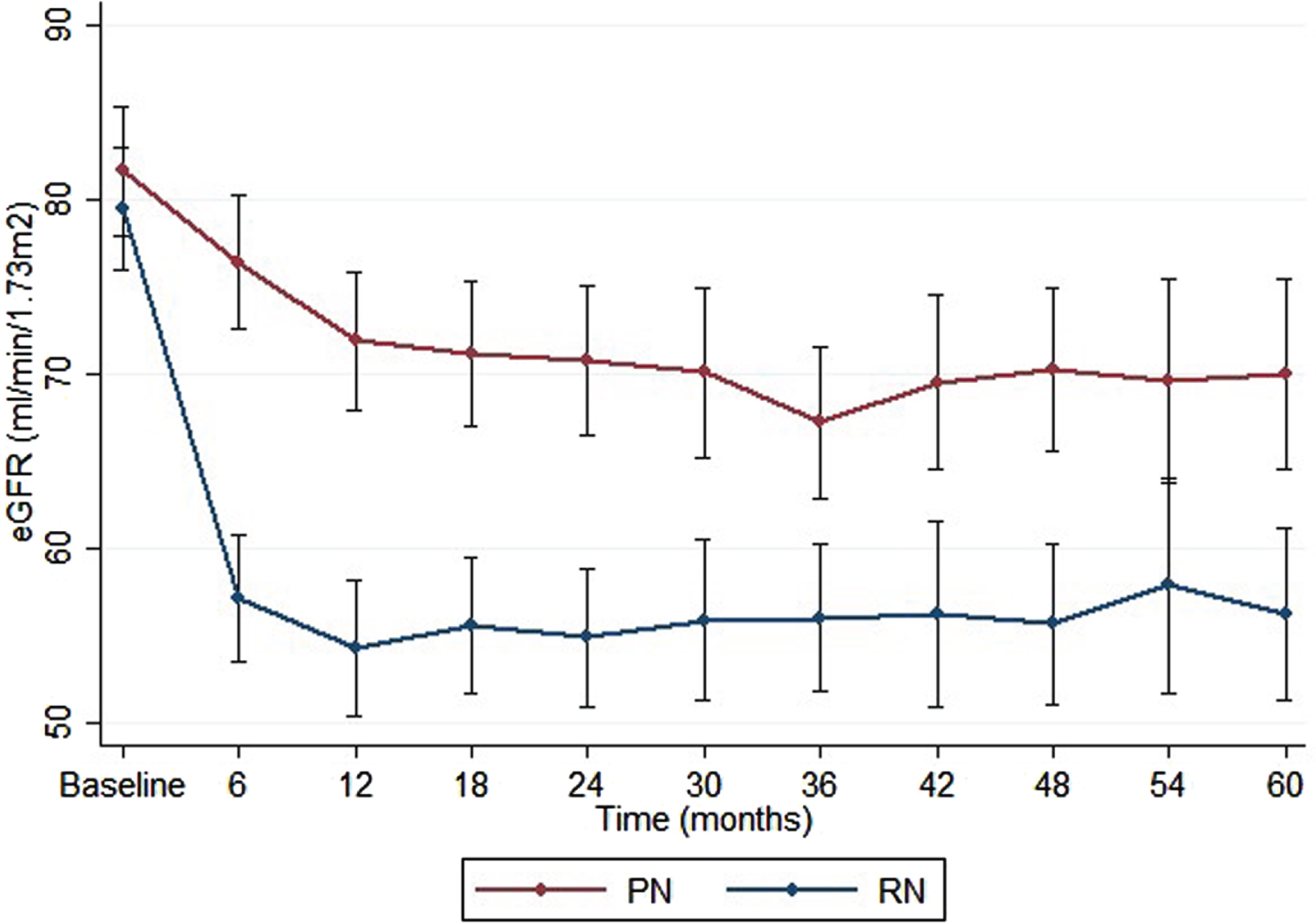
The RN group showed a significant reduction in eGFR, from a median of 83 at baseline to an eGFR of 57 at 1-year post-surgery (p < 0.001). The relative reduction of eGFR after one year was higher for RN (31%) compared to PN (13%, p < 0.001). Significantly more patients developed new-onset CKD ≥3b (defined as eGFR < 45) after RN (n = 18 [22% ]) compared to PN (n = 3 [4% ]) (p < 0.001). Of patients with a preoperative CKD stage 2, 29% developed a CKD≥3b after RN, compared to 5% after PN. Kidney function outcomes are summarized in Table 4. A relative decline of 31% in eGFR compared to baseline was seen after RN, compared to 13% after PN 1-year post-surgery and remained stable during the 5-year follow-up. At 5 years follow-up the difference in mean eGFR between the PN and RN group was 15 (Fig. 3).
Table 4
Renal function outcomes after RN vs. PN for cT1b tumours, including migration of CKD stage post-surgery
| RN | PN | |||||
| Pre-op | 1-year post-op | p-value | Pre-op | 1-year post-op | p-value | |
| New-onset CKD≥3b n (%) | 18 (22) | 3 (4) | <0.001 | |||
| Pre-op CKD1 (eGFR≥90) | 0 (0) | 0 (0) | ||||
| Pre-op CKD2 (eGFR 60–89) | 12 (29) | 2 (5) | ||||
| Pre-op CKD3a (eGFR 45–59) | 6 (100) | 1 (11) | ||||
| eGFR median (IQR) | 83 (69–95) | 57 (44–65) | <0.001 | 84 (72–95) | 73 (60–87) | <0.001 |
| sCr median (IQR) | 81 (69–92) | 107 (92–127) | <0.001 | 79 (71–94) | 88 (73–104) | <0.001 |
| Change eGFR (%) | 31 | 13 | <0.001 | |||
| Median change eGFR (IQR) | 28 (18–34) | 8 (2–16) | <0.001 | |||
| Change sCr (%) | 27 | 10 | <0.001 | |||
| Median change sCr (IQR) | 32 (22–41) | 8 (1–16) | <0.001 | |||
eGFR in ml/min/1.73 m2, sCr: serum creatinine (mg/dl), CKD: chronic kidney disease.
Fig. 3
Renal function during 5-year follow-up period after RN vs. PN. Mean eGFR during 5-year follow-up, error bars represent 95% confidence interval (CI).
DISCUSSION
Our matched analysis showed that PN has superior renal function compared to RN in a 5-year follow-up of patients with suspected T1b RCC. The eGFR stabilized at 6–12 months after surgery in both groups, though, eGFR remained significantly reduced after RN compared with PN. These results are similar to the findings described by others [11, 21]. The EORTC 30904 trial showed that PN reduced the incidence of at least moderate renal dysfunction (eGFR < 60), though this was not the case for advanced kidney disease (eGFR < 30) or kidney failure (eGFR < 15) [11]. Some retrospective studies suggest preserved kidney function can lower the risk of cardiovascular events, improving the quality of life (QoL) and improving survival compared to RN [22–24]. Despite the significant decline in renal function and higher rates of new-onset CKD in the RN group, we did not observe a worse OS. This aligns with Lane et al., who concluded that surgical-induced CKD may have better survival outcomes than medically-induced CKD [25]. However, they did observe an impaired survival in case of postoperative eGFR <45. This highlights the importance of accurate prediction of postoperative eGFR. Our study revealed that 22% of patients who underwent RN had a postoperative eGFR of <45, all of whom had a preoperative eGFR <90. Moreover, this suggests that RN is a viable option for patients with good preoperative kidney function. Nevertheless, information on QoL, previous medical history or cardiovascular events was not measured in this study and must be considered when choosing between PN and RN [26]. The current PARTIAL randomized controlled trial analyses renal function and QoL after PN versus RN in patients with tumours ≤7 cm and might lead to new insights [27].
Previous studies comparing RN and PN for T1b tumours showed contradictory results regarding survival. The EORTC trial showed a worse OS for PN compared to RN, however this effect was no longer significant in the targeted RCC population [12, 28]. Two meta-analyses comparing PN and RN for T1b tumours showed similar CSS, RFS and OS rates [13, 14]. We also found comparable results: OS, CSS and RFS rates did not differ between PN and RN, suggesting that PN is a justified treatment option when considering oncological outcomes while maintaining kidney function. Even though we did not find a significant difference in RFS between RN and PN, local recurrences were significantly more frequent in the low-risk group after PN compared to RN. There were no differences in recurrence rates in the intermediate and high-risk groups. Seven recurrences in the low-risk group were seen, of which six were local recurrence. The remaining patient developed retrocaval lymph node metastases. The reason for this higher (local) recurrence rate remains unclear. Only one of the six patients with PSM had a local recurrence during follow-up, thus this does not justify the higher recurrence rate in the PN group. This patient had an intraperitoneal tumour deposition one year post-surgery for which he underwent a metastasectomy. Currently the patient is under surveillance without active treatment. In the current study the exact value of PSM remains unclear and therefore not justifies a secondary RN, as it was not predictive of CSS or RFS. No predictive factors were found for PSM. Another secondary outcome of this study was the postoperative complication rate. PN is a more complex intervention than RN with a higher complication rate [6]. There were significantly more complications in the PN group compared to the RN group. This is in accordance with other studies that looked at surgical outcomes for T1b tumours [29, 30]. There were two complications classified as CD grade 4. In one case the patient had to be re-intubated immediately after surgery due to swelling of the adenoids, in the other case the patient developed an ileus for which a laparotomy was indicated, in addition to urine leakage and an abscess that had to be drained. It is crucial to critically assess the elevated risk of complications following PN, since the increased risk may not always outweigh the benefits of kidney function preservation. The estimated blood loss was also significantly higher in the PN group, though its clinical significance is debatable.
This study is not devoid of limitations. The most important limitation is the retrospective design. We attempted to minimize treatment selection bias by using propensity score matching to adjust for confounding variables. For example, both nephrometry scores were significantly higher in the RN group, despite propensity score matching. These higher scores confirm RN as the preferred method for more complex tumours in daily practice. Nonetheless, recent data shows that increasing RENAL score does not compromise oncological outcomes [31]. Furthermore, unmeasured confounding variables may still be present. Moreover, despite all surgeries being conducted by experienced urologists, a learning curve for the PN procedure as well as the transition from open and laparoscopic to the robotic approach may have influenced the results. Lastly, survival analyses should be interpreted with caution, as the event numbers of CSS are small.
CONCLUSION
Survival rates are similar for patients with cT1b tumours undergoing PN or RN. Despite more local recurrences in the PN group, the comparable survival estimates suggest that local salvage options are effective. PN has superior preservation of renal function compared to RN, though it could be argued that RN is preferable when maintenance of renal function is not a priority.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
All authors contributed to this manuscript.
LB, NG, DK and PZ were responsible for the study design. The acquisition of data and analysis were done by DK and LB. LB wrote the manuscript and AB, HB and JM edited the paper. All authors approved the final version of the manuscript prior to submission.
CONFLICTS OF INTEREST
AB is an Associate Editor and PZ is an Editorial Board Member of this journal, but they were not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review. LB, DK, NG, HB and JM have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-230019.
REFERENCES
[1] | Ferlay J , Colombet M , Soerjomataram I , Dyba T , Randi G , Bettio M , et al., Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. (2018) ;103: :356–87. |
[2] | Bukavina L , Bensalah K , Bray F , Carlo M , Challacombe B , Karam JA , et al., Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur Urol. (2022) ;82: (5):529–42. |
[3] | Karakiewicz PI , Lewinshtein DJ , Chun FK , Briganti A , Guille F , Perrotte P , et al., Tumor size improves the accuracy of TNM predictions in patients with renal cancer. Eur Urol. (2006) ;50: (3):521–8; discussion 9. |
[4] | Ljungberg B , Albiges L , Abu-Ghanem Y , Bedke J , Capitanio U , Dabestani S , et al., European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update.. Eur Urol. (2022) ;82: (4):399–410. |
[5] | Mir MC , Derweesh I , Porpiglia F , Zargar H , Mottrie A , Autorino R . Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol. (2017) ;71: (4):606–17. |
[6] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A , et al., A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. (2007) ;51: (6):1606–15. |
[7] | Fero K , Hamilton ZA , Bindayi A , Murphy JD , Derweesh IH . Utilization and quality outcomes of cT1a, cT1b and cT2a partial nephrectomy: analysis of the national cancer database. BJU Int. (2018) ;121: (4):565–74. |
[8] | Wood EL , Adibi M , Qiao W , Brandt J , Zhang M , Tamboli P , et al., Local Tumor Bed Recurrence Following Partial Nephrectomy in Patients with Small Renal Masses. J Urol. (2018) ;199: (2):393–400. |
[9] | Carvalho JAM , Nunes P , Tavares-da-Silva E , Parada B , Jarimba R , Moreira P , et al., Impact of Positive Surgical Margins After Partial Nephrectomy. Eur Urol Open Sci. (2020) ;21: :41–6. |
[10] | Abu-Ghanem Y , Ramon J , Berger R , Kaver I , Fridman E , Leibowitz-Amit R , Dotan ZA . Positive surgical margin following radical nephrectomy is an independent predictor of local recurrence and disease-specific survival. World Journal of Surgical Oncology. (2017) ;15: (1):193. |
[11] | Scosyrev E , Messing EM , Sylvester R , Campbell S , Van Poppel H . Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. (2014) ;65: (2):372–7. |
[12] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A , et al., A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. (2011) ;59: (4):543–52. |
[13] | Jiang YL , Peng CX , Wang HZ , Qian LJ . Comparison of the long-term follow-up and perioperative outcomes of partial nephrectomy and radical nephrectomy for 4 cm to 7 cm renal cell carcinoma: a systematic review and meta-analysis. BMC Urol. (2019) ;19: (1):48. |
[14] | Zhang Y , Long G , Shang H , Ding B , Sun G , Ouyang W , et al., Comparison of the oncological, perioperative and functional outcomes of partial nephrectomy versus radical nephrectomy for clinical T1b renal cell carcinoma: A systematic review and meta-analysis of retrospective studies. Asian J Urol. (2021) ;8: (1):117–25. |
[15] | Levey AS , Stevens LA , Schmid CH , Zhang YL , Castro AF , Feldman HI , et al., A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) ;150: (9):604–12. |
[16] | Kutikov A , Uzzo RG . The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. (2009) ;182: (3):844–53. |
[17] | Ficarra V , Novara G , Secco S , Macchi V , Porzionato A , De Caro R , Artibani W . Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. (2009) ;56: (5):786–93. |
[18] | Leibovich BC , Blute ML , Cheville JC , Lohse CM , Frank I , Kwon ED , et al., Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. (2003) ;97: (7):1663–71. |
[19] | Stevens PE , Levin A . Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med. (2013) ;158: (11):825–30. |
[20] | Dindo D , Demartines N , Clavien PA . Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) ;240: (2):205–13. |
[21] | Jang HA , Kim JW , Byun SS , Hong SH , Kim YJ , Park YH , et al., Oncologic and Functional Outcomes after Partial Nephrectomy Versus Radical Nephrectomy in T1b Renal Cell Carcinoma: A Multicenter, Matched Case-Control Study in Korean Patients. Cancer Res Treat. (2016) ;48: (2):612–20. |
[22] | Weight CJ , Larson BT , Fergany AF , Gao T , Lane BR , Campbell SC , et al., Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. (2010) ;183: (4):1317–23. |
[23] | Capitanio U , Larcher A , Cianflone F , Trevisani F , Nini A , Mottrie A , et al., Hypertension and Cardiovascular Morbidity Following Surgery for Kidney Cancer. Eur Urol Oncol. (2020) ;3: (2):209–15. |
[24] | Capitanio U , Terrone C , Antonelli A , Minervini A , Volpe A , Furlan M , et al., Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol. (2015) ;67: (4):683–9. |
[25] | Lane BR , Demirjian S , Derweesh IH , Takagi T , Zhang Z , Velet L , et al., Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol. (2015) ;68: (6):996–1003. |
[26] | Poulakis V , Witzsch U , de Vries R , Moeckel M , Becht E . Quality of life after surgery for localized renal cell carcinoma: Comparison between radical nephrectomy and nephron-sparing surgery. Urology. (2003) ;62: (5):814–20. |
[27] | UK CR. A study looking at two different types of surery for kidney cancer (PARTIAL) London, UK: Cancer Research UK; 2023 [updated 17-03-2023. Available from: https://www.cancerresearchuk.org/about-cancer/find-aclinical-trial/a-study-looking-at-two-different-types-ofsurgery-for-kidney-cancer-partial#undefined. |
[28] | Ristau BT , Handorf EA , Cahn DB , Kutikov A , Uzzo RG , Smaldone MC . Partial nephrectomy is not associated with an overall survival advantage over radical nephrectomy in elderly patients with stage Ib-II renal masses: An analysis of the national cancer data base. Cancer. (2018) ;124: (19):3839–48. |
[29] | Roos FC , Brenner W , Thomas C , Jager W , Thuroff JW , Hampel C , Jones J . Functional analysis of elective nephron-sparing surgery vs. radical nephrectomy for renal tumors larger than 4 cm. Urology. (2012) ;79: (3):607–13. |
[30] | Deklaj T , Lifshitz DA , Shikanov SA , Katz MH , Zorn KC , Shalhav AL . Laparoscopic radical versus laparoscopic partial nephrectomy for clinical T1bN0M0 renal tumors: comparison of perioperative, pathological, and functional outcomes. J Endourol. (2010) ;24: (10):1603–7. |
[31] | Cerrato C , Patel D , Autorino R , Simone G , Yang B , Uzzo R , et al., Partial or radical nephrectomy for complex renal mass: a comparative analysis of oncological outcomes and complications from the ROSULA (Robotic Surgery for Large Renal Mass) Collaborative Group. World Journal of Urology. (2023) ;41: (3):747–55. |




