Nephrotoxicity Associated with Contemporary Renal Cell Carcinoma Regimens: A Systematic Review and Meta-Analysis
Abstract
Background:
The nephrotoxicity profile of contemporary first-line regimens for treatment of metastatic renal cell carcinoma (mRCC) has not been systematically studied in published clinical trials.
Objective:
To assess the rates of nephrotoxic events of contemporary first-line regimens for treatment of mRCC in comparison to vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI) mono-therapy.
Methods:
We performed a systematic search of the literature looking for randomized clinical trials that contemplated National Comprehensive Cancer Network (NCCN) recommended first-line regimens for treating mRCC in which the control arm was a VEGF-TKI. Selected trials could either include an experimental arm of immune checkpoint inhibitor (ICI) plus VEGF-TKI combination or ICI-ICI combination. Nephrotoxic events were defined as proteinuria, hypertension, blood creatinine increase, acute kidney failure or nephritis, which were all described separately.
Results:
Five studies satisfied our inclusion criteria. Combination of ICI with VEGF-TKI showed a statistically significant higher degree of proteinuria compared to VEGF-TKI alone. There was no statistically significant difference in rates of hypertension between ICI-TKI and VEGF-TKI alone, but VEGF-TKI alone was statistically significantly more associated with hypertension than immunotherapy alone. Other renal toxicities, such as an increase in creatinine, acute kidney injury (AKI) and nephritis, were uncommon and not consistently reported in each trial.
Conclusions:
Contemporary regimens for first-line treatment of mRCC are associated with a higher grade of proteinuria than VEGF-TKI alone, while VEGF-TKI is more associated with hypertension than an ICI-ICI combination. Description of many renal toxicities across the studies reported have been diverse and a standardized definition across clinical trials would be helpful to reliably interpret the data regarding nephrotoxicity in the setting of treatment of renal cell carcinoma.
BACKGROUND
The treatment landscape of metastatic renal cell carcinoma (mRCC) has considerably evolved in the last decade. With the advent of immune checkpoint inhibitors (ICI), several combinations of tyrosine kinase inhibitors (TKI) targeting mainly vascular endothelial growth factor (VEGF) with ICI have shown superior outcomes in clinical trials compared to single agent VEGF-TKI. Sunitinib was replaced as preferred option for first-line therapy for the combinations of a PD-1 inhibitor with a VEGF-TKI represented by pembrolizumab-lenvatinib, pembrolizumab-axitinib and nivolumab-cabozantinib, which are now the contemporary standard of care [1–3]. VEGF targeting agents in combination with PD-L1 inhibitors have failed to show clear benefits over sunitinib and therefore were not approved or equally endorsed by clinical guidelines, such as atezolizumab-bevacizumab and avelumab-axitinib [4, 5]. The ICI-ICI combination including the PD-1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab has also shown considerable advantage compared to sunitinib alone and is a standard option for IMDC intermediate or poor risk disease [6].
Toxicity profile is a major factor among others for deciding on which specific combination to choose from for first-line therapy. VEGF-TKIs share similar adverse events such as fatigue, hypertension, proteinuria, gastrointestinal disturbances and skin toxicity, all of which can be dose limiting [7]. The toxicity profile of ICI drugs is generally different, but it can overlap with those of VEGF-TKIs, mostly concerning hepatotoxicity and fatigue [8]. Nevertheless, immune-related adverse events can present with a variety of clinical scenarios and when used in combination with VEGF-TKI’s, it can be challenging to ascribe which drug may be causing the adverse event of concern [9]. For instance, proteinuria is a common complication of VEGF-TKI use, but it can also be a presenting feature of immune-mediated nephritis [10].
Nephrotoxic events are also common for mRCC patients, predisposed by age (a median age of 64 years) at diagnosis and who have more likely than not undergone nephrectomy during their disease course [11]. Nephrotoxic events in the interest of this review are described as hypertension, proteinuria, creatinine increase, acute kidney injury (AKI) or nephritis. Previous studies have individually reported on the occurrence of these adverse events resulting from either VEGF-TKI therapy or ICI therapy [12–16]. However, to our knowledge, no prior report has comprehensively examined nephrotoxic events within the context of contemporary first-line regimens for mRCC. Thus, we conducted a systematic literature review to assess the frequency of renal-related adverse events documented in clinical trials involving modern first-line regimens for mRCC in order to better understand the safety profile of the newer combination therapies with respect to nephrotoxicity.
METHODS
Inclusion and exclusion criteria
The research question was structured in alignment with the PICO framework. The primary focus was on determining the incidence of nephrotoxicity associated with modern treatment regimens for patients diagnosed with advanced or metastatic clear cell renal cell carcinoma (ccRCC). This assessment was undertaken in comparison to the utilization of VEGF-TKI as a standalone therapy. Nephrotoxicity, the key outcome of interest, was operationalized to encompass various manifestations, including hypertension, proteinuria, creatinine increase, acute kidney injury, and immune-related nephritis.
We attempted to identify (1) clinical trials that involved NCCN category 1 list of recommended first-line combination therapy for advanced or metastatic ccRCC patients, which (as of July 2023) were pembrolizumab-axitinib, pembrolizumab-lenvatinib, nivolumab-cabozantinib for all-risk disease and nivolumab-ipilimumab for intermediate or poor-risk disease only. (2) Only the most updated and published in English study report which included an adverse event section was selected. (3) Clinical trials with no comparison arm, a comparison arm not comprised of VEGF-TKI or less than 100 patients were excluded. (4) Adjuvant or neoadjuvant trials for RCC or trials involving other cancer histologies were not accepted. All procedures undertaken in this systematic review were done according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Search strategy
The literature review process was conducted using the PubMed and Embase databases on July 13, 2023, without any restrictions on publication dates. The search strategy employed for PubMed included the following terms: “Kidney Neoplasms” OR “Renal Cell Carcinoma” OR “metastatic kidney cancer” OR “metastatic renal cell carcinoma” and (”pembrolizumab” OR “Cabozantinib” OR “nivolumab” OR “Ipilimumab” OR “Lenvatinib” OR “Axitinib”). A similar strategy was implemented for Embase, utilizing the term entries: ‘kidney cancer’/de OR ‘renal cell carcinoma’/de OR ‘kidney cancer’/exp OR ‘renal cell carcinoma’/exp) AND (‘pembrolizumab’/exp OR ‘cabozantinib’/exp OR ‘nivolumab’/exp OR ‘ipilimumab’/exp OR ‘lenvatinib’/exp OR ‘axitinib’/exp). Filters were applied to include only clinical trials in both databases. The literature review was conducted by two independent authors (AD and MZ), and any discrepancies were resolved by a third author (SP).
Data collection
Additional clarity regarding the definition of nephrotoxic events, specifically encompassing “creatinine increase” and “AKI,” is essential. As outlined by Common Terminology Criteria (CTCAE) version 5.0, “blood creatinine increase” is categorized from grade 1 to 4. Grade 1 entails a creatinine level up to 1.5 times the upper limit of normal (ULN), whereas grade 2 encompasses a range of 1.5–3.0 times the ULN or 1.5–3.0 times the baseline. For grade 3, the threshold is considered to be >3.0 times the ULN or baseline, while grade 4 signifies a value exceeding >6.0 times the ULN [18].
Conversely, according to CTCAE guidelines, the grading of AKI begins from grade 3 onwards. Grade 3 entails a circumstance necessitating hospitalization, grade 4 denotes life-threatening consequences or the necessity to undergo dialysis, and grade 5 signifies a fatal outcome [18]. Despite the potential for overlap in patients experiencing either “creatinine increase” or “AKI”, their separate reporting led to the decision to treat them as distinct events.
Another frequently encountered term is “nephritis,” which is not included in the CTCAE. However, the ASCO and Society for Immunotherapy of Cancer (SITC) guidelines for managing immune-related adverse events in patients undergoing ICI therapy do provide a definition. In these guidelines, nephritis is defined by the Kidney Disease Improving Global Outcomes (KDIGO) acute kidney injury parameters, coupled with the exclusion of other plausible causes for renal failure aside from ICI use [19, 20]. As such, the reporting of nephritis in the trials was contingent on AKI being directly attributed to ICI use, thus warranting separate documentation.
In addition to these definitions, other pertinent data retrieved from all the included trials encompassed author names, publication years, study designs, number of participants, characteristics of treatment arms, as well as the numbers of treatment-related nephrotoxic events of any grade and those classified as grade 3–5. Data collection was undertaken by two independent reviewers (AD and MZ), with any discrepancies resolved by a third reviewer (SP).
Statistical analysis
In this meta-analysis, we calculated the pooled odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous outcomes of nephrotoxicity. The degree of heterogeneity was assessed by the Chi-square test and I2 statistics. If there was no significant heterogeneity (P≥0.10, I2≤50%), a fixed-effects model was used; if not, a random-effects model was used. Sensitivity analysis by excluding one trial at a time was performed to evaluate whether the overall results could be significantly affected by this trial. We performed a descriptive analysis if data could not be combined. All statistical analyses were performed with R Statistical Software, version 4.3.1.
RESULTS
Literature search
The systematic literature search identified a total of 910 study reports. Utilizing an automation tool, 199 duplicates were effectively eliminated. Subsequently, 711 reports underwent assessment, leading to the exclusion of 693 based on screening of titles and abstracts [21]. Ultimately, 17 articles were targeted for retrieval and examination of the full text. Among these, 4 articles represented trials that were still in the recruiting phase, lacking preliminary results. Furthermore, 2 articles focused on non-clear cell populations, while 3 articles were excluded due to their small sample sizes. An additional 2 studies involved trials that were not situated in the first-line ccRCC setting. Another study was excluded based on the absence of same comparator arm. Consequently, a total of 5 trials were selected for inclusion in the analysis, comprising 4 phase 3 randomized controlled trials and 1 phase 2 trial. The detailed selection process is visually depicted in Fig. 1.
Fig. 1
PRISMA flow diagram.
Study characteristics
The sum of all patients included for analysis from the 5 selected trials comprised 3416 individuals. A total of 1750 patients received an experimental arm, which for 1104 patients it consisted of an ICI-TKI combination and for 648 patients an ICI-ICI combination. There were 1664 patients who were recruited to the control arms and received VEGF-TKI single-agent as first-line treatment. Across the experimental arms of the trials, 3 different VEGF-TKIs were employed alongside nivolumab or pembrolizumab as the ICI backbone. All included studies are described in Table 1.
Table 1
Study characteristics
| Study Author (Year), Journal | Study Name | Study interventions | Sample Size | Median Age | Male (%) | IMDC score | Previous Nephrectomy (%) | |||||
| Experimental | Control | Total | Experimental | Control | F | I | P | |||||
| Choueiri et al. (2021), NEJM | Checkmate-9ER | Nivolumab 240 mg every 2 weeks + Cabozantinib 40 mg daily | Sunitinib 50 mg daily for 4 weeks every 6 week cycle | 640 | 320 | 320 | 62 (28–90) | 73.8 | 22.4 | 57.7 | 19.8 | 69.8 |
| Motzer et al. (2021), NEJM | CLEAR | Pembolizumab 200 mg + Lenvatinib | Sunitinib 50 mg daily for 4 weeks every 6 week cycle | 692 | 352 | 340 | 62 (29–88) | 74.4 | 32.8 | 63.9 | 9.8 | 83.0 |
| Powles et al. (2020), Lancet Oncology | Keynote-426 | Pembrolizumab 200 mg every 3 weeks + Axitinib 5 mg twice a day | Sunitinib 50 mg daily for 4 weeks every 6 week cycle | 861 | 432 | 429 | 62 (53–68) | 72.9 | 31.2 | 56.2 | 12.5 | 83.0 |
| Motzer et al. (2019), Lancet Oncology | Checkmate-214 | Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg every 3 weeks for 4 doses followed by Nivolumab 3 mg/kg every 2 weeks | Sunitinib 50 mg daily for 4 weeks every 6 week cycle | 1082 | 547 | 535 | 62 (21–85) | 73.7 | 22.7 | 60.8 | 16.4 | 81.2 |
| Vano et al. (2020), Lancet Oncology | BIONIKK | Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg every 3 weeks for 4 doses followed by Nivolumab 3 mg/kg every 2 weeks | VEGF-TKI (Sunitinib + Pazopanib) | 141 | 101 | 40 | 65 (54–73) | 77.3 | 32.6 | 51.7 | 15.6 | 74.4 |
IMDC: International Metastatic RCC Database Consortium. F: favorable-risk, I: intemediate-risk, P: poor-risk.
In the CLEAR trial, data from arms 1 and 3 were utilized for analysis, specifically involving the pembrolizumab plus lenvatinib combination in arm 1 and sunitinib monotherapy in arm 3. The arm combining lenvatinib with everolimus was excluded from the analysis.
Two trials shared the same experimental arm, consisting of ipilimumab plus nivolumab. These trials are the phase 3 Checkmate-214 trial and the phase 2 BIONIKK trial. Notably, the analysis did not incorporate the single-agent nivolumab arm within the BIONIKK trial.
Within the BIONIKK trial, the control arm involved a single-agent VEGF-TKI, either sunitinib or pazopanib. Among the 40 patients in this arm, 33 were administered sunitinib, while the remaining 7 received pazopanib.
Adverse event data were sourced from adverse events tables in the primary articles and supplementary materials of each paper.
Hypertension
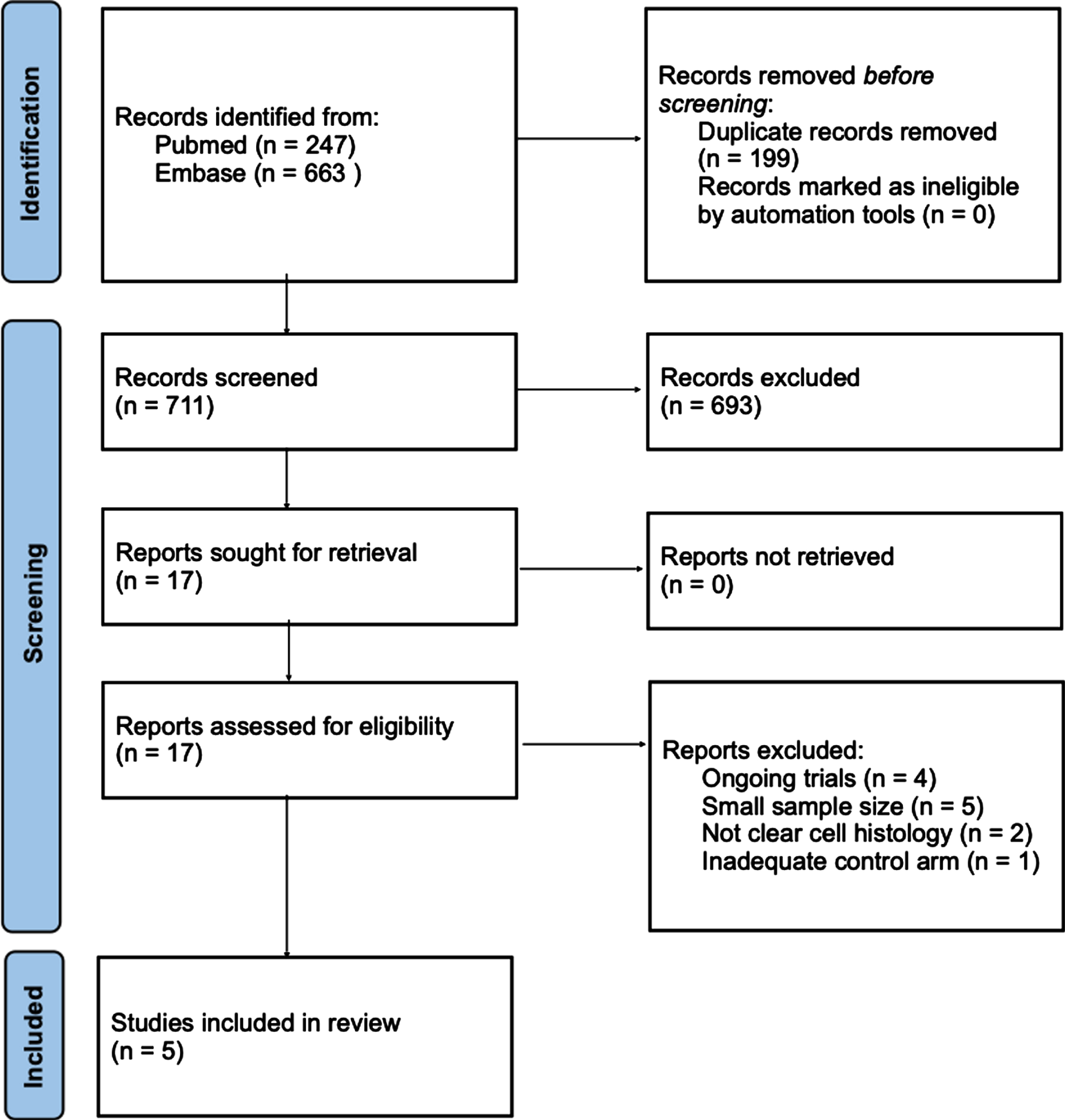
Regarding hypertension, there was no statistically significant difference observed between ICI plus VEGF-TKI versus VEGF-TKI alone, both for all-grade and grade ≥3 (Fig. 2). Despite no statistical significance, the trial that incorporated lenvatinib in its treatment regimen displayed a higher numerical incidence of both all-grade and grade ≥3 hypertension compared to sunitinib (55.4% vs 41.5% for all-grade and 27.6% vs 18.8% for grade ≥3). On the other hand, cabozantinib and axitinib exhibited no clear numerical distinction in the occurrence of hypertension across all grades when compared to sunitinib. For cabozantinib the rates of all-grade hypertension were 34.4% vs 37.2% of sunitinib while for grade ≥3 they were 12.5% vs 13.1%. For axitinib, the incidence rates of all-grade hypertension were 44% vs 45% of sunitinib and for grade ≥3 hypertension there were 22% vs 20% cases.
Fig. 2
Hypertension, all-grade and grade ≥3, for ICI-TKI vs VEGF-TKI trials. ICI-TKI: Immune checkpoint inhibitor and tyrosine kinase inhibitor combination; VEGF-TKI: vascular endothelial growth factor tyrosine kinase inhibitor.
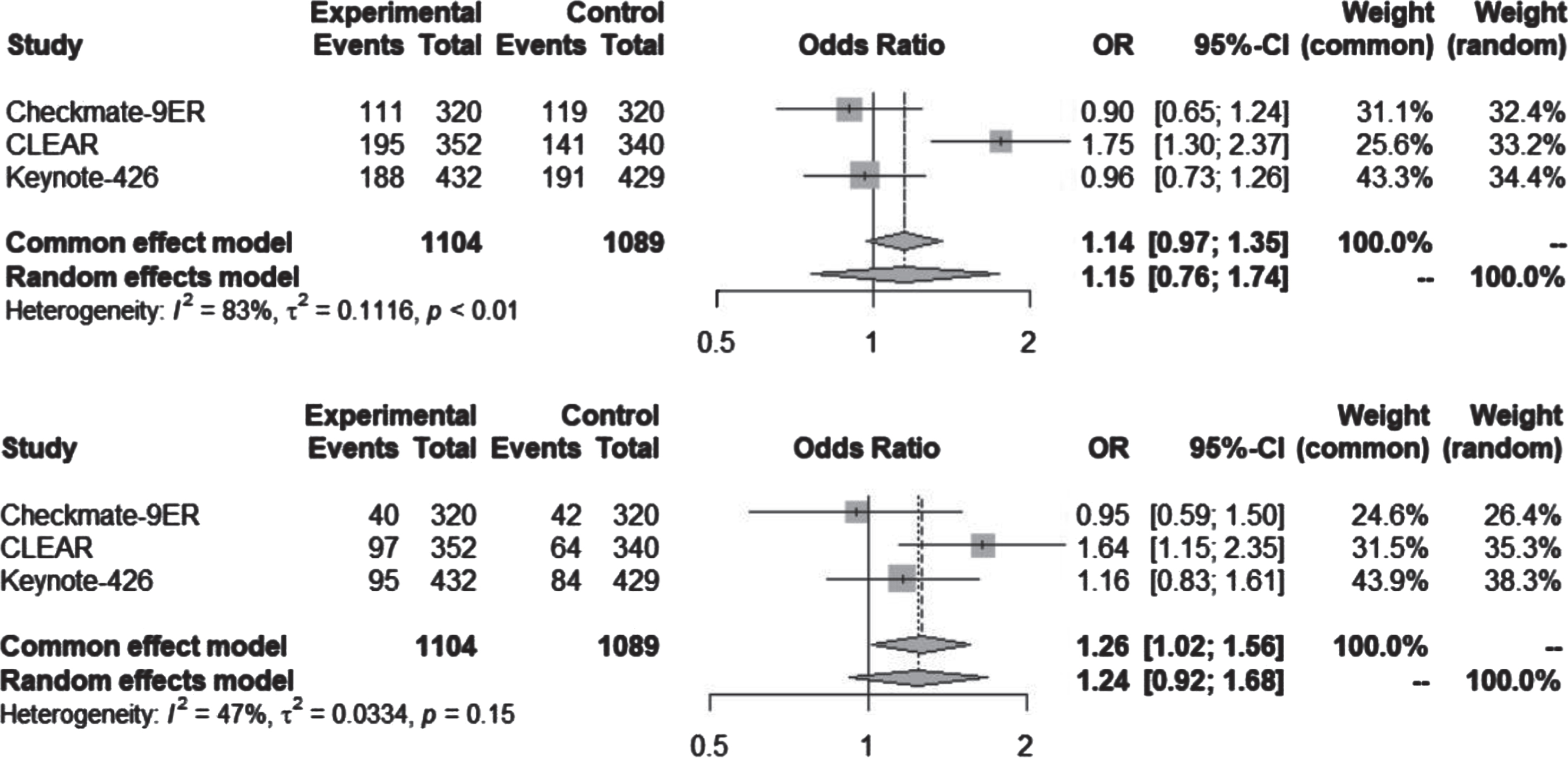
The utilization of VEGF-TKI monotherapy demonstrated a greater prevalence of hypertension in contrast to the Ipilimumab plus nivolumab (Ipi-Nivo) combination, which was statistically significant for both all-grade and grade ≥3 (Fig. 3). In the context of the Checkmate-214 trial, treatment-related cases of all-grade hypertension accounted for 2.2% (12 out of 547), while the use of sunitinib in the same trial was associated with 40.9% (218 cases) occurrence of all-grade hypertension.
Fig. 3
Hypertension, all-grade and grade ≥3, for ICI-ICI vs VEGF-TKI trials. ICI-ICI: Immune checkpoint inhibitor combination; VEGF-TKI: vascular endothelial growth factor tyrosine kinase inhibitor.
Within the BIONIKK trial, the ICI-ICI arm showcased a single case (1%) of grade 3 hypertension. In contrast, the VEGF-TKI arm exhibited a total of 6 cases (15%) of all-grade hypertension, all of which were grade 3 or more severe.
Proteinuria
Significant statistical differences were observed in the occurrence of all-grade, but not grade ≥3, proteinuria between the combinations of VEGF-TKI plus ICI and the administration of sunitinib alone (Fig. 4).
Fig. 4
Proteinuria, all-grade and grade ≥3 for ICI-TKI vs TKI trials. ICI-TKI: Immune checkpoint inhibitor and tyrosine kinase inhibitor combination; VEGF-TKI: vascular endothelial growth factor tyrosine kinase inhibitor.
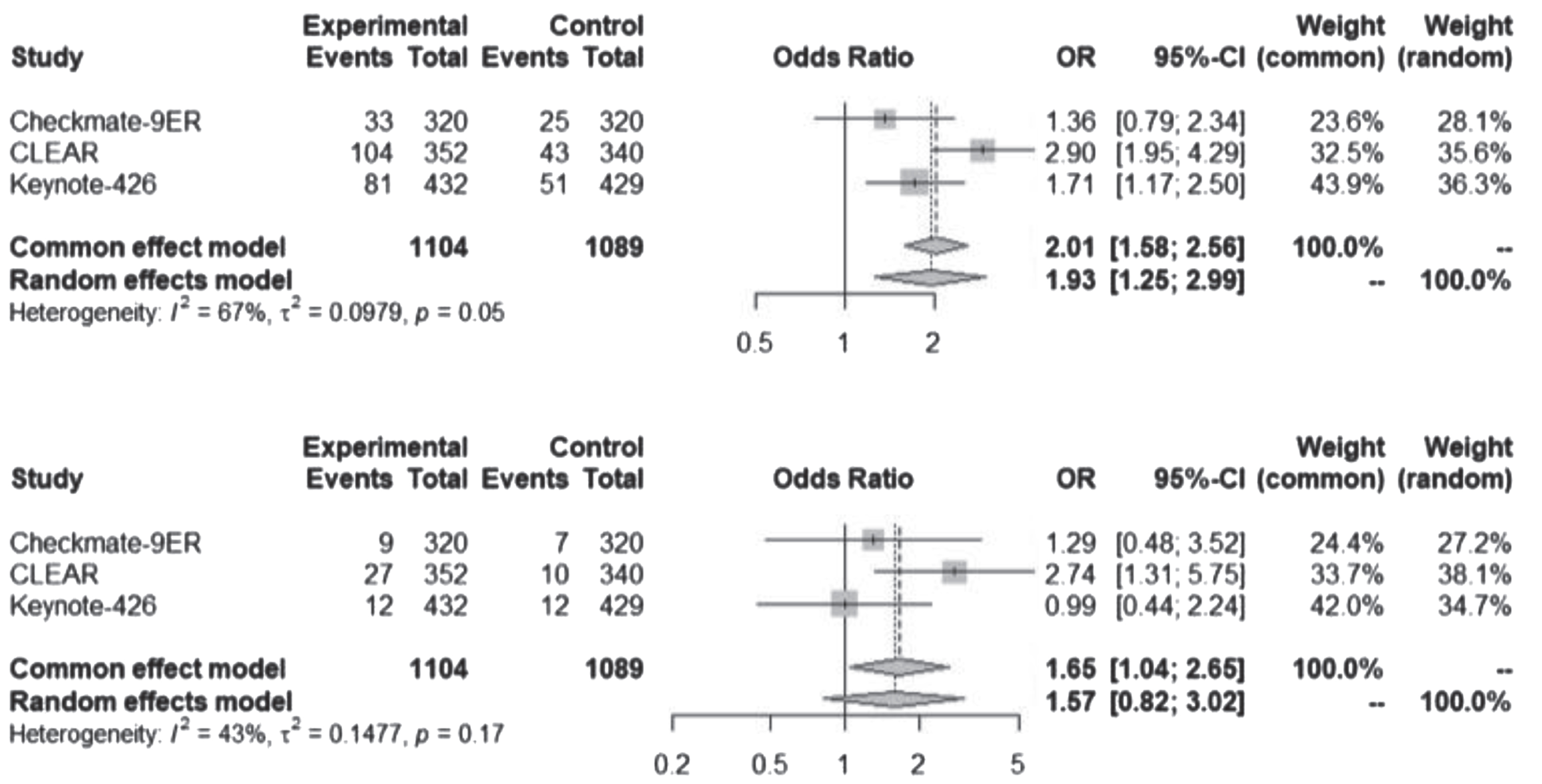
The combination of axitinib plus pembrolizumab exhibited a reported 19% (81 cases) occurrence of all-grade proteinuria, of which 3% (12 cases) were classified as grade 3 or higher. Similarly, the lenvatinib plus pembrolizumab arm was associated with 29.5% (104 cases) occurrence of all-grade proteinuria, of which 7.7% (27 cases) were of grade 3 or higher. In the case of cabozantinib plus nivolumab, there were 10.3% (33 cases) instances of proteinuria, with 2.8% (9 cases) categorized as grade 3 or more severe. Across these 3 trials, sunitinib was associated with a total of 119 cases of proteinuria, out of which 29 were classified as grade 3 or more severe.
In the context of the ICI-ICI combination as opposed to sunitinib, there could not be found any reported data for proteinuria in the Checkmate-214. In the BIONIKK trial, there were no cases of proteinuria reported in association with ICI-ICI combination. Because of this, it was opted to exclude these studies from the calculated meta-analysis for proteinuria.
Creatine increase, acute kidney injury and nephritis
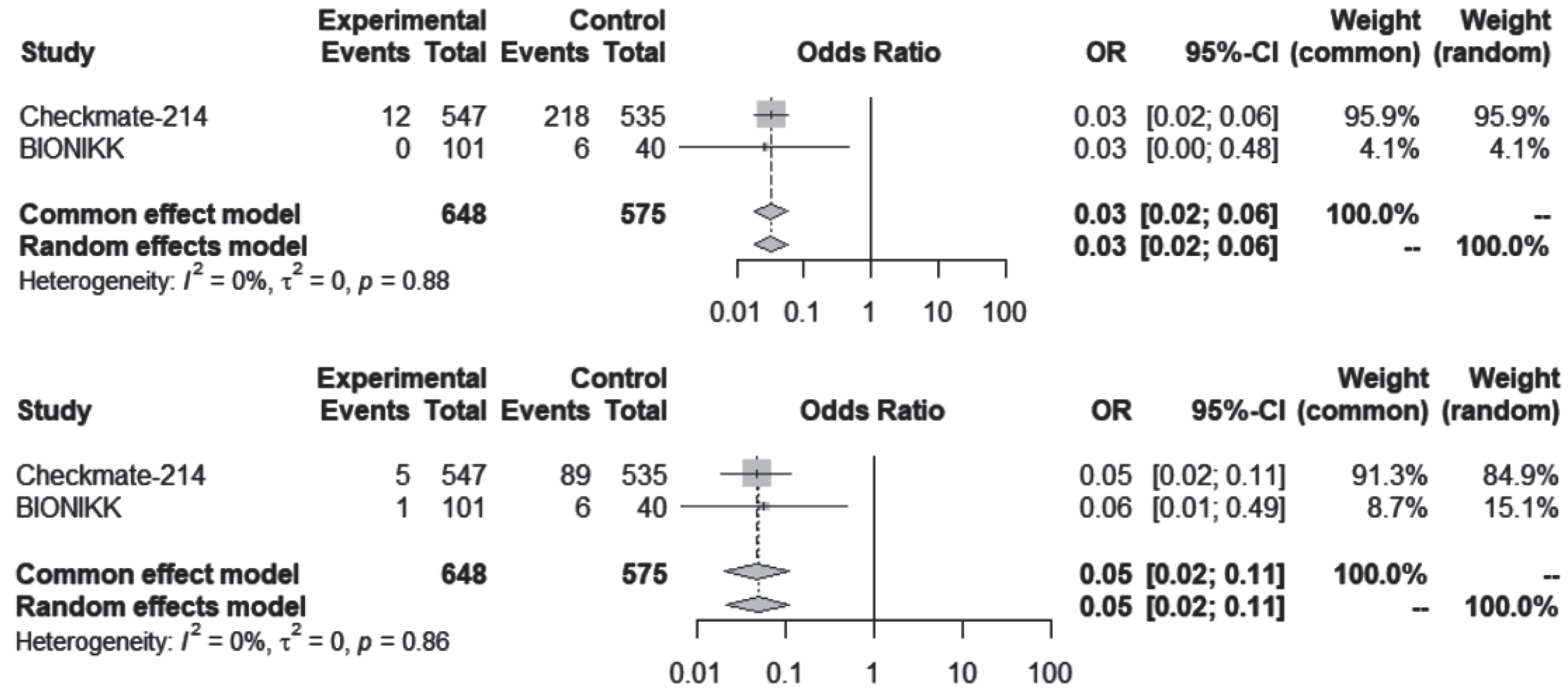
Creatinine increase, AKI and nephritis were uncommon. Despite the possibility of overlap, these events were considered separate entities and added for a combined analysis shown in Fig. 5. There was no statistically significant difference of kidney events between the contemporary regimens and VEGF-TKI alone. Also, Table 2 shows the collected data for each of these entries.
Fig. 5
Creatinine increase, acute kidney injury and nephritis, any grade and grade ≥3 for all studies.
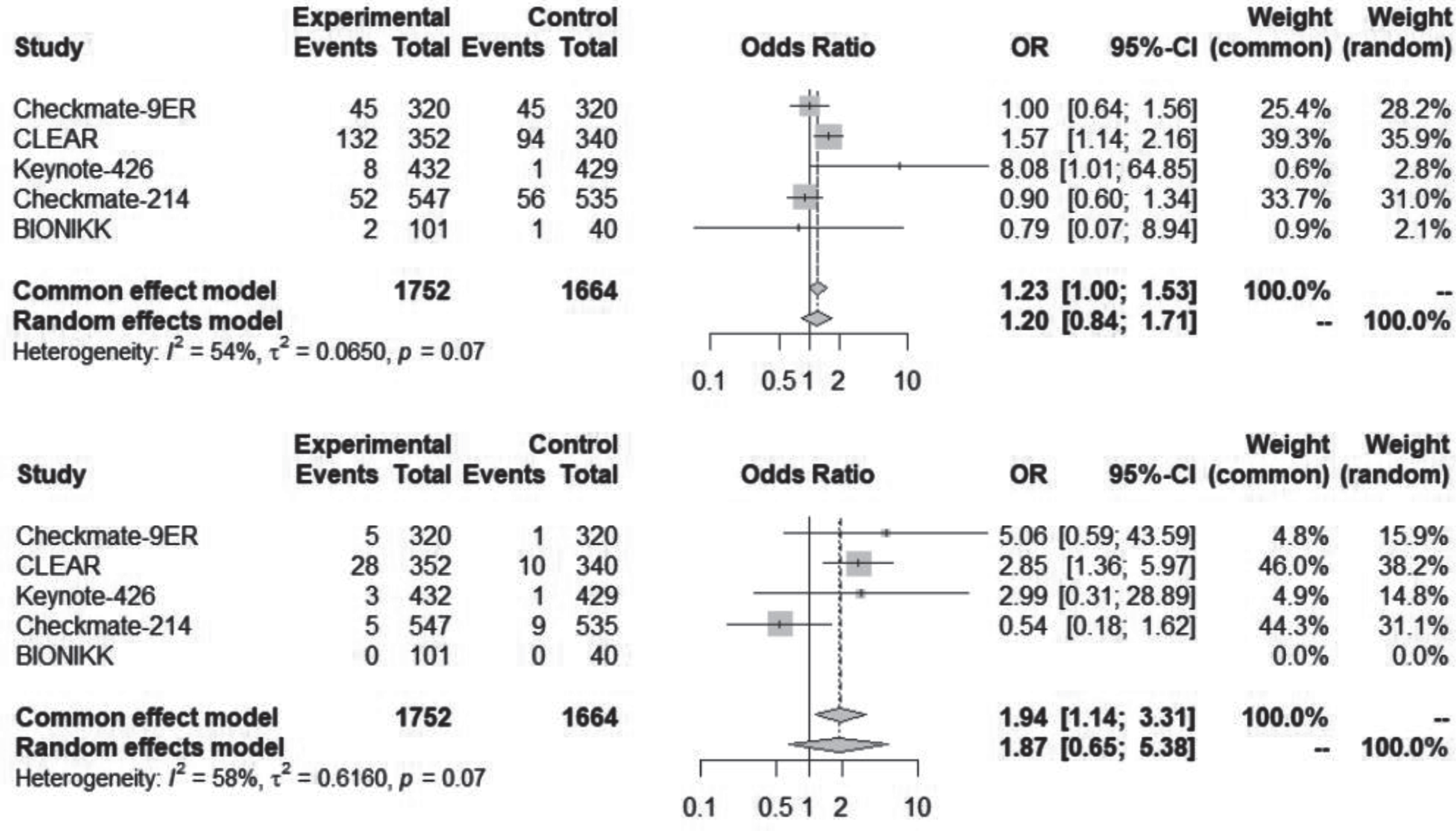
Nephritis was not always included in the adverse events list, including the Checkmate-214 study, where there was no report of nephritis. Although, in this study, there were 40 (7.3%) cases of creatinine increase and 12 (2.2%) cases of AKI. In the BIONIKK study, there were 2 cases of AKI with the ICI-ICI combination, both of grade 1-2, but there was also no report of nephritis or creatinine increase.
Table 2
Rates of all-grade and grade ≥3 creatinine increase, acute kidney injury (AKI) and nephritis
| Study name | Intervention | Sample Size | TRAE | Experimental arm | Control | ||||
| Total | Experimental | Control | Any grade (%) | ≥grade 3 (%) | Any grade (%) | ≥grade 3 (%) | |||
| Checkmate-9ER | Nivolumab + Cabozantinib | 640 | 320 | 320 | Creatinine increase | 42 (13.1) | 4 (1.2) | 43 (13.4) | 1 (0.3) |
| AKI/Renal failure | 2 (0.6) | 1 (0.3) | 2 (0.6) | 0 | |||||
| Nephritis | 1 (0.3) | 0 | 0 | 0 | |||||
| CLEAR | Pembolizumab + Lenvatinib | 692 | 352 | 340 | Creatinine increase | 48 (13.6) | 4 (1.1) | 34 (10.0) | 2 (0.6) |
| AKI/“Renal events” | 78 (22.2) | 20 (5.7) | 60 (17.6) | 8 (2.4) | |||||
| Nephritis | 6 (1.7) | 4 (1.1) | 0 | 0 | |||||
| Keynote-426 | Pembrolizumab + Axitinib | 861 | 432 | 429 | Creatinine increase | NR | NR | NR | NR |
| AKI | NR | NR | NR | NR | |||||
| Nephritis | 8 (2) | 3 (<1) | 1 (<1) | 1 (<1) | |||||
| Checkmate-214 | Nivolumab + Ipilimumab | 1082 | 547 | 535 | Creatinine increase | 40 (7.3) | 1(<1) | 47 (8.8) | 6 (1.1) |
| AKI | 12 (2.2) | 4 (<1) | 9 (1.7) | 3 (<1) | |||||
| Nephritis | NR | NR | NR | NR | |||||
| BIONIKK | Nivolumab + Ipilimumab | 141 | 101 | 40 | Creatinine increase | NR | NR | NR | NR |
| AKI | 2 | 0 | 1 | 0 | |||||
| Nephritis | NR | NR | NR | NR | |||||
TRAE: treatment related adverse event, AKI: Acute kidney injury, NR: not reported.
Creatinine increase was not reported in Keynote-426, which tested the combination of pembrolizumab plus axitinib, while it was reported on all other trials.
AKI was not always equally reported across the studies. In Checkmate-9ER it could have been described both as AKI or renal failure. In the CLEAR trial, adverse events were classified in a broader category of “renal events”. Although it is not clear if all “renal events” could be interpreted as “AKI”, for the purpose of this review, they were analyzed together. In Keynote-426, there was no report of AKI events.
Except for Checkmate-214 and BIONIKK, all other trials reported on the rate of “nephritis”. Taking in consideration only the experimental arm of these 3 trials, there were 15 (1,3%) reported cases of all-grade nephritis, of which 7 were grade 3 or higher. There was also 1 case of nephritis reported with the use of sunitinib, in Keynote-426.
Grade 5 events
There were 16 reported deaths in the ICI-TKI combination arms compared to 10 in the sunitinib arm and there were 9 reported deaths in the ICI-ICI combination arms compared to 6 in the VEGF-TKI arm of those trials. From these 16 reported deaths in all VEGF-TKI plus ICI combination trials, 2 (12.5%) were caused by nephrotoxic events. Both cases were reported in the CLEAR trial, of lenvatinib plus pembrolizumab. One case was reported to be secondary to AKI and the other due to uncontrolled hypertension. Also in the CLEAR study, sunitinib was related to the occurrence of 1 (16.6%) nephrotoxic related death, reported as secondary to both respiratory failure and AKI. There were no ICI-ICI nephrotoxic related deaths reported. One patient of the BIONIKK VEGF-TKI arm had a thrombotic microangiopathy grade 5 event, which was not counted as a nephrotoxic event.
DISCUSSION
This systematic review and meta-analysis represents the first evaluation of nephrotoxic events linked with modern systemic treatments for ccRCC. The study adopted an inclusive definition of nephrotoxic events, encompassing a range of distinct terms. Significantly, a substantial portion of the compiled data was exclusively available within the supplementary materials, particularly when it was absent from the information provided in the main articles.
There was a statistically significant higher rate of grade ≥3 proteinuria associated with the ICI-TKI combinations when compared to VEGF-TKI alone. However, this observation might not necessarily indicate a synergistic effect between immunotherapy and VEGF-TKI leading to an increased incidence of proteinuria. Notably, the presence of proteinuria induced by ICI alone, particularly in ICI-ICI combinations, was notably less pronounced than when using VEGF-TKI as a monotherapy. An alternative explanation could be that the high VEGF receptor affinity of lenvatinib and axitinib, as compared to other VEGF-TKIs, could potentially contribute to their propensity for inducing higher rates of proteinuria. These drugs have previously demonstrated elevated occurrences of proteinuria, a factor frequently linked to dose modifications or interruptions in their administration [13, 22, 23].
Hypertension did not exhibit any statistically significant difference in occurrence between ICI-TKI combinations and VEGF-TKI alone. Nevertheless, there was a tendency of a higher frequency of grade ≥3 hypertension cases within the ICI-TKI combination group compared to VEGF-TKI alone. If a distinction were to emerge, it might potentially be attributed to the same rationale as previously mentioned for proteinuria: the heightened potency of axitinib and lenvatinib, which could make them more prone to inducing hypertension [9, 24].
The ICI-ICI combination arms showcased only minimal instances of hypertension specifically associated with immunotherapy treatment and it was both evident and statistically significant that VEGF-TKI causes more instances of hypertension than the use of immunotherapy alone.
It is important to note that the limited usage of pazopanib in the BIONIKK trial is unlikely to exert a significant influence on the studied outcomes. Although pazopanib and sunitinib might be interchangeable in terms of efficacy, they undoubtedly exhibit distinct profiles of adverse events. In the COMPARZ trial, of first-line setting ccRCC, 548 patients received sunitinib and 554 patients were treated with pazopanib [25]. Within this context, pazopanib yielded 3% (17 cases) of proteinuria, while sunitinib presented 1% (6 cases). For all-grade hypertension, the incidence was 46% (257 cases) with pazopanib and 41% (223 cases) with sunitinib [25]. Consequently, the COMPARZ trial indicated a different profile of adverse events for pazopanib and sunitinib. However, concerning hypertension and proteinuria, the differentiation between the two agents appears less pronounced [26]. In any scenario, the influence of pazopanib on the outcomes of our study is likely to be minimal due to the relatively small size of the group under consideration, in relation to the overall study population.
The descriptions of various renal abnormalities leading to an eventual increase in creatinine were notably diverse across the different trials. The terminology employed encompassed a wide spectrum, ranging from the broadest term “creatinine increase” to more specific terms like “nephritis,” which denotes a kidney injury with an immune-related mechanism. When reviewing the reports, it remains challenging to estimate the extent of overlap that might exist among these different categories. Notably, a grade 3 increase in creatinine could likely result in hospitalization due to acute kidney injury (AKI), essentially making them equivalent adverse events. Certain trials opted for an even broader classification, employing the term “renal and urinary disorders,” a broader category from CTCAE. This category encompasses various adverse events, including those included here, but also extends to conditions like “chronic kidney disease,” “hematuria,” and “nephrotic syndrome” [18]. For clinical trial investigators, reporting on nephrotoxic events can prove particularly challenging, especially in contexts where these events are infrequent. Furthermore, fluctuations in participants’ creatinine levels may not always be solely attributable to the treatment administered, and such variations might not necessarily warrant major changes in their management. On the other hand, in patients with underlying chronic kidney disease (CKD), minor increases in serum creatinine could represent a major complication. Therefore, although measurement of serum creatinine alone is not a reliable marker of kidney function, it is still the main form of assessment either for including or excluding patients in clinical trials or during the study’s follow-up [27]. This lack of harmonization of kidney function assessment and report has been previously noticed as it encompasses both early phase and phase III clinical trials [28, 29]. Consequently, our ability to properly interpret how toxic novel oncologic treatments are to the kidney and how to manage them adequately is quite limited [30]. The IRMA study has previously shown the prevalence of CKD in patients with cancer undergoing treatment, although this retrospective analysis was mostly limited to cytotoxic agents and did not include various cancer types [31]. Despite these complexities, future trials could aim to enhance the reporting of nephrotoxic events, potentially incorporating a classification system for different types of injuries, akin to the approach taken with nephritis and reporting baseline kidney function by creatine clearance estimates with the proper formulas. Further insight could be gained with the inclusion of nephrologists as partners in the planning and conduction of oncological clinical trials [32].
Nephritis unequivocally signifies a type of kidney injury that would not likely manifest without the use of immune checkpoint inhibitors (ICIs) [33]. It is estimated that these ICIs can directly contribute to acute kidney injury (AKI) in approximately 2.2% of treated patients [15, 34]. In our data, we found a 1,3% rate of all-grade nephritis, which is not distant from other reports. Moreover, previous data have indicated a correlation between the potential heightened risk of AKI development in patients undergoing ICI therapy, particularly if they are concurrently taking proton pump inhibitors or non-steroidal anti-inflammatory drugs [35]. The predominant manifestation of immune-related kidney injury typically takes the form of acute tubulo-interstitial nephritis (ATIN) [36, 37]. Instances of immune-related ATIN may present with sterile pyuria, mild proteinuria, and eosinophilia. Empirical corticosteroid treatment is often considered for suspected cases; however, prudent consideration should be given to conducting a kidney biopsy. Such a biopsy not only aids in confirming the cause of AKI but also assists in differentiation from other types of immune-related injuries, beyond ATIN, that can lead to nephritis [38–40].
Nephrotoxic events constituted a considerable proportion of all fatalities attributed to the interventions studied. The emergence of immune checkpoint inhibitor (ICI) combinations and their expanded application across various tumor types has heightened investigators’ awareness of adverse events, prompting proactive interventions to avert fatal consequences [20]. However, it is important to underscore that nephrotoxic events stemming from ICI-TKI combinations can indeed lead to lethal outcomes.
CONCLUSIONS
Combination of ICI with VEGF-TKI has statistically higher degree of proteinuria compared to VEGF-TKI alone. No statistically significant difference in rates of hypertension was observed in the trials included in our study between ICI-TKI and VEGF-TKI alone. On the other hand, VEGF-TKI alone is associated with higher rates of all grade and grade ≥3 hypertension, compared to immunotherapy alone. Renal injury (increase in creatinine, AKI and nephritis) were uncommon. Description of renal injuries (increase in creatinine, AKI and nephritis) in the studies we report here have been diverse and a standardized definition of this term across clinical trials will be helpful to reliably interpret the data regarding nephrotoxicity in the setting of treatment of renal cell cancer.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
AD collected data, wrote, and analyzed. XL collected, wrote, and analyzed. SKP designed, reviewed, and revised. MZ designed, collected, wrote, and revised.
CONFLICTS OF INTEREST
Dr. Pal is an Editorial Board Member of this journal, but was not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review.
Dr. Pal has received travel expenses from CRISPR and Ipsen.
Drs. Dukkipati, Li and Zugman have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are derived from articles published in scientific journals and are publicly available within the respective publications.
Researchers interested in accessing the specific data used in this study can refer to the cited sources, and the data can also be provided upon request.
REFERENCES
[1] | Motzer R , Alekseev B , Rha SY , Porta C , Eto M , Powles T , et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma, New England Journal of Medicine (2021) ;384: (14):1289–1300. |
[2] | Powles T , Plimack ER , Soulières D , Waddell T , Stus V , Gafanov R , et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial, Lancet Oncol (2020) ;21: (12):1563–1573. |
[3] | Choueiri TK , Powles T , Burotto M , Escudier B , Bourlon MT , Zurawski B , et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma, New England Journal of Medicine (2021) ;384: (9):829–841. |
[4] | Rini BI , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial, The Lancet (2019) ;393: (10189):2404–2415. |
[5] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma, New England Journal of Medicine (2019) ;380: (12):1103–1115. |
[6] | Motzer RJ , Tannir NM , McDermott DF , Arén Frontera O , Melichar B , Choueiri TK , et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma, New England Journal of Medicine (2018) ;378: (14):1277–1290. |
[7] | Schmidinger M Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. In: European Journal of Cancer, Supplement. 2013:172-191. |
[8] | Amin A , Plimack ER , Ernstoff MS , Lewis LD , Bauer TM , McDermott DF , et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: The CheckMate 016 study, J Immunother Cancer (2018) ;6: (1). |
[9] | Rini BI , Atkins MB , Choueiri TK , Thomaidou D , Rosbrook B , Thakur M , et al. Time to resolution of axitinib-related adverse events after treatment interruption in patients with advanced renal cell carcinoma, Clin Genitourin Cancer (2021) ;19: (5):e306–e312. |
[10] | Franzin R , Netti GS , Spadaccino F , Porta C , Gesualdo L , Stallone G , et al. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Vol. 11, Frontiers in Immunology. Frontiers Media S.A.; 2020. |
[11] | Siegel RL , Miller KD , Wagle NS , Jemal A Cancer statistics, CA Cancer J Clin (2023) ;73: (1):17–48. |
[12] | Semeniuk-Wojtás A , Lubas A , Stec R , Szczylik C , Niemczyk S Influence of tyrosine kinase inhibitors on hypertension and nephrotoxicity in metastatic renal cell cancer patients. Vol. 17, International Journal of Molecular Sciences. MDPI AG; 2016. |
[13] | Zhang W , Feng LJ , Teng F , Li YH , Zhang X , Ran YG Incidence and risk of proteinuria associated with newly approved vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: An up-to-date meta-analysis of randomized controlled trials, Expert Rev Clin Pharmacol (2020) ;13: (3):311–320. |
[14] | Mielczarek Ł , Brodziak A , Sobczuk P , Kawecki M , Cudnoch-Jedrzejewska A , Czarnecka AM Renal toxicity of targeted therapies for renal cell carcinoma in patients with normal and impaired kidney function. Vol. 87, Cancer Chemotherapy and Pharmacology. Springer Science and Business Media Deutschland GmbH; 2021, pp. 723-742. |
[15] | Seethapathy H , Zhao S , Chute DF , Zubiri L , Oppong Y , Strohbehn I , et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors, Clinical Journal of the American Society of Nephrology (2019) ;14: (12):1692–1700. |
[16] | Cantini L , Merloni F , Rinaldi S , Lenci E , Marcantognini G , Meletani T , et al. Electrolyte disorders in advanced non-small cell lung cancer patients treated with immune check-point inhibitors: A systematic review and meta-analysis. Vol. 151, Critical Reviews in Oncology/Hematology. Elsevier Ireland Ltd; 2020. |
[17] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Vol. 372, The BMJ. BMJ Publishing Group; 2021. |
[18] | Cancer Institute N. Common terminology criteria for adverse events (CTCAE) common terminology criteria for adverse events (CTCAE) v5.0 [Internet]. 2017. Available from: https://www.meddra.org/ |
[19] | Brahmer JR , Abu-Sbeih H , Ascierto PA , Brufsky J , Cappelli LC , Cortazar FB , et al. Society for immunotherapy of cancer (sitc) clinical practice guideline on immune checkpoint inhibitor-related adverse events, J Immunother Cancer. (2021) ;9: (6). |
[20] | Schneider BJ , Naidoo J , Santomasso BD , Lacchetti C , Adkins S , Anadkat M , et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update, J Clin Oncol [Internet] (2021) ;39: :4073–4126. Available from: https://doi. |
[21] | Ouzzani M , Hammady H , Fedorowicz Z , Elmagarmid A Rayyan-a web and mobile app for systematic reviews, Syst Rev (2016) ;5: (1). |
[22] | Motzer R , George S , Merchan JR , Hutson TE , Song X , Perini RF , et al. Characterization and management of adverse reactions from the CLEAR study in advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab, Oncologist (2023) ;28: (6):501–509. |
[23] | Srinivas S , Stein D , Teltsch DY , Tao S , Cisar L , Ramaswamy K . Real-world chart review study of adverse events management in patients taking tyrosine kinase inhibitors to treat metastatic renal cell carcinoma, Journal of Oncology Pharmacy Practice (2018) ;24: (8):574–583. |
[24] | Shyam Sunder S , Sharma UC , Pokharel S Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Vol. 8, Signal Transduction and Targeted Therapy. Springer Nature; 2023. |
[25] | Motzer RJ , Hutson TE , Cella D , Reeves J , Hawkins R , Guo J , et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma, New England Journal of Medicine (2013) ;369: (8):722–731. |
[26] | Escudier B , Porta C , Bono P , Powles T , Eisen T , Sternberg CN , et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study, Journal of Clinical Oncology (2014) ;32: (14):1412–1418. |
[27] | Elyan BMP , Rankin S , Jones R , Lang NN , Mark PB , Lees JS Kidney disease patient representation in trials of combination therapy with VEGF-signaling pathway inhibitors and immune checkpoint inhibitors: A systematic review, Kidney Med (2023) ;5: (7). |
[28] | Rudek MA , Graham RA , Ratain MJ Harmonization of renal function assessment is needed during early clinical development of oncology drugs, Journal of Clinical Oncology. American Society of Clinical Oncology (2016) ;34: :103–104. |
[29] | Porta C , Cosmai L , Gallieni M , Perazella MA . Harmonization of renal function assessment is needed throughout the whole process of anticancer drug development, Journal of Clinical Oncology. American Society of Clinical Oncology (2016) ;34: :2429. |
[30] | Porta C , Cosmai L , Gallieni M , Pedrazzoli P , Malberti F . Renal effects of targeted anticancer therapies, Nature Reviews Nephrology. Nature Publishing Grou (2015) ;11: :354–370. |
[31] | Launay-Vacher V , Oudard S , Janus N , Gligorov J , Pourrat X , Rixe O , et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: The renal insufficiency and anticancer medications (IRMA) study, Cancer (2007) ;110: (6):1376–1384. |
[32] | Cosmai L , Porta C , Gallieni M , Perazella MA Onconephrology: A decalogue. Vol. 31, Nephrology Dialysis Transplantation. Oxford University Press; 2016, pp. 515-519. |
[33] | Curran CS , Kopp JB PD-1 immunobiology in glomerulonephritis and renal cell carcinoma. BMC Nephrology. BioMed Central Ltd; 2021;22. |
[34] | Cortazar FB , Marrone KA , Troxell ML , Ralto KM , Hoenig MP , Brahmer JR , et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors, Kidney Int (2016) ;90: (3):638–647. |
[35] | Ullur AR , Côté G , Pelletier K , Kitchlu A . Immunotherapy in oncology and the kidneys: A clinical review of the evaluation and management of kidney immune-related adverse events, Clin Kidney J 2023. |
[36] | Irifuku T , Satoh A , Tani H , Mandai K , Masaki T Acute tubulointerstitial nephritis and IgM deposits on glomerular capillary walls after immunotherapy with nivolumab for metastatic renal cell carcinoma, CEN Case Rep (2020) ;9: (1):48–54. |
[37] | Jung K , Zeng X , Bilusic M Nivolumab-associated acute glomerulonephritis: A case report and literature review, BMC Nephrol (2016) ;17: (1):1–6. |
[38] | Gupta S , Short SAP , Sise ME , Prosek JM , Madhavan SM , Soler MJ , et al. Acute kidney injury in patients treated with immune checkpoint inhibitors, J Immunother Cancer (2021) ;9: (10). |
[39] | Isik B , Alexander MP , Manohar S , Vaughan L , Kottschade L , Markovic S , et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events, Kidney Int Rep (2021) ;6: (4):1022–1031. |
[40] | Perazella MA , Shirali AC . Immune checkpoint inhibitor nephrotoxicity: What do we know and what should we do? Kidney International, Elsevier B.V (2020) ;97: :62–74. |




