Systematic Review of Comparative Studies of 3D Models for Preoperative Planning in Minimally Invasive Partial Nephrectomy
Abstract
BACKGROUND:
The employment of 3-dimensional (3D) virtual models of the organs and tumors, obtained from conventional 2-dimensional (2D) imaging (i.e. computed tomography scan and magnetic resonance imaging) have already demonstrated an outstanding potential in urology, especially in renal surgery.
OBJECTIVES:
The aim of this systematic review is to provide an updated focus on the results obtained from the preoperative employment of 3D virtual imaging reconstructions in nephron sparing oncological surgery.
METHODS:
A systematic literature search was conducted in April 2022 using Medline (via PubMed), Embase (via Ovid), Scopus, and Web of Science. The search strategy used PICO criteria and article selection was conducted in accordance with the PRISMA guidelines. The risk of bias and the quality of the articles included were assessed. A dedicated data extraction form was used to collect the data of interest.
RESULTS:
The initial electronic search identified 471 papers, of which 13 ultimately met the inclusion criteria and were included in the review. 11 studies reported outcomes of virtual models, 2 studies focused on printed 3D models. In these studies, the application of 3D models for preoperative planning has been reported to increase the selective clamping rate and reducing the opening of collecting system, blood loss and loss of renal function.
CONCLUSIONS:
3D virtual models seem to provide some surgical benefits for preoperative planning especially for complex renal masses. In the future the continuous evolution of this technology may further increase its field of application and its potential clinical benefit.
INTRODUCTION
Minimally invasive techniques revolutionized the concept of surgery over the last years, improving intra- and postoperative results along with patient’s satisfaction. Nowadays, we are taking a step forward to “Precision Medicine Era”, where each treatment strategy is tailored on the specific clinical case in order to achieve the therapy goal minimizing any impact on the patient. On the same line, “Precision Surgery” aims to target the surgical strategy on the specific disease, trying to spare all the healthy tissue in order to improve functional outcomes. Several tools have been introduced to maximize the performance of minimally invasive strategies, both in preoperative and postoperative setting. Among them, the employment of 3-dimensional (3D) virtual models of the organs and tumors, obtained from conventional 2-dimensional (2D) imaging (i.e. computed tomography scan and magnetic resonance imaging) have already demonstrated an outstanding potential in urology, especially in renal surgery, thanks to a better visualization of target anatomy with higher resolution of important details not visualized with conventional imaging [1–4]. This tool may help surgeons in maximize their effort in order to guarantee a safe and effective treatment.
In the setting of nephron sparing surgery, the use this technology aims to improve patient’s counselling and surgical strategy planning [5–7], including resection strategy (i.e. enucleation vs. enucleoresection of the tumor) and vascular clamping (i.e. selective vs. global arterial clamping), minimizing the impact of the surgery on kidney function without affecting oncological safety. Especially for complex renal masses, this advantages could help surgeons in reducing the rate of radical nephrectomies, postoperative complications, renal function impairment and local recurrences.
The aim of this systematic review is to provide an updated focus on the results obtained from the preoperative employment of 3D virtual imaging reconstructions in nephron sparing oncological surgery.
METHODS - Evidence aquisition
Research strategy
A systematic search of the literature was conducted through Medline (via PubMed), Embase (via Scopus) and Web of Science databases in April 2022.
The research strategy was built according to the “PICOS” criteria [8] (Patient-Intervention-Control-Outcome-Study design): we searched for patient undergoing partial nephrectomy (P) using preoperative 3D virtual models (I) or conventional partial nephrectomy (C) in order to assess peri operative, functional and oncological outcomes (O). Only comparative studies (S) were considered for the review.
No publication date restriction was applied.
Article selection
Article were selected in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
Literature records were independently screened for eligibility by two of the authors (G.V. and J.M.), disagreements were solved by a third reviewer (D.A.) until consensus was reached.
Only English written full text comparative studies reporting outcomes of the employment of 3D virtual models in the preoperative setting of partial nephrectomy were selected. Non comparative studies, review articles, comments, editorials, and congress abstracts were excluded from the evidence synthesis as well as studies on animals or cadaver.
References of the selected articles were manually reviewed to identify additional studies of interest.
Risk of bias assessment
The risk of bias was independently assessed by 2 authors (S.D.C. and F.P.) using The Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool [10] and the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [11] for of comparative studies and randomized controlled trials (RCTs), respectively.
Assessment of Study Quality
For non-randomized controlled trials (RCTs) the study quality was assessed using the Newcastle–Ottawa scale [12] (total score of≤5 was low quality, 6–7 as intermediate quality, and 8–9 as high quality). The RCTs were evaluated with Jadad scale [13] (0: very poor quality –5: rigorous quality. Moreover, the level of evidence of each study was assessed according to the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence [14].
RESULTS - Evidence Synthesis
471 papers were identified after the initial electronic search. After duplicates removal and title and abstract review, a total of 282 studies were identified for full text review. Finally, 13 studies [15–27] were found to meet the inclusion criteria and included in the review (Fig. 1). Among them 6 were prospective studies, 5 were retrospective and 2 were prospective RCTs. 6 studies evaluate the impact of 3D planning prior to Laparoscopic partial nephrectomy (LPN), 6 reported results in case of robot-assisted partial nephrectomy (RAPN) and only one study apply the use of 3D models reconstruction in both techniques. Considering the type of 3D technology applied, 11 studies reported outcomes of virtual models, 2 studies focused on printed 3D models. All characteristics al involved studies are summarized in Table 1.
Fig. 1
PRISMA 2020 flow diagram.
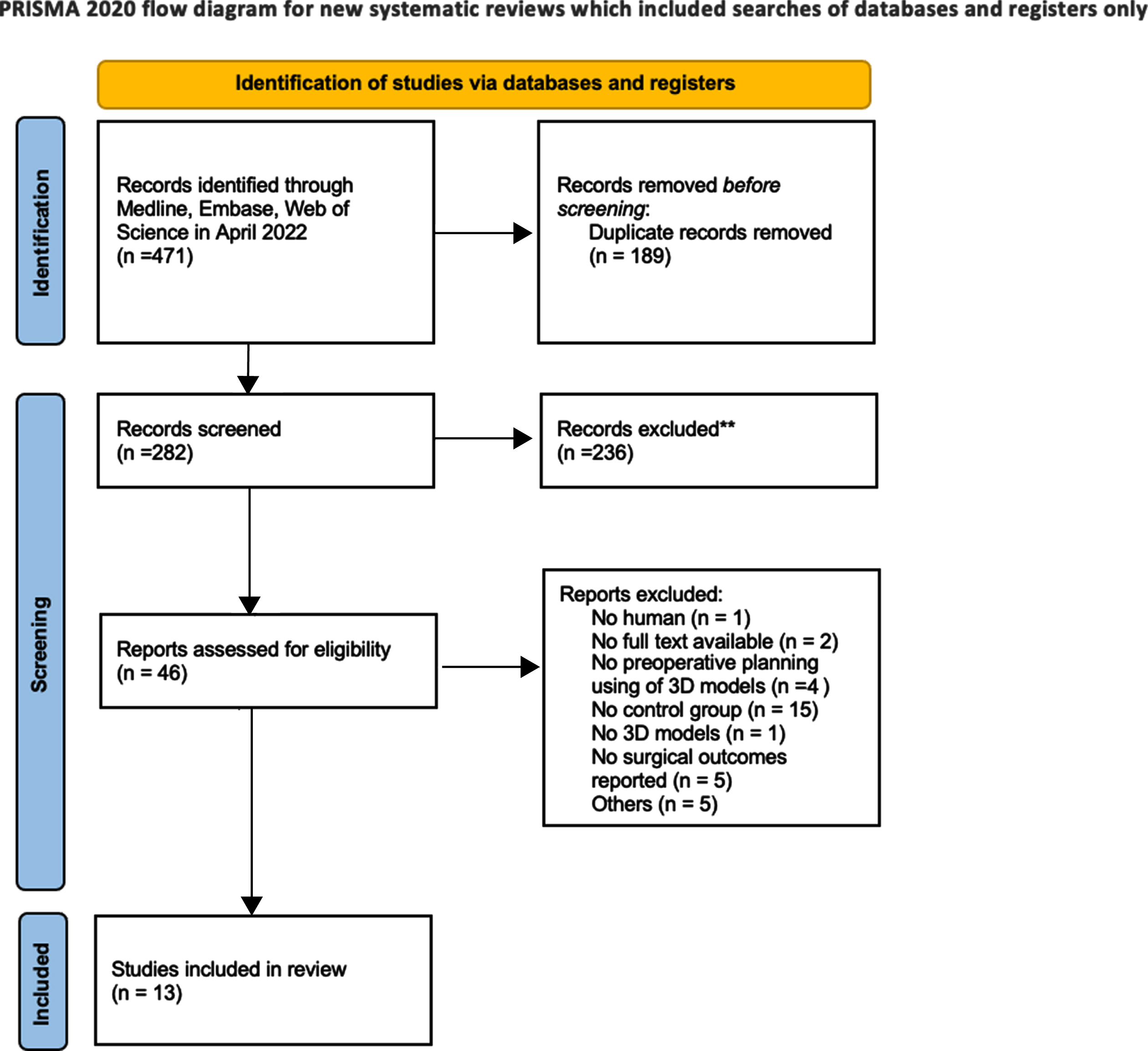
Table 1
Characteristics and level of evidence and quality assessment of involved studies
| First Author | Title | Year | Type of study | Surgical approach | Number of patients | 3D models application | Assessed Outcomes | SQ | LE | |
| 3D | No3D | |||||||||
| Haijie Zhang | Computed Tomography Image under Three-Dimensional | 2021 | RCT | LPN | 15 | 15 | Virtual reality | renal arteriovenous variability | Jadad 3 | 1b |
| Reconstruction Algorithm Based in Diagnosis of Renal Tumors | operation time, estimated | |||||||||
| and Retroperitoneal Laparoscopic Partial Nephrectomy | blood loss, intraoperative blood transfusion number/rate, | |||||||||
| incidence of complication, postoperative hemoglobin value | ||||||||||
| tumor recurrence number/rate | ||||||||||
| Cl.ment Michiels | 3D-Image guided robotic-assisted partial nephrectomy: | 2021 | Retrospective study | RAPN | 157 | 157 | Virtual reality | Intra-operative variables | 8 | 3 |
| a multi-institutional propensity score-matched analysis | Trifecta: negative | High Quality | ||||||||
| surgical margins | ||||||||||
| (UroCCR study 51) | 90% preservation of eGFR at first postoperative clinical visit | |||||||||
| (3– 6 post-operative month), no perioperative complication | ||||||||||
| Lorenzo Bianchi | The Impact of 3D Digital Reconstruction on the | 2020 | Prospective study | PN | 32 | 25 | Virtual reality | arterial clamping | 8 | 2b |
| Surgical Planning of Partial Nephrectomy: A | High Quality | |||||||||
| Case-control Study. Still Time for a Novel | ||||||||||
| Surgical Trend? | ||||||||||
| Jipeng Wang | The role of three-dimensional | 2019 | Retrospective study | LPN | 21 | 28 | Virtual rerality | Preoperative and postoperative | 7 Intermediate Quality | 3 |
| reconstruction in laparoscopic partial | ipsilateral parenchymal mass volume | |||||||||
| nephrectomy for complex renal tumors | GFR | |||||||||
| Michael M. Maddox | 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study | 2016 | feasibility study (retrospective control group) | RAPN | 7 | Prospectively maintained database | Printed | Ischemic time EBL | 6 Intermediate Quality | 3 |
| Length of hospital | ||||||||||
| Stay | ||||||||||
| Complications | ||||||||||
| Tumor histology | ||||||||||
| Margin status | ||||||||||
| Gang Fan | Three-dimensional printing | 2019 | Retrospective study | LPN | 69 | 58 | Printed | operative time, | 6 | 3 |
| for laparoscopic partial | estimated intra-/postoperative blood loss, | Intermediate Quality | ||||||||
| nephrectomy in patients | GFR, complications | |||||||||
| with renal tumors | ||||||||||
| Xiaorong Wu | Comparison of three dimensional | 2020 | Retrospective study | LPN | 30 | 30 | Virtual reality | perioperative variables | 6 | 3 |
| reconstruction and conventional computer | tumor feeding artery orientation | Intermediate Quality | ||||||||
| tomography angiography in patients | ||||||||||
| undergoing zero-ischemia laparoscopic | ||||||||||
| partial nephrectomy | ||||||||||
| Zhi Wang | Application of Three-Dimensional Visualization | 2017 | Retrospective study | LPN | 49 | 45 | Virtual reality | blood loss volume | 6 | 3 |
| Technology in Laparoscopic Partial Nephrectomy | postoperative complication | Intermediate Quality | ||||||||
| of Renal Tumor: A Comparative Study | selective | |||||||||
| clamping success rate, postoperative renal function, | ||||||||||
| operative and ischemic time | ||||||||||
| Joseph D. Shirk | The Use of 3-Dimensional, Virtual | 2019 | Prospective study (Retrospective control group) | RAPN | 30 | 30 | Virtual reality | operative | 7 Intermediate Quality | 3 |
| Reality Models for Surgical Planning of | time | |||||||||
| Robotic Partial Nephrectomy | clamp time | |||||||||
| estimated blood loss | ||||||||||
| hospital stay complications margin status | ||||||||||
| Joseph D. Shirk | Effect of 3-Dimensional Virtual Reality Models for Surgical Planning | 2019 | RCT | RAPN | 44 | 48 | Virtual reality | operative time | Jadad | 1b |
| of Robotic-Assisted Partial Nephrectomy on Surgical Outcomes | clamp time | 3 | ||||||||
| A Randomized Clinical Trial | estimated blood loss | |||||||||
| length of hospital stay. | ||||||||||
| Francesco Porpiglia | Hyperaccuracy Three-dimensional Reconstruction Is Able to | 2017 | Prospective study | RAPN | 21 | 31 | Virtual reality | renal arterial pedicle management | 8 | 2b |
| Maximize the Efficacy of Selective Clamping During Robotassisted | and success rate of its planned management | High Quality | ||||||||
| Partial Nephrectomy for Complex Renal Masses | ||||||||||
| Dongwen Wang | Preoperative planning and real-time assisted navigation by | 2014 | Prospective study (Retrospective control group) | LPN | 21 | 14 | Virtual reality | operative time | 6 | 3 |
| three-dimensional individual digital model in partial nephrectomy | segmental renal artery clamping time, | Intermediate Quality | ||||||||
| with three-dimensional laparoscopic system | estimated blood loss | |||||||||
| postoperative hospitalization | ||||||||||
| GFR | ||||||||||
| 6-month follow up | ||||||||||
| Daniele Amparore | Three-dimensional Virtual Models’ _Assistance During Minimally Invasive Partial Nephrectomy Minimizes the Impairment of Kidney Function | 2021 | Prospective study (Retrospective control group | RAPN | 100 | 251 | Virtual reality | loss of renal function | 7 | 3 |
| renal nuclear scintigraphy– derived postoperative outcomes | Intermediate Quality | |||||||||
Bias assessment, Level of Evidence and Study Quality
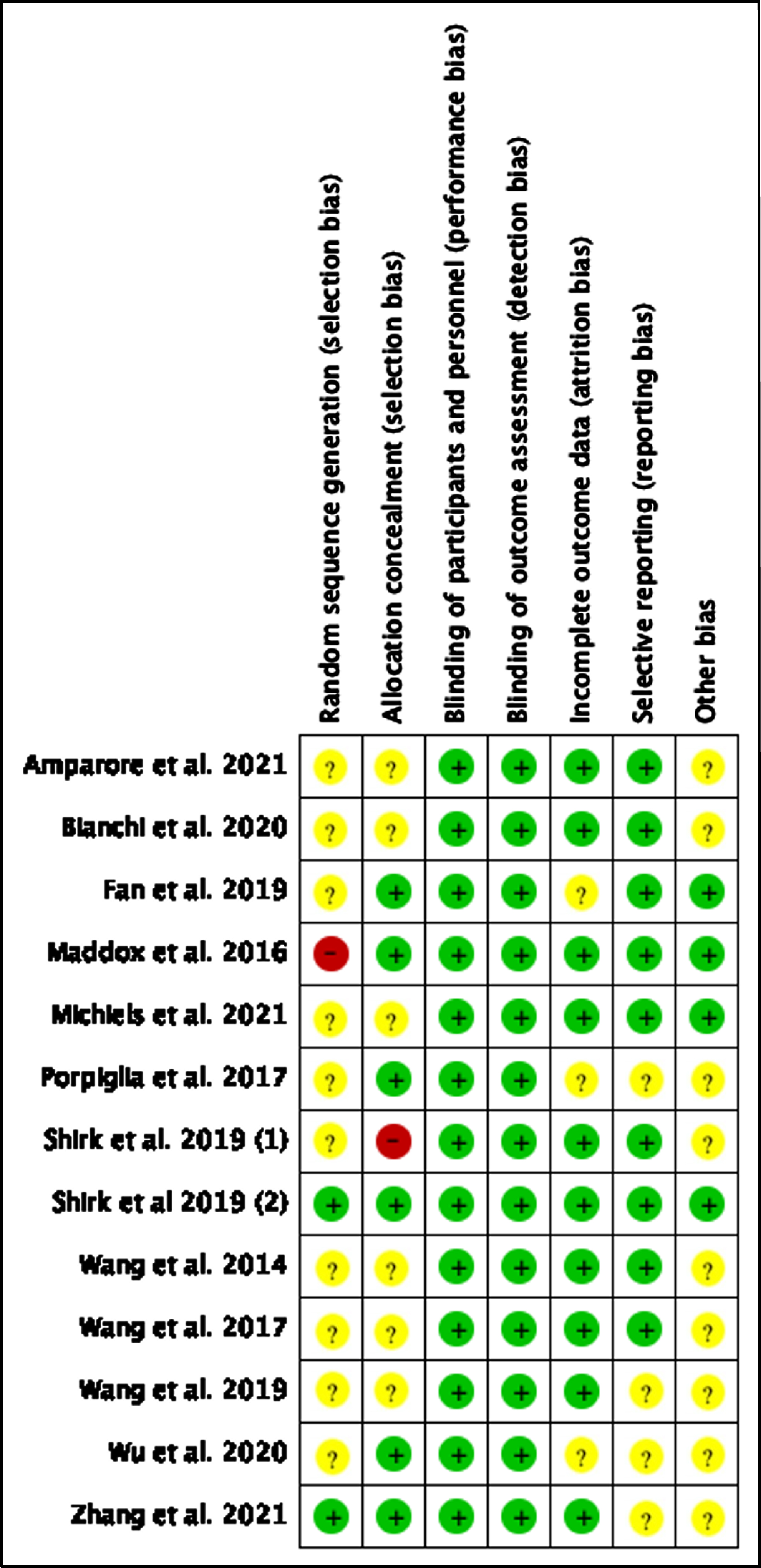
All the non-RCT studies included in our analysis ranged from intermediate to high quality according to the New- castle-Ottawa scale [16–26]. Both the RCTs were deemed to be of acceptable quality, receiving 3 points according to the Jadad scale [15, 27]. The quality assessment and level of evidence are summarized in Table 1. All the included studies revealed a risk of bias ranging from moderate to low, both for retrospective and randomized studies (Fig. 2).
Fig. 2
Risk of Bias of included studies.
3D virtual models preoperative planning
In 2015, Wang et al. [18] reported their preliminary experience with 3D guided LPN both for planning and intraoperative surgical navigation and compared their first 23 cases with 14 standard LPN. All the surgical staff members underwent meeting in which they discuss the clinical cases and explore the 3D virtual model appreciating tumors features and vascular anatomy. Moreover, for each case a selective clamping strategy was planned according to vascular anatomy. All the procedure were successfully completed with an effective selective clamping. No positive surgical margins were recorded. A lower operative time (159.0 vs. 193.2 min; p < 0.001), and estimated blood loss (148.1 vs. 176.1 mL; p < 0.001) were reported for the 3D group indicating a higher awareness of the surgical strategy. Following this concept some years later Porpiglia et al. [17] published their experience with 3D virtual model assistance for the preoperative planning of vascular strategy. This prospective study analyzed 52 patients scheduled for RAPN due to complex renal tumors and divided in two group (3D virtual model planning vs standard CT-scan based planning). In 90% of the 3D based planning group, intraoperative management of the renal pedicle was performed as preoperatively planned against only the 60% of control group (p = 0.04). Moreover, a lower rate of global ischemia was recorded in the 3D group (24% vs 80%, p < 0.01). Similar findings were reported later by Bianchi et al. in 2020 [20] with a 86% of pedicle management performed as preoperatively planned. The correspondence between 3D virtual models and real vascular anatomy was described also by Wu et al. [24]: 3D virtual models revealed a higher rate of feeding arteries identified respect the conventional CT-scan examination. The accuracy of that findings were directly correlated with intraoperative surgical findings during clampless LPN. Authors conclude that 3D virtual model assistance for preoperative identification of feeding arteries may facilitate clampless LPN.
In 2017 Wang et al. [25] underline as the 3D model assistance was more effective in reducing operative time (p = 0.018) and postoperative urinary leak (p = 0.033) in case of complex renal tumor. This finding was supported by another group in 2019 who focused the analysis on highly complex renal masses [21]. The use of 3D models for preoperative planning allows to reduce warm ischemia time (p = 0.003) and functional impairment (p = 0.01).
Concerning the impact of 3D virtual models on renal function, Amparore et al. [19] recently reported their experience by using renal scan to evaluate the functional drop after surgery. They analyzed 351 patients (100 with 3D model based planning and 251 performed with standard CT-scan preoperative planning) undergone minimally invasive PN with renal scan evaluation performed both pre- and postoperatively. The loss of renal function was significantly lower in thos procedures assisted by 3D virtual models, moreover at multivariable logistic regression the use of 3D models was the only protective factor against a significant functional damage in both intermediate (PADUA 8-9) and high complex tumor (PADUA≥10).
Regarding safety of 3D models, in a multi institutional propensity-score matched analysis adjusted for tumor complexity, Michiels et al. [16] reported a lower postoperative complication rate in the 3D group than the standard control group (3.8% vs. 9.5%, P = 0.04). they recorded also a better functional outcome (lower eGFR reduction: –5.6% vs. –10.5%, p = 0.002) and trifecta achievement (55.7% vs. 45.1% ; P = 0.005) in the 3D group.
In all the above-mentioned experiences the virtual models were usually navigable PDF, displayed on PC, laptop or tablet. A different way to use 3D virtual model for preoperative planning was described by Shirk et al. [26] in 2019. In their preliminary experience surgeon use Google Cardboard headset to visualize the 3D model in a more engaging setting.
Concerning the two RCT retrieved by our search their findings are consistent with those presented above. In particular Shirk et al. [27] randomized 92 patients in receiving either standard 2D preoperative planning [48] or 3D model based one [44]. The 3D model group showed shorter operative (odds ratio [OR], 1.00; 95% CI, 0.37– 2.70; estimated OR, 2.47) and ischemia time (OR, 1.60; 95% CI, 0.79– 3.23; estimated OR, 11.22), with lower blood loss (OR, 1.98; 95% CI, 1.04– 3.78; estimated OR, 4.56) and earlier hospital discharge (OR, 2.86; 95% CI, 1.59– 5.14; estimated OR, 5.43). Another group lead by H. Zhang [15] published few years later a similar experience randomizing 30 patients. Even in this experience 3D virtual models showed to be able to improve surgeon’s performance when applied for preoperative planning, reducing operative time (p < 0.05). Notwithstanding the relatively small sample size, these are the first RCTs demonstrating the efficacy of 3D based preoperative planning and providing reliable research basis for the clinical application of 3D virtual models in our daily practice.
3D printed model preoperative planning
Beside virtual reality experiences, in the last years also 3D printed models have been developed and tested their efficacy in enhance preoperative surgical planning. In particular, kidney surgery was one of the most attractive thanks to a higher anatomical and tumors variation. Maddox et al. [22] were one of the first group reporting their experience with 3D printed soft tissue models. The printed models were used not only to plan the surgeries but also to perform them allowing surgeon to simulate pretty the same conditions of the real interventions. Although this was a feasibility experience including only 7 procedures, the perioperative results was in favor of 3D printed use (even if not statistically significant). Another experience in 3D printed models was reported by Fan et al. in 2019 [23]. They analyzed 69 cases with the assistance of 3D printed models (made by Materialise, Leuven, Belgium) to appreciate the tumors’ relationship with surrounding anatomical structures and planned the most suitable surgical strategy. No differences were retrieved between the 3D group and the control group (CT-scan based only planning) except for a shorter ischemia time (24.1+ 5.1 vs 26.6 + 4.2 min, p < 0.05).
DISCUSSION
This critical review analyzed 13 Studies which investigated the use of 3D printed, augmented reality and virtual reality in clinical care. It is, therefore, one of the first reviews integrating the three-dimensional renal model and its surgical and functional results.
As stated by all the recent guidelines, the gold standard for the treatment of localized renal tumors is partial nephrectomy (PN) whenever it is technically feasible. One of the proposed way to assess PN perioperative outcomes is the “Trifecta Renal”, a term introduced by Hung et al. [28] which consists of free margins, absence of surgical complications and reduced ischemia time (<25 minutes). With technological development and the progressive introduction of technology in the operating room, sophisticated and minimally invasive means have been used to achieve Renal Trifecta, such as robotic surgery, intraoperative ultrasound and indocyanine (assessing renal perfusion and selective clamping). Such procedures allowed the introduction of a new era in renal surgery, the “Precision Era” [29]. In this context, the use of three-dimensional renal models for preoperative assessment and intraoperative navigation began [17], and this could represent a tool to further decrease the rate of radical nephrectomies performed for localized renal masses.
The results obtained will be discussed below, considering: type of study, surgical modality, software used, imaging techniques, operative time, estimated blood loss (EBL), renal clamping, functional outcome, associated complications, process costs and number of surgeons involved. Finally, limitations and future perspectives are highlighted.
Type of Study and Surgery
The studies were predominantly nRCT (n = 11, 84.6% ), being six retrospective [16, 21–25] and five prospective [17–20, 26]. It is noteworthy that in three of the prospective studies [18, 19, 26] the control group was built retrospectively, a modality also adopted in the feasibility study [22]. The retrospective predominance is possibly due to the difficulty in assembling large cohorts for intervention and control.
The two RCT studies [15, 27] were assessed on a level 3 Jadad scale. Of the nRCT studies, according to the Newcastle– Ottawa scale, eight showed Intermediate Quality [18, 19, 21–26] and three showed High Quality [16, 17, 20]. Regarding the level of evidence [14], the vast majority of studies (n = 9, 69.9% ) were classified as level 3 [16, 18, 19, 21–26], while two obtained a level 2b [17, 20] and two other studies conferred evidence 1b [15, 27].
As for the surgical modality of the articles reviewed, the distribution was homogeneous. Six of them were RAPN [16, 17, 19, 22, 26, 27], six LPN [15, 18, 21, 23–25] and only one study [20] included open surgery, LPN and RAPN. This distribution shows the reality that robotic surgery has gained irreversible ground in the field of uro-oncological surgery [30].
Image, Software and Use
Mixed Reality models are the product of an intense collaboration between radiologists, urologists and bioengineers, as three-dimensional virtual objects are developed from an additive manufacturing of thousands of 2D images [17]. The genesis of the formation of objects with HA3D begins with high resolution image files in DICOM format - Digital Imaging and Communications in Medicine - obtained through thin sections of magnetic resonance imaging or computed tomography. DICOM files are then converted, using different digital software, into Standard Mosaic Language (STL) codes, forming a virtual 3D object [31]. From this stage, there is an artistic work between the urologist and the biomedical engineer in delineating the virtual object according to the study demand through specific software. When planning three-dimensional renal models, the accuracy of the study is essential, not only of the arterial and venous tree, but also of the collecting system and the relationship between the renal tumor and the structures described.
Once the virtual object is finalized, it can be used in Mixed, Augmented or Virtual Reality and can also be printed on three-dimensional printers [32]. In the case of 3D kidney printing, technologies such as TangoPlus or PolyJet print artificial kidneys using printers such as Stratasys and MakerBotresinas. Printing is done on photopolymers, a material that confers biodynamic properties to mimic real tissue, enabling the replication of complete tissues andorgans.
Technological advances are also reflected in the number of software and the quality of its three-dimensional reconstructions. Of the nine software used, five (55.5% ) were developed in the United States (3DSystems, FreeForm Modeling System, IQQA, D2P software, Ceevra), one in Belgium (Mimics), one in Italy (M3DICS), one in Japan (Synapse) and one in China (3D-MIRGS). This reveals the diversity of centers and countries that have invested in this technology.
After rendering, the three-dimensional plans were used in Virtual Reality, applied in Augmented Reality [17, 19], Mixed Reality [18, 27] or 3D printing [22, 23]. These terms were first described by Paul Miligram in 1994, who also defined the concept of Mixed Reality as a spectrum of AR and VR [33].
Three-dimensional objects were used for preoperative planning in all studies, and in ten of them [15–20, 23, 24, 26, 27] they were also used for intraoperative planning. In the study by Maddox et al. [22], the application of 3D printing in preoperative planning led to an improvement in intraoperative decision-making and a considerable increase in the surgeon’s confidence to perform the proposed procedure. Likewise, Cacciamani et al. [31] claim that the creation of such specific models avoids complications that could not be foreseen in 2D representations.
Interestingly, Maddox et al. [22] and Wang et al. [25] also used their models for training and surgical simulation. The literature shows that generic training models allow students, residents and inexperienced surgeons to practice both technical and decision-making skills in an environment that does not cause harm to the patient [e.g. 34].
Estimated Blood Loss
Regarding blood loss, a relevant factor in partial nephrectomy, in the present review five authors reported a statistically significant decrease in blood loss in the groups that had three-dimensional perioperative planning for surgery [18, 21, 22, 24, 26, 27], when compared to groups with two-dimensional planning.
Operative Time
Reduced operative time was one of the most common findings in most studies in this review. Wang et al. [18, 25], Shirk et al [19], Wu et al. [24], Zang et al. [15] reported a statistically significant decrease in the operative time of surgeries with 3D planning. On the other hand, Bianchi et al. [20] and Michiels et al. [16] presented a longer operative time in partial nephrectomies with three-dimensional planning in their results. However, this fact can be explained by the fact that the patients included in their three-dimensional group are known to have larger tumors (4.3 cm [1.2–12 cm] vs. 3.5 cm [1–18 cm] p < 0.001) and with a complexity larger anatomical.
Selective Clamping
When we talk about renal clamping, surgeons usually clamp the main renal artery (approximately 80% of cases) [17]. Lieberman et al. [35] reported that global ischemia is preferred even when not necessary. Interestingly, studies investigating their benefit on the renal functional outcome did not reveal clinically significant difference with global ischemia [36, 37].
Regarding selective clamping, Porpiglia et al. [17] showed a significant reduction in the percentage of global clamping when using a three-dimensional template for three-dimensional planning (24% , p < 0.01). In their study, Bianchi et al. [20] reported a much higher rate of selective clamping of patients undergoing three-dimensional planning (57.1% vs. 13.3% , p = 0.01). Similar data were evidenced by Michiels et al. [16], where 35.2% of the group with 3D planning underwent selective clamping, with this rate being reduced in the control group (3.4% , p < 0.001). Likewise, the accurate tumor feeding artery dissection was higher in the 3D group in the study by Wu et al. [24]. The 3D-guided plan therefore allows for a higher percentage of selective arterial clamping compared to two-dimensional computed tomography and magnetic resonanceimaging.
Warm Ischemia Time and Zero Ischemia
The warm ischemia timeout is still a topic of debate in the literature, with the 30-minute tolerance period described by Ward et al. [38] and Novick et al. [39] being widely accepted as non-pejorative on the long term kidney function preservation [40]. Non global ischemia techniques have been proposed in recent times [41]. Shirk et al. [27] observed a significant decrease in clamping time in their 3D group compared to the group without three-dimensional planning. In their study, in addition to a reduction in WIT, there was a shorter hospital stay, EBL and OT. Likewise, Fan et al. [23] found an abbreviated ischemia time for their 3D group, whose tumors were classified as RENAL occurs > = 8.
Of the other strategies to minimize the loss of renal function, partial nephrectomy without clamping or zero-ischemia is one of the options adopted. Described by Gill et al. in 2011 [42], the procedure tends to be challenging, on a fine line between renal preservation and hemodynamic decline, which includes anatomical tumor devascularization with selective branch microdissection of the renal artery. In the present review, Michiels et al. [16] described a higher significant percentage of clampless procedures with the group in which three-dimensional perioperative planning and navigation were performed (54.3×4.8% p < 0.001).
Although some randomized trials have not shown a significant difference in perioperative surgical outcomes [43], partial nephrectomy is a tool to be considered and the use of three-dimensional reconstruction may have been a factor that allowed the surgeon to have a greater understanding of the tumor and a safety when managing the resection without arterial clamping.
3D PN Guided×Functional Outcome
Although many of the studies do not show a significant difference in the variation in the decline in renal function in kidneys undergoing partial nephrectomies with or without the use of three-dimensional reconstructions, Amparore et al. [19] described a significant reduction in the decline in renal function in the planning group. three-dimensional compared to the control group (– 10% ×19.6% , p = 0.02). In their series of 351 patients (3D = 100, n3D = 251), patients were sequentially studied with renal scintigraphy and renal function for 3 years after partial nephrectomy. The outcome was observed both in patients with intermediate PADUA classification 8– 9 and in high risk patients (PADUA> = 10). In turn, Wang et al. [21] described a higher percentage of preserved kidney when patients underwent partial nephrectomy with three-dimensional planning. Michiels et al. [16] also showed better functional results in their multicentric review.
Since the amount of renal parenchyma preserved after partial nephrectomy is one of the most important predictors of good maintenance of renal function [44], the use of 3D renal planning can be adopted as a protective factor against a decrease in renal function.
Complications
In the present review, fewer complications were found in surgeries with 3D planning compared to surgeries without three-dimensional planning. Wang et al. [25] reported a lower number of urinary fistula (0×4% ) and Porpiglia et al. [17] showed a smaller opening of the collecting system (41.9×14.3, p = 0.05). In accordance with these data, Michiels et al. [16] showed lower rates of perioperative complications in the three-dimensional group compared to the control group, including lower rates of blood transfusions.
Cost
The cost of reconstructions is a topic rarely addressed in the studies, with only Fan et al. [23] mentioning the value of creating a three-dimensional model, which is $500.00 (in 2019). Shirk et al. [26], on the other hand, cite the value of the device for displaying mixed reality, the Google Card, estimated at $15.00.
The main limitations to obtain a reliable cost assessment is due to the continuous development of this technology with a constant improvement and add of supporting technologies and details. For example, the 3D models can be used in different ways, from virtual to printed reality [2], each of them with different extra costs. Moreover, since it is still a research field it is also hard to quantifies the costs related to personnel and commercialization that companies will probably consider.
We believe that it is important to estimate the cost of three-dimensional renal models so that we can assess their use in the future, their impact on health policies and encourage, as far as possible, the introduction of these technologies in centers that do not yet have them.
Surgeons
The number of surgeons who performed partial nephrectomies in the evaluated studies was one [17–19, 21, 23, 24], three [16, 20, 26] and 11 [27]. The conclusions of the studies pointed out that nephrographic models have similar clinical outcomes, regardless of the number of surgeons who participated in each study. Importantly, multiple studies [16, 17, 21, 23, 26, 27] were performed with surgeons with high surgical volume (>150 procedures).
Limitations
The present review has its limitations. First, the number (n) of patients is limited in most studies, which is possibly due to the difficulty of setting up large cohorts on this topic. Moreover, they are not randomized and the control groups data are often retrospectively collected.
Secondly, relatively low-level evidence was recorded, even if that should not be considered as a limitation in the methodology but in the existing literature.
Thirdly, many of the cut off used by Authors are subjective and sometimes not meaningful, especially for estimated blood loos and ischemia time.
Fourth, the cost of three-dimensional products is rarely reported in the works, which could provide a quantitative value for the introduction of this technology in the daily routine of the operating room. Furthermore, there were few multicenter studies in the review, which could fine-tune a different reality on the same study. Finally, the studies are carried out with different software and different reconstructions, which can be used in different applications.
Future
We hope that the advent of three-dimensional renal reconstruction technology can increasingly assist the surgeon in surgical planning, surgical training and medical education. Further more, the continuous evolution of this technology may further increase its field of application and its potential clinical benefit.
CONCLUSION
The available literature evidences showed 3D virtual models may have some surgical benefits for preoperative planning especially for complex renal masses, reducing bleeding, surgical and ischemia time. Notwithstanding this promising results, further prospective randomized studies are needed to clarify their role and impact in our clinical practice.
ACKNOWLEDGMENTS
We would like to thank Dr. Nicoletta Colombi for her help during the systematic review.
FUNDING
None.
AUTHORS’ CONTRIBUTION
Protocol/project development: F. Porpiglia, F. Piramide, D. Duarte, E. Checcucci
Data collection or management: S. De Cillis, G. Volpi, J. Meziere,
Data analysis: S. De Cillis, F. Piramide, A. Piana
Manuscript writing/editing: F.Piramide, D. Duarte, D. Amparore
Supervision: C. Fiori, F. Porpiglia, E. Checcucci
CONFLICT OF INTEREST
Federico Piramide, Dorival Duarte, Daniele Amparore, Alberto Piana, Sabrina De Cillis, Gabriele Volpi, Juliette Meziere, Cristian Fiori, Francesco Porpiglia and Enrico Checcucci have nothing to declare.
REFERENCES
[1] | Porpiglia F , Bertolo R , Checcucci E , Amparore D , Autorino R , Dasgupta P , et al. Development and validation of 3D printed virtual models for robot-assisted radical prostatectomy and partial nephrectomy: urologists’ and patients’ perception. World J Urol. (2018) ;36: :201–7. https://doi.org/10.1007/s00345-017-2126-1 |
[2] | Porpiglia F , Amparore D , Checcucci E , Autorino R , Manfredi M , Iannizzi G , et al. Current Use of Three-dimensional Model Technology in Urology: A Road Map for Personalised Surgical Planning. Eur Urol Focus. (2018) ;4: :652–6. https://doi.org/10.1016/j.euf.2018.09.012 |
[3] | Checcucci E , Amparore D , Pecoraro A , Peretti D , Aimar R , De cillis S , et al. 3D mixed reality holograms for pre-operative surgical planning of nephron-sparing surgery: evaluation of surgeons’ perception. Minerva Urol e Nefrol 2019. https://doi.org/10.23736/S0393-2249.19.03610-5 |
[4] | Amparore D , Piramide F , De Cillis S , Verri P , Piana A , Pecoraro A , et al. Robotic partial nephrectomy in 3D virtual reconstructions era: is the paradigm changed? World J Urol. (2022) ;40: :659–70. https://doi.org/10.1007/s00345-022-03964-x |
[5] | AMPARORE D , PECORARO A , CHECCUCCI E , DE CILLIS S , PIRAMIDE F , VOLPI G , et al. 3D imaging technologies in minimally invasive kidney and prostate cancer surgery: which is the urologists’ perception? Minerva Urol Nephrol. (2022) ;74: . https://doi.org/10.23736/S2724-6051.21.04131-X |
[6] | Wang Y , Chen M , Li Y , Zhao C , Tong S , Cai Y , et al. Clinical implications of 3D printing technology in preoperative evaluation of partial nephrectomy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2022) ;47: :328–33. https://doi.org/10.11817/j.issn.1672-7347.2022.210586 |
[7] | Yoshitomi KK , Komai Y , Yamamoto T , Fukagawa E , Hamada K , Yoneoka Y , et al. Improving Accuracy, Reliability, and Efficiency of the RENAL Nephrometry Score With 3D Reconstructed Virtual Imaging. Urology. 2022. https://doi.org/10.1016/j.urology.2022.01.024 |
[8] | Cumpston M , Li T , Page MJ , Chandler J , Welch VA , Higgins JP , et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.ED000142 |
[9] | Moher D , Liberati A , Tetzlaff J , Altman DG . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. (2010) ;8: :336–41. https://doi.org/10.1016/j.ijsu.2010.02.007 |
[10] | Sterne JA , Hernán MA , Reeves BC , Savović J , Berkman ND , Viswanathan M , et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) ;i4919: . https://doi.org/10.1136/bmj.i4919 |
[11] | Sterne JAC , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) ;l4898: . https://doi.org/10.1136/bmj.l4898 |
[12] | Wells GA , Shea B , O’Connell D , Peterson J , Welch V , Losos MPT . The Newcastle Ottawa 1 Scale (NOS) for Assessing the Quality of Non-randomized Studies in Meta- Analyses. n.d. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp |
[13] | Jadad AR , Moore RA , Carroll D , Jenkinson C , Reynolds DJM , Gavaghan DJ , et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. (1996) ;17: :1–12. https://doi.org/10.1016/0197-2456(95)00134-4 |
[14] | Guyatt GH , Oxman AD , Vist GE , Kunz R , Falck-Ytter Y , Alonso-Coello P , et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) ;336: :924–6. https://doi.org/10.1136/bmj.39489.470347.AD |
[15] | Zhang H , Yin F , Yang L , Qi A , Cui W , Yang S , et al. Computed Tomography Image under Three-Dimensional Reconstruction Algorithm Based in Diagnosis of Renal Tumors and Retroperitoneal Laparoscopic Partial Nephrectomy. J Healthc Eng. (2021) ;2021: :1–9. https://doi.org/10.1155/2021/3066930 |
[16] | Michiels C , Khene ZE , Prudhomme T , Boulenger de Hauteclocque A , Cornelis FH , Percot M , et al. 3D-Image guided robotic-assisted partial nephrectomy: a multi-institutional propensity score-matched analysis (UroCCR study 51). World J Urol. 2021. https://doi.org/10.1007/s00345-021-03645-1 |
[17] | Porpiglia F , Fiori C , Checcucci E , Amparore D , Bertolo R . Hyperaccuracy Three-dimensional Reconstruction Is Able to Maximize the Efficacy of Selective Clamping During Robot-assisted Partial Nephrectomy for Complex Renal Masses. Eur Urol. (2018) ;74: :651–60. https://doi.org/10.1016/j.eururo.2017.12.027 |
[18] | Wang D , Zhang B , Yuan X , Zhang X , Liu C . Preoperative planning and real-time assisted navigation by three-dimensional individual digital model in partial nephrectomy with three-dimensional laparoscopic system. Int J Comput Assist Radiol Surg. (2015) ;10: :1461–8. https://doi.org/10.1007/s11548-015-1148-7 |
[19] | Amparore D , Pecoraro A , Checcucci E , Piramide F , Verri P , De Cillis S , et al. Three-dimensional Virtual Models’ Assistance During Minimally Invasive Partial Nephrectomy Minimizes the Impairment of Kidney Function. Eur Urol Oncol. (2022) ;5: :104–8. https://doi.org/10.1016/j.euo.2021.04.001 |
[20] | Bianchi L , Barbaresi U , Cercenelli L , Bortolani B , Gaudiano C , Chessa F , et al. The Impact of 3D Digital Reconstruction on the Surgical Planning of Partial Nephrectomy: A Case-control Study. Still Time for a Novel Surgical Trend? Clin Genitourin Cancer. (2020) ;18: :e669–78. https://doi.org/10.1016/j.clgc.2020.03.016 |
[21] | Wang J , Lu Y , Wu G , Wang T , Wang Y , Zhao H , et al. The role of three-dimensional reconstruction in laparoscopic partial nephrectomy for complex renal tumors. World J Surg Oncol. (2019) ;17: :159. https://doi.org/10.1186/s12957-019-1701-x |
[22] | Maddox MM , Feibus A , Liu J , Wang J , Thomas R , Silberstein JL . 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J Robot Surg. (2017) ;12: :27–33. https://doi.org/10.1007/s11701-017-0680-6 |
[23] | Fan G , Meng Y , Zhu S , Ye M , Li M , Li F , et al. Three-dimensional printing for laparoscopic partial nephrectomy in patients with renal tumors. J Int Med Res. (2019) ;47: :4324–32. https://doi.org/10.1177/0300060519862058 |
[24] | Wu X , Jiang C , Wu G , Shen C , Fu Q , Chen Y , et al. Comparison of three dimensional reconstruction and conventional computer tomography angiography in patients undergoing zero-ischemia laparoscopic partial nephrectomy. BMC Med Imaging. (2020) ;20: :47. https://doi.org/10.1186/s12880-020-00445-8 |
[25] | Wang Z , Qi L , Yuan P , Zu X , Chen W , Cao Z , et al. Application of Three-Dimensional Visualization Technology in Laparoscopic Partial Nephrectomy of Renal Tumor: A Comparative Study. J Laparoendosc Adv Surg Tech. (2017) ;27: :516–23. https://doi.org/10.1089/la2016.0645 |
[26] | Shirk JD , Kwan L , Saigal C . The Use of 3-Dimensional, Virtual Reality Models for Surgical Planning of Robotic Partial Nephrectomy. Urology. (2019) ;125: :92–7. https://doi.org/10.1016/j.urology.2018.12.026 |
[27] | Shirk JD , Thiel DD , Wallen EM , Linehan JM , White WM , Badani KK , et al. Effect of 3-Dimensional Virtual Reality Models for Surgical Planning of Robotic-Assisted Partial Nephrectomy on Surgical Outcomes. JAMA Netw Open. (2019) ;2: :e1911598. https://doi.org/10.1001/jamanetworkopen.2019.11598 |
[28] | Hung AJ , Cai J , Simmons MN , Gill IS . “Trifecta” in partial nephrectomy. J Urol. (2013) ;189: (1):36–42. doi: 10.1016/j.juro.2012.09.042. Epub 2012 Nov 16. PMID: 23164381. https://doi.org/10.1089/la2016.0645 |
[29] | Autorino R , Porpiglia F , Dasgupta P , et al. Precision surgery and genitourinary cancers. Eur J Surg Oncol. (2017) ;43: :893–908. |
[30] | Rassweiler Jens J ; Autorino, Riccardo ; Klein, Jan ; Mottrie, Alex ; Goezen, Ali Serdar ; Stolzenburg, Jens-Uwe ; Rha, Koon H. ; Schurr, Marc ; Kaouk, Jihad ; Patel, Vipul ; Dasgupta, Prokar ; Liatsikos, Evangelos . (2017). Future of robotic surgery in urology. BJU International, (), –.doi: 10.1111/bju.13851 |
[31] | Cacciamani GE , Okhunov Z , Meneses AD , Rodriguez-Socarras ME , Rivas JG , Porpiglia F , Liatsikos E , Veneziano D . Impact of Three-dimensional Printing in Urology: State of the Art and Future Perspectives. A Systematic Review by ESUT-YAUWP GrouEur Urol. (2019) ;76: (2):209–21. doi:10.1016/j.eururo.2019.04.044. Epub 2019 May 18. PMID: 31109814 |
[32] | Bertolo R , Autorino R , Fiori C , Amparore D , Checcucci E , Mottrie A , Porter J , Haber GP , Derweesh I , Porpiglia F . Expanding the Indications of Robotic Partial Nephrectomy for Highly Complex Renal Tumors: Urologists’ Perception of the Impact of Hyperaccuracy Three-Dimensional Reconstruction. J Laparoendosc Adv Surg Tech A. (2019) ;29: (2):233–9. doi:10.1089/la2018.0486. Epub 2018 Nov 3. PMID: 30394820. |
[33] | Milgram P , Takemura H , Utsumi A , Kishino F . Augmented reality: a class of displays on the reality-virtuality continuum, Telemanipulator and Telepresence Technologies (1994) 2351. |
[34] | Silberstein JL , Maddox MM , Dorsey P , Feibus A , Thomas R , Lee BR . Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology. (2014) ;84: :268–72. |
[35] | Lieberman L , Barod R , Dalela D , et al. Use of main renal artery clamping predominates over minimal clamping techniques during robotic partial nephrectomy for complex tumors. J Endourol. (2017) ;31: :149–52. |
[36] | Desai MM , de Castro Abreu AL , Leslie S , et al. Robotic partial nephrectomy with superselective versus main artery clamping: a retrospective comparison. Eur Urol. (2014) ;66: :713–9. |
[37] | Long JA , Fiard G , Giai J , Teyssier Y , Fontanell A , Overs C , Poncet D , Descotes JL , Rambeaud JJ , Moreau-Gaudry A , Ittobane T , Bouzit A , Bosson JL , Lanchon C . Superselective Ischemia in Robotic Partial Nephrectomy Does Not Provide Better Long-term Renal Function than Renal Artery Clamping in a Randomized Controlled Trial (EMERALD): Should We Take the Risk? Eur Urol Focus. 2021:S2405-4569(21)00115-2. doi: 10.1016/j.euf.2021.04.009. Epub ahead of print. PMID: 33931361. |
[38] | Ward JP . Determination of the Optimum temperature for regional renal hypothermia during temporary renal ischaemia. Br J Urol. (1975) ;47: (1):17–24. doi: 10.1111/j.1464-410x.1975.tb03913.x. PMID: 236801. |
[39] | Novick AC . Renal hypothermia: in vivo and ex vivo. Urol Clin North Am. (1983) ;10: (4):637–44. PMID: 6356550. |
[40] | Greco , Francesco Autorino , Riccardo Altieri , Vincenzo Campbell , Steven Ficarra , Vincenzo Gill , Inderbir Kutikov , Alexander Mottrie , Alex Mirone , Vincenzo van Poppel , Hendrik . (2018). Ischemia Techniques in Nephron-sparing Surgery: A Systematic Review and Meta-Analysis of Surgical, Oncological, and Functional Outcomes. European Urology, (), S0302283818307450–. doi: 10.1016/j.eururo.2018.10.005 |
[41] | Simone G , Gill IS , Mottrie A , et al. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial ne- phrectomy: a systematic review of the literature. Eur Urol. (2015) ;68: :632–40. |
[42] | Inderbir Gill S , Manuel Eisenberg; S. , Monish Aron; , Andre Berger; , Osamu Ukimura; , Mukul Patil; B. , Vito Campese; , Duraiyah Thangathurai; , Mihir Desai M. . ((2011) ). “Zero Ischemia” Partial Nephrectomy: Novel Laparoscopic and Robotic Technique., 59: (1), 128–34. doi: 10.1016/j.eururo.2010.10.002 |
[43] | Antonelli , Alessandro Cindolo , Luca Sandri , Marco Bertolo , Riccardo Annino , Filippo Carini , Marco Celia , Antonio D’Orta , Carlo De Concilio , Bernardino Furlan , Maria Giommoni , Valentina Ingrosso , Manuela Mari , Andrea Muto , Gianluca Nucciotti , Roberto Porreca , Angelo Primiceri , Giulia Schips , Luigi Sessa , Francesco Simeone , Claudio Veccia , Alessandro Minervini , Andrea (2019) . (2019). Safety of on- vs off-clamp robotic partial nephrectomy: per-protocol analysis from the data of the CLOCK randomized trial. World Journal of Urology, (), –. doi: 10.1007/s00345-019-02879-4 |
[44] | Marconi L , Desai MM , Ficarra V , Porpiglia F , Van Poppel H . Renal preservation and partial nephrectomy: patient and surgical factors. Eur Urol Focus. (2016) ;2: :589–600. |




