Disparities in Clinical Care and Research in Renal Cell Carcinoma
Abstract
Disparities in cancer screening, prevention, therapy, clinical outcomes, and research are increasingly recognized and pervade all malignancies. In response, several cancer research and clinical care organizations have issued policy statements to acknowledge and address barriers to achieving health equity in cancer care. The increasingly specialized nature of oncology warrants a disease-focused appraisal of existing disparities and potential solutions. Although clear improvements in clinical outcomes have been recently observed for patients with renal cell carcinoma (RCC), these improvements have not been equally shared across diverse populations. This review describes existing RCC cancer disparities and their potential contributing factors and discusses opportunities to improve health equity in clinical research for all patients with RCC.
INTRODUCTION
Cancer mortality has declined over the past decade, an improvement largely driven by advances in cancer prevention, earlier detection, and novel therapies [1]. However, this improvement in clinical outcomes has unfortunately not been equally shared across diverse cancer populations. Indeed, differences in race/ethnicity, age, gender identity, socioeconomic status, and health literacy have each been associated with disparate outcomes among cancer patients [2]. Recently, such disparities have been increasingly recognized and have ignited an ardent call for improved strategies to improve equity, diversity, and inclusion across cancer care [3].
While disparities in cancer care stem from long-standing systemic inequities in screening, prevention, therapy, and research, and pervade all malignancies, the increasingly specialized nature of oncology warrants a disease-focused appraisal of existing disparities and potential solutions. In particular, renal cell carcinoma (RCC) represents a malignancy that is rapidly rising in incidence and suffers from notable disparities in cancer outcomes [4]. From 2014–2018, the overall incidence of kidney cancer was 17.1 per 100,000, and varied considerably across ethnic groups (White: 17.3, Black: 18.9, Asian/Pacific Islander: 8.1, American Indian/Alaska Native: 29.6, Hispanic: 17.0 per 100,000) [1]. In 2022, there is estimated to be 79,000 newly diagnosed invasive kidney cancers (50,290 among men and 28,710 among women) and 13,920 associated deaths [1]. While recent improvements in systemic therapy have led to near-doubling of survival outcomes for patients with advanced RCC, several care gaps persist in the presentation, clinical outcomes, and access to care for patients. For example, kidney cancer mortality rates are higher among Blacks, Hispanics, and American Indians/Alaska Natives when compared to White patients [5–11]. Furthermore, while five-year survival rates for RCC have improved over time among White patients, survival outcomes have remained stagnant for Black patients, thus contributing to widening health disparities [12].
In response to these observed widening gaps in cancer care and outcomes, several cancer research and clinical care organizations, including the American Society of Clinical Oncology, have issued policy statements to acknowledge and address barriers to achieving health equity in cancer care. Importantly, health equity is defined within this context as an ethical and human rights principle ensuring a fair opportunity for achieving the highest possible standard of health [13, 14]. In keeping with this effort, this review article will describe existing RCC cancer disparities, including across RCC disease epidemiology, treatment, and access to clinical research, and examine potential contributing factors. Additionally, we will discuss opportunities to improve health equity in clinical research for all patients with RCC. Study selection for inclusion in this review was conducted via a PubMed biomedical library search index of original research studies and review articles published between 1996 through 2022 and using the search terms “renal cell carcinoma”, “disparity”, “race,” “gender,” “socioeconomic status,” and “clinical trials.”
RACE/ETHNICITY AND GENDER-BASED DISPARITIES IN RCC
Epidemiology and clinical outcomes
Racial/ethnic differences in the incidence and burden of chronic diseases have been well-characterized, with non-Hispanic Black adults developing a greater burden of multi-morbidity chronic disease at an earlier age than non-Hispanic White adults [15]. Similarly, important differences in the initial presentation, diagnosis, and survival outcomes for RCC patients have been observed across race/ethnicity groups. Compared to Non-Hispanic White patients, minority groups –Non-Hispanic Blacks, Hispanic Americans, American Indian and Alaska Natives, and Asian Americans –are more likely to be diagnosed at a younger age [11], and while Black patients are more likely to present with lower American Joint Committee on Cancer (AJCC) stage, several studies have reported inferior survival outcomes relative to White patients [4, 16, 17]. For example, in a population-based cohort study, Black patients with RCC had worse survival outcomes when compared to White patients, particularly among younger patients (<65 years at diagnosis) (HR 1.46, 95% CI 1.06–2.01) and those with smaller tumor size (<4cm) (HR 1.67, 95% CI 1.20–2.33) [18]. When accounting for renal-related medical comorbidities (hypertension, diabetes, chronic renal failure) and socioeconomic factors, these observed survival differences were largely mitigated (HR = 1.14, 95% CI 0.71–1.85 for patients <65 years; HR = 1.15, 95% CI 0.67–1.98 for patients with tumor size <4cm), thus indicating that the observed disparity in clinical outcome may have been primarily attributed to renal-relevant comorbidities and socioeconomic deprivation. Similarly, in a Surveillance, Epidemiology, and End Results (SEER) cancer registry analysis of patients with invasive kidney cancer diagnosed from 1992–2007, the 5-year survival rates for White and Black patients were 72.6% and 68.0%, respectively, resulting in a White/Black survival rate ratio of 1.07 (95% CI 1.04–1.10) [19]. This survival advantage of White patients over Black patients remained persistent across both clear cell RCC and papillary RCC subtypes, as well as across nearly all stage and tumor size categories in a restricted population of surgically-treated clear cell RCC patients aged 50–74 [19].
Limited data exists regarding the presentation and clinical outcomes for other race/ethnicity groups. To date, American Indians and Alaska Natives, in particular, are notably underrepresented in epidemiologic studies of kidney cancer [10]. However, per available data, this group has been observed to have the highest kidney cancer incidence and mortality rate [5]. In the National Cancer Database, American Indians and Alaska Natives had greater than twofold higher odds of early-onset kidney cancer compared to Non-Hispanic White patients (OR 2.21, 95% CI 1.88–2.59) [11] and a twofold increased kidney cancer mortality rate [7].
Similarly, comparatively less data is available regarding gender-based differences in RCC clinical outcomes. While clear cell RCC is nearly twice as common in men than women, sex-specific differences in clinical outcomes have also been observed, potentially reflecting differences in risk factor prevalence and/or tumor biology [20, 21]. For example, in an analysis of metastatic RCC patients in the National Cancer Database, women had a worse prognosis than men that was not explained by demographic differences over each of the pre-tyrosine kinase inhibitor (TKI) (2004–2005), TKI (2006–2014), and immune checkpoint inhibitor (ICI) (2015–2016) RCC treatment eras [22]. However, in addition to these potential biologic differences, gender-based disparities in care access may also contribute to these disparate outcomes. For example, studies of older, yet effective and expensive, RCC systemic therapies, including interleukin-2 (IL-2), indicated less treatment access for female patients, potentially reflecting provider and/or patient perceptions regarding fitness for aggressive RCC treatment interventions [23].
Treatment access as a contributing factor to race/ethnicity and gender-based disparities in RCC
While numerous factors, including rates of early detection, differences in cancer biology, and comorbid medical conditions can each contribute to the aforementioned disparities in cancer outcomes, it is clear that access to timely and effective RCC therapy is a major contributor to observed RCC disparities and critical for improving health equity. In particular, disparities in access to both surgical resection and increasingly effective systemic therapies for advanced RCC have been observed.
Rates of receiving surgical treatment for kidney cancer are notably lower in minority-serving hospitals (i.e. hospitals treating a RCC patient population with the highest proportion of Black and Hispanic patients) (OR 0.93, 95% CI 0.89–0.98, p = 0.002) [24]. Similarly, among Medicare patients, Black patients with RCC were less likely to undergo nephrectomy than White patients, after accounting for demographic characteristics, cancer stage, tumor size, and comorbidities [25]. While many factors may contribute to these lower observed nephrectomy rates, study authors hypothesized that an underestimation of socioeconomic factors (including childcare, costs of discharge medications, potential loss of income/work during hospitalization), differences in access and quality of care, and variation in patients’ attitudes about surgery may have contributed [25]. Indeed, similarly lower rates of surgery among Black patients have been described in multiple other cancer types [26–28]. As surgical nephrectomy remains the mainstay curative-intent therapy strategy for clinical localized disease, such differences in the rates of nephrectomy may have prognostic implications, and the contributing factors warrant further examination. For example, the chronic medical comorbidities more common to Black adults may result in decreased nephrectomy rates and/or prolonged surgical wait times required for pre-operative medical management, thus resulting in inferior long-term outcomes [29, 30]. Indeed, in an institutional experience of over 1400 patients undergoing nephrectomy for RCC, the most common cause for a surgical delay >3 months was evaluation and treatment of medical comorbidities [30].
Notably, while lack of access to nephrectomy may represent an example of an “undertreatment” race/ethnicity disparity in RCC, additional inequities in RCC “overtreatment” may also exist with similar adverse consequences. For example, in a retrospective study utilizing the National Cancer Database, Black and Hispanic patients with localized kidney cancer had higher adjusted odds of both undertreatment and overtreatment, as compared to National Comprehensive Cancer Network consensus guidelines as a reference care standard [31]. This overtreatment included higher rates of radical nephrectomy for patients potentially eligible for nephron-sparing interventions (such as partial nephrectomy, percutaneous ablation, or active surveillance), which may adversely compromise renal function and future comorbid risks. The study authors hypothesized that this finding may have resulted from either the greater medical resources needed for partial nephrectomy or other nephron-sparing strategies, or from an adverse provider perception of patient comorbidities, thereby resulting in a preference for radical versus partial nephrectomy.
For patients with advanced RCC, race/ethnicity and gender-based disparities in access to life-prolonging systemic therapies have similarly been observed, thereby likely contributing to the reported differences in clinical outcomes. Although systemic therapy options have dramatically transformed for metastatic RCC over the last two decades, such disparities in treatment access have unfortunately remained consistent. As previously noted, although IL-2 was the first systemic therapy to offer the potential for complete clinical response in metastatic RCC, women (OR 0.79, 95% CI 0.64–0.98, p = 0.03) and Black patients (OR 0.55, 95% CI 0.34–0.87, p = 0.01) were significantly less likely to receive this therapy [23]. In more recent years, with the advent of anti-angiogenic vascular endothelial growth factor receptor (VEGFR) TKI therapies, an analysis of advanced RCC patients in the National Cancer Database from 2004–2015 (“TKI era”) similarly demonstrated that female patients were at lower odds of receiving systemic therapy and at greater odds of receiving no treatment [32]. Black patients were also at reduced odds of receiving systemic therapy, increased odds of no treatment, and increased risk of death relative to Non-Hispanic White patients. Notably, these survival differences disappeared after accounting for receipt of RCC therapy, thus indicating the importance of therapy access as a primary factor for observed disparities in clinical outcomes [32]. Although combination ICI-based therapies remain the current first-line care standard for the majority of patients with advanced RCC [33], limited data currently exists regarding potential differences in the access and timing of ICI use in race/ethnicity and gender RCC patient subgroups. However, given the increasing expense and resources required for cancer immunotherapies, similar access disparities are likely to emerge (as has been observed in other advanced cancers) [34]. Finally, disparities in RCC care access may extend beyond surgery and systemic therapy to include other care domains affecting symptom management and quality of life. Indeed, Black and Hispanic metastatic RCC patients have been found to have decreased odds of receiving palliative care services (OR 0.83, 95% CI 0.75–0.89; OR 0.59, 95% CI 0.54–0.67, respectively) [35].
SOCIOECONOMIC STATUS AND GEOGRAPHY-BASED DISPARITIES IN RCC
Clinical outcomes
Inequities resulting from differences in socioeconomic status, including insurance coverage and neighborhood/geography, also contribute to RCC disparities. Utilizing SEER data from over 18,000 patients with kidney cancer and adjusting for patient and disease characteristics, uninsured and Medicaid patients were more likely to present with advanced disease and were less likely to receive treatment compared to privately insured patients [36]. These vulnerable patients also suffered worse mortality rates (13.6% uninsured vs. 12.5% Medicaid vs. 6.4% private insurance). Similarly, in a state-wide cancer registry study evaluating patients during the RCC TKI era (2004–2015), patients insured with Medicare alone had lower overall survival than those with any private insurance, even after accounting for known confounders, including age, polypharmacy, frailty, and comorbidities [37]. Neighborhood-level socioeconomic factors, resulting from residential segregation as a form of structural inequality, can also clearly adversely affect cancer mortality [38, 39]. Such neighborhood-level socioeconomic factors may include educational attainment level, median household income, and distance to resourced healthcare settings, and can arise from structural racism, which refers to the multitude of ways in which societies foster racial discrimination through systems of housing, employment, education, credit, and criminal justice [40]. Not surprisingly, these factors contribute across race/ethnicity subgroups to adverse RCC clinical outcomes, including lower rates of receipt of treatment and surgical nephrectomy and inferior overall survival [41].
Treatment access as a contributing factor for socioeconomic status and geography-based disparities in RCC
The description of these disparities presents the critical opportunity to better understand the factors contributing to these adverse clinical outcomes. Similar to observed race/ethnicity and gender disparities, treatment access emerges as a clearly implicated factor. Socioeconomic status, as reflected through insurance status and neighborhood-level factors, may limit access to initial and subsequent systemic therapies, as well as less cancer supportive services. Patients without health insurance were more likely to be undertreated (as compared to clinical consensus RCC treatment guidelines) relative to those with private insurance (OR 2.63, p < 0.001), potentially reflecting the limited availability of therapies in resource-scarce medical settings [31]. Notably, however, these differences in treatment access have carried over to even higher volume cancer hospitals with more diverse patient/payor populations. Among patients undergoing surgery for genitourinary malignancies at high-volume hospitals, private insurance was similarly associated with increased odds of treatment when compared with no insurance (OR 1.86, 95% CI 1.77–1.97) [42]. Providers practicing solely in urban areas were more likely to initiate oral anticancer agents for metastatic RCC than providers practicing in both urban and rural areas, after controlling for patient-level factors (RR 1.37; 95% CI 1.09–1.73), indicating additional provider-led influences across urban / rural geographic settings [37].
These socioeconomic differences in treatment access assume increasing urgency, given the burgeoning costs of cancer care. In particular, the economic burden of RCC for patients in the United States is substantial, with annual estimates ranging from $0.60 billion to $5.19 billion, an expansion primarily driven by the costs of oral anticancer agents [43]. Although increasingly used as a component of ICI-based combination treatment strategies, oral anticancer agents remain a standard, effective component of advanced RCC systemic therapy across consensus clinical guidelines [33]. However, the monthly cost to Medicare of a patient’s oral anticancer therapy for metastatic RCC doubled between 2007 and 2015, with a 6% increase in cost per year beyond inflation [44]. Thus, in 2015, over 50% of patients paid more than $1000 in out-of-pocket cost for a 30 day supply of oral anticancer therapy [44].
These prohibitive costs have clear implications for the treatment selection and clinical outcomes of RCC patients of lower socioeconomic status. In particular, patients under Medicare insurance alone are vulnerable, given potentially higher coinsurance costs for oral anticancer agents, limited or no caps on out-of-pocket prescription drug spending, and resulting prohibitive patient-related costs [45–47]. In a retrospective study examining treatment costs for patients with a new diagnosis of metastatic RCC, Medicare Part D patients with low out-of-pocket costs, also known as low-income subsidies (LIS) beneficiaries, were responsible for ≤$6.60 for their initial 30 day oral prescription in comparison to ≥$2,800 among non-LIS patients [48]. The authors found that fewer non-LIS patients started oral therapies compared to their counterparts (20.7% vs. 33.9%, OR 0.49, p < 0.001) and any targeted therapies compared to their counterparts (26.7% vs. 40.4%, OR 0.52, p < 0.001). Thus, escalating out-of-pocket costs affects RCC treatment initiation and places a significant financial toxicity on patients, which may ultimately lead to higher rates of pre-mature treatment discontinuation and ultimately higher mortality risks [49, 50].
OPPORTUNITIES FOR IMPROVING HEALTH EQUITY IN PRACTICE-INFORMING RCC CLINICAL RESEARCH
Limited diversity, inclusion, and equity in cancer clinical trials
Clinical trial results form the basis for evidence-based practice in cancer care, and trial participation is universally supported by oncology consensus guideline committees. Yet, clear disparities in access and participation in clinical trials are present [51, 52], and clinical trials in the United States have historically enrolled lower rates of Black and Hispanic patients when compared to White patients [53]. In an analysis of the enrollment fraction (EF, defined as the number of trial enrollees divided by the 2013 SEER database cancer prevalence) from cancer therapeutic clinical trials completed between 2003 and 2016, non-Hispanic Whites were more likely to be enrolled in clinical trials (EF, 1.2%) than African Americans (EF, 0.7%; P < .001) and Hispanics (EF, 0.4%; P < .001) [54]. Similarly, in a review of 168 phase 3 clinical trials derived from ClinicalTrials.gov and exclusively enrolling patients in the United States, the median absolute difference between trial enrollment and corresponding cancer incidence by race and ethnicity, as determined by SEER data, was +6.8% for Whites (interquartile range [IQR] = +1.8% to +10.1%; p < .001), –2.6% for Blacks (IQR = –5.1% to +1.2%; p = .004), –4.7% for Hispanics (IQR = –7.5% to –0.3%; p < .001), and –4.7% for Asians (IQR = –5.7% to –3.3%; p < .001), thus demonstrating overrepresentation of Whites in cancer clinical trials and continued underrepresentation of racial and ethnic minority subgroups [55]. More recently, despite several federal efforts to improve racial and ethnic diversity in cancer clinical trials, significant differences between cancer trial enrollment and cancer incidence among race/ethnicity groups have persisted. In fact, an observed decrease in African American (6% v 9.2%) and Hispanic (2.6% v 3.1%) cancer therapeutic trial enrollment was observed between 2003 and 2016 when compared with historical data (1996 to 2002) [54].
Given the rapid pace of cancer clinical trial conduct and the resulting regulatory approvals and changes in treatment standards, diverse participant recruitment acquires mounting importance. However, among 230 reported clinical trials that led to U.S. Food and Drug Administration oncology approvals granted between 2008–2018, a large percentage of trials (37%) did not discuss race [56], and only 7% of trials discussed four races (White, Asian Americans, Blacks, and Hispanics). White patients had a higher odds ratio of participating in practice-informing phase 3 randomized multi-arm trials compared with Blacks, Asians, and Hispanics; the latter groups were more likely to participate in phase 2, nonrandomized, single-arm trials. Overall, Black and Hispanic patients were significantly underrepresented in practice-informing clinical trials when compared to their actual cancer incidence and mortality rates nationally [56]. These trends unfortunately are underscored within recent practice-informing clinical trials for patients with advanced RCC. Among five phase 3 clinical trials leading to recent regulatory approvals of immune-based combination therapy strategies for advanced RCC or for adjuvant immune therapy for high risk resected RCC, race/ethnicity enrollment demographics are not primarily reported in any of the studies (Table 1) [57–61].
Table 1
Participant demographic data reported in five phase 3 clinical trials leading to recent renal cell carcinoma regulatory approvals
| Treatment Groups | Median Age (years) | Male Sex (percentage) | Geographic Region of Enrollment (percentage) | Race/Ethnicity |
| Pembrolizumab/Axitinib vs. Sunitinib | 62 vs. 61 | 71.3 vs. 74.6 | North America/Europe: 48.6 vs. 48.2 | – |
| Rest of the World: 51.4 vs. 51.7 | ||||
| Lenvatinib/Pembrolizumab vs. Lenvatinib/Everolimus vs. Sunitinib | 64 vs. 62 vs. 61 | 71.8 vs. 74.5 vs. 77.0 | North America/Europe: 55.8 vs. 56.0 vs. 55.7 | – |
| Rest of the World: 44.2 vs. 44.0 vs. 44.3 | ||||
| Nivolumab/Cabozantinib vs. Sunitinib | 62 vs. 61 | 77.1 vs. 70.7 | North America/Europe: 48.9 vs. 49.1 | – |
| Rest of the World: 51.1 vs. 50.9 | ||||
| Nivolumab/Ipilimumab vs. Sunitinib | 62 vs. 62 | 75 vs. 72 | North America/Europe: 65 vs. 64 | – |
| Rest of the World: 35 vs. 36 | ||||
| Pembrolizumab vs. Placebo | 60 vs. 60 | 70.0 vs. 72.1 | North America/Europe: 64.7 vs. 62.7 | – |
| Rest of the World: 35.3 vs. 37.3 |
This table highlights the demographic characteristics of the patients involved in five phase 3 clinical trials leading to recent regulatory approvals of immune-based combination treatment strategies for advanced RCC or for adjuvant immune therapy for high risk resected RCC. Notably, race/ethnicity enrollment demographics are not primarily reported [57–61].
Notably, minority groups experience greater barriers to clinical trial participation, such as lower income and lack of transportation to visits [62, 63]. Furthermore, minority populations may be more willing to participate in clinical trials when presented with the opportunity through good communication by a trusted professional. However, Black patients are often not made aware of participation in clinical trials, and medical providers from minority backgrounds remain largely underrepresented in the oncology physician workforce [64–66]. Finally, one must acknowledge the history of cultural and historical injustices toward minority communities, including but not limited to the Tuskegee experiment and the Henrietta Lacks cell line, which undoubtedly contribute adversely to clinical research engagement today [64].
EFFORTS TO IMPROVE DIVERSITY AND EQUITY IN CLINICAL TRIALS
In 1993, the National Institutes of Health (NIH) Revitalization Act directed the NIH to establish guidelines and to conduct or support outreach programs for the inclusion of women and minorities in clinical research. Now, thirty years later, these efforts continue with further interventions to expand recruitment of minority populations into clinical trials, increase racial diversity among oncologic providers, provide diversity training in the educational setting, and improve outreach to minority communities [64].
Community outreach and engagement
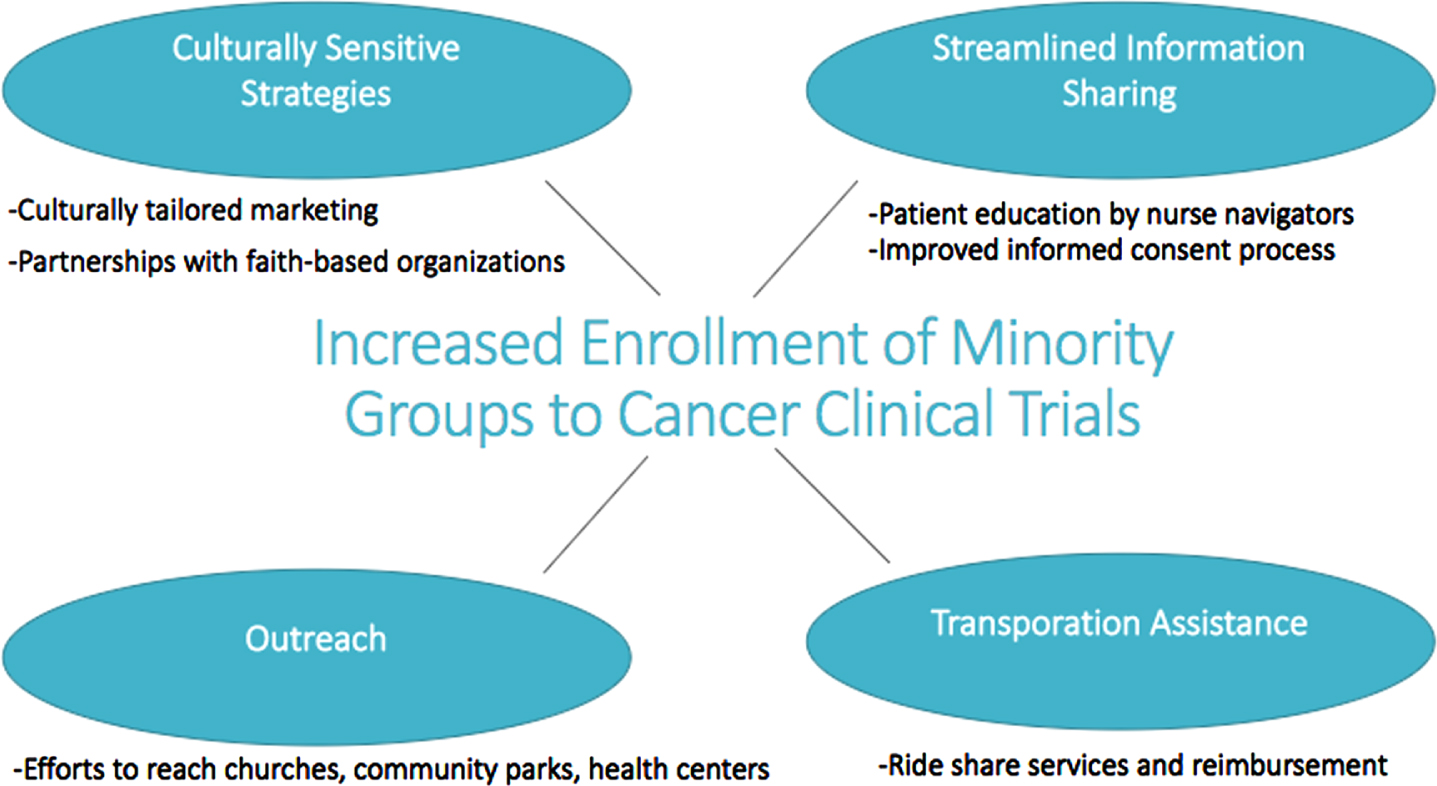
Within our own institution at the University of Pennsylvania Abramson Cancer Center, a five-year initiative of community outreach and engagement was conducted to improve enrollment of adult Black participants to oncology clinical trials. This center-wide initiative entailed 1) culturally tailored marketing strategies for cancer clinical trials; 2) plans for each protocol to facilitate Black participant enrollment; 3) new partnerships with faith-based organizations serving Black communities to conduct educational events about clinical trials; 4) pilot programs with ride share programs to address transportation barriers; 5) patient education by nurse navigators regarding cancer and clinical trials; and 6) an improved informed consent process (Fig. 1). Following implementation of these measures, the percentage of Black patients seen at our cancer center at the end of the study period matched the percentage of Black cancer patients among all cancer cases in the catchment area (16.2% vs. 16.5%). Further, the percentages of Black participants accrued onto treatment, non-therapeutic interventional, and non-interventional trials improved from 12.2%, 8.3%, and 13.0% to 23.9%, 33.1%, and 22.5%, respectively –representing between a 1.7–4.0 fold increase over five years [67].
Fig. 1
Strategies to Improve Minority Enrollment in Cancer Clinical Trials. This figure highlights the key elements of a five-year initiative of community engagement to improve enrollment of adult Black participants to clinical trials at the Abramson Cancer Center at the University of Pennsylvania. The percentage of Black participants accrued onto treatment, non-therapeutic interventional, and non-interventional clinical trials saw a 1.7–4 fold increase over five years. Guerra CE, Sallee V, Hwang W-T, et al. Accrual of Black participants to cancer clinical trials following a five-year prospective initiative of community outreach and engagement. Journal of Clinical Oncology. 2021;39(15_suppl):100-100.
Medicaid expansion and clinical trial cost subsidies
Patient-level financial barriers are an increasingly recognized deterrent to trial participation [68–72]. Up to a quarter or more of patients who decline to participate in cancer clinical trials cite financial concerns [73, 74]. Moreover, clinical trial participants may be particularly vulnerable to the financial toxicities of cancer care given that trials require more frequent clinic visits, time away from work, and travel-related expenses [75, 76]. In order to increase enrollment of racial and ethnic minority patients in cancer clinical trials — both to improve the generalizability of research findings and to promote equitable access to state-of-the-art therapies — policies and programs have sought to address patient financial barriers to participation.
Federal legislation mandates that Medicare and private payers cover the so-called “routine costs” of clinical trial participation, such as the fees associated with physician visits, hospital stays, diagnostic tests, and other standard clinical services that would have been covered absent the patient’s participation in a trial. Until recently, however, Medicaid beneficiaries were excluded from these federal measures, an omission that left states to legislate their own coverage policies. Consequently, state Medicaid programs have varied in the degree to which they cover the routine costs associated with trial participation, with only 16 states explicitly mandating coverage through statute or other written policy as of 2020 [77]. These mandates have been associated with a short-term increase in enrollment of Black participants on cancer clinical trials [78]. Effective this year, the Clinical Treatment Act extends federally mandated coverage of the routine costs of trial participation to Medicaid beneficiaries for the first time and may help ameliorate long-standing financial barriers to participation among historically disadvantaged groups, though questions remain about its implementation at the state level.
The Patient Protection and Affordable Care Act (ACA) expanded Medicaid coverage to more than 20 million U.S. residents, leading to dramatic improvements in coverage for racial and ethnic minority patients. These expansions are associated with improved access to care, affordability, and health outcomes for certain conditions [79–81], with mixed effects on racial and socioeconomic disparities for patients with cancer [82–85]. It is hypothesized that Medicaid expansion might increase trial participation by extending mandated coverage of trial enrollment costs to previously uncovered patients, and more generally, by improving coverage, access, and treatment options for patients who were previously uninsured. Preliminary research suggests that Medicaid expansion was associated with nearly a threefold increase in the proportion of patients using Medicaid participating in cancer clinical trials [86].
An additional initiative demonstrating early success in improving minority participation and equitable access to cancer clinical trial participation is the IMproving Patient Access to Cancer Clinical Trials (IMPACT) program, developed by the Lazarex Cancer Foundation [87]. This nationwide effort involves a financial reimbursement program that covers travel and lodging costs associated with cancer clinical trial participation. Eligible participants include adult patients with solid or hematologic malignancies being considered for a therapeutic clinical trial, and the program at least partially covers out-of-pocket travel- and lodging-related expenses (e.g., ride share, mileage, air fare, hotel rooms) tied to clinical trial participation for patients who have a household income <700% of the 2022 Health and Human Services Poverty Guidelines.
Informed consent and disease-specific registries
Language barriers may serve as an additional hurdle to enrollment of minority groups to cancer clinical trials. The informed consent process for cancer clinical trials has become increasingly complex, and federal regulations do not currently provide adequate protection to ensure the rights of non-English speaking participants. While federal regulations mandate that a participant receives information about a clinical trial “in language understandable to the subject or the legally authorized representative,” a researcher may utilize a “short-form” consent when a “long-form” translation in the subject’s native language is unavailable, and there are no formal guidelines requiring the use of a translator nor ensuring the translator’s competency [88]. Furthermore, a standardized process for consenting non-English speaking participants is lacking. In a single institution study aiming to assess the knowledge of proper consenting procedures among researchers working with low English fluency patients and following required completion of online training, research members responded correctly to only 65% of questions [89]. Therefore, in order to ensure proper informed consent among non-English speakers and mitigate this enrollment barrier, universal federal policies detailing the translation process, the necessary capabilities of the translator, and the use of additional language resources are needed.
The development of disease-specific registries or consortiums to address key questions in RCC diversity research could also further help decrease disparities in clinical trial access and participation. As an example, the Prostate Cancer Precision Medicine Multi-Institutional Collaborative Effort (PROMISE) provides a platform that links clinical data to patient outcomes and includes a diversity/inclusion committee to address clinical questions related to racial disparities [90]. Similarly, the International Registry for Men with Advanced Prostate Cancer (IRONMAN), which is a global population-based study that has collected clinical and patient-reported outcomes and epidemiological data, was created to increase minority engagement in clinical research and decrease prostate cancer disparities. Similar registries can be built to improve minority population engagement in clinical research and tackle the observed disparities present for patients with RCC [91].
CONCLUSION
In conclusion, racial/ethnic and socioeconomic disparities are increasingly recognized in cancer care. These disparities extend across the presentation, treatment, and clinical outcomes for patients with RCC. Clinical trials, including seminal practice-informing studies for RCC, continue to lack representation from many minority groups, thus limiting the access and generalizability of key findings. The use of focused community engagement efforts, expanded coverage and cost reimbursement programs, facilitated informed consent processes, and disease-specific registries and consortiums can help address critical diversity and inclusion needs and improve health equity in RCC clinical care.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
DP contributed to conception and writing the article. ST contributed to writing the article. VN contributed to conception and writing the article.
CONFLICT OF INTEREST
DP has no conflict of interest to report. ST has no conflict of interest to report. VN reports research grants from Merck, Janssen, Pfizer, and Xencor, and consulting fees for Merck, Janssen, Pfizer, Exelixis, and Myovant Sciences.
REFERENCES
[1] | Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer statistics, CA Cancer J Clin (2022) ;72: (1):7–33. |
[2] | Cancer Disparities was originally published by the National Cancer Institute. National Cancer Institute. https://www.cancer.gov/about-cancer/understanding/dis-parities. Published 2018. Updated March 28, 2022. Accessed2022. |
[3] | The Lancet O . Racial disparities in cancer care: Can we close the gap? Lancet Oncol (2021) ;22: (12):1643. |
[4] | Stafford HS , Saltzstein SL , Shimasaki S , Sanders C , Downs TM , Sadler GR . Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival, J Urol (2008) ;179: (5):1704–1708. |
[5] | Siegel RL , Miller KD , Jemal A . Cancer statistics, CA Cancer J Clin (2019) ;69: (1):7–34. |
[6] | White MC , Espey DK , Swan J , Wiggins CL , Eheman C , Kaur JS . Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States, Am J Public Health (2014) ;104: (Suppl 3):S377–S387. |
[7] | Li J , Weir HK , Jim MA , King SM , Wilson R , Master VA . Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990-2009, Am J Public Health (2014) ;104: (Suppl 3):S396–S403. |
[8] | Foote M , Strickland R , Lucas-Pipkorn S , Williamson A , Lamers L . The high burden of cancer among American Indians/Alaska natives in wisconsin, Wmj (2016) ;115: (1):11–16. |
[9] | Saad AM , Gad MM , Al-Husseini MJ , Ruhban IA , Sonbol MB , Ho TH . Trends in renal-cell carcinoma incidence and mortality in the United States in the last 2 decades: A SEER-Based study, Clin Genitourin Cancer.46-57.e (2019) ;17: (1):45. |
[10] | Batai K , Bergersen A , Price E , Hynes K , Ellis NA , Lee BR . Clinical and molecular characteristics and burden of kidney cancer among hispanics and native Americans: Steps toward precision medicine, Clin Genitourin Cancer.e535-e (2018) ;16: (3):541. |
[11] | Batai K , Harb-De la Rosa A , Zeng J , Chipollini JJ , Gachupin FC , Lee BR . Racial/ethnic disparities in renal cell carcinoma: Increased risk of early-onset and variation in histologic subtypes, Cancer Med (2019) ;8: (15):6780–6788. |
[12] | Eisenberg MS , Leibovich BC , Kim SP . Racial disparity in renal cell carcinoma patient survival according to demographic and clinical characteristics, Cancer (2013) ;119: (16):3100. |
[13] | Patel MI , Lopez AM , Blackstock W , et al., Cancer disparities and health equity: A policy statement from the American society of clinical oncology, J Clin Oncol (2020) ;38: (29):3439–3448. |
[14] | Braveman P . What are health disparities and health equity? We need to be clear, Public Health Rep (2014) ;129: (Suppl 2):5–8. |
[15] | Quiñones AR , Botoseneanu A , Markwardt S , et al., Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults, PLoS One (2019) ;14: (6):e0218462. |
[16] | Vaishampayan UN , Do H , Hussain M , Schwartz K . Racial disparity in incidence patterns and outcome of kidney cancer, Urology (2003) ;62: (6):1012–1017. |
[17] | Chow WH , Devesa SS . Contemporary epidemiology of renal cell cancer, Cancer J (2008) ;14: (5):288–301. |
[18] | Schwartz K , Ruterbusch JJ , Colt JS , Miller DC , Chow WH , Purdue MP . Racial disparities in overall survival among renal cell carcinoma patients with young age and small tumors, Cancer Med (2016) ;5: (2):200–208. |
[19] | Chow WH , Shuch B , Linehan WM , Devesa SS . Racial disparity in renal cell carcinoma patient survival according to demographic and clinical characteristics, Cancer (2013) ;119: (2):388–394. |
[20] | Peired AJ , Campi R , Angelotti ML , et al., Sex and gender differences in kidney cancer: Clinical and experimental evidence, Cancers (Basel) (2021) ;13: (18). |
[21] | Mamtani R , Wang XV , Gyawali B , et al. Association between age and sex and mortality after adjuvant therapy for renal cancer, Cancer (2019) ;125: (10):1637–1644. |
[22] | Grigg C , Trufan S , Clark PE , et al. Survival trends of men and women with metastatic clear cell renal cell carcinoma, Journal of Clinical Oncology (2021) ;39: (15_suppl):4566–4566. |
[23] | Saigal CS , Deibert CM , Lai J , Schonlau M . Disparities in the treatment of patients with IL-2 for metastatic renal cell carcinoma, Urol Oncol (2010) ;28: (3):308–313. |
[24] | Calderon* LP , Awamlh BAHA , Khan AI , et al., MP21-10&#xrenal surgery rates for kidney cancer at minority serving hospitals, Journal of Urology (2020) ;203: (Supplement 4):e321–e322. |
[25] | Berndt SI , Carter HB , Schoenberg MP , Newschaffer CJ . Disparities in treatment and outcome for renal cell cancer among older black and white patients, J Clin Oncol (2007) ;25: (24):3589–3595. |
[26] | Bach PB , Cramer LD , Warren JL , Begg CB . Racial differences in the treatment of early-stage lung cancer, N Engl J Med (1999) ;341: (16):1198–1205. |
[27] | Tripathi RT , Heilbrun LK , Jain V , Vaishampayan UN . Racial disparity in outcomes of a clinical trial population with metastatic renal cell carcinoma, Urology (2006) ;68: (2):296–301. |
[28] | Cooper GS , Yuan Z , Landefeld CS , Rimm AA . Surgery for colorectal cancer: Race-related differences in rates and survival among Medicare beneficiaries, Am J Public Health (1996) ;86: (4):582–586. |
[29] | Zini L , Perrotte P , Capitanio U , et al., Race affects access to nephrectomy but not survival in renal cell carcinoma, BJU Int (2009) ;103: (7):889–893. |
[30] | Mano R , Vertosick EA , Hakimi AA , et al. The effect of delaying nephrectomy on oncologic outcomes in patients with renal tumors greater than 4cm, Urol Oncol (2016) ;34: (5):239.e231–238. |
[31] | Howard JM , Nandy K , Woldu SL , Margulis V . Demographic factors associated with non-guideline–based treatment of kidney cancer in the united states, JAMA Network Open (2021) ;4: (6):e2112813–e2112813. |
[32] | Metcalf MR , Peña VN , Cheaib JG , Srivastava A , Pierorazio PM , Patel HD . Disparities in the treatment and survival of metastatic renal cell carcinoma. Urology. 2021. |
[33] | NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. |
[34] | Ahn JC , Lauzon M , Luu M , et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022. |
[35] | Wadiwala J , Patel M , Li C , Maraboyina S , Safar A , Kim T . Health care disparities and barriers to palliative care among metastatic renal cell carcinoma patients: An NCDB analysis, Journal of Clinical Oncology (2021) ;39: (15_suppl):4545–4545. |
[36] | Tan H-J , Chuang RJ , Shirk JD , Laviana AA , Hu JC . Health insurance status and disparities in kidney cancer care, Urology Practice (2016) ;3: (1):18–24. |
[37] | Spees LP , Dinan MA , Jackson BE , et al.Patient- and provider-level predictors of survival among patients with metastatic renal cell carcinoma initiating oral anticancer agents. Clin Genitourin Cancer. 2022. |
[38] | Ellis L , Canchola AJ , Spiegel D , Ladabaum U , Haile R , Gomez SL . Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics, J Clin Oncol (2018) ;36: (1):25–33. |
[39] | Landrine H , Corral I , Lee JGL , Efird JT , Hall MB , Bess JJ . Residential segregation and racial cancer disparities: A systematic review, J Racial Ethn Health Disparities (2017) ;4: (6):1195–1205. |
[40] | Bailey ZD , Krieger N , Agénor M , Graves J , Linos N , Bassett MT . Structural racism and health inequities in theUSA: Evidence and interventions, Lancet (2017) ;389: (10077):1453–1463. |
[41] | Cruz A , Dickerson F , Pulling KR , et al . Impacts of neighborhood characteristics and surgical treatment disparities on overall mortality in stage I renal cell carcinoma patients, Int J Environ Res Public Health (2022) ;19: (4). |
[42] | Berg S , Tully KH , Sahraoui A , et al. Inequity in selective referral to high-volume hospitals for genitourinary malignancies, Urol Oncol (2020) ;38: (6):582–589. |
[43] | Shih YC , Chien CR , Xu Y , Pan IW , Smith GL , Buchholz TA . Economic burden of renal cell carcinoma: Part I–anupdated review, Pharmacoeconomics. (2011) ;29: (4):315–329. |
[44] | Wilson LE , Spees L , Pritchard J , et al. Real-world utilization of oral anticancer agents and related costs in older adults with metastatic renal cell carcinoma in the united states, Kidney Cancer (2021) ;5: (3):115–127. |
[45] | Dean LT , George M , Lee KT , Ashing K . Why individual-level interventions are not enough: Systems-level determinants of oral anticancer medication adherence, Cancer (2020) ;126: (16):3606–3612. |
[46] | Narang AK , Nicholas LH . Out-of-pocket spending and financial burden among medicare beneficiaries with cancer, JAMA Oncol (2017) ;3: (6):757–765. |
[47] | Dusetzina SB , Keating NL . Mind the gap: Why closing the doughnut hole is insufficient for increasing medicare beneficiary access to oral chemotherapy, J Clin Oncol (2016) ;34: (4):375–380. |
[48] | Li P , Wong YN , Jahnke J , Pettit AR , Doshi JA . Association of high cost sharing and targeted therapy initiation among elderly Medicare patients with metastatic renal cell carcinoma, Cancer Med (2018) ;7: (1):75–86. |
[49] | Shih YT , Xu Y , Liu L , Smieliauskas F . Rising prices of targeted oral anticancer medications and associated financial burden on medicare beneficiaries, J Clin Oncol (2017) ;35: (22):2482–2489. |
[50] | Ramsey SD , Bansal A , Fedorenko CR , et al.Financial insolvency as a risk factor for early mortality among patients with cancer, J Clin Oncol (2016) ;34: (9):980–986. |
[51] | Hamel LM , Penner LA , Albrecht TL , Heath E , Gwede CK , Eggly S . Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer, Cancer Control (2016) ;23: (4):327–337. |
[52] | Niranjan SJ , Martin MY , Fouad MN , et al. Bias and stereotyping among research and clinical professionals: Perspectives on minority recruitment for oncology clinical trials, Journal of Clinical Oncology (2019) ;37: (27_suppl):152–152. |
[53] | Murthy VH , Krumholz HM , Gross CP . Participation in cancer clinical trials: Race-, sex-, and age-based disparities, Jama (2004) ;291: (22):2720–2726. |
[54] | Duma N , Vera Aguilera J , Paludo J , et al. Representation of minorities and women in oncology clinical trials: Review of the past 14 years, J Oncol Pract (2018) ;14: (1):e1–e10. |
[55] | Grant SR , Lin TA , Miller AB , et al. Racial and ethnic disparities among participants in US-based phase 3 randomized cancer clinical trials, JNCI Cancer Spectr (2020) ;4: (5):pkaa060. |
[56] | Loree JM , Anand S , Dasari A , et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from to 2008 to 2018, JAMA Oncol (2019) ;5: (10):e191870. |
[57] | Rini BI , Plimack ER , Stus V , et al., Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma, N Engl J Med (2019) ;380: (12):1116–1127. |
[58] | Motzer R , Alekseev B , Rha SY , et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma, N Engl J Med (2021) ;384: (14):1289–1300. |
[59] | Choueiri TK , Powles T , Burotto M , et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma, N Engl J Med (2021) ;384: (9):829–841. |
[60] | Motzer RJ , Tannir NM , McDermott DF , et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma, N Engl J Med (2018) ;378: (14):1277–1290. |
[61] | Choueiri TK , Tomczak P , Park SH , et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma, N Engl J Med (2021) ;385: (8):683–694. |
[62] | Guidry JJ , Aday LA , Zhang D , Winn RJ . Transportation as a barrier to cancer treatment, Cancer Pract (1997) ;5: (6):361–366. |
[63] | Silver D , Blustein J , Weitzman BC . Transportation to clinic: Findings from a pilot clinic-based survey of low-income suburbanites, J Immigr Minor Health (2012) ;14: (2):350–355. |
[64] | Woods-Burnham L , Johnson JR , Hooker SE , Bedell FW , Dorff TB , Kittles RA . The role of diverse populations in US clinical trials, Med (2021) ;2: (1):21–24. |
[65] | Winkfield KM , Flowers CR , Mitchell EP . Making the case for improving oncology workforce diversity, Am Soc Clin Oncol Educ Book (2017) ;37: :18–22. |
[66] | Manana AIV , Leibrandt R , Duma N . Trainee and workforce diversity in hematology and oncology: Ten years later what has changed? Journal of Clinical Oncology (2020) ;38: (15_suppl):11000–11000. |
[67] | Guerra CE , Sallee V , Hwang W-T , et al. Accrual of Black participants to cancer clinical trials following a five-year prospective initiative of community outreach and engagement, Journal of Clinical Oncology (2021) ;39: (15_suppl):100–100. |
[68] | Ford JG , Howerton MW , Lai GY , et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review, Cancer (2008) ;112: (2):228–242. |
[69] | Lara PN Jr , Higdon R , Lim N , et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment, J Clin Oncol (2001) ;19: (6):1728–1733. |
[70] | Weckstein DJ , Thomas CA , Emery IF , et al. Assessment of perceived cost to the patient and other barriers to clinical trial participation, Journal of Oncology Practice (2011) ;7: (5):330–333. |
[71] | Winkfield KM , Phillips JK , Joffe S , Halpern MT , Wollins DS , Moy B . Addressing financial barriers to patient participation in clinical trials: ASCO policy statement. J Clin Oncol. 2018:Jco1801132. |
[72] | Unger JM , Gralow JR , Albain KS , Ramsey SD , Hershman DL . Patient income level and cancer clinical trial participation: A prospective survey study, JAMA Oncol (2016) ;2: (1):137–139. |
[73] | Comis RAC , Stovall E , Krebs L , Risher P , Taylor H . AQuantitative Survey of Public Attitudes towards Cancer Clinical Trials. Coalition of National Cancer Cooperative Groups, Cancer Research Foundation of America, Cancer Leadership Council and Oncology Nursing Society; 2000. |
[74] | Unger JM , Hershman DL , Albain KS , et al.Patient income level and cancer clinical trial participation, J Clin Oncol (2013) ;31: (5):536–542. |
[75] | Zafar SY . Financial toxicity of cancer care: It’s time to intervene, J Natl Cancer Inst (2016) ;108: (5). |
[76] | Nipp RD , Hong K , Paskett ED . Overcoming barriers to clinical trial enrollment, American Society of Clinical Oncology Educational Book (2019) ;(39):105–114. |
[77] | Takvorian SU , Guerra CE , Schpero WL . A hidden opportunity — medicaid’s role in supporting equitable access to clinical trials, New England Journal of Medicine (2021) ;384: (21):1975–1978. |
[78] | Takvorian SU , Chatterjee P , Mamtani R , et al. Association between state Medicaid policies and accrual of Black participants to cancer clinical trials, Journal of Clinical Oncology (2022) ;40: (16_suppl):1501–1501. |
[79] | Loehrer AP , Chang DC , Scott JW ,et al. Association of the affordable care act medicaid expansion with access to and quality of care for surgical conditions, JAMA Surg.e (2018) ;153: (3):175568. |
[80] | Swaminathan S , Sommers BD , Thorsness R , Mehrotra R , Lee Y , Trivedi AN . Association of medicaid expansion with 1-year mortality among patients with end-stage renal disease, Jama (2018) ;320: (21):2242–2250. |
[81] | Khatana SAM , Bhatla A , Nathan AS , et al. Association of medicaid expansion with cardiovascular mortality, JAMA Cardiology (2019) ;4: (7):671–679. |
[82] | Han X , Yabroff KR , Ward E , Brawley OW , Jemal A . Comparison of insurance status and diagnosis stage among patients with newly diagnosed cancer before vs after implementation of the patient protection and affordable care act, JAMA Oncology (2018) ;4: (12):1713–1720. |
[83] | McDermott J , Zeymo A , Chan K , et al. Affordable care act’s medicaid expansion and use of regionalized surgery at high-volume hospitals, Journal of the American College of Surgeons (2018) ;227: (5):507–520.e509. |
[84] | Adamson BJS , Cohen AB , Estevez M , et al. Affordable Care Act (ACA) Medicaid expansion impact on racial disparities in time to cancer treatment, Journal of Clinical Oncology (2019) ;37: (18_suppl):LBA1–LBA1. |
[85] | Crocker AB , Zeymo A , McDermott J , et al. Expansion coverage and preferential utilization of cancer surgery among racial and ethnic minorities and low-income groups, Surgery (2019) ;166: (3):386–391. |
[86] | Unger JM , Xiao H , Vaidya R , Hershman DL . The medicaid expansion of the affordable care act and participation of patients with Medicaid in cancer clinical trials, Journal of Clinical Oncology (2022) ;40: (16_suppl):6505–6505. |
[87] | Improving Patient Access to Cancer Clinical Trials. https://pc3i.upenn.edu/projects/improving-patient-access-to-cancer-clinical-trials/. Accessed. |
[88] | Mistretta S . Amending federal regulations to counteract language barriers in the informed consent process. Voices in Bioethics. 2022;8. |
[89] | Staples JN , Lester J , Li A , et al. Language as a barrier to cancer clinical trial accrual: Assessing consenting team knowledge and practices for cancer clinical trial consent among low English fluency patients, Applied Cancer Research (2018) ;38: (1):14. |
[90] | Koshkin VS , Patel VG , Ali A , et al. PROMISE: A realworld clinical-genomic database to address knowledge gaps in prostate cancer. Prostate Cancer and Prostatic Diseases. 2021. |
[91] | George DJ , Mucci LA , Kantoff PW , et al. IRONMAN: The international registry for men with advanced prostate cancer, Journal of Clinical Oncology (2022) ;40: (6_suppl):TPS190–TPS190. |




