What We Have Learnt from CARMENA and SURTIME and What Should Be Done Differently in Future Trials on Cytoreductive Nephrectomy
Abstract
Upfront cytoreductive nephrectomy (CN) was the standard treatment for selected patients with metastatic Renal Cell Carcinoma (RCC) in the cytokine era for many years. In the recent ‘targeted therapy era’ it has been re-challenged by both the CARMENA and SURTIME trials. As first-line therapy for treatment-naive metastatic clear-cell RCC has now changed to immune checkpoint inhibitor combination therapy (ICI), and previous studies concerning CN were built in the targeted therapy era, the role and sequence of CN needs to be revisited. Here we address what we have learnt from both trials and how future trials should be designed to investigate CN.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 3–5% of all adult cancers. Approximately 15–20% of patients have distant metastases with the primary tumor in place at time of diagnosis [1]. While upfront cytoreductive nephrectomy (CN) was the standard treatment for selected patients in the cytokine era for a long time [2, 3] it has recently been re-challenged by the CARMENA (The Clinical Trial to Assess the Importance of Nephrectomy) trial in the targeted therapy era (TT) [4]. Patients were randomized to receive either vascular endothelial growth factor receptor (VEGFR)-targeted therapy with sunitinib alone or upfront CN followed by sunitinib. Ultimately, median overall survival (OS) with sunitinib alone was non-inferior to the upfront CN approach followed by sunitinib. Nevertheless, deferred CN was still an option in the sunitinib only arm and was performed in 17% of the patients. The timing of CN - upfront or deferred - was investigated in the SURTIME (Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients with Metastatic Kidney Cancer) trial [5]. Patients with metastatic RCC (mRCC) were randomised to sunitinib therapy, followed by CN in the absence of progression, versus immediate CN followed by sunitinib. Unfortunately, due to poor accrual, results were mainly exploratory, however, results suggested that in the deferred CN group more patients received sunitinib and had an increased OS. As endpoints were previously reported [6], the hazard ratio of the secondary endpoint OS, favoured deferred CN [0.57(CI: 0.34–0.95, p = 0.032)]. Immediate CN showed a median OS of only 15.0 months versus 32.4 in the deferred group. The SURTIME trial concluded that pre-treatment with sunitinib may identify patients with inherent resistance to systemic therapy (ST) before planned CN and therefore avoid unnecessary surgery [5]. Meanwhile, first-line therapy for treatment-naive metastatic clear-cell RCC, on which the previous studies were built, has changed to immune checkpoint inhibitor combination therapy (ICI). Therefore, the role and sequence of CN needs to be revisited. In this review we address what we learnt from both trials and how future trials should be designed to investigate CN after the paradigm shift to more effective combination immunotherapies.
EVIDENCE ACQUISITION
We searched the relevant publication resources (MEDLINE (PubMed), Embase (Ovid) and the Cochrane library) on the following topics: CN, CN in combination with ICI, ICI trials including patients with synchronous mRCC, CN trials completed or in progress and the website of clintrials.gov. Key words were: renal cell carcinoma, cytoreductive surgical procedure, cytoreductive nephrectomy, upfront nephrectomy, immediate nephrectomy, deferred nephrectomy, nephrectomy, systemic treatment, immune checkpoint inhibitors, immune checkpoint inhibitor therapy, immune checkpoint inhibitor combination therapy, (VEGFR)-targeted therapy, CARMENA, SURTIME, Controlled trials as topic, randomized controlled trial, neoadjuvant, adjuvant. Only controlled trials and studies published in the English language were included. The information from the publication was used for a narrative review.
WHAT HAVE WE LEARNT FROM CARMENA AND SURTIME?
Comparing upfront CN to either sunitinib only (CARMENA) or to deferred CN after 3 months of sunitinib, provided the disease would not progress at metastatic sites, (SURTIME) showed, despite differences in design and hypothesis, that patients with primary metastatic clear-cell RCC requiring ST with sunitinib had no additional benefit from upfront CN. In CARMENA, deferred CN was performed in 17% of the patients in the sunitinib-only arm, mainly due to near-complete responses of metastasis [4]. Unsurprisingly, both EAU [6] and ESMO RCC guidelines [7] recommended ST with sunitinib for patients with primary mRCC and progressive metastatic disease, with the option to consider deferred CN in those responding at metastatic sites. In the meantime, first-line ST for primary mRCC changed from single-agent VEGF-targeted therapy to immune checkpoint inhibitor combination therapies. The role of CN in the new treatment paradigm of ICI-based combination therapy remains undetermined due to a lack of evidence from randomised trials and needs further analysis. The sole data available, is that from the pivotal ICI combination trials which led to the approval of the combinations of nivolumab and ipilimumab, pembrolizumab and axitinib, pembrolizumab and lenvatinib, avelumab and axitinib as well as nivolumab and cabozantinib for the treatment of IMDC intermediate and poor risk metastatic clear-cell RCC. In addition, results from those trials showed that the amount of metastatic patients treated with their primary tumour in place was up to 30% (Table 1). Interestingly, the available subgroup HRs suggest better outcomes for the ICI combination compared to sunitinib monotherapy. Despite the lack of RCTs that evaluate the role and sequence of CN in the ICI era, the data from pivotal ICI combination trials do suggest that ICI combination therapies could lead to superior outcomes when following the recommendations established in the TT era to treat patients with synchronous metastatic RCC with their primary tumour in place.
Table 1
Key trials on immune checkpoint inhibitor combinations for primary metastatic disease
| Trial | Drug combination | Number and % of patients treated with primary tumour in place | Number of patients treated with the primary tumour in place (ICI combination vs. sunitinib) | Subgroup analyses (HR with 95% CIs) | ||
| ICI | Sunitinib | PFS | OS | |||
| combination | ||||||
| CheckMate 214 | ipilimumab + | 187/847 (22%) | 84 | 103 | NA | 0.63 |
| [8] | nivolumab | (0.42–0.94) | ||||
| CheckMate 9ER | cabozantinib + | 196/651 (30.1%) | 101 | 95 | 0.63 | 0.79 |
| [9] | nivolumab | (0.43–0.92) | (0.48–1.29) | |||
| Javelin 101 | axitinib + | 179/886 (20.2%) | 90 | 89 | 0.75 | NA |
| [10] | avelumab | (0.48–1.65) | ||||
| KEYNOTE-426 | axitinib + | 143/861 (16.6%) | 73 | 70 | 0.68 | 0.57 |
| [11] | pembrolizumab | (0.45–1.03) | (0.36–0.89) | |||
| CLEAR [12] | Lenvatinib + pembrolizumab | 175/712 (24.6%) | 93 | 82 | 0.44 (0.28–0.68) | 0.52 (0.31–0.86) |
The EAU guideline recommendation thus acknowledges the lesson learnt from CARMENA and SURTIME - to treat patients who require systemic therapy with the primary tumour in place - as a proof-of-principle. A recent post-hoc analysis of exposure to sunitinib in both arms of the SURTIME trial may lend further support to this principle and provides a potential explanation for the observed survival benefit after upfront systemic therapy followed by deferred CN, compared to immediate CN followed by sunitinib in the postoperative setting [13]. The authors concluded that immediate CN impairs administration, onset, and duration of sunitinib. The data demonstrated that starting with systemic therapy not only results in longer exposure to sunitinib, but leads to early and more profound disease control and identification of progression prior to planned CN, which may have contributed to the observed OS benefit. Contrary to CARMENA, in the SURTIME trial the deferred CN group consisted of 89% patients with a MSKCC intermediate risk based on more than 1 factor, which corresponds largely to the IMDC intermediate risk group. Interestingly, a recent post-hoc analysis of CARMENA data showed a very similar OS difference between patients with IMDC intermediate risk based on 2 factors who underwent upfront CN followed by sunitinib versus sunitinib only [14] (Table 2).
Table 2
Comparison of OS data of SURTIME and the IMDC 2-factor risk subgroup of CARMENA
| Median OS, months | Arm A: | Arm B: | HR (95% CI) | P value |
| (95% CI) | Nephrectomy+ | Sunitinib alone/ | ||
| Sunitinib | deferred CN | |||
| CARMENA IMDC 2 risk factors | 17.6 (13.7–21.5) N = 64 | 31.2* (20.5–40.4) N = 76 | 0.65 (0.44–0.97) | 0.033 |
| SURTIME 89% MSKCC (deferred | 15.0 (9.3–29.5) N = 50 | 34.2 (14.5–65.3) N = 49 | 0.57 (0.34–0.95) | 0.032 |
| CN-group) intermediate risk |
*Includes an unreported number of patients who had a deferred CN.
Emerging real-world data suggest that CARMENA and SURTIME left a legacy to treat patients with primary metastatic RCC without immediate CN if they require systemic therapy.
Up to 16% of patients treated with combination immunotherapy achieve complete responses (CR) at metastatic sites [12]. These patients are increasingly being advised deferred CN to achieve surgical complete remissions. In addition, a retrospective analysis showed that 10% of 20 patients had a complete pathological response in the primary tumour of their deferred CN following ICI therapy [15]. Recent retrospective real-world data from 3 European referral centres of 71 patients with treatment-naïve mRCC who received first-line nivolumab and ipilimumab with the primary in place showed that irrespective of IMDC risk, patients with a partial response in the primary had an 89% 1-year overall survival (OS) rate versus 67% in patients without (p = 0.012) [16]. The overall response rate (ORR) for primary and metastatic sites was analysed in which 33.3% (23 patients) had a RECIST 1.1 partial response (PR) in the primary tumour. The mean baseline diameter of the primary tumour was 10.14 cm [range 2.9–15.3 cm]) and median time-to-response was 4.8 months (IQR 2.5–6). Of those with a PR in the primary tumour, 91.3% (20/23) achieved responses at metastatic sites of which 17.3 % (4/23) complete response (CR). These data are comparable to post-hoc analyses of patients treated in the pivotal trials with their primary tumour in place.
Data from the CheckMate 214 trial of 55 patients treated with their primary tumour in place with nivolumab and ipilimumab showed a median progression free survival (PFS) and OS of 8.1 months [95% CI 5.5–20.9] and 26.1 months [95% CI 13.9–25.4] months, respectively [17]. Including the primary tumour in the RECIST target lesions, ORR was 34% with none of the patients achieving a CR. Data from the Javelin 101 trial showed similar results for the combination of avelumab and axitinib in 55 patients without prior nephrectomy [18]. A PR in the primary occurred in 34.5% after a median of 4.4 months. The agreement rate between patients with PR in the primary and ORR in all target lesions was 83.6% [19].
In some of these patients deferred CN may result in no evidence of disease (NED) with the main advantage of at least a time-out for ST, and therefore avoiding from side-effects. As a consequence, indications for deferred CN are now increasingly being discussed at multidisciplinary tumour boards but lack high-level evidence.
WHAT SHOULD BE DONE DIFFERENTLY IN FUTURE TRIALS ON CYTOREDUCTIVE NEPHRECTOMY?
To answer to this clinical unmet need, two randomised controlled trials have been initiated to investigate deferred CN in the era of immune checkpoint inhibitor combination therapy and have started accrual (NORDICSUN; NCT03977571 and PROBE; NCT04510597). They are testimony that upfront CN for patients requiring systemic therapy is not only no longer regarded standard-of-care but also not a relevant question to be investigated in conjunction with immunotherapy [Table 3]. Translational data from other tumour entities such as melanoma support using immunotherapy with the tumour antigens in place to prime the immune response, [20, 21] but it is unclear if this is also required in the setting of metastatic RCC. A recent publication including patients with synchronous clear-cell mRCC who were pre-treated with nivolumab and underwent either a biopsy or CN suggests that nivolumab maintains a pre-existing T-cell mediated immune response in the tumour tissue of patients responding to therapy [22]. A higher level of T cells was found in responders both pre- and post-treatment. In addition, maintenance of highly similar clusters of T-cell receptors post-treatment predicted response. An explanation for this could be an ongoing antigen engagement and survival of families of T cells likely recognizing the same antigens. The authors suggested that nivolumab drives both maintenance and replacement of previously expanded T cell clones. Only maintenance correlated with response [22].
Table 3
Suggestions in what future trials on CN should do differently
| •For the intermediate and poor risk group there is no role for upfront CN. |
| •Allowing all systemic treatment combinations to be eligible will lead to more rapid accrual |
| •Sample size calculation should be adapted accordingly and not be based on efficacy data from VEGFR-TKI trials |
| •Including patients with no progression at metastatic sites will give longer median OS and therefore larger sample sizes are required |
NORDICSUN AND PROBE Trial
NORDICSUN was the first trial to be designed by the Scandinavian DARENCA and NORENCA groups to test the hypothesis that deferred CN after initial nivolumab combined with ipilimumab will improve OS in patients with synchronous metastatic RCC and ≤3 IMDC risk features (Fig. 1). Starting from the principle that patients who require ST should receive it first and should only undergo deferred CN if they meet general surgical criteria and have no more than 3 IMDC factors, the trial will assess surgical feasibility and IMDC factor status after a period of pre-treatment with nivolumab and ipilimumab. This rationale is based on current data that suggest that only for intermediate risk patients a survival benefit may be achieved by performing CN, and not for poor risk patients.
Fig. 1
The design of the NORDICSUN.
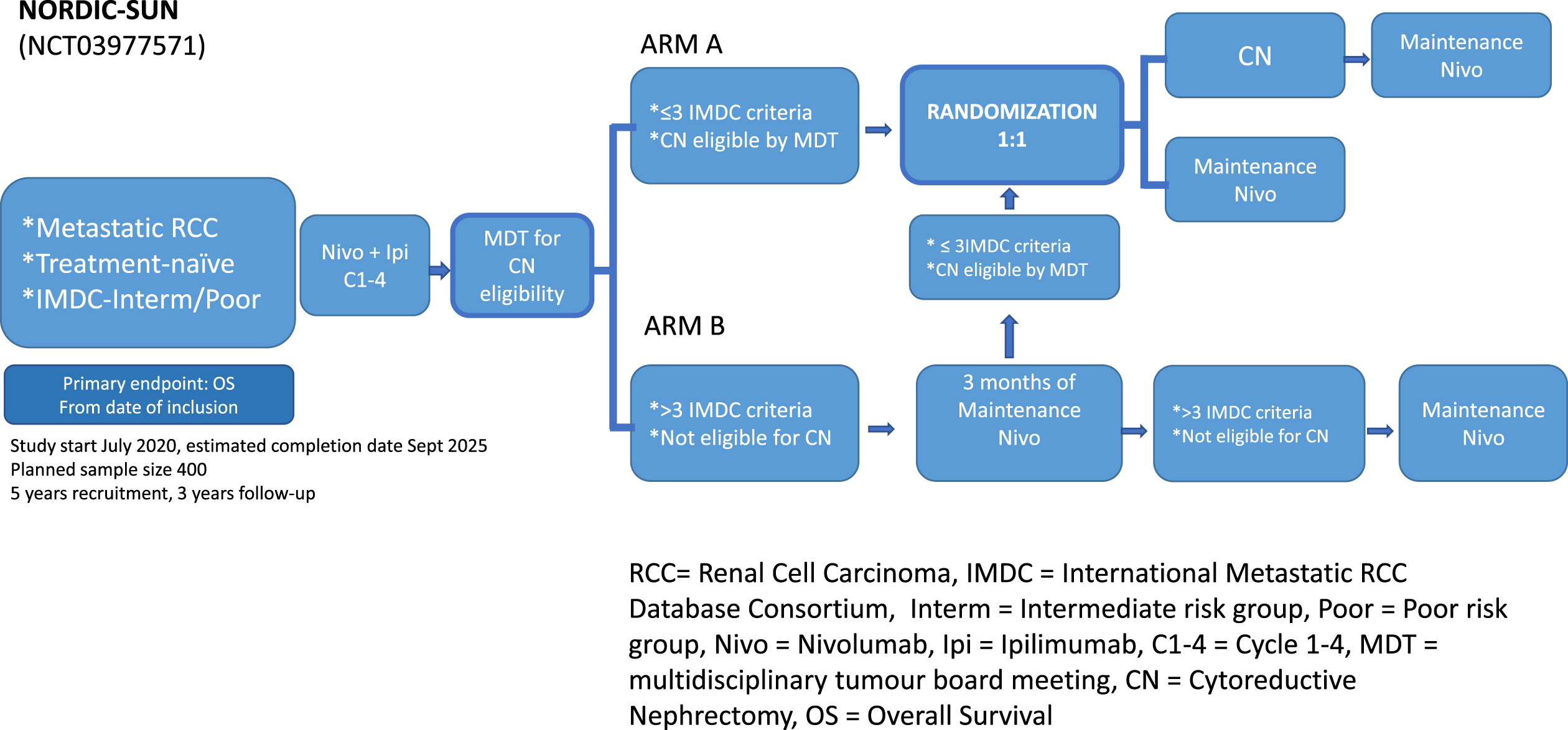
All patients will receive induction checkpoint immunotherapy immediately after inclusion. After 3 months or a total of 4 series of nivolumab combined with ipilimumab, depending on which comes first, the patient will be discussed for CN at the multidisciplinary tumour board meeting (MDT). Whether the patient is eligible for CN is left to the discretion of the urologist at the local MDT. Patients with ≤3 IMDC risk factors and deemed suitable for CN will subsequently undergo randomization. Patients deemed not suitable for surgery or have > 3 IMDC risk features at the 3-month evaluation continue systemic therapy for another 3 months, after which a second similar evaluation will take place (Fig. 1). Nivolumab may continue until unacceptable toxicity or total treatment length of 2 years from inclusion. The main eligibility criteria require core needle biopsy proven primary metastatic RCC of any histologic subtypes which is a novelty compared to CARMENA and SURTIME. The planned sample size for this study is 400 patients, based on the assumption that 240 patients (60%) will meet the criteria for randomisation. At the time of randomisation, patients will be randomly assigned on a 1:1 basis with 120 patients in each of the two treatment arms. Analysis for the primary endpoint is scheduled after 168 deaths or 70% of the events. A duration of five years is planned for randomisation, continued with a minimum of 3 years follow up.
Patients will undergo tumour tissue, blood, and stool collection at baseline, 3 and 6 months, for planned translational research.
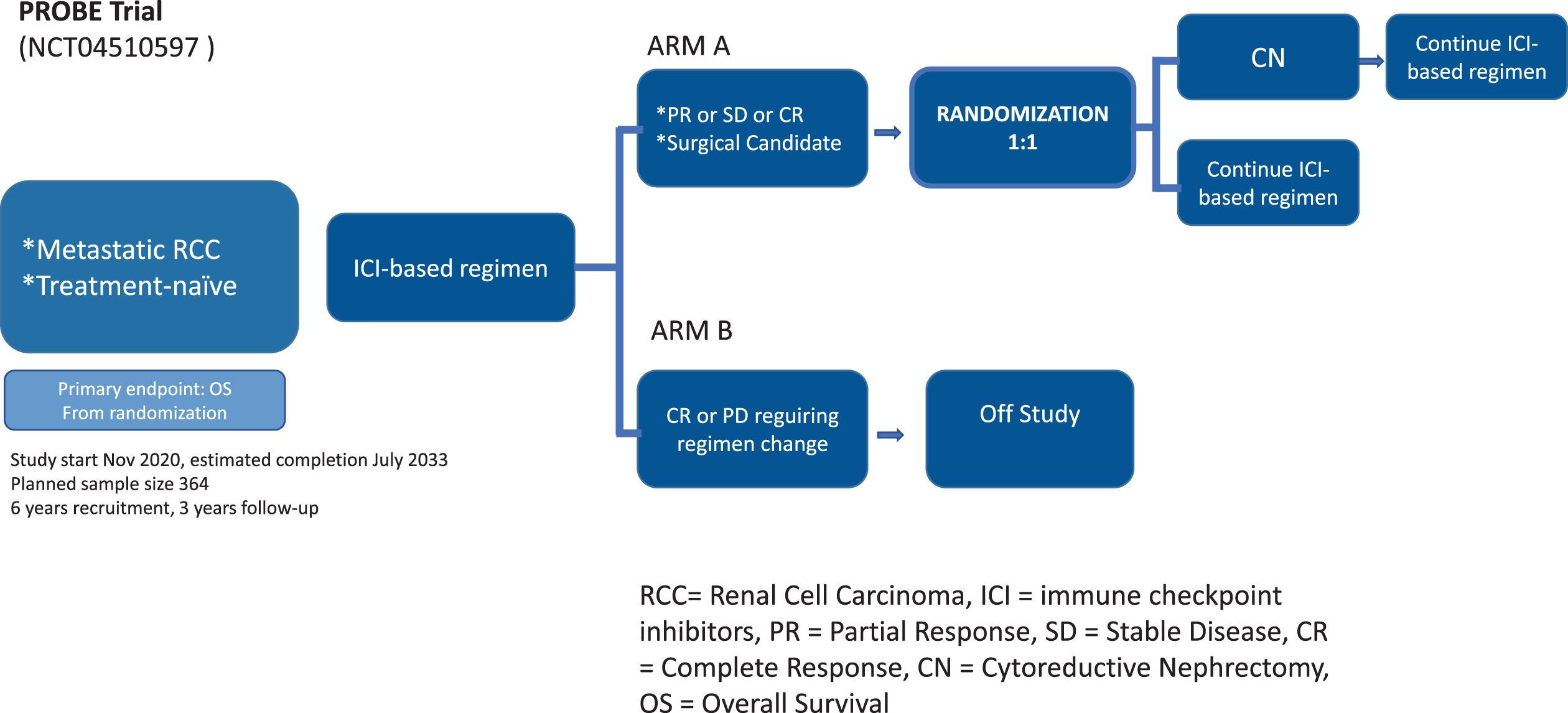
Contrary to NORDICSUN, the US-based PROBE phase III trial led by the SWOG compares the effect of adding CN to a standard of care immunotherapy-based drug combination versus a standard of care immunotherapy-based drug combination alone in treating patients with primary metastatic RCC (Fig. 2). Immunotherapy includes nivolumab and ipilimumab, pembrolizumab and axitinib, and avelumab and axitinib. In PROBE, patients are pre-treated for 3 months and all subtypes are eligible after biopsy proven metastatic RCC. Only patients with an objective RECIST 1.1 response or stable disease at metastatic sites will be randomised to deferred CN and continuation of therapy or continuation of therapy without deferred CN (Fig. 2). The planned sample size includes 364 patients based on the hypothesis that deferred CN in the intention to treat population has a superiority at a one-sided level of 0.005. The study is 85% powered to detect a 47% improvement in median survival. A six-year duration of randomisation is planned with three years follow up, assuming exponential survival. As in NORDICSUN, the trial will have secondary and translational exploratory endpoints.
Fig. 2
The design of the PROBE Trial. Studies any U.S. FDA-regulated ICI combination.
Trials investigating CN in the current era of immune checkpoint inhibitor combination therapies will be challenging to perform considering the rapid evolvement of new treatment paradigms. Like the previous SWOG and EORTC trials in the cytokine era, both CARMENA and SURTIME took 8 years to recruit enough patients for academically valid information on how these patients should be best managed. Both trials however, did not reach full accrual which was especially apparent in the more complex designed SURTIME trial. With new treatment options replacing sunitinib as first line treatment of metastatic RCC, the continued conduct of both CARMENA and SURTIME would have introduced ethical dilemmas and poor accrual. NORDICSUN has recently been amended to accommodate other immune checkpoint inhibitor combinations to answer to the increasing variety of first line options. This aspect is important to render these trials future proof and acknowledge that the efficacy of these combinations is a class-effect. Without head-to-head comparison, there is no evidence as to the superiority of one combination over the other and allowing all treatment combinations to be eligible will lead to more rapid accrual [Table 3].
A potentially more serious threat to the trials reporting on time could be a sample size calculation based on efficacy data from the VEGFR-TKI trials [Table 3]. The recently presented 5-year data from CheckMate 214 suggest that for IMDC intermediate and poor risk patients, who classically include patients with synchronous metastatic RCC, the progression-free survival curve plateaus at 30% beginning at 24 months, continuing past the five-year mark [23]. The current reported median OS for the combined intermediate and poor IMDC risk group is 47 (95% CI 35.4–57.4) months [20] compared to 26.6 (22.1–33.5) months for sunitinib. The PROBE study based the sample size calculation on OS data from the VEGFR-TKI era which are similar to the sunitinib arm in the intermediate and poor IMDC risk in CheckMate 214.
What has also not been accounted for is the fact that both trials only randomise patients who had no progression at metastatic sites. The impressive median OS achieved in CheckMate 214 with the combination arm, however, includes patients with progressive disease. Excluding these patients, median OS could be longer suggesting that, based on the current sample size calculations, the event rates will be lower. Consequently, this would probably require larger sample sizes which may even be prohibitive and render these trials outdated by the time they complete accrual [Table 3].
Furthermore, OS as an endpoint should be meaningful for patients and deferred CN should at least lead to an improvement of OS of 12 months or longer.
Recently, at ASCO-GU 2022, the 30-month follow-up of the KEYNOTE-564 was presented. This double-blind, multicenter, randomized trial (NCT03142334) of adjuvant pembrolizumab for patients with RCC at intermediate-high or high risk of recurrence after nephrectomy or nephrectomy and resection of metastatic lesions resulted in a statistically significant improvement in disease-free survival (DFS) vs placebo during 24 months of follow-up (HR 0.68, 95% CI 0.53–0.87; P = 0.0010 [one-sided])[24]. This trial included a small subgroup of patients after metastasectomy (M1) to no-evidence-of-disease (NED)< / = 1 year after surgery (5.8%). This subgroup contained both primary metastatic (synchronous) and metachronous patients but no distinction between both groups is made in the publication. With only 29 patients per arm and a further unknown ratio of primary mRCC who underwent upfront CN and metastasectomy to NED it is difficult to draw any firm conclusions from Keynote-564 at this stage. The data however suggest that a supgroup of patients may benefit from upfront CN and metastasectomy to NED followed by adjuvant pembrolizumab in terms of DFS.
Even though the role of adjuvant versus upfront ST in locally advanced RCC has yet to be determined, the topic generates discussions during current multidisciplinary tumour board meetings. Future data will help in consolidating and structuring treatment strategies for timing of (cytoreductive) nephrectomy within the landscape of locally advanced and metastatic RCC.
CONCLUSIONS
Upfront CN for intermediate and poor-risk patients with mRCC is no longer standard-of-care. The lesson learnt from CARMENA and SURTIME is that patients who require systemic therapy should be treated with the primary tumour in place as a proof-of-principle. Deferred CN may subsequently be offered to those without progression during ST and randomised controlled trials are ongoing to investigate if this approach yields OS benefits in the era of immune checkpoint inhibition. In view of the rapid evolvement of new treatment paradigms, future trials should allow all ST combinations which will lead to more rapid accrual. Sample size calculation should not be based on efficacy data from VEGFR-TKI trials as OS data are longer for ICI. In addition, randomising only patients without progression at metastatic sites will result in longer median OS, which will require larger sample sizes.
ACKNOWLEDGMENTS
We would like to thank Luna van den Brink for grammar control as a native speaker.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
a. AB and PZ both have made substantial contributions to the work.
b. AB and PZ both have been writing the article.
c. AB and PZ both approved the final version to be published.
d. AB and PZ both agreed to be accountable for the accuracy and integrity of the work.
CONFLICT OF INTEREST
Patricia Zondervan is an Editorial Board Member of this journal, but was not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review.
Axel Bex is an Editorial Board Member of this journal, but was not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review. He received an educational grant from Pfizer for a neoadjuvant trial and is steering committee member of two adjuvant trials of BMS and Roche/Genentech.
REFERENCES
[1] | Wong MCS , Goggins WB , Yip BHK , Fung FDH , Leung C , Fang Y , Wong SYS , Ng CF , Incidence and mortality of kidney cancer: temporal patterns and global trends in 39 countries, Sci Rep (2017) ;7: (1):15698. |
[2] | Flanigan RC , Salmon SE , Blumenstein BA , Bearman SI , Roy V , McGrath PC , Caton JR Jr , Munshi N , Crawford ED , Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer, N Engl J Med (2001) ;345: (23):1655–9. |
[3] | Mickisch GH , Garin A , van Poppel H , de Prijck L , Sylvester R ;European Organisation for Research and Treatment of Cancer (EORTC)Genitourinary GrouRadical nephrectomy plus interferon-alfa-basedimmunotherapy compared with interferon alfa alone in metastaticrenal-cell carcinoma: a randomised trial. Lancet, (2001) ;358: 966–70. |
[4] | Méjean A , Ravaud A , Thezenas S , Colas S , Beauval JB , Bensalah K , Geoffrois L , Thiery-Vuillemin A , Cormier L , Lang H , Guy L , Gravis G , Rolland F , Linassier C , Lechevallier E , Beisland C , Aitchison M , Oudard S , Patard JJ , Theodore C , Chevreau C , Laguerre B , Hubert J , Gross-Goupil M , Bernhard JC , Albiges L , Timsit MO , Lebret T , Escudier B , Sunitinib Alone or after Nephrectomy in MetastaticRenal-Cell Carcinoma, N Engl J Med (2018) ;379: (5):417–27. |
[5] | Bex A , Mulders P , Jewett M , Wagstaff J , van Thienen JV , Blank CU , van Velthoven R , Del Pilar Laguna M , Wood L , van Melick HHE , Aarts MJ , Lattouf JB , Powles T , de Jong Md PhD IJ , Rottey S , Tombal B , Marreaud S , Collette S , Collette L , Haanen J , Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial, JAMA Oncol (2019) ;5: (2):164–70. |
[6] | Ljungberg B , Albiges L , Abu-Ghanem Y , Bensalah K , Dabestani S , Fernández-Pello S , Giles RH , Hofmann F , Hora M , Kuczyk MA , KuuskT , Lam TB , Marconi L , Merseburger AS , Powles T , Staehler M , TahbazR , Volpe A , Bex A , European Association of Urology Guidelines onRenal Cell Carcinoma: The Update, Eur Urol (2019) ;75: (5):799–810. |
[7] | Escudier B , Porta C , Schmidinger M , Rioux-Leclercq N , Bex A , Khoo V , Grünwald V , Gillessen S , Horwich A; ESMO Guidelines Committee , Electronic address: clinicalguidelines@esmo, org. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) ;30: (5):706–20. |
[8] | Motzer RJ , Tannir NM , McDermott DF , Arén Frontera O , Melichar B , Choueiri TK , Plimack ER , Barthélémy P , Porta C , George S , Powles T , Donskov F , Neiman V , Kollmannsberger CK , Salman P , GurneyH , Hawkins R , Ravaud A , Grimm MO , Bracarda S , Barrios CH , Tomita Y , Castellano D , Rini BI , Chen AC , Mekan S , McHenry MB , Wind-Rotolo M , Doan J , Sharma P , Hammers HJ , Escudier B; CheckMate 214Investigators , Nivolumab plus Ipilimumab versus Sunitinib inAdvanced Renal-Cell Carcinoma, N Engl J Med (2018) ;378: (14):1277–90. |
[9] | Choueiri TK , Powles T , Burotto M , Escudier B , Bourlon MT , Zurawski B , Oyervides Juárez VM , Hsieh JJ , Basso U , Shah AY , SuárezC , Hamzaj A , Goh JC , Barrios C , Richardet M , Porta C , Kowalyszyn R , Feregrino JP , Zołnierek J , Pook D , Kessler ER , Tomita Y , Mizuno R , Bedke J , Zhang J , Maurer MA , Simsek B , Ejzykowicz F , Schwab GM , Apolo AB , Motzer RJ , CheckMate 9ER Investigators, Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-CellCarcinoma. N Engl J Med (2021) ;384: (9):829–41. |
[10] | Choueiri TK , Motzer RJ , Rini BI , Haanen J , Campbell MT , Venugopal B , Kollmannsberger C , Gravis-Mescam G , Uemura M , Lee JL , Grimm MO , Gurney H , Schmidinger M , Larkin J , Atkins MB , Pal SK , Wang J , Mariani M , Krishnaswami S , Cislo P , Chudnovsky A , Fowst C , Huang B , di Pietro A , Albiges L , Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma, Ann Oncol.-9 (2020) ;31: (8):1030–9. doi: 10.1016/j.annonc.2020.04.010. Epub 2020 Apr 25. PMID: 32339648; PMCID: PMC8436592. |
[11] | Soulières D , et al., Pembrolizumab Plus Axitinib Versus Sunitinib as First-Line Therapy for Advanced Renal Cell Carcinoma (RCC): Subgroup Analysis From KEYNOTE 426 by Prior Nephrectomy 19th annual meeting of the International Kidney Cancer 524 Symposium, 2020. A Virtual Experience. https://www.urotoday.com/conferencehighlights/asco-2021/asco-2021-kidney-cancer/130133. |
[12] | Motzer R , Alekseev B , Rha SY , Porta C , Eto M , Powles T , GrünwaldV , Hutson TE , Kopyltsov E , Méndez-Vidal MJ , Kozlov V , AlyasovaA , Hong SH , Kapoor A , Alonso Gordoa T , Merchan JR , Winquist E , Maroto P , Goh JC , Kim M , Gurney H , Patel V , Peer A , Procopio G , Takagi T , Melichar B , Rolland F , De Giorgi U , Wong S , Bedke J , Schmidinger M , Dutcus CE , Smith AD , Dutta L , Mody K , Perini RF , Xing D , Choueiri TK ; CLEAR Trial Investigators. Lenvatinib plusPembrolizumab or Everolimus for Advanced Renal Cell Carcinoma, NEngl J Med (2021) ;384: (14):1289–300. |
[13] | Abu-Ghanem Y , van Thienen JV , Blank C , Aarts MJB , Jewett M , de Jong IJ , Lattouf JB , van Melick HHE , Wood L , Mulders P , Rottey S , Wagstaff J , Zondervan P , Powles T , Neven A , Collette L , Tombal B , Haanen J , Bex A , Cytoreductive nephrectomy and exposure to sunitinib - a post hoc analysis of the Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients With Metastatic Kidney Cancer (SURTIME) trial. BJU Int. 2021 Oct 27. doi: 10.1111/bju.15625. Epub ahead of print. PMID: 34706141. |
[14] | Méjean A , Ravaud A , Thezenas S , Chevreau C , Bensalah K , Geoffrois L , Thiery-Vuillemin A , Cormier L , Lang H , Guy L , Gravis G , Rolland F , Linassier C , Lechevallier E , Oudard S , Laguerre B , Gross-Goupil M , Bernhard JC , Colas S , Albiges L , Lebret T , Treluyer JM , Timsit MO , Escudier B , Sunitinib Alone or After Nephrectomy forPatients with Metastatic Renal Cell Carcinoma: Is There Still a Rolefor Cytoreductive Nephrectomy? Eur Urol (2021) ;80: (4):417–24. |
[15] | Singla N , Hutchinson RC , Ghandour RA , Freifeld Y , Fang D , Sagalowsky AI , Lotan Y , Bagrodia A , Margulis V , Hammers HJ , Woldu SL , Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: An analysis of the National Cancer Database, Urol Oncol (2020) ;38: (6):604.e9–604.e17. |
[16] | Meerveld-Eggink A , Graafland N , Wilgenhof S , Van Thienen JV , Lalezari F , Grant M , Szabados B , Abu-Ghanem Y , Kuusk T , Boleti E , Blank CU , Haanen JBAG , Powles T , Bex A , Primary Renal Tumour Response in Patients Treated with Nivolumab and Ipilimumab for Metastatic Renal Cell Carcinoma: Real-world Data Assessment, Eur Urol Open Sci (2022) ;35: :54–8. |
[17] | Albiges L , Tannir NM , Burotto M , McDermott D , Plimack ER , Barthélémy P , Porta C , Powles T , Donskov F , George S , Kollmannsberger CK , Gurney H , Grimm MO , Tomita Y , Castellano D , Rini BI , Choueiri TK , Leung D , Saggi SS , Lee CW , McHenry MB , Motzer RJ , First-line Nivolumab plus Ipilimumab Versus Sunitinib in PatientsWithout Nephrectomy and With an Evaluable Primary Renal Tumor in theCheckMate 214 Trial, Eur Urol (2022) ;81: (3):266–71. |
[18] | Albiges L , Rini BI , Haanen JBAG , Motzer RJ , Kollmannsberger CK , Negrier S , et al., Primary renal tumour shrinkage in patients (pts) who did not undergo upfront cytoreductive nephrectomy (uCN): Subgroup analysis from the phase III JAVELIN Renal 101 trial of first-line avelumab + axitinib (A + Ax) vs sunitinib (S) for advanced renal cell carcinoma (aRCC), Annals of Oncology (2019) ;30: :v359–v60. |
[19] | Albiges L , Rini BI , Haanen JBAG , Motzer RJ , Kollmannsberger CK , Negrier S , Nole F , Bedk8 J , Bilen MA , Nathan P , Tomita Y , Huang B , Chin KA , Chudnovsky A , Robbins PB , di Pietro A , Thomaidou D , Choueiri TK , Primary renal tumour shrinkage in patients (pts) whodid not undergo upfront cytoreductivenephrectomy (uCN): Subgroupanalysis fromthe phase III JAVELIN Renal 101 trial of first-line avelumab 1axitinib (A 1 Ax) vs sunitinib (S) for advanced renal cell carcinoma(aRCC), Ann Oncol (2019) ;30: (suppl 5):v359–v360. |
[20] | Rozeman EA , Hoefsmit EP , Reijers ILM , Saw RPM , Versluis JM , Krijgsman O , Dimitriadis P , Sikorska K , van de Wiel BA , Eriksson H , Gonzalez M , Torres Acosta A , Grijpink-Ongering LG , Shannon K , Haanen JBAG , Stretch J , Ch’ng S , Nieweg OE , Mallo HA , Adriaansz S , Kerkhoven RM , Cornelissen S , Broeks A , Klop WMC , Zuur CL , van Houdt WJ , Peeper DS , Spillane AJ , van Akkooi ACJ , Scolyer RA , Schumacher TNM , Menzies AM , Long GV , Blank CU , Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma, Nat Med (2021) ;27: (2):256–63. doi: 10.1038/s41591-020-01211-7. Epub 2021 Feb 8. PMID: 33558721. |
[21] | Blank CU , Rozeman EA , Fanchi LF , Sikorska K , van de Wiel B , Kvistborg P , Krijgsman O , van den Braber M , Philips D , Broeks A , van Thienen JV , Mallo HA , Adriaansz S , Ter Meulen S , Pronk LM , Grijpink-Ongering LG , Bruining A , Gittelman RM , Warren S , van Tinteren H , Peeper DS , Haanen JBAG , van Akkooi ACJ , Schumacher TN , Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma, Nat Med (2018) ;24: (11):1655–61. doi: 10.1038/s41591-018-0198-0. Epub 2018 Oct 8. PMID: 30297911. |
[22] | Au L , Hatipoglu E , Robert de Massy M , Litchfield K , Beattie G , Rowan A , Schnidrig D , Thompson R , Byrne F , Horswell S , Fotiadis N , Hazell S , Nicol D , Shepherd STC , Fendler A , Mason R , Del Rosario L , Edmonds K , Lingard K , Sarker S , Mangwende M , Carlyle E , Attig J , Joshi K , Uddin I , Becker PD , Sunderland MW , Akarca A , Puccio I , Yang WW , Lund T , Dhillon K , Vasquez MD , Ghorani E , Xu H , Spencer C , López JI , Green A , Mahadeva U , Borg E , Mitchison M , Moore DA , Proctor I , Falzon M , Pickering L , Furness AJS , Reading JL , Salgado R , Marafioti T , Jamal-Hanjani M ; PEACE Consortium, Kassiotis G , Chain B , Larkin J , Swanton C , Quezada SA , Turajlic S ; TRACERx Renal Consortium Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma, Cancer Cell (2021) ;39: (11):1497–1518.e11. |
[23] | Motzer RJ , McDermott DF , Escudier B , Burotto M , Choueiri TK , Hammers HJ , Barthélémy P , Plimack ER , Porta C , George S , Powles T , Donskov F , Gurney H , Kollmannsberger CK , Grimm MO , Barrios C , Tomita Y , Castellano D , Grünwald V , Rini BI , McHenry MB , Lee CW , McCarthy J , Ejzykowicz F , Tannir NM , Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022 Apr 5. doi: 10.1002/cncr.34180. Epub ahead of print. PMID: 35383908. |
[24] | Choueiri TK , Tomczak P , Park SH , Venugopal B , Ferguson T , Chang YH , Hajek J , Symeonides SN , Lee JL , Sarwar N , Thiery-Vuillemin A , Gross-Goupil M , Mahave M , Haas NB , Sawrycki P , Gurney H , Chevreau C , Melichar B , Kopyltsov E , Alva A , Burke JM , Doshi G , Topart D , Oudard S , Hammers H , Kitamura H , Bedke J , Perini RF , Zhang P , Imai K , Willemann-Rogerio J , Quinn DI , Powles T ; KEYNOTE-564 Investigators Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma, N Engl J Med (2021) ;385: (8):683–94. doi: 10.1056/NEJMoa2106391. PMID: 34407342. |
[25] | Bex A , van Thienen JV , Schrier M , Graafland N , Kuusk T , Hendricksen K , Lagerveld B , Zondervan P , van Moorselaar JA , Blank C , Wilgenhof S , Haanen J A Phase II, single-arm trial ofneoadjuvant axitinib plus avelumab in patients with localized renalcell carcinoma who are at high risk of relapse after nephrectomy(NEOAVAX), Future Oncol.-9 (2019) ;15: (19):2203–9. doi: 10.2217/fon-2019-0111. Epub 2019 Apr 26. Erratum in: Future Oncol.2019;15(22):2667. PMID: 31023100. |
[26] |




