First-Line Immunotherapy Combinations in Advanced Renal Cell Carcinoma: A Rapid Review and Meta-Analysis
Abstract
BACKGROUND:
Combination or multi-agent therapy including immune checkpoint inhibitors has shifted the landscape of the treatment of advanced/metastatic renal cell carcinoma. There are several approved immune checkpoint inhibitor (ICI) combinations featuring antibodies against programmed cell death protein 1 (PD-1) receptor or its ligand 1 (PD-L1) combined with other immune checkpoint inhibitors, multi-targeted tyrosine kinase inhibitors (TKIs), or other agents active in renal cell carcinoma.
OBJECTIVE:
This study aims to compile the evidence of available first-line combination therapies compared to sunitinib monotherapy in advanced renal cell carcinoma.
METHODS:
A systematic literature search was conducted according to the PRISMA statement to identify all randomized Phase III clinical trial data in previously untreated metastatic renal cell carcinoma featuring an immune checkpoint inhibitor combination compared against sunitinib. A two-stage selection process was utilized to determine eligible studies. Of a total of 124 studies and 94 additional abstracts, 6 studies were considered for final analysis. These studies were evaluated for progression free survival (PFS), overall survival (OS), Grade III or higher adverse events (AEs), objective response rate (ORR), and complete response rate (CRR).
RESULTS:
6 studies with 5,121 patients met our search criteria. For OS, ICI combination therapy was favored over sunitinib with an estimated combined hazard ratio of 0.74 (0.67–0.81 95% CI). For PFS, ICI combination therapy was favored over sunitinib with an estimated combined hazard ratio of 0.65 (0.52–0.82, 95% CI). The combination of nivolumab and ipilimumab had the longest duration of response and less incidence of grade III or higher adverse events compared to the combination of anti-PD-1/PD-L1 with TKI. The combination of anti-PD-1/PD-L1 with TKI had higher rates of overall response and longer PFS than the combination of nivolumab/ipilimumab.
CONCLUSIONS:
This meta-analysis supports the recommendation of immune checkpoint inhibitor combination therapy over sunitinib monotherapy for previously untreated advanced renal cell carcinoma by virtue of improved PFS and OS. The choice of which ICI combination therapy to use may be guided by patient-specific characteristics including IMDC risk status, adverse effect profile, and need for early response.
INTRODUCTION
The treatment landscape for advanced or metastatic renal cell carcinoma (aRCC) has shifted considerably in the past five years. With increased understanding of the pathophysiology of the most common histologic variant, clear cell renal cell carcinoma, targeted therapies have been developed and studied with improvements in overall survival [1]. Targeting vascular endothelial growth factor (VEGF) and other angiogenic signals through monoclonal antibodies or multi-targeted tyrosine kinase inhibitors (TKIs) led to improved outcomes in aRCC [2–4]. Identified mutations in the mammalian target of rapamycin (mTOR) pathway led to studies featuring mTOR inhibitors, which were also approved for the systemic treatment of aRCC [5]. Lastly, the immunogenic nature of kidney cancer has allowed for the use immunotherapies including interferon-alfa, IL-2, and immune checkpoint inhibitors (e.g., antibodies against programmed cell death protein 1 (PD-1) receptor or its ligand 1 (PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)) [6–9].
The standard of care therapy for much of the past two decades has generally been monotherapy with one of the aforementioned agents, typically a multi-targeted TKI such as sunitinib, pazopanib, or cabozantninib. More recently, randomized, phase III trials have shifted the paradigm of front-line treatment to combination therapies including immune checkpoint inhibitors (ICI) [9–14]. The combinations feature a PD-1/PD-L1 inhibitor paired with either a CTLA-4 inhibitor, an anti-VEGF antibody, or a multi-targeted VEGF TKI. With new phase III data on novel ICI combinations being published, a significant clinical question has become not just whether to use ICI combinations in the first-line setting, but which combination should be preferred. In the absence of direct comparisons, a systematic review may be able to identify patterns to help guide future head-to-head studies. Thus, we aimed to summarize the available evidence for ICI combinations in the first line setting and perform a meta-analysis that may generate additional hypotheses and studies.
MATERIAL AND METHODS
Search strategy
We sought to identify all English-language, randomized Phase III clinical trial data in previously untreated metastatic renal cell carcinoma featuring an immune checkpoint inhibitor combination compared against sunitinib. A systematic literature search was conducted in accordance with PRISMA recommendations [15]. PubMed search terms included combinations of advanced and/or metastatic renal cell carcinoma as well as the phrase “previously untreated”. Full search terms are available in the appendix. A supplementary search was performed manually to identify congress abstracts published in American Society of Clinical Oncology (ASCO) Annual Meeting, ASCO Genitourinary Cancers Symposium (ASCO-GU) and European Society for Medical Oncology (ESMO) Annual Meeting from January 2020 to February 2021.
Inclusion criteria necessitated that studies investigated advanced/metastatic clear-cell renal cell carcinoma patients undergoing first-line treatment with an immunotherapy combination compared to patients undergoing first-line treatment with sunitinib. Included studies needed to report PFS, OS, objective response, and AEs in phase III randomized clinical trials only.
Exclusion criteria for records included non-English studies, non-original articles (reviews, meta-analyses), letters, editorials, and case reports. In cases of multiple publications on the same cohort, the most recent publication was selected. References of all papers included were scanned for additional studies of interest.
Outcome measures and statistical analyses
Multiple outcomes were extracted for the purposes of this study, including progression-free survival (PFS), overall survival (OS), objective response rate (ORR), complete response rate (CRR) and adverse events (AE).
PFS was defined as time from randomization to first radiographic progression or death due to any cause, censoring individuals without progression at the last disease evaluation. OS was defined as time from randomization to death due to any cause, censoring individuals at the date last known alive. Objective response was defined as the percentage of enrolled patients who achieved either a complete or partial response, based on investigator assessment. Adverse events were defined by the total incidence of any or treatment-related grade 3 or higher adverse event as defined in the respective study.
For PFS and OS, estimated log HRs and standard errors were calculated from the published HRs and CIs. Outcomes for OS and PFS are presented as HR with 95% confidence interval (CI). Subgroup analyses on PFS and OFS were additionally performed for favorable and intermediate/poor-risk disease as categorized by the International mRCC Database Consortium risk categorization (IMDC) [16]. For evaluation, of AE, ORR, and CRR, odds ratios (with 95% confidence intervals) were calculated from the available summarized data presented in the selected studies.
Review Manager (RevMan) software version 5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen) was utilized for this meta-analysis. Fixed and random effect models were used according to the I2 value of heterogeneity; for I2≤50%, a fixed effect model was applied, whereas for I2 > 50% a random model was used. A two-tailed p-value < 0.05 was considered statistically significant.
RESULTS
A CONSORT diagram for the selection process of included studies is provided in Fig. 1. Initial database search identified 124 records of interest with an additional 94 records coming from abstract search of the most recent relevant genitourinary oncology conferences, summing 218 records initially screened. After screening per the above inclusion and exclusion criteria, 6 prospective, randomized Phase III clinical trials were analyzed and compiled for meta-analysis. In sum, the 6 studies included 5,121 subjects. The most recent published or presented update to the study was used for the purposes of analysis [14, 17–21]. The included studies evaluated nivolumab (anti-PD-1) with ipilimumab (anti-CTLA-4), atezolizumab (anti-PD-L1) with bevacizumab (anti-VEGF), avelumab (anti-PD-L1) with axitinib (TKI), pembrolizumab (anti-PD-1) with axitinib, nivolumab with cabozantinib (TKI), and pembrolizumab with lenvantinib (TKI). Study demographics and design are presented in Table 1.
Fig. 1
CONSORT Diagram outlining the selection process of included studies.
Notable results from each of the included studies are presented in Table 2 including median OS, median PFS, ORR, CRR, duration of response, and median follow up.
Intention to treat population: Overall survival
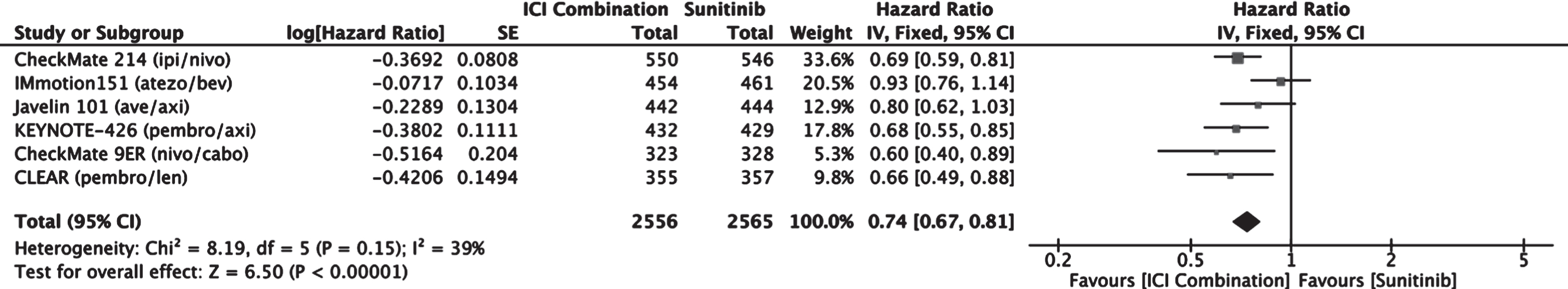
A meta-analysis comparing OS between patients treated with an ICI combination therapy versus sunitinib was performed (Fig. 2). ICI combination therapy resulted in improved OS compared to sunitinib with an estimated combined hazard ratio of 0.74 (0.67–0.81 95% CI).
Fig. 2
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (ITT), outcome: Overall Survival.
Intention to treat population: Progression free survival
A meta-analysis comparing PFS between patients treated with immune checkpoint inhibitor (ICI) combination therapy versus sunitinib was performed (Fig. 3). The results favored the use ICI combination therapy over sunitinib for progression free survival with an estimated combined hazard ratio of 0.65 (0.52–0.82, 95% CI). Of the treatment options, the combination of two checkpoint inhibitors nivolumab + ipilimumab was the only option whose hazard ratio did not reach statistical significance.
Fig. 3
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (ITT), outcome: Progression Free Survival.
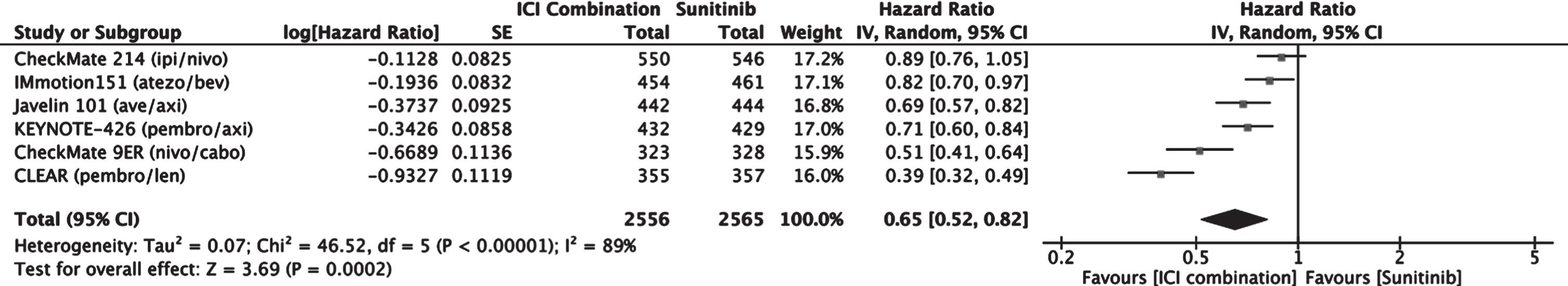
Table 1
Study Demographics
| CheckMate 214 | IMmotion151 | JAVELIN 101 | KEYNOTE-426 | CheckMate 9ER | CLEAR | |
| Year | 2018 | 2019 | 2019 | 2019 | 2020 | 2021 |
| Treatment | Nivolumab 3 mg/kg | Atezolizumab 1200 mg | Avelumab 10 mg/kg | Pembrolizumab 200 mg | Nivolumab 240 mg | Pembrolizumab 200 mg |
| Ipilimumab 1 mg/kg | Bevacizumab 15 mg/kg | Axitinib 5 mg | Axitinib 5 mg | Cabozantinib 40 mg | Lenvatinib 20 mg | |
| Control | Sunitinib 50 mg | Sunitinib 50 mg | Sunitinib 50 mg | Sunitinib 50 mg | Sunitinib 50 mg | Sunitinib 50 mg |
| N (T/C) | 550/546 | 550/546 | 442/444 | 432/429 | 323/328 | 355/357 |
| Median Age (T/C) | 62/62 | 62/62 | 62/61 | 62/61 | 62/61 | 64/61 |
| % Male (T/C) | 75/72 | 75/72 | 71.5/77.5 | 71.3/74.6 | 77/71 | 71.8/77.0 |
| Favorable (T/C) | 125/124 (23% /23%) | 125/124 (23% /23%) | 94/96 (21.3% /21.6%) | 138/131 (31.9% /30.5%) | 74/72 (23% /22%) | 110/124 (31% /34.7%) |
| Intermediate (T/C) | 334/333 (61% /61%) | 334/333 (61% /61%) | 271/276 (61.3% /62.1%) | 238/246 (55.1% /57.3%) | 187/187 (58% /57%) | 210/192 (59.2% /53.8%) |
| Poor (T/C) | 91/89 (17% /16%) | 91/89 (17% /16%) | 72/71 (16.2% /16.0%) | 56/52 (13% /12.1%) | 61/69 (19% /21%) | 33/37 (9.3% /10.4%) |
T indicates treatment arm, C indicates control arm.
Table 2
Notable results from the 6 included studies
| CheckMate 214 | IMmotion 151 | JAVELIN 101 | KEYNOTE-426 | CheckMate 9ER | CLEAR | |
| (Nivo/Ipi), | (Atezo/Bev), | (Ave/Axi), | (Pembro/Axi), | (Nivo/Cabo), | (Pembro/Len), | |
| (n = 550 vs n = 546) | (n = 454 vs n = 461) | (n = 442 vs n = 444) | (n = 432 vs n = 429) | (n = 323 vs n = 328) | (n = 355 vs n = 357) | |
| mOS, months | NR vs 38.4 | 33.6 vs 34.9 | NR vs NR | NR vs 35.7 | NR vs NR | NR vs NR |
| HR (CI) | 0.69 (0.59–0.81) | 0.93 (0.76–1.14) | 0.80 (0.62–1.03) | 0.68 (0.55–0.85) | 0.66 (0.40–0.89) | 0.66 (0.49–0.88) |
| mPFS, months | 12.2 vs 12.3 | 11.2 vs 8.4 | 13.3 vs 8.0 | 15.4 vs 11.1 | 16.6 vs 8.3 | 23.9 vs 9.2 |
| HR (CI) | 0.89 (0.76–1.05) | 0·83 (0.70–0.97) | 0.69 (0.57–0.83) | 0.71 (0.60–0.84) | 0.51 (0.41–0.64) | 0.39 (0.32–0.49) |
| ORR % (CI) | 39.1% (35 to 43) | 37% (32 to 41) | 52.5% (47.7–57.2) | 60% (55.4–64.8) | 55.7% (50.1–61.2) | 71.0% (66.3–75.7) |
| CRR, % | 10.7% | 5% | 3.8% | 9% | 8.0% | 16.1% |
| Duration of Response, months (CI) | NR (49.5 –NR) | 16.6 (15 –NR) | 18.5 (17.8-NR) | 23.5 (19.4–29.0) | 20.2 (not reported) | 25.8 (22.1–27.9) |
| Median Follow up, months | 55 | 24 | 19.3 | 30.6 | 18.1 | 26.6 |
Nivo: nivolumab, Ipi: ipilimumab, Atezo: atezolizumab, Bev: bevacizumab, Ave: avelumab, Axi: axitinib, Pembro: pembrolizumab, Cabo: cabozantinib, Len: lenvantinib, NR: not reached. All confidence intervals are 95%.
Intention to treat population: Objective response rate
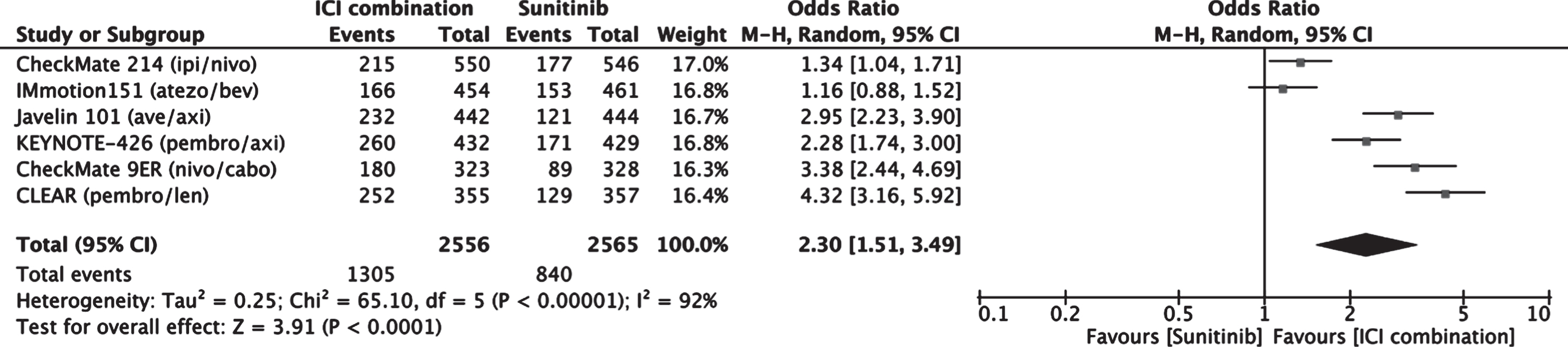
A meta-analysis comparing objective response rate between patients treated with an ICI combination therapy versus sunitinib was performed (Fig. 4). Patients receiving ICI combination had a higher objective response rate than those receiving sunitinib with a combined estimated odds ratio of 2.30 (1.51–3.49, 95% CI).
Fig. 4
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (ITT), outcome: Objective Response Rate.
Intention to treat population: Complete response rate
A meta-analysis comparing complete response rate between patients treated with an ICI combination therapy versus sunitinib was performed (Fig. 5). Patients receiving ICI combination had a higher complete response rate than those receiving sunitinib with a combined estimated odds ratio of 3.07 (2.36–4.01, 95% CI).
Fig. 5
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (ITT), outcome: Complete Response Rate.
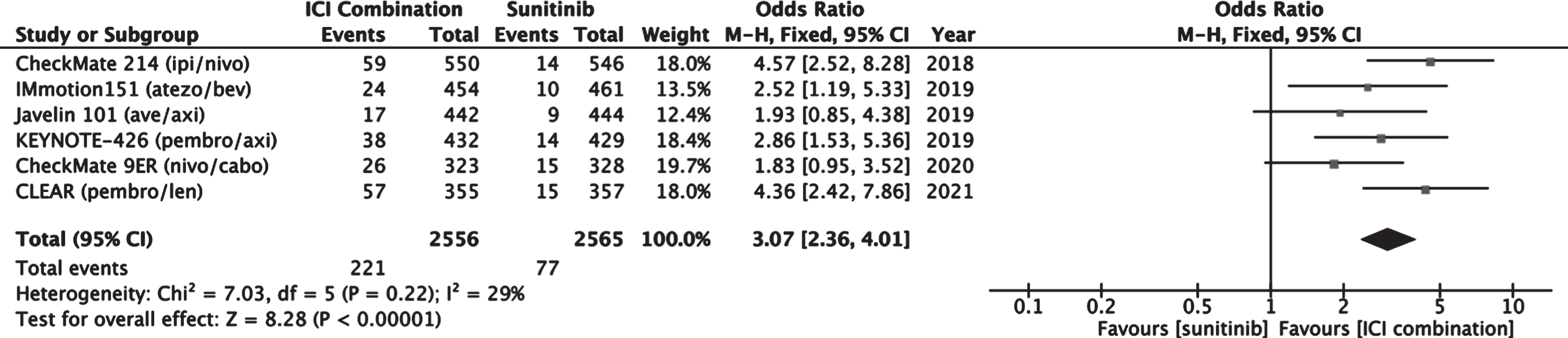
Intention to treat population: Adverse events
A meta-analysis comparing incidence of Grade 3 or higher adverse events between patients treated with an ICI combination therapy versus sunitinib was performed (Fig. 6). In this analysis, an OR > 1 measures an increase in the likelihood of adverse events for immune checkpoint combination. Overall, the incidence of grade 3 or higher adverse events was comparable between sunitinib and ICI combination therapy with a combined estimated odd ratio of 1.01 (0.67–1.52 95% CI). Therapies including a TKI appeared to have similar or more incidence of grade 3 adverse events compared to sunitinib alone (OR < 1). Meanwhile, therapies without a VEGF TKI demonstrated less likelihood of adverse effects compared to sunitinib (OR > 1).
Fig. 6
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (ITT), outcome: Adverse Events (Grade 3 or higher).
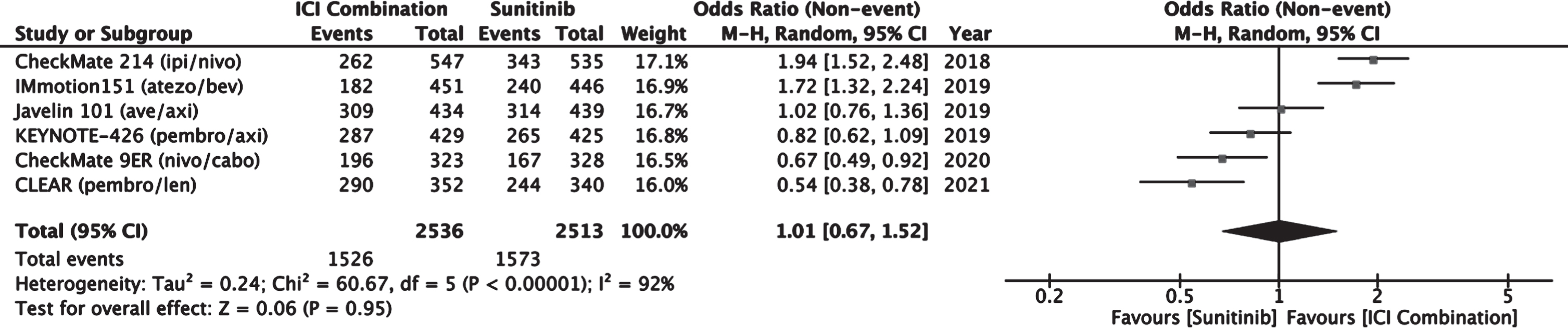
IMDC favorable risk population: PFS and OS
Meta-analyses comparing PFS and OS in patients receiving ICI combination versus sunitinib was performed specifically on the IMDC favorable risk subgroup. Although subgroup analysis of PFS was reported in IMmotion 151, it did not include reported confidence intervals and so was not included in the metanalysis of PFS. Subgroup analysis of OS was not reported in IMmotion151. Progression-free survival seems to favor ICI combination therapy as a group with a combined estimated HR of 0.75 (0.45–1.26, 95% CI) compared to sunitinib (Fig. 7). The effect is driven by the combination of ICI and TKI, as sunitinib demonstrated longer PFS compared to nivolumab/ipilimumab in CheckMate 214. Excluding CheckMate 214 from the analysis adjusted the HR to 0.6 (0.45–0.81, 95% CI) in studies comparing immune checkpoint inhibitor with TKI to sunitinib alone.
Fig. 7
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (IMDC Favorable Risk), outcome: Progression Free Survival.
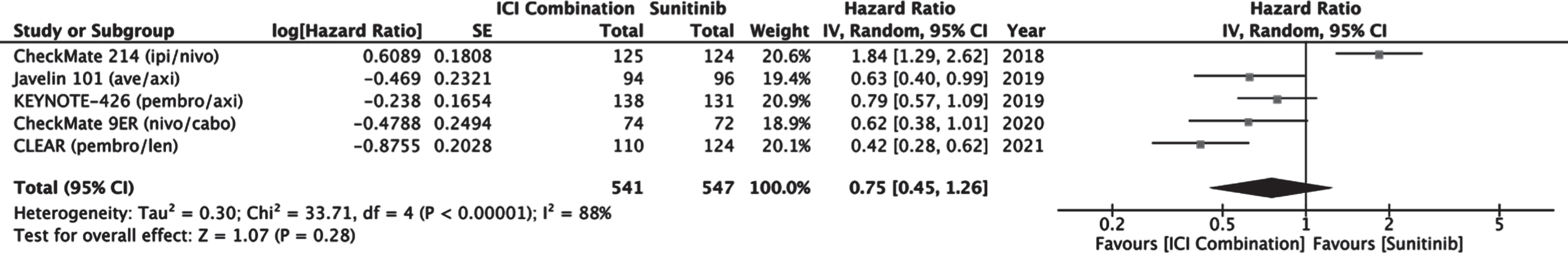
Meta-analysis of overall survival in the favorable risk group treated with either ICI combination or sunitinib did not reveal a significant difference with a combined estimated HR of 0.96 (0.73–1.26, 95% CI) favoring ICI combination (Fig. 8). The true effect on OS may be elucidated as the data matures from these recent trials.
Fig. 8
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (IMDC Favorable Risk), outcome: Overall Survival.
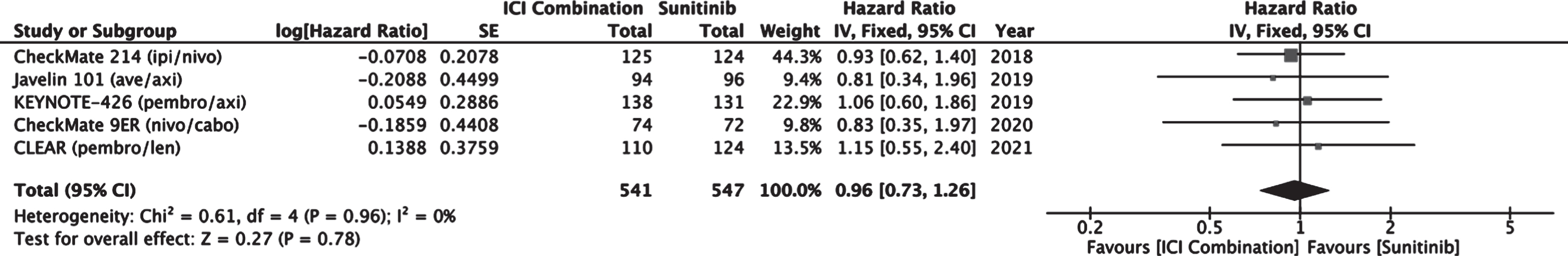
IMDC intermediate/poor risk population: PFS and OS
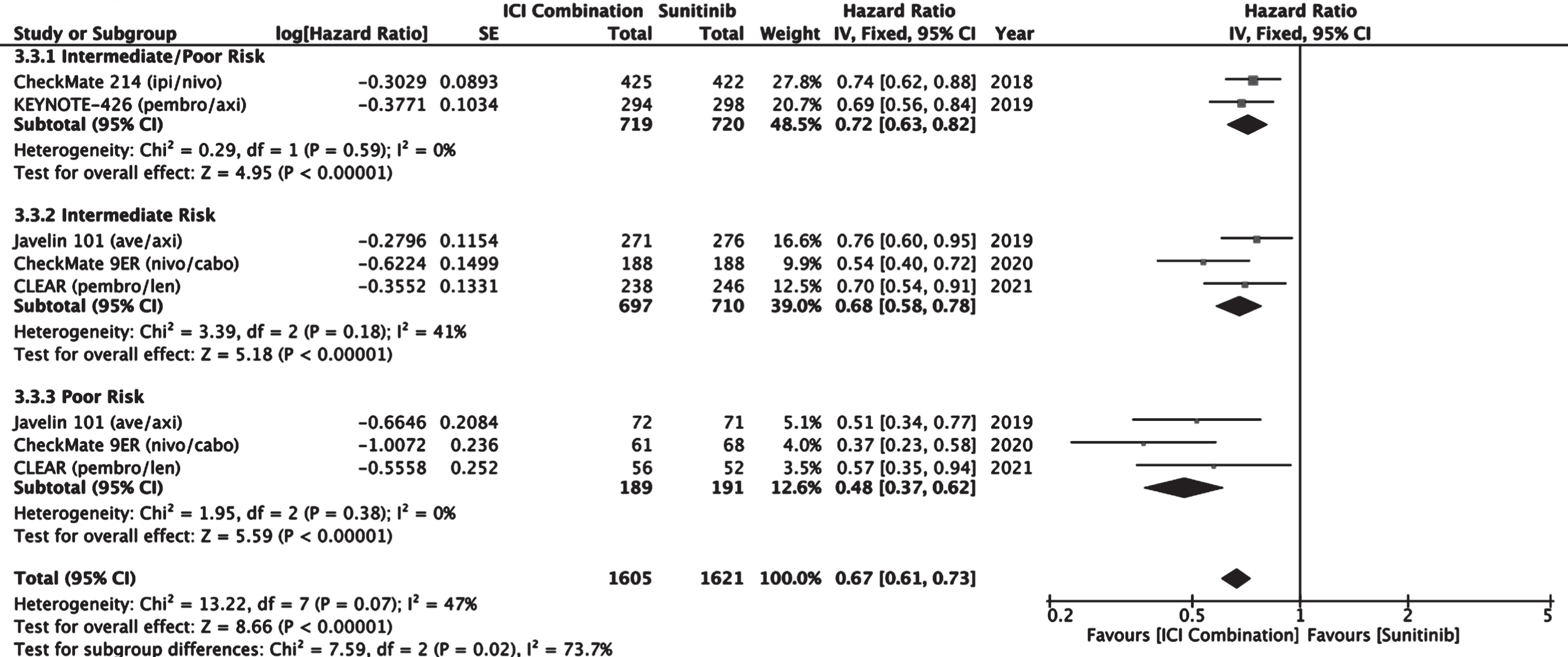
Meta-analyses comparing PFS and OS in patients receiving ICI combination versus sunitinib was performed specifically on the IMDC intermediate and poor risk subgroup. CheckMate 214 and KEYNOTE-426 reported PFS and OS in a combined intermediate/poor risk group. Javelin 101, CheckMate 9ER, and CLEAR reported PFS and OS in distinct intermediate and poor subgroups. Although subgroup analysis of PFS was reported in IMmotion 151, it did not include reported confidence intervals and so was not included in the metanalysis of PFS. Subgroup analysis of OS was not reported in IMmotion151. Overall, treatment with immune checkpoint combination therapy demonstrated an improved PFS compared to sunitinib with an overall estimated HR of 0.67 (0.61–0.73) in intermediate and poor risk patients (Fig. 9).
Fig. 9
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (IMDC Intermediate/Poor Risk), outcome: Progression Free Survival.
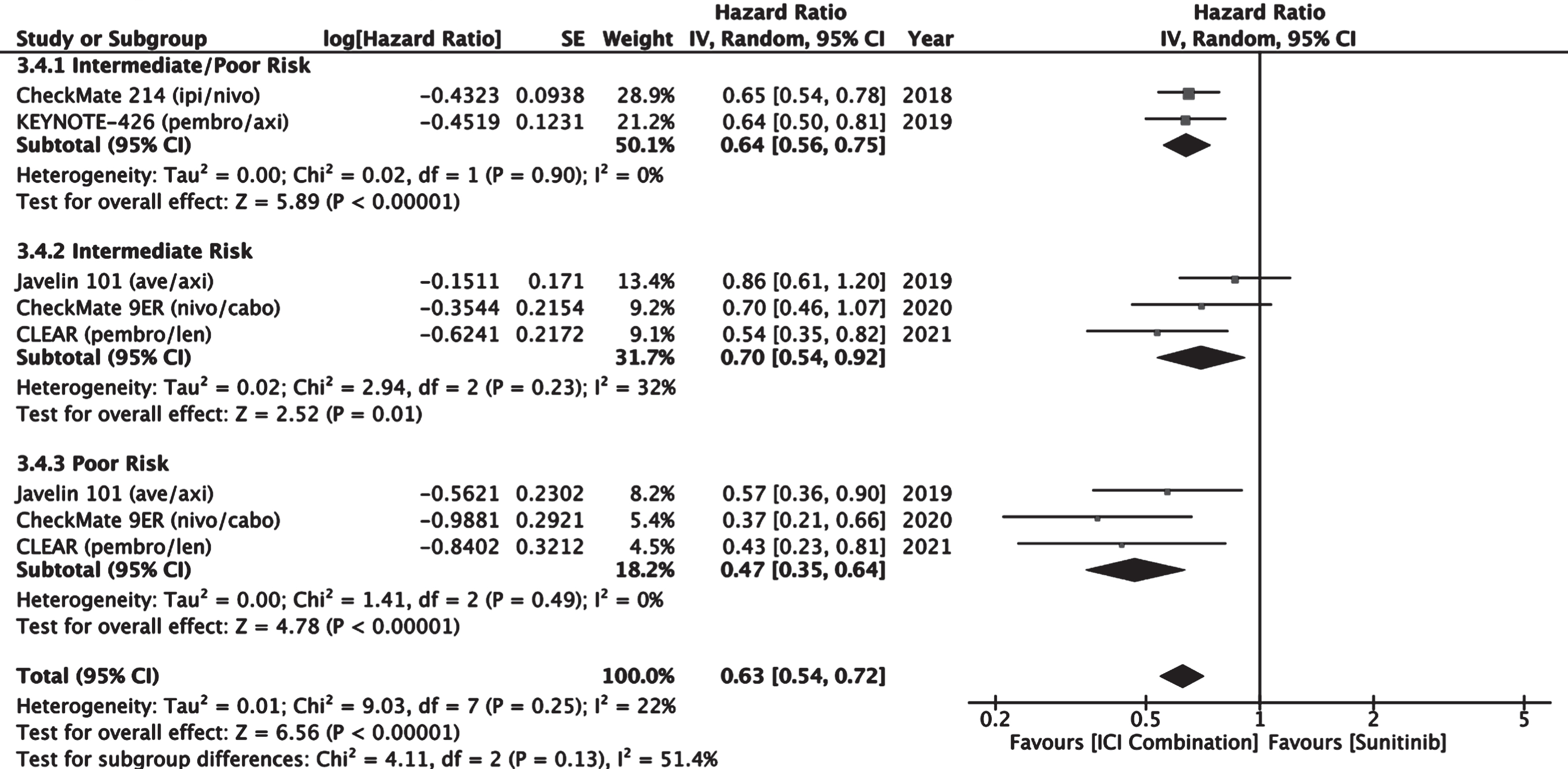
Overall survival was improved with ICI combination therapy compared to sunitinib in this patient population with a combined estimated HR of 0.63 (0.54–0.72, 95% CI) favoring ICI combination (Fig. 10).
Fig. 10
Forest plot of comparison Immunotherapy Combinations versus Sunitinib (IMDC Intermediate/Poor Risk), outcome: Median Overall Survival.
DISCUSSION
Recent advances in the treatment of aRCC have seen the study and approval of multiple immune checkpoint inhibitors in combination with other immune checkpoint inhibitors or multi-targeted VEGF TKIs in the first-line treatment setting. A rapid systematic review and meta-analysis was performed to evaluate and compile the most recent data regarding the use of ICI combination therapy compared against sunitinib. Other reviews and meta-analyses have previously established the efficacy and preference for ICI combination therapies, but with the recent publication of several new trials, an up-to-date evaluation of the most commonly used combinations in previously untreated aRCC is justified [22–25]. The findings of this study confirm the superiority of ICI combination therapy over sunitinib and suggests that a combination option be selected ahead of TKI monotherapy for untreated aRCC. While some national guidelines (i.e., Society for Immunotherapy in Cancer) clearly rank ICI combinations ahead of TKI monotherapy, others (National Comprehensive Center Network) only show a preference for ICI combination in the poor/intermediate risk group [26, 27].
In the intention to treat population, ICI combination therapy demonstrated improved progression free survival and overall survival over sunitinib. An interesting observational trend is the temporal improvement in PFS in the studies since 2018. With respect to overall survival, the two combinations with a PD-L1 monoclonal antibody did not meet statistical significance: atezolizumab with bevacizumab and avelumab with axitinib. The other combinations featuring an anti-PD-1 monoclonal antibody (nivolumab or pembrolizumab) met the threshold for statistically significant improvement in OS.
In the meta-analysis, there was no significant difference in the incidence of grade 3 or higher adverse events. However, there appeared to be a dichotomy between combinations including a multi-targeted TKI and those that did not. All therapies featuring a TKI either had similar toxicity or worse toxicity compared to TKI monotherapy with sunitinib. The combinations of nivolumab with ipilimumab and atezolizumab with bevacizumab had lower likelihood of grade 3 or higher adverse events compared to sunitinib. Objective response rates and complete response rates favored ICI combination therapy over sunitinib. In particular, combinations including a TKI trended toward higher objective response rates. The evidence suggests that for previously untreated patients with advanced renal cell carcinoma a combination featuring an immune checkpoint inhibitor should be considered first-line over monotherapy with a multi-targeted TKI.
In the IMDC favorable risk subgroup analysis, treatment with an ICI combination therapy trended towards, but did not show statistically significant improved progression-free survival over sunitinib. However, a meta-analysis of exclusively the combination PD-L1 blockade and TKI did yield a significant hazard reduction. This correlates with current approvals in which the combination of nivolumab and ipilimumab does not have FDA approval for first-line treatment in favorable risk aRCC. Meta-analysis of overall survival was not statistically different between treatment with all ICI combination therapies or sunitinib in the favorable risk group. Much of the data for overall survival remains immature and may further reach significance with extended follow up. In favorable risk aRCC patients, many clinicians may favor a combination of immune checkpoint inhibition combined with a TKI given the advantage of improved PFS, consistent with published guidelines.
In the IMDC intermediate/poor risk subgroup analysis, treatment with an ICI combination therapy improved both PFS and OS when compared to sunitinib. Without clear efficacy differences among the ICI combination therapies, it can be difficult to select the optimal therapy. We can glean some information from the above meta-analyses that guides this challenging dilemma. It appears that immune checkpoint inhibition with a PD-1/PD-L1 inhibitor combined with a TKI generates more early responses by virtue of higher overall response rates and longer median PFS. This may be the optimal choice for patients needing early response on account of symptomatic metastases. At the same time, the combination of two checkpoint inhibitors, anti-PD-1 nivolumab and anti-CTLA-4 ipilimumab had less objective response rate, but a significantly longer duration of said response. Additionally, the combination of nivolumab and ipilimumab had less Grade 3 or higher toxicity as compared to sunitinib. Thus, for asymptomatic IMDC intermediate/poor risk patients or those looking to minimize adverse events of TKI, the combination of ipilimumab and nivolumab could provide long-term response. In fact, the 4-year follow up of CheckMate 214 demonstrates the feasibility of treatment-free survival, defined as time between protocol therapy discontinuation until subsequent therapy initiation. Long-term follow up data for studies with immune checkpoint inhibitors with TKI will likely inform further clinical decision making. A clinical trial directly comparing nivolumab with ipilimumab against an anti-PD-1 with TKI combination would allow definitive evidence for selection of the optimal first-line therapy.
This systematic review and meta-analysis feature the most recent published phase III studies for previously untreated aRCC all compared against sunitinib monotherapy. The meta-analyses herein demonstrate the superiority of ICI combination therapy over sunitinib and begin to address the current clinical dilemma facing physicians: which ICI combination therapy should be considered first-line? This study is limited in its ability to answer the question given the absence of comparative trials or access to patient level data. This study is purposefully limited in its scope to focus on the most recent advances in front-line treatment of aRCC. Other limitations include those common to systematic reviews and include reporting bias, differences in patient characteristics among the studies, and reliance on published as opposed to patient-level data.
Ultimately, the optimal sequencing of therapies in the aRCC armamentarium can only be decided by direct comparison in clinical trials. Ongoing studies have begun to use ICI combination therapy as the standard of care control arm. The COSMIC-313 trial is investigating the combination of nivolumab, ipilumab, and cabozantninib compared to the combination of nivolumab and ipilimumab [28]. The PDIGREE trial is investigating the sequencing of nivolumab with ipilumab followed by either nivolumab or nivolumab with cabozantinib [29]. In the absence of randomized clinical trials, further information may be gathered by centralized collection and analysis of patient level data from the available studies. Furthermore, the study and development of certain biomarkers may further elucidate subsets of aRCC that respond more favorably to antiangiogenic medications or immune checkpoint inhibition [30, 31]. This systematic review and meta-analysis cannot replace head-to-head analysis but may allow for more informed clinical decision making. Specifically, this study supports the use of ICI combination therapy over that of TKI monotherapy in previously untreated aRCC patients.
CONCLUSIONS
This meta-analysis supports the recommendation of immune checkpoint inhibition combination therapy (anti-PD-1/PD-L1 + either anti-CTLA-4 or TKI) over sunitinib monotherapy for previously untreated advanced renal cell carcinoma by virtue of improved PFS and OS. The choice of which ICI combination therapy to use may be guided by patient-specific characteristics including IMDC risk status, adverse effect profile, and need for early response. Direct comparative trials are further needed to define the optimal sequencing of available therapies.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding for this study.
AUTHOR CONTRIBUTIONS
JS and DM performed the literature review. JS performed the statistical analysis which was reviewed by PC. JS and DM interpreted the data. All authors helped write and edit the manuscript.
CONFLICT OF INTEREST
Jason Shpilsky and Paul J Catalano have no conflicts of interest or financial disclosures. David F McDermott discloses the following:
Consulting honoraria from Bristol Myers Squibb, Pfizer, Merck, Alkermes, Inc., EMD Serono, Eli Lilly and Company, Iovance, Eisai Inc., Werewolf Therapeutics, Calithera Biosciences. Research support from Bristol Myers Squibb, Merck, Genentech, Pfizer, Exelixis, X4 Pharma, and Alkermes, Inc.
REFERENCES
[1] | Choueiri TK , Motzer RJ . Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med. (2017) ;376: :354–66. https://doi.org/10.1056/NEJMra1601333. |
[2] | Motzer RJ , Hutson TE , Tomczak P , Michaelson MD , Bukowski RM , Rixe O , et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N Engl J Med. (2007) ;356: :115–24. https://doi.org/10.1056/NEJMoa065044. |
[3] | Sternberg CN , Davis ID , Mardiak J , Szczylik C , Lee E , Wagstaff J , et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. (2010) ;28: :1061–8. https://doi.org/10.1200/JCO.2009.23.9764. |
[4] | Choueiri TK , Hessel C , Halabi S , Sanford B , Michaelson MD , Hahn O , et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer. (2018) ;94: :115–25. https://doi.org/10.1016/j.ejca.2018.02.012. |
[5] | Hudes G , Carducci M , Tomczak P , Dutcher J , Figlin R , Kapoor A , et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N Engl J Med. (2007) ;356: :2271–81. https://doi.org/10.1056/NEJMoa066838. |
[6] | Motzer RJ , Bacik J , Murphy BA , Russo P , Mazumdar M . Interferon-Alfa as a Comparative Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma. J Clin Oncol. (2002) ;20: :289–96. https://doi.org/10.1200/JCO.2002.20.1.289. |
[7] | Fyfe G , Fisher RI , Rosenberg SA , Sznol M , Parkinson DR , Louie AC . Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol.. (1995) ;13: :688–96. https://doi.org/10.1200/JCO.1995.13.3.688. |
[8] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med.. (2015) ;373: :1803–13. https://doi.org/10.1056/NEJMoa1510665. |
[9] | Motzer RJ , Tannir NM , McDermott DF , Frontera OA , Melichar B , Choueiri TK , et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018. https://doi.org/10.1056/NEJMoa1712126. |
[10] | Motzer RJ , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. IMmotion A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). J Clin Oncol. (2018) ;36: :578–578. https://doi.org/10.1200/JCO.2018.36.6_suppl.578. |
[11] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med.. (2019) ;380: :1103–15. https://doi.org/10.1056/NEJMoa1816047. |
[12] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med.. (2019) ;380: :1116–27. https://doi.org/10.1056/NEJMoa1816714. |
[13] | Choueiri TK , Powles T , Burotto M , Bourlon MT , Zurawski B , Oyervides Juárez VM , et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol. (2020) ;31: :S1159. https://doi.org/10.1016/j.annonc.2020.08.2257. |
[14] | Motzer R , Alekseev B , Rha S-Y , Porta C , Eto M , Powles T , et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. (2021) ;0: :null. https://doi.org/10.1056/NEJMoa2035716. |
[15] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) ;372: :n71. https://doi.org/10.1136/bmj.n71. |
[16] | Ko JJ , Xie W , Kroeger N , Lee J , Rini BI , Knox JJ , et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. (2015) ;16: :293–300. https://doi.org/10.1016/S1470-2045(14)71222-7. |
[17] | Albiges L , Tannir NM , Burotto M , McDermott D , Plimack ER , Barthélémy P , et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. (2020) ;5: :e001079. https://doi.org/10.1136/esmoopen-2020-001079. |
[18] | Rini BI , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. The Lancet. (2019) ;393: :2404–15. https://doi.org/10.1016/S0140-6736(19)30723-8. |
[19] | Choueiri TK , Motzer RJ , Rini BI , Haanen J , Campbell MT , Venugopal B , et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. (2020) ;31: :1030–9. https://doi.org/10.1016/j.annonc.2020.04.010. |
[20] | Powles T , Plimack ER , Soulières D , Waddell T , Stus V , Gafanov R , et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. (2020) ;21: :1563–73. https://doi.org/10.1016/S1470-2045(20)30436-8. |
[21] | Choueiri TK , Powles T , Burotto M , Escudier B , Bourlon MT , Zurawski B , et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) ;384: :829–41. https://doi.org/10.1056/NEJMoa2026982. |
[22] | Hahn AW , Klaassen Z , Agarwal N , Haaland B , Esther J , Ye XY , et al. First-line Treatment of Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur Urol Oncol. (2019) ;2: :708–15. https://doi.org/10.1016/j.euo.2019.09.002. |
[23] | Monteiro FSM , Soares A , Debiasi M , Schutz FA , Maluf FC , Bastos DA , et al. First-line Treatment of Metastatic Renal Cell Carcinoma in the Immuno-oncology Era: Systematic Review and Network Meta-analysis. Clin Genitourin Cancer. (2020) ;18: :244–251.e4. https://doi.org/10.1016/j.clgc.2020.02.012. |
[24] | Hofmann F , Hwang EC , Lam TB , Bex A , Yuan Y , Marconi LS , et al. Targeted therapy for metastatic renal cell carcinoma. Cochrane Database Syst Rev 2020. https://doi.org/10.1002/14651858.CD012796.pub2. |
[25] | Mori K , Mostafaei H , Miura N , Karakiewicz PI , Luzzago S , Schmidinger M , et al. Systemic therapy for metastatic renal cell carcinoma in the first-line setting: a systematic review and network meta-analysis. Cancer Immunol Immunother. (2021) ;70: :265–73. https://doi.org/10.1007/s00262-020-02684-8. |
[26] | Rini BI , Battle D , Figlin RA , George DJ , Hammers H , Hutson T , et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer. (2019) ;7: :354. https://doi.org/10.1186/s40425-019-0813-8. |
[27] | Motzer RJ , Jonasch E , Boyle S , Carlo MI , Manley B , Agarwal N , et al. NCCN Guidelines Insights: Kidney Cancer, Version 1. Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. (2020) ;18: :1160–70. https://doi.org/10.6004/jnccn.2020.0043. |
[28] | Choueiri TK , Albiges L , Powles T , Scheffold C , Wang F , Motzer RJ . A phase III study (COSMIC-313) of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in patients (pts) with previously untreated advanced renal cell carcinoma (aRCC) of intermediate or poor risk. J Clin Oncol. (2020) ;38: :TPS767–TPS767. https://doi.org/10.1200/JCO.2020.38.6_suppl.TPS767. |
[29] | Zhang T , Ballman KV , Choudhury AD , Chen RC , Watt C , Wen Y , et al. PDIGREE: An adaptive phase III trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). J Clin Oncol. (2020) ;38: :TPS5100–TPS5100. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS5100. |
[30] | McDermott DF , Huseni MA , Atkins MB , Motzer RJ , Rini BI , Escudier B , et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. (2018) ;24: :749–57. https://doi.org/10.1038/s41591-018-0053-3. |
[31] | Motzer RJ , Robbins PB , Powles T , Albiges L , Haanen JB , Larkin J , et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. (2020) ;26: :1733–41. https://doi.org/10.1038/s41591-020-1044-8. |
Appendices
APPENDIX: SEARCH TERMS
((”carcinoma, renal cell” [MeSH Terms] OR (“carcinoma” [All Fields] AND “renal” [All Fields] AND “cell” [All Fields]) OR “renal cell carcinoma” [All Fields] OR (“renal” [All Fields] AND “cell” [All Fields] AND “carcinoma” [All Fields]) OR (“carcinoma, renal cell” [MeSH Terms] OR (“carcinoma” [All Fields] AND “renal” [All Fields] AND “cell” [All Fields]) OR “renal cell carcinoma” [All Fields] OR (“renal” [All Fields] AND “cell” [All Fields] AND “cancer” [All Fields]) OR “renal cell cancer” [All Fields]) OR (“carcinoma, renal cell” [MeSH Terms] OR (“carcinoma” [All Fields] AND “renal” [All Fields] AND “cell” [All Fields]) OR “renal cell carcinoma” [All Fields] OR (“kidney” [All Fields] AND “carcinoma” [All Fields]) OR “kidney carcinoma” [All Fields]) OR (“kidney neoplasms” [MeSH Terms] OR (“kidney” [All Fields] AND “neoplasms” [All Fields]) OR “kidney neoplasms” [All Fields] OR (“kidney” [All Fields] AND “cancer” [All Fields]) OR “kidney cancer” [All Fields])) AND (“advance” [All Fields] OR “advanced” [All Fields] OR “advancement” [All Fields] OR “advancements” [All Fields] OR “advances” [All Fields] OR “advancing” [All Fields] OR (“metastatically” [All Fields] OR “metastatics” [All Fields] OR “metastatization” [All Fields] OR “metastatize” [All Fields] OR “metastatized” [All Fields] OR “metastatizing” [All Fields] OR “secondary” [MeSH Subheading] OR “secondary” [All Fields] OR “metastatic” [All Fields])) AND “clinical trial” [Publication Type] AND “previously untreated” [All Fields]) AND (clinicaltrial[Filter])
= 124.
ASCO GU search “Advanced renal cell carcinoma” = 7
ASCO search “Advanced renal cell carcinoma” = 75
ESMO search “Advanced renal cell carcinoma” = 12




