Extended Disease Control with Unconventional Cabozantinib Dose Increase in Metastatic Renal Cell Carcinoma1
Abstract
BACKGROUND:
Cabozantinib is among the most potent tyrosine kinase inhibitors (TKIs) FDA-approved for metastatic renal cell carcinoma (mRCC). Effective treatments after progression on cabozantinib salvage therapy are limited. Dose escalation for other TKIs has been shown to afford added disease control.
OBJECTIVE:
We sought to evaluate whether dose escalation of cabozantinib (Cabometyx®) from conventional doses in select patients with limited treatment options offered additional disease control. We asked how cabozantinib dose increases may affect circulating drug levels.
METHODS:
We identified patients with mRCC at the University of Texas Southwestern Medical Center who were treated with cabozantinib dose escalation to 80 mg after progressing on conventional cabozantinib 60 mg. We then queried leading kidney cancer investigators across the world to identify additional patients. Finally, we reviewed pharmacokinetic (PK) data to assess how higher doses impacted circulating levels by comparison to other formulations (Cometriq® capsules).
RESULTS:
We report six patients treated at two different institutions with cabozantinib-responsive disease and good tolerability, where cabozantinib was dose escalated (typically to 80 mg, but as high as 120 mg) after progression on 60 mg, a strategy that resulted in added disease control (median duration, 14 months; 95% Confidence Interval [CI]: 8 –Not Estimable[NE]). Four patients (66.7%) had disease control lasting at least 1 year. No grade III/IV adverse events were identified in this small, select, cohort. A comparison of PK data to FDA-approved cabozantinib 140 mg capsules suggests that cabozantinib 80 mg tablets results in comparable exposures.
CONCLUSIONS:
mRCC patients with cabozantinib responsive disease and reasonable tolerability may benefit from dose escalation at progression.
INTRODUCTION
Renal cell carcinoma (RCC) is among the top ten diagnosed malignancies in the United States, with an estimated ∼75,000 new cases in 2021 [1]. The treatment landscape for metastatic RCC (mRCC) has evolved dramatically over the past decade and there are over a dozen FDA approved agents and combinations [2]. Response rates to new frontline combination therapies can reach 70% with durable complete responses in ∼10% of patients [3–8]. Despite this, most patients ultimately experience disease progression necessitating additional lines of therapy. Each additional line of therapy tends to yield diminishing benefits, especially among targeted therapies [9]. Strategies to maximize activity of existing therapies are therefore warranted.
Cabozantinib is a tyrosine kinase inhibitor (TKI) FDA approved in RCC as a single agent and most recently in combination with nivolumab, an immune checkpoint inhibitor (ICI) targeting programmed death protein 1 (PD1) [7, 10]. Cabozantinib targets VEGFR2 (vascular endothelial growth factor receptor 2), as well as MET (hepatocyte growth factor receptor) and AXL (GAS6 receptor) among other kinases. MET and AXL targeting differentiates cabozantinib from other TKIs approved for mRCC [11]. Response rates to cabozantinib monotherapy are ∼35% in the front line setting, and reach 57% when used in combination with nivolumab [7, 12, 13]. In previously treated disease, response rates to cabozantinib monotherapy are ∼20% [14] with a median progression free survival (PFS) 7-8 months, though this may be higher in patients who are TKI naïve [15, 16]. For patients who progress on salvage cabozantinib, therapeutic options include lenvatinib/everolimus [17], but are otherwise limited. After progression on cabozantinib, there is limited benefit from other TKIs [18].
Herein, we report six cases from two institutions in which cabozantinib dose escalation beyond the FDA recommended dose overcame acquired resistance, and propose that cabozantinib dose escalation may be a viable strategy to prolong clinical benefit in carefully selected patients with cabozantinib responsive disease and good tolerability.
METHODS
All patients with mRCC at University of Texas Southwestern (UTSW) from January 1st, 2015 to October 30th, 2021 who were prescribed cabozantinib at a dose greater than 60 mg were identified and included in this report. Medical records were reviewed independently by two investigators (RE, AS). Written consent was obtained from all participants or their next of kin. Tumor burden was defined as the sum of the longest diameter of all measurable lesions and was assessed by a qualified radiologist (IP) retrospectively using RECIST v1.1 principals. We queried providers at leading kidney cancer programs in the world (n = 14), in order to identify centers who adopted a similar treatment strategy. A second institution was found, where a similar approach was attempted and their patients are also included.
RESULTS
We identified three patients at UTSW (Cases 1–3) in whom cabozantinib was increased from 60 mg to 80 mg. All 3 patients had cabozantinib responsive metastatic RCC and good tolerability. A multi-institutional query revealed an additional three patients (cases 4–6) which were treated with a similar strategy at the Sunnybrook Odette Cancer Centre (SOCC) (Table 1). Cabozantinib dose escalation after progression on 60 mg resulted in added disease control for a median duration of 14 months (95% CI: 8 –NE). Four patients (66.7%) had disease control lasting at least 1 year. Remarkably, no grade III/IV adverse events were identified albeit the cohort is small and highly selected. A comparison of pharmacokinetic (PK) data between the two cabozantinib formulations revealed a Cmax (% CV) which was higher for cabozantinib 80 mg tablets compared to 140 mg capsule (647 ng/mL; Coefficient of variation [CV] 30 vs. 554 ng/mL; CV 43), but an AUC0hboxlast which was similar, 55,800 ng*hr/mL; CV 25 vs. 54,900 ng*hr/mL; CV 37) (Table 2). Detailed case summaries are presented below.
Table 1
Case overview
| Case | Site | Age | Sex | Subtype | NG | Prior Therapies | TNT60 mg (months) | Cabozantinib Maximal Dose | Schedule (On/Off) | TNTHD (months) |
| 1 | UTSW | 65 | M | ccRCC | 4 | pazopanib, nivolumab, C60, L/E | 9 | 80 mg | Daily | 18 |
| 2 | UTSW | 46 | M | ccRCC | 4 | HD-IL2, pazopanib, N/I, N/C40, N/C60 | 15a | 80 mg | Daily | 30b |
| 3 | UTSW | 75 | M | ccRCC | 2 | N/I, pazopanib, axitinib, investigational agentx2, | 18 | 80 mg | Daily | 12 |
| 4 | SOCC | 47 | M | ccRCC | 4 | sunitinib, nivolumab, L/E | 2 | 120 mg | 28 / 7 | 6 |
| 5 | SOCC | 62 | M | pRCC | NA | sunitinib, nivolumab | 4 | 120 mg | 16 / 7 | 16 |
| 6 | SOCC | 48 | M | ccRCC | 4 | sunitinib, nivolumab, N/I | 9 | 100 mg | 14 /7 | 8 |
aCabozantinib was given in combination with Nivolumab and Ipilimumab. bTreatment ongoing. Abbreviations: C, Cabozantininb (Dose); ccRCC, clear cell RCC; pRCC, papillary RCC; HD-IL2, High dose interleukin-2; L/E, lenvatinib and everolimus; M, male; N/C, nivolumab and cabozantinib; N/I, nivolumab and ipilimumab; NA, not applicable; NG, nuclear grade; TNT60 mg, Time to next treatment on standard cabozantinib dose; TNTHD, Time to next treatment (or death) after initiation of higher cabozantinib dose; SOCC, Sunnybrook Odette Cancer Centre; UTSW, University of Texas Southwestern.
Table 2
Cabozantinib PK with Dose and Formulationa
| Dose (mg) | Cmax [ng/mL] (% CV) | AUC0hboxlast [ng*hr/mL] (% CV) |
| 20 (tablet) | 117 (72) | 9,290 (50) |
| 40 (tablet) | 239 (56) | 19,800 (42) |
| 60 (tablet) | 343 (41) | 29,800 (38) |
| 80 (tablet)b | 647 (30) | 55,800 (25) |
| 140 (tablet) | 702 (54) | 61,900 (44) |
| 140 (capsule) | 554 (43) | 54,900 (37) |
| 175 (capsule)c | 544 (47) | 76,800 (37) |
aValues shown represent mean (% CV) after a single dose of cabozantinib in healthy volunteers and are taken from Nguyen et al. (2016) [24] except where indicated. bData from NDA 208692 Clinical Pharmacology Review –Cabozantinib [27]. cData from Kurzrock et al. (2011) in MTC patients after a single dose [26].
UTSW Experience
Case 1
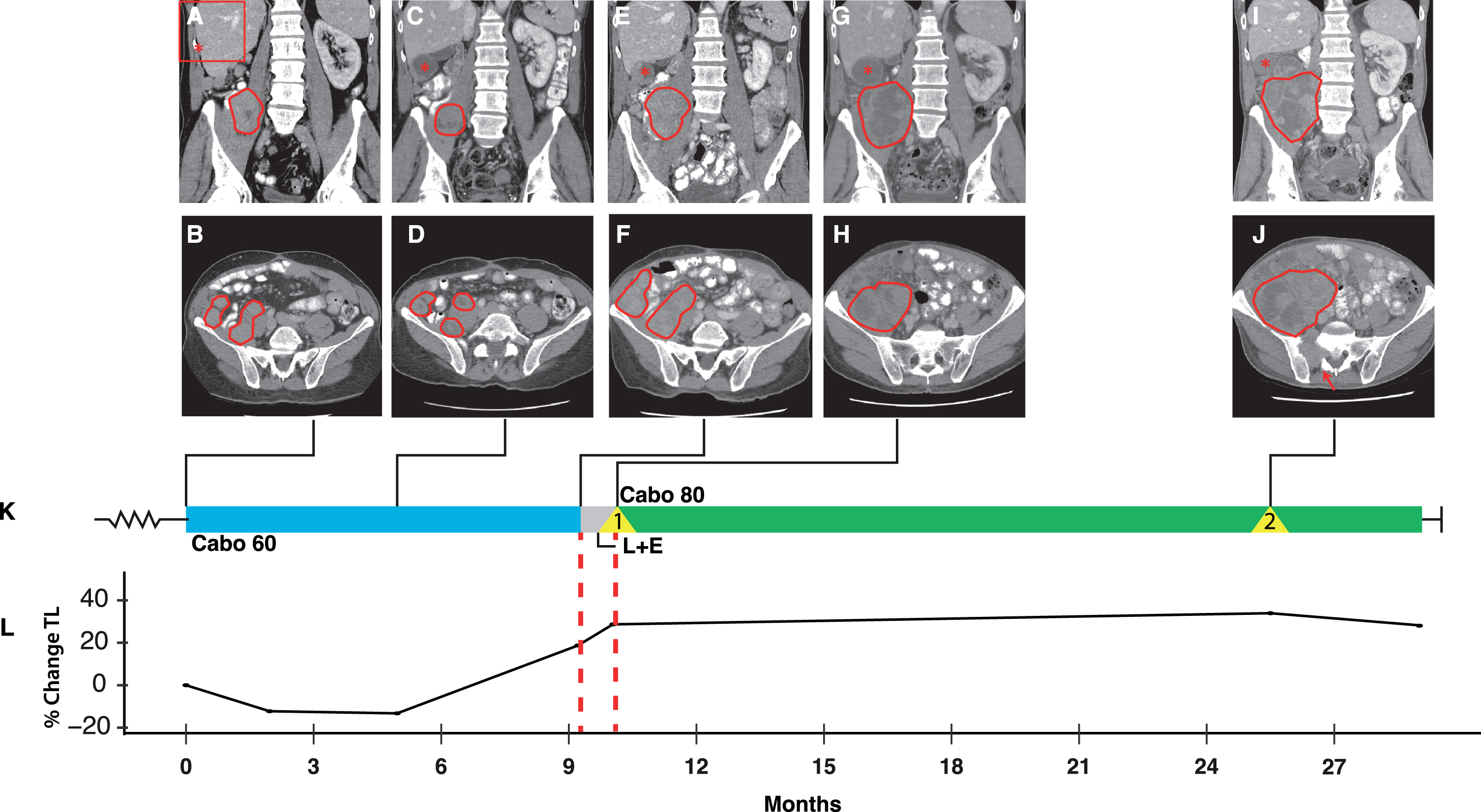
A 65-year-old man presented with an 18 cm right kidney mass in 2013. Radical nephrectomy and lymphadenectomy revealed a pT3aN0 clear cell RCC (ccRCC), nuclear grade (NG) 4, with focal rhabdoid morphology, and BAP1 loss on immunohistochemistry. Two years later, the disease recurred in the nephrectomy bed, and a resection was performed. However, shortly thereafter lung and liver metastases were identified. Following biopsy confirmation of mRCC, the patient was started on pazopanib 800 mg daily. He had stable disease lasting 6 months followed by enlargement of liver metastases and abdominal implants, and was switched to nivolumab 3 mg/kg every 2 weeks. Imaging 3 months later revealed significant enlargement at multiple disease sites within the abdomen/pelvis. At this point, he was switched to cabozantinib 60 mg daily (Fig. 1). He developed moderate palmar plantar erythrodysesthesia (PPE) and oral discomfort, both of which were manageable with over the counter (OTC) remedies. Imaging 2 months later revealed shrinkage and decreased enhancement of multiple hepatic, perihepatic, abdominal and pelvic metastases (compare Fig. 1C,D to A,B). Nine months after starting cabozantinib, the patient developed progressive disease (Fig. 1E,F,K and L) and was transitioned to a combination of lenvatinib 18 mg and everolimus 5 mg daily. Three weeks later there was marked clinical progression with abdominal discomfort, hiccups reminiscent of initial presentation, fatigue, and anorexia. Palpable growth of abdominal lesions was noted on exam, and progressive disease was confirmed by imaging (Fig. 1G,H, and L). The patient had a good performance status but limited treatment options (this was prior to the approval of ICI/ICI or TKI/ICI combination therapies). Given that cabozantinib had shown the most activity of all prior therapies, a discussion was held with the patient about attempting unconventional doses of cabozantinib in the 5th line. In addition, a perihepatic lesion thought to be contributing to the patient’s hiccups was treated with stereotactic body radiation therapy (SBRT) (Fig. 1K, Event 1). Within 5 weeks of cabozantinib at 80 mg, the patient reported increased energy and appetite. He developed grade II diarrhea and hypertension but therapy was otherwise well tolerated. At this dose he was diagnosed with paroxysmal atrial fibrillation, though an earlier onset cannot be excluded and the patient had a history of palpitations. Overall, there was a meaningful improvement in his quality of life. Fourteen months after starting cabozantinib 80 mg, he developed hip pain secondary to a new right sacral lesion, which was treated with SBRT (Fig. 1J [arrow] and K [Event 2]). Progression was otherwise modest. As there were no other treatment options available, scans were foregone and the patient remained on cabozantinib 80 mg for a total of 18 months. Eventually, he developed acute gastrointestinal bleeding, which was attributed to progression of his abdominal disease, and transitioned to hospice care.
Fig. 1
Integrated clinical overview containing key images, tumor burden, and treatment history for patient case 1. Red tracings (A-J) depict representative abdominal masses at key timepoints during the patient’s clinical course: At start of cabozantinib 60 mg (A and B); at response to cabozantinib 60 mg (C and D); after 9 months, at the time of acquired resistance to cabozantinib 60 mg (E and F); at the time of rapid progression following transition to lenvatinib and everolimus (G and H); and one year after starting cabozantinib 80 mg (I and J). Red Asterix (A,C,E,G,I) indicates a subcapsular hepatic metastases (upper right inlet in A). Radiographic images A-J are integrated into the treatment timeline (K) which depicts systemic therapies (cabo 60 mg in blue; L + E in grey; and cabo 80 mg in green) and events (yellow triangles). The proportional change in tumor burden, reported as the sum of the longest diameters of measurable lesions is depicted in (L). Events: 1, progressive perihepatic lesion treated with SBRT; 2, new right sacral lesion (arrow in J) treated with SBRT. Abbreviations: Cabo, cabozantinib; L + E, lenvatinib and everolimus; TL, target lesions.
Case 2
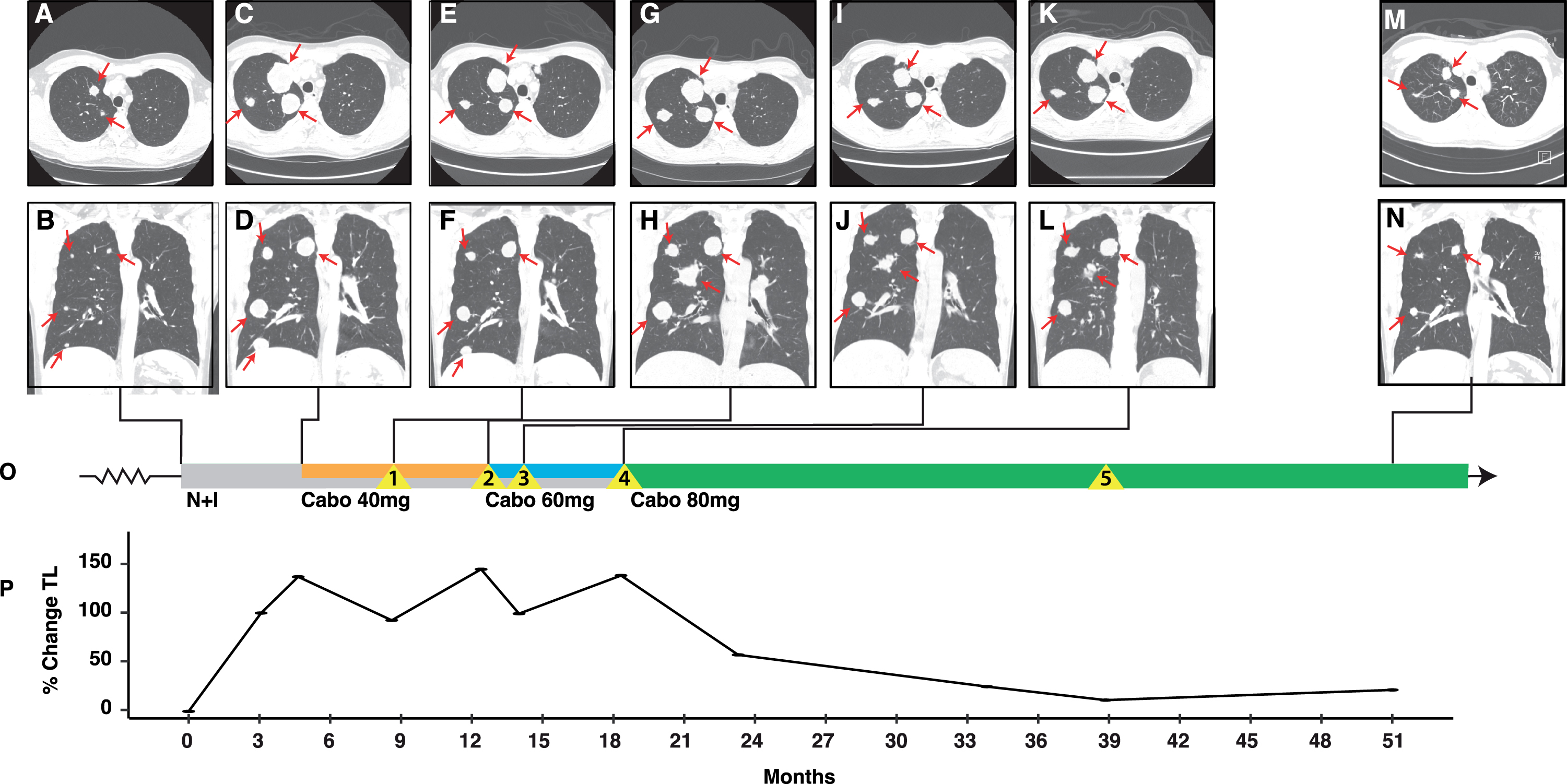
The patient is a 46-year-old male who initially presented in 2017 with a 15cm right renal mass. Radical nephrectomy revealed a ccRCC of NG 4 with rhabdoid features infiltrating the renal sinus and ipsilateral adrenal gland but without lymph node involvement (pT4N0). A few months later, lung metastases were diagnosed (confirmed by biopsy), and he was started on high dose interleukin-2 (HD-IL2) in combination with SBRT (to a lung lesion) in a clinical trial (NCT01896271). Imaging studies after two cycles of HD-IL2 revealed progressive lung lesions, and the decision was made to switch therapy to pazopanib 800 mg daily on which the patient experienced stable disease lasting 6 months. At this point, he had progression of existing pulmonary metastases, a new scalp metastasis, and two brain metastases. His scalp and a right parietal brain metastasis were resected, a smaller left parietal metastasis was treated with gamma knife (GK), and systemic therapy was started with nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) (Fig. 2A and B). Imaging studies after completing four cycles showed progression and the decision was made to add cabozantinib 40 mg to nivolumab 480 mg every 4 weeks (Fig. 2C,D,O and P). Imaging two months later revealed shrinkage of pulmonary and liver metastases (Fig. 2E and F), but isolated progression in a gluteal mass, which was surgically resected (Fig. 2O, Event 1). After 6 months on cabozantinib/nivolumab, imaging revealed growth of pulmonary and hepatic lesions (Fig. 2G,H, and P) as well as two new intracranial metastases which were treated with GK radiotherapy (Fig. 2O, Event 2). At this point, the decision was made to increase the dose of cabozantinib from 40 mg to 60 mg daily and continue nivolumab. The patient demonstrated a mixed response with shrinkage of some pulmonary metastases but enlargement of two hepatic metastases (which were treated with SBRT [Fig. 2O, Event 3]), followed by enlargement of existing lesions and a new brain metastasis (which was irradiated using GK, Fig. 2O [Event 4]). The decision was made to switch to cabozantinib alone at 80 mg daily. Two months later the patient reported improved energy and overall quality of life. As with case 1, he developed grade II diarrhea and hypertension, but cabozantinib was otherwise well tolerated. Imaging demonstrated significant shrinkage of his lung masses. Over the course of the following six months the patient continued to report improved quality of life, appetite, and energy level. Thirty months since starting cabozantinib 80 mg the patient continues to experience disease control and excellent quality of life with the exception a 1cm local recurrence of the resected gluteal metastasis which was treated with consolidative SBRT (Fig. 2O, Event 5). Overall, his disease burden is significantly reduced compared to when he was on cabozantinib 60 mg (compare Fig. 2M,N to K,L). In addition, since starting cabozantinib 80 mg, he has not had any symptoms of recurrent or new brain metastases.
Fig. 2
Integrated clinical overview containing key images, tumor burden, and treatment history for patient case 2. Representative lung lesions (red arrows) at key timepoints during the patient’s disease course including: At the start of N + I (A and B); at progression following 4 cycles of N + I with the addition of cabozantinib 40 mg to nivolumab (C and D); at time of initial response to nivolumab/cabozantinib 40 mg (E and F); at time of progression with new brain lesions and transition to nivolumab/cabozantinib 60 mg (G and H); after initial response to nivolumab/cabozantinib 60 mg (I and J); at the time of acquired resistance with development of new brain lesion (K and L); and after prolonged therapy on cabozantinib 80 mg (M and N). Radiographic images are integrated into the treatment timeline (O) which depicts systemic therapies (N + I in grey; cabo 40 mg in orange; cabo 60 mg in blue; and cabo 80 mg in green), and clinical events (yellow triangles). The proportional change in tumor burden, reported as the sum of the longest diameters of target lesions is depicted in (P). Events: 1, isolated progression in a gluteal mass which was surgically resected; 2, development of two new brain metastases treated with gamma-knife (GK); 3, isolated progression of two hepatic metastases which were treated with SBRT; 4, new brain metastasis treated with GK; 5, recurrence of resected gluteal metastasis treated with consolidative SBRT. Abbreviations: Cabo, cabozantinib; N + I, nivolumab and ipilimumab; TL, target lesion.
Case 3
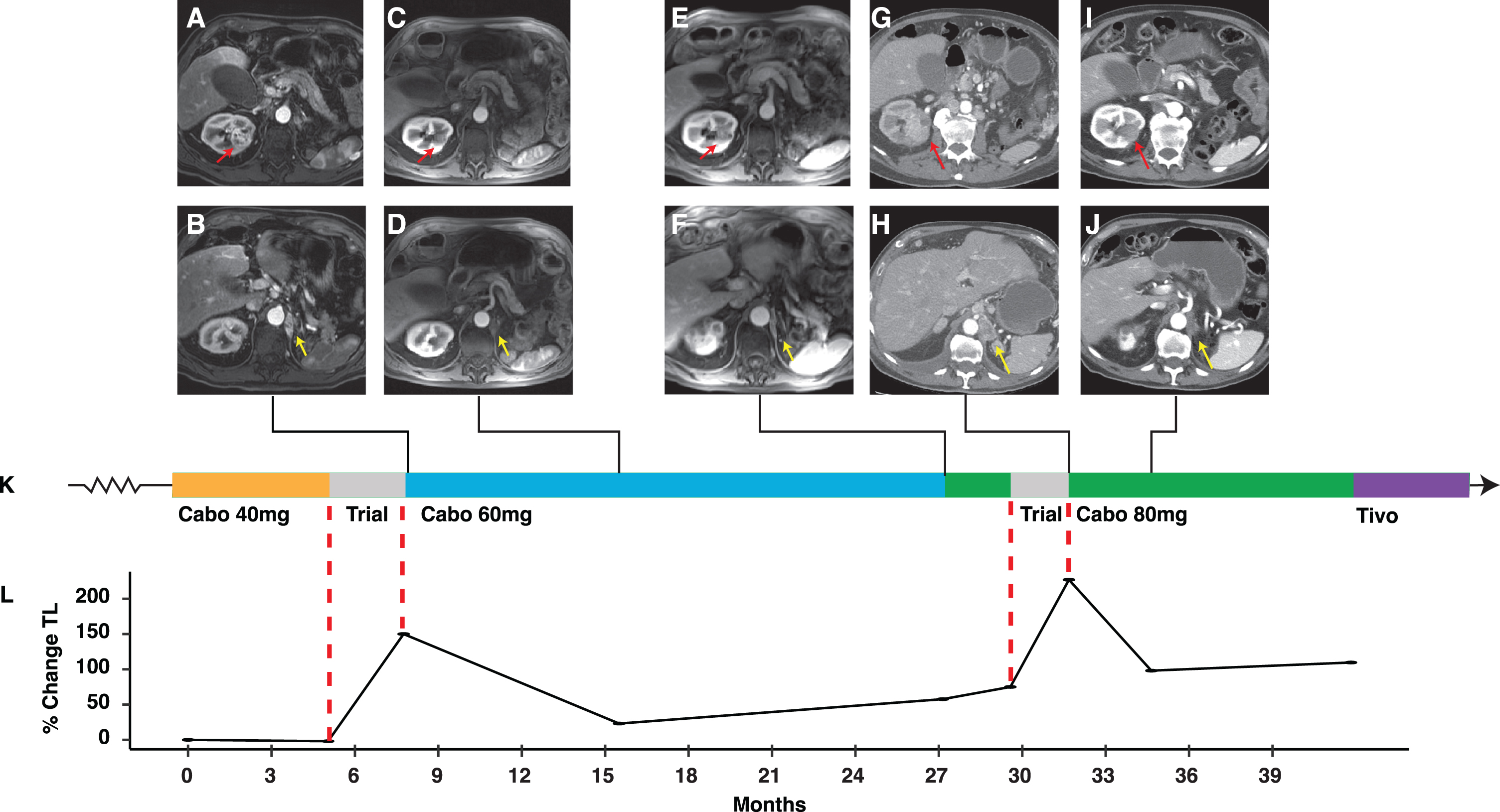
A 75-year-old man was initially diagnosed with a left renal mass in 2013. Left radical nephrectomy revealed a 9cm ccRCC, NG 2 (pT2aNx). Metastatic disease to the diaphragmatic crus (biopsy proven) and left adrenal bed were diagnosed 18 months later, and the patient was started on a combination of ipilimumab and nivolumab on a clinical trial (NCT02231749). He had progressive disease after completing 4 cycles and was started on pazopanib 800 mg. The patient remained on pazopanib for 18 months, at which point progressive disease prompted a change to axitinib 5 mg which provided disease control for an additional year. Imaging revealed enlargement of several known sites of disease, including two calvarial lesions and several abdominal implants. He enrolled in a second clinical trial (NCT03435640), but while temporizing, started cabozantinib at 40 mg (Fig. 3). Baseline imaging for the clinical trial two months later revealed stable findings. Within three months of enrollment, the patient developed progressive disease manifesting by enlargement of existing lesions and a new left diaphragmatic crus lesion (Fig. 3A,B and K). Cabozantinib was resumed, this time at 60 mg daily. He had a response with resolution of liver and other soft tissue metastases as well as shrinkage of pancreatic and adrenal metastases (Fig. 3C and D). Diarrhea and gastroesophageal reflux disease were grade II and managed with OTC remedies. Eighteen months after starting cabozantinib 60 mg, imaging revealed six new subcentimeter liver nodules (Fig. 3K). At this point, he was switched to cabozantinib 80 mg daily, but shortly thereafter enrolled in a third clinical trial (NCT04169711) (Fig. 3K). Imaging two months after starting the trial demonstrated overt progression, with enlargement of known lesions and new liver metastases (Fig. 3G,H and K) prompting resumption of cabozantinib at 80 mg. The patient noted rapid clinical improvement. Cabozantinib 80 mg was relatively well tolerated (grade II HTN and diarrhea). Imaging 3 months since resuming cabozantinib 80 mg revealed a deep partial response, with shrinkage and devascularization of known lesions and improvement of bilateral pleural effusions (Fig. 3I and J). The patient remained on cabozantinib 80 mg for a total of 12 months before the development of multiple new liver lesions prompted a change to tivozanib (Fig. 3K).
Fig. 3
Integrated clinical overview containing key images, tumor burden, and treatment history for patient case 3. Representative renal lesion (red arrow) and diaphragmatic crus lesion (yellow arrow) at key timepoints during the patient’s clinical course including: At progression to an investigational agent combination (A and B); at initial response to cabozantinib 60 mg (C and D); at the time of acquired resistance to cabozantinib 60 mg with development of new liver lesions (E and F); at the time of progression with development of a new liver lesion following another clinical trial (G and H); and at first response following cabozantinib 80 mg (I and J). Radiographic images are integrated into the treatment timeline (K) which depicts systemic therapies (cabo 40 mg in orange; clinical trials in grey; cabo 60 mg in blue; and cabo 80 mg in green, tivozanib in purple). The proportional change in tumor burden, reported as the sum of the longest diameters of measurable lesions is depicted in (L). Abbreviations: Cabo, cabozantinib; TL, target lesion; Tivo, tivozanib.
SOCC Experience (Cases 4–6)
Case 4
A 47-year-old man presented in late 2016 with hematuria. A CT scan showed a 14 cm left kidney mass with extension into the renal vein and bilateral pulmonary nodules. Left radical nephrectomy revealed a ccRCC NG 4 with both renal vein and lymphovascular invasion. He initiated therapy with sunitinib with an initial response followed by progression in lung metastases and new liver metastases 6 months later. This was followed by nivolumab for an additional 6 months. At this point the patient received lenvatinib and everolimus on a clinical trial (NCT03173560), on which he remained for 14 months. He developed progressing lung and mediastinal metastases as well as peritoneal carcinomatosis. Cabozantinib 60 mg was started as 4th line therapy. Surveillance imaging 2 months later revealed a mixed response with shrinkage of lung lesions but progression in mesenteric and liver metastases. Cabozantinib was then increased to 80 mg. After 4 weeks, he had no toxicity and cabozantinib was further escalated to 100 mg following a 7 day break. After 4 weeks on cabozantinib 100 mg, imaging again revealed shrinkage in lung metastases but progression in the liver and peritoneal carcinomatosis. He had no cabozantinib toxicity, and after a 7 day break, he was escalated to a dose of 120 mg. He continued cabozantinib 120 mg on a 28 days on/ 3–7 days off schedule for ∼3 months. Imaging showed improvement across all sites of disease. After an additional 6 weeks, however, a CT scan showed a mixed response with worsening peritoneal carcinomatosis and ascites. He was started on gemcitabine and 5-flourouracil (Gem/5-FU) but progressed and died a few months later. In total, this patient remained on escalated doses of cabozantinib for 6 months after progressing on standard doses.
Case 5
A 62-year-old man presented in 2018 with a 7 cm left kidney mass and multiple retroperitoneal nodes up to 3.8 cm. Radical nephrectomy was aborted due to tumor proximity to the aorta, but biopsies revealed a papillary RCC. Imaging shortly thereafter revealed pulmonary metastases as well as progression of the primary tumor and retroperitoneal disease. The patient’s disease was refractory to initial therapy with sunitinib on a clinical trial (NCT02761057). Second line nivolumab resulted in progressive disease in the primary site, retroperitoneal lymphadenopathy, and lungs as well as new hepatic metastases. The patient was started on 3rd line cabozantinib in early 2019 and imaging two months later showed a partial response which lasted an additional 2 months. At this point a mixed response prompted dose escalation to 80 mg. One month later, the patient continued to have no toxicities and cabozantinib was escalated to 100 mg after a 7 day break. Of note, imaging at this time demonstrated a response in lung metastases and otherwise stable disease. The patient continued to be free of treatment related adverse events and cabozantinib was escalated to 120 mg following a 7 day break. At this dose the patient developed PPE, hypertension, and heartburn, and his treatment schedule was modified to 120 mg 14 days on/ 7 days off. The patient remained on this regimen with a favorable response for 5 months when there was isolated progression in a few nodal areas in the mediastinum and retroperitoneum which were treated with SBRT. Otherwise cabozantinib was continued for an additional 5 months. At this point, imaging revealed mild progression in isolated areas, and the schedule was intensified to 16 days on/ 7 days off. The patient remained on this regimen for an additional 3 months at which point he developed progressive disease. In total, this patient was treated with 16 months of cabozantinib at escalated doses after progression on cabozantinib 60 mg. The patient was treated with Gem/5-FU in the 4th line but unfortunately progressed and passed away a few months later.
Case 6
A 48-year-old man presented with an 8 cm left renal mass, 1.2cm right renal lesion, and extensive bone metastasis in 2018. A left nephrectomy revealed a ccRCC NG 4. The patient’s painful bone lesions were treated with SBRT and he was started on systemic therapy with sunitinib. He had a favorable response and remained on sunitinib for 21 months. At this point, 2nd line nivolumab was initiated but he progressed shortly thereafter and ipilimumab was added. Unfortunately, the patient had extensive progression in addition to immune-mediated pneumonitis and hepatitis requiring steroids. He was started on cabozantinib at 60 mg with an initial response followed by progression 9 months later. At this point cabozantinib was dose escalated to 80 mg with a mixed response 3 months later. The patient was then escalated to cabozantinib at 100 mg (14 days on / 7 days off) on which he experienced stable disease for an additional 5 months. In total, the patient was on elevated doses of cabozantinib for 8 months.
DISCUSSION
We report six cases in which dose escalation of cabozantinib above the FDA recommended dose in the salvage setting overcame acquired resistance leading to meaningful clinical benefit. In all cases, cabozantinib dose escalation occurred late in the treatment course when there were limited treatment options. Overall, these patients had in common: (i) cabozantinib-responsive disease as determined by disease control at standard doses for an extended period of time; (ii) tolerability of cabozantinib 60 mg daily; and (iii) limited other treatment options. All patients were aware that the dose recommended exceeded the FDA recommended dose, and that there could be added risks.
Cabozantinib responsiveness implies disease control generally for at least 4–6 months, but this depends on multiple factors including line of therapy and previous therapies (in particular other TKIs). Furthermore, the assessment of responsiveness is also contextual, and as such, a patient that appears to benefit from cabozantinib to a greater extent than other drugs, may be considered as having preferentially cabozantinib responsive disease. Of note, treatment related adverse events in all cases were manageable with symptom-directed therapy and there were no grade III/IV events. This may reflect, however, a highly selected patient cohort that tolerated lower doses of cabozantinib well.
The notion that increasing cabozantinib dose at progression may improve disease control is supported by studies of other TKIs. A study reported a progression-free survival (PFS) benefit in 17 patients where sunitinib was dose escalated to 62.5 mg or 75 mg after early progression [19]. Another retrospective analysis of 25 patients progressing on sunitinib 50 mg, reported a benefit from dose escalation to 62.5 mg and 75 mg [20]. The activity and safety of sunitinib dose escalation was confirmed in a prospective phase II trial of sunitinib individualization where ∼20% of the study patients were dose escalated to 62.5 mg (12 pts) and ∼15% to 75 mg (8 pts) [21]. These results are consistent with experiments in mice, where sunitinib-induced resistance was ameliorated by increasing the dose, and a potential role of epigenetic changes was suggested [19].
There are two formulations of cabozantinib which have received FDA approval, the tablet formulation (CABOMETYX®), which is approved at the 60 mg dose for RCC and hepatocellular carcinoma [22], and the capsule formulation (COMETRIQ®), which is currently approved at the 140 mg dose in medullary thyroid cancer (MTC) [23]. These formulations failed to meet predefined criteria for bioequivalence [24]. The steady-state concentration between cabozantinib 140 mg (capsule) in MTC patients and 60 mg (tablet) in patients with RCC (and other cancers) appears comparable though there appeared to be increased clearance in the MTC population, possibly due to diarrhea [25]. The AUC for the 140 mg capsule is most comparable to that attained by the 80 mg tablet formulation (Table 2). Furthermore, the AUC for cabozantinib 80 mg tablet is lower than for cabozantinib 175 mg capsule, which was the maximally tolerated dose (MTD) in dose escalation studies in MTC patients [26, 27]. However, the Cmax is higher for the 80 mg tablet. Overall, these data suggest that cabozantinib 80 mg (tablet formulation) results in overall drug exposures that are not dissimilar to those in MTC patients on 140 mg capsule. Nevertheless, a higher Cmax could be associated with higher toxicities, and dose reductions secondary to adverse events are reported in up to 60% of patients in RCC trials [12, 14].
It is also worth noting that cabozantinib clearance is highly variable across patients [25]. Among participants in the METEOR study, a phase III study of cabozantinib vs sunitinib in mRCC [14], patients in the cabozantinib arm could be stratified into low (1.3 h/L), typical (2.3L/hr), and high (3.3L/hr) clearance groups [28]. As expected, high clearance groups exhibited improved cabozantinib tolerability (i.e, less frequent dose reductions). Additionally, some patients had clearance rates as high as ∼7L/h, and these patients are most likely underdosed. While PK analyses are not routinely performed to guide dosing, given the correlation between exposure and tolerability, one approach is to uptitrate cabozantinib until adverse effects develop.
Consistent with the notion that higher exposures may be associated with higher activity, two recent retrospective studies of cabozantinib in RCC noted improved outcomes in patients requiring a dose reduction for toxicity. The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) analyzed real world data for 413 RCC patients treated with cabozantinib between 2011 and 2019. Roughly 50% (129/258) of patients required dose reductions. Across all lines of therapy, the time to treatment failure was significantly longer for patients who required dose reductions vs. patients who did not, with an adjusted hazard ratio (HR) of 0.37 (95% CI: 0.202–0.672; p < 0.01) [29]. This correlated also with an improved median overall survival (OS) (HR: 0.46; 95% CI: 0.215–0.980; p = 0.04). The CABOREAL study reported treatment patterns and outcomes of 410 mRCC patients treated with cabozantinib through the French Early Access Program. Initiating cabozantinib at a dose of 60 mg vs 40 mg or 20 mg led to similar treatment duration; however, patients initiating at 60 mg had a longer median OS (15.4 versus 11.8 months, respectively; p = 0.03) [30]. In addition, patients requiring a dose reduction for toxicity (57%, 233/410) had a longer median OS (17.5 vs 8.9 months, p < 0.0001) than those without. Thus, using toxicity-based dose reductions as surrogates of exposure, higher exposures appear to be associated with more prolonged disease control.
The notion that cabozantinib drug exposures (and the need for dose reductions) are associated with clinical benefit is consistent with observations for other TKIs and may be a class effect. Both retrospective series as well as analyses of phase III trials of sunitinib and pazopanib show that patients experiencing toxicities necessitating a dose reduction have improved outcomes [31–34]. The concept of individualized dosing and dose escalation in patients with minimal toxicity has been tested in prospective phase II trials not only for sunitinib [21] but also for axitinib [35], where it was found to be both safe and effective. By using toxicity as a surrogate for optimal drug exposure for each patient, one may be able to account for the many complex variables affecting TKI pharmacokinetics including interindividual differences in absorption and metabolism, drug-drug interactions, and genomic polymorphisms [33].
Cabozantinib has been recently shown to have activity against brain metastases [36]. An added benefit of higher doses of cabozantinib may be higher control rates of brain metastases, where drug levels are often lower than at extracranial sites. This may explain the prolonged intracranial disease control observed in case 2 after initiating cabozantinib 80 mg.
Taken together, these data suggest that drug exposures with cabozantinib 60 mg tablets are lower than FDA-approved cabozantinib 140 mg capsules, that some patients are likely underdosed due to rapid clearance, and that as determined by the need for dose reductions, drug exposure may be associated with clinical benefit.
While this study represents a small number of patients, similar results were observed by two independent oncologists (J.B. and G.B.) and the results are overall consistent with the notion that higher drug exposures may result in improved clinical benefit, which is supported by studies with related TKIs. For both investigators, this was a strategy deployed in the salvage setting, when few other options were available. One difference between the investigators was dose escalation beyond 80 mg, which often required treatment breaks. Overall, this series suggests that in mRCC patients with cabozantinib-responsive disease and good tolerability, cabozantinib may be escalated yielding additional clinical benefit. A clinical trial is being planned to test this notion prospectively.
ACKNOWLEDGMENTS
The authors acknowledge the patients and their families, and Exelixis for providing cabozantinib pharmacokinetic and pharmacodynamic data.
FUNDING
This research was supported by the UTSW Kidney Cancer SPORE grant P50CA196516 (J.B. and I.P.). R. Elias receives support from an institutional award from the Burroughs Wellcome Fund.
AUTHOR CONTRIBUTIONS
Authors AS and RE contributed equally to this work.-
Conception and design: AS, RE, JB.
Development of methodology: AS, RE, AC, JB.
Acquisition of data: AS, RE, AC, NW, IP, GB, JB.
Analysis and interpretation of data: AS, RE, NW, IP, GB, JB.
Writing, review, and/or revision of manuscript: AS, RE, AC, NW, IP, GB, JB.
CONFLICTS OF INTEREST
G.A. Bjarnason and J. Brugarolas are Editorial Board Members of this journal, but were not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review.
I. Pedrosa reports personal fees from Bayer Healthcare, personal fees from Health Tech International, other from Philips Healthcare, outside the submitted work. G.A. Bjarnason is a paid consultant for Pfizer, Novartis, Bristol-Myers Squibb, Eisai, Ipsen; and reports receiving commercial research grants from Pfizer and Merck. J. Brugarolas is a paid consultant for Exelixis, Arrowhead, Calithera, Eisai, and Johnson & Johnson; reports patent applications, outside the submitted work.
The remaining authors have no relevant competing interests.
CONSENT TO PARTICIPATE
All UTSW participants or their next of kin provided written approval to be included in this study. Retrospective clinical data was collected in compliance with institutional guidelines after approval of the UTSW Institutional Review Board (IRB), protocol number STU 012011-190 and the SOCC IRB, protocol number 160-2009/2008.
REFERENCES
[1] | Siegel RL , et al., Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. . (2021) ;71: (1):7–33. |
[2] | NCCN. Kidney Cancer (Version 1.2021). 1/20/21]; 597 Available from: https://www.nccn.org/professionals/physiciangls/pdf/kidney_blocks.pdf |
[3] | Motzer RJ , et al., Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine. (2018) ; 378: (14):1277–1290. |
[4] | Motzer RJ , et al., Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: (12):1103–1115. |
[5] | Rini BI , et al., Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. (2019) ;380: (12):1116–1127. |
[6] | Motzer RJ , et al., Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. The Lancet Oncology. (2019) ;20: (10):1370–1385. |
[7] | Choueiri T , et al., 696O_PR Nivolumab+cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Annals of Oncology. (2020) ;31: :S1159. |
[8] | Motzer R , et al., Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. New England Journal of Medicine. (2021) ;384: (14):1289–1300. |
[9] | Naito S , et al., Effect of third- and fourth-line systemic therapies for metastatic renal cell carcinoma. Scientific Reports. (2019) ;9: (1):15451–15451. |
[10] | Singh H , et al., U, S. Food and Drug Administration Approval: Cabozantinib for the Treatment of Advanced Renal Cell Carcinoma. Clinical Cancer Research. (2017) ;23: (2):330–335. |
[11] | Rathi N , et al., Mini-Review: Cabozantinib in the Treatment of Advanced Renal Cell Carcinoma and Hepatocellular Carcinoma. Cancer Management and Research. (2020) ;12: :3741–3749. |
[12] | Choueiri TK , et al., Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A03 CABOSUN Trial. Journal of Clinical Oncology. (2017) ;35: (6):591–597. |
[13] | Choueiri TK , et al., Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. European Journal of Cancer. (2018) ;94: :115–125. |
[14] | Choueiri TK , et al., Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine. (2015) ;373: (19):1814–1823. |
[15] | Iacovelli R , et al., Cabozantinib After a Previous Immune Checkpoint Inhibitor in Metastatic Renal Cell Carcinoma: A Retrospective Multi-Institutional Analysis. Target Oncol. (2020) ;15: (4):495–501. |
[16] | McGregor BA , et al., Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma. Eur J Cancer. (2020) ;135: :203–210. |
[17] | Motzer RJ , et al., Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. The Lancet Oncology. (2015) ;16: (15):1473–1482. |
[18] | Cerbone L , et al., Activity of Systemic Treatments After Cabozantinib Failure in Advanced Metastatic Renal Cell Carcinoma. Clinical Genitourinary Cancer. 2021. |
[19] | Adelaiye R , et al., Sunitinib dose escalation overcomes transient resistance in clear cell renal cell carcinoma and is associated with epigenetic modifications. Mol Cancer Ther. (2015) ;14: (2):513–22. |
[20] | Raphael J , Thawer A , Bjarnason GA . Sunitinib dose-escalation after disease progression in metastatic renal cell carcinoma. Urol Oncol. (2018) ;36: (1):12.e1–12.e6. |
[21] | Bjarnason GA , et al., The efficacy and safety of sunitinib given on an individualised schedule as first-line therapy for metastatic renal cell carcinoma: A phase 2 clinical trial. Eur J Cancer. (2019) ;108: :69–77. |
[22] | FDA. CABOMETYX HIGHLIGHTS OF PRESCRIBING INFORMATION. 1/21/2020]; Available from: https://www.accessdata.fda.gov/drugsatfda docs/label/2019/208692s003lbl.pdf |
[23] | FDA. COMETRIQ HIGHLIGHTS OF PRESCRIBING INFORMATION. 1/21/21]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203756s008lbl.pdf |
[24] | Nguyen L , et al., Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anti-Cancer Drugs. (2016) ;27: (7):669–678. |
[25] | Lacy S , et al., A population pharmacokinetic model of cabozantinib in healthy volunteers and patients with various cancer types. Cancer Chemotherapy and Pharmacology. (2018) ;81: (6):1071–1082. |
[26] | Kurzrock R , et al., Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. (2011) ;29: (19):2660–2666. |
[27] | USFDA, Center for drug evaluation and research. Application number: 206162Orig1s000. Clinical pharmacology and biopharmaceutics review (s). 2014. |
[28] | Castellano D , et al., Exposure-response modeling of cabozantinib in patients with renal cell carcinoma: Implications for patient care. Cancer Treat Rev. (2020) ;89: :102062. |
[29] | Gan CL , et al., Cabozantinib real-world effectiveness in the first-through fourth-line settings for the treatment of metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer Medicine. (2021) ; 10: (4):1212–1221. |
[30] | Albiges L , et al., Real-world evidence of cabozantinib in patients with metastatic renal cell carcinoma: Results from the CABOREAL Early Access Program. Eur J Cancer. (2021) ;142: :102–111. |
[31] | Motzer RJ , et al., Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. (2007) ;356: (2):115–124. |
[32] | Motzer RJ , et al., Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. (2013) ;369: (8):722–731. |
[33] | Parmar A , Bjarnason GA . Individualization of dose and schedule based on toxicity for oral VEGF drugs in kidney cancer. Kidney Cancer. (2019) ;3: (4):213–225. |
[34] | Sternberg CN , et al., COMPARZ Post Hoc Analysis: Characterizing Pazopanib Responders With Advanced Renal Cell Carcinoma. Clin Genitourin Cancer. (2019) ;17: (6):425–435.e4. |
[35] | Rini BI , et al., Overall Survival Analysis From a Randomized Phase II Study of Axitinib With or Without Dose Titration in First-Line Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer. (2016) ;14: (6):499–503. |
[36] | Hirsch L , et al., Clinical Activity and Safety of Cabozantinib for Brain Metastases in Patients With Renal Cell Carcinoma. JAMA Oncology. 2021. |




