Real-Word Experience of Cabozantinib in Metastatic Renal Cell Carcinoma (mRCC): Results from the Canadian Kidney Cancer information system (CKCis)
Abstract
BACKGROUND:
Cabozantinib is an oral multitargeted tyrosine kinase inhibitor (TKI) that has demonstrated efficacy in metastatic renal-cell carcinoma (mRCC) randomized trials.
OBJECTIVE:
To explore the real-world effectiveness of cabozantinib in pretreated patients with mRCC, including patients who progressed on immune-oncology checkpoint inhibitor (ICI) therapy.
METHODS:
Using the Canadian Kidney Cancer information system (CKCis), patients with mRCC treated with cabozantinib monotherapy as second-line or later from January 1, 2011 to September 1, 2019 were identified. Patients were stratified based on line of cabozantinib received. We reported overall survival (OS), time to treatment failure (TTF) and disease control rate (DCR). Prognostic variables were analyzed using multivariable analysis.
RESULTS:
157 patients received cabozantinib (median TTF 8.0 months; median OS 15.8 months): 37 (24%) in the second line (median TTF 10.4 months; median OS 18.9 months) 66 (42%) in third line (median TTF 5.9 months; median OS 13.3 months) and 54 (34%) in either 4th or 5th line (median TTF 9.4 months; median OS 16.8 months). One hundred sixteen patients (74%) received cabozantinib after prior ICI therapy (median TTF of 7.6 months; median OS of 15.8 months). DCR in all patients was 63% with 46%, 65% and 72% in 2nd line, 3rd line and 4th/5th line patients respectively. DCR in patients who received cabozantinib after prior ICI therapy was 64%.
CONCLUSIONS:
Cabozantinib is effective in a real-world, unselected population of mRCC patients, including in those who have progressed on prior ICI therapy, and in those exposed to multiple lines of therapy.
INTRODUCTION
Renal-cell carcinoma (RCC) is the most common form of kidney cancer, with an estimated 403,000 new cases and 175,000 deaths worldwide in 2018 [1]. One third of patients present with metastatic RCC (mRCC) at diagnosis [2]. Relapse occur in about one third of patients treated with localized disease [3].
Treatments for mRCC have evolved significantly over the past decade. Multiple tyrosine kinase inhibitors (TKIs) directed against vascular endothelial growth factor receptors (VEGFRs) have been approved, including sunitinib, pazopanib, sorafenib and axitinib [4]. Immune checkpoint inhibitors (ICI) have revolutionized the treatment of mRCC. Specifically, single agent nivolumab has been shown to be beneficial for mRCC patients who were previously treated with other systemic therapies [5]. Combination nivolumab and ipilimumab has been shown to be superior to sunitinib in the first-line setting for mRCC patients with intermediate/poor risk disease as per International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria [6]. Moreover, combination of the oral TKI axitinib and the ICI agents showed promising results for progression-free survival (PFS) and overall survival (OS) in the treatment of mRCC in the first-line setting [7, 8].
Cabozantinib is a TKI with activity against multiple kinases involved in the pathogenesis of RCC, including VEGFR-2, MET and AXL [9, 10]. Resistance to VEGF pathway inhibition is associated with activation of MET and AXL signaling, and preclinical models have demonstrated that cabozantinib may overcome sunitinib resistance [9]. As per the phase 3 METEOR trial, cabozantinib was found to be superior to everolimus in patients with mRCC following VEGFR TKI therapy, for both PFS (7.4 months versus 3.8 months) and OS (21.4 months versus 16.5 months) [11, 12]. Objective response rate (ORR) was 17% versus 3%. Based on this, cabozantinib was approved for treatment of patients with mRCC who progressed on prior VEGFR TKI therapy.
There is a paucity of prospective data with regard to the activity of cabozantinib in patients previously exposed to ICI. The majority of patients in the METEOR study were ICI-naïve [11]. Furthermore, as various treatment options exist, patients are often treated with multiple lines of systemic therapy. In this context, the effectiveness of cabozantinib in heavily-pretreated mRCC patients is unclear. To answer these questions, we explored the real-world effectiveness of cabozantinib in pre-treated mRCC patients across Canada, including those who had been previously treated with ICI.
MATERIALS AND METHODS
Study design
Data was retrieved from a prospectively maintained cohort in the Canadian Kidney Cancer Information System (CKCis) database, which consists of patients from 14 academic centres across Canada, from January 2011 to September 2019. The CKCis data from these centres has been shown to be representative of the Canadian kidney cancer population and thus felt to appropriately capture the national practice pattern. Key patient and tumour characteristics from CKCis are also in line with U.S. Surveillance, Epidemiology, and End Results (SEER) database, and the results from CKCis likely can be extrapolated to settings beyond Canadian academic centres [13]. All centres had research ethics board approval for CKCis projects. All research was conducted according to the principles of the Declaration of Helsinki. Included patients had to be > 18 years old with mRCC treated with cabozantinib monotherapy as second-line or later.
Baseline demographic, clinical, and laboratory data were collected. For the analysis, patients were grouped based on line of cabozantinib (2nd line, 3rd line, and 4th and 5th line), and whether patients received prior ICI.
Treatment outcomes
OS was defined as the time from initiation of cabo-zantinib to death from any cause. Time to treatment failure (TTF) was defined as time from initiation of cabozantinib to date of discontinuation or death from any cause, whichever came first. Disease-control rate (DCR) is defined as summation of rates of best response to cabozantinib (investigator-assessed; complete response (CR) + partial response (PR) + stable disease (SD)). Per-protocol, patient information updates occurred at least every 3 months.
Statistical analysis
Descriptive statistics were reported for the study variables. Mean and standard deviations were reported for continuous variables. Frequencies and proportions were reported for categorical variables. The objectives were to assess OS, TTF and DCR in patients with mRCC treated with cabozantinib monotherapy in the second-line or later. Distribution of OS and TTF were calculated using the Kaplan-Meier method censoring at last follow-up. Median OS and TTF, along with 95% confidence intervals were reported. One and two-year estimated survival was reported for the entire cohort and the subgroups. Multivariable Cox regression analysis was performed for OS and TTF. The prognostic variables used in the multivariable analysis include line of systemic therapy treatment (> = 3 vs. < 3), IMDC risk group (poor vs. favorable; intermediate vs. favorable), presence of liver and/or brain metastases, clear cell histology vs. non-clear cell histology, and age (< = 65 vs. > 65 years). Hazard ratios and the corresponding 95% confidence intervals were reported. Variables significant at p≤0.05 were considered for the final multivariate model. IMDC risk factors include lower performance status (Karnofsky performance score [KPS] < 80%), low hemoglobin, elevated corrected calcium, elevated neutrophils, elevated platelets, and time from diagnosis to treatment < 1 year [14].
All statistical analysis were conducted using SAS (SAS Institute Inc., Cary, NC) version 9.3 software. A p-value≤0.05 was used for statistical significance and two-sided tests were used. No adjustment was made for multiple testing.
RESULTS
Patient demographic and baseline characteristics
Our cohort consisted of 157 patients as shown in Table 1. The median age was 61 years and the majority of patients were male with good performance status (KPS > = 80%). Most patients had clear-cell histology (73%). Twenty-four percent of patients had liver metastases and 46% patients had bone metastases. Over half of patients (53%) had 3 or more metastatic organ sites. The majority of patients had nephrectomy (82%) and 29% of patients had metastatectomy. With regards to previous therapy, 74% received ICI prior to cabozantinib. Twenty-four percent of patients received cabozantinib in 2nd line, 42% of patients received cabozantinib in 3rd line and 34% of patients received cabozantinib as 4th or 5th line systemic therapy.
Table 1
Patient Demographic and Baseline Characteristics (n = 157)
| Characteristics | Variables | Value (n (%)) |
| Age | Median (range) | 61 (21–84) |
| ≤65 | 107 (68) | |
| > 65 | 50 (32) | |
| Gender | Female | 39 (25) |
| Male | 118 (74) | |
| KPS | ≥80 | 111 (71) |
| < 80 | 27 (17) | |
| NA | 19 (12) | |
| Pathology | Clear Cell | 115 (73) |
| Papillary | 12 (8) | |
| Chromophobe | 3 (2) | |
| RCC Unclassified | 12 (8) | |
| NA | 15 (10) | |
| Sarcomatoid Feature | Present | 10 (6) |
| Not Present | 69 (44) | |
| NA | 78 (50) | |
| Metastatic Locations | Brain | 17 (11) |
| Lung | 98 (62) | |
| Liver | 38 (24) | |
| Bone | 72 (46) | |
| Lymph Node | 86 (55) | |
| Other | 31 (20) | |
| No. of Metastatic Organ Sites | 1 | 27 (17) |
| 2 | 39 (25) | |
| > = 3 | 83 (53) | |
| NA | 8 (5) | |
| Therapy Prior to Cabozantinib | Nephrectomy | 129 (82) |
| Metastectomy | 45 (29) | |
| ICI | 116 (74) | |
| Received Cabozantinib | 2nd Line | 37 (24) |
| 3rd Line | 66 (42) | |
| 4th + 5th Line | 54 (34) |
Abbreviations: KPS = Karnofsky Performance Status; ICI = im-mune-checkpoint inhibitors.
IMDC risk groups by line of cabozantinib
A minority of patients (15%) in the entire cohort had IMDC favorable-risk disease upon initiation of cabozantinib as shown in Table 2. Almost half of all patients (48%) have IMDC intermediate-risk disease, and 18% have IMDC poor-risk disease. A minority of patients had missing IMDC risk group status (18%). Among patients who received cabozantinib in 2nd line, 35% of patients have IMDC favorable-risk disease whereas 11% have IMDC favorable-risk disease in 3rd line cabozantinib patients. More than half of 4th line and 5th line cabozantinib patients (63%) had either IMDC favorable-risk disease (7%) or IMDC intermediate-risk disease (56%).
Table 2
IMDC Risk Groups by Line of Cabozantinib
| All Patients | Second-Line Cabozantinib | Third-Line Cabozantinib | Fourth-Line & Fifth-Line Cabozantinib | |
| (n(%)) | (n(%)) | (n(%)) | (n(%)) | |
| Favourable-Risk | 24 (15) | 13 (35) | 7 (11) | 4 (7) |
| Intermediate-Risk | 75 (48) | 13 (35) | 32 (48) | 30 (56) |
| Poor-Risk | 29 (18) | 6 (16) | 16 (24) | 7 (13) |
| NA | 29 (18) | 5 (14) | 11 (17) | 13 (24) |
Abbreviations: IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
Systemic therapy prior to cabozantinib
Among patients who received cabozantinib in 2nd line (n = 37), 6 patients received an ICI-based regimen prior. Specifically, 2 patients received ipilimumab +nivolumab, and 4 patients received axitinib + pembrolizumab. For patients who received 2nd line cabozantinib after TKI (n = 31), 42% received pazopanib and 58% received sunitinib.
Among patients who received cabozantinib in 3rd line (n = 66), 51 patients received single agent nivolumab in the 2nd line setting. Among these 51 patients, 82% received 1st line sunitinib and 18% received 1st line pazopanib. Seven patients who received cabozantinib in the 3rd line setting received 1st line ICI-based regimen, which include avelumab + axitinib (n = 3), pembrolizumab + axitinib (n = 2), and atezolizumab + bevacizumab (n = 2). Among these 7 patients, 2 received pazopanib and 5 received sunitinib prior to 3rd line cabozantinib. Among the 8 patients who received 2 lines of TKI prior to 3rd line cabozantinib, 6 received sunitinib and 2 received pazopanib in the 1st line setting. All of them received 2nd line axitinib.
Among patients who received cabozantinib in the 4th and 5th line, majority (96%) of patients have received ICI at one point during the previous lines of therapy.
Outcomes
In the overall cohort, the median time of follow-up was 9.6 months. The investigator-assessed DCR was 63%, with 44% of patients achieving SD, 17% achieving PR and 1% achieving CR as best response to cabozantinib, as shown in Table 3. Median TTF was 8.0 months (range 6.2 –10.8 months) and median OS was 15.8 months (range 11.7 –21.5 months) for the entire cohort. At two years, 33% of patients were alive. DCR for patients who previously received ICI prior to initiating cabozantinib was 64%, with 19% achieving PR and 1% achieving CR. In this group with prior ICI exposure, the median OS was similar to the overall cohort with a value of 15.8 months (range 10.3 –20.7 months) and 28% of patients were alive at two years.
Table 3
Best Response to Cabozantinib, TTF and OS
| n | CR | PR | SD | PD | DCR | mTTF | mOS | One-year | Two-year | ||
| (%) | (%) | (%) | (%) | (%) | (mos) | (mos) | OS (%) | OS (%) | |||
| All | 157 | 1 | 17 | 44 | 36 | 63 | 8.0 (6.2 –10.8) | 15.8 (11.7 –21.5) | 57% | 33% | |
| Prior ICI | 116 | 1 | 19 | 42 | 35 | 64 | 7.6 (5.9 –10.8) | 15.8 (10.3 –20.7) | 56% | 28% | |
| 2nd Line Cabozantinib | 37 | 0 | 8 | 38 | 54 | 46 | 10.4 (5.7 –13.6) | 18.9 (11.1 –36.6) | 65% | 40% | |
| TKI - >Cabozantinib | 31 | 0 | 10 | 39 | 52 | 48 | 10.6 (3.9 –19.5) | 18.9 (11.8 –36.7) | 71% | 43% | |
| ICI - >Cabozantinib | 6 | 0 | 0 | 33 | 67 | 33 | - | - | - | - | |
| 3rd Line Cabozantinib | 66 | 2 | 20 | 41 | 33 | 65 | 5.9 (4.5 –8.8) | 13.3 (8.3 –22.6) | 52% | 21% | |
| TKI- >ICI- >Cabozantinib | 51 | 2 | 20 | 43 | 29 | 69 | 6.5 (4.9–12.0) | 13.3 (8.3 –20.7) | 52% | 27% | |
| ICI- >TKI- >Cabozantinib | 7 | 0 | 29 | 43 | 29 | 71 | - | - | - | - | |
| TKI- >TKI- >Cabozantinib | 8 | 0 | 13 | 25 | 63 | 38 | - | - | - | - | |
| *4th + 5th Line Cabozantinib | 54 | 0 | 18 | 53 | 27 | 72 | 9.4 (7.1 –19.4) | 16.8 (10.4 –27.8) | 58% | 36% |
*Majority (52/54) of patients in the 4th and 5th line group have received ICI at one point during the previous lines of therapy. Abbreviations: ICI = immune-checkpoint inhibitors; TKI = tyrosine-kinase inhibitor; CR = complete response; PR = partial response; SD = stable disease; DCR = disease-control rate; OS = overall survival; TTF = time to treatment failure.
Median TTF of patients based on line of therapy is shown in Fig. 1 and Table 3, and median OS for these groups of patients is shown in Fig. 2 and Table 3. The sequence of systemic agents received is shown in Table 3.
Fig. 1
Kaplan-Meier Curve for TTF for patient groups based on line of cabozantinib received.
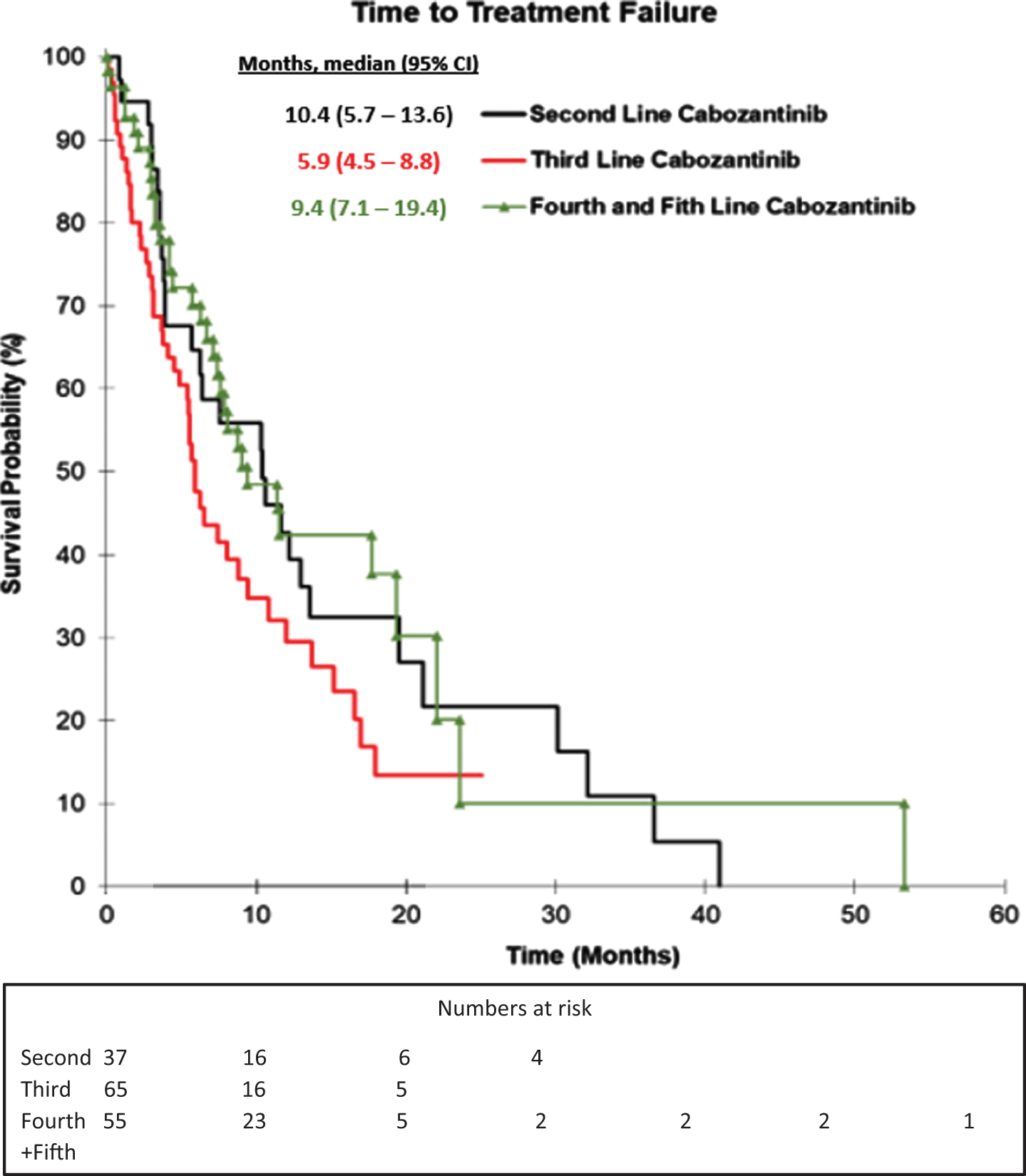
Fig. 2
Kaplan-Meier Curve for OS for patient groups based on line of cabozantinib received.
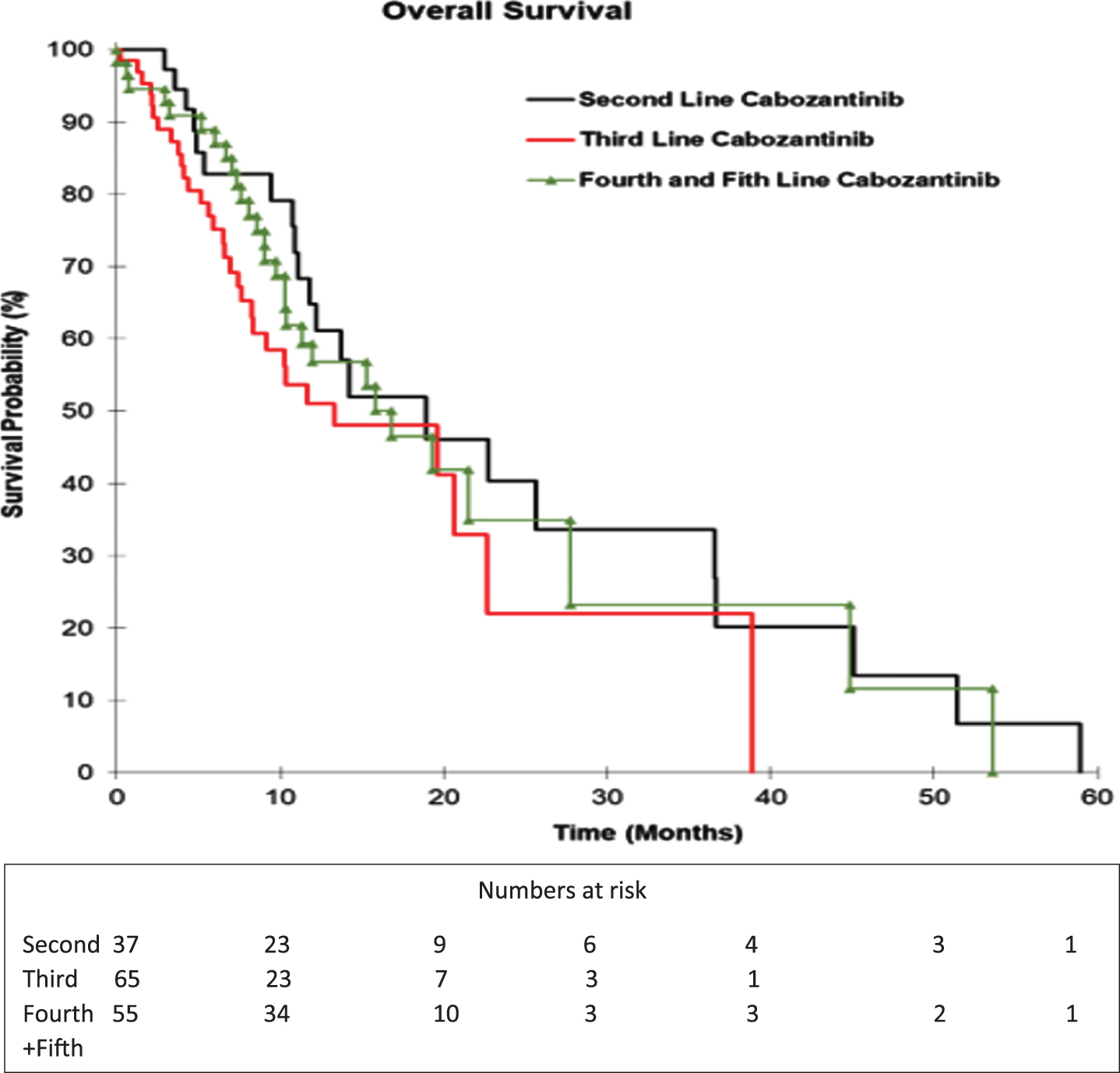
The majority of patients (31/37) in the 2nd line cabozantinib group received TKI prior to cabozantinib, with a DCR of 48%, and a two-year OS rate of 43%. For the 6 patients in the 2nd line group (6/37) who received cabozantinib immediately after first-line ICI, the DCR was 33%. The DCR for 3rd line cabozantinib was 65%, with a median TTF (5.9 months vs. 10.4 months) and a median OS (13.3 months vs. 18.9 months) compared to 2nd line. One patient in the group who received cabozantinib immediately after ICI achieved a CR as best response. Fourth and 5th line cabozantinib patients had a DCR of 72% with an associated median TTF of 9.4 months (range 7.1 –19.4 months) and median OS of 16.8 months (range 10.4 months –27.8 months). The majority (52/54 or 96%) of patients in the 4th and 5th line group received ICI therapy at one point during the previous lines of therapy.
The DCR of cabozantinib in patients with bone metastases and in patients with non-clear cell histology were 56% and 40%, respectively.
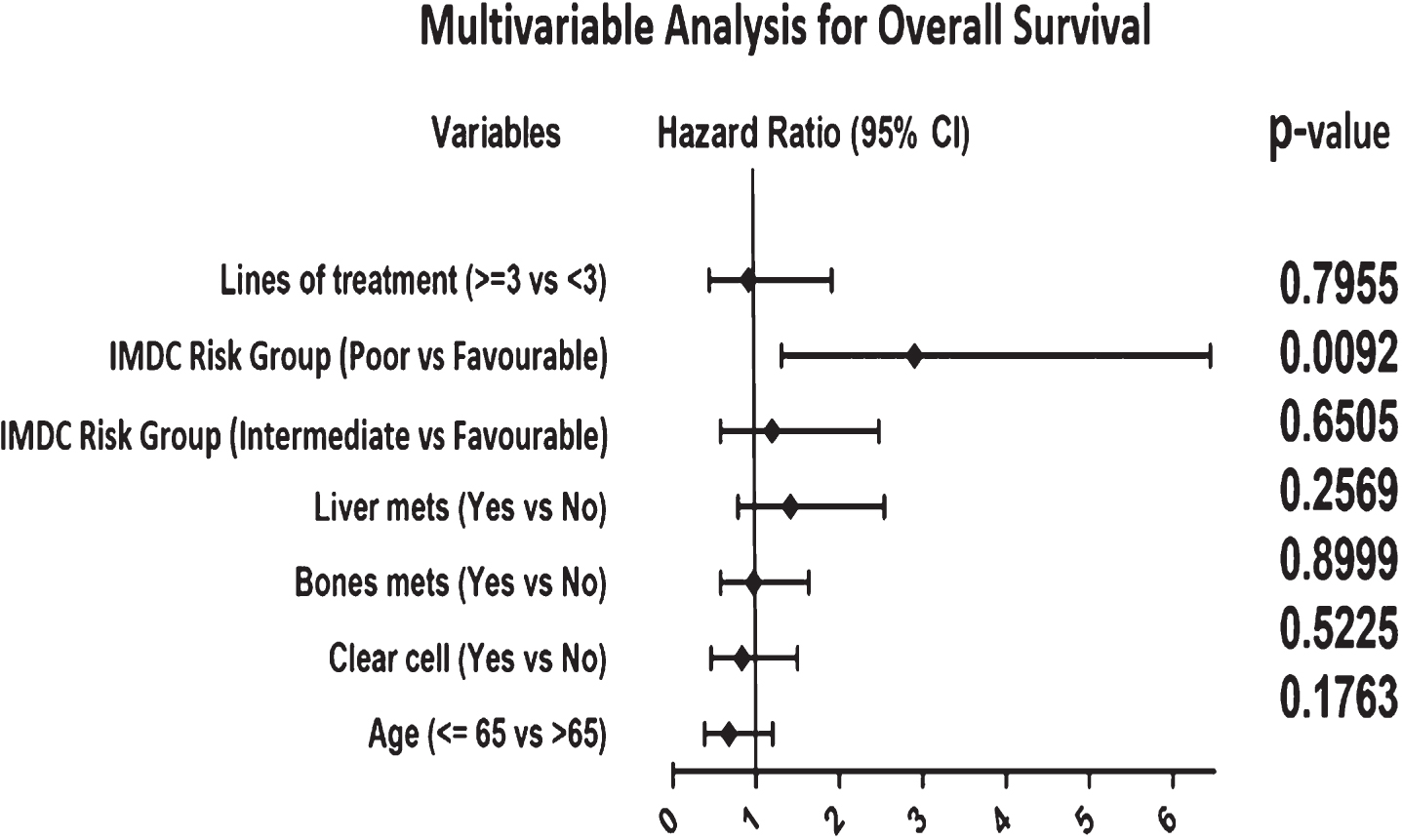
In multivariable analysis for TTF and OS shown in Fig. 3 and 4, initial IMDC poor-risk disease was independently associated with shorter survival (P = 0.0092), and a trend towards shorter TTF which was not statistically significant (P = 0.4272).
Fig. 3
Forest Plots illustrating result of multivariable analyses of variables associated with TTF. Hazard ratio greater than 1 is associated with shorter TTF. Abbreviations: IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
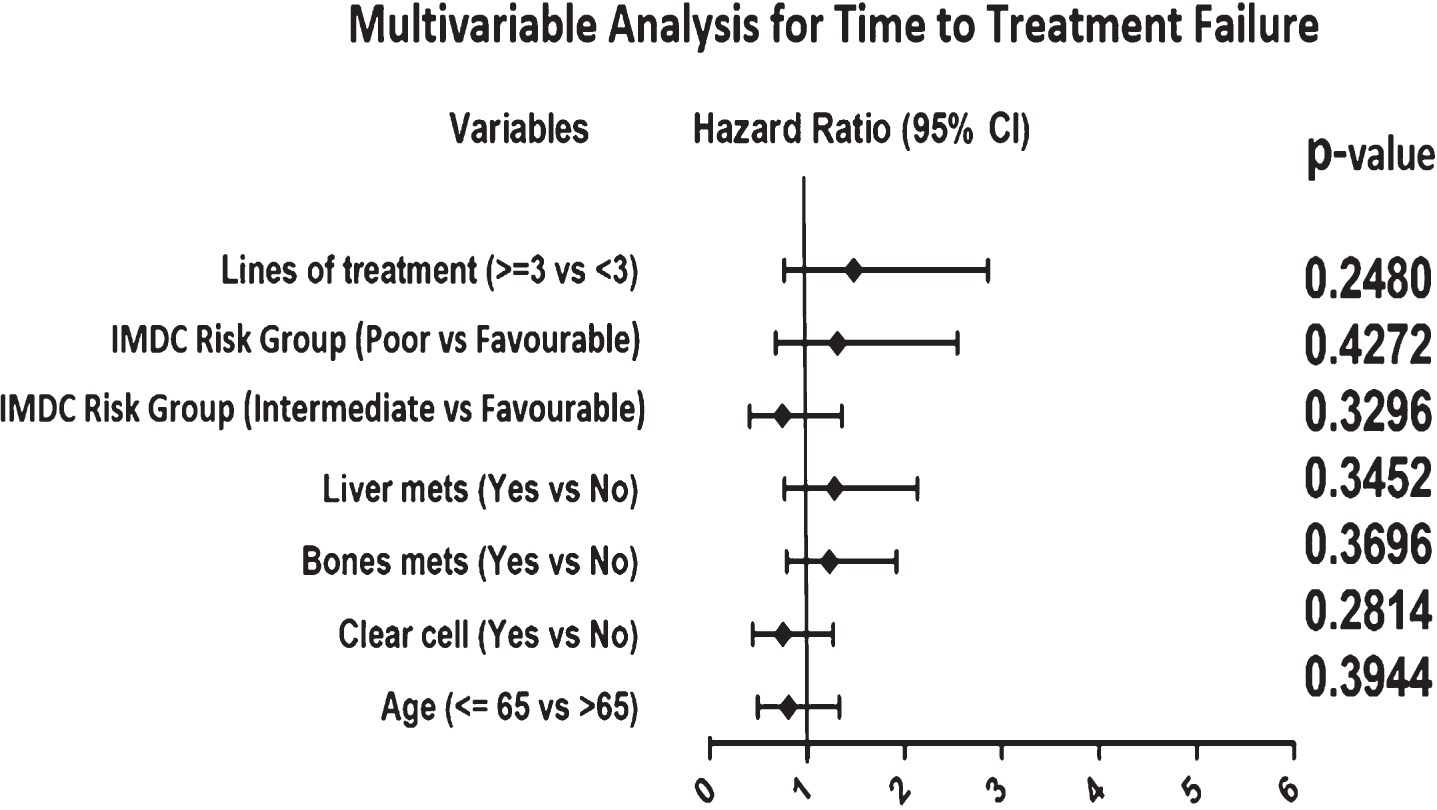
Fig. 4
Forest Plots illustrating result of multivariable analyses of variables associated with OS. Hazard ratio greater than 1 is associated with shorter OS. Abbreviations: IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
DISCUSSION
In this study, we aimed to characterize the effectiveness of cabozantinib in the treatment of mRCC in a real-world setting. The CKCis database used in this analysis contains prospectively collected data and is a valuable resource for evaluating clinical characteristics and outcomes of kidney cancer. Real-world data in this space is important to confirm the efficacy of novel drugs in a less selective and more diverse patient population.
In this analysis of 157 patients, we demonstrated that patients with mRCC treated with cabozantinib in the second or later-line setting appear to derive clinical benefit in terms of DCR (63%) as well as TTF and OS. The median OS observed in in this cohort who received cabozantinib in the 2nd line setting was 18.9 months, which is comparable to that of the METEOR study of 21.4 months [12]. Median TTF seen in those who received cabozantinib in the 2nd line setting was 10.4 months, which was longer than the median PFS observed in the METEOR study of 7.4 months [11]. However, 31% of the study population in the METEOR study were treated with two or more prior regimens [11, 12].
Similar findings were presented by Gan et al. in a retrospective analysis of the IMDC database of 413 mRCC patients who received cabozantinib in the 1st to 4th line setting. This study revealed a median TTF of 7.3 months and median OS of 17.8 months in the second-line setting and median TTF of 7.0 months and median OS of 12.6 months in the third-line [15]. CABOREAL is a real-world retrospective French multicentre study of 410 mRCC patients who received > 1 dose of cabozantinib. Nearly all patients (99%) received cabozantinib in the second or later-line setting with 41% of patients having had > 3 lines of prior therapy. Consistent with our results, this study revealed a median treatment duration of 7.6 months and a median OS of 14.4 months [16]. In contrast to our analysis, the CABOREAL study did not report response rates or TTF outcomes.
In patients who received 2nd line cabozantinib after a first-line TKI agent (pazopanib or sunitinib), survival outcomes were very similar to those reported in the subgroup analyses of the METEOR study [17]. Particularly, we saw a median TTF of 10.6 months and median OS of 18.9 months in this group of patients, compared to the METEOR study of 9.1 months median PFS and 21.4 months median OS in those who received sunitinib first-line, and 7.4 months median PFS and 22.0 months median OS in those who received pazopanib first-line. Our results support the hypothesis that cabozantinib can overcome resistance to first-line VEGFR TKIs by combined inhibition of VEGFRs and additional targets including MET and AXL [9].
We saw impressive response rates and survival in the heavily pre-treated group who received cabozantinib as 4th or 5th line systemic therapy, with DCR of 72%, median TTF of 9.4 months (range 7.1 –19.4 months) and median OS of 16.8 months (range 10.4 –27.8 months). Similarly, the IMDC retrospective analysis also reported long median TTF of 8.0 months (range 5.0 –9.4 months) and median OS of 14.9 months (range 10.2 –21.7 months) in patients who received cabozantinib in the 4th line setting [15]. We postulate that this could be impacted by selection bias, as RCC is a heterogenous disease in which some tumours display a more indolent natural history which may allow patients to receive multiple lines of treatment [18]. Based on these data, cabozantinib remains a viable option in patients who have progressed on multiple lines of therapy.
As ICI based therapies enter clinical treatment algorithms, an area of active investigation is determining the optimal treatment strategy in those who progress on ICI. We observed activity of cabozantinib in patients who had previously received ICI, with a DCR of 64% and median OS of 15.8 months (range 10.3 –20.7 months). Similar findings were seen in the METEOR study as well as other retrospective series [16–20]. Importantly, we included patients treated immediately after first-line ICI-based combination therapy (Table 3), which represents a group where data is lacking on the optimal treatment strategy. Based on preclinical studies, resistance to PD-1/PD-L1 inhibition has been associated with increased expression of pro-angiogenic cytokines and transcription factors that promote epithelial-mesenchymal transition [21]. It is hypothesized that cabozantinib may have clinical activity after prior anti-PD-1/PD-L1 therapy by inhibiting mediators of both angiogenesis (VEGF receptors) and epithelial-mesenchymal transition (MET and AXL) [17].
Bone metastases are common in mRCC (approximately 30% of patients), and are associated with high morbidity and a poor prognosis [22]. There is evidence to suggest that bone metastases do not respond well to VEGF inhibitors [23] . However, the CABOSUN study suggested that cabozantinib may have particular efficacy in patients with bone metastases [24]. In our real-word study, we saw clinical activity in patients with bone metastases, with a DCR of 56%. Similar results were seen in the subgroup analysis of the METEOR trial, in which cabozantinib was superior to everolimus in patients with bone metastases in terms of higher ORR (17% vs. 0%) [25]. In preclinical models, osteoblasts appear to promote bone metastases via HGF-MET signalling pathway as well as VEGF-VEGFR pathway [26, 27]. It is postulated that cabozantinib is active against bone metastases in mRCC by inhibiting both MET and VEGRF signalling pathways [28].
There is also limited data regarding the activity of cabozantinib in non-clear cell mRCCs due to the rarity of these subtypes and clinical heterogeneity [29]. In our study, we found a DCR of 40% (PR + SD) in the non-clear cell patients (n = 27). In an international retrospective study involving patients with non-clear cell mRCCs treated with cabozantinib during any treatment line (n = 112), an ORR of 27% was reported across various non-clear cell mRCCs [30]. However, due to the limited sample size of this group in our study, we cannot draw conclusions regarding the activity of cabozantinib for specific non-clear cell subtypes.
A unique aspect of our study is that we reported outcomes based on the sequence of therapies received prior to initiation of cabozantinib. We found that response rates appear high regardless of sequence and class of therapy received prior to cabozantinib. However, due to the small sample size of patients in some of these subgroups, it is challenging to determine the optimal sequencing strategies based on this data alone.
Real-world evidence plays an important role in expanding our knowledge on the treatment of mRCC, as it provides a better understanding of groups that are traditionally excluded from randomized controlled trials [31]. However, results need to be interpreted in light of a number of limitations. These include the retrospective nature of the cohort, heterogeneity of patient population, missing data, and selection bias [32] In the multivariable analysis, most of the subgroups were underpowered to assess prognostic factors. Furthermore, data on safety outcomes is not available for this manuscript.
There is emerging evidence to support cabozantinib in the first-line setting. Specifically, the phase 3 Checkmate 9ER trial demonstrated superiority of combination nivolumab and cabozantinib compared to sunitinib as first-line treatment for clear cell mRCC [33]. Additionally, Health Canada has approved cabozantinib monotherapy for the first-line treatment of patients with mRCC based on the CABOSUN study. Despite regulatory approval, it is currently not funded as a first-line treatment option for mRCC in Canada. Hence, our study did not report on the activity of cabozantinib in the first-line setting.
CONCLUSIONS
In summary, we demonstrated that cabozantinib appears to be effective in a real-world population of mRCC patients, including those who have progressed on prior ICI, and also in those who have had multiple lines of prior therapy. These results are consistent with those from randomized trials and other observational studies. Our results support the incorporation of cabozantinib into contemporary treatment algorithms. Future directions include prospectively expanding our cohort of patients in each subgroup and longer follow-up is needed in order to assess differences in effectiveness and survival between these different sequencing strategies. Toxicities of cabozantinib as well as dosing strategies are topics of interest for future studies within this cohort.
FUNDING
The Canadian Kidney Cancer information system (CKCis) is funded by the Kidney Cancer Research Network of Canada which receives funding support from industry sponsors.
AUTHOR CONTRIBUTIONS
Project development: HZ, NSB, JG. Data collection or management: HZ, IJ, SG. Data analysis: HZ, SG, JG. Manuscript writing/editing: HZ, NSB, SG, AAL, ARH, DYH, VC, CKK, EW, LW, GAB, RHB, AK, JG.
CONFLICT OF INTERESTS
All authors have no conflicts of interest to declare related to this manuscript. For COI unrelated to this manuscript: HZ: Hororarium - Merck. NSB: Honoraria - Astellas Pharma; Eisai; Ipsen; Janssen; Merck; Pfizer. Consulting or Advisory Role - Astellas Pharma; AstraZeneca; Bristol-Myers Squibb; Eisai; Ipsen; Janssen; Merck; Pfizer; Roche Canada; Bayer. Travel, Accommodations, Expenses - Eisai; Janssen. SG: None. IJ: None. AAL: Honoraria - Astellas Pharma; Bayer; Bristol-Myers Squibb; Merck; Novartis; Pfizer; Roche/Genentech; Tersera. Consulting or Advisory Role - Abbvie; Astellas Pharma; Bayer; Bristol-Myers Squibb; Eisai; Ipsen; Janssen; Merck; Pfizer; Roche/Genentech; Tersera. Research Funding - Bristol-Myers Squibb (Inst); Ipsen; Novartis; Roche. ARH: Honoraria - AstraZeneca/MedImmune; Bristol-Myers Squibb; GlaxoSmithKline/Novartis; Merck; Pfizer. Consulting or Advisory Role - Boehringer Ingelheim; Boston Biomedical; Bristol-Myers Squibb; Genentech/Roche; GlaxoSmithKline; Merck; Novartis. Research Funding - Boehringer Ingelheim; Bristol-Myers Squibb (Inst); GlaxoSmithKline (Inst); Janssen (Inst); Karyopharm Therapeutics (Inst); Merck (Inst); Novartis (Inst); Roche/Genentech (Inst). DYH: Consulting or Advisory Role - Astellas Pharma; Bristol-Myers Squibb; Eisai; Ipsen; Janssen; Merck; Novartis; Pfizer. Research Funding - Bristol-Myers Squibb (Inst); Exelixis (Inst); Ipsen (Inst); Novartis (Inst); Pfizer (Inst). VC: Consulting or Advisory Role - AstraZeneca; Bristol-Myers Squibb; Celgene; Janssen; Novartis. CKK: Honoraria - Bristol-Myers Squibb; Novartis; Pfizer. Consulting or Advisory Role - Astellas Pharma; Bristol-Myers Squibb; Eisai; Ipsen; Janssen; Novartis; Pfizer. Travel, Accommodations, Expenses - Eisai; Novartis; Pfizer. EW: Honoraria - Amgen; Bayer; Eisai; Merck; Roche. Research Funding - AstraZeneca/MedImmune (Inst); Bristol-Myers Squibb (Inst); Eisai (Inst); Merck (Inst); Pfizer (Inst); Roche/Genentech (Inst). LW: Research Funding - Aragon Pharmaceuticals (Inst); AstraZeneca (Inst); Bristol-Myers Squibb (Inst); Exelixis (Inst); Merck (Inst); Novartis (Inst); Pfizer (Inst); Roche Canada (Inst). GAB: Honoraria - Bristol-Myers Squibb; Eisai; Ipsen; Novartis; Pfizer. Consulting or Advisory Role - Bristol-Myers Squibb; Eisai; Ipsen; Novartis; Pfizer. Research Funding - Merck (Inst); Pfizer (Inst). Travel, Accommodations, Expenses - Novartis; Pfizer. RHB: None. AK: Consulting or Advisory Role - Amgen; Bristol-Myers Squibb; Eisai; Ipsen; Janssen Oncology; Novartis; Pfizer. Research Funding - Bristol-Myers Squibb (Inst), EMD Serrano. JG: Consulting or Advisory Role - Ipsen; Janssen Oncology.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) ;68: (6):394–424. |
[2] | Gupta K , Miller JD , Li JZ , Russell MW , Charbonneau C . Epidemiologic and socio-economic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. (2008) ;34: (3):193–205. |
[3] | Janzen NK , Kim HL , Figlin RA , Belldegrun AS . Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. (2003) ;30: (40):843–52. |
[4] | Choueiri TK , Motzer RJ . Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. (2017) ;376: (4):354–66. |
[5] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) ;373: (19):1803–13. |
[6] | Motzer RJ , Tannir NM , McDermott DF , Frontera OA , Melichar B , Choueiri TK . Nivolumab plus ipilimumab versus sunitinib in advanced renal cell carcinoma. N Engl J Med. (2018) ;378: (14):1277–90. |
[7] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal cell carcinoma. N Eng J Med. (2019) ;380: (12):1116–27. |
[8] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell M , et al. Avelumab plus axitinib versus sunitinib for advanced renal cell carcinoma. N Eng J Med. (2019) ;380: (12):1103–15. |
[9] | Tannir NM , Schwab G , Grunwald V . Cabozantinib: an active novel multi-kinase inhibitor in renal cell carcinoma. Curr Oncol Rep. (2017) ;19: (2):14. |
[10] | Yakes FM , Chen J , Tan J , Yamaguchi K , Shi Y , Yu P , et al: Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. (2011) ;10: (12):2298–308. |
[11] | Choueiri TK , Escudier B , Powles T , Mainwaring P , Rini BI , Donskov F , et al. Cabozantinib versus everolimus in advanced renal cell carcinoma. N Engl J Med. (2015) ;373: (19):1814–23. |
[12] | Choueiri TK , Escudier B , Powles T , Tannir NM , Mainwaring PN , Rini BI , et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomized, open-label, phase 3 trial. Lancet Oncol. (2016) ;17: (7):917–27. |
[13] | Tajzler C , Tanguay S , Mallick R , Ahrens B , Ly TL , Breau RH , et al. Determining generalizability of the Canadian Kidney Cancer information system (CKCis) to the entire Canadian kidney cancer population. Can Urol Assoc J. (2020) ;14: (10):E499–506. |
[14] | Heng D , Xie W , Regan M , Harshman LC , Bjarnason GA , Vaishampayan UN , et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. (2013) ;14: (2):141–8. |
[15] | Gan CL , Dudani S , Wells C , Donskov F , Pal SK , Dizman Z , et al. Cabozantinib real-world effectiveness in first through fourth-line settings for the treatment of metastatic renal cell carcinoma (mRCC): results from the international mRCC database consortium (IMDC) [abstract]. J Clin Oncol. (2020) ;38: (Suppl 6):639. |
[16] | Albiges L , Fléchon A , Chevreau C , Topart D , Gravis G , Oudard S , et al. Real-world evidence of cabozantinib in patients with metastatic renal cell carcinoma: Results from the CABOREAL Early Access Program. Eur J Cancer. (2021) ;142: :102–11. |
[17] | Powles T , Motzer R , Escudier B , Pal S , Kollmannsberger C , Pikiel J , et al. Outcomes based on therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. (2018) ;119: (6):663–9. |
[18] | Fisher R , Pender A , Thillai K , Chowdhury S , Pickering L , Khabra K , et al. Observation as a treatment strategy for advanced renal cell carcinoma –a call for prospective validation. Front Oncol. (2012) ;2: :155. |
[19] | Procopio G , Prisciandaro M , Iacovelli R , Cortesi E , Fornarini G , Facchini G , et al. Safety and efficacy of cabozantinib in metastatic renal-cell carcinoma: real-world data from an Italian managed access program. Clin. Genitourin. Cancer. (2018) ;16: (4):945–51. |
[20] | Santoni M , Heng DY , Bracarda S , Procopio G , Milella M , Porta C , et al. Real-world data on cabozantinib in previously treated patients with metastatic renal cell carcinoma: focus on sequences and prognostic factors. Cancers. (2020) ;12: (1):84. |
[21] | Jenkins RW , Barbie DA , Flaherty KT . Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. (2018) ;118: (1):9–16. |
[22] | Bianchi M , Sun M , Jeldres C , Shariat SF , Trinh QD , Briganti A , et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann Oncol. (2012) ;23: (4):973–80. |
[23] | Plimack E , Tannir N , Lin E , Bekele BN , Jonasch E . Patterns of disease progression in metastatic renal cell carcinoma patients treated with antivascular agents and interferon: impact of therapy on recurrence patterns and outcome measures. Cancer. (2009) ;115: (9):1859–66. |
[24] | Choueiri TK , Halabi S , Sanford BL , Hahn O , Michaelson MD , Walsh MK , et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A03 CABOSUN trial. J Clin Oncol. (2017) ;35: (6):591–7. |
[25] | Escudier B , Powles T , Motzer R , Olencki T , Frontera OA , Oudard S , et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J Clin Oncol. (2018) ;36: (8):765–72. |
[26] | Tsai S , Huang Y , Yang W , Tang CH . Cabozantinib, Hepatocyte growth factor-induced BMP-2 expression is mediated by c-MET receptor, FAK, JNK, Runx2, and p300 pathways in human osteoblasts. Int Immunopharmacol. (2012) ;13: (2):156–62. |
[27] | Deckers M , Karperien M , Van Der Bent C , Yamashita T , Papapoulos SE , Lowik CW , et al. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. (2000) ;141: (5):1667–74. |
[28] | Ruiz-Morales JM , Heng DY . Cabozantinib in the treatment of advanced renal cell carcinoma: clinical trial evidence and experience. Ther Adv Urol. (2016) ;8: (6):338–47. |
[29] | Giles RH , Choueiri TK , Heng DY , Albiges L , Hsieh JJ , Linehan WM , et al. Recommendations for the management of rare kidney cancers. Eur Urol. (2017) ;72: (6):974–983. |
[30] | Chanza NM , Xie W , Bilen MA , Dzimitrowicz H , Burkart J , Geynisman DM , et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. (2019) ;20: (4):581–90. |
[31] | Graham J , Heng DY . Real-world evidence in metastatic renal cell carcinoma. Tumori. (2018) ;104: (2):76–82. |
[32] | Nazha B , Yang J , Owonikoko TK . Benefits and limitations of real-world evidence: lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol [Internet]. 2020 Nov [cited 2021 Feb 7]; Available from: https://www.futuremedicine.com/doi/pdf/10.2217/fon-2020-0951 |
[33] | Choueiri TK , Powles T , Burotto M , Bourlon MT , Zurawski B , Oyervides Juárez VM , et al. Nivolumab+cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase III CheckMate 9ER trial [abstract]. Ann Oncol. (2020) ;31: (Suppl 4):S1159. |



