A Systematic Review of Immune Checkpoint Inhibitors in Non-Clear-Cell Renal Cancer
Abstract
BACKGROUND:
Immune checkpoint inhibitors (ICI) have emerged as active therapies in the management of advanced RCC. While multiple studies have shown clinical activity of ICIs in clear cell histologies, the evidence to support their use in non-clear cell (ncc) subtypes is based on smaller prospective trials and retrospective analyses.
OBJECTIVE:
The objective of this review is to summarize the clinical outcomes of ICI-based therapies in ncc-subtypes and in tumors with sarcomatoid/rhabdoid features.
METHODS:
We performed a systematic literature search using PubMed, Google Scholar and ASCO databases. The keywords “renal cell cancer” and “immune checkpoint inhibitors” and equivalents were used and all original publications between July 2016 and July 2021 were included.
RESULTS:
We included a total of 14 publications, including two clinical trials and 12 case series. The most frequent histologies were papillary (up to 75-100%), unclassified (up to 34%) and chromophobe (up to 28%). ICI monotherapy showed some activity in both 1st and 2nd line with response rates up to 27%. ICI combination regimens yielded better activity than ICI monotherapy but, overall, a heterogeneous efficacy was noted across histologies. Overall, outcomes of ICIs were superior in tumors with sarcomatoid/rhabdoid features.
CONCLUSION:
The observed activity of ICI-based therapies was heterogeneous. Combination regimens, papillary subtype and sarcomatoid/rhabdoid features were associated with higher responses. These findings might help treatment decisions and require further validation.
INTRODUCTION
Renal cell cancer (RCC) is among the top-10 most frequent cancers in the United States in 2021. It is the sixth most frequent cancer among men, representing 5% of new cancers and 46,780 cases, and the ninth most frequent among women, representing 3% of new cancers and 27,300 cases [1].
According to WHO (2016) [2], RCC pathology reveals a clear cell (cc) tumor in 80% of cases. A different histology, referred to as non-clear-cell (ncc) RCC, occurs in the remaining 20% of cases. Among nccRCC patients, papillary and chromophobe tumors account for most cases (∼80%) [3]. nccRCC includes rare histologies such as medullary, acquired cystic disease-associated RCC, collecting ducts carcinoma, and translocation RCC. Sarcomatoid and rhabdoid dedifferentiation is associated with a more aggressive course of disease [4] and can occur in a varying percentage of both ccRCC or nccRCC.
The standard of care for the treatment of advanced RCC has been changing rapidly. In the last few years, immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced RCC. By blocking interactions between surface proteins on cancer cells (programmed death-ligand 1 - PD-L1) or antigen-presenting cells (B7-1, B7-2) and receptors on T cells (programmed cell death 1 protein - PD-1, cytotoxic T-lymphocyte-associated protein 4 -CTLA-4), ICIs help T cells recognize and kill cancer cells [4]. Nivolumab (an anti-PD1 ICI) was initially approved in monotherapy in second or further lines of treatment [5]. More recently, combination therapies including ICIs have demonstrated superior results as first-line therapy, either using an antiPD1/PDL-1 ICI plus an antiCTLA4 ICI (nivolumab plus ipilimumab [6]) or using an antiPD1/PDL1 ICI plus a thyrosine kinase inhibitor (TKI) (avelumab plus axitinib [7], pembrolizumab plus axitinib [8], nivolumab plus cabozantinib [9] and pembrolizumab plus lenvatinib [10]).
Most data supporting the use of ICIs for the treatment of advanced RCC has resulted from trials including only ccRCC. Some therapeutic options are currently recommended for nccRCC, such as sunitinib [11–13], platinum-based chemotherapy for medullary cancer [4], or cabozantinib for papillary RCC [14]. However, the real efficacy of ICIs in nccRCC remains unknown. The objective of this review is to summarize the reported activity of ICIs as monotherapy or combined with other therapeutic agents in nccRCC histologic subtypes.
METHODS
Search strategy
We conducted a systematic literature search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15] to identify studies reporting outcomes of ICIs in advanced nccRCC in PubMed, Google Scholar, and the American Society of Clinical Oncology (ASCO) database between 2016 and 2021. The search strategy used the following keywords: “non-clear cell renal cell cancer”, “nccRCC”, “papillary renal cell cancer”, “translocation renal cell cancer”, “sarcomatoid renal cell cancer”, “unclassified renal cell cancer”, “immunotherapy”, “Immune checkpoint inhibitor”, “ICI”, “IO”, and “ICP.”
The first and second authors independently conducted the selection process in three stages. The initial inclusion of articles in the first stage was performed by screening the content of the title and abstract. The second stage included full-text reading of the remaining articles. For all articles identified in the second stage, we manually searched their bibliographies for potentially relevant articles. In this third stage, we also searched the chains of references using a “snowball” type approach by searching their bibliographies until no new references could be found. The first and second authors individually performed all stages of the selection process. Any discrepancies or omissions were resolved by discussion and consensus. Where consensus was not achieved, the last author made the final decision.
Eligibility criteria
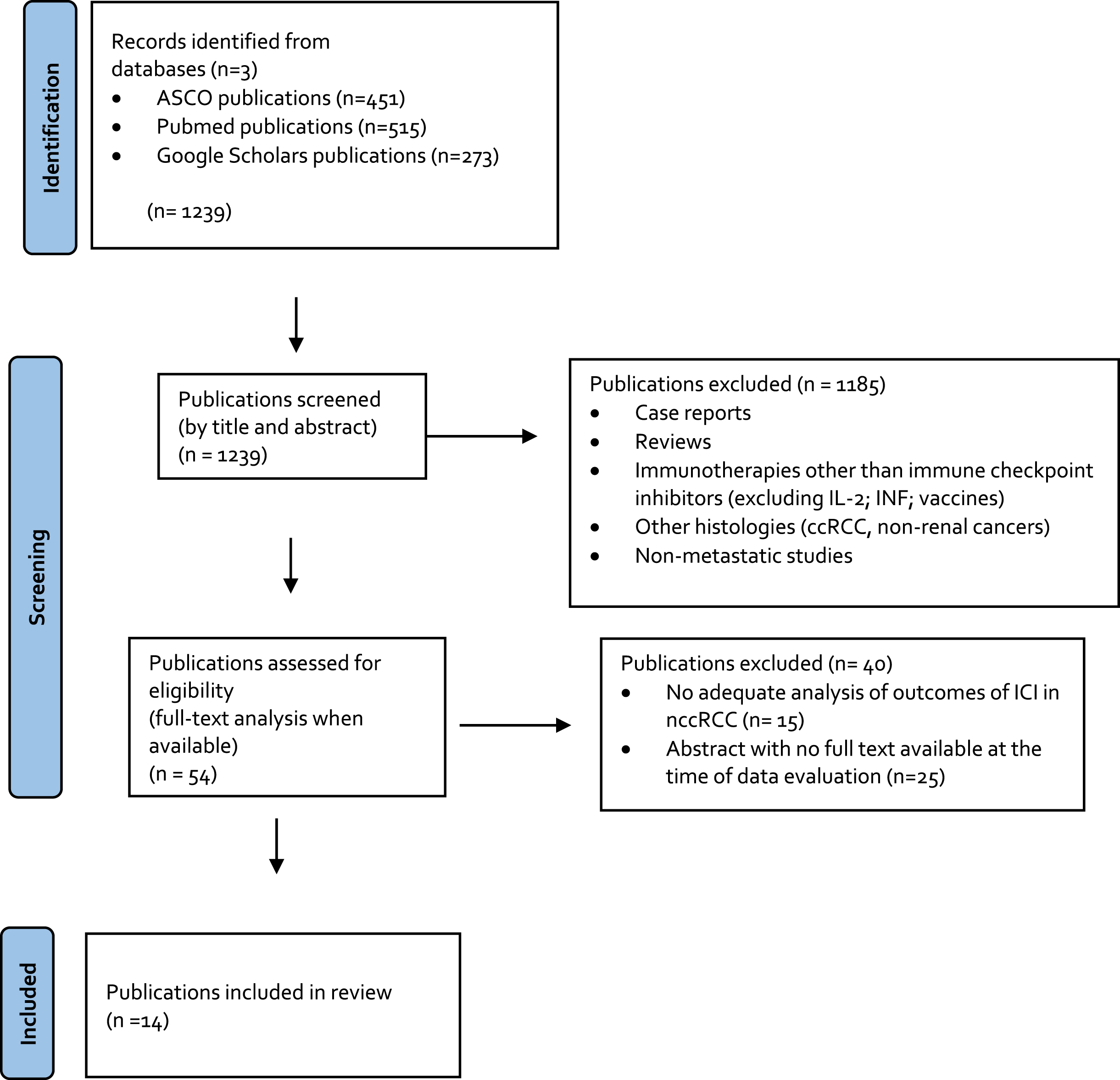
All original articles with full text available reporting results of the treatment of nccRCC or sarcomatoid dedifferentiated advanced RCC with an ICI were included, independent of the line of therapy. Non-English articles, non-original articles (i.e. review articles with or without systematic review or meta-analysis, editorials, opinions, commentaries, case reports, etc.), abstracts, and repeated publications on the same cohort were excluded. Whenever the full text was not available or the absolute number of nccRCC was <5 patients, the publication was excluded. Publications that did not report the outcomes of the subset of nccRCC or sarcomatoid patients apart from the intention-to-treat (ITT) population were excluded (Fig. 1).
Fig. 1
PRISMA flow chart.
Data extraction
The following variables were extracted: type of study; number of patients; type of nccRCC included; use of ICIs either as monotherapy or combination therapy (either with an ICI-ICI or with an ICI-TKI); proportion of use of ICIs as a first-line or second/third-line treatment; objective response rate (ORR) to ICI; progression-free survival (PFS); overall survival; duration of response (DOR) to ICI; and differences in ICI outcomes between different subsets of tumors (e.g. nccRCC versus ccRCC or between subtypes of nccRCC).
Data synthesis
The outcome measures were not combined because of the heterogeneity of the studies in terms of inclusion of different histologies, different contexts of treatment such as first-line versus second/third-line, and different types of studies, such as retrospective versus prospective and single-arm versus case-control. Data were summarized and main findings organized in five different contexts: ICI-TKI (immunotherapy agent with a tyrosine kinase inhibitor) in nccRCC, ICI-ICI (two immunotherapy agents) in nccRCC, ICI monotherapy in nccRCC, efficacy of ICI in special histologies, and ICI in sarcomatoid or rhabdoid dedifferentiated RCC.
RESULTS
The initial search yielded 1,239 articles. Of these 1,185 were excluded in the first screening stage and 40 were excluded in the second screening stage, leaving 14 full text articles that were included in this analysis (Fig. 1).
Immune checkpoint inhibitors in nccRCC
•ICI-TKI combination
The only study with full-text availability at the time of this systematic review on the use of the ICI-TKI combination in nccRCC was by McGregor et al. [16]. The study used atezolizumab plus bevacizumab with acceptable efficacy and tolerable toxicity. It included a total of 60 patients with either nccRCC or any histology with > 20% sarcomatoid dedifferentiation. The study included a total of 42 patients (70%) with variant histology RCC. The majority (65%) of patients were treatment-naïve. Results were convincing, with an intention to treat (ITT) population ORR of 33%, median PFS (mPFS) of 8.3 months, and median overall survival (mOS) that was not reached. Importantly, there was no statistically significant difference in the ORR associated with histology, although the ORR for nccRCC was 26% and for ccRCC with sarcomatoid features it was 50%. Toxicity was as expected, with no new adverse events comparable to what is reported with the same combination in ccRCC [17].
•ICI-ICI combination
The only study with full-text availability at the time of this systematic review that reported outcomes of an ICI-ICI combination in a population exclusively of nccRCC patients was by Gupta et al. [18]. This retrospective study showed promising results of nivolumab plus ipilimumab, with acceptable toxicity. However, it was a small sized population from two institutions (total n = 18). The included histologies were various (papillary, chromophobe, unclassified, renal adenocarcinoma, translocation and medullary). Of the 18 patients, 72% were therapy-naïve. The reported ORR was 33.3%, mPFS was 7.1 months, and 12-months OS was 64.2%. Although the number of patients receiving all four doses of ipilimumab was less than 50% and lower than in CheckMate 214 trial of nivolumab plus ipilimumab in advanced RCC [6], all grade (G) and G3/4 adverse events were as expected for this combination.
In contrast, Tachibana et al. [19], also a retrospective study, showed lower outcomes with nivolumab plus ipilimumab combination as first-line therapy for a nccRCC subset constituted of only a total of seven papillary tumors compared to ccRCC patients. The population was small, with 30 patients: 23 with ccRCC and seven with papillary RCC. The ORR for papillary RCC was significantly lower compared with ccRCC (14.2% versus 52.1%, respectively), and mPFS was significantly lower as well (2.4 months versus 28.1 months, respectively). However, mOS was not significantly different between the two subsets.
•ICI monotherapy
The outcomes of studies in which ICI monotherapy was used to treat advanced nccRCC are summarized in this section and presented in Table 1. However, some of these studies included a minor subset of patients on combination therapy and this will be highlighted when relevant.
Table 1
Outcomes of ICI monotherapy in nccRCC
| Design | Histology | Line (%) | Combination | Outcomes* | |
| Koshkin et al. (2018) | Retrospective multicentric, nivolumab in nccRCC (n = 41) | Papillary (39%), Unclassified (34%), Chromophobe (12%). Others: collecting duct, translocation, MTSCC. | 1st (8%)≥2nd (92%) | 0% | (20/3.5/NR) † |
| McKay et al. (2018) | Retrospective multicentric, nivolumab in nccRCC (n = 43) | Papillary (33%), Chromophobe (23%), Unclassified (21%). Others: translocation. | ≥2nd (100%) | 30.3% | (13/4.6/12.9) ‡ |
| Chahoud et al. (2019) | Retrospective unicentric, nivolumab in nccRCC and ccRCC with > 20% rhabdoid dedifferentiation (n = 40) | Papillary (30%), Unclassified (27.5%), Chromophobe (12.5%). Others: translocation, MTSCC. | 1st (15%)≥2nd (85%) | 22.5% | (13/4.3/11.6) ‡ |
| Vogelzang et al. (2020) | Prospective, nivolumab in nccRCC (n = 44) | Papillary (54.5%), Unclassified (18.2%), Chromophobe (15.9%), Others: collecting duct, translocation, medullary | 1st (65.9%)≥2nd (34.1%) | 0% | (13.6/2.2/16.3) ‡ |
| Hinata et al. (2020) | Retrospective multicentric analysis of nivolumab in Japanese patients with nccRCC or ccRCC (n = 208) | Clear (76.9%), Papillary (20.8%), MTSCC (10.4%), Others: chromophobe, other. | 1st (1%)≥2nd (99%) | 0% | 22.6/7.1/NR† |
| Barata et al. (2020) | Retrospective multicentric, antiPD1/PDL1 in nccRCC and ccRCC (n = 27) | Clear cell (56%), Papillary (26%), Unclassified (11%). Others: chromophobe, translocation. | 1st (100%) | 0% | (5/NS/NS) ‡ |
| McDermott et al. (2021) | Prospective, pembrolizumab in nccRCC (n = 165) | Papillary (71.5%), Unclassified (15.8%), Chromophobe (12.7%). | 1st (100%) | 0% | (26.7/4.2/28.9) † |
| Overall Range | ORR 5–26.7% /mPFS 2.2-7.1 months / mOS 12.9-NR |
NR: not reached; NS: not specified. *Outcomes for nccRCC treated with ICI monotherapy and organized as ORR (%)/mPFS in months (m)/mOS (m). ‡ICI monotherapy treatment in nccRCC with outcomes overall considered as numerically inferior to what is reported in the literature for ccRCC (Checkmate-025). †ICI monotherapy treatment in nccRCC considered overall in the same range of what is reported in the literature in ccRCC (Checkmate-025).
Seven studies were included in this pre-defined context, five of which were retrospective. Two other studies were, to our knowledge, the only prospective trials that reported the outcomes of ICIs in nccRCC to date: the phase II KEYNOTE-427 Study (Cohort B) [20] and the phase IIIb/IV CheckMate 374 Study [21].
Overall, the use of ICI monotherapy has a variable degree of efficacy in nccRCC across the included series. Some studies report outcomes in the range of what has been reported for the use of ICI monotherapy in the treatment of ccRCC [20, 22, 23] while others have poorer results [21, 24–26]. The expected efficacy of ICI monotherapy among ccRCC was considered to be near the one reported in CheckMate-025 (ORR 25%, mPFS 4.6 months; mOS 25 months) [5].
To date, KEYNOTE-427 was the largest prospective study in this context, including a total of 165 patients. Strict criteria selected a naïve advanced RCC population, with the majority of patients (75.2%) scoring 90–100% on the Karnofsky Performance Scale (KPS) and all of the patients scoring > 70% on the KPS. Moreover, although chromophobe and unclassified RCC were included, the majority (72%) of the population had papillary RCC. The outcomes were promising for pembrolizumab first line monotherapy, with an ORR of 26.7%, median duration of response (mDOR) of 29 months (with more than half of responses lasting longer than a year), mPFS of 4.2 months, and mOS of 28.9 months.
CheckMate 374 was the remaining trial that was included but it reported a range of outcomes that were poorer than what would be expected with ICI monotherapy in ccRCC. Although cross-trial comparison is not possible, outcomes of efficacy of nivolumab monotherapy (ORR of 13.6%, DOR of 10.2 months, mPFS of 2.2 months, and mOS of 16.3 months) were numerically lower than outcomes obtained for pembrolizumab monotherapy in KEYNOTE-427. However, some aspects of CheckMate 374 differed from KEYNOTE-427. It was a smaller prospective study with 44 patients. Although all patients also scored > 70% on the KPS, only around half of the patients scored 90–100% on the KPS. This study included approximately a third of patients in later lines (27.3% in the second line and 6.8% in the third line). Only 54.5% of the population had papillary RCC, with the inclusion of a variety of other nccRCC subtypes, namely chromophobe, unclassified, translocation, collecting duct, and medullary.
In Koshkin et al. [22], the outcomes were considered positive and to be in the range of values expected for ccRCC in this same context, with an ORR of 20%, mPFS of 3.5 months, and mOS that was not reached). These results are noteworthy because nivolumab monotherapy was administered in the second (62%) or third line of therapy (20%) to most of the population. Moreover, variable subtypes of nccRCC were included, with only 39% of papillary and 34% of unclassified nccRCC. Chromophobe, collecting duct, translocation, and mucinous tubular and spindle cell carcinoma (MTSCC) subtypes were also included.
Hinata et al. [23] was a Japanese real-world data analysis using nivolumab in previously treated advanced RCC. The study design was similar to CheckMate-025 but the eligibility criteria were broader with inclusion of a large population (208 evaluable patients) and nccRCC patients were also included. Since there was a ccRCC comparator-arm, a subset direct comparison was possible. Outcomes were not statistically different in nccRCC (48 patients) versus ccRCC (160 patients) and the ITT population results were globally positive, with an ORR of 22.6%, mPFS of 7.1 months, a 12-month survival rate of 75.6% and a mOS that was not reached.
Two other studies, McKay et al. [24] and Chahoud et al. [25], had a subset of patients under combination therapy (30.3% and 22.5% of the population, respectively). In both studies, the outcomes of ICI for the ITT population were not obviously lower than what would be expected in the ccRCC context. However, outcomes were numerically lower for the subset of patients treated with ICI monotherapy. McKay et al. reported an ORR of 13% and a time to treatment failure of 4.6 months for patients on ICI monotherapy and an overall mOS of 12.9 m. Chahoud et al. reported very similar results for patients on ICI monotherapy, with an ORR of 13%, mPFS of 4.3 months, and mOS of 11.6 months. In these two studies the apparent benefit of ICIs in nccRCC may be driven by a smaller subset of patients that is on combination therapy rather than by the majority of patients on ICI monotherapy.
Finally, Barata et al. [26] conducted a study that also showed only modest efficacy of ICI monotherapy in nccRCC, poorer than what would be expected among ccRCC. It included a real-life frail metastatic population of 27 patients with 33% of patients with Performance Status (PS) 2-3. Treatment was first-line PD1/PDL1 monotherapy. The ITT population consisted of both ccRCC (n = 15) and nccRCC (n = 12). Therefore, a direct subset comparison was possible and, although the ITT population ORR was 33%, there was a significant difference between ccRCC and nccRCC, with ORRs of 29% and 5%, respectively; mPFS in the ITT was 6.3 months but was also significantly longer in ccRCC; mOS was 31 months. The inferiority of ORR and mPFS in nccRCC may not be completely explained by the worse baseline PS among the ITT population, since that was similar for both the ccRCC and nccRCC subsets of patients.
Immune checkpoint inhibitors in special histologies
The range of the main outcomes (ORR, mPFS, and mOS) per each specific histology is presented in Table 2. The general tendencies across trials will be summarized in this section.
Table 2
Outcomes* of ICI on special histologies
| Study/Histology | Design | Papillary | Chromophobe | Unclassified | Translocation | Collecting duct | Medullary | MTSCC | AC | MITF | Sarcomatoid/ | ITT |
| Rhabdoid | ||||||||||||
| Koshkin et al. (2018) | Retrospective | n = 16 14/NS/NS | n = 5 0/NS/NS | n = 14 36/NS/NS | n = 1 0/NS/NS | n = 4 25/NS/NS | — | n = 1 0/NS/NS | — | — | — | n = 41 20/3.5/NR |
| McKay et al. (2018) | Retrospective | n = 14 28/4.8/NS | n = 10 0/4.3/NS | n = 9 0/2.8/NS | n = 3 33/NS/NS | — | — | — | — | — | n = 7cc/n = 4 ncccc43/4/NS ncc0/4/NS | n = 43 19/4/12.9 |
| Boliève et al. (2018) | Retrospective | — | — | — | — | — | — | — | — | n = 24 16.7/2.5/24 | — | n = 24 16.7/2.5/24 |
| Chahoud et al. (2019) | Retrospective | n = 12 Type 1:25/3.1/3.6 Type 2:0/9.7/NR | n = 5 0/4.3/6.9 | n = 11 44.4/5.5/17.3 | n = 3 0/NS/NS | — | — | n = 1 0/7.4/7.8 | — | — | n = 8 cc 28.6/4.8/14.9 | n = 40 21.6/4.9/21.7 |
| McGregor et al. (2019) | Prospective | n = 12 25/NS/NS | n = 10 10/NS/NS | n = 9 33/NS/NS | n = 5 20/NS/NS | n = 5 40/NS/NS | n = 1 100/NS/NS | — | — | — | n = 18cc/n = 8 ncccc50/4/NS ncc38/4/NS | n = 60 33/8.3/NR |
| Brilland et al. (2020) | Retrospective | n = 57 Type 1:14/5/11.4 Type2:9/2.9/ 14.6 Unclassified: 14/4.1/17.6 | — | — | — | — | — | — | — | — | — | n = 57 11/3.1/14.6 |
| Vogelzang et al. (2020) | Prospective | n = 24 NS | n = 7 NS | n = 8 NS | n = 2 NS | n = 1 NS | n = 1 NS | — | — | — | n = 4 ncc | n = 44 13.6/2.2/16.3 |
| Hinata et al. (2020) | Retrospective | n = 10 12.5/NS/NS | n = 2 0/NS/NS | — | — | — | — | n = 5 40/NS/NS | — | — | — | n = 208 (ccn = 160/nccn = 48) 22.6/7.1/NR |
| Barata et al. (2020) | Retrospective | n = 7 5/NS/NS | n = 1 NS/NS/NS | n = 3 0/NS/NS | n = 1 0/NS/NS | — | — | — | — | — | — | n = 27 33/6.3/31 |
| Tachibana et al. (2020) | Retrospective | n = 7 14.2/2.4/NS | — | — | — | — | — | — | — | — | — | n = 30 (ccn = 23/nccn = 7) cc52.1/28.1/NR ncc14.2/2.4/NR |
| Gupta et al. (2020) | Retrospective | n = 6 Type 2:50/NS/NS | n = 5 20/NS/NS | n = 3 66.7/NS/NS | n = 1 0/NS/NS | — | n = 1 0/NS/NS | — | n = 2 50/NS/NS | — | — | n = 18 33.3/7.1/NR |
| McDermott et al. (2021) | Prospective | n = 118 28.8/5.5/ 31.5 | n = 21 9.5/3.9/23.5 | n = 26 30.8/2.8/17.6 | — | — | — | — | — | — | n = 38 ncc 42.1/6.9/25.5 | n = 165 26.7/4.2/28.9 |
| Main Outcomes (Range) | — | ORR 5–50% | ORR 0–20% | ORR 0–66.7% | ORR 0–33% | ORR 25–40% | ORR 0–100% | ORR 0–40% | ORR 50% | ORR 16.7% | nccORR 0–42.1% | — |
| mPFS 2.4–9.7m | mPFS 3.0–4.3m | mPFS 2.8–5.5m | mPFS NS | mPFS NS | mPFS NS | mPFS NS | mPFS NS | mPFS 2.5m | nccmPFS 2.3–6.9m | |||
| mOS 3.6–17.6m | mOS 6.9–23.5m | mOS 17.3–17.6m | mOS NS | mOS NS | mOS NS | mOS NS | mOS NS | mOS 24m | nccmOS 25.5–NRm |
cc = clear cell RCC; MITF = microphthalmia transcription factor family translocation renal cell carcinoma; MTSCC = mucinous tubular and spindle cell carcinoma; n = number of pts included in that specific context; NR = not reached; NS = non-specified; ITT = Intention to Treat Population; — = not included. *Outcomes are organized as ORR (%)/mPFS in months (m)/mOS (m).
By far, a higher number of patients had the most frequent nccRCC histologies: papillary, unclassified, and chromophobe.
Overall, the higher ORR percentages were obtained in patients with an unclassified histology, with values as high as 44.4–66.7% [18, 25]. Unclassified RCC includes different types of tumors with different behaviors, which can possibly explain the variability of outcomes between studies, with a lack of response and an ORR of 0% obtained in some series with a low number of these tumors [24, 26].
Papillary tumors were consistently more responsive to ICIs across different series of nccRCC, although when a subset analysis of type 1 and type 2 papillary RCC was performed some differences were encountered. Type 1 papillary RCC had an ORR range between 14% [27] and 25% [25] and type 2 papillary had a lower ORR with some series reporting outcomes as low as 0% [25] or 9% [27]. Despite the tendency for lower ORR in papillary type 2 tumors, a higher ORR (of up to 50%) was reported with the use of ICI-ICI combination therapy [18]. Moreover, in one study, complete responses were reported among the papillary type 2 patients that did respond [27].
Nevertheless, chromophobe patients had poorer results, with no documented responses to ICI monotherapy and an ORR of 0% across most studies [22–26] and with an ORR of 9.5% in one of the included studies using ICI monotherapy [20]. However, the ORR obtained among chromophobe patients improved with the use of combination regimens, with a value of 10% obtained with ICI-TKI [16] and a value of 20% obtained with an ICI-ICI scheme [18].
As for rarer tumors, their inclusion was only occasional and in very low numbers, which limits interpretation of any specific tendency.
Namely, translocation RCC tumors included across studies were 16 with the highest number per study being 5, collecting duct RCC tumors included across studies were 10 with the highest number per study being 5, the mucinous tubular and spindle cell carcinoma (MTSCC) RCC tumors included across studies were 7 with the highest number per study being 5, the medullary RCC tumors included across studies were 3 with the highest number per study being 1 and the adenocarcinoma (AC) tumors were only included in one study and in a total number of 2. Moreover, a single multicentric analysis was able to gather 24 microphthalmia transcription factor family translocation (MITF) RCC tumors.
The ORR in these histologies has been very variable. Translocation RCC ORR varied between 0% [18, 22, 25, 26] and 33% [24]. The ORR for collecting duct carcinoma was 25–40% [16, 22]. Similarly, MTSCC RCC has varied considerably, from 0% [22, 25] to 40% [23]. Outcomes of ICIs in medullary tumors were only reported for two patients in two different studies: one responded [16] and the other did not [18]. Similarly, of the two cases of AC RCC included in a case series [18], one responded and the other did not.
Boliève et al. [28] conducted a retrospective multicentric analysis of antiPD1/PDL1 in previously treated patients with MITF RCC and results were compatible with the efficacy of these drugs in this subset of tumors, with an ORR of 16.7%, mPFS of 3 m, and mOS of 24 m. Although these outcomes are compatible with clinical benefit they are inferior to the efficacy that would be expected among ccRCC tumors.
Immune checkpoint inhibitors in tumors with sarcomatoid and rhabdoid dedifferentiation
Sarcomatoid dedifferentiation is found in 5–8% of RCC, both in nccRCC or ccRCC [29]. It associates with a worse prognosis and more aggressive disease and is present in a higher percentage in the metastatic setting (up to 20%) [29]. Rhabdoid features are the second most common form of dedifferentiation and were reported to happen in 4.7% of tumors, with associated metastatic disease in percentages as high as 51% [30].
Moreover, an inferior survival has been reported among tumors with sarcomatoid features that were treated with a TKI. A study including different anti vascular endothelial growth factor receptor (VEGF) TKIs also reported that sarcomatoid dedifferentiated tumors had worse results (with reported responses only in ccRCC with <20% sarcomatoid component and ORR of 19%, mPFS 5.3 m and mOS 11.8 m) [31]. Although results for rhabdoid tumors aren’t separately reported, a similar behaviour could be expected, since the molecular features of sarcomatoid, rhabdoid and mixed sarcomatoid/rhabdoid tumors was not found to be significantly different in a multi-trial analysis of Ziad Bakouny et al. [32].
Recent post-hoc analysis of six phase III trials of combination therapy with ICI-ICI [6] or ICI-TKI [7–10, 17] have highlighted a tendency for higher efficacy of ICIs in this group of tumors comparing with standard sunitinib. The range of reported outcomes is an ORR of 46.8–60.8%, mPFS of 7–26.5 m, and mOS of 21.3m-NR (See Table 3). Three meta-analyses have confirmed this tendency [33–35]. These findings support the preference of an ICI-based therapy in sarcomatoid dedifferentiated tumors. When series also include tumors with rhabdoid or mixed sarcomatoid/rhabdoid features a similar superiority of ICI based therapies was encountered [36].
Table 3
Outcomes of combination ICI-ICI or ICI-TKI Phase III front-line advanced ccRCC with sarcomatoid features
| Trial | Investigational drug | ITT (n) | Sarcomatoid tumors (n) | (%) sarcomatoid | Outcomes | NCT |
| CheckMate-214 | Nivolumab + Ipilimumab | 1096 | 139* | 12,68% | ORR 60.8% mPFS 26.5m mOS NR (52% alive at 42 m) | NCT02231749 |
| Keynote-426 | Pembrolizumab + Axitinib | 578 | 105* | 18,17% | ORR 58.8% mPFS NR mOS NR (83.4% alive at 12 m) | NCT02853331 |
| IMmotion-151 | Atezolizumab + Bevacizumab | 915 | 142 | 15,52% | ORR 49% mPFS 8.3m mOS 21.7m | NCT02420821 |
| CheckMate-9ER | Nivolumab + Cabozantinib | 651 | 75 | 11,52% | ORR 55.9% mPFS 10.9m mOS NR | NCT03141177 |
| JAVELIN Renal- 101 | Avelumab + Axitinib | 886 | 108 | 12,19% | ORR 46.8% mPFS 7m mOS NR (83% alive at 12 m) | NCT02684006 |
| CLEAR trial | Pembrolizumab + Lenvatinib | 1069 | 73 | 6,82% | ORR 60.7% mPFS 11,1m mOS NR | NCT02811861 |
*Only intermediate and poor risk patients; NR: not Reached.
There are few studies that reported the outcomes of the use of ICI based therapies in nccRCC patients with sarcomatoid dedifferentiated histology, therefore it remains unclear whether the benefit of ICIs among sarcomatoid and rhabdoid tumors is verified equally in ccRCC and nccRCC histology. Main results of ICI in nccRCC patients will be presented in this section.
Park et al. [37] verified that the choice of an ICI-based therapy for the treatment of sarcomatoid dedifferentiated RCC was associated with better outcomes and a statistically significant better survival, even in mixed populations, including ncc and cc tumors. Results were of an ORR of 35.3% and mOS of 57.6 m with ICI therapy comparing with an ORR of 0% and an mOS of 6.6 m with non-ICI therapy. Nevertheless, in that study, the proportion of included nccRCC patients was low (5.9% of patients in the ICI arm and 30% of patients in the non-ICI arm). The benefit in the sarcomatoid nccRCC subset was not separately reported.
Accordingly, across most of the included studies, nccRCC with sarcomatoid features was responsive to ICI-based therapies, with an ORR as high as 38% [16] or 42.1% [20]. However, this aspect was variable, with one of study reporting an ORR of 0% among the four included patients with nccRCC tumors and sarcomatoid features [24].
Furthermore, a tendency for lower results of ICI-based therapy in nccRCC compared to ccRCC has been suggested in one study. Chahoud et al. [38] reported a statistically significant superiority of ICI based therapies in sarcomatoid tumors of cc versus ncc histology, respectively with an ORR of 39% versus 14.3%, an mPFS of 8.9 m versus 2.9 m, and an mOS of 30.1 m versus 6.7 m. However, the number of nccRCC patients was low in this study, with only seven patients with nccRCC and 41 with ccRCC.
Moreover, in two of the included studies using ICI based therapies and including a subset of RCC tumors with sarcomatoid features, a tendency for a superior ORR among cc versus ncc tumors was also noticed, respectively with an ORR of 50% versus 38% in one study [16] and an ORR of 43% versus 0% in the other [24].
DISCUSSION
This review mainly included retrospective analysis, with only two prospective studies, which reflects the less than desirable level of evidence and the unmet need in nccRCC. The pool of patients from these studies was heterogeneous, so the conclusions for specific histologies are limited.
Overall, ICI-based therapy in nccRCC was reported to elicit a variable degree of response and disease control across all the included studies (Table 2).
The available data is consistent with the concept that ICI monotherapy is a valid option for the treatment of advanced nccRCC. In fact, the reported outcomes of the recommended therapy with sunitinib [4] for nccRCC in trials such as ASPEN [11] and ESPN [12] are in the same numeric range of the outcomes obtained with ICI monotherapy.
Importantly, ICI monotherapy use in nccRCC yielded results in the same range as for ccRCC in some studies [20, 22, 23], considering CheckMate-025 [5] as the benchmark of benefit in the ccRCC context. However, other studies [21, 24–26] had poorer results with ICI monotherapy in nccRCC tumors than what was reported for ccRCC tumors. Therefore, it remains unclear if the efficacy of ICI monotherapy is equivalent among nccRCC and ccRCC.
One of the main factors influencing the outcomes of ICI among nccRCC was the histology. ICI-based therapy elicited higher responses in papillary and sarcomatoid dedifferentiated tumors, with an ORR as high as 50% [18] and 42.1% [20], respectively. Potentially, the lower proportion of papillary tumors in CheckMate 374 in comparison with KEYNOTE-427 could have contributed to the worse results in that trial. However, papillary histology does not have a homogeneous response to ICI based therapies and a noticeable tendency for lower response rates for papillary type 2 tumors has been highlighted [25, 27]. Unclassified tumors are a broad group and as such they seem to have highly variable rates of response, from 0% [24, 26] to 44.4–66.7% [18, 25]. Chromophobe tumors have poor responses to ICIs [22–26]. Other nccRCC subtypes were too scarce to proceed to considerations about their ORR to ICI-based therapy. However, it is important to acknowledge that responses to ICIs were documented in rarer nccRCC subtypes, such as MITF [28], as well as in aggressive subtypes, such as collecting duct tumors [16, 22].
Other factors appeared to influence outcomes of ICI monotherapy treatment among nccRCC. As in other contexts, worse PS/ KPS score was associated with worse outcomes, not only in nccRCC patients but also in ccRCC patients, when included [26]. Moreover, the worse PS of the included population in one study [26] was correlated with a higher toxicity to ICI monotherapy. Correspondingly, in a Japanese nivolumab real-life data study, the ICI benefit had a positive association with a better PS [23]. Also, the line of therapy was associated with outcomes. ICIs administered as a 1st line therapy were associated with a higher rate of response among nccRCC [24, 25]. Worse results in CheckMate 374 comparing with KEYNOTE-427 might have been partially influenced by a lower number of treatment-naïve patients. Moreover, in some of the included studies, a higher rate of complete and partial responses in the first evaluations of response, was associated with a longer lasting response and disease control [20, 22, 26].
In general, more prospective trials are needed to further clarify ICI monotherapy’s real level of efficacy in the nccRCC population. Until then, therapeutic choices should take into account factors such as the tumor histologic subtype and the patient’s PS, co-morbidities, and cancer symptoms.
For ICI combination therapy, outcomes were only reported for a separate population of nccRCC in two of all the included studies. The combination regimens used were either ICI-ICI (ipilimumab and nivolumab) [18] or ICI-TKI combinations (atezolizumab and bevacizumab) [16]. Numeric outcomes pointed towards a higher efficacy than with ICI monotherapy, but there was no comparison group. However, when a direct comparison between the treatment of nccRCC with ICI monotherapy versus ICI combination therapy was possible, outcomes were better with ICI combination regimens. These comparisons were performed by subset analysis in two studies that included only a minor proportion of patients on ICI combination therapy, with numerically and statistically significant superiority for combination regimens [24, 25]. Moreover, ICI combination schemes were overall well tolerated by nccRCC patients.
Importantly, better outcomes with ICI combination therapy may not be consistently verified across all subtypes of nccRCC. For example, a study with a nivolumab-ipilimumab combination reported poorer outcomes for papillary cancers than for ccRCC patients [19], although it included only seven papillary tumors. This aspect requires further clarification.
Unfortunately, only up to a third of patients with nccRCC will respond to ICIs. Therefore, adequate selection of which nccRCC patients might benefit the most from a given drug is a current unmet need. Some of the included studies searched for possible biomarkers of response to ICI-based therapy. Among possible histological biomarker findings, there was an association of efficacy and a higher expression of PDL-1 in some studies [16, 20] but not in others [21]. Moreover, the tumor mutational burden did not seem to associate with response in nccRCC [24]. There was an association of efficacy to some potential laboratory biomarkers (namely, platelets under the upper limit of normal (ULN) and LDH under the ULN) [23], but it remains exploratory. Interestingly, occurrence of immune-related adverse events was associated with positive outcomes in one study [23]. Future trials with larger populations and exploratory analyses may help finding biomarkers for the adequate selection of nccRCC cases in which to use ICIs.
Reassuringly, multiple trials involving ICI-based therapies in nccRCC are ongoing (Table 4). These should answer key outstanding questions about nccRCC treatment strategies, including the efficacy and safety of different ICI-TKI and ICI-ICI combinations in nccRCC, the difference of efficacy between ICI-TKI and TKI alone, the efficacy and safety of a triple combination of TKI-ICI-ICI and the outcomes of a strategy of individualized treatment based on genomic alterations found in patients with advanced nccRCC.
Table 4
Ongoing nccRCC trials including ICIs registered in Clinical Trials.gov
| ClinicalTrials.gov identifier | Phase | Estimated enrollment | Intervention arm | Primary endpoint | Estimated completion date |
| TKI-ICI | |||||
| NCT04958473 | Phase 2 | 40 | Sintilimab plus axitinib | ORR | 1/8/2025 |
| NCT04385654 | Phase 2 | 40 | Toripalimab plus axitinib as neoadjuvant therapy | MPR, pCR, pNR | 06/2022 |
| NCT04704219 | Phase 2 | 152 | First-line pembrolizumab plus lenvatinib in participants | ORR | 22/10/2025 |
| NCT04267120 | Phase 2 | 34 | Lenvatinib plus pembrolizumab | ORR, CR, PR | 31/7/2025 |
| NCT03635892 | Phase 2 | 97 | Nivolumab plus cabozaninib | ORR | 8/2022 |
| NCT02724878 | Phase 2 | 65 | Atezolizumab plus bevacizumab | ORR, CR, PR | 10/2023 |
| ICI-ICI | |||||
| NCT03177239 | Phase 2 | 85 | Single-agent nivolumab, then ipilimumab plus nivolumab | ORR | 12/2022 |
| NCT03075423 | Phase 2 | 306 | Nivolumab plus ipilimumab versus standard of care (SUNIFORECAST) | OS (at 12 months) | 31/12/2023 |
| OTHER TRIALS | |||||
| NCT04644432 | Phase 2 | 30 | Individualized treatment strategy for patients with metastatic non-clear cell renal cell carcinoma | ORR TTF | 6/9/2022 |
| NCT04413123 | Phase 2 | 40 | Cabozantinib plus nivolumab and Ipilimumab | ORR | 20/12/2022 |
| NCT04338269 | Phase 3 | 500 | Atezolizumab plus cabozantinib versus cabozantinib alone | PFS; OS | 11/12/2024 |
PRACTICAL CONSIDERATIONS FOR NCC RCC TREATMENT
Currently, standard targeted therapies for metastatic nccRCC tumors include cabozantinib [14], sunitinib [11–13], and levantinib plus everolimus combination [39]. Nivolumab and pembrolizumab are also possible therapeutic options. Cytotoxic treatment like platinum-based chemotherapy can be considered for patients with medullary cancer [4]. ICI based therapies achieve better responses in papillary tumors or in tumors with sarcomatoid/rhabdoid features. Combination regimens (either with ICI-ICI or with ICI-TKI combinations) are associated with better outcomes and should be considered in these histologies. Patients diagnosed with advanced nccRCC should be always offered a clinical trial, whenever available.
CONCLUSION
Immune checkpoint inhibitor-based therapy might be a valid option for the treatment of advanced RCC of non-clear-cell histology, but the level of evidence to support it is low. Combination therapy might increase the overall activity, but more trials with larger populations are needed to confirm these observations. In addition, the overall response rate varies widely across tumor subtypes, with papillary subtype and tumors with sarcomatoid features being associated with higher activity. Mixed responses have been observed with unclassified tumors while chromophobe tumors seem to be resistant to ICI therapy. Other tumors were included in insufficient numbers to understand their pattern of response. Biomarkers that adequately predict the efficacy of ICI based therapies among nccRCC are lacking and their inclusion in future trials is warranted.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Conception or design of the study
Ana Filipa Palma dos Reis, Pedro C. Barata
Acquisition, analysis, or interpretation of data
Ana Filipa Palma dos Reis, Diana Simão, Pedro C. Barata
Writing (original draft or revision for important intellectual content)
Ana Filipa Palma dos Reis, Diana Simão, Thomas Odeny, Chiara Rodrigues, Mário Fontes, Ricardo da Luz, Rajasree Pia Chowdry, Sarah J Welsh, Channing Paller, Pedro C. Barata
Supervision and/or final approval of the version to be submitted
Ana Filipa Palma dos Reis, Diana Simão, Thomas Odeny, Chiara Rodrigues, Mário Fontes, Ricardo da Luz, Rajasree Pia Chowdry, Sarah J Welsh, Channing Paller, Pedro C. Barata
All authors have had access to the data.
CONFLICT OF INTERESTS
Ana Filipa Palma dos Reis (has no conflict of interests to report), Diana Simão (has no conflict of interests to report), Thomas Odeny (has no conflict of interests to report), Chiara Rodrigues (has no conflict of interests to report), Mário Fontes (speaking role/advisory boards: Bristol Myers Squibb, Daiichi Sankyo, Gilead, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Servier), Ricardo da Luz (has no conflict of interests to report), Rajasree Pia Chowdry (has no conflict of interests to report), Sarah J Welsh (has no conflict of interests to report), Channing Paller (has no conflict of interests to report), Pedro C. Barata consultant (Institution): Astellas; Eisai; Janssen, EMD Serono; Dendreon; Pfizer, Seattle Genetics, BMS, Bayer, Guardant Health; contracted research (Institution): AstraZeneca, Merck; research grant (Institution): BlueEarth Diagnostics; speaker’s bureau (Institution): Bayer, Caris, Pfizer.
REFERENCES
[1] | Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. (2021) ;71: :7–33. https://doi.org/10.3322/caac.21654 |
[2] | Moch H , Cubilla AL , Humphrey PA , Reuter VE , Ulbright TM . The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. (2016) ;70: :93–105. https://doi.org/10.1016/j.eururo.2016.02.029 |
[3] | Article S . Renal cell carcinoma : ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. 2019:706-20. https://doi.org/10.1093/annonc/mdz056 |
[4] | Professionals S-O . EAU Guidelines: Renal Cell Carcinoma. Uroweb n.d. https://uroweb.org/guideline/renal-cellcarcinoma/ (accessed August 30, 2021). |
[5] | Motzer RJ , Escudier B , Mcdermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015:1803-13. https://doi.org/10.1056/NEJMoa1510665 |
[6] | Motzer R.J. , Tannir N.M. , McDermott D.F. , Arén Frontera O. , Melichar B. , Choueiri T.K. , Plimack E.R. , Barthélémy P. , Porta C. , George S. , Powles T. , Donskov F. , Neiman V. , Kollmannsberger C.K. , Salman P. , Gurney H. RH , Ravaud A. , Grimm M.-O. , Bracarda S. , Barrios C.H. , Tomita Y. , Castellano D. , Rini B.I. , Chen A.C. , Mekan S. , McHenry M.B. , Wind-Rotolo M. , Doan J. , Sharma P. , Hammers H.J. . Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma R.J. NEngl J Med. (2018) ;378: :1277–90. |
[7] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: :1103–15. https://doi.org/10.1056/NEJMoa1816047 |
[8] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: :1116–27. https://doi.org/10.1056/NEJMoa1816714 |
[9] | Choueiri TK , Powles T , Burotto M , Escudier B , Bourlon MT , Zurawski B , et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) ;384: :829–41. https://doi.org/10.1056/NEJMoa2026982 |
[10] | Grünwald V , Powles T , Choueiri TK , Hutson TE , Porta C , Eto M , et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: study design and rationale. Future Oncology. (2019) ;15: :929–41. https://doi.org/10.2217/fon-2018-0745 |
[11] | Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial - The Lancet Oncology n.d. https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(15)00515-X/fulltext (accessed August 30, 2021). |
[12] | Tannir NM , Jonasch E , Albiges L , Altinmakas E , Ng CS , Matin SF , et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol. (2016) ;69: :866–74. https://doi.org/10.1016/j.eururo.2015.10.049 |
[13] | Record-3: Phase II randomized trial comparing sequential first-line everolimus (EVE) and second-line sunitinib (SUN) versus first-line SUN and second-line EVE in patients with metastatic renal cell carcinoma (mRCC). | Journal of Clinical Oncology n.d. https://ascopubs.org/doi/abs/10.1200/jco.2013.31.15_suppl.4504 (accessed August 30, 2021). |
[14] | Testing Cabozantinib, Crizotinib, Savolitinib and Sunitinib in Kidney Cancer Which Has Progressed - Full Text View - ClinicalTrials.gov n.d. https://www.clinicaltrials.gov/ct2/show/NCT02761057 (accessed August 30, 2021). |
[15] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. https://doi.org/10.1136/bmj.n71 |
[16] | McGregor BA , McKay RR , Braun DA , Werner L , Gray K , Flaifel A , et al. Results of a Multicenter Phase II Study of Atezolizumab and Bevacizumab for PatientsWith Metastatic Renal Cell Carcinoma With Variant Histology and/or Sarcomatoid Features. JCO. (2020) ;38: :63–70. https://doi.org/10.1200/JCO.19.01882 |
[17] | Rini BI , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): amulticentre, open-label, phase 3, randomised controlled trial. The Lancet. (2404) ;393: :2404–15. https://doi.org/10.1016/S0140-6736(19)30723-8 |
[18] | Gupta R , Ornstein MC , Li H , Allman KD , Wood LS , Gilligan T , et al. Clinical Activity of Ipilimumab Plus Nivolumab in PatientsWith Metastatic Non–Clear Cell Renal Cell Carcinoma. Clinical Genitourinary Cancer. (2020) ;18: :429–35. https://doi.org/10.1016/j.clgc.2019.11.012 |
[19] | Tachibana H , Kondo T , Ishihara H , Fukuda H , Yoshida K , Takagi T , et al. Modest efficacy of nivolumab plus ipilimumab in patients with papillary renal cell carcinoma. Japanese Journal of Clinical Oncology. (2021) ;51: :646–53. https://doi.org/10.1093/jjco/hyaa229 |
[20] | McDermott DF , Lee J-L , Ziobro M , Suarez C , Langiewicz P , Matveev VB , et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as FirstLine Therapy in Patients With Advanced Non–Clear Cell Renal Cell Carcinoma. JCO. (2021) ;39: :1029–39. https://doi.org/10.1200/JCO.20.02365 |
[21] | Vogelzang NJ , Olsen MR , McFarlane JJ , Arrowsmith E , Bauer TM , Jain RK , et al. Safety and Efficacy of Nivolumab in PatientsWith Advanced Non–Clear Cell Renal Cell Carcinoma: Results From the Phase IIIb/IV CheckMate 374Study. Clinical Genitourinary Cancer. (2020) ;18: :461–468.e3. https://doi.org/10.1016/j.clgc.2020.05.006 |
[22] | Koshkin VS , Barata PC , Zhang T , George DJ , Atkins MB , Kelly WJ , et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunotherapy Cancer. (2018) ;6: :9. https://doi.org/10.1186/s40425-018-0319-9 |
[23] | Hinata N , Yonese J , Masui S , Nakai Y , Shirotake S , Tatsugami K , et al. A multicenter retrospective study of nivolumab monotherapy in previously treated metastatic renal cell carcinoma patients: interim analysis of Japanese real-world data. Int J Clin Oncol. (2020) ;25: :1533–42. https://doi.org/10.1007/s10147-020-01692-z |
[24] | McKay RR , Bossé D , Xie W , Wankowicz SAM , Flaifel A , Brandao R , et al. The Clinical Activity of PD 1/PD-L1 Inhibitors in Metastatic Non–Clear Cell Renal Cell Carcinoma. Cancer Immunol Res. (2018) ;6: :758–65. https://doi.org/10.1158/2326-6066.CIR-17-0475 |
[25] | Chahoud J , Msaouel P , Campbell MT , Bathala T , Xiao L , Gao J , et al. Nivolumab for the Treatment of Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma (nccRCC): A Single-Institutional Experience and Literature Meta-Analysis. The Oncol. (2020) ;25: :252–8. https://doi.org/10.1634/theoncologist.2019-0372 |
[26] | Barata P , Hatton W , Desai A , Koshkin V , Jaeger E , Manogue C , et al. Outcomes With First-Line PD-1/PD-L1 Inhibitor Monotherapy for Metastatic Renal Cell Carcinoma (mRCC): A Multi-Institutional Cohort. Front Oncol. (2020) ;10: :581189. https://doi.org/10.3389/fonc.2020.581189 |
[27] | de Vries-Brilland M , Gross-Goupil M , Seegers V , Boughalem E , Beuselinck B , Thibault C , et al. Are immune checkpoint inhibitors a valid option for papillary renal cell carcinoma? A multicentre retrospective study. European Journal of Cancer. (2020) ;136: :76–83. https://doi.org/10.1016/j.ejca.2020.02.019 |
[28] | Boilève A , Carlo MI , Barthélémy P , Oudard S , Borchiellini D , Voss MH , et al. Immune checkpoint inhibitors in MITF family translocation renal cell carcinomas and genetic correlates of exceptional responders. Journal for ImmunoTherapy of Cancer. (2018) ;6: :159. https://doi.org/10.1186/s40425-018-0482-z |
[29] | Outcomes of Patients with Renal Cell Carcinoma and Sarcomatoid Dedifferentiation Treated with Nephrectomy and Systemic Therapies: Comparison between the Cytokine and Targeted Therapy Eras - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/28411072/ (accessed September 5, 2021). |
[30] | Renal cell carcinoma with rhabdoid features - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/11023094/ (accessed November 10, 2021). |
[31] | Golshayan AR , George S , Heng DY , Elson P , Wood LS , Mekhail TM , et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. (2009) ;27: :235–41. https://doi.org/10.1200/JCO.2008.18.0000 |
[32] | Bakouny Z , Braun DA , Shukla SA , Pan W , Gao X , Hou Y , et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. (2021) ;12: :808. https://doi.org/10.1038/s41467-021-21068-9 |
[33] | Iacovelli R , Ciccarese C , Bria E , Bracarda S , Porta C , Procopio G , et al. Patients with sarcomatoid renal cell carcinoma - re-defining the first-line of treatment: A meta-analysis of randomised clinical trials with immune checkpoint inhibitors. Eur J Cancer. (2020) ;136: :195–203. https://doi.org/10.1016/j.ejca.2020.06.008 |
[34] | Outcomes Associated with First-Line anti-PD-1/ PD-L1 agents vs. Sunitinib in Patients with Sarcomatoid Renal Cell Carcinoma: A Systematic Review and Meta-Analysis n.d. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7072485/#B20-cancers-12-00408 (accessed September 25, 2021). |
[35] | Immune Checkpoint Inhibitors for the FirstLine Treatment of Patients With Sarcomatoid Renal Cell Carcinoma | PracticeUpdate n.d. https://www.practiceupdate.com/content/immune-checkpoint-inhibitors-for-the-first-line-treatment-of-patients-with-sarcomatoid-renal-cell-carcinoma/104123 (accessed September 25, 2021). |
[36] | Bakouny Z , Vokes N , Gao X , Nassar A , Abou Alaiwi S , Flippot R , et al. Efficacy of immune checkpoint inhibitors (ICI) and genomic characteriza-tion of sarcomatoid and/or rhabdoid (S/R) metastatic renal cell carcinoma (mRCC). JCO. (2019) ;37: :4514–4514. https://doi.org/10.1200/JCO.2019.37.15_suppl.4514 |
[37] | Park JJ , Kellezi O , Hamasha R , Ali A , Alva AS . Immunotherapy in metastatic sarcomatoid renal cell carcinoma: A single institution experience. Cancer Treat Res Commun. (2020) ;25: :100251. https://doi.org/10.1016/j.ctarc.2020.100251 |
[38] | Chahoud J , Msaouel P , Ross JA , McCormick BZ , Bathala TK , Gao J , et al. Outcomes of patients with metastatic renal cell carcinoma with sarcomatoid dedifferentiation to immune checkpoint inhibitors. Urol Oncol. (2021) ;39: :134.e9–134.e16. https://doi.org/10.1016/j.urolonc.2020.10.019 |
[39] | Hutson TE , Michaelson MD , Kuzel TM , Agarwal N , Molina AM , Hsieh JJ , et al. A phase II study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma (nccRCC). JCO. (2020) ;38: :685–685. https://doi.org/10.1200/JCO.2020.38.6_suppl.685 |




