Cost-Effectiveness of Immunotherapy Treatments for Renal Cell Carcinoma: A Systematic Review
Abstract
BACKGROUND:
Kidney cancer exerts significant disease burden in the United States and possesses a rapidly evolving treatment landscape. The expansion of novel systemic treatment approaches and the use of immunotherapy has been accompanied by increased costs over time. However, the cost-effectiveness of immunotherapy in renal cell carcinoma (RCC) has not been fully assessed. The current study presents a systematic review of cost-effectiveness studies of immunotherapy-based treatment in the context of RCC.
METHODS:
A literature search utilizing PubMed, Embase, Web of Science, and the Cochrane Library was undertaken to find articles related to the cost-effectiveness of immunotherapy treatment in renal cell carcinoma (RCC). The inclusion criteria for articles were as follows: English, published between 1983 and 2020 and evaluated cost-effectiveness in any of the currently approved immunotherapies for RCC. Exclusion criteria included being a review article, commentary or editorial, as well as possessing no specific cost-effectiveness evaluation or analysis relevant to the current review.
RESULTS:
The current review identified 23 studies, published between 2008 and 2020, across 9 different countries. The studies identified tended to focus on patients with locally advanced or metastatic RCC and examined the cost-effectiveness of immunotherapy across various lines of treatment (first-line treatment (n = 13), second-line treatment (n = 8), and first-line and beyond (n = 2). Eight studies examined the use of interferon-alpha (IFN-alpha), with some reports supporting the cost-effectiveness of these agents and an equal number of studies demonstrating the opposite, with sunitinib often demonstrating superior cost bases. The majority, fourteen studies, included the use of novel immune checkpoint inhibitors (nivolumab, ipilimumab, pembrolizumab), half of which found that checkpoint inhibitors were more cost-effective when compared to oral systemic therapies (sunitinib, everolimus, axitinib, pazopanib, and cabozantinib).
DISCUSSION:
Novel immune checkpoint inhibitors constituted the most frequently examined agents and were likely to be deemed cost-effective as compared to other treatments; although this often required higher willingness-to-pay (WTP) thresholds or healthcare systems that possessed more cost-constraints. These observations have clinical and health system applicability, with the ability to potentially reduce the cost of treatment for locally advanced or metastatic RCC.
BACKGROUND
Kidney cancer is a major disease burden in the United States. In 2021 alone, it is estimated that 76,080 new cases and 13,780 cancer-related deaths will occur, the majority of which will be classified as renal cell carcinoma (RCC) [1]. While the incidence of kidney cancer has increased at a rate of 0.5% per year among males, deaths are trending down overall due to the development of advanced diagnostics that enable earlier detection of disease as well as novel therapeutics. Localized disease is typically treated with resection for curative intent [2], however metastatic disease is considered incurable and typically requires lifelong systemic therapy.
RCC has been a pioneer in early utilization of immunotherapy approaches due to poor response to chemotherapy-based approaches. Therefore, utilization of immunotherapy based approaches such interferon-alpha (IFN-alpha) or interleukin-2 (IL-2) were the standard of care until canonical phase III clinical trials demonstrated activity of targeted oral therapeutics, known as tyrosine kinase inhibitors (TKIs) [3]. Today, novel immune checkpoint inhibitors, such as programmed cell death protein 1 (PD-1), programmed death ligand 1 (D-L1), and cytotoxic T lymphocyte associated protein 4 (CTLA-4) inhibitors are employed in the frontline setting across all risk categories alone or in combination with TKIs for patients with metastatic disease [4, 5].
The rapid expansion of available therapies has been accompanied by an increase in treatment cost over time, especially in patients with more advanced disease [6]. Due to the availability of multiple systemic treatment options, understanding the costs associated with treatment would be beneficial in order to minimize patient financial toxicity. Effective therapies are rarely compared head to head and choosing a particular treatment can be difficult for patients, physicians, and health systems [7].
The cost-effectiveness of immunotherapy approaches across tumor types has been evaluated extensively. However, analyzing cost-effectiveness of immunotherapy treatment in advanced RCC is useful to guide health systems on preferred treatment options. Cost-effectiveness, usually measured in incremental cost-effectiveness ratio (ICER = ratio of cost difference vs. clinical efficacy for two compared therapies), is often calculated using data from phase III studies and real world cohort data [8]. Using this calculation, a particular country’s health system might decide to offer certain treatments based on their willingness to pay (WTP) threshold. The current paper presents the results of a systematic review of cost-effectiveness studies of immunotherapy in the context of RCC.
METHODS
Data sources and searches
In order to obtain the most comprehensive results, a literature search utilizing PubMed, Embase, Web of Science, and the Cochrane Library was conducted to find articles related to the cost-effectiveness of therapies in renal cell carcinoma (RCC). Both keywords and index terms (MeSH and Emtree) to develop these searches. The concept of RCC was combined with concepts of selected drugs and surveillance, along with that of cost-effectiveness. The full search strategies for each database are included as a supplement. Besides the database searches, the gray literature was searched by reviewing conference abstracts downloaded from Embase, and by hand-searching the literature of our most relevant articles for additional references. Among all identified manuscripts, only studies employing immunotherapy in the review were included.
Study selection criteria & data extraction
The inclusion criteria for articles were as follows: English, published between 1983 and 2020, evaluated cost-effectiveness in any of the currently approved immunotherapies for RCC. Exclusion criteria included being a review article, commentary or editorial, as well as possessing no specific cost-effectiveness evaluation or analysis relevant to the current review. Three phases of review were undertaken, including title, abstract and then full-text review of identified articles. At each stage, a dual consensus was reached for each inclusion and exclusion, with five reviewers (EP, AB, DK, SZ, FW) independently reviewing and assessing articles. Based on inclusion and exclusion criteria and dual review consensus, a total of 23 articles met criteria for data extraction and review.
RESULTS
As presented in Fig. 1 and detailed in the subsequent tables, the current review included 23 studies published between 2008 and 2020. Studies reviewed aimed to evaluate the cost and cost-effectiveness of various immunotherapies in the treatment of advanced or metastatic RCC. The studies were performed in 9 different countries (Italy, Canada, United Kingdom, Singapore, United States, China, Sweden, Israel, & Spain) and were sponsored by government grants, national organizations, and private companies. Although not a specific inclusion criterion, the studies identified in this review focused on patients with mRCC or advanced RCC and examined the cost-effectiveness of immunotherapy across various lines of treatment. Included studies utilized four different methodological approaches in their analysis of the cost and cost-effectiveness of RCC treatment. Sensitivity analyses were not always performed, but when utilized, included probabilistic, deterministic, univariate/one-way sensitivity analyses. The study horizons ranged from 11 months to patients’ lifetime but was not always specified in the included papers.
Fig. 1
PRISMA Flow chart.
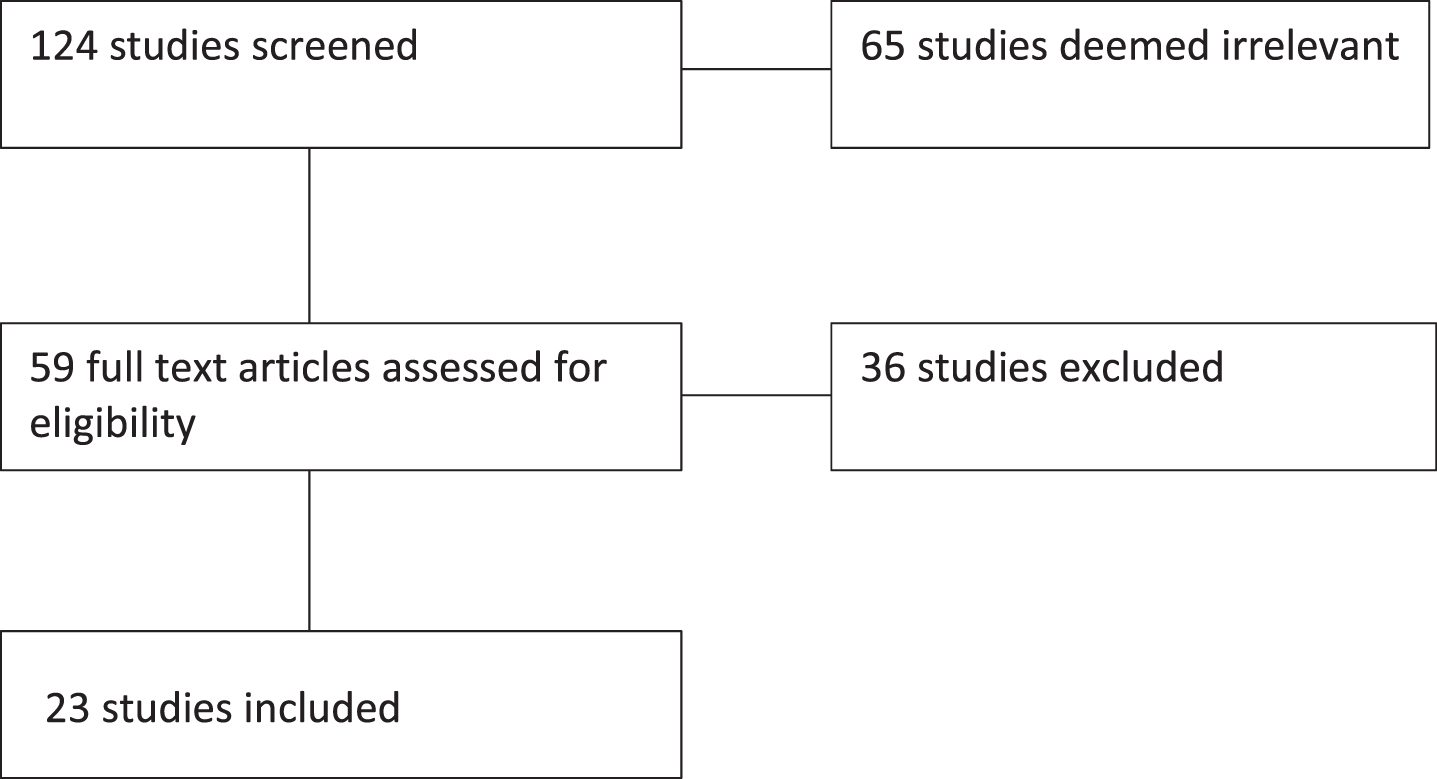
Table 1
Study Characteristics
| Author (Year) | Country | Study sponsorship | Patient population | Sample size | Clinical data source for analysis | Line of therapy | Methodological approach | Sensitivity analysis | Primary study objective |
| Remak (2008) [14] | US | Pfizer | mRCC* | N/A | Data drawn from previous clinical trials/studies | 1st | Cost-effectiveness analysis (CEA) and cost utility | Yes | To assess the cost-effectiveness and cost utility of sunitinib malate compared to IFN-α and IL-2 |
| Hoyle (2010) [15] | UK | Grant funded | mRCC or recurrent RCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To estimate the cost-effectiveness of temsirolimus compared to IFN-α |
| Ravasio (2011) [16] | Italy | Roche | Advanced or mRCC | 649/750 | Direct comparison of two clinical trials | 1st | CEA | Yes | To evaluate the incremental costs of bevacizumab + IFN-α versus sunitinib |
| Benedict (2011) [17] | US/Sweden | Pfizer | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To assess the economic value of sunitinib vs sorafenib in the US and bevacizumab plus IFN-α in the US and Sweden |
| Calvo Aller (2011) [18] | Spain | Pfizer | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To assess the economic value of sunitinib compared to sorafenib or bevacizumab + IFN-α |
| Kilonzo (2012) [19] | UK | Grant funded | Advanced or mRCC | N/A | Base Case economic model and Evidence Review Group (ERG) analysis | 1st | CEA | Yes | ERG analysis of pazopanib compared to sunitinib, IFN-α and supportive care |
| Wu (2012) [20] | China | Grant Funded | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | Comparison of five first-line strategies: sunitinib, bevacizumab + IFN-α, IFN-α, IL-2 and IL-2 plus IFN-α |
| Shi (2014) [21] | China | None/Unknown | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | No | To evaluate the clinical and economic consequences of sunitinib compared with sorafenib and IFN-α |
| Wan (2017) [10] | US/China | Grant funded | Advanced RCC | 1096 | Data drawn from single clinical trial | 2nd | CEA | Yes | This study evaluated the cost-effectiveness of nivolumab plus ipilimumab as compared to sunitinib |
| Meng (2018) [22] | England | Ipsen Pharma | Advanced RCC | N/A | Data drawn from previous clinical trials/studies | 2nd | CEA | Yes | To compare the cost-effectiveness of cabozantinib with everolimus |
| Swallow (2018) [23] | US | Novartis | mRCC | N/A | Data drawn from previous clinical trials/studies | 2nd and beyond | CEA | Yes | To compare the additional cost per month, overall survival and of progression-free survival associated with cabozantinib, nivolumab, and axitinib with everolimus |
| Sarfaty (2018) [9] | Israel/US | None/Unknown | Advanced RCC | N/A | Data drawn from previous clinical trials/studies | 2nd | CEA | Yes | To estimate the cost-effectiveness of nivolumab versus everolimus versus placebo |
| Raphael (2018) [24] | Canada | None/Unknown | mRCC | N/A | Data drawn from previous clinical trials/studies | 2nd | CEA | Yes | To compare the effectiveness and estimated lifetime costs associated with nivolumab compared to everolimus |
| Wu (2018) [25] | US, UK, and China | Grant funded | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To assess the cost-effectiveness of nivolumab plus ipilimumab compared to sunitinib |
| McCrea (2018) [26] | US | Bristol-Myers Squibb | Advanced or mRCC | N/A | Data drawn from previous clinical trials/studies | 2nd and beyond | CEA | Yes | To evaluate the cost-effectiveness of nivolumab compared to everolimus |
| Giuliani (2019) [27] | Italy | None/Unknown | mRCC | N/A | Data drawn from previous clinical trials/studies | 2nd | Cost per PFS and OS | No | To assess the pharmacologic costs of everolimus, axitinib, nivolumab, and cabozantinib |
| Deniz (2019) [28] | US | Bristol-Myers Squibb | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st and 2nd | CEA | Yes | To evaluate the economic impact of treatment sequences: pazopanib or sunitinib as first-line treatment, followed by nivolumab, cabozantinib, axitinib, pazopanib or everolimus |
| Chen (2019) [29] | China | Grant funded | Advanced RCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To evaluate the cost-effectiveness of pembrolizumab plus axitinib as compared to sunitinib |
| Pruis (2019) [30] | Singapore | None/Unknown | Advanced or mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To evaluate the cost-effectiveness of sunitinib as compared to IFN-α |
| Wan (2019) [31] | US | Grant Funded | mRCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To evaluate the cost-effectiveness of nivolumab plus ipilimumab as compared to sunitinib |
| Giuliani (2018) [32] | Italy | None/Unknown | mRCC, | N/A | Data drawn from previous clinical trials/studies | 2nd | Cost per PFS and OS | No | To assess the pharmacologic costs associated with treatment in trials involving everolimus, axitinib, sorafenib, nivolumab and temsirolimus |
| Reinhorn (2019) [33] | US | None/Unknown | Advanced RCC | N/A | Data drawn from previous clinical trials/studies | 1st | CEA | Yes | To estimate the cost-effectiveness of nivolumab and ipilimumab as compared with sunitinib |
| Ambavane (2020) [34] | US | Bristol-Myers Squibb and ONO Pharmaceutical Company Ltd. | Advanced RCC | N/A | Data drawn from previous clinical trials/studies | 1st and beyond | CEA | Yes | To assess the cost-effectiveness of nivolumab + ipilimumab-initiated sequences as compared to tyrosine kinase inhibitor-initiated sequences |
*mRCC = metastatic renal cell carcinoma, †ICER = incremental cost-effectiveness ratio, ‡ICUR = incremental cost-utility ratio, §QALY = quality-adjusted life-year, ∥ IFN-α= interferon-alpha, ¶IL-2 = interleukin-2.
As noted, there were four methodological approached employed in the reviewed studies: (1) Cost-Effectiveness based on Clinical Trials, (2) Cost-Effectiveness based on Hypothetical Cohorts from Clinical Trials, (3) Cost per progression-free-survival and overall-survival, and (4) Base Case Economic Model and Evidence Review Group (ERG) Analysis. Our review primarily focused on quality adjusted life years (QALY), incremental cost-effectiveness ratio (ICER), and willingness-to-pay (WTP) thresholds to determine the cost-effectiveness of each therapy. Given that four studies did not include these specific measures, we used costs associated with therapy as another proxy to assess its cost-effectiveness for these studies.
Eight of the twenty-three studies included examined the use of IFN-alpha (Table 2a). Four of these studies showed IFN-alpha to be the more cost-effective option when compared to prior standards of care (IL2, temsirolimus and sunitinib). In contrast, the remaining four reported IFN-alpha to be the less cost-effective option when compared with sunitinib and pazopanib. Two studies included the use of cytokine therapy (IL-2); one of which reported cytokine therapy to be more cost-effective when compared with sunitinib or bevacizumab, while the second noted cytokine therapy to be less cost-effective as compared to IFN-alpha or sunitinib.
Table 2a
Summary of cost analyses for IL-2 & IFN-α
| Author | Intervention | Intervention dose | Comparator | Comparator dose | Line of therapy | Study horizon | Intervention cost | Comparator cost | QALY/ICER† | Key findings |
| IL-2, IFN-α | ||||||||||
| Remak (2008) | Sunitinib malate | 50 mg oral daily for 4 weeks, followed by 2 weeks off-treatment in 6-week cycles | IFN-α and IL-2 | Subcutaneous injection 3 nonconsecutive days each week | 1st | 10 years | $224,970 | $217,436 | •Incremental cost per progression-free year gained = $18,611 | •Both IFN-α and sunitinib treatments dominate IL-2 treatment with an ICUR per QALY gained at a 5% discount rate. |
| •ICER = $67,215 per LY gained | ||||||||||
| •ICUR = $52,593 per QALY gained | ||||||||||
| Hoyle (2010) | Temsirolimus | 25 mg IV once per week | IFN-α | 3MU subcutaneous three times per week in the first week The dose was raised to 9MU three times per week in the second week and 18MU three times per week in week 3 if this dose was tolerated. | 1st | 10 years | £28,849 | £ 6,519 | •For temsirolimus compared to IFN-α we estimate an incremental gain of 0.24 QALYs, at an additional cost of £22,331 per patient, giving an | •ICERs do not support the use of temsirolimus when considered against published guidance. •Treatment with temsirolimus may not be regarded as a cost-effective use of resources in some health care settings |
| •ICER =£94,632 per QALY for general patient group | ||||||||||
| •ICER =£154,752 per QALY for the patient subgroups | ||||||||||
| Ravasio (2011) | Bevacizumab + IFN-α | Bevacizumab (10 mg/kg) IV + IFN-α (9, 6 or 3 million IU) subcutaneous | Sunitinib | 50 mg oral | 1st | 11 months | 9 MIU € 32065.99 | € 34118.46 | •Unknown | •Treatment with bevacizumab + IFN-α was associated with cost savings in comparison to sunitinib, regardless of dosage of IFN-α used. |
| Benedict (2011) | Bevacizumab + IFN-α | Bevacizumab 10 mg/kg IV every 2wks + IFN-α 3 MU in the first week, 6 MU in the second week and 9 MU subcutaneously thereafter | Sunitinib, Sorafenib | Sunitinib (full dose –50 mg/day or 37.5 mg/day oral, 4wks on + 2wks off for full dose or [reduced dose - 37.5 mg/day for 4 weeks followed by 2 weeks off treatment]) Sorafenib (400 mg oral twice daily) | 1st | Lifetime | Bevacizumab + IFN-alpha , 437,144.46 | Sunitinib ($369,346.80) Sorafenib ($382,922.67) | US QALY | •Sunitinib was dominant in both US and Sweden. |
| •Sunitinib = 1.876 | ||||||||||
| •Sorafenib = 1.706 | ||||||||||
| •Bevacizumab + IFN-α= 1.714 Sweden QAL | ||||||||||
| •Sunitinib = 1.862 | ||||||||||
| •Sorafenib = N/A | ||||||||||
| •Bevacizumab + IFN-α= 1.703 | ||||||||||
| CalvoAller (2011) | Sunitinib | 50 mg oral daily, 4 weeks on, 2 weeks off dosing schedule | Sorafenib, Bevacizumab/IFN-α | Sorafenib at 400–600 mg oral twice daily | 1st | 10 years | € 118,417 | Sorafenib (€ 119,541) | •Incremental net bene-fit (INB) = € 9,717 compared with SFN | •Sunitinib is a cost-effective alternative to other targeted therapies as first-line mRCC therapy in the Spanish healthcare setting. |
| Bevacizumab/IFN-α at 10 mg/kg every 2 weeks | Bevacizumab/IFN-α (€ 141,634) | •INB = € 31,211 compared with BEV/IFN-α | ||||||||
| Kilonzo (2012) | Pazopanib | Unknown | Sunitinib, IFN-α, Supportive Care | Unknown | 1st | None | Unknown | £36,301 | ICER =£38,925 | •Pazopanib is recommended as first line treatment for advanced renal cell carcinoma who have not received prior cytokine therapy due to its potential cost-effectiveness with manufacturer discount and future rebate. |
| Wu (2012) | 1 Bevacizumab + IFN-α 2 IFN-α 3 IL-2 + IFN-α 4 Sunitinib | Bevacizumab 10 mg/kg or placebo (IV) every 2 weeks. | IL-2 | IL-2 (IV) as 18 MU6body-surface area (m2) daily for 5 days, once every 3 weeks. | 1st | 10 years | Bevacizumab + IFN-α ($178,864.96) | IL-2 ($27.441.92) | ICER without sunitinib patient assistant program (SPAP) compared to IL2: | ICERs of sunitinib and bevacizumab plus IFN-α compared to cytokine therapies are far greater than the societal willingness-to-pay ($13,290 Yuan for China). |
| IFN-α subcutaneous injection 3 times per week in first cycle at 3 MU/dose in the first week, 6 MU/dose in the second week, and 9 MU/dose thereafter. The subsequent cycles involved three 9-MU/dose injections. Sunitinib (oral) at 50 mg once daily for 4 weeks, followed by 2 weeks off treatment. | IFN-α ($32,620.30) | •Bevacizumab + IFN-α= 1,021,196.49 | ||||||||
| IL-2 + IFN-alpha ($35.623.41) Sunitinib ($95,978.35) | •IFN-α= 177,724.92 | |||||||||
| •IL-2 + IFN-α= 5,872,545.72 | ||||||||||
| •Sunitinib = 220,384.01 | ||||||||||
| •ICER with SPAP compared to IL2: | ||||||||||
| •Bevacizumab + IFN-α= 1,024,876.36 | ||||||||||
| •IFN-α= 152,038.42 | ||||||||||
| •IL-2 + IFN-α= 5,478,038.63 | ||||||||||
| •Sunitinib = 16,992.29 | ||||||||||
| •Traditional cytokine therapy is the cost-effective option in the Chinese healthcare setting. •In some relatively developed regions, sunitinib with SPAP may be a favorable cost-effective alternative for mRCC | ||||||||||
| Pruis (2019) | Sunitinib | 50 mg and 37.5 mg oral | IFN-α | IFN-α dose not stated | 1st | 10 years | Progression free state: SG$83,890 (US$61,364) | Progression free state: SG$13,776 (US$10,077) | •ICER = SGD191,061 (USD139,757) per QALY | •In the absence of any price reduction, sunitinib had an exceedingly high ICER and was not considered a cost-effective use of healthcare resources in Singapore’s context for the first-line treatment of advanced RCC. |
| Post-progression state: SG$72,976 (US$53,380) | Post-progression state: SG$83,164 (US$60,833) | |||||||||
| Shi (2014) | Sunitinib | Unknown | Sorafenib, IFN-α | Unknown | 1st | 5 years | RMB 217,038.50 | Unknown | •Incremental cost per progression free life year between sunitinib and IFN-α was –RMB78,562.10 and RMB 22,501.03 between sunitinib and sorafenib. | •Sunitinib was dominant compared to IFN-α. •Sunitinib was cost effective compared to sorafenib based on the threshold recommended by the World Health Organization. |
| •The incremental cost per life year between sunitinib and IFN-α was –RMB168,633.00 and RMB 21,022.38 between sunitinib and sorafenib. | ||||||||||
| •The incremental cost per QALY between sunitinib and IFN-α was –RMB184,825.00 and RMB 29,493.42 between sunitinib and sorafenib. |
Fourteen studies included the use of novel immune checkpoint inhibitors (nivolumab, ipilimumab, pembrolizumab) (Table 2b). Seven of these studies found that checkpoint inhibitors were more cost-effective when compared to oral TKIs (sunitinib, everolimus, axitinib, pazopanib, and cabozantinib), while three studies found that they were less cost-effective when compared with cabozantinib and everolimus. Two of the fourteen studies found that the cost-effectiveness of these agents was dependent on the WTP threshold employed, though in general, if WTP was greater than $150,000, immune checkpoint inhibitors were the more cost-effective option when compared to everolimus. A single study showed that immune checkpoint inhibitors were more cost-effective in China than the US when compared to everolimus. Finally, one study reported inconclusive findings regarding the cost-effectiveness of nivolumab compared with everolimus. Among the 14 studies examining novel immune checkpoint agents, they were relatively evenly split between those examining cost in a first-line setting as compared to those in the second-line setting. Overall, the results suggested that regional differences and consequent disparities in pricing may be an important factor in determining the benefit of first- versus second-line treatment with immune checkpoint inhibitors.
Table 2b
Summary of Cost Analyses for novel immune-checkpoint inhibitor (PD-1, PD-L1, and CTLA-4 inhibitors)
| Author | Intervention | Intervention dose | Comparator | Comparator dose | Line of therapy | Study horizon | Intervention cost | Comparator cost | QALY/ICER† | Key findings |
| PD-1 inhibitors, PD-L1 inhibitors, CTLA-4 inhibitors | ||||||||||
| Wan (2017) | Nivolumab | 240 mg IV every 2 weeks | Everolimus | 10 mg daily oral | 1st | 20 years | •US: Nivolumab $25.62/mg •China: Nivolumab $7.90/mg –$9.70/mg | •US: Everolimus $27.41/mg •China: $5.43/mg | In the United States, nivolumab provided an additional 0.29 QALYs at a cost of. | •In the US, nivolumab is unlikely to be a high-value treatment for mRCC at its current price. •In China, value-based prices for nivolumab should inform multilateral drug-price negotiations given the lack of official cost-effectiveness thresholds. |
| •ICER = $145,940/LY | ||||||||||
| •ICER = $151,676/QALY | ||||||||||
| When nivolumab cost $9.02 and $10.58/mg in China, the ICERs approximated the WTP thresholds of $22,785 and $48,838/QALY, respectively | ||||||||||
| When nivolumab cost $22.50/mg in the United States, the ICER approximated the WTP threshold of $100,000/QALY | ||||||||||
| Meng (2018) | Standard of care (everolimus, axitinib, nivolumab) | Everolimus (10mg) oral | Cabozantinib | 20/40/60 formulation (mg) oral | 2nd | 30 years | £4800 cost per cycle | Cost per cycle: | The health gains were 2.26 life-years (LYs) and 1.78 quality adjusted LYs (QALYs). The incremental cost-effectiveness ratios (ICERs) versus axitinib and everolimus were 98,967 £/QALY and 137,450 £/QALY, respectively. Cabozantinib was less costly and more effective than nivolumab (dominant); the incremental cost was –£6,742 GBP and the QALY difference was 0.18 | •Cabozantinib was associated with higher total costs despite being more effective than treatment with axitinib or everolimus. •Cabozantinib has nominally better efficacy and lower costs compared to ivolumab. |
| Axitinib (5 mg) oral Nivolumab (40 mg) IV | Everolimus (£2093.41), axitinib (£3587.34), nivolumab (£5146.15) | |||||||||
| Swallow (2018) | Everolimus with cabozantinib, nivolumab or axitinib | Cabozantinib (60 mg oral daily) oral Nivolumab (3 mg/kg IV infusion over 1 hour, every 2 weeks) Axitinib (5 mg oral twice daily) | Everolimus | 10 mg oral daily | 2nd and beyond | 1- and 2-year horizons | $61,955 | Total treatment costs: | None/Unknown | •Everolimus for second-line mRCC was associated with lower costs and longer overall survival than axitinib. •Everolimus was associated with lower costs than cabozantinib and nivolumab. |
| Cabozantinib ($99,458), nivolumab ($77,173), axitinib ($72,382). | ||||||||||
| The additional cost per month of overall survival (OS) was $48,773 for cabozantinib and $24,214 for nivolumab versus everolimus. | ||||||||||
| Over 2 years, the additional cost per treated patient compared with everolimus was $59,147 for cabozantinib, $54,799 for nivolumab, and $29,229 for axitinib, with an additional cost per patient $36,967 for cabozantinib and $23,826 for nivolumab for each additional month of OS. | ||||||||||
| Sarfaty (2018) | Nivolumab | 3 mg/kg IV every 3 weeks | Everolimus | 10 mg daily oral | 2nd | 10 years | $14611/month, $101,070 total | $9631/month, $50,935 total | •ICER = $146,532/QALY vs. everolimus •ICER = $226,197/QALY over placebo | •Nivolumab is not cost-effective compared to everolimus with a WTP threshold of $100,000/QALY |
| •At WTP threshold of $140,000/QALY, there is a 90% chance that it is cost effective. | ||||||||||
| Raphael (2018) | Nivolumab | 3 mg/kg IV every 2 weeks | Everolimus | 10 mg daily orally | 2nd | Lifetime | CA$8604.44 per 28-day course with wastage | CA$5503.4 per 28-day course | •The ICER for nivolumab treatment was CA$8138/QALM gained with 3% discounting. Nivolumab increased the quality-adjusted life expectancy by 4.2 QALMs over the lifetime of mRCC patients in Ontario at a net cost of CA$92,414 | •At its current price and a WTP of $50,000/QALY, nivolumab is unlikely to be a cost-effective option in the treatment of mRCC from a Canadian health care perspective. |
| •Nivolumab becomes cost-effective at a threshold of $100,000/QALY. | ||||||||||
| Wu (2018) | Nivolumab + ipilimumab | Nivolumab (3 mg/kg) IV + ipilimumab (1 mg/kg) IV, every 3 weeks for four doses (induction phase), followed by nivolumab monotherapy (3 mg/kg) every 2 weeks (maintenance phase) | Sunitinib | 50 mg/day oral | 1st | 10 years | Nivolumab (100mg): $2670 (US), $1426 (UK), $1362 (China) Ipilimumab (50 mg): $7324 (US), $4875 (UK), $4655(China) | Sunitinib (50mg): $601.9 (US), $145.7 (UK), $275.2 (China) | Nivolumab + ipilimumab was associated with a gain of 0.70–0.76 QALYs compared with sunitinib. The following ICERs for nivolumab + ipilimumab over sunitinib in first-line advanced RCC treatment were found: US $85,506 /QALY; UK $126,499/QALY; and China $4682/QALY. | •A combination of nivolumab and ipilimumab could be associated with benefit as first-line treatment for patients with advanced RCC when compared to sunitinib. |
| •Regional differences may exist given the differences in pricing. | ||||||||||
| McCrea (2018) | Nivolumab | Nivolumab 3 mg/kg IV every 2 weeks | Everolimus | 10 mg/day orally | 2nd and beyond | 25 years | $197,089 | $163,902 | •ICUR = $US51,714 per QALY gained versus everolimus, •ICER = $US44,576 per life-years gained (LYG) versus everolimus | •At a WTP threshold of $US150,000 per QALY, nivolumab was found to be cost-effective. |
| •Key drivers of cost-effectiveness were survival inputs for OS and the average weight of patients, the latter directly affects nivolumab drug acquisition cost. | ||||||||||
| Giuliani (2019) | Axitinib, nivolumab, cabozantinib | Axitinib (5 mg twice daily) oral | Everolimus | 10 mg daily oral | 2nd | None | $3812/month | Costs per month of therapy: axitinib $3315, sorafenib $3221, temsirolimus $3920, nivolumab $6450, cabozantinib $2920 (at all doses) | None | •Combining pharmacologic costs of drugs with the measure of efficacy represented by OS, cabozantinib is a cost-effective second-line treatment for patients with mRCC |
| Sorafenib (400 mg twice daily) oral | ||||||||||
| Temsirolimus (25 mg every week) IV | ||||||||||
| Nivolumab (240 mg every 2 weeks) IV | ||||||||||
| Cabozantinib (60 mg, 40 mg, 20 mg, no scheduled dosing) oral | ||||||||||
| Deniz (2019) | First line pazopanib or sunitinib followed by second line treatment with nivolumab | First line: sunitinib (50 mg oral daily, 4 weeks on, 2 weeks off) or pazopanib (800 mg oral daily) followed by nivolumab (240 mg IV every 2 weeks) | First line pazopanib or sunitinib followed by second line treatment with cabozantinib, axitinib, pazopanib or everolimus | First line: sunitinib (50 mg oral daily, 4 weeks on, 2 weeks off) or pazopanib (800 mg oral daily) followed by either pazopanib (800 mg oral daily), everolimus (10 mg oral daily), axitinib (5 mg oral twice daily) or cabozantinib (60 mg oral daily) | 1st and 2nd | 25 years | 1st line: sunitinib ($11,302/month), pazopanib ($10,886/month) 2nd line: nivolumab ($13,349/month) | 1st line: sunitinib ($11,302/month), pazopanib ($10,886/month). | Sunitinib + pazopanib was dominant, while sunitinib + nivolumab was $73,927/LY. Pazopanib + axitinib was dominant, while pazopanib + nivolumab was $49,591/LY. All other regimens were dominated | •Treatment sequences using nivolumab in the second-line setting are less costly compared with sequential use of targeted agents. |
| 2nd line: pazopanib ($10,886/month) | ||||||||||
| everolimus ($14,005/month) axitinib ($13,357/month) | ||||||||||
| cabozantinib ($15,391/month), | ||||||||||
| Chen (2019) | Pembrolizu-mab + axitinib | Pembrolizumab (IV 200 mg once every 3 weeks for a maximum of 35 doses), axitinib (5 mg oral twice daily) | Sunitinib | 50mg/daily oral for first 4 weeks of each 6-week cycle | 1st | Lifetime | Drug costs per cycle for pembrolizumab was $10659.77, $2586.11 for axitinib and $2581.95 for sunitinib | Sunitinib $2581.95 | Pembrolizumab + axitinib was associated with 2.461 additional life years and 1.650 QALYs. Total cost = $178,725, with incremental ICER = $55,185/QALY. Sunitinib: Total cost = $87,693. ICER remained greater than $32,000/QLY across all patient subgroups. | •Pembrolizumab + axitinib is unlikely to be cost effective versus sunitinib for patients with previously untreated advanced RCC at threshold value of $29,306/QALY. |
| Wan (2019) | Nivolumab + ipilimumab | Nivolumab 3 mg/kg*70kg IV every 3 weeks for 4 doses. | Sunitinib | Sunitinib 50 mg/d oral for 4 weeks followed by 2 weeks off treatment | 1st | Lifetime | $350,646 | $246,573 | The use of nivolumab plus ipilimumab cost an additional $104 072, resulting in an ICER of $82 035 per life-year, or $108 363 per QALY compared with sunitinib | •Nivolumab plus ipilimumab would be considered cost-effective at a willingness-to-pay threshold of $100,000 to $150,000 per QALY. |
| Ipilimumab 1 mg/kg*70 kg IV every 3 weeks for 4 doses | ||||||||||
| Giuliani (2019) | Nivolumab | Nivolumab 3 mg/kg IV | Temsirolimus, sorafenib, axitinib, everolimus | Temsirolimus (25 mg 1x/week) IV | 2nd | PFS and OS | Nivolumab € 4512 per month | Temsirolimus € 3920, sorafenib € 3221, axitinib € 3315, everolimus € 3812 (all per month) | None reported | •Nivolumab was a cost-effective second-line treatment for patients with mRCC in terms of OS with a € 1772 difference in cost per month of OS gained, compared to everolimus (€ 28590 difference in cost per month of OS gained) |
| Sorafenib (400 mg oral twice daily) | ||||||||||
| Axitinib (5 mg oral twice daily) | ||||||||||
| Everolimus 10 mg oral once daily | ||||||||||
| Reinhorn (2019) | Nivolumab + ipilimumab | Nivolumab 3 mg/kg IV and ipilimumab 1 mg/kg IV every 3 weeks for four doses followed by nivolumab 3 mg/kg IV every 2 weeks until progression | Sunitinib | Sunitinib 50 mg oral once daily, in 6 week cycles of 4 weeks on and 2 weeks off | 1st | 10 years | $292,308 | $169,287 | ICER: $125,739/QALY | •Base case ICER in the model for nivolumab and ipilimumab versus sunitinib is below the upper limit of the theoretical WTP threshold in the U.S. ($150,000/QALY) and is thus estimated to be cost effective. |
| Ambavane (2020) | Nivolumab + ipilimumab-initiated sequences | Recommended dose and dosing frequency based on FDA labels and clinical trials | Tyrosine Kinase Inhibitor-initiated sequences | Recommended dose and dosing frequency based on FDA labels and clinical trials | 1st and beyond | 40 years | First line drug costs: Nivolumab + ipilimumab: $32,485 (induction), $13,887 (maintenance) | First line drug Costs: Cabozantinib: $18,633 Sunitinib: $12,950 Pazopanib: $13, 269 | ICER calculated as incremental costs per QALY gained were lowest for nivolumab + ipilimumab followed by axitinib, pazopanib, and cabozantinib sequences ($66,357, $72,927, and $73, 237 respectively). | •The estimated average QALYs gained was the highest on nivolumab + ipilimum-ab-initiated sequences versus TKI-initiated sequences. |
| •Incremental cost per QALY gained for nivolumab + ipilimum-ab-initiated sequences was below the willingness-to-pay threshold of $150,000 versus other sequences | ||||||||||
Of the 23 studies included, thirteen examined first-line therapy, eight focused on second-line therapy, and two studies examined first-line therapy and beyond. The drugs most commonly used in first line therapy included sunitinib (12 studies), IFN-alpha (8 studies), and immune checkpoint inhibitors (4 studies). Across studies, the agents most commonly used in second-line treatment included immune checkpoint inhibitors (8 studies), everolimus (8 studies), and axitinib (3 studies). Finally, agents examined in studies of first-line therapy and beyond included sunitinib, nivolumab, pazopanib, axitinib, and cabozantinib.
DISCUSSION
The current study was a systematic review of a global literature to identify studies examining the cost and cost-effectiveness of immunotherapy agents in the treatment of RCC. As newer therapeutic agents and combination regimens gain approval in this space, it is critical that the economic consequences of treatment decision-making be examined more fully. This review identified 23 studies across nine countries that met inclusion criteria and reported on the cost or cost-effectiveness of an immunotherapy agent in the treatment of RCC. These high-level findings provide insight into the relative economic advantages of these newer therapeutic agents and can help guide the refinement of treatment guidelines and shared decision making among various stakeholders in the RCC field.
The majority of studies focused on the costs associated with immune checkpoint inhibitors, including nivolumab, ipilimumab and pembrolizumab, with most suggesting that these agents were more cost-effective when compared to oral TKIs sunitinib, everolimus, axitinib, pazopanib or cabozantinib. Importantly however, the region of study and WTP threshold were critical contributors to the eventual cost-effectiveness assessment, with these agents often requiring a higher WTP threshold (e.g. [9]) or a non-US based healthcare system (e.g. [10]). As shown in Table 2b, immune checkpoint inhibitors were compared to oral TKIs and observed to demonstrate a gain in QALYs. Interestingly, these data demonstrated that regional differences in benefit of first- versus second-line treatment with immune checkpoint inhibitors may occur due to differences in pricing.
There was no clear consensus among those studies that examined the costs associated with IL-2 & IFN-α, with some reports supporting the cost-effectiveness of these agents and an equal number of studies demonstrating the opposite, with sunitinib often demonstrating superior cost bases. These results make it difficult to draw firm conclusions, and may once again suggest that the geographic location, and subsequent healthcare system, in which a study is conducted may play a role in guiding such results.
Implications for healthcare systems
Cost-effective analyses (CEA) aim to promote health equity by prioritizing distributive benefits for the entire health system [11]. Observations from this systematic review suggest that immunotherapy approaches are more cost-effective, however, may require higher WTP thresholds. While standard CEA assume interventions are independent [12, 13], this assumption may not hold valid for low-income countries that require alteration of health delivery platforms in order to dispense certain agents. This is especially relevant when an analysis includes an intravenous versus orally administered medication. Therefore, as healthcare systems determine the applicability of a CEA, understanding the interdependence of the intervention with other aspects of health care delivery will be required.
Strengths & limitations
The current study possesses several strengths, including the systematic nature of the review dual review of studies at each stage of analysis, and the global nature of the studies included in the final analysis. This review also possesses limitations that should be acknowledged; including restricting analysis to studies published in English and to those examining immunotherapy agents. It is possible that further studies exist that could provide insight into the cost-effectiveness and contribution of other aspects of RCC treatment, including surgical interventions, and thus warrants further research.
CONCLUSIONS
To our knowledge, this is the first contemporary systematic review examining literature of cost-effectiveness of immunotherapy approaches in RCC treatment, regardless of treatment setting. Immune checkpoint inhibitors constituted the most frequently examined agents and were likely to be deemed cost-effective as compared to other agents; although this often required higher WTP thresholds or healthcare systems that possessed more cost-constraints. Regional differences in pricing may lead to differences in measured benefit of use of immune checkpoint inhibitors in first- or second-line setting for advanced RCC. These observations have clinical and health system applicability, with the ability to potentially reduce cost of treatment for advanced RCC.
FUNDING
HB is funded by Prostate Cancer Foundation and Lazarex Cancer Foundation.
AUTHOR CONTRIBUTIONS
Study conception: EP, HB. Study review: EP, AB, DK, SZ, FW. Interpretation: EP, AB, DK, SZ, FW, HB. Manuscript development: EP, AB, DK, SZ, FW, HB. Manuscript editing and review: EP, AB, DK, SZ, FW, HB.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer statistics, CA Cancer J Clin ((2021) ;71: (1):7–33. |
[2] | National Comprehensive Cancer Network. Nccn clinical practice guidelines in oncology: Kidney cancer. National Comprehensive Cancer Network; 2020. |
[3] | Motzer RJ , Hutson TE , Tomczak P , et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. (2007) ;356: (2):115–24. |
[4] | Rini BI , Plimack ER , Stus V , et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019. |
[5] | Motzer RJ , Tannir NM , McDermott DF , et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. (2018) ;378: (14):1277–90. |
[6] | Kale HP , Mays DP , Nadpara PA , Slattum PW , Paul AK , Carroll NV . Economic burden of renal cell carcinoma among older adults in the targeted therapy era. Urol Oncol. (2019) ;37: (6):356.e19–.e28. |
[7] | Parmar A , Sander B , Bjarnason GA , Chan KKW . Systemic therapy in metastatic renal cell carcinoma: Emerging challenges in therapeutic choice. Crit Rev Oncol Hematol. (2020) ;152: :102971. |
[8] | Crespo C , Monleon A , Díaz W , Ríos M . Comparative efficiency research (comer): Meta-analysis of cost-effectiveness studies. BMC Med Res Methodol. (2014) ;14: :139. |
[9] | Sarfaty M , Leshno M , Gordon N , et al. Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol. (2018) ;73: (4):628–34. |
[10] | Wan XM , Peng LB , Ma JA , Li YJ . Economic evaluation of nivolumab as a second-line treatment for advanced renal cell carcinoma from us and chinese perspectives. Cancer. (2017) ;123: (14):2634–41. |
[11] | Cookson R , Mirelman AJ , Griffin S , et al. Using cost-effectiveness analysis to address health equity concerns. Value Health. (2017) ;20: (2):206–12. |
[12] | Hauck K , Morton A , Chalkidou K , et al. How can we evaluate the cost-effectiveness of health system strengthening? A typology and illustrations. Soc Sci Med. (2019) ;220: :141–9. |
[13] | World Health O, Baltussen RMPM , Adam T , et al. Making choices in health :Who guide to cost-effectiveness analysis / edited by t. Tan-torres edejer... [et al]. Geneva: World Health Organization; 2003. |
[14] | Remák E , Charbonneau C , Négrier S , Kim ST , Motzer RJ . Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol. (2008) ;26: (24):3995–4000. |
[15] | Hoyle M , Green C , Thompson-Coon J , et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health.. (2010) ;13: (1):61–8. |
[16] | Ravasio R , Ortega C , Sabbatini R , Porta C . Bevacizumab plus interferon-α versus sunitinib for first-line treatment of renal cell carcinoma in italy: A cost-minimization analysis. Clin Drug Investig. (2011) ;31: (7):507–17. |
[17] | Benedict A , Figlin RA , Sandström P , et al. Economic evaluation of new targeted therapies for the first-line treatment of patients with metastatic renal cell carcinoma. BJU Int. (2011) ;108: (5):665–72. |
[18] | Calvo Aller E , Maroto P , Kreif N , et al. Cost-effectiveness evaluation of sunitinib as first-line targeted therapy for metastatic renal cell carcinoma in spain. Clin Transl Oncol. (2011) ;13: (12):869–77. |
[19] | Kilonzo M , Hislop J , Elders A , et al. Pazopanib for the first-line treatment of patients with advanced and/or metastatic renal cell carcinoma: A nice single technology appraisal. Pharmacoeconomics. (2013) ;31: (1):15–24. |
[20] | Wu B , Dong B , Xu Y , et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: A cost-effectiveness analysis in a health resource-limited setting. PLoS One.e. (2012) ;7: (3):32530. |
[21] | Shi Q , Yin H , Xuan J , Wu Y , Cheng G . Cost effectiveness of sunitinib as first-line targeted therapy for metastatic renal cell carcinoma in china. Value Health.A. (2014) ;17: (7):638. |
[22] | Meng J , Lister J , Vataire AL , Casciano R , Dinet J . Cost-effectiveness comparison of cabozantinib with everolimus, axitinib, and nivolumab in the treatment of advanced renal cell carcinoma following the failure of prior therapy in england. Clinicoecon Outcomes Res. (2018) ;10: :243–50. |
[23] | Swallow E , Messali A , Ghate S , McDonald E , Duchesneau E , Perez JR , The additional costs per month of progression-free survival and overall survival: An economic model comparing everolimus with cabozantinib, nivolumab, and axitinib for second-line treatment of metastatic renal cell carcinoma. J Manag Care Spec Pharm. (2018) ;24: (4):335–43. |
[24] | Raphael J , Sun Z , Bjarnason GA , Helou J , Sander B , Naimark DM . Nivolumab in the treatment of metastatic renal cell carcinoma: A cost-utility analysis. Am J Clin Oncol. (2018) ;41: (12):1235–42. |
[25] | Wu B , Zhang Q , Sun J . Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. (2018) ;6: (1):124. |
[26] | McCrea C , Johal S , Yang S , Doan J . Cost-effectiveness of nivolumab in patients with advanced renal cell carcinoma treated in the united states. Exp Hematol Oncol. (2018) ;7: :4. |
[27] | Giuliani J , Bonetti A . Cost-effectiveness of second-line treatments for metastatic renal-cell carcinoma. Clin Genitourin Cancer. (2019) ;17: (2):e258–e62. |
[28] | Deniz B , Ambavane A , Yang S , et al. Treatment sequences for advanced renal cell carcinoma: A health economic assessment. PLoS One. (2019) ;14: (8):e0215761. |
[29] | Chen J , Hu G , Chen Z , et al. Cost-effectiveness analysis of pembrolizumab plus axitinib versus sunitinib in first-line advanced renal cell carcinoma in china. Clin Drug Investig. (2019) ;39: (10):931–8. |
[30] | Pruis SL , Aziz MIA , Pearce F , Tan MH , Wu DB , Ng K . Cost-effectiveness analysis of sunitinib versus interferon-alfa for first-line treatment of advanced and/or metastatic renal cell carcinoma in singapore. Int J Technol Assess Health Care. (2019) ;35: (2):126–33. |
[31] | Wan X , Zhang Y , Tan C , Zeng X , Peng L . First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: A cost-effectiveness analysis. JAMA Oncol. (2019) ;5: (4):491–6. |
[32] | Giuliani J , Bonetti A . Nivolumab is a cost-effective second-line treatment for metastatic renal-cell carcinoma. Clin Genitourin Cancer. (2018) ;16: (3):e557–e62. |
[33] | Reinhorn D , Sarfaty M , Leshno M , et al. A cost-effectiveness analysis of nivolumab and ipilimumab versus sunitinib in first-line intermediate- to poor-risk advanced renal cell carcinoma. Oncologist. (2019) ;24: (3):366–71. |
[34] | Ambavane A , Yang S , Atkins MB , et al. Clinical and economic outcomes of treatment sequences for intermediate- to poor-risk advanced renal cell carcinoma. Immunotherapy. (2020) ;12: (1):37–51. |




