Adjuvant Therapy in Renal Cell Carcinoma: Current Status and Future Directions
Abstract
In recent years, incredible progress has been made in the treatment of metastatic renal cell carcinoma, with a paradigm shift from the use of cytokines to tyrosine kinase inhibitors, and more recently, immune checkpoint inhibitors (ICIs). Despite advances in the metastatic setting, effective therapies in the adjuvant setting are a largely unmet need. Currently, sunitinib (Sutent, Pfizer) is the only therapy for the adjuvant treatment of RCC included in the National Comprehensive Cancer Network guidelines, which was approved by the FDA based on the improvement in disease-free survival (DFS) seen in the S-TRAC trial. However, improvement in DFS has not translated into an overall survival (OS) benefit for patients at high-risk of relapse post-nephrectomy, illustrating the need for more effective therapies. This manuscript will highlight attributes of both historical and current drug trials and their implications on the landscape of adjuvant therapy. Additionally, we will outline strategies for selecting patients in whom treatment would be most beneficial, as optimal patient selection is a crucial step towards improving outcomes in the adjuvant setting. This is especially critical, given the financial cost and pharmacological toxicity of therapeutic agents. Furthermore, we will review the design of clinical trials including the value of utilizing OS as an endpoint over DFS. Finally, we will discuss how the incorporation of genomic data into predictive models, the use of more sensitive imaging modalities for more accurate staging, and more extensive surgical intervention involving lymph node dissection, may impact outcomes.
INTRODUCTION
Renal cell carcinoma (RCC) is estimated to ac-count for 73,750 cases of new cancer diagnoses and 14,830 cancer deaths in the United States in 2020 [1]. Of these, clear cell RCC (ccRCC) is the predominant histological subtype, accounting for approximately 85% of cases. Non-clear cell RCC (non-ccRCC) comprises the remaining 15% of RCC tumors, of which a significant proportion include papillary (pRCC) or chromophobe (chRCC) subtypes. Incidence of early stage RCC has risen in recent decades due to incidental detection secondary to the increased use of computed tomography scans in practice [2, 3]. From 2004–2015, stage I diagnoses proportionally increased and accounted for approximately 70% of cases, while stage III and IV diagnoses trended downwards to 8% and 11% respectively [4].
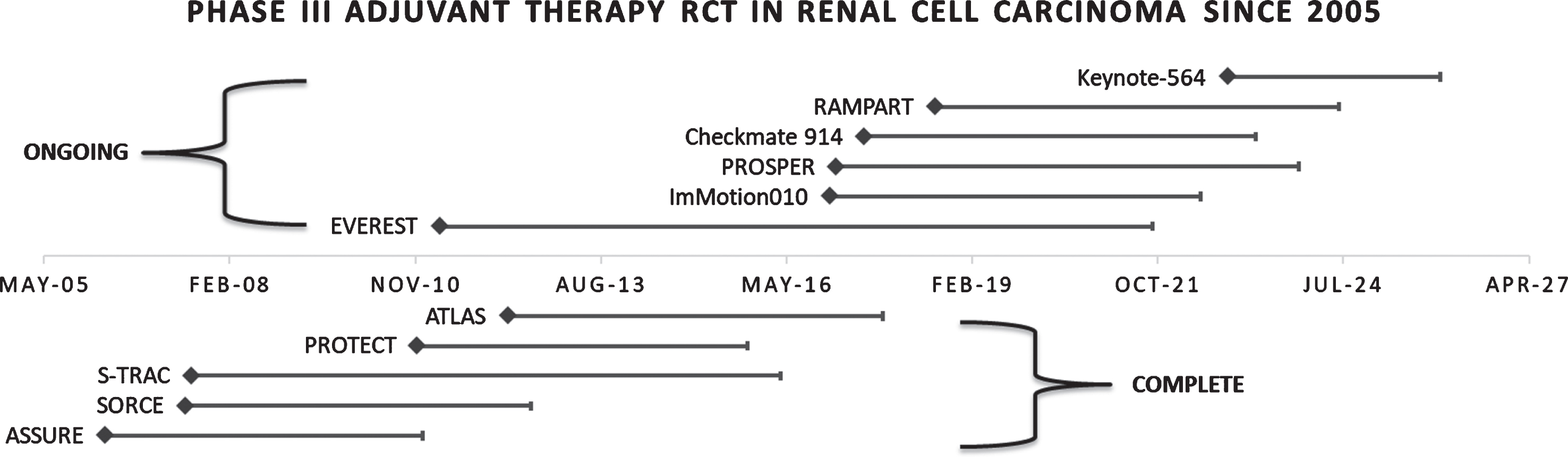
Despite statistically significant improvement in early detection, improvement in survival for stage I-III tumors has not been achieved over this time period. Nephrectomy or ablation can potentially offer patients a cure in early localized disease, however, in stage II and III RCC the risk of recurrence is significant, with 20–40% of patients experiencing a local or distant recurrence [5]. On the other hand, a statistically significant improvement in overall survival (OS) for stage IV disease has been noted, likely secondary to the major advancements in systemic therapy for mRCC [4]. Therapeutic options for mRCC have rapidly progressed from cytokines to targeted therapies with tyrosine kinase inhibitors (TKIs), with a current focus on immune checkpoint inhibitors (ICIs), and combinations thereof. The majority of trial modalities in the adjuvant setting have mirrored those in mRCC (Fig. 1). Understanding key aspects of these trials, reasons for which they may have failed, and future directions in systemic therapy is vital in improving OS within the adjuvant setting. Of note, these agents have varying levels of both pharmacologic and financial toxicities. Subjecting patients at a lower risk of relapse to these treatments must be avoided. Therefore, optimal identification of both high-risk patients, and effective adjuvant therapies remain among the great challenges of the field.
Fig. 1
Phase III trials completed and ongoing in renal cell carcinoma since the start of the tyrosine kinase era. Abbreviations: RCT, randomized clinical trial.
CYTOKINE THERAPY IN THE ADJUVANT SETTING
Initial experimental studies prior to 1990 explored cytotoxic, hormonal, and radiation therapy in mRCC with minimal and unsustained responses [6]. Soon thereafter, it was hypothesized that cytokine therapy could stimulate an immune response against cancer cells [7]. Interferon alpha (IFN-α) and high dose interleukin-2 (IL-2) quickly emerged as front runners, with the latter achieving a 5% sustained complete response in mRCC, a new milestone at the time [8, 9]. Given this response, IL-2 became an option for monotherapy, while IFN-α was utilized as an adjunctive therapy. Due to their success with mRCC, it was presumed these agents may provide benefit, leading to their exploration via formalized trials within the adjuvant setting [10–12].
Overall, across a constellation of trials, IFN-α and IL-2 did not provide a significant improvement in disease free survival (DFS) or OS. Moreover, they frequently resulted in severe side effects [7, 10]. Pizzocaro et al. investigated adjuvant IFN-α versus placebo in 247 patients with Robson stage II and III RCC, the majority of which were T3a/bN0. This study did not find a significant benefit in five-year DFS or OS [13]. The majority of treated patients developed toxicity, and over a quarter required dose suspension or reduction. Notably, a small number (n = 26) of pN2/3 patients had a statistically significant reduction in recurrence rate in the experimental arm, observed on univariate analysis. Subsequently, a study by Messing et al. failed to show a statistically significant benefit of adjuvant IFN-α after 10.4 years of follow-up in 283 patients with advanced pT3–4 RCC. Clark et al. conducted a phase III prospective trial exploring adjuvant bolus high dose IL-2 in locally advanced, pT3b-4 or N1-3, or metastatic patients following complete resection of disease prior to enrollment. The study was terminated early due to failure to meet its primary endpoint of 30% improvement in DFS [11]. At interim analysis, there was no difference in DFS or OS observed in the overall cohort, nor in the locally advanced subgroup. The Italian Oncology Group for Clinical Research explored combination therapy with low-dose IL-2 and IFN-α in 303 patients with all histologic subtypes and surgically resectable stages of RCC, which failed to show a statistically significant benefit in recurrence free survival (RFS) or OS after five years [7].The German Cooperative Renal Carcinoma Chemoimmunotherapy Group investigated high dose IL-2, IFN-α, and 5-fluorouracil combination versus observation in the adjuvant setting for 203 patients with locally advanced, pT3b/c-4N0, TXN1-2, or completely resected relapsed or solitary metastatic disease [10]. The trial showed no difference after 4.3 years of follow-up in both the overall cohort and in subset analysis with regard to DFS. The intervention cohort experienced significantly decreased survival as compared with the control arm. Another European phase III trial explored this regimen in 309 patients with T3b/c, T4, or TXN1-2 and again found no significant difference in DFS or OS after 7 years of follow-up [14]. Ultimately the cytokine approach has been largely abandoned in RCC, as drugs with more efficacy, improved safety, and tolerability have come to the forefront.
TARGETED THERAPY IN THE ADJUVANT SETTING
As the biology and tumor microenvironment of various cancers came to be better understood in the early 2000s, several molecules were developed with the aim of disrupting the molecular functions that facilitate tumor growth and invasion. Small molecules, antibodies, hormones, and interfering RNA (iRNA) are examples of the class of medications known as targeted therapy [15]. Amongst the small molecule compounds are the tyrosine kinase inhibitors (TKIs). Mutations in the Von Hippel-Lindau (VHL) gene are implicated in the majority of ccRCC tumors [16, 17]. The VHL tumor-suppressor protein is a component of the E3 ubiquitin ligase complex which under normal conditions promotes the degradation of hypoxia inducible factor 1-α (HIF1α), a transcriptional regulator of the angiogenesis pathway. HIF1-α promotes the activation of vascular endothelial growth factor (VEGF) and drives platelet derived growth factor (PDGF) production. Therefore, mutations in this pathway often lead to aberrantly persistent angiogenesis and tumorigenesis. TKIs that specifically inhibit VEGF were developed and came to be known as VEGF-TKIs [18]. The mammalian target of rapamycin (mTOR), a cell proliferation pathway frequently hyperactive in RCC, was also a subject of targeted therapy, with everolimus and temsirolimus eventually gaining FDA approval in mRCC [19].
Early stage trials established the efficacy of VEGF-TKIs, but revealed the potential toxicities seen with this class of medications. Common side effects associated with this class include hypertension, diarrhea, fatigue, mucosal inflammation, and palmar–plantar erythrodysesthesia also known as hand-foot syndrome [20]. A phase III trial comparing sunitinib with IFN-α in 750 mRCC patients showed a significant benefit in DFS and OS [21]. Further studies using VEGF inhibitors including pazopanib and axitinib were also approved for mRCC based on significant DFS [22, 23]. Although a number of trials attempted to utilize VEGF-TKIs, and mTOR inhibitors in the adjuvant setting, results have been far less fruitful.
Within the realm of targeted therapy, only S-TRAC (sunitinib vs placebo) has shown improvement in DFS, and thereby has been approved for treatment in the adjuvant setting, despite its increased incidence of grade >3 adverse effects [21]. The results of ASSURE (sunitinib vs sorafenib vs placebo), PROTECT (pazopanib vs placebo), SORCE (sorafenib vs placebo), and ATLAS (axitinib vs placebo) suggest no improvement in DFS with adjuvant therapy [19, 22–24].
The randomized phase III trial ASSURE (Sunitib Malate or Sorafenib Tosylate in Treating Patients with Kidney Cancer that was Removed by Surgery) launched soon after regulatory approval of sunitinib and sorafenib for mRCC, and sought to explore these targeted therapies in the high-risk adjuvant setting [25]. 1943 patients, of any histological subtype, were randomized 1:1:1 to receive sunitinib 50 mg daily, sorafenib 800 mg daily, or placebo. Ultimately, no significant difference was observed in DFS between treatment groups. However, there was greater toxicity seen in the treatment arms, with a significantly greater incidence of hypertension and hand-foot syndrome. Discontinuation due to adverse events or patient withdrawal occurred in 44% and 45% of participants in the sunitinib and sorafenib arms respectively, compared to 11% on placebo. This led to a protocol amendment for dose reductions from planned levels. Subsequently, a subgroup analysis found no difference in DFS regardless of dosage. The ASSURE trial included variable histology, with 20% of patients having non-ccRCC. Yet, no difference was observed in ccRCC or non-ccRCC cohorts. Despite the negative outcome seen in this trial, two subsequent studies exploring each agent individually in the adjuvant setting were launched: S-TRAC (A Clinical Trial Comparing Efficacy And Safety of Sunitinib Versus Placebo For the Treatment of Patients at High Risk of Recurrent Renal Cell Cancer) for sunitinib and SORCE (Sorafenib in Treating Patients at Risk of Relapse After Undergoing Surgery to Remove Kidney Cancer) for sorafenib [26].
In contrast to the ASSURE trial, the S-TRAC trial enrolled patients with only clear cell histology who were defined as high risk per the UCLA integrated staging system (UISS) [26]. A total of 615 patients were enrolled and randomized to receive sunitinib or placebo. Results demonstrated a significant DFS benefit with sunitinib over placebo (6.8 versus 5.6 years, (95% CI 5.8-not reached) (95% CI 3.8–6.6) respectively, p = 0.03). This was the first and only positive trial investigating TKI agents in the adjuvant setting. A significant proportion of patients required dose reductions or interruptions: 34.3% and 46.4% with sunitinib versus 2.0% and 13.2% with placebo, respectively. The observed benefit in DFS held true across various subgroup analyses. However, longer follow-up has shown that improvement in DFS did not correlate to a difference in OS benefit between sunitinib or placebo (median OS not reached at 6.6 versus 6.7 years respectively, (HR 0.92, 95% CI 0.66–1.28; p = 0.6) [26].
The S-TRAC results were particularly interesting given the lack of DFS benefit identified for sunitinib in the ASSURE trial [25, 26]. The subtle differences between both study populations were scrutinized due to their divergent outcomes. It was hypothesized that this may be due to the selection of a high-risk ccRCC cohort in the S-TRAC trial. The ASSURE authors investigated this question by analyzing the high-risk clear cell RCC cohort in a subset analysis; however, there remained no significant difference in either DFS or OS [25]. The reasons for these differing results are still not completely understood and continue to be controversial.
The SORCE trial recruited 1711 intermediate or high-risk patients, as determined by Leibovich classification of 3–11, who were randomized 2:3:3 to placebo, one year of sorafenib followed by two years of placebo, or three years of sorafenib [24]. The study included both ccRCC and non-ccRCC histology. In the most recently reported efficacy analysis of placebo versus three years of sorafenib, no difference was observed in DFS or OS for the overall, high-risk only, or clear cell RCC only cohorts [24]. As with other trials investigating targeted therapy, a significant proportion of patients experienced side effects, leading to amendment of the trial protocol to reduce the dosage of sorafenib. Despite dose reductions, over half of patients stopped treatment early and nearly a quarter experienced grade three hand-foot syndrome.
Given the mixed results of other VEGF-TKI trials in this setting, the PROTECT (A Study to Evaluate Pazopanib as an Adjuvant Treatment for Localized Renal Cell Carcinoma) trial sought to compare pazopanib versus placebo following partial or radical nephrectomy in patients at high risk for recurrence [23]. The study allowed only ccRCC patients who were pT2N0M0: pT3-4 of any grade N0M0 or any pTN1M0. Comparatively, the risk of recurrence in the PROTECT study was between that of the S-TRAC and ASSURE study populations [23, 25, 26]. The starting dosage of pazopanib was reduced from 800 mg to 600 mg because a safety review revealed unexpectedly high rates of drug discontinuation due to adverse effects [23]. The primary endpoint was DFS of pazopanib 600 mg. OS and PFS of the 800 mg dosage were assessed as secondary endpoints. The protocol allowed for increase of dosage to 800 mg after 8–12 weeks if tolerated. Primary analysis was done following 350 DFS events, which demonstrated no significant difference in DFS between the 600 mg trial arm and placebo. However, a 33.7% reduction in risk of recurrence or death was observed for those receiving 800 mg dosage (n = 189 patients), with median DFS not yet reached in the experimental group, and 54.0 months in the placebo group (p = .008). Investigators cautioned that the difference in DFS between the 600 mg and 800 mg groups may be attributable to superior DFS in the placebo arm of the former as compared to the latter. Notably, patients with higher plasma trough levels of pazopanib tended towards longer DFS. OS at the time of DFS analysis did not significantly differ at either dose level.
The ATLAS trial, another trial restricted to ccRCC patients, explored the novel TKI axitinib versus placebo in 724 patients with advanced disease with similar inclusion criteria as the PROTECT trial [22, 23]. ATLAS was discontinued due to futility, failing to show a difference in the primary endpoint of DFS [22]. A subgroup analysis was conducted evaluating low-risk versus high-risk patients. Although, a DFS benefit was seen with investigator assessed outcomes in the high-risk group (HR = 0.641, p = 0.0051), this benefit was insignificant per independent review facility assessment (HR 0.735, p = 0.0704). Survival data were not mature at the time of analysis. While the incidence of adverse events was similar between treatment and placebo groups, increased grade 3-4 events and discontinuation due to adverse events were noted in the experimental group. It is notable that the majority (73%) of the study population were of Asian heritage, which may have impacted its applicability to other patient groups.
Additionally, adjuvant VEGF-TKI therapy has been explored in the related population of patients with limited metastatic disease undergoing radical nephrectomy and metastasectomy. E2810, a phase III trial investigating 800 mg pazopanib for one year in patients with no evidence of disease following metastasectomy, failed to find a benefit in DFS, with limited data supporting a trend of worse OS in those receiving pazopanib [27]. Similarly, the phase II RESORT trial compared RFS in patients randomized to 800 mg sorafenib versus observation for one year of planned treatment. After five years resulting in 69 accrued patients, the trial was closed early and analysis failed to reveal a benefit in RFS between groups. The RESORT trial also reported toxicity data consistent with other VEGF-TKI trials, revealing an approximately eight-fold increase in grade 3 toxicities in the sorafenib arm of the study [28].
Trials exploring mTOR inhibitors in the adjuvant setting are lacking. The ongoing EVEREST trial (NCT01120249) is seeking to shed light on this approach [19]. This trial is exploring one year of adjuvant everolimus versus placebo of the same duration, in patients with any histological RCC subtypes. This study includes patients at high and intermediate risk of recurrence. The primary endpoint is DFS. A safety study has been reported that suggests a continued trend of treatment limiting toxicity in targeted therapy, as 39% of patients on experimental therapy have prematurely discontinued treatment. Medication trough levels were also measured and did correlate with the development of adverse events. Results regarding DFS are not yet mature, but when available will likely determine if any further exploration of this therapeutic class as adjuvant therapy is warranted.
Trials of targeted therapy in RCC have generally failed to show a statistical or clinical benefit for patients (Table 1). The use of these agents is frequently accompanied by the high cost of toxicity. The S-TRAC study remains an outlier amongst this group of trials and its application in clinical practice is unclear, a fact reflected in governing guidelines. The National Comprehensive Cancer Network (NCCN) recommends adjuvant sunitinib with a category 2B designation, indicating lower-level evidence and a lack of uniform consensus [29]. The European Association of Urology does not recommend adjuvant sunitinib, based on high quality systematic review and homogeneity of randomized clinical trials (level of evidence 1A) [30]. Furthermore, this therapy has not been approved by the European Medicines Agency in the adjuvant setting. A systematic review and pooled analysis of ASSURE, S-TRAC, and PROTECT supported general trends, with no consistent effect on DFS or OS of adjuvant VEGF therapy despite a significant incidence of adverse events [31]. The pooled analysis showed that out of every ten patients treated with VEGF therapy, only one was likely to experience improvement in DFS. In conclusion, while TKI therapy may continue to play a role in the management of metastatic disease, current evidence suggests that observation may better serve patients over TKI therapy in the adjuvant setting. However, the newest class of therapy in RCC, immune checkpoint inhibitors, may offer benefit in the adjuvant space.
Table 1
Randomized clinical trials investigating adjuvant tyrosine kinase inhibitors to in renal cell carcinoma
| Trial | Experimental Therapy | Therapy Duration (years) | Completion | Enrollment | Histology | Endpoint | Statistically Significant? | Eligibility |
| ASSURE | sunitinib or sorafenib | 1 | 2010 | 1943 | Any | DFS | No* | ≥T1bNXM0 |
| SORCE | sorafenib | 3 | 2012 | 1656 | Any | DFS | No | Leibovich 3–11 |
| S-TRAC | sunitinib | 1 | 2016 | 674 | ccRCC | DFS | Yes | High-risk per UISS |
| PROTECT | pazopanib | 1 | 2016 | 1538 | ccRCC | DFS | No† | pT2, pT3-4N0, or node positive disease |
| ATLAS | axitinib | 3 | 2017 | 592 | ccRCC | DFS | No | pT2, pT3-4N0, or node positive disease |
| EVEREST | everolimus | 1 | Estimated 2021 | 1545 | Any | DFS | TBD | Intermediate (pT2-3aN0) or high (pT3a-4N0-1) risk |
*Subset analysis of high risk ccRCC patients similar to S-TRAC failed to show DFS difference. †Subset analysis of patients receiving 800 mg dose showed a significant DFS benefit. Abbreviations: ccRCC, clear cell renal cell carcinoma; DFS, disease-free survival; UISS, UCLA integrated scoring system; TBD, to be determined.
THE IMMUNE CHECKPOINT ERA
Immune checkpoint inhibitors have truly transformed therapy across a number of solid tumors, most notably in RCC. Not unlike the rationale behind the cytokine era, checkpoint inhibition again involves modulation of the immune system to fight cancer. These agents block signals which perpetuate downregulation of T-cell action and prevent normal antitumor functions [32]. Two sets of checkpoint inhibitor interactions are targeted by these monoclonal antibody agents: programmed death protein 1 (PD-1) with its ligand (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) with its ligand (CD80/86). Agents in this class have rapidly progressed from investigational to first line therapy in mRCC, displaying both increased efficacy and improved side effect profiles than previous targeted agents [33–35]. Ipilimumab (Yervoy, Bristol-Meyers Squibb, a CTLA-4 inhibitor) and nivolumab (Opdivo, Bristol-Meyers Squibb, a PD-1 inhibitor) combination therapy is a preferred first line regimen in the poor prognostic group in mRCC, as well as in subsequent therapy, with a category 1 recommendation for both scenarios [29]. As with previous generations of therapies, the natural question of efficacy in the adjuvant space has emerged. Five phase III randomized clinical trials are currently exploring this very question (Table 2).
Table 2
Randomized clinical trials planned to explore checkpoint inhibitors in renal cell carcinoma
| Trial | Therapy | Inhibitor Type(s) | Therapy Duration (years) | Completion | Planned Enrollment | Histology | Endpoint |
| ImMotion010* | Atezolizumab | PD-L1 | 1 | 2022 | 664 | Any | DFS |
| Keynote-564* | Pembolizumab | PD-1 | 1 | 2022 | 950 | ccRCC | DFS |
| PROSPER* | Nivolumab | PD-1 | 0.75† | 2023 | 805 | Any | EFS |
| Checkmate 914 | Nivolumab; Nivolumab + Ipilimumab | PD-1; PD-1 + CTLA-4 | 2 | 2023 | 1600 | ccRCC | DFS |
| RAMPART | Durvalumab; Durvolumab + Tremelimumab | PD-L1; PD-L1 + CTLA-4 | 1 | 2024 | 1750 | Any | DFS; OS |
*Metastasctomy patients eligible. †Patients receive one dose neoadjuvant and 9 doses adjuvant. Abbreviations: PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ccRCC, clear cell renal cell carcinoma; DFS, disease-free survival; OS, overall survival; event-free survival, EFS.
ImMotion 010 (A Study of Atezolizumab as Adjuvant Therapy in Participants With RCC at High Risk of Developing Metastasis Following Nephrectomy) is a large trial of atezolizumab (Tecentriq, Genentech, a PD-L1 inhibitor), versus placebo, with an accrual goal of over 750 patients [36]. Eligible patients include those at high risk for recurrence following radical or partial nephrectomy or following metastasectomy without residual disease on imaging following surgery. Primary endpoint is DFS by independent review, with secondary endpoints including OS, and DFS of patients with ≥1% PD-L1 expression. Results are expected in 2022.
Keynote-564 (Safety and Efficacy Study of Pemb-rolizumab as Monotherapy in the Adjuvant Treatment of Renal Cell Carcinoma Post Nephrectomy) is investigating the potential of pembrolizumab (Keytruda, Merck, a PD-1 inhibitor), in patients with intermediate-high risk or high-risk clear cell histology, sarcomatoid features and without evidence of disease following nephrectomy [37]. Those with a few soft tissue metastatic lesions would be allowed if lesions can be removed with a synchronous or metasynchronous metastasectomy (at the time of, or one year following nephrectomy, respectively). Approximately 950 patients will be randomized to one year of pembrolizumab or placebo. Patients are stratified into intermediate or high risk, based on TNM and Fuhrman grade criteria, or completely resected metastatic disease. DFS by investigator assessment will serve as the primary endpoint. OS, safety and tolerability will be included amongst the secondary endpoints. Reported results anticipated in 2022.
PROSPER (Nivolumab in Treating Patients With Localized Kidney Cancer Undergoing Nephrectomy) is a phase 3 unblinded study of nivolumab in the perioperative setting versus nephrectomy followed by observation [38]. Patients in the experimental arm will undergo one neoadjuvant nivolumab dose, followed by nine cycles of adjuvant nivolumab followed by observation (a prior version of the protocol stated 2 neoadjuvant doses, and up to 12 cycles of adjuvant therapy). The PROSPER neoadjuvant-adjuvant combination protocol is unique among contemporary trials in kidney cancer. While the rationale for pure adjuvant therapy is to eliminate or halt the growth of residual tumor cells following surgery, the rationale behind the neoadjuvant dosing is to prime T-cells against the primary tumor in an environment rich with neoantigens [39]. Theoretically, natural immunity would thus be heightened following operation, which, in combination with further adjuvant therapy, may lead to robust clearance of residual micrometastases and a maintained immune memory for tumor antigens. Planned enrollment is 805 patients and any histology may be included. Primary endpoint is event free survival, defined as recurrence or death from any cause up to 10 years, with primary data analysis estimated for 2023.
RAMPART (Renal Adjuvant MultiPle Arm Randomised Trial) is a multi-arm trial of 1 year of observation following surgery (arm A) versus single agent durvalumab (Imfinzi, AstraZeneca, a PD-L1 inhibitor) (arm B) versus doublet therapy with durvalumab with tremelimumab (Optune, Novocure, a CTLA-4 inhibitor) (arm C) [40]. Primary endpoints are DFS and OS for arms B and C versus arm A. Patients with Leibovich score 3–11 (indicating intermediate to high risk of recurrence following nephrectomy) of any histology are eligible [40]. Notably, those with an intermediate Leibovich score of 3–5 will be enrolled up to three years of the start of the trial or until this group represents 25% of the study population. Recruitment target is 1750 patients and with results expected in 2024.
CheckMate 914 (A Study Comparing the Combination of Nivolumab and Ipilimumab Versus Placebo in Participants With Localized Renal Cell Carcinoma) is investigating nivolumab alone or combination nivolumab and ipilimumab, compared to placebo [34]. Randomization in part A of the trial will be 1:1 nivolumab+ipilimumab to placebo for 2 years. Part B randomization will be 1:1:2, with nivolumab plus ipilimumab placebo serving as the third arm. Eligible patients include those at high risk for relapse following nephrectomy. The aforementioned interventions are planned for 24 months or first DFS event, treatment limiting toxicity, or consent withdrawal. Only patients with predominantly clear cell histology are eligible. This study has a planned enrollment of 1600 with anticipated completion in 2023. Primary and secondary endpoints are DFS and OS by independent review, respectively.
The next generation of adjuvant trials are positioned to answer a number of novel questions, in addition to exploring the role of adjuvant checkpoint inhibitors. ImMotion010, Keynote-564, and PROSPER all allow enrollment of patients who are status post metastasectomy, a previously unexplored area for adjuvant therapy [36–38]. ImMotion010, PROSPER, and RAMPART have generous histology inclusion criteria, allowing for subgroup analysis of adjuvant therapy in selected non-ccRCC cohorts [36, 38, 40]. Checkmate 914 and RAMPART incorporate experimental arms with dual CTLA-4 and PD blockade, a combination with previously demonstrated efficacy in advanced RCC [34, 40]. With the structure of these trials, monotherapy and combination therapy can be directly compared.
NOVEL THERAPEUTIC APPROACHES
Parallel to the evolution of classical generations of cytokine, targeted, and checkpoint inhibitor therapy, novel approaches have been tested. A number of vaccines have been explored as adjuvant therapy for RCC. Autologous tumor cells incubated with Bacillus Calmette-Guerin (BCG) twice-injected after surgery was compared to placebo without statistically significant differences in DFS or OS at 5 years [41]. Another study explored an autologous vaccine complexly created from patient lymph tissue incubated in interferon gamma and injected in six doses adjuvant to surgery. It showed an improvement in DFS over placebo after five years of follow-up (p = 0.02) [42]. This trial has been subject to a number of criticisms, however, including the proportion of ccRCC patients being higher in the experimental group, as well as trial conduction parameters. Given the cost and complexity of the treatment, this vaccine treatment has been abandoned [40]. Another vaccine based on heat-shock protein (glycoprotein-96)-peptide complex, HSPPP-96, was investigated in a phase III trial which showed no difference in RFS [43]. Although promising in a phase II trial of mRCC, the autologous RNA-modulated dendritic cell vaccine AGS-003 phase III trial (NCT01582672) in mRCC was terminated due to lack of efficacy which lead to termination of investigation of this therapy in the neoadjuvant setting (NCT02170389) [41].
The ARISER trial investigated the monoclonal antibody girentuximab targeting carbonic anhydrase IX, a surface glycoprotein commonly expressed on ccRCC tumors [44]. The trial recruited 864 patients with ccRCC tumors with high risk for recurrence defined as T2G3 + N0M0, T3-4NXM0, or TXN+M0. No clinical benefit in DFS, with a median follow-up of 6.0 years, or OS, with median follow-up not reached, was observed. Drug-related and serious ad-verse events were comparable between experimental and placebo arms.
CURRENT CHALLENGES AND FUTURE DIRECTIONS
With the tradeoff of adverse events and minimal, if any, benefit of therapy, selecting the optimal patients in which to administer adjuvant therapy is of the utmost importance. Potential areas of focus include improvement of current predictive models, improved surgical techniques incorporating lymph node dissection, and the incorporation of more sensitive imaging in identifying patients who would benefit from lymph node dissection [45].
Previously, risk of recurrence was stratified utilizing several factors including tumor size, grade, necrosis, and lymph node involvement [46–48]. Using these elements, several predictive models have been developed. One of the initial models, the SSIGN score, developed by the Mayo Clinic, included stage, size, grade and necrosis [46]. Later, this model was modified to include LN involvement, known as the Leibovich prognosis score. The University of California Los Angeles Integrated Staging System (UISS) incorporates presence of metastasis, Fuhrman nuclear grade and the Eastern Cooperative Oncology Group (ECOG) performance status [49]. These predictive models are often utilized to select patients at high risk for recurrence thereby determining who would benefit from adjuvant therapy. Other nomograms include the Union for Initial Cancer, Memorial Sloan Kettering Cancer Center nomogram, and the GRANT score [50]. However, they often contain limitations and lack prospective validation [45].
Despite their flaws, these predictive models are often utilized as they are relatively simple and have little to no additional cost. Novel methods using genetic sequencing offer improved accuracy and potentially wider application; however, they are hindered by cost and availability. These methods largely appear to be improved when combined with current clinical scoring systems. Several studies have sought to establish gene mutational signatures as prognostic tools in RCC using next generation sequencing (NGS). Brooks et al developed a 34 gene panel, which was validated using RNA sequencing data from the Cancer Genome Atlas database. Patients in the poor risk group were found to have more frequent and earlier recurrence, were at nearly three times higher risk of disease specific death, and more than twice as likely to experience all cause-mortality [51]. Rini et al. developed a 16 gene panel to predict clinical outcomes of stage I-III ccRCC. Using reverse-transcription PCR, over 500 genes were identified that correlated with recurrence free survival (RFS) from a database of nearly 1000 patients [52]. 11 were ultimately selected via statistical analysis, with 5 other reference genes for a total of 16 genes, based upon which a recurrence score was calculated. This approach was validated using the cohort data from the S-TRAC trial, and was found to be predictive of RFS, time to recurrence, and renal cancer-specific survival. The cell cycle proliferation (CCP) score was developed by Morgan et al. by identifying genes involved in cellular proliferation using an RNA expression assay. The CCP score predicted recurrence and disease-specific mortality in these patients [53]. When used with the Karakiewicz nomogram, a composite score was developed, which reliably differentiated low-risk and high-risk patients. Notably, this study included patients with chromophobe and papillary histologies in addition to ccRCC. Long non-coding RNA (LncRNA) has been found to play key roles in tumorigenesis and tumor progression. Qu et al. identified four LncRNAs associated with recurrence in RCC that stratified patients as low or high risk for recurrence and outperformed TNM and SSIGN scores. Additionally, studies have illustrated cell tumor DNA (ctDNA) has the potential to function as a surveillance biomarker in patients with localized RCC. A study by Al-Qassab et al. found 67% of patients with RCC (n = 30) had detectable mutations in more than gene via ctDNA NGS assay, as compared with 3.1% of individuals in their healthy control group (n-32) [54]. The incorporation of genomic data in conjunction with current predictive scores in RCC has improved accuracy in identifying patients at higher risk.
Another approach requiring further exploration is the widespread use of lymph node dissection during nephrectomy, even in patients without significant risk factors for nodal involvement. The European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881, which compared nephrectomy versus nephrectomy with routine lymph node dissection, showed no difference in OS or PFS benefit between both groups [55]. Based on this study, NCCN guidelines recommend that lymph node dissection be completed only when enlarged lymph nodes are present on preoperative imaging or palpated during surgery which allows variability in nodal analysis [29]. However, the EORTC study did not look at the use of subsequent adjuvant treatment in those who were found to be node positive. By performing routine lymph node dissection, we may be able to better stratify patients and identify a larger subset which are at higher risk of distant recurrence, and accordingly may benefit from adjuvant treatments.
Currently, cross sectional imaging with CT scan is the standard imaging modality used in the staging evaluation of patients prior to nephrectomy. The potential use of more sensitive imaging techniques such as FDG-PET or 18F-FDG PET may be a useful tool in the preoperative evaluation of patients for increased detection of nodal involvement [45]. Although the utility of conventional FDG-PET imaging is debatable in RCC, novel PET methods may prove useful in identifying ideal candidates for immunotherapy. Currently under investigation are PET tracers tracking CD8, and reports exist of PET tracers labeling atezolizumab and other checkpoint inhibitors [56–58]. These novel imaging approaches may increase imaging sensitivity with downstream effects of identifying a greater number of patients with metastatic disease at diagnosis and allowing treatment optimization.
As recurrence risk in trial design evolves to incorporate genetics, so too must the primary aims be evaluated. The majority of adjuvant studies in RCC have used DFS as a primary outcome, as are many of the current adjuvant studies investigating checkpoint inhibitors. However, a meta-analysis of trials in the targeted era found that DFS correlated only moderately with OS, suggesting its ability to serve as a surrogate was not particularly strong [59].
CONCLUSION
Improving patient selection for therapy will likely position future drug trials in the adjuvant RCC setting for greater success. Further development of methods that combine clinical data with gene mutation data likely hold the most promise. However, optimal patient selection is futile without improving adjuvant therapeutic modalities. Despite conferring improved patient outcomes in mRCC, neither cytokine nor TKI agents have shown consistent benefit in the adjuvant setting. The one exception is the S-TRAC trial, which illustrated a 1-year DFS, but lacked OS benefit, and was burdened by notably high toxicity rates. Furthermore, these findings were not reproduced in a subsequent subset analysis. Other trials with various TKIs have also failed to show benefit in the adjuvant setting. VEGF-TKIs, by their very nature, may not be a fruitful strategy in this arena. Data from phase III trials testing everolimus and several checkpoint inhibitors are eagerly awaited and have the potential to provide the long-sought value of adjuvant therapy of RCC.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Austin G. Kazarian: conception, literature review, interpretation of data, revisions
Neal S. Chawla: conception, literature review, interpretation of data, revisions
Ramya Muddasani: conception, literature review, interpretation of data, revisions
Sumanta K. Pal: conception, literature review, interpretation of data, revisions
CONFLICT OF INTEREST
AGK, NSC, and RM have no disclosures.
SKP reports consultancy fees from Pfizer, Inc, Novartis, Aveo, Genentech, Exelixis, Bristol Myers Squibb, Astellas Pharmaceuticals, Eisai, Roche, and Ipsen, and honoraria from Novartis, Medivation, and Astellas Pharmaceuticals.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020. CA Cancer J Clin. (2020) ;70: :7–30. https://doi.org/10.3322/caac.21590. |
[2] | Touloupidis S , Papathanasiou A , Kalaitzis C , Fatles G , Manavis I , Rombis V . Renal cell carcinoma: The influ-ence of new diagnostic imaging techniques on the size and stage of tumors diagnosed over the past 26 years. Int Urol Nephrol. (2006) ;38: :193–7. https://doi.org/10.1007/s11255-005-4762-4. |
[3] | Kane CJ , Mallin K , Ritchey J , Cooperberg MR , Carroll PR . Renal cell cancer stage migration: Analysis of the National Cancer Data Base. Cancer. (2008) ;113: :78–83. https://doi.org/10.1002/cncr.23518. |
[4] | Patel HD , Gupta M , Joice GA , Srivastava A , Alam R , Allaf ME , et al. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur Urol Oncol. (2019) ;2: :343–8. https://doi.org/10.1016/j.euo.2018.08.023. |
[5] | Stephenson AJ , Chetner MP , Rourke K , Gleave ME , Signaevsky M , Palmer B , et al. GUIDELINES FOR THE SURVEILLANCE OF LOCALIZED RENAL CELL CARCINOMA BASED ON THE PATTERNS OF RELAPSE AFTER NEPHRECTOMY. J Urol. (2004) ;172: :58–62. https://doi.org/10.1097/01.ju.0000132126.85812.7d. |
[6] | Bloom HJG . Medroxyprogesterone Acetate (Provera) in the Treatment of Metastatic Renal Cancer. Br J Cancer. (1971) ;25: :250–65. https://doi.org/10.1038/bjc.1971.31. |
[7] | Passalacqua R , Caminiti C , Buti S , Porta C , Camisa R , Braglia L , et al. Adjuvant Low-Dose Interleukin-2 (IL-2) Plus Interferon-α (IFN-α) in Operable Renal Cell Carcinoma (RCC): A Phase III, Randomized, Multicentre Trial of the Italian Oncology Group for Clinical Research (GOIRC). J Immunother. (2014) ;37: :440–7. https://doi.org/10.1097/CJI.0000000000000055. |
[8] | Interferon-α and survival in metastatic renal carcinoma: early results of a randomised controlled trial. The Lancet. (1999) ;353: :14–7. https://doi.org/10.1016/S0140-6736(98)03544-2. |
[9] | Fyfe G , Fisher RI , Rosenberg SA , Sznol M , Parkinson DR , Louie AC . Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. (1995) ;13: :688–96. https://doi.org/10.1200/JCO.1995.13.3.688. |
[10] | Atzpodien J , Schmitt E , Gertenbach U , Fornara P , Heynemann H , Maskow A , et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Br J Cancer. (2005) ;92: :843–6. https://doi.org/10.1038/sj.bjc.6602443. |
[11] | Clark JI , Atkins MB , Urba WJ , Creech S , Figlin RA , Dutcher JP , et al. Adjuvant High-Dose Bolus Interleukin-2 for Patients With High-Risk Renal Cell Carcinoma: A Cytokine Working Group Randomized Trial. J Clin Oncol. (2003) ;21: :3133–40. https://doi.org/10.1200/JCO.2003.02.014. |
[12] | Messing EM , Manola J , Wilding G , Propert K , Fleischmann J , Crawford ED , et al. Phase III Study of Interferon Alfa-NL as Adjuvant Treatment for Resectable Renal Cell Carcinoma: An Eastern Cooperative Oncology Group/Intergroup Trial. J Clin Oncol. (2003) ;21: :1214–22. https://doi.org/10.1200/JCO.2003.02.005. |
[13] | Pizzocaro G , Piva L , Colavita M , Ferri S , Artusi R , Boracchi P , et al. Interferon Adjuvant to Radical Nephrectomy in Robson Stages II and III Renal Cell Carcinoma: A Multicentric Randomized Study. J Clin Oncol. (2001) ;19: :425–31. https://doi.org/10.1200/JCO.2001.19.2.425. |
[14] | Aitchison M , Bray CA , Van Poppel H , Sylvester R , Graham J , Innes C , et al. Adjuvant 5-flurouracil, alpha-interferon and interleukin-2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: Results of a Phase III randomised European Organisation for Research and Treatment of Cancer (Genito-Urinary Cancers Group)/National Cancer Research Institute trial. Eur J Cancer. (2014) ;50: :70–7. https://doi.org/10.1016/j.ejca.2013.08.019. |
[15] | Baudino T . Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr Drug Discov Technol. (2015) ;12: :3–20. https://doi.org/10.2174/1570163812666150602144310. |
[16] | Gnarra JR , Tory K , Weng Y , Schmidt L , Wei MH , Li H , et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. (1994) ;7: :85–90. https://doi.org/10.1038/ng0594-85. |
[17] | Nickerson ML , Jaeger E , Shi Y , Durocher JA , Mahurkar S , Zaridze D , et al. Improved Identification of von Hippel-Lindau Gene Alterations in Clear Cell Renal Tumors. Clin Cancer Res. (2008) ;14: :4726–34. https://doi.org/10.1158/1078-0432.CCR-07-4921. |
[18] | Peña C , Lathia C , Shan M , Escudier B , Bukowski RM . Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from sorafenib phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res Off J Am Assoc Cancer Res . (2010) ;16: :4853–63. https://doi.org/10.1158/1078-0432.CCR-09-3343. |
[19] | Synold TW , Plets M , Tangen CM , Heath EI , Palapattu GS , Mack PC , et al. Everolimus Exposure as a Predictor of Toxicity in Renal Cell Cancer Patients in the Adjuvant Setting: Results of a Pharmacokinetic Analysis for SWOG S0931 (EVEREST), a Phase III Study (NCT01120249). Kidney Cancer Clifton Va. (2019) ;3: :111–8. https://doi.org/10.3233/KCA-180049. |
[20] | Hayman SR , Leung N , Grande JP , Garovic VD . VEGF Inhibition, Hypertension, and Renal Toxicity. Curr Oncol Rep. (2012) ;14: :285–94. https://doi.org/10.1007/s11912-012-0242-z. |
[21] | Motzer RJ , Hutson TE , Tomczak P , Michaelson MD , Bukowski RM , Oudard S , et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. (2009) ;27: :3584–90. https://doi.org/10.1200/JCO.2008.20.1293. |
[22] | Gross-Goupil M , Kwon TG , Eto M , Ye D , Miyake H , Seo SI , et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol. (2018) ;29: :2371–8. https://doi.org/10.1093/annonc/mdy454. |
[23] | Motzer RJ , Haas NB , Donskov F , Gross-Goupil M , Varlamov S , Kopyltsov E , et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. (2017) ;35: :3916–23. https://doi.org/10.1200/JCO.2017.73.5324. |
[24] | Eisen TQG , Frangou E , Smith B , Ritchie A , Kaplan RS , Oza B , et al. Primary efficacy analysis results from the SORCE trial (RE05): Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: An international, randomised double-blind phase III trial led by the MRC CTU at UCL. Ann Oncolv. (2019) ;30: :891–2. https://doi.org/10.1093/annonc/mdz394.050. |
[25] | Haas NB , Manola J , Dutcher JP , Flaherty KT , Uzzo RG , Atkins MB , et al. Adjuvant Treatment for High-Risk Clear Cell Renal Cancer: Updated Results of a High-Risk Subset of the ASSURE Randomized Trial. JAMA Oncol. (2017) ;3: :1249. https://doi.org/10.1001/jamaoncol.2017.0076. |
[26] | Ravaud A , Motzer RJ , Pandha HS , George DJ , Pantuck AJ , Patel A , et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med. (2016) ;375: :2246–54. https://doi.org/10.1056/NEJMoa1611406. |
[27] | Appleman LJ , Puligandla M , Pal SK , Harris W , Agarwal N , Costello BA , et al. Randomized, double-blind phase III study of pazopanib versus placebo in patients with metastatic renal cell carcinoma who have no evidence of disease following metastasectomy: A trial of the ECOG-ACRIN cancer research group (E2810). J Clin Oncol. (2019) ;37: :4502–4502. https://doi.org/10.1200/JCO.2019.37.15_suppl.4502. |
[28] | Procopio G , Apollonio G , Cognetti F , Miceli R , Milella M , Mosca A , et al. Sorafenib Versus Observation Following Radical Metastasectomy for Clear-cell Renal Cell Carcinoma: Results from the Phase 2 Randomized Open-label RESORT Study. Eur Urol Oncol. (2019) ;2: :699–707. https://doi.org/10.1016/j.euo.2019.08.011. |
[29] | Kidney Cancer (Version 1.2021): National Comprehensive Cancer Network; [Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf n.d. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (accessed September 29, 2020). |
[30] | Ljungberg B , Albiges L , Abu-Ghanem Y , Bensalah K , Dabestani S , Fernández-Pello S , et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol. (2019) ;75: :799–810. https://doi.org/10.1016/j.eururo.2019.02.011. |
[31] | Sun M , Marconi L , Eisen T , Escudier B , Giles RH , Haas NB , et al. Adjuvant Vascular Endothelial Growth Factor-targeted Therapy in Renal Cell Carcinoma: A Systematic Review and Pooled Analysis. Eur Urol. (2018) ;74: :611–20. https://doi.org/10.1016/j.eururo.2018.05.002. |
[32] | Topalian SL , Drake CG , Pardoll DM . Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. (2012) ;24: :207–12. https://doi.org/10.1016/j.coi.2011.12.009. |
[33] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. (2015) ;373: :1803–13. https://doi.org/10.1056/NEJMoa1510665. |
[34] | Motzer RJ , Tannir NM , McDermott DF , Arén Frontera O , Melichar B , Choueiri TK , et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) ;378: :1277–90. https://doi.org/10.1056/NEJMoa1712126. |
[35] | Rini BI , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Lond Engl. (2019) ;393: :2404–15. https://doi.org/10.1016/S0140-6736(19)30723-8. |
[36] | Uzzo R , Bex A , Rini BI , Albiges L , Suarez C , Donaldson F , et al. A phase III study of atezolizumab (atezo) vs placebo as adjuvant therapy in renal cell carcinoma (RCC) patients (pts) at high risk of recurrence following resection (IMmotion010). J Clin Oncol. (2017) ;35: :TPS4598–TPS4598. https://doi.org/10.1200/JCO.2017.35.15_suppl.TPS4598. |
[37] | Choueiri TK , Quinn DI , Zhang T , Gurney H , Doshi GK , Cobb PW , et al. KEYNOTE- A phase 3, randomized, double blind, trial of pembrolizumab in the adjuvant treatment of renal cell carcinoma. J Clin OncolTPS-TPS. (2018) ;36: :TPS4599–TPS4599. https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS4599. |
[38] | Harshman LC , Puligandla M , Haas NB , Allaf M , Drake CG , McDermott DF , et al. PROSPER: A phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients with renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN 8143). J Clin Oncol. (2019) ;37: :TPS4597–TPS4597. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS4597. |
[39] | Broderick SR . Adjuvant and Neoadjuvant Immunotherapy in Non-small Cell Lung Cancer. Thorac Surg Clin. (2020) ;30: :215–20. https://doi.org/10.1016/j.thorsurg.2020.01.001. |
[40] | Powles T , O’Donnell PH , Massard C , Arkenau H-T , Friedlander TW , Hoimes CJ , et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. (2017) ;3: :e172411. https://doi.org/10.1001/jamaoncol.2017.2411. |
[41] | Galligioni E , Quaia M , Merlo A , Carbone A , Spada A , Favaro D , et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guèrin: five-year results of a prospective randomized study. Cancer. (1996) ;77: :2560–6. https://doi.org/10.1002/(SICI)1097-0142(19960615)77:12<2560::AID-CNCR20>3.0.CO;2-P. |
[42] | Jocham D , Richter A , Hoffmann L , Iwig K , Fahlenkamp D , Zakrzewski G ,et al.. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet Lond Engl. (2004) ;363: :594–9. https://doi.org/10.1016/S0140-6736(04)15590-6. |
[43] | Wood C , Srivastava P , Bukowski R , Lacombe L , Gorelov AI , Gorelov S ,et al.. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet Lond Engl. (2008) ;372: :145–54. https://doi.org/10.1016/S0140-6736(08)60697-2. |
[44] | Chamie K , Donin NM , Klöpfer P , Bevan P , Fall B , Wilhelm O ,et al.. Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma: The ARISER Randomized Clinical Trial. JAMA Oncol. (2017) ;3: :913–20. https://doi.org/10.1001/jamaoncol.2016.4419. |
[45] | Tsao C-K . Improvements Needed for Adjuvant Therapy in RCC 2020. |
[46] | Frank I , Blute ML , Cheville JC , Lohse CM , Weaver AL , Zincke H . An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. (2002) ;168: :2395–400. https://doi.org/10.1097/01.ju.0000035885.91935.d5. |
[47] | Zisman A , Pantuck AJ , Dorey F , Said JW , Shvarts O , Quintana D ,et al.. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol Off J Am Soc Clin Oncol. (2001) ;19: :1649–57. https://doi.org/10.1200/JCO.2001.19.6.1649. |
[48] | Leibovich BC , Blute ML , Cheville JC , Lohse CM , Frank I , Kwon ED ,et al.. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer. (2003) ;97: :1663–71. https://doi.org/10.1002/cncr.11234. |
[49] | Patard J-J , Kim HL , Lam JS , Dorey FJ , Pantuck AJ , Zisman A ,et al.. Use of the University of California Los Angeles Integrated Staging System to Predict Survival in Renal Cell Carcinoma: An International Multicenter Study. J Clin Oncol. (2004) ;22: :3316–22. https://doi.org/10.1200/JCO.2004.09.104. |
[50] | Buti S , Puligandla M , Bersanelli M , DiPaola RS , Manola J , Taguchi S ,et al.. Validation of a new prognostic model to easily predict outcome in renal cell carcinoma: the GRANT score applied to the ASSURE trial population. Ann Oncol Off J Eur Soc Med Oncol. (2017) ;28: :2747–53. https://doi.org/10.1093/annonc/mdx492. |
[51] | Brooks SA , Brannon AR , Parker JS , Fisher JC , Sen O , Kattan MW ,et al.. ClearCode A Prognostic Risk Predictor for Localized Clear Cell Renal Cell Carcinoma. Eur Urol. (2014) ;66: :77–84. https://doi.org/10.1016/j.eururo.2014.02.035. |
[52] | Rini B , Goddard A , Knezevic D , Maddala T , Zhou M , Aydin H ,et al.. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol. (2015) ;16: :676–85. https://doi.org/10.1016/S1470-2045(15)70167-1. |
[53] | Morgan TM , Mehra R , Tiemeny P , Wolf JS , Wu S , Sangale Z ,et al.. A Multigene Signature Based on Cell Cycle Proliferation Improves Prediction of Mortality Within 5 Yr of Radical Nephrectomy for Renal Cell Carcinoma. Eur Urol. (2018) ;73: :763–9. https://doi.org/10.1016/j.eururo.2017.12.002. |
[54] | Al-Qassab U , Lorentz CA , Laganosky D , Ogan K , Master V , Pattaras J ,et al.. PNFBA-12 LIQUID BIOPSY FOR RENAL CELL CARCINOMA. J Urol 2017;197. https://doi.org/10.1016/j.juro.2017.02.3241. |
[55] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A ,et al.. A Prospective, Randomised EORTC Intergroup Phase 3 Study Comparing the Oncologic Outcome of Elective Nephron-Sparing Surgery and Radical Nephrectomy for Low-Stage Renal Cell Carcinoma. Eur Urol. (2011) ;59: :543–52. https://doi.org/10.1016/j.eururo.2010.12.013. |
[56] | Lim EA , Drake CG , Mintz A . Molecular imaging for cancer immunotherapy. Immuno-Oncol Technol. (2020) ;5: :10–21. https://doi.org/10.1016/j.iotech.2020.03.001. |
[57] | Bensch F , van der Veen EL , Lub-de Hooge MN , Jorritsma-Smit A , Boellaard R , Kok IC ,et al.. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. (2018) ;24: :1852–8. https://doi.org/10.1038/s41591-018-0255-8. |
[58] | Griessinger CM , Olafsen T , Mascioni A , Jiang ZK , Zamilpa C , Jia F ,et al.. The PET-Tracer 89 Zr-Df-IAB22M2C Enables Monitoring of Intratumoral CD8 T-cell Infiltrates in Tumor-Bearing Humanized Mice after T-cell Bispecific Antibody Treatment. Cancer Res. (2020) ;80: :2903–13. https://doi.org/10.1158/0008-5472.CAN-19-3269. |
[59] | Harshman LC , Xie W , Moreira RB , Gustavo R , Sweeney C , Choueiri TK . Meta-analysis of disease free survival (DFS) as a surrogate for overall survival (OS) in localized renal cell carcinoma (RCC). J Clin Oncol. (2017) ;35: :459–459. https://doi.org/10.1200/JCO.2017.35.6_suppl.459. |



