Long-Term Outcomes in Clear-Cell Renal Cell Carcinoma Patients Treated with Complete Metastasectomy
Abstract
Background:
Complete metastasectomy is routinely performed in selected patients with metastatic clear-cell renal cell carcinoma (ccRCC).
Objectives:
To assess (1) outcomes after first and repeat metastasectomy, (2) outcomes on targeted therapy in patients who underwent previous metastasectomy and (3) compare outcomes with and without metastasectomy after correction for selection bias.
Methods:
Metastatic ccRCC patients treated with or without metastasectomy at University Hospitals Leuven were included from prospective databases. We calculated disease-free survival (DFS), time to systemic therapy and cancer-specific survival (CSS) after metastasectomy, and progression-free survival (PFS) and CSS on 1st line sunitinib/pazopanib. We calculated propensity scores to estimate a patient’s likelihood to undergo metastasectomy.
Results:
We included 113 patients who underwent complete metastasectomy and 139 who did not. (1) Median DFS after complete metastasectomy was 18 mo, time to systemic therapy was 73 mo and CSS was 101 mo. 20% did not relapse during long-term follow-up. Outcomes remained favorable after repeat metastasectomy. (2) PFS and CSS on 1st line sunitinib/pazopanib were 15 mo and 35 mo. (3) The propensity scores of patients who did and did not undergo metastasectomy showed no overlap, indicating that correction for selection bias is impossible and comparison of outcomes unreliable.
Conclusions:
Complete metastasectomy and repeat metastasectomy can result in excellent outcomes in highly selected patients, even when its causal benefit cannot be formally assessed. Previous metastasectomy does not impair outcomes on targeted therapies.
INTRODUCTION
The contemporary treatment of metastatic clear-cell renal cell carcinoma (ccRCC) consists of 1st line systemic therapy with an immune checkpoint inhibitor (ICI), combined with another ICI or an angiogenesis inhibitor [1]. Local treatments have historically played an important role, but their current position is ill-defined. Cytoreductive nephrectomy, the long-standing standard of care, did not improve overall survival (OS) in patients treated with 1st line sunitinib that were Intermediate or Poor risk according to the International Metastatic ccRCC Database Consortium (IMDC) criteria [2]. Yet its role in the ICI era is unclear. The radical local treatment of metastases has been associated with long-term OS and deferral of systemic therapy in retrospective series of patients with favorable prognostic features, such as long disease-free interval, limited number of metastases, good performance status, lung metastases, low T-stage, low Fuhrman grade and absence of sarcomatoid features [3, 4]. Radical local treatment is therefore recommended in selected patients, although its exact benefit remains unclear in the absence of randomized trials. The rapid improvement of systemic treatment options for ccRCC now poses new questions. Will metastasectomy be abandoned in favor of ICI combinations? Or will we increase its use in combination with ICI? The latter option is currently being investigated in several randomized trials (NCT03024996, NCT03055013, NCT03142334). Therefore, it is important to establish a baseline for outcomes after complete metastasectomy without systemic therapy. With this study, we contribute to establishing this baseline in a large patient series from a tertiary referral center, with long-term follow-up. Furthermore, we aimed to fill the current gaps in the literature on ccRCC metastasectomy: outcomes after repeat metastasectomy, outcomes on antiangiogenic therapy after metastasectomy and comparison of outcomes without metastasectomy, after correction for selection bias through propensity score analysis. Of note, other series that compare outcomes of patients with and without metastasectomy typically do so through multivariable Cox regression, which can take into account much less variables than a propensity score.
METHODS
Patient population
After approval by the medical ethics review board, we selected patients from two prospective records at University Hospitals Leuven.
The first record was of RCC patients undergoing metastasectomy since 1995. Inclusion criteria were: clear-cell histology, resection of the primary tumor, metastasectomy with aim of complete resection, no second cancer and no prior or adjuvant systemic therapy. Ipsilateral adrenal lesions that were spatially separated from the primary tumor were considered metastases, continuous growth into the adrenal gland was not. Patients who relapsed were treated in accordance with local practice guidelines: second metastasectomy, active surveillance or systemic therapy.
As a control group, we selected patients from ano-ther prospective record of metastatic RCC patients treated with systemic therapy, kept since 2005. Inclusion criteria were: clear-cell histology, resection of the primary tumor, no second cancer, systemic treatment started for metastatic disease and not in the (neo-)adjuvant setting. Local treatment for symptomatic reasons was allowed if it concerned <30% of metastatic tumor volume.
Statistical analysis
Survival outcomes were estimated with the Kaplan-Meier method. Disease-free survival (DFS) was calculated as time from surgery to relapse or death from any cause. Progression-free survival (PFS) on 1st line sunitinib/pazopanib was calculated as time from start of therapy and to radiological progression or death from any cause.
We calculated propensity scores to estimate the probability of patients to undergo metastasectomy. Propensity scores can take into account much more variables than classic multivariate models. They are therefore more reliable to correct for selection bias when comparing outcomes of patients who did and did not undergo metastasectomy. We used a multivariable logistic regression model to calculate propensity scores, including patient characteristics that correlated significantly with treatment assignment on univariable analysis (p < 0.05). As potential predictors at time of nephrectomy, we tested: T-stage ≥3, Fuhrman grade ≥3, lymph node positivity, synchronous metastases, sarcomatoid component and gender. As predictors at time of diagnosis of metastasis: age, multiple metastases, multiorgan metastases, disease-free interval >1 year, Karnofsky performance status <80%, anemia (hemoglobin < 14 g/dl in men, <12 g/dl in women), neutrophils >4,500/μl, platelets >450,000/μl, corrected calcium >2.55 mmol/l, C-reactive protein (CRP) >5 mg/l, lactate dehydrogenase above 1.5 upper limit of normal and location (including sites with at least 10 affected patients: lung, bone, adrenal, liver, lymph node, pleura, pancreas, local relapse).
Graphpad Prism 8.3.0 and XLstat 2020.1.3 were used for statistical analysis.
RESULTS
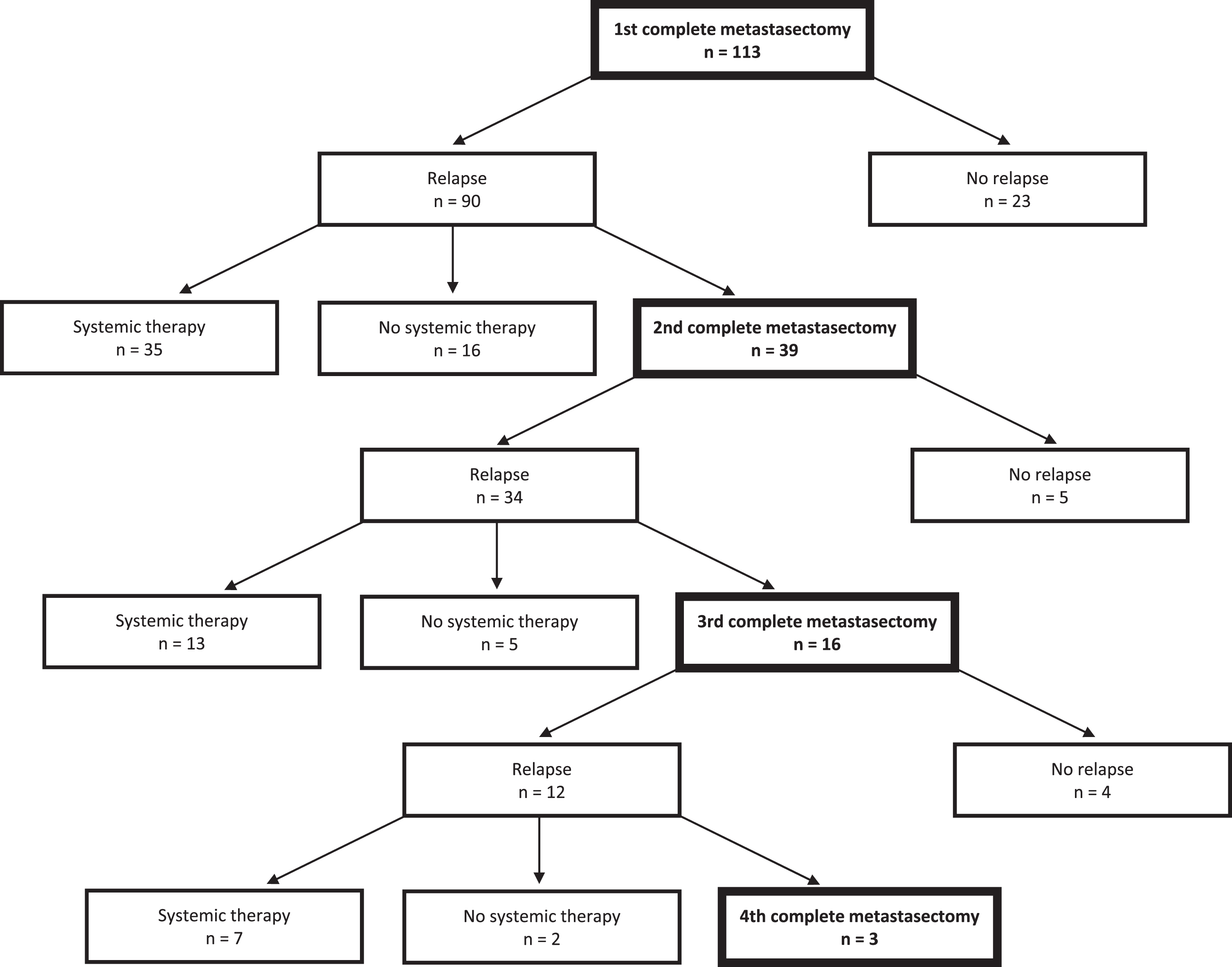
We included 113 patients who underwent complete metastasectomy and 139 patients who were treated with systemic therapy. In the metastasectomy patients, median follow-up after first metastasectomy was 78 mo (interquartile range 42–177 mo) and 53 CSS events occurred (47%). Resection margins were negative in 91% of cases. Patient characteristics are summarized in Table 1, their clinical course in Fig. 1.
Table 1
Characteristics of 113 patients that underwent complete metastasectomy
| At diagnosis of primary RCC | ||
| Male | 79/113 | 70% |
| Age (median, IQ range) | 60 | 52–69 |
| T-stage | ||
| T1 | 21/94 | 22% |
| T2 | 28/94 | 30% |
| T3 | 42/94 | 45% |
| T4 | 3/94 | 3% |
| Lymph node positive | 8/55 | 15% |
| Synchronous metastases | 26/113 | 23% |
| Fuhrman grade | ||
| 1 | 0 | |
| 2 | 22/89 | 25% |
| 3 | 34/89 | 38% |
| 4 | 33/89 | 37% |
| Sarcomatoid component | 6/35 | 17% |
| At first metastasectomy | ||
| Number of metastases | ||
| 1 | 76/113 | 67% |
| 2 | 12/113 | 11% |
| ≥3 | 25/113 | 22% |
| Organs affected | ||
| 1 | 98/113 | 87% |
| 2 | 10/113 | 9% |
| ≥3 | 5/113 | 4% |
| Site | ||
| lymph nodes | 10/113 | 9% |
| lung | 40/113 | 35% |
| liver | 3/113 | 3% |
| brain | 2/113 | 2% |
| kidney/local | 18/113 | 16% |
| adrenal | 22/113 | 19% |
| pancreas | 5/113 | 4% |
| bone | 13/113 | 12% |
| other | 13/113 | 12% |
| KPS <80% | 6/75 | 8% |
| Anemia | 42/107 | 39% |
| Elevated neutrophils | 29/89 | 33% |
| Elevated platelets | 1/106 | 1% |
| Hypercalcemia | 5/86 | 6% |
| Elevated CRP | 29/94 | 31% |
| LDH>1.5 ULN | 2/36 | 6% |
| After first metasasectomy | ||
| Negative resection margins | 95/104 | 91% |
KPS = Karnofsky performance status; Anemia = hemoglobin <14 g/dl (men) or 12.5 g/dl (women); ULN neutrophils = 4.500/μl; ULN platelets = 450.000/μl; ULN albumin-corrected calcium = 2.55 mmol/l; CRP = c-reactive protein; ULN CRP = 5 mg/l; LDH = lactate dehydrogenase; ULN = upper limit of normal.
Fig. 1
Flow chart of the clinical course of 113 included patients during follow-up.
Outcomes after first metastasectomy
Median DFS was 18 mo (Table 2, Fig. 2). A substantial number of patients achieved long-term DFS, with actuarial 2-year DFS of 41% and 10-year DFS 13%. Of note, this number is negatively influenced by patients who deceased without relapse: 23 patients (=20%) did not relapse during follow-up, of whom five died from other causes. The median follow-up of those who did not relapse was 131 mo, whereas the latest recorded relapse occurred at 107 mo. There was no clear difference in DFS according to site of metastasis in this series, but patients with multiple metastases had shorter DFS compared to those with only one lesion (24 vs 11 mo, p = 0.01).
Table 2
Outcomes after metastasectomy
| Nephrectomy | Complete metastasectomy | |||
| 1st | 2nd | 3rd | ||
| Number | 113 | 113 | 39 | 16 |
| Disease-free survival | ||||
| median | 33 mo | 18 mo | 15 mo | 18 mo |
| 2 years | 66% | 41% | 30% | 40% |
| 5 years | 32% | 20% | 13% | 25% |
| 10 years | 5% | 13% | undefined | 25% |
| Time to systemic therapy | ||||
| median | 135 mo | 73 mo | 56 mo | 43 mo |
| 2 years | 90% | 78% | 77% | 74% |
| 5 years | 79% | 51% | 39% | 26% |
| 10 years | 58% | 39% | 20% | 26% |
| Cancer-specific survival | ||||
| median | 182 mo | 124 mo | 127 mo | 90 mo |
| 2 years | 97% | 89% | 93% | 90% |
| 5 years | 81% | 67% | 68% | 69% |
| 10 years | 66% | 52% | 49% | 42% |
Fig. 2
Outcomes after first, second and third metastasectomy.
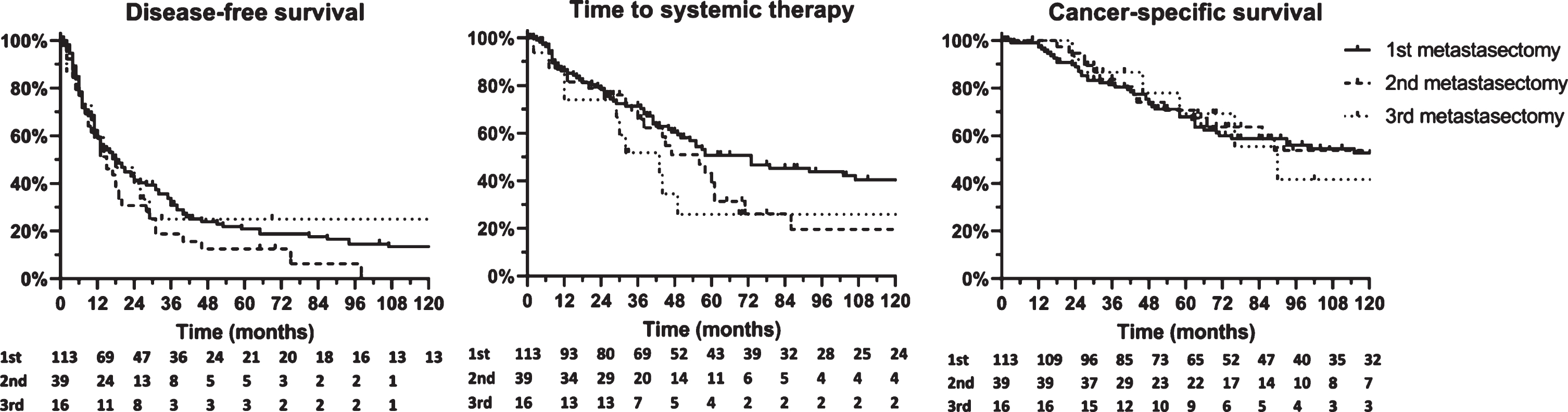
The median time to systemic therapy was 73 mo, and the median CSS 124 mo.
Outcomes after repeat metastasectomy
One third of patients (n = 39) was eligible for second metastasectomy and another 41% of these for third metastasectomy (n = 16). DFS, time to systemic therapy and CSS were numerically shorter compared to first metastasectomy, but still favorable and survival curves were largely overlapping (Table 2, Fig. 2). A substantial number of these patients did not relapse during follow-up: 5 of 39 after second metastasectomy (follow-up 28 to 70 mo) and 4 of 16 after third metastasectomy (follow-up 27 to 220 mo).
Systemic therapy after metastasectomy
The median time to systemic therapy was much longer than the DFS after metastasectomy (73 vs 18 mo), indicating that most relapsing patients were eligible for a period of active surveillance or repeat metastasectomy. The 59 patients who started systemic therapy during follow-up, did so after a median of 32 mo after relapse. This is again an indication of the overall indolent disease course in patients who are eligible for metastasectomy. 41 patients were treated with 1st line sunitinib or pazopanib. Their PFS and CSS were 18 and 35 mo, with a response rate of 50%. These outcomes are excellent compared to historical trial results, indicating that patients do not miss a window of opportunity by deferring antiangiogenic therapy in favor of metastasectomy and underscoring the indolent nature of these tumors [5, 6].
Comparison of outcomes of patients with and without metastasectomy
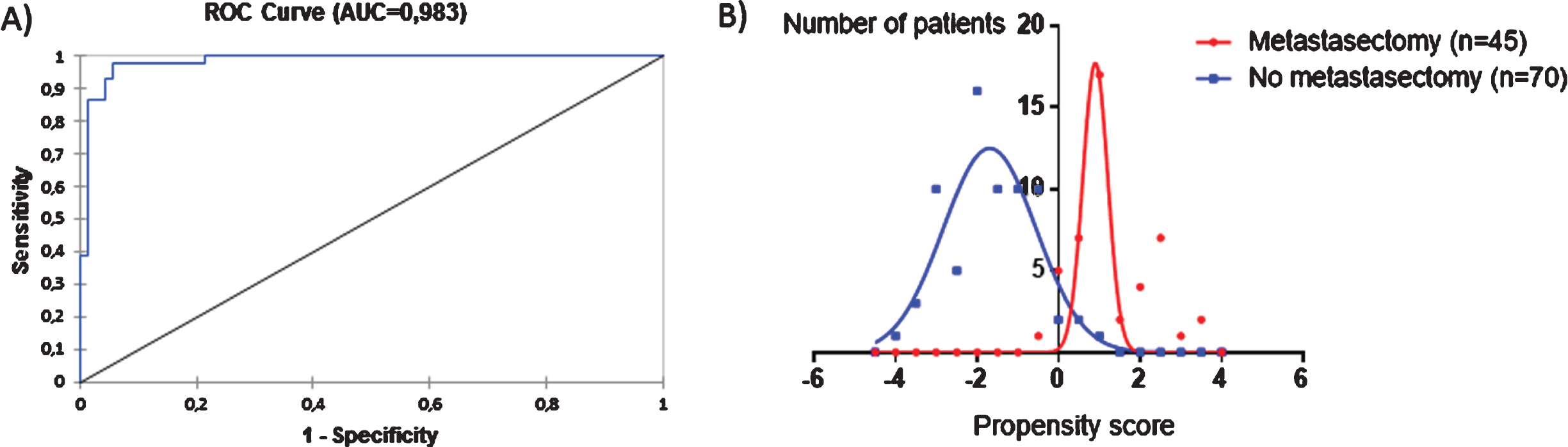
We aimed to compare outcomes in patients who did and did not undergo metastasectomy after correction for propensity score. This score indicates a patient’s probability to undergo metastasectomy and can therefore address selection bias. On univariable logistic regression, following predictors were significantly associated with higher probability of metastasectomy: T-stage ≤2, Fuhrman grade ≤2, metachronous metastases, disease-free interval >12mo, single metastasis, single organ, adrenal or liver metastasis. The following predictors were associated with lower probability: bone, lymph node or pleural metastasis, anemia, elevated neutrophils, elevated platelets, elevated CRP and hypercalcemia. After selective bivariable analyses, following predictors were selected for inclusion in the propensity model: T-stage, Fuhrman grade, synchronous metastases, multiple metastases, multiple organs, elevated CRP, hypercalcemia and four sites (bone, lymph node, adrenal gland, pleura). However, the area under the curve of the resulting propensity model was 0.98. Accordingly, the histograms of the propensity scores of the two populations were barely overlapping (Fig. 3). This implies that baseline prognostic factors determine treatment choice and that the characteristics of the patients who undergo metastasectomy vs those who do not were so different, that a meaningful comparison of outcomes is not plausible. It was therefore not possible to analyze the causal effect of metastasectomy on outcome.
Fig. 3
Propensity model that estimates a patient’s probability to undergo metastasectomy. (A) over 98% of the probability is determined by baseline characteristics. (B) There is almost no overlap between the propensity scores of patients who undergo metastasectomy and those who do not. Therefore these populations cannot be compared. A more positive score indicates a higher probability to receive metastasectomy, a more negative score a lower probability. In case of missing data for one or more predictors in a patient, the known predictors were used in the multivariable logistic regression model calculating the AUC, but the patients’ propensity score was not calculated. The characteristics of patients who did not receive metastasectomy are shown in supplemental Table 1.
DISCUSSION
In this large and homogeneous case series with long-term follow-up, we show that ccRCC patients can achieve excellent long-term outcomes after complete metastasectomy. The 10-year DFS was 13% and is probably even underestimated, as several patients were censored because of death from other cause. As the latest relapse on our series occurred at 8 years, this high probability of 10-year DFS indicates potential curative treatment for a subset of patients. The current recommendation to consider radical local treatment in selected patients remains therefore unchallenged, even in the absence of randomized trials. This makes sense, as it has been shown that metastatic lesions can metastasize themselves [7]. Indeed, a randomized trial in metastatic non-small cell lung carcinoma showed that radical local treatment could delay the onset of new metastatic lesions [8]. A novel finding we report are the favorable outcomes in patients who are eligible for repeat metastasectomy: this supports the practice of offering surgery to patients that maintain favorable features.
This series offers some interesting insights on the decision between metastasectomy or systemic therapy. First, it is often assumed that metastasectomy can defer systemic therapy and the associated toxicity, and is therefore a good option if expected morbidity is low. In our series, we report indeed a very long time to systemic therapy after metastasectomy (median 73 mo). But even after relapse, the median time before start was 32 mo: this is an effect of indolent disease course, and not metastasectomy itself. Nonetheless, metastasectomy probably contributes to the delay, as was also shown in a randomized trial setting in prostate cancer [9]. Importantly, outcomes on 1st line sunitinib/pazopanib were superior to those in historical trials (PFS 15 mo, CSS 35 mo, response rate 50%) [5, 6]. This reflects the selection of indolent tumors for metastasectomy, but importantly also indicates that patients do not miss a window of opportunity for systemic therapy by opting for metastasectomy.
The calculation of propensity scores that estimate a patient’s likelihood to undergo metastasectomy, showed very explicitly that the populations that are selected for metastasectomy and those that are not, are entirely different. Therefore, outcomes in these populations cannot be compared to estimate the causal benefit of metastasectomy, not even by multivariate regression as is done in several series [10–15]. One could argue that we should have selected a more similar control group. However, if such a control group existed, it would have been apparent by overlap of the propensity score curves, which was not the case. This has the reassuring implication that patient selection is based on rigorous clinical criteria, and not patient or physician’s preference. Indeed, the strict selection at our center is also apparent from the 91% negative resection margins and outcomes that are better than most reported series: the favorable reported outcomes should therefore be carefully interpreted in light of this stringent selection.
While the long-term follow-up and rigorous patient selection are strong points of this study, it is important to note its limitations. There is a certain time bias, as we included patients since 1995. Yet, as patient outcomes have only improved over time, this will not result in overestimation of the outcomes after metastasectomy. Of note, only 5 of 113 patients died before sunitinib became available, which will therefore not have a major influence on the results. More importantly, 1st line treatment with sunitinib or pazopanib is by now outdated and the place of metastasectomy in the ICI era is unclear. Whereas several trials testing adjuvant angiogenesis inhibitors were strictly negative, the (neo-)adjuvant use of ICI is now being investigated including in the oligometastastic setting [16, 17]. In node-positive melanomas, early results of neo-adjuvant ipilimumab-nivolumab followed by radical surgery are extremely encouraging [18]. This might be a future paradigm for oligometastastic ccRCC, rather than complete resection followed by systemic therapy at relapse. Yet this gives even more importance to the development of predictive tools that can select the population in whom metastasectomy alone may be curative. In this context, molecular subtypes may prove useful: recent work showed that among highly selected patients, primary tumors with a favorable subtype (ccrcc2 or –3) have a high probility of long DFS, whereas those with an unfavorable subtype (ccrcc1 or –4) are at risk of early relapse [19].
In conclusion, complete metastasectomy and re-peat metastasectomy can result in excellent outcomes in highly selected patients, even when its causal benefit cannot be formally assessed. Previous metastasectomy does not impair outcomes on targeted therapies. In light of upcoming combination strategies of metastasectomy with immunotherapies, it is important to identify the populations who will need immunotherapy and those for whom surgery alone may suffice.
FUNDING
Annelies Verbiest has received a grant from Kom op tegen Kanker (Stand up to Cancer) and from Research Foundation Flanders.
AUTHOR CONTRIBUTIONS
Annelies Verbiest: data collection, study conception, statistical analysis, writing of the paper; Eduard Roussel: data collection; Lorenzo Tosco: data collection; Steven Joniau: patient inclusion; Annouschka Laenen: statistical advice; Paul Clement: study conception, critical revision of the manuscript; Agnieszka Wozniak: critical revision of the manuscript; Maarten Albersen: patient inclusion; Benoit Beuselinck: data collection, study conception.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
SUPPLEMENTARY MATERIAL
[1] The supplementary Table 1 is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-200093.
REFERENCES
[1] | Escudier B , Porta C , Schmidinger M , Rioux-Leclercq N , Bex A , Khoo V , et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2019) ;30: (5):706–20. |
[2] | Méjean A , Ravaud A , Thezenas S , Colas S , Beauval J-B , Bensalah K , et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. (2018) ;379: (5):417–27. |
[3] | Dabestani S , Marconi L , Bex A . Metastasis therapies for renal cancer. Curr Opin Urol. (2016) ;26: (6):566–72. |
[4] | Zaid HB , Parker WP , Safdar NS , Gershman B , Erwin PJ , Murad MH , et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: a systematic review and meta-analysis. J Urol. (2017) ;197: (1):44–9. |
[5] | Motzer R , Hutson T , Tomczak P , Michaelson M , Bukowski R , Rixe O , et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. (2007) ;356: (2):115–24. |
[6] | Sternberg C , Davis I , Mardiak J , Cezary S , Wagstaff J , Salman P , et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Onc. (2010) ;28: (6):1061–8. |
[7] | Gundem G , Van Loo P , Kremeyer B , Alexandrov LB , Tubio JMC , Papaemmanuil E , et al. The evolutionary history of lethal metastatic prostate cancer. Nature. (2015) ;520: (7547):353–7. |
[8] | Gomez DR , Blumenschein GR Jr , Lee JJ , Hernandez M , Ye R , Camidge DR , et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. (2016) ;17: (12):1672–82. |
[9] | Phillips R , Shi WY , Deek M , Radwan N , Lim SJ , Antonarakis E . Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostatce cancer: the ORIOLE phase 2 randomized clinical trial. JAMA oncology. (2020) ;6: (5):650–9. |
[10] | Eggener SE , Yossepowitch O , Kundu S , Motzer RJ , Russo P . Risk score and metastasectomy independently impact prognosis of patients with recurrent renal cell carcinoma. J Urol. (2008) ;180: (3):873–8. |
[11] | Alt AL , Boorjian SA , Lohse CM , Costello BA , Leibovich BC , Blute ML . Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. (2011) ;117: (13):2873–82. |
[12] | Yu X , Wang B , Li X , Lin G , Zhang C , Yang Y , et al. The significance of metastasectomy in patients with metastatic renal cell carcinoma in the era of targeted therapy. Biomed Res Int. 2015:ID176373. |
[13] | Staehler MD , Kruse J , Haseke N , Stadler T , Roosen A , Karl A , et al. Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J Urol. (2010) ;28: (4):543–7. |
[14] | Petralia G , Roscigno M , Zigeuner R , Strada E , Sozzi F , Da Pozzo L , et al. 450 Complete metastasectomy is an independent predictor of cancer-specific survival in patients with clinically metastatic renal cell carcinoma. Eur Urol Suppl. (2010) ;9: (2):162. |
[15] | Kwak C , Park YH , Jeong CW , Lee SE , Ku JH . Metastasectomy without systemic therapy in metastatic renal cell carcinoma: comparison with conservative treatment. Urol Int. (2007) ;79: (2):145–51. |
[16] | Procopio G , Cognetti F , Miceli R , Milella M , Mosca A , Chiuri VE , et al. A randomized, open label, multicenter phase 2 study, to evaluate the efficacy of sorafenib (So) in patients (pts) with metastatic renal cell carcinoma (mRCC) after a radical resection of the metastases: RESORT trial. J Clin Oncol. (2018) ;36: (15_suppl):4502. |
[17] | Appleman LJ , Puligandla M , Pal SK , Harris W , Agarwal N , Costello BA , et al. Randomized, double-blind phase III study of pazopanib versus placebo in patients with metastatic renal cell carcinoma who have no evidence of disease following metastasectomy: A trial of the ECOG-ACRIN cancer research group (E2810). J Clin Oncol. (2019) ;37: (15_suppl):4502. |
[18] | Rozeman EA , Menzies AM , van Akkooi ACJ , Adhikari C , Bierman C , van de Wiel BA , et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. (2019) ;20: (7):948–60. |
[19] | Verbiest A , Couchy G , Job S , Caruana L , Lerut E , Oyen R , et al. Molecular Subtypes of Clear-cell Renal Cell Carcinoma are Prognostic for Outcome After Complete Metastasectomy. Eur Urol. (2018) ;74: (4). |




