Race/Ethnicity and Survival in Metastatic Renal Cell Carcinoma: Outcomes for Patients Receiving First Line Targeted Therapies
Abstract
BACKGROUND:
No study to date has assessed the relationship between treatment-specific therapeutic outcomes and race/ethnicity in metastatic renal cell carcinoma (mRCC). As targeted therapies have formed the backbone of first-line treatment options for mRCC until very recently, we assessed the relationship between race/ethnicity and targeted therapy-related outcomes in mRCC.
OBJECTIVE:
To retrospectively compare response rates and survival outcomes across ethnicities in patients who received first-line targeted therapies for mRCC.
METHODS:
Patients with mRCC receiving a first-line targeted therapy were identified from an institutional database encompassing consecutive patients treated between 2009 and 2019. Patient demographics, clinical characteristics and survival outcomes were recorded. The racial/ethnic groups included for analysis were Caucasian American, Hispanic American, and Asian American. Survival and response outcomes including progression-free survival (PFS), overall survival (OS), objective response rate (ORR) and disease control rate (DCR) were calculated and compared across ethnic groups using Kaplan-Meier method and Chi-square test, respectively.
RESULTS:
In total, 295 patients were included for analysis. There were 184 (62.4%) Caucasian American patients, 82 (27.8%) Hispanic American patients, and 29 (9.8%) Asian American patients. No statistically significant differences in PFS nor OS were found between groups (PFS: 5.6 vs. 4.7 vs. 4.7 months, respectively) (OS: 32 vs. 31.7 vs. 51.7 months, respectively). No significant difference was found in ORR nor DCR across groups. Univariate cox regression analyses demonstrated no independent effect of race/ethnicity on PFS or OS.
CONCLUSIONS:
The apparent lack of differences in treatment-related outcomes across racial/ethnic groups is encouraging. However, further validation is required in larger series.
INTRODUCTION
Discussions surrounding health disparities in oncology have become a preeminent focus among clinicians, researchers, and professional healthcare organizations [1, 2]. A growing volume of literature has elucidated variations in molecular, pathological, and clinical characteristics of renal cell carcinoma (RCC) based on differences in race and ethnicity. Genomic studies have delineated differences in somatic DNA alterations and gene expression across European American and African American individuals with RCC [3]. Pathological data suggests variations in the frequency of histological subtype across racial/ethnic groups, with clear cell RCC being more common among Hispanic Americans and American Indians. In contrast, a higher proportion of African American individuals had papillary RCC compared to European Americans [4–7]. With respect to clinical characteristics, earlier ages of RCC diagnoses in Hispanic, Native American and African American minority groups compared to non-Hispanic whites have been reported [7–9].
Incidence and mortality rates of RCC also vary across racial/ethnic groups with disparities noted between non-Hispanic whites and minorities (including Native Americans, African Americans, Hispanics, and Asians) [10–18]. Concordant results from these studies suggest that African Americans, Hispanics, and Native Americans experience higher incidence and mortality rates from RCC than non-Hispanic whites and Asians. Although substantial focus has been placed on epidemiological differences and clinicopathological characteristics, there has been little effort to characterize therapy-specific outcomes in metastatic RCC (mRCC) across racial/ethnic groups [19–25]. Targeted therapies continue to play a role in the first-line setting; particularly for patients with favorable risk disease based on the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk score or in combination with immune checkpoint inhibitors [26–29]. Through a single institution experience, this study explores the relationship between race, ethnicity and treatment outcomes in mRCC.
MATERIALS AND METHODS
Patients with mRCC who had received systemic treatment were retrospectively identified from the institutional database curated between 2009 and 2019 at City of Hope Comprehensive Cancer Center. Patients receiving a first-line targeted therapy agent including VEGF- and mTOR-inhibitors were included for analysis. Demographics and clinical data including gender, age, race, ethnicity, treatment received, duration on therapy, and response to treatment were collected for each patient with respect to first-line therapy. The patient population was categorized into three groups: Caucasian American, Hispanic American, or Asian American according to the definition of National Institutes of Health for racial and ethnic categories [30]. Patients from other ethnic or racial groups were excluded due to small sample size. Retrospective data collection was performed in compliance with the requirements of the institutional review board (the retrospective IRB approval number is 18486). This study was conducted based on recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary Figure S1).
Descriptive analyses were conducted for estimation of median, proportions and 95% confidence interval (95% CI) within the overall cohort and the ethnic/racial groups individually. The chi-square test was used to assess association between categorical variables. Objective response was defined as complete or partial response to therapy. Patients who had a complete response, partial response or stable disease for at least 6 months were considered to have disease control. Progression-free survival (PFS) was calculated from the initiation date of the first line targeted therapy until the date of disease progression or death. Overall survival (OS) was calculated from the initiation date of the first line targeted therapy to the date of death or last follow up. PFS and OS were analyzed across racial/ethnic groups with the Kaplan-Meier method and comparisons were tested using the log rank statistic. Cox regression models were generated to identify the effect of variables on survival outcomes. SAS ® 9.4 was used to conduct all statistical analyses and for the generation of graphs.
RESULTS
A total of 295 patients were included in the analysis. The median age of the cohort was 61 years (range 23–89). The majority of patients were male (75.6%), had clear cell histology (81%) and had nephrectomy (84.1%) prior to initiation of systemic treatment. There were 184 (62.4%) Caucasian American patients, 82 (27.8%) Hispanic American patients, and 29 (9.8%) Asian American patients. Demographics and clinical information of patients were comparable across racial/ethnic groups. Patient characteristics in the overall cohort and the individual racial/ethnic subcategories are presented in Table 1.
Table 1
Patient characteristics
| Overall | Caucasian American | Hispanic American | Asian American | p value | |
| N = 295 | N = 184 | N = 82 | N = 29 | ||
| Gender | |||||
| Male | 223 (75.6%) | 155 (84.2%) | 48 (58%) | 20 (69%) | <0.01 |
| Female | 72 (24.4%) | 29 (15.8%) | 34 (42%) | 9 (31%) | |
| Age, median (range) | 61 (23–89) | 62 (28–89) | 59 (23–85) | 65 (25–86) | 0.253 |
| Histologic subtype | 0.9 | ||||
| Clear Cell | 239 (81%) | 150 (81.5%) | 65 (79.3%) | 24 (82.8%) | |
| Non-clear cell | 56 (19%) | 34 (18.5%) | 17 (20.7%) | 5 (17.2%) | |
| Nephrectomy, yes | 248 (84.1%) | 156 (84.8%) | 68 (82.9%) | 24 (82.8%) | 0.9 |
| IMDC risk category | 0.6 | ||||
| Favorable | 89 (30.2%) | 52 (28.3%) | 27 (32.9%) | 10 (34.5%) | |
| Intermediate/Poor | 206 (69.8%) | 132 (71.7%) | 55 (67.1%) | 19 (65.5%) | |
| First line targeted therapy | 0.08 | ||||
| Sunitinib | 185 (62.7%) | 114 (62%) | 56 (68.3%) | 15 (51.7%) | |
| Temsirolimus | 30 (10.2%) | 17 (9.2%) | 7 (8.5%) | 7 (24.1%) | |
| Pazopanib | 21 (7.1%) | 11 (5.9%) | 6 (7.3%) | 5 (17.2%) | |
| Sorafenib | 16 (5.4%) | 12 (6.5%) | 4 (4.9%) | – | |
| Cabozantinib | 11 (3.7%) | 10 (5.4%) | 1 (1.2%) | 1 (3.5%) | |
| Others | 32 (10.6%) | 20 (10.9%) | 8 (9.7%) | 1 (3.5%) | |
| Metastatic Site | |||||
| Lung | 185 (62.7) | 113 (61.4%) | 49 (59.8%) | 23 (79.5%) | 0.1 |
| Lymph node | 149 (50.5%) | 94 (51.1%) | 47 (57.3%) | 8 (27.6%) | 0.02 |
| Bone | 106 (35.9%) | 61 (33.2%) | 38 (46.3%) | 7 (24.1%) | 0.04 |
| Soft tissue | 96 (32.5%) | 61 (33.2%) | 28 (34.1%) | 7 (24.1%) | 0.6 |
| Liver | 49 (16.6%) | 31 (16.8%) | 13 (15.9%) | 5 (17.2%) | 1 |
| Brain | 27 (9.2%) | 17 (9.2%) | 7 (8.5%) | 3 (10.3%) | 1 |
| Other | 74 (25.1%) | 40 (21.7%) | 19 (23.2%) | 5 (17.2) | 0.8 |
N/A: Not applicable, IMDC: International Metastatic Renal Cell Carcinoma Consortium Database Consortium.
Objective response rate and disease control rate was similar across Caucasian American, Hispanic American and Asian American groups (22.3% , 19.5% , 13.5% , p = 0.5 and 58.7% , 62.2% , 55.2% , p = 0.9, respectively). Response patterns of ethnic/racial groups with targeted therapy are detailed in Table 2.
Table 2
Response outcomes across racial/ethnic groups
| Caucasian American | Hispanic American | Asian American | p value | |
| N = 184 | N = 82 | N = 29 | ||
| Response N (%) | 0.9 | |||
| Complete response | 5 (2.7%) | 0 (0.0%) | 0 (0.0%) | |
| Partial response | 36 (19.6%) | 16 (19.5%) | 4 (13.5%) | |
| Stable disease | 74 (40.2%) | 37 (45.1%) | 14 (48.3%) | |
| Progressive disease | 53 (28.8%) | 23 (28%) | 8 (27.6%) | |
| Not Available | 16 (8.7%) | 6 (7.3%) | 3 (10.3%) | |
| Objective response rate (95% CI) | 22.3% (16.2, 28.4) | 19.5% (10.8, 28.3) | 13.8% (0.4, 27.1) | 0.6 |
| Disease control rate (95% CI) | 58.7 (51.5, 65.9) | 62.2 (51.5, 72.9) | 55.2 (35.9, 74.4) | 0.9 |
With a median follow up of 51.2 months (95% CI: 42.6, 63.8), progression free survival was 5.6 months (95% CI 4.1 –8.1), 4.7 months (95% CI 1.9 –6.4) and 4.7 months (95% CI 3.5–7.5), and overall survival was 32 months (95% CI 25.5 –37.3), 31.7 months (95% CI 25.8 –47.9) and 51.7 months (95% CI 33.3 –79.4), respectively, for Caucasian American, Hispanic American and Asian American patient groups. Neither progression free survival nor overall survival differed in a statistically significant manner across the ethnic/racial groups. Kaplan-Meier plots are provided in Fig. 1.
Fig. 1
Progression free survival (A) and overall survival (B) across racial/ethnic groups.
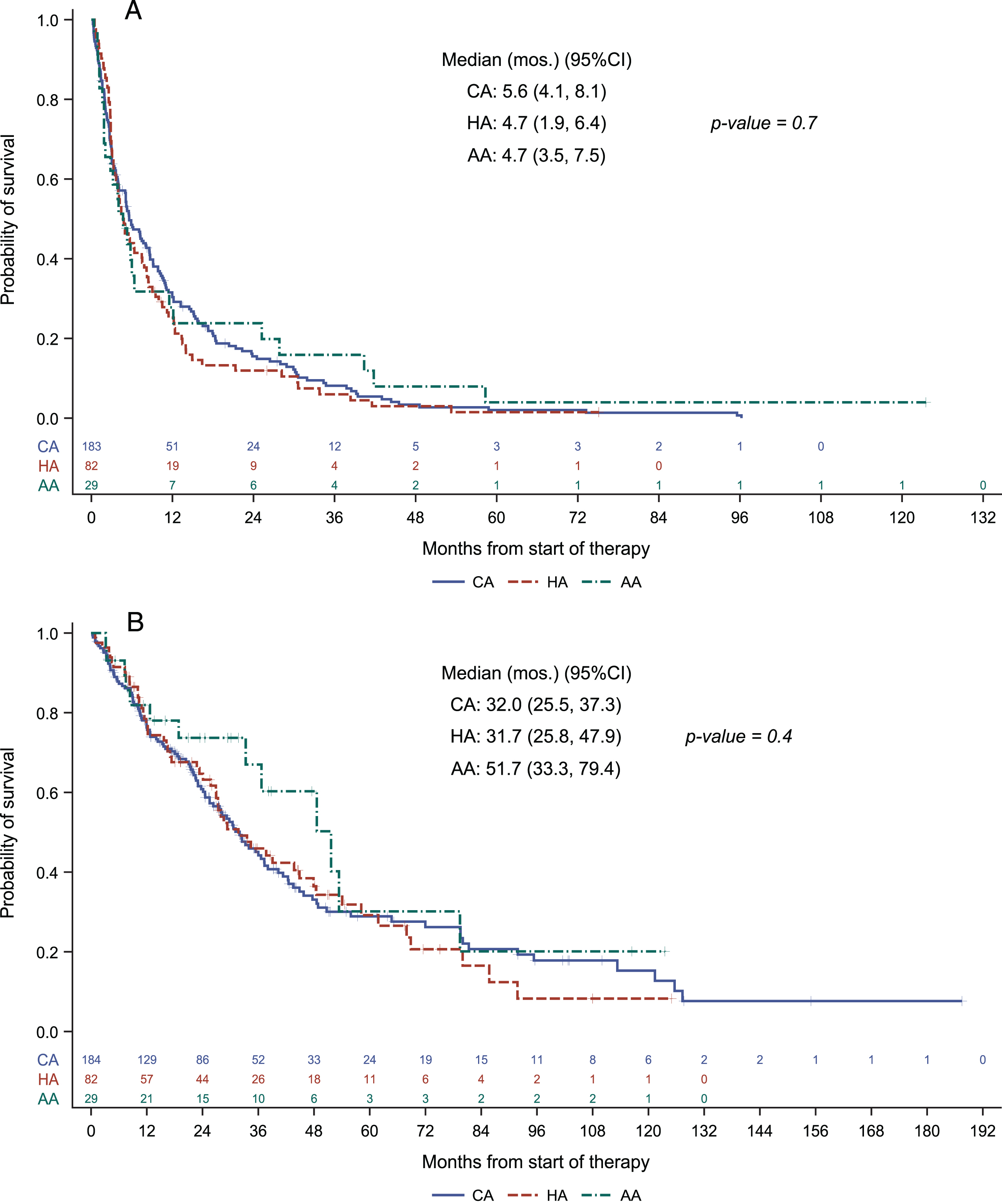
Univariate cox regression analysis demonstrated no significant relationship between ethnicity/race and progression free survival or overall survival outcomes (Table 3). Amongst the patient characteristics tested, only IMDC risk category stood as a correlate of progression free survival (HR = 1.57, 95% CI 1.21 –2.04, p = 0.001). Univariate analysis for overall survival showed that IMDC risk category and age were the parameters correlated with overall survival (HR = 2.24, 95% CI 1.60 –3.15, p < 0.0001 and HR 1.36, 95% CI 1.01 –1.84, p = 0.047) (Table 3). As in progression-free survival, race was not a predictor of overall survival. No additional variables were significant when considered in multivariate regression analyses in the presence of IMDC risk groups for prediction of progression-free or overall survival.
Table 3
Univariate Cox proportional hazards model for prediction of progression-free and overall survival
| PFS | OS | |||
| Hazard ratio | p value | Hazard ratio | p value | |
| (95% CI) | (95% CI) | |||
| Gender (Male vs. female) | 0.78 (0.59, 1.03) | 0.08 | 0.82 (0.59, 1.14) | 0.2 |
| Age (≥65 vs.<65) | 1.07 (0.84, 1.37) | 0.6 | 1.36 (1.01, 1.84) | 0.047 |
| Race | ||||
| Caucasian American (baseline) | – | 0.7 | – | 0.4 |
| Hispanic American | 1.11 (0.85, 1.45) | 1.02 (0.74, 1.42) | ||
| Asian American | 0.92 (0.61, 1.40) | 0.70 (0.39, 1.24) | ||
| Histologic subtype (ccRCC vs. other) | 0.85 (0.63, 1.15) | 0.3 | 1.04 (0.70, 1.54) | 0.8 |
| IMDC (intermediate/poor vs. favorable risk) | 1.57 (1.21, 2.04) | 0.001 | 2.24 (1.60, 3.15) | <0.001 |
PFS: Progression free survival, OS: Overall survival, CI: Confidence interval, ccRCC: Clear cell renal cell carcinoma, IMDC: International Metastatic Renal Cell Carcinoma Consortium Database Consortium.
DISCUSSION
No statistically significant differences were seen between race/ethnicity and objective response rate, race/ethnicity and progression free survival, or race/ethnicity and overall survival following first-line targeted therapy. These results, albeit with a limited sample size, are encouraging and point towards a lack of differences in clinical outcome on the basis of race/ethnicity at a tertiary cancer center. Our study adds to a growing compendium of evidence regarding health disparities in RCC by reporting the first data comparing treatment-specific outcomes in mRCC across Caucasian Americans, Hispanic Americans, and Asian Americans.
Disparities in cancer-related outcomes remain a significant issue in oncology. Aizer et al identified over two million cancer patients diagnosed from 1988–2007 and reported that racial disparities in cancer-specific mortality has not changed over that 20-year period, specifically with respect to African Americans compared to people of other race/ethnicities diagnosed with cancer [31]. A similar study from Singh and Jemal looked at racial/ethnic disparities in cancer incidence and outcomes over 60 years, from 1950–2014 [32]. The authors found higher mortality rates among African Americans and lower rates among Asian/Pacific Islanders and Hispanics compared to Caucasians. Neither study offered direct focus on RCC. Even when compared with other genitourinary malignancies, the understanding of ethnic and racial disparities is less developed in RCC than in bladder and prostate cancer, warranting further investigation [33].
The limited studies that do exist in RCC do offer critical insights. A study from Luzzago et al., included an analysis of survival across racial/ethnic groups specifically in patients receiving systemic therapy for RCC [14]. The investigators demonstrated a statistically significant difference in OS between African Americans, Caucasians, and Hispanics with clear cell disease, but not difference in those with non-clear cell histologies. These results, unlike in our study, are not controlled for disease stage or treatment-type and represent only a subset analysis of their larger works.
The generalizability of our results is narrowed by the highly-selected patient population chosen for this study, specifically in the context of treatment type. Targeted therapy was the predominant standard-of-care option for mRCC in the first line from 2005–2018, during which the overwhelming majority of patients in our database were initiated on treatment. As utilization of front-line immune therapy increases, long-term follow-up of this population will be feasible. Immune therapy has emerged as an effective treatment modality in mRCC with significantly higher costs and more frequent hospital visits that could lead to aggravation of preexisting racial and ethnic disparities [34]. Thus, as our follow up duration in immunotherapy-treated patients matures, we will repeat our analysis in the context of immune treatments and perform comparisons across different treatment types to unravel potential disparities across racial/ethnic groups [27].
The exclusion of African American cases from the analyses due to small sample size poses another limitation of this study. Analysis of The Cancer Genome Atlas clear cell kidney dataset reported that African American patients have less frequent VHL inactivation and decreased upregulation of HIF-associated genomic signatures [3]. Despite the link between HIF-associated genomic signatures and benefit from anti-angiogenic therapies directed on VEGF is yet to be studied, this, in theory, could predict poorer outcomes. Additionally, previous studies have demonstrated greater incidences of RCC diagnosis and poorer survival outcomes among African Americans, which we could not provide further evidence in support of [11–16, 18]. In addition to the limitations of not including an African American cohort, the number of the Asian American patients included in this study was relatively small. This might be a cause of the statistically nonsignificant but numerically prolonged overall survival in this cohort despite similar objective response rates.
The results of this study present a best-case scenario for the current state of race- and ethnicity-based healthcare disparities in RCC. Moving forward, we aim to utilize multi-center collaborations to better define treatment outcomes in mRCC across a wider variety of racial/ethnic groups with larger sample sizes while also comparing racial/ethnic disparities in the academic versus community settings. As the mRCC treatment algorithm now emphasizes immunotherapy in the front-line for the majority of patients, further assessment of racial/ethnic disparities in immunotherapy response will also be valuable.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
N. Dizman: Study concept and design; acquisition, analysis, or interpretation of data; drafting of the manuscript; statistical analysis; critical revision of the manuscript for important intellectual content.
N. Salgia: Study concept and design; acquisition, analysis, or interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
P. G. Bergerot: Acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content.
J. Hsu: Acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content.
N. Ruel: Acquisition, analysis, or interpretation of data; statistical analysis.
S. K. Pal: Study concept and design; acquisition, analysis, or interpretation of data; drafting of the manuscript; statistical analysis; critical revision of the manuscript for important intellectual content; study supervision.
CONFLICT OF INTEREST
N. Dizman, N. Salgia, P. G. Bergerot, and J. Hsu have no conflicts of interest to report. S. K. Pal reports consulting roles in Genentech, Aveo, Eisai, Roche, Pfizer, Novartis, Exelixis, Ipsen, BMS, Astellas.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-200092.
REFERENCES
[1] | Goss E , Lopez AM , Brown CL , Wollins DS , Brawley OW , Raghavan D . American society of clinical oncology policy statement: disparities in cancer care. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. (2009) ;27: (17):2881–5. |
[2] | Griggs J , Maingi S , Blinder V , Denduluri N , Khorana AA , Norton L , Francisco M , Wollins DS , Rowland JH . American Society of Clinical Oncology Position Statement: Strategies for Reducing Cancer Health Disparities Among Sexual and Gender Minority Populations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. (2017) ;35: (19):2203–8. |
[3] | Krishnan B , Rose TL , Kardos J , Milowsky MI , Kim WY . Intrinsic Genomic Differences Between African American and White Patients With Clear Cell Renal Cell Carcinoma. JAMA Oncology. (2016) ;2: (5):664–7. |
[4] | Batai K , Bergersen A , Price E , Hynes K , Ellis NA , Lee BR . Clinical and Molecular Characteristics and Burden of Kidney Cancer Among Hispanics and Native Americans: Steps Toward Precision Medicine. Clinical Genitourinary Cancer. (2018) ;16: (3):e535–e41. |
[5] | Lipworth L , Morgans AK , Edwards TL , Barocas DA , Chang SS , Herrell SD , Penson DF , Resnick MJ , Smith JA , Clark PE . Renal cell cancer histological subtype distribution differs by race and sex. BJU International. (2016) ;117: (2):260–5. |
[6] | Olshan AF , Kuo TM , Meyer AM , Nielsen ME , Purdue MP , Rathmell WK . Racial difference in histologic subtype of renal cell carcinoma. Cancer Medicine. (2013) ;2: (5):744–9. |
[7] | Batai K , Harb-De la Rosa A , Zeng J , Chipollini JJ , Gachupin FC , Lee BR . Racial/ethnic disparities in renal cell carcinoma: Increased risk of early-onset and variation in histologic subtypes. Cancer Medicine. (2019) ;8: (15):6780–8. |
[8] | Batai K , Harb-De la Rosa A , Lwin A , Chaus F , Gachupin FC , Price E , Lee BR . Racial and Ethnic Disparities in Renal Cell Carcinoma: An Analysis of Clinical Characteristics. Clinical Genitourinary Cancer. (2019) ;17: (1):e195–e202. |
[9] | Mafolasire A , Yao X , Nawaf C , Suarez-Sarmiento A , Chow WH , Zhao W , Corley D , Hofmann JN , Purdue M , Adeniran AJ , Shuch B . Racial disparities in renal cell carcinoma: a single-payer healthcare experience. Cancer Medicine. (2016) ;5: (8):2101–8. |
[10] | Pinheiro PS , Callahan KE , Gomez SL , Marcos-Gragera R , Cobb TR , Roca-Barcelo A , Ramirez AG . High cancer mortality for US-born Latinos: evidence from California and Texas. BMC Cancer. (2017) ;17: (1):478. |
[11] | Lin J , Zahm SH , Shriver CD , Purdue M , McGlynn KA , Zhu K . Survival among Black and White patients with renal cell carcinoma in an equal-access health care system. Cancer Causes & Control: CCC. (2015) ;26: (7):1019–26. |
[12] | Colt JS , Schwartz K , Graubard BI , Davis F , Ruterbusch J , DiGaetano R , Purdue M , Rothman N , Wacholder S , Chow WH . Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology (Cambridge, Mass). (2011) ;22: (6):797–804. |
[13] | Marchioni M , Harmouch SS , Nazzani S , Bandini M , Preisser F , Tian Z , Kapoor A , Cindolo L , Briganti A , Shariat SF , Schips L , Karakiewicz PI . Effect of African-American race on cancer specific mortality differs according to clear cell vs. non-clear cell histologic subtype in metastatic renal cell carcinoma. Cancer Epidemiology. (2018) ;54: :112–8. |
[14] | Luzzago S , Palumbo C , Rosiello G , Knipper S , Pecoraro A , Nazzani S , Tian Z , Musi G , Montanari E , Shariat SF , Saad F , Briganti A , de Cobelli O , Karakiewicz PI . Racial and ethnic differences in survival in contemporary metastatic renal cell carcinoma patients, according to alternative treatment modalities. Cancer Causes & Control. (2020) ;31: (3):263–72. |
[15] | Chow WH , Devesa SS . Contemporary epidemiology of renal cell cancer. Cancer journal (Sudbury, Mass). (2008) ;14: (5):288–301. |
[16] | Schwartz K , Ruterbusch JJ , Colt JS , Miller DC , Chow WH , Purdue MP . Racial disparities in overall survival among renal cell carcinoma patients with young age and small tumors. Cancer Medicine. (2016) ;5: (2):200–8. |
[17] | Li J , Weir HK , Jim MA , King SM , Wilson R , Master VA . Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990-2009. American Journal of Public Health. (2014) ;104: (Suppl 3):S396–403. |
[18] | Stafford HS , Saltzstein SL , Shimasaki S , Sanders C , Downs TM , Sadler GR . Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. The Journal of Urology. (2008) ;179: (5):1704–8. |
[19] | Chow LQ , Eckhardt SG . Sunitinib: from rational design to clinical efficacy. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2007) ;25: (7):884–96. |
[20] | Gore ME , Szczylik C , Porta C , Bracarda S , Bjarnason GA , Oudard S , Lee SH , Haanen J , Castellano D , Vrdoljak E , Schoffski P , Mainwaring P , Hawkins RE , Crino L , Kim TM , Carteni G , Eberhardt WE , Zhang K , Fly K , Matczak E , Lechuga MJ , Hariharan S , Bukowski R . Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. British Journal of Cancer. (2015) ;113: (1):12–9. |
[21] | Sternberg CN , Davis ID , Mardiak J , Szczylik C , Lee E , Wagstaff J , Barrios CH , Salman P , Gladkov OA , Kavina A , Zarba JJ , Chen M , McCann L , Pandite L , Roychowdhury DF , Hawkins RE . Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2010) ;28: (6):1061–8. |
[22] | Hutson TE , Al-Shukri S , Stus VP , Lipatov ON , Shparyk Y , Bair AH , Rosbrook B , Andrews GI , Vogelzang NJ . Axitinib Versus Sorafenib in First-Line Metastatic Renal Cell Carcinoma: Overall Survival From a Randomized Phase III Trial. Clinical Genitourinary Cancer. (2017) ;15: (1):72–6. |
[23] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , Tykodi SS , Sosman JA , Procopio G , Plimack ER , Castellano D , Choueiri TK , Gurney H , Donskov F , Bono P , Wagstaff J , Gauler TC , Ueda T , Tomita Y , Schutz FA , Kollmannsberger C , Larkin J , Ravaud A , Simon JS , Xu L-A , Waxman IM , Sharma P . Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine. (2015) ;373: (19):1803–13. |
[24] | Motzer RJ , Tannir NM , McDermott DF , Aren Frontera O , Melichar B , Choueiri TK , Plimack ER , Barthelemy P , Porta C , George S , Powles T , Donskov F , Neiman V , Kollmannsberger CK , Salman P , Gurney H , Hawkins R , Ravaud A , Grimm MO , Bracarda S , Barrios CH , Tomita Y , Castellano D , Rini BI , Chen AC , Mekan S , McHenry MB , Wind-Rotolo M , Doan J , Sharma P , Hammers HJ , Escudier B . Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England Journal of Medicine. (2018) ;378: (14):1277–90. |
[25] | Hudes G , Carducci M , Tomczak P , Dutcher J , Figlin R , Kapoor A , Staroslawska E , Sosman J , McDermott D , Bodrogi I , Kovacevic Z , Lesovoy V , Schmidt-Wolf IG , Barbarash O , Gokmen E , O’Toole T , Lustgarten S , Moore L , Motzer RJ . Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England Journal of Medicine. (2007) ;356: (22):2271–81. |
[26] | de Velasco G , Bex A , Albiges L , Powles T , Rini BI , Motzer RJ , Heng DYC , Escudier B . Sequencing and Combination of Systemic Therapy in Metastatic Renal Cell Carcinoma. European Urology Oncology. (2019) ;2: (5):505–14. |
[27] | Motzer RJ , Jonasch E , Michaelson MD , Nandagopal L , Gore JL , George S , Alva A , Haas N , Harrison MR , Plimack ER , Sosman J , Agarwal N , Bhayani S , Choueiri TK , Costello BA , Derweesh IH , Gallagher TH , Hancock SL , Kyriakopoulos C , LaGrange C , Lam ET , Lau C , Lewis B , Manley B , McCreery B , McDonald A , Mortazavi A , Pierorazio PM , Ponsky L , Redman BG , Somer B , Wile G , Dwyer MA , Hammond LJ , Zuccarino-Catania G . NCCN Guidelines Insights: Kidney Cancer, Version 2.2020. Journal of the National Comprehensive Cancer Network: JNCCN. (2019) ;17: (11):1278–85. |
[28] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , Pouliot F , Alekseev B , Soulieres D , Melichar B , Vynnychenko I , Kryzhanivska A , Bondarenko I , Azevedo SJ , Borchiellini D , Szczylik C , Markus M , McDermott RS , Bedke J , Tartas S , Chang YH , Tamada S , Shou Q , Perini RF , Chen M , Atkins MB , Powles T . Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England Journal of Medicine. (2019) ;380: (12):1116–27. |
[29] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , Venugopal B , Kollmannsberger C , Negrier S , Uemura M , Lee JL , Vasiliev A , Miller WH Jr . , Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England Journal of Medicine. (2019) ;380: (12):1103–15. |
[30] | Health NIo. https://grants.nih.gov/grants/guide/noticefiles/not-od-15-089.html. 2015 |
[31] | Aizer AA , Wilhite TJ , Chen M-H , Graham PL , Choueiri TK , Hoffman KE , Martin NE , Trinh Q-D , Hu JC , Nguyen PL . Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. (2014) ;120: (10):1532–9. |
[32] | Singh GK , Jemal A . Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of Environmental and Public Health. (2017) ;2017: :2819372. |
[33] | Das H , Rodriguez R . Health Care Disparities in Urologic Oncology: A Systematic Review. Urology. (2020) ;136: :9–18. |
[34] | Haque W , Verma V , Butler EB , Teh BS . Racial and Socioeconomic Disparities in the Delivery of Immunotherapy for Metastatic Melanoma in the United States. Journal of Immunotherapy. (2019) ;42: (6):228–35. |




