Trends in Initial Systemic Therapy for Elderly Patients with Metastatic Clear Cell Renal Cell Carcinoma
Abstract
BACKGROUND:
The treatment landscape for metastatic clear cell renal cell carcinoma (mRCC) is rapidly changing. It is unknown how adoption of new types of therapies may differ by patient age.
OBJECTIVE:
To compare trends in first-line therapy use for older (≥70 years) and younger (< 70) patients with mRCC before and after approval of nivolumab in 2015.
METHODS:
Using the National Cancer Database, we assessed trends in first-line therapy use by calculating the proportion of patients receiving targeted therapy, immunotherapy, or no systemic therapy by year of diagnosis. Initial systemic treatment was compared for patients diagnosed in 2016 with patients diagnosed in 2011 as a control group prior to nivolumab approval. Multivariable regression analysis was used to evaluate the interaction between year of diagnosis and elderly status for use of first-line immunotherapy or targeted therapy.
RESULTS:
From 2006 to 2016, the proportion of patients receiving any type of systemic therapy increased from 43.7% to 56.5%. On stratified multivariable regression analysis, older patients diagnosed in 2016 were 17.3 times more likely to receive first-line immunotherapy compared to those diagnosed in 2011, while younger patients were 2.3 times more likely. There was no change in targeted therapy use over this time regardless of patient age.
CONCLUSIONS:
The rate of adoption of first-line immunotherapy was particularly pronounced for elderly compared to younger patients. While first-line use of immunotherapy may have allowed elderly patients to receive systemic therapy that they otherwise would not, the efficacy of these drugs in elderly patients deserves further study.
INTRODUCTION
Kidney cancer represents approximately 4% of all new cancer diagnoses in the United States (US) and is primarily a disease of older adults with a median age at diagnosis of 64 years [1]. Of all kidney cancers, the vast majority are renal cell carcinomas (RCC) and the predominant histologic subtype of RCC is clear cell carcinoma [2]. Approximately 15– 20% of patients will have metastatic disease at diagnosis and an additional 20% of patients with initially localized disease will experience tumor recurrence [3, 4]. With the large population burden of disease in older adults, there is a great need for effective yet tolerable systemic treatments for RCC. Historically, treatment for metastatic RCC (mRCC) was characterized by substantial toxicity and modest survival benefits. High-dose IL-2 (HD-IL2) was approved by the Food and Drug Administration (FDA) for mRCC in 1992 and, along with interferon-alpha, represented a standard of care for many years. Given the low response rates and high toxicity, use of HD-IL2 was limited to younger patients with excellent performance status [5, 6].
The treatment landscape for RCC changed with the approval of targeted inhibitors of vascular endothelial growth factor receptor (VEGFR) and mammalian target of rapamycin (mTOR), which demonstrated improved survival outcomes and tolerability, allowing a greater proportion of patients to receive systemic therapy [7]. More recently, approval of immune checkpoint inhibitors (ICI) has ushered in another new generation of treatment options. The approval of nivolumab as second-line monotherapy for mRCC in 2015 preceded first-line immunotherapy combination approvals by several years, with ipilimumab plus nivolumab not approved until 2018.
This study aims to evaluate trends in the use of first-line therapies for mRCC in order to explore whether the introduction of ICIs for mRCC has changed the proportion of patients receiving any systemic treatment, and to examine age-related differences in utilization trends. We hypothesize that the approval of nivolumab in 2015 led to a higher proportion of patients receiving any first-line systemic treatment, even prior to first-line ICI approval. Furthermore, we hypothesize that early adoption of first-line ICI for mRCC disproportionately increased in elderly compared to younger patients due to the enhanced tolerability of ICI.
MATERIALS AND METHODS
This study was exempt from IRB approval as it uses a publicly available de-identified data set.
We utilized patient registry data from the National Cancer Database (NCDB) from 2006 - 2016. The NCDB contains data regarding cancer diagnosis, treatment, and outcomes from over 1,500 cancer programs across the US and Puerto Rico and captures approximately 70% of all new cancer diagnoses in the US [8]. Additional information describing the NCDB and the data collected are detailed elsewhere [8]. Patients included in this study were age 18 years or older and were diagnosed with metastatic clear cell RCC. Metastatic disease was determined using the analytic stage group, while clear cell histology was based on International Classification of Diseases for Oncology, 3rd edition, codes (8310 and 8312). Patients that underwent nephrectomy were identified using surgery of primary site codes (40, 50, 70). Oncology practice type was defined per the NCDB. Patients age 70 years or older were considered “elderly.” Patients were identified as receiving first-line chemotherapy, immunotherapy, or no systemic therapy per NCDB standard definitions. While targeted therapy is categorized as chemotherapy by the NCDB, patients coded as receiving chemotherapy were assumed to have received targeted therapy as traditional cytotoxic chemotherapy is not a standard of care for mRCC. For patients coded as receiving both targeted therapy and immunotherapy, the time from diagnosis to therapy initiation was evaluated to determine which therapy was first-line. Patients with the same time (within 30 days) from diagnosis to initiation of targeted therapy and immunotherapy were considered to receive both as first-line treatment.
Overall trends in first-line therapy use were assessed by calculating the proportion of patients receiving targeted therapy, immunotherapy, or no systemic therapy by year of diagnosis. Initial systemic treatment was then compared for patients diagnosed in 2016, the year after nivolumab approval but prior to front-line ICI combination therapy approval, with patients diagnosed in 2011, as a control group prior to nivolumab approval. Comparisons of initial treatment by year of diagnosis (2011 vs 2016) were performed using chi-square analysis. Multivariable log binomial regression analysis was used to evaluate the interaction between year of diagnosis and elderly status for use of first-line immunotherapy or targeted therapy. All models were controlled for patient gender, race, Charlson comorbidity score, nephrectomy status, insurance status, and oncology practice type. Sensitivity analyses were performed to evaluate whether outcomes differed by the age cut point used to define “elderly” or by year used as a comparator. Additionally, the Joinpoint Regression Program was used to calculate the estimated annual percent change in use of immunotherapy or targeted therapy by elderly status [9, 10]. Statistical analyses were performed using STATA v16.0 and all analyses were considered statistically significant at p < 0.05.
Table 1
Patient characteristics
| Entire Cohort | Young Patients | Elderly | p-value | |
| (n = 42,577) | (< 70 years, | Patients | ||
| n = 27,411) | (≥70 years, | |||
| n = 15,166) | ||||
| Median age (IQR) | 65 (57– 74) | 59 (53– 64) | 77 (73– 82) | < 0.001 |
| Male | 66.9% | 70.3% | 60.7% | < 0.001 |
| Race | < 0.001 | |||
| White | 86.5% | 85.6% | 88.2% | |
| Black | 9.2% | 10% | 7.7% | |
| Other | 4.3% | 4.4% | 4.1% | |
| Practice type | < 0.001 | |||
| CCP | 9.1% | 8.3% | 10.6% | |
| CCCP | 39.3% | 36.7% | 43.9% | |
| Academic | 38.9% | 42.8% | 31.9% | |
| INCP | 12.7% | 12.2% | 13.6% | |
| Charlson comorbidity score | < 0.001 | |||
| 0 | 68.8% | 72.3% | 62.4% | |
| 1 | 20.8% | 19.4% | 23.5% | |
| 2 | 6.7% | 5.4% | 9.0% | |
| ≥ 3 | 3.7% | 2.9% | 5.1% | |
| Underwent nephrectomy | 34.7% | 41.9% | 22.1% | < 0.001 |
| Insurance status | < 0.001 | |||
| Not insured | 4.7% | 6.9% | 0.8% | |
| Private insurance | 36.6% | 51.3% | 10.1% | |
| Medicaid | 7.6% | 10.9% | 1.6% | |
| Medicare | 46.8% | 25.9% | 84.6% | |
| Other | 4.3% | 5.0% | 2.9% |
Abbreviations: CCP, community cancer program; CCCP, comprehensive community cancer program; INCP, integrated network cancer program; IQR, interquartile range.
RESULTS
A total of 42,577 patients were identified for inclusion in this study. There was an increase in the absolute number of patients diagnosed with mRCC each year with 3,150 patients diagnosed in 2006, 3,765 diagnosed in 2011, and 4,577 diagnosed in 2016. The proportion of metastatic ccRCC of all ccRCC diagnoses remained stable over time (13.8% in 2006 to 14.2% in 2016). Median age at diagnosis was 65 years, with 35.6% of patients categorized as elderly (Table 1). From 2006 to 2016, the overall proportion of patients receiving any type of systemic therapy increased significantly from 43.7% to 56.5% (p < 0.001; Fig. 1). In 2016, 7.7% of all patients received initial immunotherapy and 51.1% received targeted therapy, compared to 2.6% and 49.8%, respectively, in 2011, representing a significant rise in the use of immunotherapy (p < 0.001) but unchanged use of targeted therapy (p = 0.26; Fig. 2).
Fig.1
Trends in initial systemic treatment for mRCC by year of diagnosis.
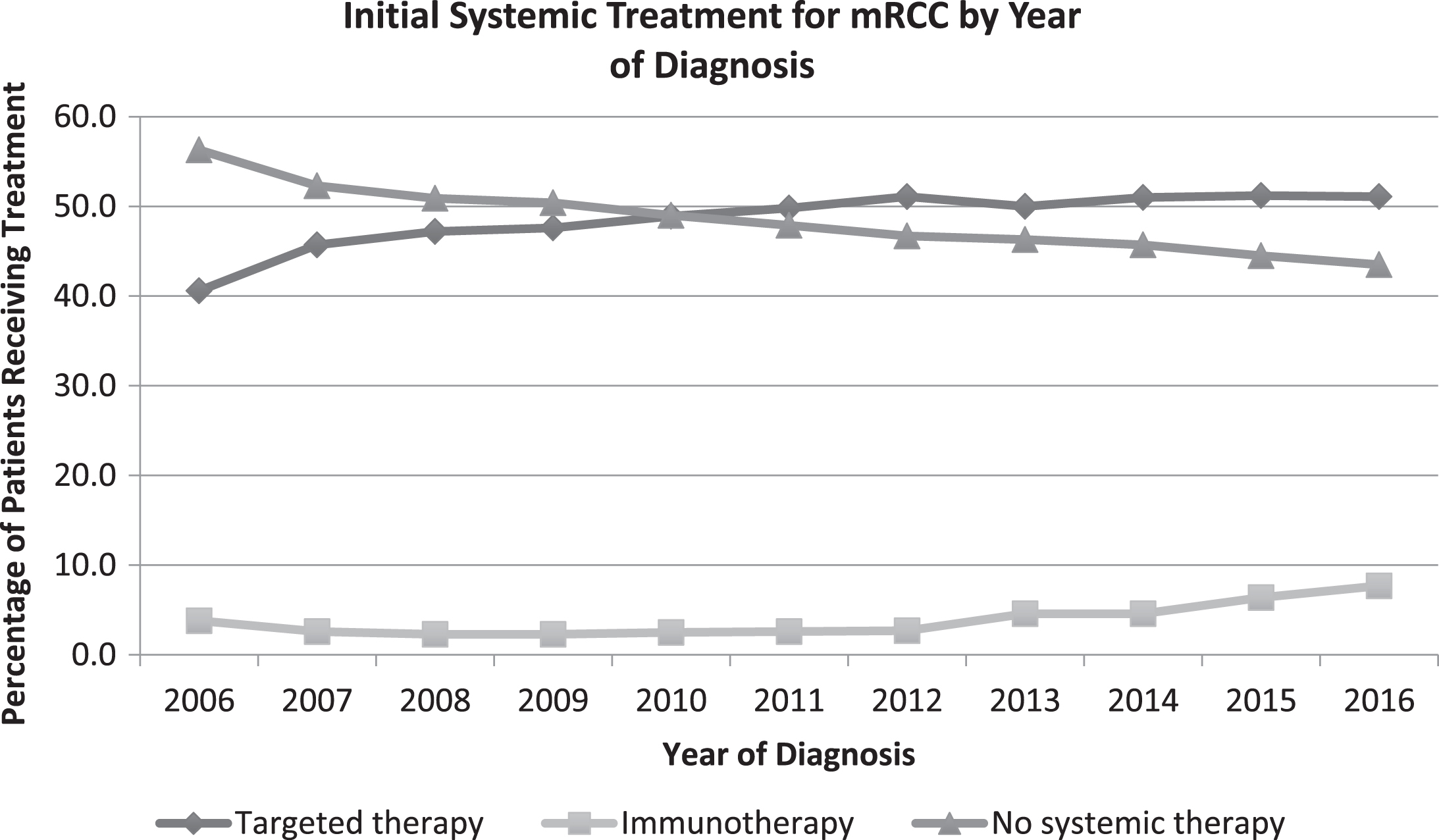
Fig.2
Trends in initial use of immunotherapy by patient age and year of diagnosis.
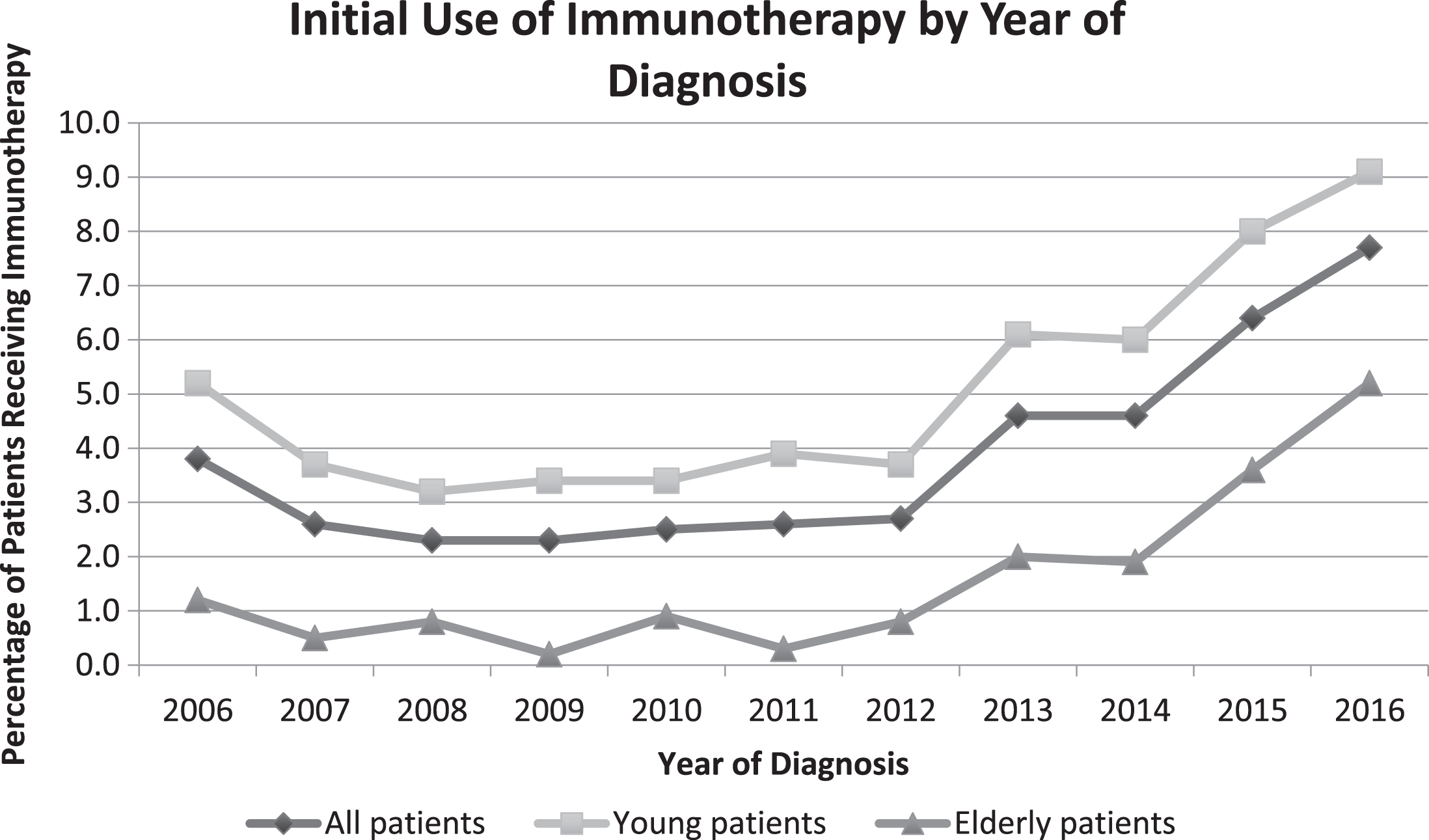
On multivariable log binomial regression analysis with immunotherapy use as the primary outcome, there was a significant interaction between year of diagnosis and elderly status (unadjusted pinteraction < 0.001). This interaction remained significant after controlling for patient gender, race, Charlson comorbidity score, prior nephrectomy, insurance status, and oncology practice type (adjusted pinteraction < 0.001). On stratified adjusted analysis, elderly patients diagnosed in 2016 were 17.3 times more likely to receive first-line immunotherapy as elderly patients diagnosed in 2011 (95% CI 6.4– 47.1, p < 0.001; unadjusted rate of immunotherapy use 5.2% vs 0.3% with relative percent change of 1633%), while younger patients diagnosed in 2016 were 2.3 times more likely to receive immunotherapy as young patients diagnosed in 2011 (95% CI 1.8– 2.9, p < 0.001; unadjusted rate of immunotherapy use 9.1% vs 3.9% with relative percent change of 133%, Table 2). The estimated annual percent change for elderly patients receiving immunotherapy was 70.7 (95% CI 38.4– 110.6) and for young patients was 20.5 (95% CI 11.1– 30.7). There was no interaction between year of diagnosis and elderly status for the first-line use of targeted therapy (unadjusted pinteraction = 0.36; adjusted pinteraction = 0.33), and the annual percent change for use of targeted therapy was 1.7 (95% CI 0.7– 2.8) and 0.2 (95% CI – 0.6– 1.0) for elderly and younger patients, respectively. Results were not different if only patients with pathologically confirmed clear cell histology were included, if the cut point for “elderly” was changed to≥60 years,≥65 years, or≥75 years, or if the comparator year was changed to 2012 or 2013.
Table 2A
Stratified multivariable regression model to predict first-line use of immune checkpoint inhibitors in patients (A) under 70 years old and (B) patients 70 years and over
| A: | ||
| Variable | Adjusted risk ratio | p Value |
| (95% CI) | ||
| Year of diagnosis | ||
| 2011 | 1.0 | |
| 2016 | 2.3 (1.8– 2.9) | < 0.001 |
| Gender | ||
| Male | 1.0 | |
| Female | 0.88 (0.69– 1.1) | 0.28 |
| Race | ||
| White | 1.0 | |
| Black | 0.77 (0.50– 1.2) | 0.22 |
| CCI | ||
| 0 | 1.0 | |
| 1 | 0.77 (0.57– 1.0) | 0.08 |
| 2 | 0.62 (0.34– 1.1) | 0.11 |
| ≥3 | 1.1 (0.69– 1.7) | 0.72 |
| Insurance | ||
| Uninsured | 1.0 | |
| Private | 1.65 (0.96– 2.9) | 0.07 |
| Medicaid | 1.1 (0.58– 2.1) | 0.77 |
| Medicare | 1.1 (0.62– 2.0) | 0.75 |
| Nephrectomy status | ||
| No nephrectomy | 1.0 | |
| Received nephrectomy | 2.0 (1.59– 2.4) | < 0.001 |
| Practice type | ||
| CCP | 1.0 | |
| CCCP | 0.82 (0.49– 1.2) | 0.39 |
| Academic/research | 1.2 (0.78– 1.8) | 0.45 |
| INCP | 1.2 (0.77– 2.0) | 0.40 |
Abbreviations: CCP, community cancer program; CCCP, comprehensive community cancer program; CI, confidence interval; INCP, integrated network cancer program.
Table 2B
Table2B
| B: | ||
| Variable | Adjusted risk ratio | p Value |
| (95% CI) | ||
| Year of diagnosis | ||
| 2011 | 1.0 | |
| 2016 | 17.3 (6.4– 47.1) | < 0.001 |
| Gender | ||
| Male | 1.0 | |
| Female | 0.73 (0.47– 1.1) | 0.17 |
| Race | ||
| White | 1.0 | |
| Black | 0.46 (0.15– 1.4) | 0.18 |
| CCI | ||
| 0 | 1.0 | |
| 1 | 1.0 (0.61– 1.7) | 0.96 |
| 2 | 0.84 (0.41– 1.7) | 0.64 |
| ≥3 | 0.53 (0.22– 1.3) | 0.17 |
| Insurance | ||
| Uninsured | 1.0 | |
| Private | 0.53 (0.07– 4.1) | 0.54 |
| Medicaid | N/Aa | |
| Medicare | 0.76 (0.11– 5.1) | 0.78 |
| Nephrectomy status | ||
| No nephrectomy | 1.0 | |
| Received nephrectomy | 1.4 (0.86– 2.1) | 0.19 |
| Practice type | ||
| CCP | 1.0 | |
| CCCP | 0.93 (0.49– 1.8) | 0.83 |
| Academic/research | 0.62 (0.30– 1.3) | 0.18 |
| INCP | 1.0 (0.49– 2.3) | 0.90 |
Abbreviations: CCP, community cancer program; CCCP, comprehensive community cancer program; CI, confidence interval; INCP, integrated network cancer program. aNo elderly patients with Medicaid were treated with immunotherapy.
DISCUSSION
Our study found that after second-line approval of nivolumab in 2015, there was an increase in the total proportion of patients who received systemic therapy for mRCC. This increase appears to be driven by the use of first-line immunotherapy, even before FDA approval of first-line ICI for mRCC, with stagnant use of targeted therapy over the same time period. Notably, the rate of adoption of first-line immunotherapy was particularly pronounced for elderly patients compared to younger patients. These trends suggest that the introduction of ICI allowed patients that were previously ineligible for systemic therapy to undergo treatment, likely partly due to the perceived increased tolerability of ICI compared to targeted therapy. Furthermore, the lack of a corresponding decline in targeted therapy use suggests that ICI did not replace targeted therapy for patients that were candidates for either treatment during this time.
These results extend prior work examining trends in first-line immunotherapy or targeted therapy use for mRCC between 2003-2011, which found a significant decrease in the use of immunotherapy over this time after the approval of targeted agents [7]. During that period, HD-IL2 was the primary immunotherapy available and so targeted therapy represented a more tolerable and efficacious alternative, whereas in the current treatment era ICIs are now seen as the more tolerable option. We also found that use of immunotherapy across all patients had a slow rise beginning in 2012. We suspect that this initial small increase reflects a combination of patient enrollment on clinical trials of immunotherapy and improvement in supportive care, thus increasing eligibility for the small subset of patients that were treated with HD-IL2. Despite this, the percentage of elderly patients receiving immunotherapy remained below 2% until 2015.
While first-line use of ICI for mRCC may have allowed many elderly patients to receive systemic therapy that otherwise would not, evidence for the efficacy of these drugs in elderly patients deserves further study and caution must be exercised when applying new treatments to untested clinical scenarios. In the CheckMate-025 study comparing nivolumab versus everolimus in previously treated mRCC, the median participant age was 62 with an overall hazard ratio (HR) for death of 0.73 (98.5% CI 0.57– 0.93). However, for patients age 75 or older, the HR was 1.23 (95% CI 0.66– 2.31) [11]. Similarly, the KEYNOTE-426 trial of pembrolizumab+axitinib versus sunitinib [12] and the JAVELIN Renal 101 trial of avelumab+axitinib versus sunitinib [13] each had a median participant age of 62. For patients under age 65, the HR for death was 0.47 (95% CI 0.30– 0.73) and 0.6 (95% CI 0.44– 0.81) for the KEYNOTE and JAVELIN trials respectively, while for patients age 65 or older the HR for death was 0.59 (95% CI 0.36– 0.97) and 0.71 (95% CI 0.46– 1.09), respectively. These studies highlight the need for increased enrollment of older patients on clinical trials to better understand the efficacy of new treatments in the geriatric population.
This study has several limitations owing to the use of a large patient database. First, NCDB data are only available through 2016, prohibiting the analysis of trends in immunotherapy use after approval of first-line ipilimumab plus nivolumab for intermediate or poor risk mRCC in 2018 and approval of combination ICI+targeted therapy in all risk groups in 2019. Information on patient risk group is also unavailable, although the approval of second-line nivolumab monotherapy applies to all risk groups. Patient nephrectomy status was included as a covariate in our model, however the NCDB specifically reports initial therapy and so we were unable to evaluate receipt of delayed cytoreductive nephrectomy by patient age. Additionally, the NCDB does not specify the exact therapy received, and so it is possible that some of the patients who received immunotherapy were treated with HD-IL2, rather than an ICI. However, HD-IL2 use in the current treatment era is very rare and is primarily restricted to younger patients [14], and so this is unlikely to have a significant effect on our findings. Future studies are required to explore the continued changes in the treatment landscape of mRCC as new therapeutic options are approved at a rapid pace and to further examine the efficacy of these therapies within the elderly population.
CONCLUSIONS
The proportion of patients receiving any systemic treatment for metastatic clear cell RCC has increased from 2006 to 2016. In particular, there has been a high rate of adoption of immune checkpoint inhibitor use among elderly patients, suggesting that the introduction of immune checkpoint inhibitors allowed patients that were previously ineligible for systemic therapy to undergo treatment.
FUNDING
CKO is supported by a National Research Service Award Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by The Cecil G. Sheps Center for Health Services Research, The University of North Carolina at Chapel Hill (grant 5T32H2000032). TLR is supported by the National Cancer Institute at the National Institute of Health K08 Clinical Investigator Award (grant 1K08CA248967-01) and Grant 2015213 from the Doris Duke Charitable Foundation.
AUTHOR CONTRIBUTIONS
Project development: CKO, TLR
Data collection or management: CKO, AMD, TLR
Data analysis: CKO, AMD, MIM, MAB, TLR
Manuscript writing/editing: CKO, AMD, MIM, MAB, TLR
CONFLICT OF INTEREST
All authors have no conflicts of interest to declare related to this manuscript. For COI unrelated to this manuscript:
CKO: None
AMD: None
MIM: Please see the following:
https://coi.asco.org/share/7UQ-6ARQ/Matthew% 20Milowsky
MAB: None
TLR: Research funding to her institution from Merck, GeneCentric
Therapeutics, Bristol-Myers Squibb, and X4 Pharmaceuticals
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | National Cancer Institute. SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer. [Internet]. [cited 2019 Jul 8]. Available from:http://seer.cancer.gov/statfacts/html/kidrhtml. |
[2] | Inamura K , Renal cell tumors: Understanding their molecular pathological epidemiology and the 2016 WHO classification, Vol. 18, International Journal of Molecular Sciences. (2017) . |
[3] | Siegel RL , Miller KD , Jemal A , Cancer statistics, 2019, CA Cancer J Clin [Internet] [cited 2019 Oct 16];69: (1):7–34. Available from: 10.3322/caac.21551. |
[4] | Dabestani S , Thorstenson A , Lindblad P , Harmenberg U , Ljungberg B , Lundstam S , Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study, World J Urol, (2016) ;34: (8):1081–6. |
[5] | Motzer RJ , New Perspectives on the Treatment of Metastatic Renal Cell Carcinoma: An Introduction and Historical Overview, Oncologist. 2011 Feb 1;16: (Supplement 2), 1–3. |
[6] | Rini BI , Campbell SC , Escudier B , Renal cell carcinoma, Lancet [Internet]. 2009 [cited 2019 Oct 16];373: :1119–51. Available from: www.thelancet.com. |
[7] | Ferry EK , Minnillo BJ , Maurice MJ , Abouassaly R , Zhu H , Trends of systemic therapy use for renal cell carcinoma in the United States, Urology, (2015) ;85: (6):1399–403. |
[8] | Mohanty S , Bilimoria KY , Comparing national cancer registries: The National Cancer Data Base (NCDB) and the surveillance, epidemiology, and end results (SEER) program. Vol. 109, Journal of Surgical Oncology. Wiley-Liss Inc:(2014) . p.629–30. |
[9] | Kim HJ , Fay MP , Feuer EJ , Midthune DN , “Permutation tests for joinpoint regression with applications to cancer rates”, Statistics in Medicine, (2000) ;19: :335–351. (correction:2001;20:655). |
[10] | Joinpoint Regression Program, Version 4.8.0.1 – April 2020: Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. |
[11] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus everolimus in advanced renal-cell carcinoma, N Engl J Med. 2015 Nov 5 ;373: (19), 1803–13. |
[12] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med [Internet]. 2019 [cited 2019 Aug 7];380: (12):,1116–,lpage>27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30779529. |
[13] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med. (2019) ;380: (12):1103–15. |
[14] | Allard CB , Gelpi-Hammerschmidt F , Harshman LC , Choueiri TK , Faiena I , Modi P , et al. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States, Urol Oncol Semin Orig Investig. 2015 Nov 1;33: (11),:496.e11–496.e16. |




