Skeletal-Related Events in Patients with Metastatic Renal Cell Carcinoma: A Systematic Review
Abstract
About one-third of advanced renal cell carcinoma (RCC) patients have bone metastases, which subsequently leads to the development of skeletal-related events (SREs), broadly defined to include surgery and radiation to bone, bone pain, pathological fracture, spinal cord compression, or hypercalcemia. The cumulative impact of SREs in RCC has not been well studied. SREs increase morbidity and mortality of RCC patients, although many interventions do significantly reduce their rates of development and improve prognosis. We performed a systematic review from the existing literature in PubMed from January 2002 through September 2019 and summarized the body of evidence regarding the development, prevention, prognosis and treatment of SREs in advanced RCC patients.
INTRODUCTION
Renal cell carcinoma (RCC) is the sixth most common cancer diagnosed in men and the tenth most common cancer diagnosed in women worldwide [1]. The World Health Organization estimates there are over 175,000 deaths annually [2]. Distant metastases are present in almost one in five newly diagnosed cases [3]. The most common sites for RCC metastasis are lung, followed by bone and lymph nodes [4].
About one-third of advanced RCC patients have bone metastases (BM) [5], most commonly to the pelvis and lower lumbar spine [6]. The biology of bone metastasis is a complex process involving tumor, bone, and the immune system, which all produce cytokines, growth factors, and hormones promoting seeding in bone [7]. Notably, the interaction of RANK (receptor activator of NF-kB), RANKL (receptor activator of NF-kB ligand), and osteoprotegerin (OPG), which are regularly involved in bone remodeling, plays a major role [8]. These factors also make most RCC BM osteolytic lesions, which decreases bone integrity [9].
Decreased bone integrity contributes to skeletal-related events (SREs). The definition of SRE is variable in reported studies, yet most authors define it as: 1) surgery to BM, 2) bone pain requiring palliative radiotherapy or surgery, 3) pathological fractures, 4) spinal cord compression (SCC), and 5) hypercalcemia. SREs from different malignancies significantly decrease mobility and impair quality of life in patients as well as increase the health-care burdens in society [10–14].
The overall impact of specific SREs in RCC patients has not been well-studied. In this systematic review from the existing literature from January 2002 through September 2019, we assess the development, prevention, prognosis and treatment of the different categories of SREs caused by RCC.
METHODS
Search strategy
A systematic literature search was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [15] to identify studies reporting on SREs of RCC between January 2002 and August 2019. The PubMed database was searched using one or combinations of the following keywords: “kidney cancer,” “renal cell carcinoma,” “bone metastases,” “skeletal metastases,” “skeletal related events,” “pathological fracture,” “impending fracture,” “bone-directed targeted therapies,” “cord compression,” and “hypercalcemia.” The selection process was conducted in two stages by the first and second authors independently: the first stage was performed via initial screening of the title and abstract to identify eligible publications. The second stage was done via full-text reading including a manual search of publications in journals not listed in PubMed to further avoid missing any eligible study.
For this systematic review, we excluded (I) non-English articles, (II) non-original articles (i.e. review articles with or without systematic review or meta-analysis), (III) editorials or case reports and (IV) repeated publications on the same cohort to avoid publication bias. The first and second authors individually performed initial screening of the title and abstract to identify eligible publications, then double-checked. Afterwards, full-text reading was performed by individual authors to narrow the number of manuscripts relevant to this review.
Data extraction
The following variables were extracted: subtype of renal cell carcinoma, palliative radiotherapy for bone pain, bone metastases and fractures requiring surgical intervention, spinal cord compression, hypercalcemia, and prevention of SREs with bone-modifying agents and molecular inhibitors.
Outcome measures
The rate of development of SREs in RCC and the outcomes of the SREs were the primary objective. A total of 187 manuscripts originally met criteria based on title, abstract, and references from other review articles. After screening, 54 manuscripts were included for this review (Fig. 1). The outcome data are reported by individual SRE; thus, each section may include data from the same publication.
Fig. 1
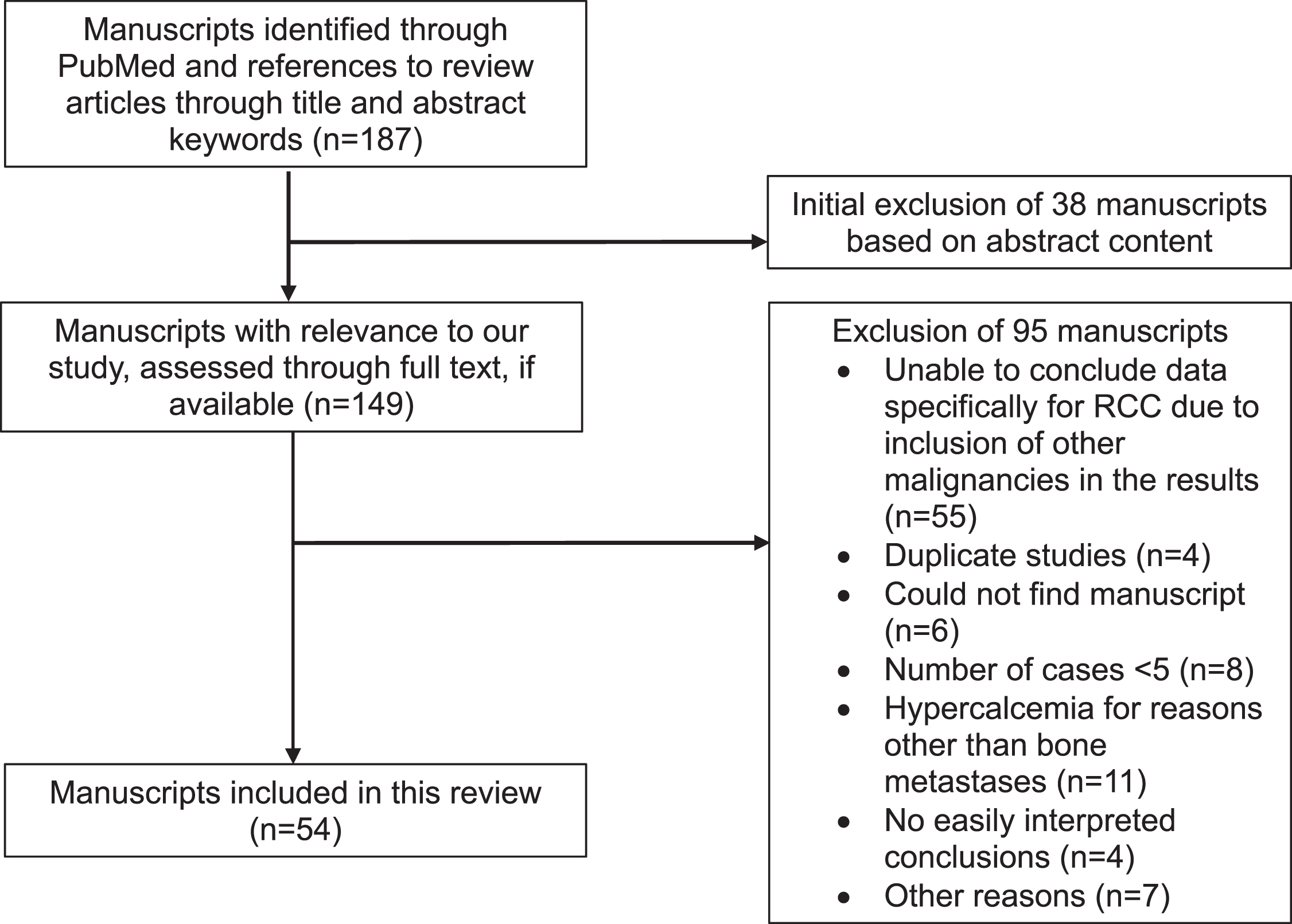
RESULTS
Bone metastases and pathological fractures requiring surgical intervention
The rate of surgical intervention for those with BM in RCC (24%) was significantly higher than in prostate and urothelial cancers from a retrospective study of 650 patients with genitourinary cancers at eleven affiliated hospitals over a ten-year period in Japan, according to Owari et al. [16]. Despite the reported increase in surgical intervention, another study found that at one tertiary care center in the United Kingdom, surgery to bone and fracture as the first SRE was associated with better prognosis [5]. Kitamura et al. [17] found those who received surgery had a significantly prolonged median overall survival (OS) of 54.3 months versus 10.4 months without surgery (p = 0.0002). Du et al. [18] further noted those who underwent BM resection (33 patients) had longer OS than resection of any other site such as other organs (22 patients), with a median OS of 39.1 months versus 8.3 months, respectively.
The prolonged OS associated with surgery for BM may have been impacted by numerous factors, including RCC histology (clear cell versus non-clear cell type), the number and location of BM, the type of surgery itself, and comorbid conditions. Higuchi et al. [19] found that surgery for non-clear cell patients carried a worse prognosis with a median OS of 28 months versus 127 months for clear cell (p = 0.01), albeit with a limited sample size of 5 non-clear cell and 49 clear cell patients. Similarly, Lin et al. [20] found that one year after surgery, the OS rate was 51% for clear cell (248 patients) versus 25% for non-clear cell (47 patients).
The nature of the BM themselves also carry prognostic value. Fottner et al. [21] found that among 27 patients with solitary bone metastasis, 20 with multiple BM, and 54 with concomitant bone and visceral metastases, the solitary bone metastasis had significantly better survival outcomes after BM surgery (60 months versus 30 months for multiple BM versus 12 months for visceral metastases, p < 0.001). Higuchi et al. [19] similarly concluded that metastasis to more than two sites were independent risk factors for a poor prognosis with a hazard ratio of 3.34 (CI 1.39 to 8.05, p = 0.007). The improved prognostic outcomes with resection of solitary BM have been corroborated through other studies [20, 22–26].
One controversial prognostic factor is the location of the BM. Higuchi et al. [19] further noted that the median OS after surgery was shortest for lesions in the pelvis compared to lesions in the spine or appendicular skeleton. Another study found that BM in the pelvic region were significant factors for both increased blood loss and poor performance status after surgery [27]. Yet, a third study found that axial location of metastases was associated with decreased OS [24]. However, two studies did not find a survival difference after surgical treatment based on the location of the BM [21, 25].
The type of operation also has debatable prognostic value. En bloc resection was described as a safe and effective treatment, with a five-year OS rate of 83% [28]. One retrospective study which included 183 patients (88 underwent metastasectomy, 54 had intralesional curettage, and 41 had stabilization only) found that the local recurrence rate was highest after stabilization only (39%), followed by intralesional curettage (22%) and metastasectomy (12%) (p = 0.003) [29]. Survival was better in patients who underwent metastasectomy (p = 0.020), which increased further with negative margins (p < 0.001); however, after considering only patients with solitary metastasis (44/183), there was no survival difference (p = 0.997). One study found no difference in OS between en bloc resection and curettage [30]. Another study also found no difference in survival between the three procedure types [23].
Blood loss is a troubling complication of surgery for BM in RCC, given the highly vascular nature of the procedure. Preoperative embolization has been traditionally used to reduce bleeding risk, but more recent studies cast doubt on its utility. Jernigan et al. [31] found that among 1285 patients with femur metastasis who underwent stabilization, there was no difference in blood transfusion percentage between preoperative embolization (41/135 = 30%) and the control group (359/1150 = 31%). Two other studies found tumor size, rather than embolization itself, was a more important indicator of intraoperative blood loss [32, 33].
Bone pain requiring intervention
Diverse strategies have been attempted to relieve bone pain, including radiotherapy (RT) (most frequently), radiofrequency ablation (RFA), stereotactic radiotherapy (SBRT), embolization, cementoplasty, and interleukin therapy, either as solo use or in different combinations.
Irradiating RCC BM could provide adequate pain relief, with average dosage generally ranging from 20–50 Gray (Gy). In Reichel et al. [34], 28 patients underwent irradiation to 36 sites (median total dosage 30 Gy) throughout the body (mostly along the spine); 25 sites did not need repeat RT, but the palliation was short term, as median time for pain to return to pre-RT levels was only two months. In Wilson et al. [35], 78 RCC patients received 143 palliative RT treatments (median dosage 20 Gy), in which 72 treatments were for bone pain at unspecified locations; overall, the authors concluded the palliative response using RT to bone was better than to other sites, such as the brain and lung. Similarly, from a phase 2 trial of palliative RT, 24 patients received RT for bone pain (unspecified locations), and 30 Gy in 10 fractions resulted in a significant response rate and pain relief measured by the modified McGill-Melzack scale [36]. Radiation (total dosage between 45–50 Gy) combined with immunochemotherapy (IL-2, IFN-alpha, and 5-fluorouracil) in 16 patients also provided remarkable pain relief [37]. However, another study of 19 RCC patients (9 were clear cell subtype) received IL-2 treatment to help relieve pain at various BM, with some patients receiving RT and/or surgery as well, and most patients continued to require analgesics afterwards [38].
Other successful methods of relieving bone pain in metastatic RCC include SBRT, embolization, cementoplasty and RFA. Jhaveri et al. [39] determined in 18 patients with clear cell RCC (total of 24 lesions to the spine, ribs, clavicles and pelvis), the most effective dose of SBRT for pain resolution was 40 Gy in 5 fractions, and there was a relationship between increased radiation dose and shorter time to stable pain relief. From a study of 107 patients receiving 163 embolizations using N-2-butyl cyanoacrylate to various sites (pelvis and vertebral column combined for over half), 157 had a clinical response with pain relief occurring within seven days [40]. Thirty transcatheter embolization procedures were used for 21 patients to treat 39 metastatic bone lesions (various locations, nearly half were pelvis), and 36 of the 39 sites achieved a clinical response lasting on average 5.5 months [41]. Five patients received cementoplasty for painful extraspinal BM (to the femur, acetabulum, and humerus), and for all cases, the visual acuity score (VAS) decreased immediately after treatment through an average of six months on follow-up [42]. Combinations of these techniques have been employed as well. Both cementoplasty and percutaneous RFA applied to 6 RCC patients with 9 painful BM (humerus, pelvis, and spine locations) had a success rate of 100% with pain relief [43]. Pellerin et al. [44] analyzed 52 patients with 58 pelvic lesions using embolization, RFA, and cementoplasty; all procedures were successful, with a significant decrease in the VAS score at discharge along with one-month and six-month follow-up compared to the pre-procedure baseline VAS score.
Finally, cryoablation can also be used in such cases; however, the data in RCC is very limited and did not meet the inclusion criteria of this analysis [45, 46].
Spinal cord compression (SCC)
Over a ten-year period, out of 254 RCC patients in Europe (212 clear cell, 19 sarcomatoid, 7 poorly/undifferentiated, 4 chromophobe, 3 papillary, 2 unclassified, 2 other, 5 diagnosed radiologically), 68 (27%) developed SCC. Three other studies were from Asia. Over a five-year period, out of 94 RCC patients with BM in Yokomizo et al. [47], 13 patients (14%) developed SCC. Owari et al. [48] tracked 43 RCC patients with BM, and 10% of them developed SCC. Huang et al. [49] specifically documented 106 clear cell RCC patients with BM, and 46 (43%) developed SCC.
Three studies tried to identify potential prognostic factors associated with SCC [50–52]. In the study by Rades et al. [51] that included 71 elderly RCC patients with SCC who had received RT for relief; the authors developed a tool to best predict six-month survival rates, incorporating data of the time interval from RCC diagnosis to SCC diagnosis, number of visceral metastases at the time of RT, gait function at the time of RT, the time to developing motor deficits before RT, and ECOG performance. Similarly, in 30 clear cell RCC patients with SCC who received surgery, a prognostic (Tokuhashi) score included the patient’s general condition, the number of spinal and extraspinal bone metastases, presence of visceral metastases, primary site of the cancer, and the severity of spinal cord palsy, with the most favorable having a score greater than 10 [52, 53].
Many different therapeutic methods have been used in SCC. Notably, 21 patients who received decompressive surgery for SCC in kidney cancer had increased survival after the operation relative to other cancers, including lung, breast, and prostate [54]. In 25 patients with SCC who received preoperative embolization, it was noted greater embolization trended towards more blood loss, which suggested more extensive cord compression [55]. Six non-ambulatory patients, who received transcatheter arterial embolization then decompressive surgery plus stabilization of the vertebrae involved, had safely and significantly improved ambulatory function [56]. Another study by Rades et al. [57] compared 25 patients receiving short-course RT versus 87 receiving long-course RT; because both had a similar functional outcome, the short course of 1×8 Gy was the most recommended. Wilson et al. [35] analyzed 143 palliative RT treatment sites, 10 of which were for SCC, with variable success ranging from complete response to no response.
Hypercalcemia
Four manuscripts collected information on the number of cases of hypercalcemia secondary to BM in RCC. Woodward et al. [5] noted 31 patients (12%) of 254 RCC patients with BM (212 were clear cell) to have hypercalcemia. Yokomizo et al. [47] reviewed records of 511 genitourinary cancers with BM, including 94 RCC patients (56 of which were clear cell), and 10 of the 94 patients (11%) developed hypercalcemia, compared to metastatic prostate cancer (8/351 = 2%), bladder cancer (2/41 = 5%), and urothelial cancer (3/25 = 12%). Owari et al. [48] reviewed records of 180 genitourinary cancers with BM (43 RCC, 111 prostate cancer, 26 urothelial cancer). Up to 87 of the 180 received either zoledronic acid or denosumab, but it was unclear how many RCC patients received this intervention. As a whole, the frequency of hypercalcemia in the RCC subgroup was 10% (compared to 3% for prostate cancer, and 20% for urothelial cancer). Guillot et al. [58] reported 41 RCC patients (40 of which were clear cell carcinoma) treated with denosumab and an anti-angiogenic therapy; five cases (12%) developed hypercalcemia (one of whom also developed osteonecrosis of the jaw).
Prevention of SREs
Multiple studies have concluded potential benefits of bisphosphonates to reduce incidence of SREs and to prolong OS in bone metastatic RCC. Woodward et al. [5] conducted a comprehensive study that found a decrease of approximately 24% in the number of SREs in 53 patients with metastatic RCC who received multiple doses of bisphosphonate (pamidronate, zoledronic acid, clodronate, or alendronate) compared to 28 patients who received a single dose. Similarly, Lipton et al. [59] conducted a study with 74 RCC patients and compared outcomes of zoledronic acid versus placebo in addition to antineoplastic treatment and found that zoledronic acid significantly reduced the number of SREs. The mean skeletal morbidity rate (events per year), time to first SRE event, and median time to progression also improved. According to Santini et al. [60], RCC patients who received zoledronic acid had a median survival time of 15 months after BM diagnosis compared to seven months for patients without this agent, and there was a significant delay in time to first SRE from diagnosis in patients who received bisphosphonate compared to those who did not receive treatment. Yasuda et al. [61] found a longer OS in 23 patients who had received zoledronic acid compared to 22 patients who did not (80.8% versus 59.1% survival at one year, p = 0.0034).
Zoledronic acid has additionally been studied along with other treatment agents to evaluate its efficacy. Zoledronic acid was found to improve outcomes of patients who received this treatment in addition to RT compared to RT alone (although not always statistically significant) [62–64]. Hosaka et al. [64] demonstrated that treatment with sunitinib with the bisphosphonate improved the post-irradiation SRE-free rate. Zoledronic acid was also shown to work synergistically with everolimus, increasing median progression-free survival and median time to first SRE [65]. Other regimen combinations included zoledronate with statins, which did not demonstrate any significant changes in SREs in an eleven-patient group [66], and zoledronate, thalidomide, and interferon-gamma, which together was well-tolerated and potentially clinically beneficial [67].
However, other studies achieved different conclusions regarding efficacy of bisphosphonates. In a study by McKay et al. [68], in 2,749 patients who were treated with different angiogenic therapies (sunitinib, sorafenib, axitinib), temsirolimus or interferon-alpha, 28% had bone metastasis. The use of bisphosphonates was not associated with improved OS (13.3 versus 13.1 months, respectively; p = 0.3801), improved progression free survival (5.1 versus 4.9 months, respectively; p = 0.1785), or decreased rate of SREs (8.6% versus 5.8%, respectively; p = 0.191). Bisphosphonate use was also noted to be associated with increased hypocalcemia, renal insufficiency, and osteonecrosis of the jaw (p < 0.0001). The combination of a bisphosphonate and targeted therapy (sunitinib, sorafenib, bevacizumab, temsirolimus, everolimus, pazopanib, or IL-2–based immunotherapy) did provide clinical efficacy [69, 70], although it was observed to cause higher rates of osteonecrosis of the jaw and therefore warrants consideration of oral and maxillofacial exams before and during treatment [70].
Besides bisphosphonates, other agents have been studied that decrease the rate of SREs, such as denosumab. Denosumab, which has been used to reduce SREs in other malignancies including breast cancer, might have a more pronounced toxic profile when combined with anti-angiogenic therapy, as noted in Guillot et al. where 7 of 41 RCC patients receiving denosumab and a tyrosine kinase inhibitor together developed osteonecrosis of the jaw [58].
Finally, in a post hoc analysis of the METEOR trial, where 253 RCC patients receiving cabozantinib were compared to 263 patients treated with everolimus, those who received cabozantinib presented with a lower rate of SREs (23% compared to 29%) [71].
DISCUSSION
Overall, the diversity and number of studies included in our literature review limits the ability to definitively conclude the rate of development of SREs in patients with metastatic RCC to the bone, but it is evident that SREs play a major role in morbidity and quality of life for these patients. There may be an increased rate in the development of SREs in RCC relative to other genitourinary cancers [16, 47], suggesting an increased awareness for these events is needed for these patients and their health care providers. Many different interventions for SREs including surgery, radiation, cementoplasty, embolization, bone modifying-agents, and molecular inhibitors have been investigated.
For patients with BM and pathological fractures, surgical intervention may provide a better prognosis and longer median OS under favorable conditions. Factors that improved surgical outcomes include clear-cell histology and solitary bone metastasis. Conversely, non-clear cell histology and metastasis to multiple sites were poor risk factors. The location of the BM and type of surgery has debatable prognostic value based on current literature.
Clear cell RCC appears to have a better prognosis than non-clear cell RCC from the standpoint of outcomes from SREs. The data is sparse, as few studies included in this review broke down the subtypes of RCC, and even fewer commented on the differences in outcomes between clear cell versus non-clear cell RCC. More research needs to be done in this area tailored towards distinguishing the many subtypes of RCC in terms of the rate of BM, prognosis and treatment outcomes.
Successful strategies implemented to counteract bone pain in RCC include irradiation of BM by SBRT, RFA, embolization, and cementoplasty. Radiation implemented together with other strategies including interleukins and surgery also have demonstrated successful pain management. However, most of these studies had small sample sizes, and studies with larger cohorts should be performed to better assess these methods.
Similarly, the use of RT, bisphosphonates, small-molecule inhibitors, surgery, and embolization have all been attempted to prevent the number of cases of malignant SCC or to emergently relieve them when they occur, with mixed results. It is possible these may be applied to decrease their morbidity and mortality, but more studies need to be done on preventing SCC in RCC patients.
Hypercalcemia is a known negative prognostic risk factor by the Memorial Sloan-Kettering Cancer Center (MSKCC) and the International Metastatic RCC Database Consortium (IMDC) [72, 73]. Bone-modifying agents such as bisphosphonates have been used in the treatment of malignant hypercalcemia and BM in several carcinomas including lung, breast, and prostate cancers, but they may be underused in patients with RCC [74]. In addition, in other solid tumors such as breast cancer, there is data suggesting an association between the expression of RANK on circulating tumor cells and denosumab effectiveness [75]. Despite the absence of definitive efficacy data and potentially significant side effects –such as osteonecrosis of the jaw –their use in RCC is associated with a reduction in the incidence of SREs and may be associated with a survival benefit.
Research continues for the molecular mechanisms of metastatic tumors reaching bone, which may lead to better treatments against the formation of BM in the first place. Metastatic bone lesions occur through a complex process in which cancer cells occupy bone erythropoietic system and induces immune cells to release factors that attract and stimulate osteoclasts [74]. Bisphosphonates oppose this process to some extent by reducing osteoclast development from precursors, disrupting bone resorption, inhibiting angiogenesis, and reducing interleukin-6 production from bone stromal cells [66]. The receptor c-MET has been noted to be overexpressed in clear cell RCC [76], and c-MET signaling appears to play a role in bone metastasis [77]. The use of a c-MET inhibitor on RCC stem cells injected into bone inhibited the development of bone metastasis [78]. Cabozantinib is a small molecule kinase inhibitor against MET, VEGFR2, and other receptor tyrosine kinases [79]. As mentioned previously, cabozantinib decreased the number of SREs compared to everolimus in the METEOR trial, likely through affecting osteoblast and osteoclast activity through RANK, RANKL, and OPG [80]. Because RCC has been found to have RANKL and RANK mRNA correlate positively with primary tumor stage, and elevated RANKL and RANK expression was found to be a significant predictor for bone metastasis, additional insight into their interactions may shed further light into new inhibitors [81].
In addition, further research will continue to explore new treatment combinations of available drugs and strategies along with novel therapies to combat the development and minimizing the poor prognoses of SREs in RCC. Several clinical studies specifically investigating these questions are currently underway. A phase 2 study (RadiCaL study, NCT04071223) is testing the addition of radium-223 dichloride (an α-emitting radionuclide that successfully delays time to SREs in metastatic prostate cancer [82, 83]) to cabozantinib in metastatic advanced RCC to the bone. A different phase 2 study (KEYPAD, NCT03280667) is combining pembrolizumab and denosumab in clear cell RCC patients. A larger phase 3 study (MOSCAR, NCT03408652) will assess the efficacy and safety of denosumab and zoledronic acid for BM in RCC patients treated with targeted therapies. Other studies include bone pain as an endpoint in their clinical designs (e.g., NCT00920816).
The strengths of this literature review include an exhaustive search to examine the different broad categories of SREs specifically focused on RCC in peer-reviewed published literature over the last two decades. We also reviewed therapies and medications that increase the time to development of SREs and prevent their incidence and summarize the areas that need further research in order to gain a better understanding of these SREs.
By contrast, the limitations of this study include the heterogeneity of the patient population, the lack of prospective randomized studies, and the paucity of data specifically in RCC. Manuscripts that studied the rate of development of SREs or the rate for needing intervention (specifically for SCC and hypercalcemia) were included, but if specific studies analyzed fewer than five cases of the SRE of interest (especially surgical or bone pain relief cases), these were excluded due to the low significance. Finally, the financial burden of SREs in patients with RCC was not addressed, including the length of stay of hospital admissions and costs of procedures and medications.
In summary, SREs negatively impact RCC patients with BM. Diverse therapies exist for their prevention and treatment, but improved clinical outcomes must be weighed against adverse effects. More studies are needed as several gaps of knowledge exist, including the prognoses of different subtypes of RCC leading to SREs and the continued search for better prevention and treatment strategies. Current clinical trials are underway to study novel ideas and different combinations to minimize occurrences of SREs and improve prognosis.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
AJ, SRC: performance, interpretation of data; JX, MAB: interpretation of data; PCB: conception, interpretation of data.
CONFLICT OF INTEREST
MAB has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Genomic Health, Nektar, and Sanofi and has received grants to his institution from Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peleton Therapeutics, and Pfizer for work performed as outside of the current study.
PCB has served in a consulting or advisory role for Bayer, Bristol-Myers Squibb, Pfizer, EMD Sorono, Eisai, Caris Life Sciences, Clovis, and Dendreon. PB has also received research funding from Blue Earth Diagnostics.
The other authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-200087.
REFERENCES
[1] | Capitanio U , Bensalah K , Bex A , Boorjian SA , Bray F , Coleman J , et al. Epidemiology of Renal Cell Carcinoma. Eur Urol. (2019) ;75: (1):74–84. |
[2] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) ;68: (6):394–424. |
[3] | Capitanio U , Montorsi F . Renal cancer. Lancet. (2016) ;387: (10021):894–906. |
[4] | Bianchi M , Sun M , Jeldres C , Shariat SF , Trinh QD , Briganti A , et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. (2012) ;23: (4):973–80. |
[5] | Woodward E , Jagdev S , McParland L , Clark K , Gregory W , Newsham A , et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. (2011) ;48: (1):160–6. |
[6] | Forbes GS , McLeod RA , Hattery RR . Radiographic manifestations of bone metastases from renal carcinoma. AJR Am J Roentgenol. (1977) ;129: (1):61–6. |
[7] | Chen SC , Kuo PL . Bone Metastasis from Renal Cell Carcinoma. Int J Mol Sci. (2016) ;17: (6). |
[8] | Ando K , Mori K , Redini F , Heymann D . RANKL/RANK/OPG: key therapeutic target in bone oncology. Curr Drug Discov Technol. (2008) ;5: (3):263–8. |
[9] | Roodman GD . Mechanisms of bone metastasis. N Engl J Med. (2004) ;350: (16):1655–64. |
[10] | Yuasa T , Urakami S . Kidney cancer: decreased incidence of skeletal-related events in mRCC. Nat Rev Urol. (2014) ;11: (4):193–4. |
[11] | Kinnane N . Burden of bone disease. Eur J Oncol Nurs. (2007) ;11: Suppl 2:S28–31. |
[12] | Antczak C , Trinh VQ , Sood A , Ravi P , Roghmann F , Trudeau V , et al. The health care burden of skeletal related events in patients with renal cell carcinoma and bone metastasis. J Urol. (2014) ;191: (6):1678–84. |
[13] | Hagiwara M , Delea TE , Saville MW , Chung K . Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. (2013) ;16: (1):23–7. |
[14] | Roghmann F , Antczak C , McKay RR , Choueiri T , Hu JC , Kibel AS , et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol. (2015) ;33: (1):17.e9–.e8. |
[15] | Moher D , Shamseer L , Clarke M , Ghersi D , Liberati A , Petticrew M , et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement. Syst Rev. (2015) ;4: :1. |
[16] | Owari T , Miyake M , Nakai Y , Hori S , Tomizawa M , Ichikawa K , et al. Clinical benefit of early treatment with bone-modifying agents for preventing skeletal-related events in patients with genitourinary cancer with bone metastasis: A multi-institutional retrospective study. Int J Urol. (2019) ;26: (6):630–7. |
[17] | Kitamura H , Takahashi A , Takei F , Hotta H , Miyao N , Shindo T , et al. Molecular-targeted Therapy and Surgery May Prolong Survival of Renal Cell Carcinoma Patients with Bone Metastasis: A Multi-institutional Retrospective Study in Japan. Anticancer Res. (2016) ;36: (10):5531–6. |
[18] | Du Y , Pahernik S , Hadaschik B , Teber D , Duensing S , Jager D , et al. Survival and prognostic factors of patients with renal cell cancer with bone metastasis in the era of targeted therapy: A single-institution analysis. Urol Oncol. (2016) ;34: (10):433e1–8. |
[19] | Higuchi T , Yamamoto N , Hayashi K , Takeuchi A , Abe K , Taniguchi Y , et al. Long-term patient survival after the surgical treatment of bone and soft-tissue metastases from renal cell carcinoma. Bone Joint J. (2018) ;100-b(9):1241–8. |
[20] | Lin PP , Mirza AN , Lewis VO , Cannon CP , Tu SM , Tannir NM , et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am. (2007) ;89: (8):1794–801. |
[21] | Fottner A , Szalantzy M , Wirthmann L , Stahler M , Baur-Melnyk A , Jansson V , et al. Bone metastases from renal cell carcinoma: patient survival after surgical treatment. BMC Musculoskelet Disord. (2010) ;11: :145. |
[22] | Petteys RJ , Spitz SM , Rhee J , Goodwin CR , Zadnik PL , Sarabia-Estrada R , et al. Tokuhashi score is predictive of survival in a cohort of patients undergoing surgery for renal cell carcinoma spinal metastases. Eur Spine J. (2015) ;24: (10):2142–9. |
[23] | Fuchs B , Trousdale RT , Rock MG . Solitary bony metastasis from renal cell carcinoma: significance of surgical treatment. Clin Orthop Relat Res. (2005) (431):187–92. |
[24] | Jung ST , Ghert MA , Harrelson JM , Scully SP . Treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop Relat Res. (2003) (409):223–31. |
[25] | Szendroi A , Dinya E , Kardos M , Szasz AM , Nemeth Z , Ats K , et al. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol Oncol Res. (2010) ;16: (1):29–38. |
[26] | Kato S , Murakami H , Demura S , Nambu K , Fujimaki Y , Yoshioka K , et al. Spinal metastasectomy of renal cell carcinoma: A 16-year single center experience with a minimum 3-year follow-up. J Surg Oncol. (2016) ;113: (5):587–92. |
[27] | Yoshiyama A , Morii T , Susa M , Morioka H , Kobayashi E , Asano N , et al. Preoperative evaluation of renal cell carcinoma patients with bone metastases on risks for blood loss, performance status and lethal event. J Orthop Sci. (2017) ;22: (5):924–30. |
[28] | Assouad J , Masmoudi H , Berna P , Steltzlen C , Radu D , Riquet M , et al. Isolated rib metastases from renal cell carcinoma. Interact Cardiovasc Thorac Surg. (2010) ;10: (2):172–5. |
[29] | Langerhuizen DW , Janssen SJ , van der Vliet QM , Raskin KA , Ferrone ML , Hornicek FJ , et al. Metastasectomy, intralesional resection, or stabilization only in the treatment of bone metastases from renal cell carcinoma. J Surg Oncol. (2016) ;114: (2):237–45. |
[30] | Fukushima H , Hozumi T , Goto T , Nihei K , Karasawa K , Nakanishi Y , et al. Prognostic significance of intensive local therapy to bone lesions in renal cell carcinoma patients with bone metastasis. Clin Exp Metastasis. (2016) ;33: (7):699–705. |
[31] | Jernigan EW , Tennant JN , Esther RJ . Not All Patients Undergoing Stabilization of Impending Pathologic Fractures for Renal Cell Carcinoma Metastases to the Femur Need Preoperative Embolization. Clin Orthop Relat Res. (2018) ;476: (3):529–34. |
[32] | Rehak S , Krajina A , Ungermann L , Ryska P , Cerny V , Talab R , et al. The role of embolization in radical surgery of renal cell carcinoma spinal metastases. Acta Neurochir (Wien). (2008) ;150: (11):1177–81; discussion 81. |
[33] | Ratasvuori M , Sillanpaa N , Wedin R , Trovik C , Hansen BH , Laitinen M . Surgery of non-spinal skeletal metastases in renal cell carcinoma: No effect of preoperative embolization? Acta Orthop. (2016) ;87: (2):183–8. |
[34] | Reichel LM , Pohar S , Heiner J , Buzaianu EM , Damron TA . Radiotherapy to bone has utility in multifocal metastatic renal carcinoma. Clin Orthop Relat Res. (2007) ;459: :133–8. |
[35] | Wilson D , Hiller L , Gray L , Grainger M , Stirling A , James N . The effect of biological effective dose on time to symptom progression in metastatic renal cell carcinoma. Clin Oncol (R Coll Radiol). (2003) ;15: (7):400–7. |
[36] | Lee J , Hodgson D , Chow E , Bezjak A , Catton P , Tsuji D , et al. A phase II trial of palliative radiotherapy for metastatic renal cell carcinoma. Cancer. (2005) ;104: (9):1894–900. |
[37] | Brinkmann OA , Bruns F , Gosheger G , Micke O , Hertle L . Treatment of bone metastases and local recurrence from renal cell carcinoma with immunochemotherapy and radiation. World J Urol. (2005) ;23: (3):185–90. |
[38] | Adiga GU , Dutcher JP , Larkin M , Garl S , Koo J . Characterization of bone metastases in patients with renal cell cancer. BJU Int. (2004) ;93: (9):1237–40. |
[39] | Jhaveri PM , Teh BS , Paulino AC , Blanco AI , Lo SS , Butler EB , et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta Oncol. (2012) ;51: (5):584–8. |
[40] | Rossi G , Mavrogenis AF , Casadei R , Bianchi G , Romagnoli C , Rimondi E , et al. Embolisation of bone metastases from renal cancer. Radiol Med. (2013) ;118: (2):291–302. |
[41] | Forauer AR , Kent E , Cwikiel W , Esper P , Redman B . Selective palliative transcatheter embolization of bony metastases from renal cell carcinoma. Acta Oncol. (2007) ;46: (7):1012–8. |
[42] | Basile A , Giuliano G , Scuderi V , Motta S , Crisafi R , Coppolino F , et al. Cementoplasty in the management of painful extraspinal bone metastases: our experience. Radiol Med. (2008) ;113: (7):1018–28. |
[43] | Toyota N , Naito A , Kakizawa H , Hieda M , Hirai N , Tachikake T , et al. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. (2005) ;28: (5):578–83. |
[44] | Pellerin O , Medioni J , Vulser C , Dean C , Oudard S , Sapoval M . Management of painful pelvic bone metastasis of renal cell carcinoma using embolization, radio-frequency ablation, and cementoplasty: a prospective evaluation of efficacy and safety. Cardiovasc Intervent Radiol. (2014) ;37: (3):730–6. |
[45] | Susa M , Kikuta K , Nakayama R , Nishimoto K , Horiuchi K , Oguro S , et al. CT guided cryoablation for locally recurrent or metastatic bone and soft tissue tumor: initial experience. BMC Cancer. (2016) ;16: (1):798. |
[46] | Gardner CS , Ensor JE , Ahrar K , Huang SY , Sabir SH , Tannir NM , et al. Cryoablation of Bone Metastases from Renal Cell Carcinoma for Local Tumor Control. J Bone Joint Surg Am. (2017) ;99: (22):1916–26. |
[47] | Yokomizo A , Koga H , Shinohara N , Miyahara T , Machida N , Tsukino H , et al. Skeletal-related events in urological cancer patients with bone metastasis: a multicenter study in Japan. Int J Urol. (2010) ;17: (4):332–6. |
[48] | Owari T , Miyake M , Nakai Y , Morizawa Y , Itami Y , Hori S , et al. Clinical Features and Risk Factors of Skeletal-Related Events in Genitourinary Cancer Patients with Bone Metastasis: A Retrospective Analysis of Prostate Cancer, Renal Cell Carcinoma, and Urothelial Carcinoma. Oncology. (2018) ;95: (3):170–8. |
[49] | Huang Z , Du Y , Zhang X , Liu H , Liu S , Xu T . Clear cell renal cell carcinoma bone metastasis: What should be considered in prognostic evaluation. Eur J Surg Oncol. (2019) ;45: (7):1246–52. |
[50] | Morgen SS , Lund-Andersen C , Larsen CF , Engelholm SA , Dahl B . Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of patients. Spine (Phila Pa 1976). (2013) ;38: (16):1362–7. |
[51] | Rades D , Conde-Moreno AJ , Cacicedo J , Szegedin B , Schild SE . Estimating the Survival of Elderly Patients with Renal Cell Carcinoma Presenting with Malignant Spinal Cord Compression. Anticancer Res. (2016) ;36: (1):409–13. |
[52] | Tokuhashi Y , Matsuzaki H , Toriyama S , Kawano H , Ohsaka S . Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). (1990) ;15: (11):1110–3. |
[53] | Han S , Wang T , Jiang D , Yu Y , Wang Y , Yan W , et al. Surgery and survival outcomes of 30 patients with neurological deficit due to clear cell renal cell carcinoma spinal metastases. Eur Spine J. (2015) ;24: (8):1786–91. |
[54] | Chaichana KL , Pendleton C , Sciubba DM , Wolinsky JP , Gokaslan ZL . Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J Neurosurg Spine. (2009) ;11: (1):56–63. |
[55] | Quraishi NA , Purushothamdas S , Manoharan SR , Arealis G , Lenthall R , Grevitt MP . Outcome of embolised vascular metastatic renal cell tumours causing spinal cord compression. Eur Spine J. (2013) ;22: Suppl 1:S27–32. |
[56] | Suzuki H , Kondo T , Kuwatsuru R , Wada K , Kubota M , Kobayashi H , et al. Decompressive surgery in combination with preoperative transcatheter arterial embolization: successful improvement of ambulatory function in renal cell carcinoma patients with metastatic extradural spinal cord compression. Int J Urol. (2011) ;18: (10):718–22. |
[57] | Rades D , Walz J , Stalpers LJ , Veninga T , Schulte R , Obralic N , et al. Short-course radiotherapy (RT) for metastatic spinal cord compression (MSCC) due to renal cell carcinoma: results of a retrospective multi-center study. Eur Urol. (2006) ;49: (5):846–52; discussion 52. |
[58] | Guillot A , Joly C , Barthelemy P , Meriaux E , Negrier S , Pouessel D , et al. Denosumab Toxicity When Combined With Anti-angiogenic Therapies on Patients With Metastatic Renal Cell Carcinoma: A GETUG Study. Clin Genitourin Cancer. (2019) ;17: (1):e38–e43. |
[59] | Lipton A , Zheng M , Seaman J . Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. (2003) ;98: (5):962–9. |
[60] | Santini D , Procopio G , Porta C , Ibrahim T , Barni S , Mazzara C , et al. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis survey. PLoS One. (2013) ;8: (12):e83026. |
[61] | Yasuda Y , Fujii Y , Yuasa T , Kitsukawa S , Urakami S , Yamamoto S , et al. Possible improvement of survival with use of zoledronic acid in patients with bone metastases from renal cell carcinoma. Int J Clin Oncol. (2013) ;18: (5):877–83. |
[62] | Takeda N , Isu K , Hiraga H , Shinohara N , Minami A , Kamata H . Zoledronic acid enhances the effect of radiotherapy for bone metastases from renal cell carcinomas: more than a 24-month median follow-up J Orthop Sci. (2012) ;17: (6):770–4. |
[63] | Kijima T , Fujii Y , Suyama T , Okubo Y , Yamamoto S , Masuda H , et al. Radiotherapy to bone metastases from renal cell carcinoma with or without zoledronate. BJU Int. (2009) ;103: (5):620–4. |
[64] | Hosaka S , Katagiri H , Niwakawa M , Harada H , Wasa J , Murata H , et al. Radiotherapy combined with zoledronate can reduce skeletal-related events in renal cell carcinoma patients with bone metastasis. Int J Clin Oncol. (2018) ;23: (6):1127–33. |
[65] | Broom RJ , Hinder V , Sharples K , Proctor J , Duffey S , Pollard S , et al. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin Genitourin Cancer. (2015) ;13: (1):50–8. |
[66] | Manoukian GE , Tannir NM , Jonasch E , Qiao W , Haygood TM , Tu SM . Pilot trial of bone-targeted therapy combining zoledronate with fluvastatin or atorvastatin for patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. (2011) ;9: (2):81–8. |
[67] | Tannir N , Jonasch E , Pagliaro LC , Mathew P , Siefker-Radtke A , Rhines L , et al. Pilot trial of bone-targeted therapy with zoledronate, thalidomide, and interferon-gamma for metastatic renal cell carcinoma. Cancer. (2006) ;107: (3):497–505. |
[68] | McKay RR , Lin X , Perkins JJ , Heng DY , Simantov R , Choueiri TK . Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol. (2014) ;66: (3):502–9. |
[69] | Ivanyi P , Koenig J , Trummer A , Busch JF , Seidel C , Reuter CW , et al. Does the onset of bone metastasis in sunitinib-treated renal cell carcinoma patients impact the overall survival? World J Urol. (2016) ;34: (7):909–15. |
[70] | Smidt-Hansen T , Folkmar TB , Fode K , Agerbaek M , Donskov F . Combination of zoledronic Acid and targeted therapy is active but may induce osteonecrosis of the jaw in patients with metastatic renal cell carcinoma. J Oral Maxillofac Surg. (2013) ;71: (9):1532–40. |
[71] | Escudier B , Powles T , Motzer RJ , Olencki T , Aren Frontera O , Oudard S , et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J Clin Oncol. (2018) ;36: (8):765–72. |
[72] | Motzer RJ , Mazumdar M , Bacik J , Berg W , Amsterdam A , Ferrara J . Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. (1999) ;17: (8):2530–40. |
[73] | Heng DY , Xie W , Regan MM , Warren MA , Golshayan AR , Sahi C , et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. (2009) ;27: (34):5794–9. |
[74] | Umer M , Mohib Y , Atif M , Nazim M . Skeletal metastasis in renal cell carcinoma: A review. Ann Med Surg (Lond). (2018) ;27: :9–16. |
[75] | Pantano F , Rossi E , Iuliani M , Facchinetti A , Simonetti S , Ribelli G , et al. Dynamic changes of Receptor activator of nuclear factor-kappaB expression in Circulating Tumor Cells during Denosumab predict treatment effectiveness in Metastatic Breast Cancer. Sci Rep. (2020) ;10: (1):1288. |
[76] | Gibney GT , Aziz SA , Camp RL , Conrad P , Schwartz BE , Chen CR , et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. (2013) ;24: (2):343–9. |
[77] | Whang YM , Jung SP , Kim MK , Chang IH , Park SI . Targeting the Hepatocyte Growth Factor and c-Met Signaling Axis in Bone Metastases. Int J Mol Sci. (2019) ;20: (2). |
[78] | D’Amico L , Belisario D , Migliardi G , Grange C , Bussolati B , D’Amelio P , et al. C-met inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget. (2016) ;7: (29):45525–37. |
[79] | Yakes FM , Chen J , Tan J , Yamaguchi K , Shi Y , Yu P , et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. (2011) ;10: (12):2298–308. |
[80] | Fioramonti M , Santini D , Iuliani M , Ribelli G , Manca P , Papapietro N , et al. Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions. Oncotarget. (2017) ;8: (12):20113–21. |
[81] | Mikami S , Katsube K , Oya M , Ishida M , Kosaka T , Mizuno R , et al. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J Pathol. (2009) ;218: (4):530–9. |
[82] | Sartor O , Coleman R , Nilsson S , Heinrich D , Helle SI , O’Sullivan JM , et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. (2014) ;15: (7):738–46. |
[83] | Deshayes E , Roumiguie M , Thibault C , Beuzeboc P , Cachin F , Hennequin C , et al. Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. (2017) ;11: :2643–51. |




